Abstract
Traditional metrics of smoke exposure in cigarette smokers are derived either from self-report, biomarkers, or puff topography. Methods involving biomarkers measure concentrations of nicotine, nicotine metabolites, or carbon monoxide. Puff-topography methods employ portable instruments to measure puff count, puff volume, puff duration, and inter-puff interval. In this study, we propose smoke exposure metrics calculated from the breathing signal and describe a novel algorithm for the computation of these metrics. The Personal Automatic Cigarette Tracker v2 (PACT-2) sensors, puff topography devices (CReSS), and video observation were used in a study of 38 moderate to heavy smokers in a controlled environment. Parameters of smoke inhalation including the start and end of each puff, inhale and exhale cycle, and smoke holding were computed from the breathing signal. From these, the traditional metrics of puff duration, inhale-exhale cycle duration, smoke holding duration, inter-puff interval, and novel Respiratory Smoke Exposure Metrics (RSEMs) such as inhale-exhale cycle volume, and inhale-exhale volume over time were calculated. The proposed RSEM algorithm to extract smoke exposure metrics named generated interclass correlations (ICCs) of 0.85 and 0.87 and Pearson’s correlations of 0.97 and 0.77 with video observation and CReSS, respectively, for puff duration. Similarly, for the inhale-exhale duration, an ICC of 0.84 and Pearson’s correlation of 0.81 was obtained with video observation. The RSEMs provided measures previously unavailable in research that are proportional to the depth and duration of smoke inhalation. The results suggest that the breathing signal may be used to compute smoke exposure metrics.
Keywords: breathing signal analysis, cigarette smoking, respiratory inductive plethysmography, smoke exposure, smoking topography
I. Introduction
SMOKING is one of the major causes of death in the United States[1]–[9]. The well-documented major consequences of cigarette smoking on health underscore the importance of focused research on smoking behavior and smoke exposure. To understand the health risks of cigarette smoking, it is crucial to examine the amount of smoke a smoker is exposed to due to his or her smoking.
The methods to measure smoke exposure include self-report, use of puff topography devices, and biomarkers [10]. Self-report is one of the most commonly used and least expensive methods to understand smoking behavior. However, this method can be influenced by recall biases, memory lapses, distortions, and lack of awareness of an individual’s smoking behavior[11]. Also, studies have suggested only a modest correlation between self-reported and objectively determined components of smoking topography and no significant association between self-reported topography and cotinine (a biomarker of nicotine exposure)[12]. Thus, self-report does not offer a comprehensive measure of smoking behavior [13].
Another method for assessment of smoke exposure is to assess puff topography metrics using computerized devices or portable devices (such as CReSS). Puff topography devices measure several elements of smoking topography including puff count, puff interval, puff volume, time to first puff, and inter-puff interval [14]. More than 30 studies that have been conducted using the CReSS pocket device[15], which appears to provide a valid and reliable index of smoking topography and an indirect measure of smoke exposure[16]. Topography measurements from mouthpiece based computerized devices and direct observation differ a little from each other, however both methods show adequate levels of reliability [17]. Smoking activity is highly individualistic, which makes it difficult to characterize. Smoking topography parameters can vary as a function of sex, ethnicity, body mass index, nicotine yield, cigarette type, and stress level, [18]. Smoking behavior can also differ as a function of setting. In clinical and laboratory settings, participants tend to take more puffs with longer puff durations and shorter smoking duration as compared to natural settings [19].
Biomarker-based methods use concentrations of nicotine, cotinine, expelled carbon monoxide, and blood CO as measures of smoke exposure [20]. These biomarkers can be variously obtained from urine, plasma, erythrocytes, saliva, and expired air. From the complexity of the exposure agent (tobacco smoke contains several thousand constituents) and the diversity of the biological endpoints (various smoking-related diseases such as cancer, coronary heart disease, chronic obstructive pulmonary disease), it is evident that more than a single biomarker is needed to adequately reflect exposure and the risk associated with the use of tobacco products [21]. Furthermore, the validity of a biomarker depends on the accuracy of measurement which may be influenced by the individual’s physiological characteristics, the presence of a certain chemical in one’s body (such as food constituent), or uncompensated variations in static characteristics of measuring instruments and methods [22].
Self-reports, puff-topography devices, and biomarkers do not fully capture the intra-cigarette smoking behavior that could provide more precise information about smoke exposure. For the accurate measurement of this behavior, researchers have introduced wearable sensor systems that may include a combination of IMU and breathing sensors. IMU sensor systems typically utilize a gyroscope and accelerometer to capture hand-to-mouth gestures. Respiratory inductive plethysmograph (RIP) sensors are used to record breathing signals. Smoking inhalations can be detected with classification methods such as SVM, decision tree ensembles, and hidden Markov models (HMM) [23]–[31] applied to breathing and hand accelerometer signals. Wearable sensors offer a useful non-invasive method for the assessment of smoking behavior [32].
Most research to date has focused on understanding behavior during puffing with information obtained using self-report, puff-topography devices and biomarkers. However, little is known about the contribution of post-puff behavior to the health consequences of smoking. There is a need for a new system that can assess post-puff behavior and volume-related information. Post-puff behavior can be characterized by parameters such as duration of inhale-exhale, duration of smoke hold, the total smoke-cycle duration, tidal volume, and volume over time. These parameters may assist in better understanding the effect of smoking behavior on health.
This paper describes the Respiratory Smoke Exposure Metrics (RSEMs) and proposes an algorithm for their computation from the breathing signal captured by a wearable sensor system. The wearable sensor system consisted of a RIP sensor that measured the distinct pattern of smoke inhalation. The key metrics computed from the breathing pattern represented smoke exposure parameters including the number of puffs, puff duration, inter-puff interval, inhale-exhale duration, tidal volume, integral of inhale-exhale volume over time, and other metrics. The proposed metrics were compared to observations collected from video annotation and a puff topography device to evaluate the reliability and validity of RSEM.
II. Methods
A. Wearable Sensors
The Personal Automatic Cigarette Tracker v2 (PACT2.0) is a multi-sensory wearable system that included an instrumented lighter, a hand module, and an instrumented shirt with a chest module [25]. This paper is focused solely on the analysis of breathing signals collected using the RIP sensor of the chest module. The chest module used an STM32L151RD Cortex-M3 ARM processor with 32 MHz CPU, a 4GB micro-SD card to store sensor data and a micro-USB for the interface. The chest module contained a RIP sensor, RF proximity sensor, ECG, and bio-impedance respiratory sensor. CReSS (Clinical Research Support System for Laboratories) Pocket device (Borgawaldt Körber Solutions) was also used as a part of the study. This device automatically measured smoking such as date, time, start and end of smoking, puffs per cigarette, puff volume, and puff duration.
Fig. 1 shows the chest module used in this study. This chest module was attached to a T-shirt with RIP belt sewn on it at the chest level. Fig. 2 demonstrates the PACT 2.0 system used in this study.
Fig. 1.
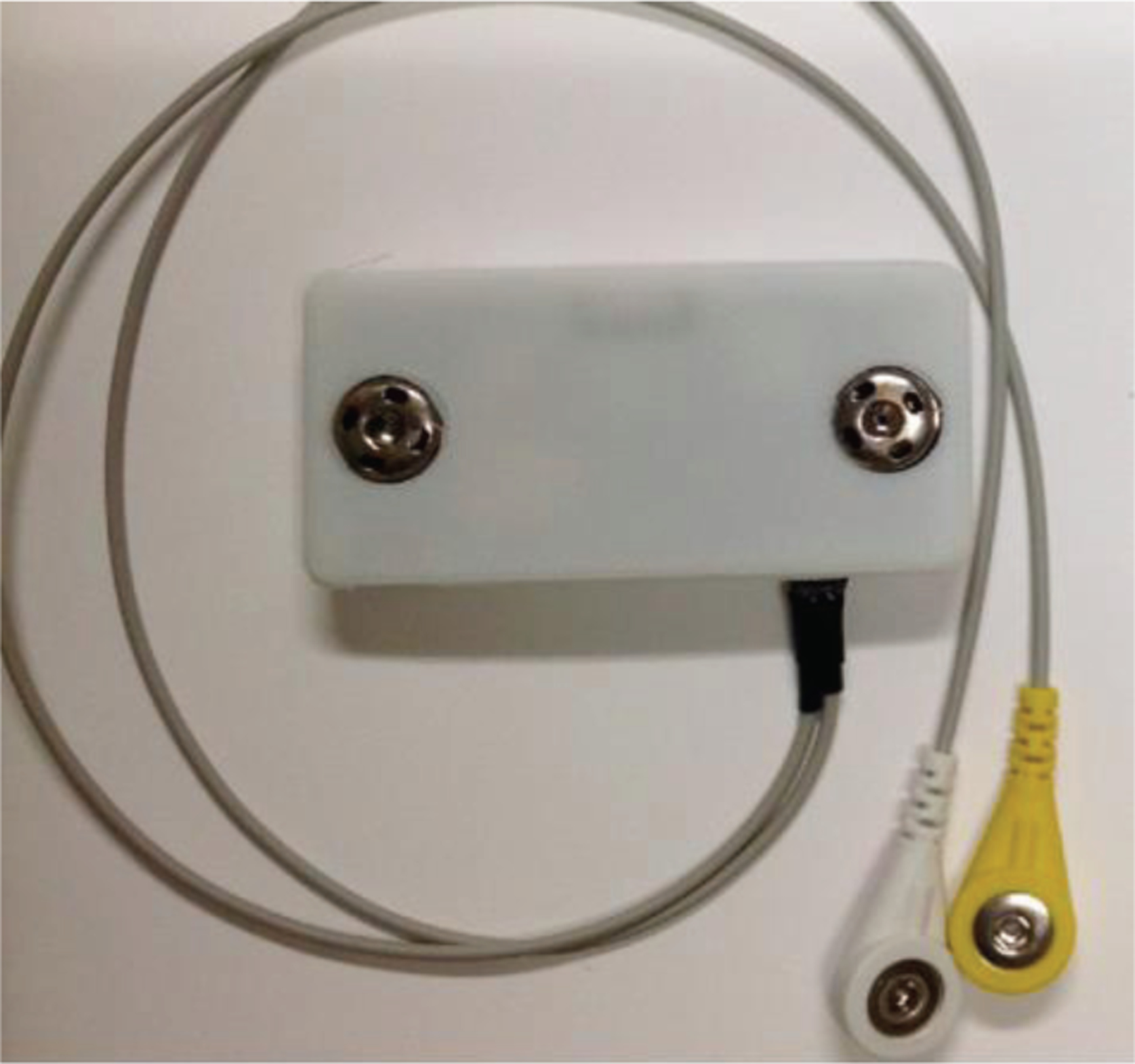
Chest Module [25]
Fig. 2.
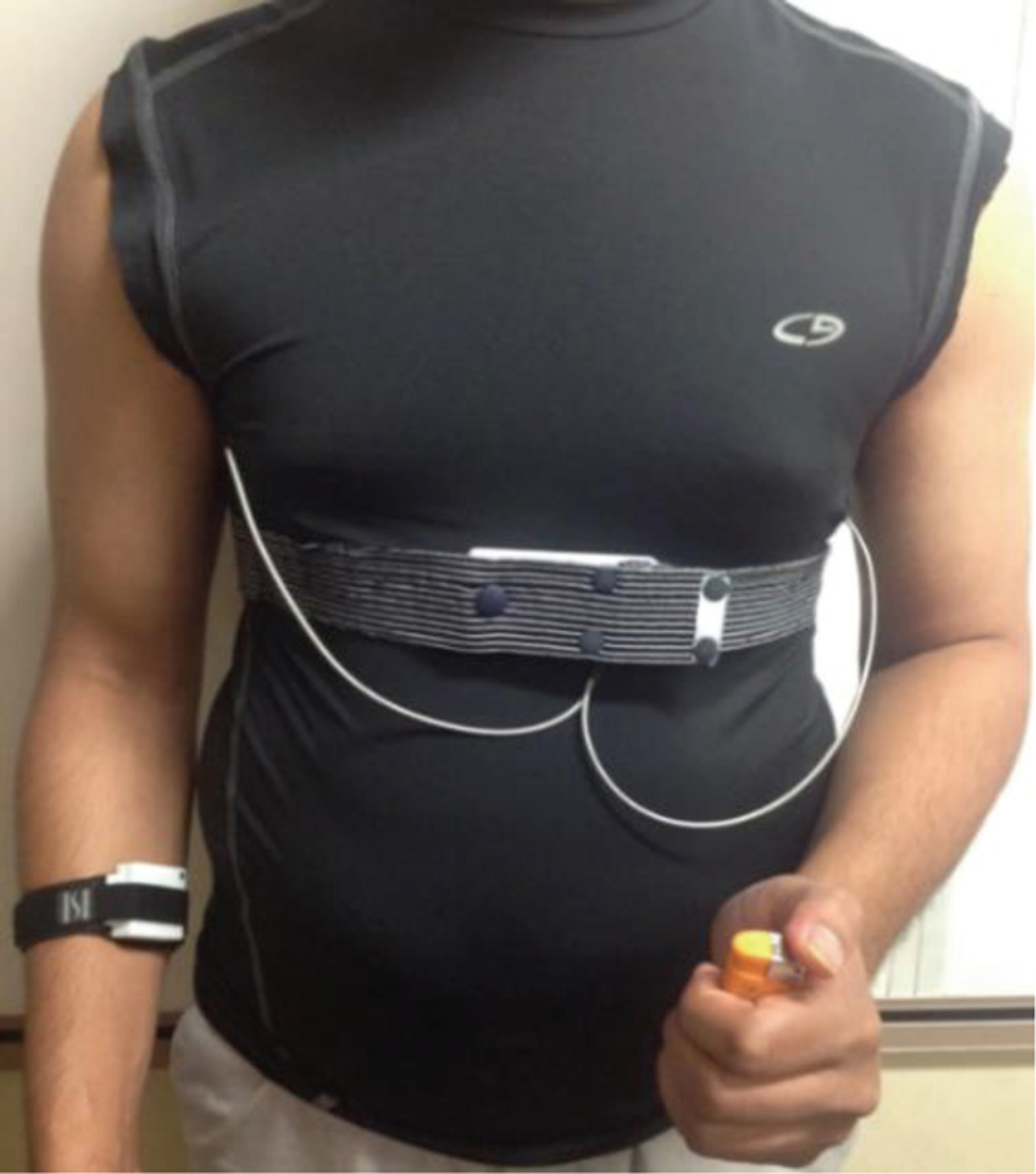
PACT 2.0 System
RIP is traditionally applied using two bands (thoracic and abdominal); however, the system becomes more cumbersome and obtrusive over extended durations. Further, the abdominal band tends to loosen over the data collection period. To address this issue, one thoracic belt was used for RIP, which provided reasonably accurate measurements and good stability over 24 hrs [33]. The volume measurements using one band were validated using bag calibration method (volume of bag 800 ml) mentioned in Section II. The results from our system showed similar volume measurements (0.79 ± 0.05 L) and RMSE as small as 0.03 ± 0.03 L [33]. This model with only one degree of freedom might have diminished accuracy, but the system has the advantage of being more stable than use of an abdomen belt and provides a linear relationship between volume change and thoracic perimeter change [34]. Since, the values obtained from the use of one thoracic belt correlates well with those obtained from bag calibration process, we might argue that even with a one thoracic belt an acceptable degree of accuracy can be achieved [33]. The performance of one degree of freedom with using thoracic belt was found to be reliable in several other studies as well [35]–[37]. There are multiple studies that utilized thoracic belt only for the respiratory measurements [27], [37]–[39].
B. Data Collection
Participants had to be between the ages of 19–70, report smoking at least 5 cigarettes per day, provide a breath carbon monoxide sample of > 5 parts per million (measured using a BreathCO vitalograph), report smoking for >1 year, asymptomatic, and have no acute or chronic respiratory problems. Participants provided informed consent prior to engaging in study activities. All study procedures were approved by the Institutional Review Board at the University of Alabama. This dataset was a subset of data used in [25]. Table I provides the summary of demographic and smoking characteristics of the participants. In addition, spirometry values such as expiratory forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and peak expiratory flow (PEF) were recorded for each subject.
Table I.
DEMOGRAPHIC AND SMOKING CHARACTERISTICS OF PARTICIPANTS
| Number of Participants | 38 |
|---|---|
| Gender | |
| Male | 24 |
| Female | 14 |
| Age | 25.24 ± 10.7years |
| BMI | 24.60 ± 6.13 kg/m2 |
| Cigarette Consumption per Day | 10.76 ± 5.5 |
| Packs per Year (estimated) | 180 ± 33.4 |
| Carbon Monoxide Level | 12.65 ± 5.98 ppm |
| Salivary Cotinine Level | 25.58 +/− 18.95 ng/mL |
| Spirometry Values | |
| FVC | 4.62 ± 1.22 L |
| FEV1 | 3.72 ± 0.98 L |
| PEF | 7.60 ± 1.44 L/min |
The study procedures were divided into two parts: the controlled portion (2~3 hours), conducted at the University of Alabama, and the free-living portion (~21 hours), where the participants followed their routine under free living conditions. Because of the availability of the reference measurement (CReSS and video observation), only data from the controlled portion of the study were used in development of smoke exposure metrics. All participants were studied individually on different days. When participants first arrived for the study, they were provided with the instrumented PACT 2.0 system. Participants then performed the activities as per the study protocol (see Table II). The start and end of all activities in the controlled portion were stored in an app, aTimeLogger, available for Android and iPhone devices.
Table II.
Study Protocol
| Activities | Duration |
|---|---|
| Spirometer test | Self-paced |
| Bag calibration | Self-paced |
| Read an article | 5 minutes |
| Walk on treadmill at self-paced slow speed | 5 minutes |
| Walk on treadmill at self-paced fast speed | 5 minutes |
| Unconstrained rest | 5 minutes |
| Sit and smoke without talking | Self-paced |
| Talk on cell phone | 5 – 10 minutes |
| Eat | Self-paced |
| Walk, talk, and smoke | Self-paced |
| Unconstrained activity | 15 minutes |
| Stand, talk and smoke | Self-paced |
| Unconstrained activity | 15 minutes |
| Walk and smoke without talking | Self-paced |
| Bag calibration | Self-paced |
| Free-Living | ~ 21 hours |
| Bag calibration | Self-paced |
| Sit and smoke (with CReSS device) without talking | Self-paced |
C. Signal Pre-processing
The raw breathing signal had noise, baseline wander, and motion artifacts. These were removed using a first-order high-pass Butterworth filter with a cut-off frequency of 0.1Hz and an average Gaussian filter of 10 points. The high pass filter was used to account for the motion artifacts. The high pass filtering did not affect the smoke inhalation or normal breathing pattern; this can be seen in Fig. 4. The smoke inhalation cycle has a distinct pattern consisting a puff, followed by smoke inhalation, smoke holding (optional), and smoke exhalation. During a puff, the smoke is drawn into the mouth but not into the lungs, thus, the puff is seen as an apnea (i.e. no breathing) or no change in volume. However, significant changes in lung volume can be observed during inhale-exhale followed by the puff. We have also validated the lung volume measurements using a bag calibration method described in Section II. The filtered signal was then calibrated using the calibration coefficient computed from the bag calibration procedure.
Fig. 4.
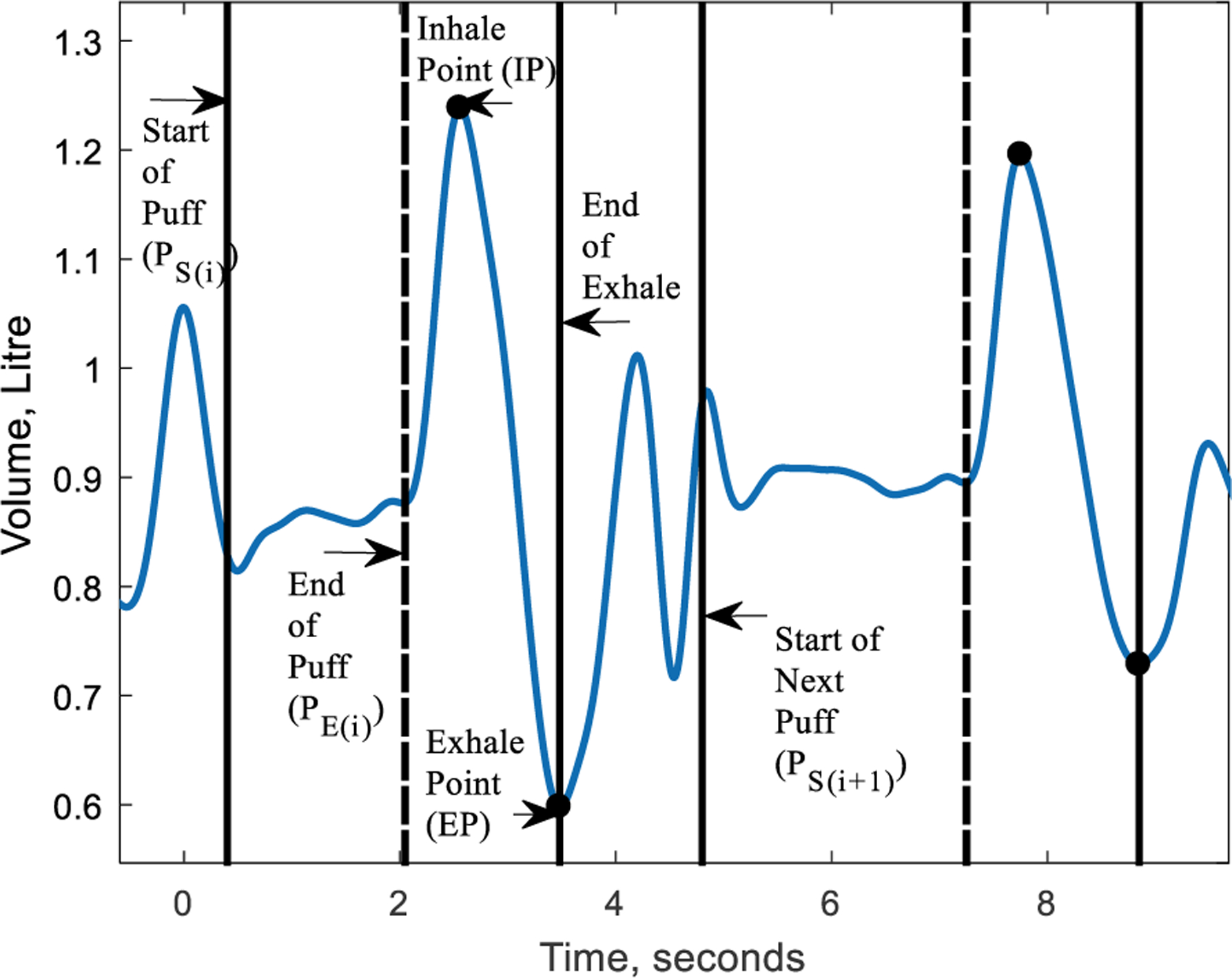
Smoke Exposure Metrics Computation using RSEM Algorithm
D. Calibration
A custom built 800ml calibration bag was used to calibrate the signal of the RIP sensor to a known volume. Calibration was conducted three times. The first calibration was performed after the spirometer test. The second calibration was the last activity during the controlled portion. The third calibration was performed when the participants returned from free-living portion of the study. During each calibration procedure, participants were asked to inflate and deflate the bag 14 times in three separate attempts (42 breathing cycles total). The two breathing cycles at the beginning and two at the end were discarded in case of extraneous events when starting and ending the collection of the sample. The remaining ten breathing cycles were used for the computation of the calibration coefficient. From these cycles, the maximum inhalation and minimum exhalation points in the rip signal were automatically detected; the absolute difference of these points provided the stretch of the breathing cycles. On average, the volume of the custom-built bag was 0.80 L with a standard deviation of 0.01 L. The calibration coefficient was computed from the first bag procedure, and the validation was done on 2nd and 3rd bag procedure. A total 3 bag samples, i.e. 30 breathing cycles, were used for calculating the calibration parameter, and 6 bag samples. i.e. 60 breathing cycles, were used to validate the calibration parameter. This calibration coefficient was then used to convert the breathing signal pulse counts into liters.
The equation (1) shows the formula to convert the breathing pulse counts into liters, SCal is the calibrated signal (in liter) and SOrg is the original signal (pulse count), EAmp is the exhale amplitude, RV is the reference volume i.e. 800 ml, γ is calibration coefficient.
| (1) |
E. Video Annotation
During the controlled portion of the study, a handheld camera was used to video record participants. This video was used to extract puff duration, inhale-exhale duration, and inhalation duration for individual smoking inhalation. Puff duration was defined as the time interval between the participant placing the cigarette in the mouth and removing it. Inhale-exhale duration was defined as the time interval between the participant removing the cigarette from the mouth and then completely exhaling the smoke i.e. no more smoke was expelled. The inhalation duration was defined as the duration of smoking cycle. The timestamps for the start and end of puff, start, and end of inhale-exhale were recorded to calculate durations. The annotations were used as a gold standard for evaluation of RSEM.
F. Algorithm for RSEM computation
The breathing signal has a characteristic pattern during a smoke inhalation (Fig. 3 (a), (b)). This pattern consists of an apnea/puff followed by an inhale, optional smoke holding, and an exhale. Our aim was to identify the start and stop of puff, the end inhale and exhale, start and stop of the smoke holding from the breathing signal. The end of inhale and exhale could be found by using a peak detection algorithm on the breathing signal. However, to find the end of puff duration and smoke holding, the rate of change of the breathing signal needed to be analyzed. This was done by taking the first-order derivative of the breathing signal. The end of puff was the zero-crossing point preceding the inhale point. Similarly, the end smoke holding/start of exhale was a zero-crossing point on the derivative signal preceding the exhale point. Once these parameters were identified, the smoke exposure metrics (puff duration, inhale-exhale duration, inhale-exhale volume, volume over time, etc.) could be computed. The definition of each parameter of smoke exposure metrics is given below. Fig. 3. (a), (b) shows these parameters with respect to a breathing signal.
Fig. 3.
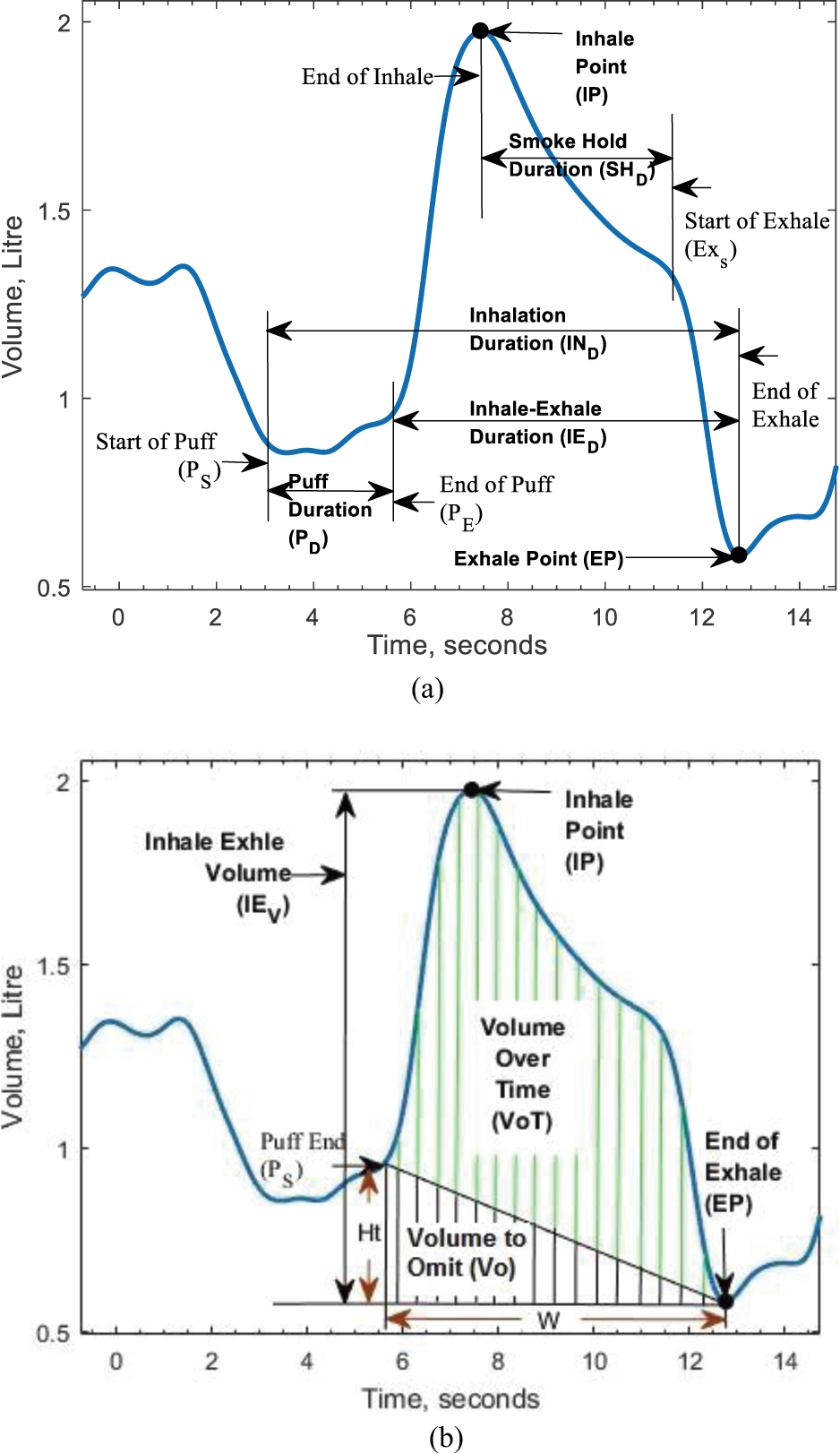
Smoke Exposure Metrics to be extracted from Breathing Signal (a) Shows the Puff Duration, Inhale-Exhale Duration, Smoke Hold Duration and Inhalation Duration, (b) Shows the Inhale-Exhale Volume, Volume over Time
1. Puff Duration (PD):
The duration for which a smoker drew smoke from the cigarette into the mouth. In the breathing signal, this part was seen by an apnea. Equation (2) was used to calculate the puff duration, with PE the end of the puff and PS the start of the puff.
| (2) |
2. Inhale Point (IP):
The point where a smoker has inhaled the smoke completely in his lungs. It is the end of the smoke inhale.
3. Exhale Point (EP):
The point where a smoker has exhaled the smoke completely. It is the end of the smoke exhale.
4. Inhale-Exhale Duration (IED):
The duration for which a smoker inhaled the smoke drawn from the cigarette into the lungs and then exhaled the smoke partially or fully through mouth/nose.
| (3) |
5. Smoke Hold Duration (SHD):
The duration for which smoke is held in the lungs after inhalation. The smoke hold was an intermediate step between the inhale and exhale point and was optional for a smoker. Equation (4) was used to calculate the smoke hold duration, where ExS was the start of exhale.
| (4) |
6. Inhalation Duration (IND):
The duration of one complete smoke cycle; that is, from the start of the puff till the end of exhale.
| (5) |
7. Inter-puff Interval (IPI):
The duration between two consecutive puffs. Equation (6) was used to calculate the inter-puff interval, where the start of next puff and the end of current puff.
| (6) |
8. Inhale-Exhale Volume (IEV):
The volume of smoke displaced between inhalation and exhalation.
| (7) |
9. Volume over Time (VoT):
The volume of smoke passing through the lungs of a smoker per unit time was measured as volume over time. This was calculated by using Simpson’s rule of integration between end of puff and exhale point. Simpson’s rule of integration calculated the area in green as well as the area in black as shown in Fig. 3(b). However, the volume over time was the area in green calculated by using Eq. (10).
| (8) |
| (9) |
| (10) |
G. Statistical Analysis
Smoking metrics collected from video annotations and CReSS were used to validate the RSEM algorithm. Not all methods used in the comparison produced the same breathing metrics (see Table III). The RSEM algorithm computed puff duration, inhale-exhale duration, inhale-exhale volume, inhale-exhale volume over time, smoke holding duration, inter-puff interval, and inhalation duration. The video annotations produced puff duration, inhale-exhale duration, inhalation duration, and inter-puff interval. The CReSS device yielded puff duration, puff volume, puff flow, and inter-puff interval.
Table III.
Available Smoke Exposure Metrics
| Metric | RSEM (PACT) | Puff topography (CReSS) | Video |
|---|---|---|---|
| Number of Puffs | ✓ | ✓ | ✓ |
| Puff Duration | ✓ | ✓ | ✓ |
| Inhale-Exhale Duration | ✓ | ✗ | ✓ |
| Inhale-Exhale Volume | ✓ | ✗ | ✗ |
| Volume over Time | ✓ | ✗ | ✗ |
| Smoke Holding Duration | ✓ | ✗ | ✗ |
| Inter-puff Interval | ✓ | ✓ | ✓ |
| Puff Volume | ✗ | ✓ | ✗ |
The inhale-exhale volume, inhale-exhale volume over time, and smoke holding were novel metrics that could not be computed from the video or CReSS, so we could not compare them across methods. For the validation of number of puffs, puff duration, inhale-exhale duration, and inter-puff interval obtained from the RSEM algorithm, ANOVAs were performed between CReSS, video annotations, and the respective values obtained from breathing signal. Interclass correlations (ICCs) and Pearson correlation coefficients were calculated for puff duration and inhale-exhale duration obtained from the algorithm with video and CReSS measurements.
III. Results
Fig. 4 shows the computation of the smoke exposure metrics from breathing signals using the RSEM algorithm. The solid black lines mark a smoke inhalation start and end. The dotted lines denote the end of the puff, the pair of black circles at the peak and valley denote the inhale and exhale points, respectively.
Table IV shows the average values for each parameter of smoke exposure computed from the RSEM algorithm, CReSS, and the video annotations. An ANOVA comparing the number of puffs per cigarette derived from video and CReSS showed no significant difference between the two methods [Fcric(1, 74) = 3.97, F = 0.25 at p = 0.05]. An ANOVA on the average puff duration computed across the three methods was not significant [Fcric(2, 1408) = 3.00, F = 0.58 at p = 0.05]. Similarly, a comparison of the inhale-exhale duration computed from the RSEM algorithm and the video was not significant [Fcric(1, 922) = 3.85, F = 2.29 at p = 0.05]. Analysis of the inter-puff interval for video, CReSS and RSEM algorithm showed no significant differences across the three methods [Fcric(2, 1330) = 3.00, F = 2.76 at p = 0.05].
Table IV.
Smoke Exposure Summary
| RSEM Algorithm | Video | CReSS | |
|---|---|---|---|
| Number of Puffs per Cig | 17.73±6.81 | 17.73±6.81 | 18.52 ±6.80 |
| Puff Duration (s) | 2.31±2.04 | 2.75±1.86 | 2. 42±1.37 |
| Inhale Exhale Duration (s) | 4.18±1.98 | 4.42±2.12 | - |
| Inhale-Exhale Volume (L) | 0.75±0.38 | - | - |
| Volume Over Time (L/s) | 3.14±1.60 | - | - |
| Smoke Holding Duration (s) | 1.17±0.19 | - | - |
| Inter-puff Interval (s) | 14.11±22.18 | 12.16±8.24 | 11.62±7.15 |
| Puff Volume (ml) | - | - | 80.15±62.36 |
The associations (ICCs and Pearson correlation coefficients) between shared smoke exposure parameters generated from the RSEM algorithm and the video and CRESS methods are shown in Table V and Table VI. In general, these associations were fairly robust with the exception of the Pearson correlation coefficient between puff duration from the RSEM algorithm and CReSS. This correlation (r12 = 0.7735), though strong, was significantly less (p<0.05) than the correlation between puff duration from the RSEM algorithm and the video (r13 = 0.9781).
Table V.
Inter-Class Correlation Of Values Calculated From Rsem Algorithm With Video And Cress Measurements
| Inter-class Correlation Coefficient | ||||||
|---|---|---|---|---|---|---|
| Activities | Sit Quiet Smoke | Walk, Talk Smoke | Stand Talk Smoke | Walk Quiet Smoke | Sit Quiet Smoke using CReSS | |
| Correlation between | RSEM and Video | RSEM and Video | RSEM and Video | RSEM and Video | RSEM and Video | RSEM and CReSS |
| Puff Duration | 0.84 | 0.80 | 0.83 | 0.81 | 0.85 | 0.87 |
| Inhale Exhale Duration | 0.79 | 0.76 | 0.81 | 0.77 | 0.81 | N/A |
Table VI.
Pearson Correlation Of Values Calculated From Rsem Algorithm With Video And Cress Measurements
| Pearson Correlation Coefficient | ||||||
|---|---|---|---|---|---|---|
| Activities | Sit Quiet Smoke | Walk, Talk Smoke | Stand Talk Smoke | Walk Quiet Smoke | Sit Quiet Smoke using CReSS | |
| Correlation between | RSEM and Video | RSEM and Video | RSEM and Video | RSEM and Video | RSEM and Video | RSEM and CReSS |
| Puff Duration | 0.93 | 0.90 | 0.94 | 0.92 | 0.97 | 0.77 |
| Inhale Exhale Duration | 0.81 | 0.78 | 0.80 | 0.79 | 0.84 | N/A |
IV. Discussion
Although PACT 2.0 consists of an instrumented lighter, hand device, and chest device, the goal of this research was to develop new smoke exposure metrics and to generate deeper insights about cigarette smoke exposure based on detailed analyses of breathing patterns (collected using the RIP sensor of the chest module) during smoking. In order to enhance understanding of smoking patterns and exposure, we proposed novel metrics of smoke exposure, RSEM. The RSEM algorithm was applied to breathing data from 38 participants. The RSEMs were compared with metrics computed from video coding of smoking and a computerized hand-held topography device, CReSS.
Puff topography provides the information about the number of puffs, puff duration, puff volume, and, inter-puff interval, i.e., information about what happens during the puff. However, information about post-puff behavior is not available from puff topography, self-report, or biomarkers. Post-puff behavior includes the inhale-exhale duration, smoke holding duration, tidal volume, and inhale-exhale volume over time. These post-puff behavior metrics may help in better understanding the relationship between smoke exposure and biomarkers.
The post-puff parameters (inhale-exhale duration, inhalation duration) were also found reliable when compared with the video annotations. The results support the use of metrics computed from the breathing signal, as the smoke exposure metrics of puff duration, number of puffs, and inter-puff interval showed similar values to those provided by conventional puff topography. This comparison was made only to show that, in addition to post puff behavior, RSEM can index conventional puff metrics as well. Moreover, the metrics obtained from the breathing signal were richer as they provided post-puff information and included measures not available from the topography devices (e.g., duration of smoke holding, inhale-exhale duration). Thus, these metrics may provide new insights into smoke exposure. Furthermore, the smoke exposure metrics computed using RSEM could be used to understand the relationship between post-puff behavior and biomarkers of smoke exposure. Thus, these metrics may provide new insights into smoking behavior and the health consequences of smoke exposure. The post-puff metrics need further evaluation to characterize their contribution to overall smoke exposure.
Previous studies have suggested analysis of breathing signals to classify smoking and non-smoking inhalation and to detect smoking episodes, but systematic analysis of smoke exposure metrics from the breathing signal have not been conducted. Research to date has focused on topography devices that give information about the puff duration, puff count, puff volume, and inter-puff interval. Such devices do not generate information about parameters after the puff is taken, i.e. the inhale-exhale duration, inhale-exhale volume, smoke holding, and volume over time. The PACT device and RSEM algorithm estimate smoke exposure without altering natural patterns of smoking behavior. As seen in Fig. 4, the RSEM algorithm accurately detected the boundaries of puff start-stop, inhale and exhale point. The detection of these events was verified with the data from video. The RSEM algorithm also identified whether smoke holding was present or absent and quantified the duration of this metric.
The RSEM algorithm computed smoke exposure metrics for 38 participants in the controlled portion of the study including five smoking activities as described in Section II. Table IV shows the average of parameters obtained from each RSEM, video annotation, and CReSS device. The average number of puff per cigarette obtained from the video was 17.73; CReSS calculated 18.52 puffs. Typically, the CReSS system counted one or more extra puffs. CReSS detects a puff based on the pressure change in the mouthpiece as a result of inhalation. Sometimes when the participant took a single long puff, the device recorded it as multiple puffs due to changes in flow rate. This could be the source of the difference in the number of puffs from video and CReSS [17]. The average puff duration obtained from the RSEM algorithm, video and CReSS was 2.56±2.06 sec, 2.75±1.86 sec, and 2.38±0.90 sec, respectively. The average inhale-exhale duration obtained from the RSEM algorithm and video was 4.18±1.98 sec and 4.42±2.12 sec, respectively. The validity of the RSEM algorithm for computation of puff duration and inhale-exhale duration was supported by the absence of significant differences in average values across methods. Furthermore, the strong cross-method correlation computed from the RSEM algorithm, video and CReSS supported the accuracy of the RSEM algorithm.
The performance of the algorithm across different smoking activities (listed in Table II) indicates that the computation of smoke exposure metrics was generally acceptable. However, the algorithm’s performance in computing puff duration and inhale-exhale duration during walk, talk and smoke activity, though acceptable, was lower than during other activities. This may be attributed to artifacts from body motion. In general, the inter-class correlations and Pearson correlations suggest the system has reasonable accuracy. The system may dependably be used even in free-living environment to calculate smoke exposure metrics across different smoking activities with some adjustment to account for motion artifacts during walk, talk and smoke activities.
PACT 2.0 consists of several modalities including an instrumented lighter, hand device, and chest device. These modalities can be used to detect smoking, but, in this particular paper, only characterized breathing during smoking as a biometric of smoking behavior is studied. PACT 2.0 might be used to identify smoking events using the integration of information from an instrumented lighter and a 6-axis Inertial Measurement Unit (IMU) on the wrist [40]. Validation findings from free-living conditions demonstrated 84.9% agreement with self-report cigarettes showing the potential use of an IMU and instrumented lighter for smoking detection [40]. Also, the regularity of hand gestures estimated using a one axis accelerometer on the wrist of the dominant hand can be used to detect smoking events [41]. The further evaluation of combined patterns of subsets of sensor data to characterize smoking behavior should be a focus of future work.
One limitation of the RSEM algorithm was that metric computation was semi-automatic, as the start/end of smoke inhalation was taken from the video as a reference point. Thus, the algorithm was applicable only in the controlled portion of the study. To address this issue, the RSEM algorithm could be further improved to identify individual smoke inhalations automatically and then compute the smoke exposure metrics. This algorithm would then no longer require manual input and could be used to analyze smoke exposure in free-living scenarios.
V. Conclusion
In this work, we presented novel metrics of smoke exposure (RSEM) and an algorithm for their computation. The RSEM algorithm was able to identify novel, previously unreported smoke exposure metrics from the breathing signal. We obtained high correlations between the smoke exposure parameters computed from breathing signal and the ground truth obtained from video and a computerized topography device. Moreover, the analysis comparing the three methods (video, CReSS and RSEM algorithm) supported the accuracy of computation of the smoke exposure metrics from the breathing signal. The RSEMs also provides deeper insights into smoking behavior than available through a widely used portable topography device or manual annotation of video recorded during smoking. This approach encourages further research on smoking exposure by allowing the measurement of a range of potentially important parameters including inhale-exhale duration, inhale-exhale volume, smoke holding, and volume over time. This work can be extended to free-living conditions by automatic identification of smoke inhalation and computation of smoke exposure metrics. Future research examining relationships between these breathing metrics and biomarkers of smoke exposure will be useful for evaluating the validity of these metrics and for generating a deeper understanding of the relationships between these micro-components of smoking behavior and the health consequences of cigarette smoking.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award R01DA035828. The content is solely the responsibility of the authors and does not necessarily represent official views of NIH. This work was also approved by IRB under Protocol 14-025-ME Validation of a System for Noninvasive Monitoring of Cigarette Smoking.
Contributor Information
Masudul H Imtiaz, Department of Electrical and Computer Engineering, University of Alabama, Tuscaloosa, AL 35487 USA..
Stephen Tiffany, Department of Psychology, University at Buffalo, The State University of New York, Buffalo, NY 14260 USA..
Edward Sazonov, Department of Electrical and Computer Engineering, the University of Alabama, Tuscaloosa, AL 35487 USA..
References
- [1].C. O. on S. and Health, “Smoking and Tobacco Use; Fact Sheet; Adult Cigarette Smoking in the United States;,” Smoking and Tobacco Use, 24-September-2018. [Online]. Available: http://www.cdc.gov/tobacco/data_statiscal/fact_sheets/adult_data/cig_smoking/. [Google Scholar]
- [2].“WHO | WHO report on the global tobacco epidemic 2011,” WHO. [Online] Available: http://www.who.int/tobacco/global_report/2011/en/. [Google Scholar]
- [3].World Health Organization, Ed., Global health risks: mortality and burden of disease attributable to selected major risks. Geneva, Switzerland: World Health Organization, 2009. [Google Scholar]
- [4].Siegel RL, Miller KD, and Jemal A, “Cancer statistics, 2018: Cancer Statistics, 2018,” CA: A Cancer Journal for Clinicians, vol. 68, no. 1, pp. 7–30, January 2018. [DOI] [PubMed] [Google Scholar]
- [5].Siegel RL et al. , “Deaths Due to Cigarette Smoking for 12 Smoking-Related Cancers in the United States,” JAMA Intern Med, vol. 175, no. 9, pp. 1574–1576, September 2015. [DOI] [PubMed] [Google Scholar]
- [6].Centers for Disease Control and Prevention (US), National Center for Chronic Disease Prevention and Health Promotion (US), and Office on Smoking and Health (US), How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US), 2010. [PubMed] [Google Scholar]
- [7].“Smoking and Cardiovascular Disease,” p. 2. [Google Scholar]
- [8].United States Surgeon General, “The Health Consequences of Smoking -- 50 Years of progress: A Report of the Surgeon General: (510072014–001).” American Psychological Association, 2014. [Google Scholar]
- [9].Jamison DT et al. , Eds., “Chapter 44. Prevention of Chronic Disease by Means of Diet and Lifestyle Changes,” in Disease Control Priorities in Developing Countries (2nd Edition), World Bank Publications, 2006, pp. 833–850. [Google Scholar]
- [10].Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, and Koren G, “Methods for Quantification of Exposure to Cigarette Smoking and Environmental Tobacco Smoke: Focus on Developmental Toxicology:,” Therapeutic Drug Monitoring, vol. 31, no. 1, pp. 14–30, February 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Warthen MW and Tiffany ST, “Evaluation of cue reactivity in the natural environment of smokers using ecological momentary assessment,” Exp Clin Psychopharmacol, vol. 17, no. 2, pp. 70–77, April 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shahab L et al. , “The reliability and validity of self-reported puffing behavior: Evidence from a cross-national study,” Nicotine & Tobacco Research, vol. 10, no. 5, pp. 867–874, May 2008. [DOI] [PubMed] [Google Scholar]
- [13].Stelmach R et al. , “Comparison between objective measures of smoking and self-reported smoking status in patients with asthma or COPD: are our patients telling us the truth?,” J Bras Pneumol, vol. 41, no. 2, pp. 124–132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Behar RZ, Hua M, and Talbot P, “Puffing Topography and Nicotine Intake of Electronic Cigarette Users,” PLOS ONE, vol. 10, no. 2, p. e0117222, February 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Jesus S, Hsin A, Faulkner G, and Prapavessis H, “A systematic review and analysis of data reduction techniques for the CReSS smoking topography device,” Journal of Smoking Cessation, vol. 10, no. 01, pp. 12–28, June 2015. [Google Scholar]
- [16].Pickworth W, Lee E, Malson J, Moolchan E, and Waters A, “Smoking topography: Reliability and validity in dependent smokers,” Nicotine & Tobacco Research, vol. 5, no. 5, pp. 673–679, October 2003. [DOI] [PubMed] [Google Scholar]
- [17].Blank MD, Disharoon S, and Eissenberg T, “Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation,” Nicotine & Tobacco Research, vol. 11, no. 7, pp. 896–903, July 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frederiksen LW, Miller PM, and Peterson GL, “Topographical components of smoking behavior,” Addictive Behaviors, vol. 2, no. 1, pp. 55–61, January 1977. [DOI] [PubMed] [Google Scholar]
- [19].Ossip-Klein DJ, Martin JE, Danley Lomax B, Prue DM, and Jo Davis C, “Assessment of smoking topography generalization across laboratory, clinical, and naturalistic settings,” Addictive Behaviors, vol. 8, no. 1, pp. 11–17, January 1983. [DOI] [PubMed] [Google Scholar]
- [20].Chang CM, Edwards SH, Arab A, Del Valle-Pinero AY, Yang L, and Hatsukami DK, “Biomarkers of Tobacco Exposure: Summary of an FDA-sponsored Public Workshop,” Cancer Epidemiol Biomarkers Prev, vol. 26, no. 3, pp. 291–302, March 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Benowitz NL, “Biomarkers of Environmental Tobacco Smoke Exposure,” Environmental Health Perspectives, vol. 107, p. 7, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Benowitz NL, “Cotinine as a Biomarker of Environmental Tobacco Smoke Exposure,” Epidemiologic Reviews, vol. 18, no. 2, pp. 188–204, January 1996. [DOI] [PubMed] [Google Scholar]
- [23].Berry D, Bell J, and Sazonov E, “Detection of cigarette smoke inhalations from respiratory signals using decision tree ensembles,” in SoutheastCon 2015, 2015, pp. 1–4. [Google Scholar]
- [24].Patil Y, Lopez-Meyer P, Tiffany S, and Sazonov E, “Detection of cigarette smoke inhalations from respiratory signals using reduced feature set,” in 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2013, pp. 6031–6034. [DOI] [PubMed] [Google Scholar]
- [25].Imtiaz M, Ramos-Garcia R, Senyurek V, Tiffany S, and Sazonov E, “Development of a Multisensory Wearable System for Monitoring Cigarette Smoking Behavior in Free-Living Conditions,” Electronics, vol. 6, no. 4, p. 104, November 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lopez-Meyer P, Tiffany S, and Sazonov E, “Identification of cigarette smoke inhalations from wearable sensor data using a Support Vector Machine classifier,” in 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2012, pp. 4050–4053. [DOI] [PubMed] [Google Scholar]
- [27].Ali AA, Hossain SM, Hovsepian K, Rahman MM, Plarre K, and Kumar S, “mPuff: Automated detection of cigarette smoking puffs from respiration measurements,” in 2012 ACM/IEEE 11th International Conference on Information Processing in Sensor Networks (IPSN), 2012, pp. 269–280. [Google Scholar]
- [28].Mittelmark MB, Murray DM, Luepker RV, Pechacek TF, Pirie PL, and Pallonen UE, “Predicting experimentation with cigarettes: the childhood antecedents of smoking study (CASS).,” American Journal of Public Health, vol. 77, no. 2, pp. 206–208, February 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saleheen N et al. , “puffMarker: a multi-sensor approach for pinpointing the timing of first lapse in smoking cessation,” in Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing - UbiComp ‘15, Osaka, Japan, 2015, pp. 999–1010. [PMC free article] [PubMed] [Google Scholar]
- [30].Ramos-Garcia RI, Sazonov E, and Tiffany S, “Recognizing cigarette smoke inhalations using hidden Markov models,” in 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2017, pp. 1242–1245. [DOI] [PubMed] [Google Scholar]
- [31].Patil Y, Tiffany S, and Sazonov E, “Understanding smoking behavior using wearable sensors: Relative importance of various sensor modalities,” in 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2014, pp. 6899–6902. [DOI] [PubMed] [Google Scholar]
- [32].Sazonov E, Lopez-Meyer P, and Tiffany S, “A Wearable Sensor System for Monitoring Cigarette Smoking,” Journal of Studies on Alcohol and Drugs, vol. 74, no. 6, pp. 956–964, November 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ramos-Garcia RI, Imtiaz MH, Sazonov E, and Tiffany ST, “Evaluation of RIP sensor calibration stability for daily estimation of lung volume,” in 2017 Eleventh International Conference on Sensing Technology (ICST), 2017, pp. 1–5. [Google Scholar]
- [34].White BM, Zhao T, Lamb J, Bradley JD, and Low DA, “Quantification of the thorax-to-abdomen breathing ratio for breathing motion modeling,” Medical Physics, vol. 40, no. 6Part1, p. 063502, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Leutheuser H, Heyde C, Roecker K, Gollhofer A, and Eskofier BM, “Reference-Free Adjustment of Respiratory Inductance Plethysmography for Measurements during Physical Exercise,” IEEE Transactions on Biomedical Engineering, vol. 64, no. 12, pp. 2836–2846, December 2017. [DOI] [PubMed] [Google Scholar]
- [36].Liu S, Gao R, He Q, Staudenmayer J, and Freedson P, “Improved regression models for ventilation estimation based on chest and abdomen movements,” Physiol Meas, vol. 33, no. 1, pp. 79–93, January 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Retory Y, Niedzialkowski P, de Picciotto C, Bonay M, and Petitjean M, “New Respiratory Inductive Plethysmography (RIP) Method for Evaluating Ventilatory Adaptation during Mild Physical Activities,” PLoS ONE, vol. 11, no. 3, p. e0151983, March 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rétory Y, David P, de Picciotto C, Niedzialkowski P, Bonay M, and Petitjean M, “Validity of thoracic respiratory inductive plethysmography in high body mass index subjects,” Respiratory Physiology & Neurobiology, vol. 242, pp. 52–58, August 2017. [DOI] [PubMed] [Google Scholar]
- [39].Retory Y, de Picciotto C, Niedzialkowski P, Petitjean M, and Bonay M, “Body Mass Index-Dependent Ventilatory Parameters From Respiratory Inductive Plethysmography During 6-Minute Walk Test,” Respiratory Care, vol. 61, no. 4, pp. 521–528, April 2016. [DOI] [PubMed] [Google Scholar]
- [40].Senyurek V, Imtiaz M, Belsare P, Tiffany S, and Sazonov E, “Cigarette Smoking Detection with an Inertial Sensor and a Smart Lighter,” Sensors (Basel), vol. 19, no. 3, January 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Senyurek VY, Imtiaz MH, Belsare P, Tiffany S, and Sazonov E, “Smoking detection based on regularity analysis of hand to mouth gestures,” Biomed Signal Process Control, vol. 51, pp. 106–112, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


