Abstract
Up till now, research on inflammatory bowel disease [IBD] has mainly been focused on the immune cells present in the gastrointestinal tract. However, recent insights indicate that stromal cells also play an important and significant role in IBD pathogenesis. Stromal cells in the intestines regulate both intestinal epithelial and immune cell homeostasis. Different subsets of stromal cells have been found to play a role in other inflammatory diseases [e.g. rheumatoid arthritis], and these various stromal subsets now appear to carry out also specific functions in the inflamed gut in IBD. Novel potential therapies for IBD utilize, as well as target, these pathogenic stromal cells. Injection of mesenchymal stromal cells [MSCs] into fistula tracts of Crohn’s disease patients is already approved and used in clinical settings. In this review we discuss the current knowledge of the role of stromal cells in IBD pathogenesis. We further outline recent attempts to modify the stromal compartment in IBD with agents that target or replace the pathogenic stroma.
Keywords: Stromal cells, inflammatory bowel disease, MSCs, fibroblasts, stroma
1. Introduction
Inflammatory bowel disease [IBD] incidence is still increasing worldwide, mostly due to an accelerating incidence in newly industrialized countries.1 Although clear progress has been made, the exact pathogenesis of IBD is still poorly understood. The current working model of IBD pathogenesis proposes a dysfunctional epithelial barrier that finally leads to an aberrant immune response to the intestinal bacteria. Recent research demonstrates that, in addition to intestinal epithelial and inflammatory cells, stromal cells play an important role in IBD pathogenesis. So far, therapies for IBD have been mainly focused on the targeting of immune cells, and this has given rise to the development and therapeutic application of a number of biologic therapies, small molecules (like janus kinases inhibitors), and other immunomodulators. Biologic therapies such as anti-TNF-α and anti-IL-23/12 therapies have been successfully introduced into the clinic. However, attempts to block a number of additional cytokine networks, like for example blockage of interferon-γ 2 [IFN-γ] or IL-17A,3 were rather disappointing. With immune-modulating therapies, mucosal healing in Crohn’s disease [CD] is only achieved in ≤45% of patients.4,5 Subsequently, the risk of surgery within 10 years after diagnosis is still 46.6% and 15.6% for, respectively, CD and ulcerative colitis [UC].6 In addition, a definite curative treatment for IBD patients has not yet been discovered. It might be important to develop alternative therapies that target pathogenic stromal cells in IBD, which could probably intervene earlier in the inflammatory cascade and thereby have a better chance of delaying disease progression.
This review will focus on the role that stromal cells, in particular fibroblasts, play in the pathogenesis of IBD, thereby focusing on their role in the inflamed, non-fibrotic intestinal tissue. First, we will describe the current knowledge regarding the function of stromal cells in the healthy intestine. Thereafter, we will discuss the role of activated stromal cells in diseased tissue and highlight the findings in the current literature on stromal cells in IBD, focusing on their interaction with both epithelial cells and immune cells. Finally, the recently discovered opportunities for developing potential therapies pertaining to targeting stromal cells and replacement of stromal cells, via mesenchymal stromal cell [MSC] therapy, will be highlighted.
1.1. Definitions
There seems to be a lack of consensus pertaining to the nomenclature of stromal cells in general. Terms such as ‘stromal cell’, ‘mesenchymal cell’, ‘fibroblast’, and ‘fibroblast-like cell’ are used seemingly interchangeably within and between studies. In this review, we will refer to ‘stromal cells’ as non-hematopoietic, non-epithelial, and non-endothelial cells.7 In general, the most abundant stromal cells are fibroblasts, followed by myofibroblasts, smooth muscle cells, pericytes, and mesenchymal stromal cells. In the human intestine, stromal cells can be detected in all layers of the gut wall, from the mucosa to the serosa. Mostly, stromal cells are defined as being negative for cell surface markers, such as cluster of differentiation [CD]31 [endothelial cells], CD45 [immune cells], keratins, or epithelial cell adhesion molecule [EpCAM; epithelial cells],8–10 while they are positive for the cytoskeletal marker vimentin. Fibroblasts, more specifically, are mostly reported to be positive for collagen [COL] types I and -III, CD90, and fibroblast activation protein [FAP].11–13 However, as we will discuss later in detail, subsets of fibroblasts have been identified that are negative for FAP and CD90, indicating that fibroblasts also form a heterogenous group of cells. Furthermore, fibroblasts are recognizable, through their distinct morphology in vitro, as spindle-shaped cells with a flat nucleus and slender cytoplasmic processes.8 However their morphological properties are more difficult to detect in tissues. MSCs, known for their therapeutic capacity after culture, are defined as CD105-, CD73-, and CD90-positive cells that are able to differentiate [in vitro] into osteoblasts, chondrocytes, and adipocytes.14 For pericryptic myofibroblasts, which show properties of both fibroblasts and smooth muscle cells,15 there is consensus in the nomenclature, since these cells are defined as cells that are vimentin- and alpha smooth muscle actin [α-SMA]–positive, but do not express the smooth muscle cell marker desmin.12
2. Stromal Cells in Intestinal Homeostasis
Most stromal cells in the gut wall derive from the serosal mesothelium, which originates from the mesoderm, during embryonic development.16,17 Furthermore, stromal cells in the inflamed gut may also develop from other cell types through the process of epithelial-to-mesenchymal transition [EMT] or endothelial-to-mesenchymal transition [EndoMT].18–21 Finally, stromal cells, and especially MSCs and circulating fibrocytes, are able to migrate from the bone marrow towards the intestines.22
2.1. The gut stroma
The gut stroma provides structure and form, and primarily consists of stromal cells and extracellular matrix [ECM]. Within the stroma, fibroblasts are mainly known for their role in the production of the ECM by secreting types I, II, and V collagens, and fibronectin, and matrix remodelling through proteolytic enzymes, including matrix metallopreinases [MMPs].23 A well-known complication of excessive ECM production by fibroblasts in IBD is fibrosis. In this review, we will not focus on fibrosis, since excellent reviews have already been published on the role of fibroblasts in fibrosis.24–26 It is, however, an oversimplification to see fibroblasts only as passive matrix-depositing cells, thereby providing epithelial support and tissue structure. Recent literature shows that fibroblasts also play an important role in maintaining tissue homeostasis by their interaction with both epithelial and immune cells.
2.2. Epithelial cell homeostasis
The intestine is covered by a monolayer of epithelial cells. These cells are generated from stem cells in the base of intestinal crypts and then migrate along the crypt lining, while they differentiate into specialized epithelial cells like absorptive enterocytes, goblet cells, enteroendocrine cells, tuft cells, M cells, and Paneth cells.27 They have a rapid turnover, and eventually the mature epithelial cells are shed at the top of the crypt into the lumen, renewing the crypt every 4–5 days.28 Epithelial cell homeostasis is important because epithelial cells form the first line of defence against pathogens, and they are also responsible for the absorption of nutrients.
Myofibroblasts are described as the stromal cells that are important for maintaining epithelial homeostasis. In the human intestine, myofibroblasts are found along the crypts, and they also surround the intestinal stem cell niche is comprised of Lgr5+ stem cells and Paneth cells.29 These myofibroblasts have an important role in the process of intestinal epithelial cell renewal via paracrine interactions.30 Various pathways, such as the Wnt and bone morphogenetic protein [BMP] pathways, are able to modulate stem cell function and differentiation in these intestinal niches.11 Wnt signaling is necessary for maintaining non-differentiated proliferating Lgr5+ stem cells, while BMP signaling antagonizes Wnt signaling signature genes and induces differentiation of epithelial cells.31–34 Multiple studies have shown that myofibroblasts play an important role in both of these pathways by secreting, for example, Wnt ligands and BMP antagonists.11,35,36 Myofibroblasts, specifically in the basal part of the colon crypt, express the BMP antagonists gremlin and noggin, suggesting that they inhibit BMP signaling in the basal crypt regions, yet allow BMP signaling to take place in the upper crypt regions.36 This differential expression of BMP signaling in specific places in the intestinal crypt suggests heterogeneity within the myofibroblast population. Degirmenci and colleagues identified Gli1pos fibroblasts with a close relation to the bases of intestinal crypts in mice to be important for epithelial integrity by production of Wnt and thereby stem cell renewal.37 Another study further subdivided the Gli1pos cells into CD90-positive and -negative fibroblasts.38 Those authors found that CD90pos fibroblasts, in contrast to CD90neg fibroblasts, produce BMP antagonists and Wnt ligands, like gremlin and Wnt2b, and support organoid growth.38 Interestingly, the CD90pos fibroblasts could be further divided in an α-SMA–positive and –negative population. Since myofibroblasts are defined as being α-SMA–positive cells, this suggests that fibroblasts also play a role in epithelial homeostasis and barrier function, which is often disturbed in IBD.37 Moreover, in human samples it was also found that a specific fibroblast population contributes to the maintenance of the epithelial homeostasis.39 This population, identified by CD142 expression, was found close to the epithelial monolayer, and single-cell RNA-sequencing [scRNA-seq] revealed the expression of different BMP and Wnt ligands. Overall, evidence of the specific physical location of these intestinal [myo]fibroblasts, close to the epithelial layer, and their expression of relevant markers, shows that they are able to regulate the function and fate of epithelial progenitors and thereby intestinal epithelial homeostasis.
2.3. Immune cell homeostasis
Besides epithelial cell homeostasis, stromal cells also influence intestinal immune cell homeostasis in the intestine. This is the process in which immune cell responses are in a steady-state condition, because pathogens are recognized and cleared at an early stage without immunogenic responses towards non-pathogenic peptides. The intestinal mucosal immune system consists of a variety of immune cells that reside in the healthy gut, either organized in Peyer’s patches, in lymph nodes, or scattered in the various layers of the gut. Upon encountering foreign proteins, antigen-presenting cells, such as dendritic cells, present the peptides to lymphocytes in the organized immune structures in the gut, which activates and attracts other lymphocytes to the gut.40
Stromal cells influence immune cell homeostasis via direct cell–cell contact with immune cells or through the production of chemokines and cytokines.41 Intestinal fibroblasts are able to produce, for example, interleukin [IL]-6, IL-8, chemokine ligand 2 [CCL2/MCP-1],41–44 and chemokine ligand 5 [CCL5/RANTES].45 CCL2 binds to chemokine receptor 2 [CCR2], mainly expressed by monocytes, whereas CCL5 binds to several receptors, mainly expressed by T cells, and thereby fibroblasts facilitate the recruitment of both myeloid cells and lymphocytes to the site of inflammation. Myofibroblasts and fibroblasts are also able to affect mucosal T cells via direct cell–cell contact. In non-diseased human colonic lamina propria, these stromal cells express programmed death-ligand 1 [PD-L1] and PD-L246, which are immune checkpoints that bind PD1 on T cells during antigen presentation.47 Fibroblasts are able to suppress the proliferation of CD4pos T cells via PD-L1 and PD-L2 and thereby prevent autoimmunity.46 Colonic fibroblasts can also indirectly affect T cells by induction of retinoic acid production in dendritic cells,48 which is able to block T helper [Th]1 and Th17 differentiation and to enhance regulatory T cell [Treg] differentiation. Furthermore, fibroblasts have been described as being part of the innate immune system because of their ability to recognize pathogen invasion or cell damage.13,49,50 They can detect pathogen-associated molecular patterns [PAMPs] and damage-associated molecular patterns [DAMPs] through toll-like receptors [TLRs], which triggers the release of chemokines.51 Indeed, CD90pos fibroblasts are known to express various TLRs.50 By the expression of MHC class II molecules, colonic myofibroblasts are, upon activation, also able to act as non-professional antigen-presenting cells.13,52 Through both MHC class-II expression and the production of prostaglandin E2, human colonic [myo]fibroblasts from non-diseased mucosa have been reported as contributing to the maintenance of colonic immunological tolerance by promoting the expansion of regulatory FOXP3pos T cells [Tregs].53 Together, these observations show that intestinal stromal cells are able to modify the mucosal immune landscape via different pathways. However, some caution and careful interpretation of the data is needed, since most of these studies used allogeneic immune cells and in vitro–cultured stromal cells, which could have gained their activated immunoregulatory phenotype through culturing.
3. Stromal Cells in Diseased Tissue
Upon organ damage, resident stromal cells become activated. In inflammatory diseases, especially in rheumatoid arthritis [RA], there has been more focus on the role of stromal cells in the last decade. In this review, we will use current literature in RA on stromal cells to understand more about the role and function that stromal cells might have in other inflammatory conditions and thereby IBD. RA, characterized by painful swellings of joints that will eventually lead to bone erosion and joint deformation,54 shows immunological similarities with IBD and many immunomodulating therapies currently used in IBD were initially explored and approved in RA. In the inflamed joints, leukocytes and a variety of innate effector cells accumulate in the synovium, which is similar to what occurs in the bowel of IBD patients, together with expansion of the already present lining of fibroblast-like synoviocytes [FLSs].55 Hyperplasia of this specific type of fibroblast, found in the synovium, is one of the hallmarks of RA, and therefore several studies have been performed to identify and characterize the potential pathogenicity of FLSs in RA. Both the activation of the immune system and disrupted matrix production by the hyperplastic FLSs contribute to cartilage damage and bone erosion.56 In addition to RA, we will also shortly touch on stromal subsets identified in cancer.
3.1. Stromal cell subsets in RA
In RA, several attempts have been undertaken to identify different subtypes of FLSs in the inflamed joint. scRNA-seq of RA synovial knee tissue revealed the presence of at least two main fibroblasts clusters.57 CD55pos fibroblasts, defining subset 1, were mainly found in the synovial lining and showed expression of hyaluronan synthase 1, which is important for the production of synovial fluid.57 On the other hand, CD90pos fibroblasts, defining subset 2, were found in the synovial sub-lining of the joint and showed high expression of C-X-C motif chemokine 12 [CXCL12]. In accordance, another group showed that, within the FAPα pos fibroblasts population in the mouse synovium, CD90-positive and -negative fibroblasts were also found to have different functions and location.58 Interestingly, the severity of the joint inflammation correlated with the number of FAPα posCD90pos cells and not with the number of FAPα posCD90neg cells. In the murine intestine, similar to the situation described above, these CD90pos fibroblasts were also identified, and found to be specifically located at the base of the crypt,38 which could indicate that CD90pos fibroblasts have an organ-specific cellular location. Another recent study in RA identified three major stromal subsets defined by CD90 and CD34 expression.59 One of these subsets, CD34negCD90pos cells, was a specific expanded FLS subset in RA-affected synovium. This population of FLSs showed involvement in bone destruction in RA by high tumor necrosis factor ligand superfamily member 11 [TNFSF11] expression levels; TNFSF11 is a key factor for osteoclast differentiation and activation. In contrast, CD34negCD90neg fibroblasts were less abundant in RA-affected tissue, and especially in swollen RA joints. Most of the fibroblasts detected in RA-affected joints also showed podoplanin [PDPN] expression.59,60 Although PDPN was first identified as a lymphatic vessel marker, cancer-associated fibroblasts [CAFs] were also found to express PDPN. PDPN expression on CAFs was associated with enhanced tumor progression61 and inhibition of T cell proliferation.62
3.2. Stromal cell subsets in cancer
Given the immunosuppressive environment in tumors, cancer can be seen as the counterpart of IBD, which is defined by an overactive immune response. The role of CAFs in cancer has already been discussed in various excellent recent reviews.63–65 In the present review, we will only highlight the most important findings, which have relevance for the role of stromal cells in IBD. CAFs have been associated with increased cancer cell proliferation, cell invasion, and the formation of distant metastasis.63,66 Transforming growth factor [TGF]-β1 is one of the most abundant cytokines produced by CAFs. It was shown that high TGF-β1 levels, which are associated with a poor prognosis,67 are an immunosuppressive mechanism of CAFs, promoting T cell exclusion and the blocking of the T helper 1 [Th1]-effector phenotype acquisition.68–71 Interestingly, dual treatment with anti-TGF-β and anti-PD-L1 in a murine breast cancer model changed peritumoral stromal fibroblasts and increased cytotoxic T cell counts in the tumor, leading to a significant reduction in tumor burden only in mice treated with both antibodies.72 This would indicate that most CAFs are tumor promoting, and that targeting them inhibits tumor progression. However, targeting all α-SMApos CAFs in mice with pancreatic cancer increased the number of Tregs in the tumors and led to more aggressive tumors and decreased survival.73 This indicates that different subpopulations exist, with distinct roles in tumor progression. In colorectal cancer, scRNA-seq profiling of the tumor and matched non-tumor samples revealed the presence of three clusters of fibroblasts, of which two were defined as CAFs.74 CAF-A, which was the only CAF population showing FAP expression, showed high expression of MMP2 and COL1A2. In contrast, CAF-B had a more myofibroblast-like phenotype, with high expression of α-SMA. Two different CAF types were also found in pancreatic cancer tissue by using FAP and α-SMA staining, and defined as inflammatory [i]CAFs and myofibroblastic [my]CAFs.75 iCAFs were described as activated stellate cells, forming the dense tumor stroma and being the main source of IL-6 and IL-11, whereas myCAFs were defined by high α-SMA expression and their periglandular location. Besides α-SMA, many other markers have been proposed as distinguishing certain subtypes of fibroblasts. CD14676 or CD29,77 among others, have been associated with breast cancer CAF subpopulations. Periostin [POSTN], myosin [MYH]-11, and PDPN78 have been associated with pancreatic cancer CAF subpopulations. These non-overlapping markers show that, at least up till now, robust markers identifying specific CAF subsets have not been established. The CAF subpopulations exert different functions, both on cancer and immune cells. Two studies demonstrated the effect of a CAF subpopulation, defined by expression of CD10/GPR77 or fibroblast growth factor 5 [FGF5], respectively, on the promotion of cancer stem cells.79,80 Givel et al.,81 on the other hand, observed that in ovarian cancers that are enriched for the α-SMA–expressing CAF-S1 subset, there is increased accumulation of Tregs. These CAFs were able to recruit, retain, and increase survival of CD4posCD25pos T cells and then promote differentiation of these T cells into Tregs. CXCL12β was highly expressed in this CAF subset compared with other CAF subsets, and knockdown of CXCL12 in CAF-S1 reduces CD4posCD25pos recruitment in vitro. In summary, it seems plausible, that as in the healthy colon, in cancer there are different types of stromal cells that have distinct effects on tumor cell growth and/or immune cell homeostasis.
4. Stromal Cell Subsets in IBD
Although stromal cell research in IBD is in its infancy, various mechanisms have been discovered through which stromal cells affect wound healing and modulate the immune milieu in the inflamed intestine. Three major contributions towards understanding the role of stromal cells in IBD were the recent studies from Kinchen,39Smillie,82 and Martin,83 in which the stromal cell subsets in the colon of IBD patients were analysed using scRNA-seq39,82,83 and mass cytometry time-of-flight [CyTOF].39,83 In the study from Kinchen and colleagues, 12 different non-epithelial and non-immune cell clusters could be detected in the colon of patients with UC. In addition to the myofibroblasts, four different clusters of fibroblast-like cells could be defined [S1-4]. Cluster S1 was characterized by the expression of non-fibrillar collagens and elastic fibres, whereas cluster S2 showed high CD142 expression, cluster S3 showed high CD55 and COX-2 expression, and cluster S4, which was barely detectable in the healthy gut, yet expanded in UC, showed PDPN and IL-33 upregulation. Smillie and colleagues found eight fibroblast clusters in UC tissue, which also included one myofibroblast population. The clusters mainly differed by expression of Wnt and BMP signaling genes, suggesting their different positions along the intestinal crypt. They also identified one fibroblast population, termed inflammation-associated fibroblasts, that was expanded in inflamed tissue of UC patients and showed enrichment for genes like IL-11, FAP, and IL-13RA2. In contrast, Martin and colleagues analysed lamina propria cells from ileal tissue from CD patients and identified four stromal clusters; pericytes, smooth muscle cells, fibroblasts, and activated fibroblasts.83 The two fibroblast subtypes were characterized by expression of platelet-derived growth factor receptors and genes encoding for ECM proteins. Interestingly, activated fibroblasts strongly expressed CD90 and also PDPN. The different functions assigned to the various stromal clusters are discussed below, and the most important changes in stromal cells in IBD are summarized in Figure 1.
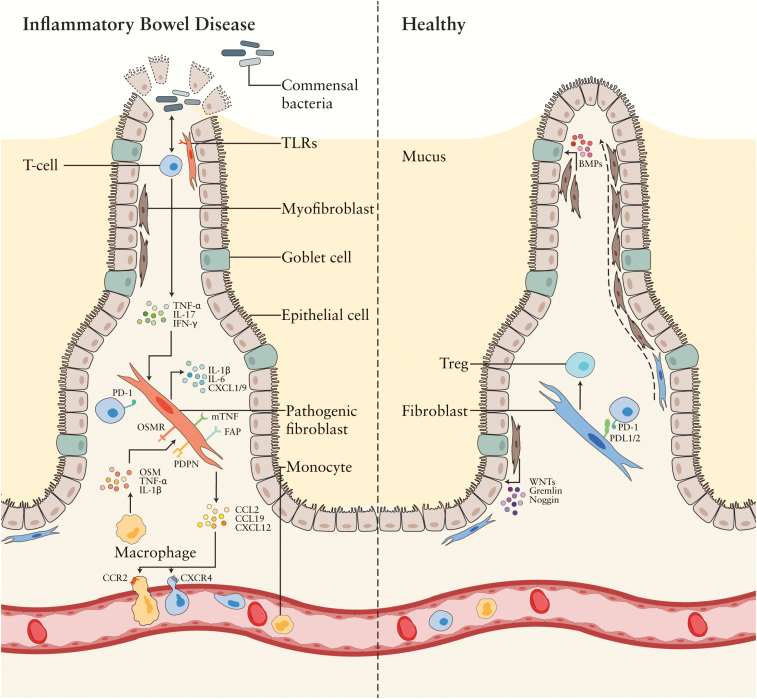
Figure 1.
Stromal cells in the intestine of IBD patients versus healthy individuals. Different stromal subsets are present in the inflamed bowel. Diminished migration capacity in fibroblasts and less stromal cells [green] supporting epithelial cells are found in IBD. Stromal cells directly [via TLRs] and indirectly [via microbiota-reactive memory T cells] respond to microbiota by the production of several pro-inflammatory factors. Pathogenic fibroblasts [pink] show expression of PDPN, OSMR, mTNF, and FAP, while they produce among others IL-6, IL-13, TNFSF14, and IL-1β. Through for example CCL2 and CXCL12, they recruit, respectively, monocytes and T cells towards the inflamed tissue.
Treg – regulatory T cell, PD-L – programmed death-ligand, PDPN – podoplanin, OSMR – oncostatin M receptor, FAP – fibroblast activation protein, IFN-y – interferon gamma, CXCL – C-X-C motif chemokine, IL-– interleukin, TNFSF-14 – tumor necrosis factor superfamily 14, mTNF – membrane-bound tumor necrosis factor, CCL – chemokine ligand, BMP – bone morphogenetic protein. Some of the figure components are derived from the Servier Medical Art library.
4.1. Wound healing by IBD stromal cells
In order to restore the damaged epithelium in IBD, the migration of fibroblasts, collagen deposition, and controlled rebuilding of the epithelial layer is essential.84 Already some years ago, it was found that the migratory capacity of human colonic lamina propria fibroblasts is altered in IBD. In vitro studies showed reduced migratory capacity of fibroblasts from IBD patients compared with control intestinal fibroblasts.85 This is even further decreased in fibroblasts derived from CD fistula patients.86 Furthermore, fibroblasts derived from CD or UC inflamed intestines proliferated faster and produced an increased amount of collagen in vitro compared with fibroblasts from healthy individuals.87 This might explain the increased risk of fibrosis in IBD patients, although proliferation and collagen production is also needed for epithelial layer repair. Regarding the role of stromal cells in restoring the epithelial cell layer, it was shown that the CD142pos fibroblast-like subpopulation S2, which is located next to the epithelial monolayer and characterized by the expression of sheet collagens and different Wnt and BMP ligands, was diminished in the colon of UC patients.39 Previously, it has been shown that in CD inflamed small intestines the fibroblastic sheath surrounding the crypt contained less SMApos and Tenascin-Cpos cells in comparison with controls.88 These observations suggest dysregulation in the fibroblasts surrounding the crypts in both forms of IBD. In addition, after induction of dextran sodium sulfate [DSS] colitis in mice, increased numbers of Gli1pos mesenchymal cells, the previously mentioned Wnt-secreting subtype of stromal cells surrounding the crypts, were found, suggesting their contribution to restoration of epithelial homeostasis.37 Together, these studies show the mutual interaction between epithelial and stromal cells in wound-healing responses in the inflamed intestine.
4.2. IBD stromal cell responses to microbiota
When the epithelial barrier is not intact, intestinal fibroblasts are able to directly respond to microbial stimuli, like lipopolysaccharides or lipoteichoic acid through expression of TLRs. Activation of TLRs increases, among other cytokines, production of IL-8, IL-6, and IL-1β by intestinal fibroblasts.89,90 Besides TLRs, the expression of nucleotide-binding oligomerization domain–containing protein 2 [NOD2] on fibroblasts renders them able to recognize bacterial products, in particular peptidoglycan-derived molecules containing muramyl dipeptide that are produced by both Gram-negative and Gram-positive bacteria.91 Loss-of-function mutations in NOD2 were one of the first risk factors identified for ileal CD.92,93 More recently, Kim and colleagues indicated colonic stromal cells as important producers of CCL2 in response to C. rodentium infection by activation of NOD244. CCL2 is in turn responsible for the recruitment of monocytes. Whether NOD2 signaling in IBD stromal cells is altered in response to bacteria is not elucidated as yet. On the other hand, intestinal fibroblasts upregulate IL-17– and IFN-γ–induced cytokines, like IL-6, CXCL1, and CXCL9, upon stimulation with cell-free supernatants of microbiota-reactive memory T cells [CD4 posCFSElowICOShigh] from IBD patients in vitro.94 These studies show both the direct and indirect impact of the intestinal microbiota on stromal cells.
4.3. Immunoregulation by IBD stromal cells
Alongside the effects of intestinal stromal cells on wound healing and their response towards microbiota, their role in immunoregulation has also been investigated in IBD. Diminished capacity of IBD human colon–derived [myo]fibroblasts to induce FOXP3posCD127neg Treg differentiation has been reported. Instead, a FOXP3posCD127pos T cell phenotype was generated, which showed a decreased expression of TGF-β1 and no expression of IL-10 and thereby reduced immunosuppressive capacities.53 Another way in which IBD-derived stromal cells are able to affect T cells was highlighted by a recent study showing that expression of the immune checkpoint PD-L1 by [myo]fibroblasts is significantly decreased in inflamed CD colon compared with that in non-inflamed matched colon samples and colons from healthy controls.95 The decreased PD-L1 expression could lead to a decreased suppression of IFN-γ production by Th cells. Surprisingly, PD-L1 expression by [myo]fibroblasts in UC tissue was increased compared with that in healthy controls, which has been linked to an increased capacity to suppress Th1 cell activity in the inflamed colon. This observation also suggests a different role for stromal cells in UC and CD. Unfortunately, in contrast to UC, no stromal subset cell analysis has as yet been performed in colonic CD, only in ileal CD. In the inflamed colon in UC, the abundance of both the S2, already described above, and S4 fibroblast-like population was changed.39 While the S4 stroma subset was barely detectable in the healthy colon, it was markedly expanded in UC and was found to be involved in leukocyte migration, with the expression of markers like CCL19, lysyl oxidases, IL-33, and TNFSF14. This was confirmed in another recent paper, showing a comparable expanded fibroblast population [inflammation-associated fibroblasts] in UC,82 which showed enrichment for inflammation-associated genes like IL-1R1, TNFSF11, and IL-13RA2. Interestingly, the expanded S4 population,39 activated fibroblasts,83 and inflammation-associated fibroblasts were associated with high expression of PDPN, a marker which has been identified to be abundantly present in the affected tissue of patients with CD or UC,96 as reported in RA.
Stromal cells both produce and respond to cytokines and chemokines. The recent scRNA-seq dataset of IBD tissue revealed that fibroblasts in the inflamed bowel produce, among other factors, monocyte chemoattractant factors [like CCL2, CCL7],83 T cell recruitment factors [like CXCL2, CCL19, CCL21, and CXCL12],39,82 neutrophil attractants [like CXCL2, CXCL8, and CXCL1],82,83 and factors involved in fibrosis [like IL-11, which is also part of the IL-6 family].82,83,97 Fibroblasts in the inflamed murine colon start producing CXCL12 in response to epithelial damage, which will recruit lymphocytes towards the mucosa.98 The importance of fibroblast-derived CXCL12 on immune cell recruitment has not only been shown in intestinal epithelial damage, but also in cancer and RA. In RA, the CD34pos subset of stromal cells defined by Mizoguchi and colleagues expressed CXCL12 and also other inflammatory genes like CCL2 and IL-6.59 The CD90pos subset found in RA by Stephenson and colleagues was also characterized by high expression of CXCL12 in comparison with the CD90neg subset.57 In contrast, a recent paper from Smillie and colleagues showed higher expression of CXCL12 by fibroblasts in the healthy colon compared with in UC inflamed colon,82 highlighting the need to further explore these findings in follow-up studies.
One of the cytokines that stromal cells can respond to is oncostatin M [OSM], by expression of its receptor OSMR or leukemia inhibitory factor receptor [LIFR] and GP130. OSM is produced by hematopoietic cells and was shown to regulate stromal cells in the bone marrow by suppressing their differentiation into adipocytes.5,99,100 In peripheral tissues, OSM induces a wide range of inflammatory factors in stromal cells, like cytokines, chemokines, and leukocyte adhesion factors.97 The OSM axis is one of the pathogenic stromal signaling pathways in IBD and is implicated in anti-TNF drug resistance.96 OSM mRNA expression is significantly increased in both CD and UC intestinal mucosal biopsies compared with in non-IBD controls, and its receptor, OSMR, which is mainly expressed in fibroblasts, is also highly expressed in IBD tissue.96 A close correlation between OSM/OSMR expression and histopathological disease severity has been reported for IBD.96 In particular, the inflammation-associated fibroblasts, which expanded during inflammation in the UC colon, showed high OSMR expression.82 Interestingly, cardiac fibroblasts showed increased CXCL12 production in response to OSM stimulation101 and could thereby stimulate the recruitment of immune cells by fibroblasts. Unpublished data from our group showed high OSM levels in CD-associated perianal fistulas, indicating the importance of this cytokine in severe complications of IBD as well. In addition to OSMR, intestinal fibroblasts also express the IL-17 receptor, which upon stimulation has been shown to induce expression of NF-κβ inhibitor zeta and CXCL1 in CD colonic fibroblasts, leading to their pro-inflammatory phenotype.102 IL-17 was indeed found to be increased in the intestinal mucosa of patients with IBD,103 thereby potentially modifying the activity and chemotaxis of immune cells by fibroblasts. The importance of the NF-κβ pathway in stromal cells has also been elucidated in a model of colitis-associated cancer, in which a specific knockout of IKKβ, an upstream regulator of NF-κβ pathway, in COL-VI stromal cells, caused reduced colitis and dysplasia development.104 Interestingly, deletion of the same gene in COL1α2 stromal cells increased the susceptibility to dysplasia and was accompanied by accumulation of Tregs in the tumors.105 This clearly shows the differential role of certain pathways in disease progression in stromal subsets. Although IL-17 can induce some pro-inflammatory pathways in stromal cells,106 it was also suggested that IL-17 is able to downregulate the TNF-α–induced CCL5 secretion by subepithelial myofibroblasts and thereby immune cell recruitment.45 The most well-studied cytokine in IBD is TNF-α, since it is the main target of the effective and often prescribed anti-TNF therapy. Although macrophages are the main TNF-α producers, myofibroblasts also signal through transmembrane TNF. CD- and UC-derived myofibroblasts from actively inflamed areas expressed more transmembrane TNF compared with non-inflamed cells or myofibroblasts from healthy controls.107 Thereby CD and UC myofibroblasts pose a direct target for anti-TNF-α therapy [as discussed in the chapter below]. Furthermore, TNF-α–induced genes, like CXCL1, CXCL6, and CCL2, were highly expressed by activated fibroblasts found in inflamed CD tissue.83 In addition to cytokines, stromal cells also produce the enzyme COX-2, which is important for the conversion of arachidonic acid into prostaglandin E2. COX-2 expression is, compared with in healthy controls, enhanced in the S3 fibroblast subset of UC patients.39 Upregulation of COX-2 was also shown before in ileum-derived CD fibroblasts.102 Specific COX-2 ablation in intestinal myofibroblasts increased susceptibility to DSS-induced colitis, especially in the initiation phase.108 These data suggest that COX-2 upregulation by myofibroblasts is a regulatory mechanism for controlling inflammation. However, for many markers expressed by stromal cells in IBD, their role in stimulating or inhibiting ongoing inflammatory responses is as yet unknown.
The analysis by Martin and colleagues of inflamed ileal tissue from CD patients revealed that the presence of activated fibroblasts was highly correlated with the presence of inflammatory macrophages, activated dendritic cells, strongly activated T cells, IgG-producing plasma cells, and atypical chemokine receptor 1–activated endothelial cells.83 They also showed that the inflammatory macrophages [CD68posCD206neg] were always in close vicinity of PDPNpos fibroblasts. This cell profile associated with high levels of activated fibroblasts was only found in a subset of patients and did not correlate with, for example, pathologic severity or disease duration. The activated fibroblasts strongly expressed CCL2 and CCL7, ligands for CCR2, which are expressed by circulating classical monocytes and facilitates their recruitment in tissues. On the other hand, the inflammatory macrophages, likely derived from these monocytes, produced inflammatory cytokines like TNF-α, IL-1β, OSM, and IL-6, which are all cytokines associated with the activation of fibroblasts.83,96
These data show the complexity of versatile, sometimes reciprocal, cytokine interactions thereby fine-tuning the function of immune and stromal cells. Taken together, it seems that particular subsets of fibroblasts in inflammatory [bowel] diseases can affect the immune system both by the production of soluble factors but also by direct cell-to-cell contact. The first evidence for subpopulations of immunoregulatory fibroblasts, identified by for example CD90 and CD55 expression, and characteristics of pathogenic fibroblasts, identified by for example PDPN or CXCL12 expression, are arising.
5. Therapeutic Modalities to Modify the Stromal Compartment in IBD
The involvement of stromal cells in the pathogenesis of IBD also makes them an interesting therapeutic target. The ultimate goal of stromal IBD therapy would be to normalize the stromal cell compartment in the inflamed gut, which could be performed in two ways [summarized in Figure 2]. The first way is to directly target the pathogenic stromal cells that play a role in immune cell recruitment and activation. The identification of these pathogenic stromal cell subsets is still ongoing, but several potential subset targets have been identified, which we will discuss in more detail below. However, because most target molecules will not be organ specific but found on stromal cells throughout the whole body, severe side effects form a potential risk, and therefore it might be a safer approach to normalize the stroma in another way. This could be circumvented via the introduction of ‘healthy’ stromal cells, in order to inhibit the inflammatory immune response and restore the epithelial cell layer. The development of clinical applications using ‘healthy’ allogeneic MSCs has been an important field of research in several inflammatory diseases, including IBD, in recent years.
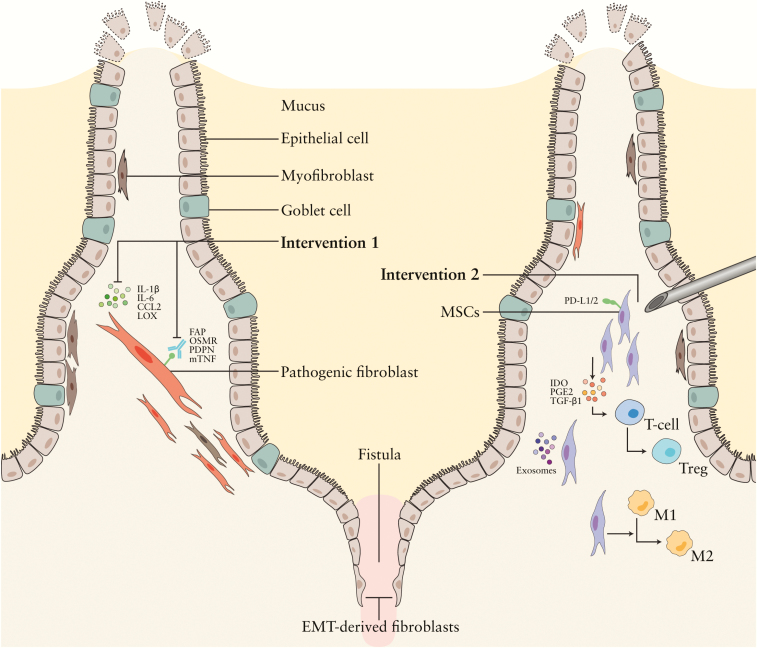
Figure 2.
Targeting stromal subsets in luminal IBD- and CD-associated perianal fistulas. 1: Targeting stromal subsets in IBD. Pathogenic stromal cells could be directly targeted via surface markers like OSMR, mTNF, PDPN, and FAP, or indirectly by blocking the soluble factors pathogenic stromal cells produce, like LOX. 2: Local MSC therapy. MSCs modulate immune cell responses, thereby reducing the number of proliferating T cells and stimulating the conversion of T cells into regulatory T cells and immunosuppressive ‘M2’ macrophages. Furthermore, they support epithelial regeneration. In these processes, soluble factors like IDO, VEGF, HGF, PGE2, and surface markers like PD-L1, ICAM, and MSC-derived exosomes are involved.
Treg – regulatory T cell, IL- interleukin, LOX – lysyl oxidase, CCL2 – chemokine ligand 2, PDPN – podoplanin, OSMR – oncostatin M receptor, mTNF – membrane-bound tumor necrosis factor, FAP – fibroblast activation protein, PGE2 – prostaglandin E2, IDO – indoleamine, PD-L1 – programmed death-ligand 1, TGF-β – transforming growth factor β. Some of the figure components are derived from the Servier Medical Art library.
5.1. Targeting stromal cells
Before defining new therapies to target stromal cells, currently applied IBD medication may also be able to target stromal cells. The presence of transmembrane TNF-α on fibroblasts makes them a target for anti-TNF-α therapy as well. Anti-TNF-α treatment with infliximab on CD-myofibroblasts in vitro increased tissue inhibitors of metalloproteinase [TIMP]-1 myofibroblast expression and thereby stimulated the migratory potential of the CD myofibroblasts.107 In this way, anti-TNF therapy could restore the wound-healing potential of stromal cells in IBD. Next to directly inhibiting TNF-α function, anti-TNF-α therapy is able to induce [indirect] apoptosis in immune cells.109 Interestingly, CD myofibroblasts revealed to be resistant to infliximab-induced apoptosis in vitro, which could be explained by the fact that peripheral blood mononuclear cells [PBMCs] are needed for induction of anti-TNF therapy–induced apoptosis in fibroblasts.110 In RA, it was found that the TNF-α targeting antibodies infliximab and adalimumab, were less efficient in inducing apoptosis in fibroblasts in the presence of PBMCs than etanercept via upregulating the anti-apoptotic molecule B cell lymphoma [Bcl]-2.110 In IBD patients, the TNFRII-Fc fusion protein etanercept [binding only soluble and not transmembrane TNF-α] showed, in contrast to the monoclonal antibodies infliximab and adalimumab, no clinical efficacy,111 which could suggest that targeting of stromal cells by anti-TNF therapy is different in IBD compared with in RA. It will be important to unravel to what extent anti-TNF-α therapy is affecting stromal cells in IBD patients and to elucidate a potential subtype of patients that would benefit more from etanercept, perhaps in adjunct to infliximab or adalimumab, since it is thought to have a higher apoptotic potential for fibroblasts. Interestingly, the intestinal cell profile detected in some of the CD patients in association with high levels of activated fibroblasts, was enriched in non-responders to anti-TNF therapy in a paediatric CD cohort.83,112 This suggests that a subtype of activated fibroblasts could play a role in resistance to anti-TNF therapy. Also, in the inflamed colon of UC patients, it was found that the inflammation-associated fibroblasts were especially enriched in pre-treatment samples from patients who did not respond to anti-TNF therapy.82 So, the presence of activated fibroblasts in CD, [characterized by CD90, PDPN, and increased IL-6, IL-11, and CCL283], inflammation-associated fibroblasts in UC, [showing IL-11, IL-25, and IL-13RA2 expression82], and OSMR tissue expression96 was associated with resistance to anti-TNF therapy. Characterizing fibroblasts in inflamed tissue at diagnosis could therefore be helpful in selecting which patient is likely to respond to anti-TNF therapy and in which patients other therapeutic strategies should be used.
Potentially pathogenic [myo]fibroblasts in the intestine of IBD have been shown to express OSMR,82,96,113 PDPN,39,96 and the S4 subset39 markers in UC: CCL19, LOX, IL-13, and TNFSF14. LOX was also found to be overexpressed by CD stenotic myofibroblasts.114 LOX inhibition restored both MMP3 activity in stenotic myofibroblasts and prevented aberrant ECM contraction. In vivo, the Lox/Loxl1 inhibitor β-aminopropionitrile [BAPN] resulted in reduced disease severity in a mouse model for colitis.39 Interestingly, the sequencing data of the pathogenic S4 subpopulation39 and inflammation-associated fibroblasts82 showed that FAP is also upregulated in UC stroma. FAP is a proline-selective protease, involved in the procession of other proteins and peptides.115 FAP can directly enhance proliferation, migration, and invasion of the cells by which it is expressed. Interestingly, CAFs with high expression of FAP produced more CCL2.116 Thus, targeting FAP could stop IBD-associated fibroblast proliferation and reduce the production of CCL2 by fibroblasts, and thereby the recruitment of myeloid cells. Anti-FAP therapy to target CAFs has already been tested in clinical trials for several malignancies and could also be a potential therapy to target the S4 fibroblasts/inflammation-associated fibroblasts in UC. The feasibility and safety of targeting FAP in the stroma of patients was demonstrated by Phase I clinical studies, applying monoclonal antibodies to advanced FAP-positive cancer patients.117,118 No major safety concerns were detected in humans, although ablation of FAP-expressing bone marrow stromal cells was observed in mice treated with anti-FAP.119,120 In the meantime, many different approaches to potentially blocking FAP via low molecular weight compounds, immunoliposomes, vaccines, and chimeric antigen receptor [CAR] T cells119 have been developed. Also in a mouse model for RA, FAP depletion, even when only depleted in the joints, showed resolution of the disease.58 Within the FAPpos cell population, PDPNposCD90pos cells seemed to contribute the most to the inflammation, since injection of this specific subpopulation in the joints resulted in more severe and sustained joint swelling, compared with in the PDPNposCD90neg subpopulation. Ex vivo inhibition of FAP in CD strictures demonstrated reduced production of type I collagen and TIMP-1,113 which suggest that anti-FAP therapy could be also targeting [IBD-related] fibrosis.
Next to FAP and LOX targeting, the correlation between OSMR on fibroblast-like cells and disease activity in IBD patients gives rise to exploring OSMR targeting. OSMR targeting by a Fc-tagged soluble OSMR-gp130 fusion protein was shown to significantly attenuate colitis in an IBD mouse model resistant to anti-TNF therapy.96 Furthermore, adenoviral transfer of OSM also reduced the severity of DSS-induced colitis.121 A Phase II clinical trial was performed for an anti-OSM humanized monoclonal antibody [GSK315234] in RA. The data from this study did not show potent clinical efficacy, but it demonstrated the safety of the drug.122 Further exploring the role of the OSMR on stromal cells in IBD might optimize patient selection for anti-OSM therapy in IBD. In this regard the effectiveness of JAK inhibitors in IBD is interesting, since the JAK pathway is downstream of the OSMR and therefore the effects of JAK inhibitors on stromal cells could teach us more about the OSM–OSMR pathway.123 As described before, PDPN is also upregulated in pathogenic IBD stromal cells. PDPN regulates cell shape and movement, and is thereby involved in cell migration.124,125 In the meantime, it is also the ligand for C-type lectin-like receptor 2, expressed by platelets and some subtypes of myeloid cells, and involved in chemokine and cytokine production.126 Targeting PDPN could therefore potentially block the interaction with myeloid cells. In preclinical studies targeting PDPN, using CAR-T cells, antibodies, and lectins, successfully inhibited the growth of PDPNpos tumor cells.127 In RA, it was shown that anti-PDPN antibodies protected mice from collagen-induced arthritis by targeting the PDPN-expressing synovial fibroblasts.128 It would be interesting to unravel whether this process is mediated by decreased fibroblast migration or the interaction with platelets or myeloid cells. No studies to target PDPN in mouse models of experimental colitis have been reported yet. Interestingly, Th17 cells also express PDPN,129 suggesting anti-PDPN is able to target both pathogenic stromal and immune cells.
5.2. MSC therapy
MSCs are multipotent stromal cells that are able to differentiate, at least in vitro, into a variety of cell types and are capable of immunomodulation and tissue regeneration.130 MSCs can be isolated from different tissues, but are mostly derived from adipose tissue and the bone marrow. In fistulizing CD, treatment with MSCs has been shown to be safe and effective [Table 1]. Perianal fistulas, which are abnormal passageways between the colon and skin around the anus, are a serious complication of CD.131 A study from our group132 showed that local application of bone marrow–derived MSCs led to fistula healing in 80% [4/5] of the patients. In accordance with these results, a double-blind placebo-controlled, multicentre study showed that local treatment with adipose-derived MSCs [Cx601/ darvadstrocel] led to significantly improved fistula closure in MSC-treated patients compared with placebo-treated patients after 24 weeks.133 Accordingly, darvadstrocel has now been approved as a treatment for refractory CD–associated perianal fistulas in Europe. Importantly, the clinical effects of MSCs seem to remain for a longer period of time, as we were recently able to show in our 4-year follow-up study.134 The treatment of luminal IBD with MSC therapy has also been investigated in pre-clinical models135–137 and Phase I/II clinical trials [Table 2]. Systemically applied MSCs are able to alleviate experimental colitis in mice,135,136 but in humans no convincing clinical responses upon systemic administration were observed. Therefore, we focused on local MSC therapy for luminal IBD. In pre-clinical experiments, local administration of MSCs in the inflamed bowel during endoscopy in DSS-induced colitis in mice showed attenuation of colitis,138 and mucosal injections of colon derived MSCs were more effective in preventing ulcer development compared with intravenously injected MSCs in a colonic wound model.139 Recently, a phase I clinical trial started in the Leiden University Medical Center [https://www.trialregister.nl/trial/6949; EudraCT number: 2017-003524-75] to determine the safety of local MSC injections in the bowel of patients with refractory ulcerative proctitis.
Table 1.
Clinical trials in IBD applying local injection of MSCs. Garcia-Olmo et al.,147 Garcia-Olmo et al.,148 Guadalajara et al.,149 Cho et al.,150 Lee et al.,151 Dietz et al.,152 Ciccocioppo et al.,153 Ciccocioppo et al.,154 De La Portilla et al.,155 Park et al.,156 Garcia-Arranz et al.,157 Panes et al.,133 Panes et al.,158 Molendijk et al.,132 Barnhoorn et al.134
| Local MSC administration – fistulising CD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication | n | Placebo-controlled | Cell type | Dosage | Evaluation | Efficacy | Placebo response rates | Follow-up | Safety | Clinical trial | Year | Study |
| CD fistulas [perianal, rectovaginal, entero-enteric] | 4 | no | adipose autologous | 3–30 × 106 | 8 w | healing in 6/8; partial closure in 2/8 | – | 12–22 m | no AEs | Phase I | 2005 | Garcia-Olmo et al.147 |
| perianal fistulas [cryptoglandular and CD] | 49 [24 MSCs] | yes | adipose autologous | 20 × 106 + F / second dose [40 × 106 + F] if incomplete closure after 8 w | 8 w | healing in 17/24 [11 with single injection, 6 after 2nd injection] | healing in: 4/25 | 12 m [38 m†] | 2 SAEs [not MSC-related] | Phase IIb | 2009 | Garcia-Olmo et al.148 †Guadalajara et al.149 |
| perianal CD fistulas | 10 | no | adipose autologous | 10 × 106, 20 × 106 or 40 × 106 MSCs/ml [proportional to fistula size – total number: 30–400 × 106] | 8 w | healing in 3/10; partial closure in 7/10 | – | 8 m | no AEs | Phase I | 2013 | Cho et al.150 |
| perianal CD fistulas | 43 [completed 33] | no | adipose autologous | 30–60 × 106 MSCs/cm [proportional to fistula size] + F / second dose [1.5× more MSCs] if incomplete closure after 8 w | 8 w | healing in 27/33, incomplete closure in 6/33 | – | 12 m | no AEs | Phase II | 2013 | Lee et al.151 |
| perianal CD fistulas | 12 | no | adipose autologous | 20 × 106 | 6 m | healing in 10/12 | – | 6 m | no AEs | Phase I | 2017 | Dietz et al.152 |
| CD fistulas [perianal, enterocutaneous] | 10 | no | bone marrow autologous | 15–30 × 106 / monthly [total 2–5×] | at each treatment [monthly] and 3, 6 and 12 months later | healing in 7/10, incomplete closure in 3/10 | – | 12 m [60 m‡] | no AEs | Phase I | 2011 | Ciccocioppo et al.153 ‡ 154 |
| perianal CD fistulas | 24 [completed 16] | no | adipose allogeneic | 20 × 106 / second dose [40 × 106] if incomplete closure after 12 w | 12 w and 24 w | healing in 8/16 | – | 6 m | 5 MSC-related AEs | Phase I/ IIa | 2013 | De La Portilla et al.155 |
| perianal CD fistulas | 6 | no | adipose allogeneic | 10 × 106 or 30 × 106 MSCs/ml [proportional to fistula size] | 8 w | healing in 3/6 | – | 8 m | no AEs | Phase I | 2015 | Park et al.156 |
| CD fistulas [rectovaginal] | 10 [completed 5] | no | adipose allogeneic | 20 × 106 / second dose [40 × 106] if incomplete closure after 12 w | 3 m and 12 m | healing in 3/5 | – | 12 m | no AEs | Phase I | 2015 | Garcia- Arranz et al.157 |
| perianal CD fistulas | 212 [107 MSC] | yes | adipose allogeneic | 120 × 106 | 24 w | healing in 53/107 | healing in: 36/105 | 12 m | 5 MSC-related SAEs | Phase III | 2016 | Panes et al.133, 158 |
| perianal CD fistulas | 21 [15 MSC] | yes | bone marrow allogeneic | 10 × 106, 30 × 106 or 90 × 106 | 6 w, 12 w and 24 w | healing in 9/15 | healing in: 2/6 | 6 m [48 m*] | 2 SAEs [not MSC-related*] | Phase IIa | 2015 | Molendijk et al.132 *Barnhoorn et al.134 |
MSC: mesenchymal stromal cell; CD: Crohn’s disease; F: fibrin glue; AE: adverse event; SAE: serious adverse event; d: days; w: weeks, m: months.
Table 2.
Clinical trials in IBD applying intravenous injection of MSCs. Duijvestein et al.159 Dhere et al.160 Liang et al.161 Forbes et al.162 Mayer et al.163 Melmed et al.164 Zhang et al.165
| Intravenous MSCs administration – luminal IBD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication | n | Placebo- controlled | Cell type | Dosage | Evaluation | Efficacy | Placebo response rates | Follow-up | Safety | Clinical trial | Year | Study |
| CD | 9 | no | bone marrow autologous | 2× 1–2 × 106 MSCs/kg, 7 days apart | 6 w and 14 w | no clinical remission, but clinical response in 3/9; though in 4/9 disease worsening | – | 14 w | no AEs | Phase I | 2010 | Duijvestein et al.159 |
| CD | 12 | no | bone marrow autologous | 2 × 106, 5 × 106 or 10 × 106 MSCs/kg | 2 w | clinical response in 5/11 | – | 9 w | 7 SAEs [2 MSC- related] | Phase I | 2016 | Dhere et al.160 |
| CD/UC | 7 [4 CD / 3 UC] | no | bone marrow allogeneic [or umbilical cord] | 1 × 106 MSCs/kg | 3 m | clinical remission in 5/7 [CD 2/4; UC 3/3] | – | 6–32 m | no AEs | Phase I | 2012 | Liang et al.161 |
| CD | 16 [completed 15] | no | bone marrow allogeneic | 4× 2 × 106MSCs/ kg once per week | 6 w | clinical remission in 8/15 [clinical response in 12/15] | – | – | no AEs related to MSCs | Phase II | 2014 | Forbes et al.162 |
| CD | 12 | no | placenta allogeneic | 2× 2 × 108 or 8 × 108 once per week | 6 m | clinical remission in 3/12 [clinical response in 8] | – | 24 m | no AEs | Phase I | 2013 | Mayer et al.163 |
| CD | 50 [34 MSCs] | yes | placenta allogeneic | 2× 1.5 × 108, 6 × 108 [or 12 × 108] once per week | 4 w and 6 w | clinical remission in 4/28 [clinical response in 10/28] | clinical remission in 0/16 [clinical response in 0/16] | 24 m | 10 MSC-related SAEs | Phase Ib/ IIa | 2015 | Melmed et al.164 |
| CD | 82 [41 MSCs] | [yes] – normal treatment | umbilical cord allogeneic | 4× 1 × 106 MSCs/ kg once per week | 12 m | no clinical remission, but improved clinical and endoscopic scores | no clinical remission | 12 m | no SAEs | 2018 | Zhang et al.165 | |
MSC: mesenchymal stromal cell; IBD: inflammatory bowel disease; CD: Crohn’s disease; UC: ulcerative colitis; AE: adverse event; SAE: serious adverse event; d: days; w: weeks, m: months.
MSC therapy could be seen as an approach to normalize the intestinal stroma by the introduction of healthy allogeneic MSCs. Our unpublished data showed, for example, that MSCs express much lower levels of the pathogenic fibroblast marker PDPN, compared with IBD-derived fibroblasts, which demonstrates their ‘healthy’ phenotype. Like fibroblasts, MSCs are able to modulate local inflammation as well as to support epithelial regeneration. It has been suggested that MSCs are able to suppress immune cell responses through secretion of paracrine factors and by cell–cell contacts.90 Furthermore, it has been postulated that the therapeutic effects of MSCs in perianal fistulizing CD is partly due to their PD-L1 expression.34 When focusing on the effects of MSCs on epithelial repair, we showed the ability of MSCs to enhance epithelial proliferation and migration via secreted soluble factors, but also to some extent via MSC-derived exosomes.140 Next to pro-regenerative and direct immune suppressive functions, recently a new hypothesis regarding the workings mechanism has been postulated, in which MSCs upon intravenous injection undergo apoptosis and effect immunosuppression via modulation of the monocytes by which they have been phagocytosed.141,142 However, there are no data available yet that show that local MSC therapy works in a comparable manner, and our published data show at least the engraftment and survival of locally injected MSCs up to 6 days post-injection.138
While stromal cell therapy is mainly focused on the use of MSCs, other stromal cells, like fibroblasts may also be capable of stimulating tissue repair and suppressing immune responses. In a Phase II trial, spray-applied allogeneic neonatal keratinocytes and fibroblasts successfully treated chronic venous leg ulcers.143 Furthermore, transplantation of autologous skin fibroblasts and adipose tissue,144,145 including stromal cells, has also been suggested for the treatment of CD perianal fistulas.146
6. Conclusion
Although unraveling the role of stromal cells in IBD pathogenesis has just started, current research is already showing a considerable role for the various subsets of intestinal stromal cells. In this review, we focused on their heterogeneity and the role of stromal subtypes on epithelial repair and immune homeostasis.
There are several challenges investigating and reporting on stromal cells in IBD. One of the difficulties in stromal research is the lack of agreement on the exact and uniform definition of stromal cell subtypes. Although there seems to be agreement on general fibroblast markers, the use of these markers varies between studies. This makes it difficult to generate a clear overall picture of the recent findings on the various subtypes of stromal cells, as it is unclear whether all studies were actually examining the same cell type. Furthermore, certain subtype definitions do not withstand close scrutiny. For example, the α-SMApos myofibroblast was always thought to be important for epithelial homeostasis; however, several recent studies also showed that α-SMAneg stromal cells surround the epithelial crypt and produce factors important for epithelial homeostasis. Based on the relatively low number of published studies so far, it seems there is high heterogeneity between individuals, organs, and diseases. In addition, the different isolation and analysis techniques used resulted in the identification of different subtypes. Addressing these problems and setting a stricter definition of stromal cell types would allow a more accurate and representative subclassification.
Many of the studies discussed in this review have analysed cultured stromal cells, which might have changed phenotype and functions compared with their in vivo counterparts. For example, the immunomodulatory properties of healthy intestinal stromal cells were shown in many studies using cultured fibroblasts. However, in freshly isolated cells in viv , only a subpopulation of fibroblasts expressed factors that could potentially affect immune cells.39,82 In addition, in most studies the effects of medication used by the patient on the function and expression profile of stromal cells has not yet been taken into account. This could have biased results, since for example anti-TNF therapy might also directly influence fibroblasts, as indicated above.
Although IBD is mentioned as one disease entity, there are interesting differences between UC and CD, and also between stromal cells in CD and UC, which need to be studied in more detail in the future. More generally, it will also be important to unravel which changes in stromal cell subsets are ‘inflammation’-mediated and which changes are ‘IBD-specific’. Data from other inflammatory disease of the gut, like infectious or microscopic colitis, should shed light on this. New technological advances, allowing the analysis of non-cultured fibroblasts and the screening of many samples in depth, for both RNA and protein expression profiles, are expected to extend the knowledge of stromal cells in the inflamed and non-inflamed gut. However, in addition to the phenotype of stromal cells, their function needs to be elucidated further, and therefore more advanced three-dimensional culture systems and transgenic rodent systems will be needed to unravel the complex and mutually interactive role of human intestinal stromal cells in contact with immune cells and epithelial cells.
Direct targeting of pathogenic stromal cells in IBD is still difficult, since the specific pathogenic subtypes are not yet well defined. The challenge lies in restoration of the stromal cells that support the epithelial cells, while targeting the stromal cells that attract and aberrantly activate immune cells. For now, the introduction of local MSCs seems to be a safer option in order to modify the stromal component in IBD, since many potential stromal targets would also be targeted for healthy stromal cells in other organs. Furthermore, since stromal cells seems to be involved in anti-TNF resistance, the characterization of stromal cells in inflamed tissue at diagnosis could be helpful in predicting disease course and therapeutic responses. In conclusion, the field of stromal IBD research is developing and will improve knowledge of the pathogenesis of both UC and CD in the coming decade, hopefully providing novel insights and therapeutic approaches.
Acknowledgments
We would like to thank Prof. Dr Hein Verspaget and Dr Andrea van der Meulen-de Jong for allowing and supporting the collaboration and for providing critical feedback on the manuscript. We would like to thank Ruben Kamphuis for editing the figures.
Funding
RSB is supported by an ECCO fellowship.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Author Contributions
MCB conducted literature searching, wrote the manuscript, and designed the figures; SKH conducted literature searching and critically revised the manuscript; RSB conducted literature searching, designed the tables, and critically revised the manuscript; GR critically revised the manuscript; LJACH and MS supervised the writing process and critically revised the manuscript. All authors approved the final version of the article.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 2. Reinisch W, Hommes DW, Van Assche G, et al. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn’s disease. Gut 2006;55:1138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-il-17a monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- 5. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012;142:1102–11.e2. [DOI] [PubMed] [Google Scholar]
- 6. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 7. Owens BM. Inflammation, innate immunity, and the intestinal stromal cell niche: opportunities and challenges. Front Immunol 2015;6:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roulis M, Flavell RA. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 2016;92:116–31. [DOI] [PubMed] [Google Scholar]
- 9. Lertkiatmongkol P, Liao D, Mei H, Hu Y, Newman PJ. Endothelial functions of platelet/endothelial cell adhesion molecule-1 [CD31]. Curr Opin Hematol 2016;23:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Altin JG, Sloan EK. The role of CD45 and CD45-associated molecules in T cell activation. Immunol Cell Biol 1997;75:430–45. [DOI] [PubMed] [Google Scholar]
- 11. Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 2011;73:213–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinchuk IV, Mifflin RC, Saada JI, Powell DW. Intestinal mesenchymal cells. Curr Gastroenterol Rep 2010;12:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saada JI, Pinchuk IV, Barrera CA, et al. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol 2006;177:5968–79. [DOI] [PubMed] [Google Scholar]
- 14. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–7. [DOI] [PubMed] [Google Scholar]
- 15. Skalli O, Schürch W, Seemayer T, et al. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest 1989;60:275–85. [PubMed] [Google Scholar]
- 16. Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 2005;132:5317–28. [DOI] [PubMed] [Google Scholar]
- 17. McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology 2009;136:2074–91. [DOI] [PubMed] [Google Scholar]
- 18. Flier SN, Tanjore H, Kokkotou EG, et al. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem 2010;285:20202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006;172:973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mintet E, Rannou E, Buard V, et al. Identification of endothelial-to-mesenchymal transition as a potential participant in radiation proctitis. Am J Pathol 2015;185:2550–62. [DOI] [PubMed] [Google Scholar]
- 21. Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease – current knowledge and future perspectives. J Crohns Colitis 2008;2:279–90. [DOI] [PubMed] [Google Scholar]
- 22. Brittan M, Hunt T, Jeffery R, et al. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut 2002;50:752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Latella G, Rieder F. Intestinal fibrosis: ready to be reversed. Curr Opin Gastroenterol 2017;33:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawrance IC, Rogler G, Bamias G, et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis 2017;11:1491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allaire JM, Crowley SM, Law HT, et al. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol 2018;39:677–96. [DOI] [PubMed] [Google Scholar]
- 28. van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 2009;71:241–60. [DOI] [PubMed] [Google Scholar]
- 29. Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–7. [DOI] [PubMed] [Google Scholar]
- 30. De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 2008;123:2229–38. [DOI] [PubMed] [Google Scholar]
- 31. Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell 2013;154:274–84. [DOI] [PubMed] [Google Scholar]
- 32. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017;169:985–99. [DOI] [PubMed] [Google Scholar]
- 33. He XC, Zhang J, Tong WG, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet 2004;36:1117–21. [DOI] [PubMed] [Google Scholar]
- 34. Qi Z, Li Y, Zhao B, et al. BMP restricts stemness of intestinal Lgr5+ stem cells by directly suppressing their signature genes. Nat Commun 2017;8:13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Powell D, Mifflin R, Valentich J, et al. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 1999;277:C183–C201. [DOI] [PubMed] [Google Scholar]
- 36. Kosinski C, Li VS, Chan AS, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A 2007;104:15418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018;558:449–53. [DOI] [PubMed] [Google Scholar]
- 38. Karpus ON, Westendorp BF, Vermeulen JLM, et al. Colonic CD90+ crypt fibroblasts secrete semaphorins to support epithelial growth. Cell Rep 2019;26:3698–708.e5. [DOI] [PubMed] [Google Scholar]
- 39. Kinchen J, Chen HH, Parikh K, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 2018;175:372–86.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut 2013;62:1653–64. [DOI] [PubMed] [Google Scholar]
- 41. Nowarski R, Jackson R, Flavell RA. The stromal intervention: regulation of immunity and inflammation at the epithelial–mesenchymal barrier. Cell 2017;168:362–75. [DOI] [PubMed] [Google Scholar]
- 42. Strong SA, Pizarro TT, Klein JS, Cominelli F, Fiocchi C. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology 1998;114:1244–56. [DOI] [PubMed] [Google Scholar]
- 43. Gelbmann CM, Leeb SN, Vogl D, et al. Inducible CD40 expression mediates NFkappaB activation and cytokine secretion in human colonic fibroblasts. Gut 2003;52:1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim YG, Kamada N, Shaw MH, et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 2011;34:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andoh A, Fujino S, Bamba S, et al. IL-17 selectively down-regulates TNF-alpha–induced RANTES gene expression in human colonic subepithelial myofibroblasts. J Immunol 2002;169:1683–7. [DOI] [PubMed] [Google Scholar]
- 46. Pinchuk IV, Saada JI, Beswick EJ, et al. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology 2008;135:1228–37, 1237.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity 2018;48:434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vicente-Suarez I, Larange A, Reardon C, et al. Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol 2015;8:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boots AM, Wimmers-Bertens AJ, Rijnders AW. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology 1994;82:268–74. [PMC free article] [PubMed] [Google Scholar]
- 50. Owens BM, Steevels TA, Dudek M, et al. Cd90+ stromal cells are non-professional innate immune effectors of the human colonic mucosa. Frontiers in Immunology 2013;4:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 2014;21:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koyama M, Kuns RD, Olver SD, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med 2011;18:135–42. [DOI] [PubMed] [Google Scholar]
- 53. Pinchuk IV, Beswick EJ, Saada JI, et al. Human colonic myofibroblasts promote expansion of CD4+ CD25high Foxp3+ regulatory T cells. Gastroenterology 2011;140:2019–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 55. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 56. Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 2013;9:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stephenson W, Donlin LT, Butler A, et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat Commun 2018;9:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Croft AP, Campos J, Jansen K, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019;570:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mizoguchi F, Slowikowski K, Wei K, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun 2018;9:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ekwall AK, Eisler T, Anderberg C, et al. The tumour-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res Ther 2011;13:R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shindo K, Aishima S, Ohuchida K, et al. Podoplanin expression in cancer-associated fibroblasts enhances tumor progression of invasive ductal carcinoma of the pancreas. Mol Cancer 2013;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cremasco V, Astarita JL, Grauel AL, et al. FAP delineates heterogeneous and functionally divergent stromal cells in immune-excluded breast tumors. Cancer Immunol Res 2018;6:1472–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–98. [DOI] [PubMed] [Google Scholar]
- 64. Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov 2019;18:99–115. [DOI] [PubMed] [Google Scholar]
- 65. Kobayashi H, Enomoto A, Woods SL, et al. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol 2019;16:282–95. [DOI] [PubMed] [Google Scholar]
- 66. Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science 2002;296:1046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors 2011;29:140–52. [DOI] [PubMed] [Google Scholar]
- 68. Tauriello DVF, Palomo-Ponce S, Stork D, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018;554:538–43. [DOI] [PubMed] [Google Scholar]
- 69. Calon A, Lonardo E, Berenguer-Llergo A, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet 2015;47:320–9. [DOI] [PubMed] [Google Scholar]
- 70. Hawinkels LJ, Verspaget HW, van Duijn W, et al. Tissue level, activation and cellular localisation of TGF-beta1 and association with survival in gastric cancer patients. Br J Cancer 2007;97:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hawinkels LJ, Verspaget HW, van der Reijden JJ, et al. Active TGF-beta1 correlates with myofibroblasts and malignancy in the colorectal adenoma–carcinoma sequence. Cancer Sci 2009;100:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li H, Courtois ET, Sengupta D, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet 2017;49:708–18. [DOI] [PubMed] [Google Scholar]
- 75. Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brechbuhl HM, Finlay-Schultz J, Yamamoto TM, et al. Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clin Cancer Res 2017;23:1710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Costa A, Kieffer Y, Scholer-Dahirel A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018;33:463–79.e10. [DOI] [PubMed] [Google Scholar]
- 78. Neuzillet C, Tijeras-Raballand A, Ragulan C, et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol 2019;248:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cazet AS, Hui MN, Elsworth BL, et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun 2018;9:2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Su S, Chen J, Yao H, et al. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 2018;172:841–56.e16. [DOI] [PubMed] [Google Scholar]
- 81. Givel AM, Kieffer Y, Scholer-Dahirel A, et al. miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun 2018;9:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Smillie CS, Biton M, Ordovas-Montanes J, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 2019;178:714–30.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Martin JC, Chang C, Boschetti G, et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell 2019;178:1493–508.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut 2007;56:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Leeb SN, Vogl D, Gunckel M, et al. Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology 2003;125:1341–54. [DOI] [PubMed] [Google Scholar]
- 86. Meier JK, Scharl M, Miller SN, et al. Specific differences in migratory function of myofibroblasts isolated from Crohn’s disease fistulae and strictures. Inflamm Bowel Dis 2011;17:202–12. [DOI] [PubMed] [Google Scholar]
- 87. Lawrance IC, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis 2001;7:226–36. [DOI] [PubMed] [Google Scholar]
- 88. Francoeur C, Bouatrouss Y, Seltana A, et al. Degeneration of the pericryptal myofibroblast sheath by proinflammatory cytokines in inflammatory bowel diseases. Gastroenterology 2009;136:268–77.e3. [DOI] [PubMed] [Google Scholar]
- 89. Pang G, Couch L, Batey R, Clancy R, Cripps A. GM-CSF, IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1 alpha and TNF-alpha. Clin Exp Immunol 1994;96:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology 2003;124:1866–78. [DOI] [PubMed] [Google Scholar]
- 91. Girardin SE, Travassos LH, Herve M, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem 2003;278:41702–8. [DOI] [PubMed] [Google Scholar]
- 92. Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 93. Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411:603–6. [DOI] [PubMed] [Google Scholar]
- 94. Hegazy AN, West NR, Stubbington MJT, et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology 2017;153:1320–37.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beswick EJ, Grim C, Singh A, et al. Expression of programmed death-Ligand 1 by human colonic CD90+ stromal cells differs between ulcerative colitis and Crohn’s disease and determines their capacity to suppress Th1 cells. Front Immunol 2018;9:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. West NR, Hegazy AN, Owens BMJ, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor–neutralizing therapy in patients with inflammatory bowel disease. Nat Med 2017;23:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. West NR. Coordination of immune–stroma crosstalk by IL-6 family cytokines. Front Immunol 2019;10:1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Westendorp BF, Büller NVJA, Karpus ON, et al. Indian hedgehog suppresses a stromal cell-driven intestinal immune response. Cell Mol Gastroenterol Hepatol 2018;5:67–82.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Walker EC, McGregor NE, Poulton IJ, et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Investig 2010;120:582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Guihard P, Danger Y, Brounais B, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012;30:762–72. [DOI] [PubMed] [Google Scholar]
- 101. Lafontant PJ, Burns AR, Donnachie E, et al. Oncostatin M differentially regulates CXC chemokines in mouse cardiac fibroblasts. Am J Physiol Cell Physiol 2006;291:C18–C26. [DOI] [PubMed] [Google Scholar]
- 102. Kerami Z, Duijvis NW, Vogels EW, et al. Effect of interleukin-17 on gene expression profile of fibroblasts from Crohn’s disease patients. J Crohns Colitis 2014;8:1208–16. [DOI] [PubMed] [Google Scholar]
- 103. Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003;52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Koliaraki V, Pasparakis M, Kollias G. IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer. J Exp Med 2015;212:2235–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pallangyo CK, Ziegler PK, Greten FR. IKKβ acts as a tumor suppressor in cancer-associated fibroblasts during intestinal tumorigenesis. J Exp Med 2015;212:2253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hata K, Andoh A, Shimada M, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 2002;282:G1035–44. [DOI] [PubMed] [Google Scholar]
- 107. Di Sabatino A, Pender SL, Jackson CL, et al. Functional modulation of Crohn’s disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology 2007;133:137–49. [DOI] [PubMed] [Google Scholar]
- 108. Roulis M, Nikolaou C, Kotsaki E, et al. Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc Natl Acad Sci U S A 2014;111:E4658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Levin AD, Wildenberg ME, van den Brink GR. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J Crohns Colitis 2016;10:989–97. [DOI] [PubMed] [Google Scholar]
- 110. Pattacini L, Boiardi L, Casali B, Salvarani C. Differential effects of anti-TNF-alpha drugs on fibroblast-like synoviocyte apoptosis. Rheumatology 2010;49:480–9. [DOI] [PubMed] [Google Scholar]
- 111. Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2001;121:1088–94. [DOI] [PubMed] [Google Scholar]
- 112. Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017;389:1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Truffi M, Sorrentino L, Monieri M, et al. Inhibition of fibroblast activation protein restores a balanced extracellular matrix and reduces fibrosis in Crohn’s disease strictures ex vivo. Inflamm Bowel Dis 2018;24:332–45. [DOI] [PubMed] [Google Scholar]
- 114. de Bruyn JR, van den Brink GR, Steenkamer J, et al. Fibrostenotic phenotype of myofibroblasts in Crohn’s disease is dependent on tissue stiffness and reversed by LOX inhibition. J Crohns Colitis 2018;12:849–59. [DOI] [PubMed] [Google Scholar]
- 115. Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein [FAP]: substrates, activities, expression and targeting for cancer therapy. Proteom Clin Appl 2014;8:454–63. [DOI] [PubMed] [Google Scholar]
- 116. Puré E, Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene 2018;37:4343–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Scott AM, Wiseman G, Welt S, et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res 2003;9:1639–47. [PubMed] [Google Scholar]
- 118. Welt S, Divgi CR, Scott AM, et al. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol 1994;12:1193–203. [DOI] [PubMed] [Google Scholar]
- 119. Busek P, Mateu R, Zubal M, Kotackova L, Sedo A. Targeting fibroblast activation protein in cancer – prospects and caveats. Front Biosci [Landmark Ed] 2018;23:1933–68. [DOI] [PubMed] [Google Scholar]
- 120. Roberts EW, Deonarine A, Jones JO, et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med 2013;210:1137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sanchez AL, Langdon CM, Akhtar M, et al. Adenoviral transfer of the murine oncostatin M gene suppresses dextran-sodium sulfate-induced colitis. J Interferon Cytokine Res 2003;23:193–201. [DOI] [PubMed] [Google Scholar]
- 122. Choy EH, Bendit M, McAleer D, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of an anti- oncostatin M monoclonal antibody in rheumatoid arthritis: results from phase II randomized, placebo-controlled trials. Arthritis Res Ther 2013;15:R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. West NR, Owens BMJ, Hegazy AN. The oncostatin M-stromal cell axis in health and disease. Scand J Immunol 2018;88:e12694. [DOI] [PubMed] [Google Scholar]
- 124. Suchanski J, Tejchman A, Zacharski M, et al. Podoplanin increases the migration of human fibroblasts and affects the endothelial cell network formation: a possible role for cancer-associated fibroblasts in breast cancer progression. PLoS One 2017;12:e0184970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Neri S, Ishii G, Hashimoto H, et al. Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int J Cancer 2015;137:784–96. [DOI] [PubMed] [Google Scholar]
- 126. Lowe KL, Navarro-Nunez L, Benezech C, et al. The expression of mouse CLEC-2 on leucocyte subsets varies according to their anatomical location and inflammatory state. Eur J Immunol 2015;45:2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Krishnan H, Rayes J, Miyashita T, et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Sci 2018;109:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Desanti G, Saghir A, Naylor A, et al. O014 Podoplanin [GP38], a marker of synovial inflammation, is an excellent therapeutic target in mouse collagen-induced arthritis. Ann Rheum Dis 2018;77:A7–A8. [Google Scholar]
- 129. Miyamoto Y, Uga H, Tanaka S, et al. Podoplanin is an inflammatory protein upregulated in Th17 cells in SKG arthritic joints. Mol Immunol 2013;54:199–207. [DOI] [PubMed] [Google Scholar]
- 130. Schepers K, Fibbe WE. Unraveling mechanisms of mesenchymal stromal cell–mediated immunomodulation through patient monitoring and product characterization. Ann N Y Acad Sci 2016;1370:15–23. [DOI] [PubMed] [Google Scholar]
- 131. Siegmund B, Feakins RM, Barmias G, et al. Results of the fifth scientific workshop of the ECCO [II]: pathophysiology of perianal fistulizing disease. J Crohns Colitis 2016;10:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Molendijk I, Bonsing BA, Roelofs H, et al. Allogeneic bone marrow–derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology 2015;149:918–27.e6. [DOI] [PubMed] [Google Scholar]
- 133. Panes J, Garcia-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells [Cx601] for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016;388:1281–90. [DOI] [PubMed] [Google Scholar]
- 134. Barnhoorn MC, Wasser M, Roelofs H, et al. Long-term evaluation of allogeneic bone marrow–derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J Crohns Colitis 2020;14:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Duijvestein M, Wildenberg ME, Welling MM, et al. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011;29:1549–58. [DOI] [PubMed] [Google Scholar]
- 136. Sala E, Genua M, Petti L, et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology 2015;149:163–76.e20. [DOI] [PubMed] [Google Scholar]
- 137. González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009;136:978–89. [DOI] [PubMed] [Google Scholar]
- 138. Barnhoorn M, de Jonge-Muller E, Molendijk I, et al. Endoscopic administration of mesenchymal stromal cells reduces inflammation in experimental colitis. Inflamm Bowel Dis 2018;24:1755–67. [DOI] [PubMed] [Google Scholar]
- 139. Manieri NA, Mack MR, Himmelrich MD, et al. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J Clin Investig 2015;125:3606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barnhoorn MC, Plug L, Jonge ESMM, et al. Mesenchymal stromal cell-derived exosomes contribute to epithelial regeneration in experimental inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2020; pii: S2352-345X(20)30013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. de Witte SFH, Luk F, Sierra Parraga JM, et al. Immunomodulation by therapeutic mesenchymal stromal cells [MSC] is triggered through phagocytosis of MSC by monocytic cells. Stem Cells 2018;36:602–15. [DOI] [PubMed] [Google Scholar]
- 142. Galleu A, Riffo-Vasquez Y, Trento C, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med 2017;9:eaam7828. [DOI] [PubMed] [Google Scholar]
- 143. Kirsner RS, Marston WA, Snyder RJ, et al. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2012;380:977–85. [DOI] [PubMed] [Google Scholar]
- 144. Serrero M, Grimaud F, Philandrianos C, et al. Long-term safety and efficacy of local microinjection combining autologous microfat and adipose-derived stromal vascular fraction for the treatment of refractory perianal fistula in Crohn’s disease. Gastroenterology 2019;156:2335–7.e2. [DOI] [PubMed] [Google Scholar]
- 145. Dige A, Hougaard HT, Agnholt J, et al. Efficacy of injection of freshly collected autologous adipose tissue into perianal fistulas in patients with Crohn’s disease. Gastroenterology 2019;156:2208–16.e1. [DOI] [PubMed] [Google Scholar]
- 146. Ascanelli S, de Tullio D, Gregorio C, Azzena G, Occhionorelli S. Autologous fibroblasts transplant after infliximab administration: a new approach in Crohn’s perianal fistulas? Brief clinical report. Int J Colorectal Dis 2007;22:1135–6. [DOI] [PubMed] [Google Scholar]
- 147. Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 2005;48:1416–23. [DOI] [PubMed] [Google Scholar]
- 148. Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 2009;52:79–86. [DOI] [PubMed] [Google Scholar]
- 149. Guadalajara H, Herreros D, De-La-Quintana P, et al. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis 2012;27:595–600. [DOI] [PubMed] [Google Scholar]
- 150. Cho YB, Lee WY, Park KJ, et al. Autologous adipose tissue–derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant 2013;22:279–85. [DOI] [PubMed] [Google Scholar]
- 151. Lee WY, Park KJ, Cho YB, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells 2013;31:2575–81. [DOI] [PubMed] [Google Scholar]
- 152. Dietz AB, Dozois EJ, Fletcher JG, et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology 2017;153:59–62.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow–derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 2011;60:788–98. [DOI] [PubMed] [Google Scholar]
- 154. Ciccocioppo R, Gallia A, Sgarella A, et al. Long-term follow-up of Crohn disease fistulas after local injections of bone marrow–derived mesenchymal stem cells. Mayo Clin Proc 2015;90:747–55. [DOI] [PubMed] [Google Scholar]
- 155. de la Portilla F, Alba F, Garcia-Olmo D, et al. Expanded allogeneic adipose-derived stem cells [EASCs] for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis 2013;28:313–23. [DOI] [PubMed] [Google Scholar]
- 156. Park KJ, Ryoo SB, Kim JS, et al. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: a pilot clinical trial. Colorectal Dis 2016;18:468–76. [DOI] [PubMed] [Google Scholar]
- 157. Garcia-Arranz M, Herreros MD, Gonzalez-Gomez C, et al. Treatment of Crohn’s-related rectovaginal fistula with allogeneic expanded-adipose derived stem cells: a phase I–IIa clinical trial. Stem Cells Transl Med 2016;5:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Panes J, Garcia-Olmo D, Van Assche G, et al. Long-term efficacy and safety of stem cell therapy [Cx601] for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology 2018;154:1334–42.e4. [DOI] [PubMed] [Google Scholar]
- 159. Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow–derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 2010;59:1662–9. [DOI] [PubMed] [Google Scholar]
- 160. Dhere T, Copland I, Garcia M, et al. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn’s disease – a phase 1 trial with three doses. Aliment Pharmacol Ther 2016;44:471–81. [DOI] [PubMed] [Google Scholar]
- 161. Liang J, Zhang H, Wang D, et al. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut 2012;61:468–9. [DOI] [PubMed] [Google Scholar]
- 162. Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol 2014;12:64–71. [DOI] [PubMed] [Google Scholar]
- 163. Mayer L, Pandak WM, Melmed GY, et al. Safety and tolerability of human placenta-derived cells [PDA001] in treatment-resistant Crohn’s disease: a phase 1 study. Inflamm Bowel Dis 2013;19:754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Melmed GY, Pandak WM, Casey K, et al. Human placenta-derived cells [PDA-001] for the treatment of moderate-to-severe Crohn’s disease: a phase 1b/2a study. Inflamm Bowel Dis 2015;21:1809–16. [DOI] [PubMed] [Google Scholar]
- 165. Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical cord mesenchymal stem cell treatment for Crohn’s disease: a randomized controlled clinical trial. Gut Liver 2018;12:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]