Introduction
The vertebrate lung is the organ with the largest surface area presented to the external environment. The combined alveolar surface area of both adult human lungs is about 100 m2 [1], and typically 10,000 to 20,000 liters of air are inhaled per day [2]. Coupled with the fact that fungal spore densities of 10 to 50 spores per liter of air are common [3, 4], the average person inhales up to 100,000 or more fungal spores daily. Lung surface areas and inhalation volumes for small mammals are comparable to those of humans when scaled for size. Moreover, many small mammals live in microenvironments (notably burrows and understories) where they are exposed to high densities of airborne spores derived from the growth of fungi on substrates in soil and litter. While lung tissues have physical and immunological defenses against infection, it is also the case that the air and blood-vessel interface is by necessity fragile. Taken together, these factors make it unsurprising that many fungi have adaptations that permit commensal, pathogenic, and perhaps, yet to be discovered, mutualistic relationships with lungs.
The title of this article references the fact that the study of interactions between lungs and certain fungi began in the first half of the 20th century. As we discuss here, modern molecular methods combined with culture-based approaches are revealing that lung mycobiomics is a field rich in opportunities for studying interactions of fungi across the vertebrate tree of life. Moreover, next-generation sequencing efforts now provide important new contexts for the study of lung-inhabiting fungi that began more than seven decades ago. Here, we briefly cover the historical context of the lung mycobiome, discuss future directions and questions, and propose that the lung mycobiome may form a reservoir for opportunistic fungal mycoses caused by commensal lung-adapted fungi. We discuss the following points: (1) Human lungs have a mycobiome. (2) Lungs of nonhuman mammals commonly possess a diversity of fungi. (3) Studies of fungi associated with lungs have implications for the ecology, distribution, and pathogenicity of specific fungi, especially members of the Onygenales and species of Pneumocystis. (4) A framework is needed to distinguish among lung-adapted fungi (some of which can be opportunistic pathogens), members of the general mammalian mycobiome, and fungi that are present in lungs because of incidental inhalation. (5) Museum research collections provide an important resource for addressing these issues.
Human lungs possess a mycobiome
The Human Microbiome Project launched by the National Institutes of Health (NIH) in 2007 ultimately resulted in recognition that microbes can alter the physiology, immunity, and neurological development of their hosts [5]. The traditional thought that lung tissues are sterile, left unchallenged by the difficulties of sampling lungs, resulted in lung microbiome studies lagging other aspects of human microbiomics. Innovations in specimen collection and advances in deep sequencing and bioinformatics have now established a genuine lung microbiome [6]. Studies of the lung microbiome have focused primarily on bacteria, but studies of the fungal component of the lung microbiome (mycobiome) are beginning to emerge.
Initial investigations of the human lung mycobiome involved individuals with lung diseases such as cystic fibrosis (CF) [7], asthma, and chronic obstructive pulmonary disease. From these studies, it appears that the mycobiome of unhealthy lungs can become dominated by one or few fungal species but may have a greater fungal burden [8]. Species of Candida, especially Candida albicans, can be dominant fungi in the lungs of CF patients and are associated with reduced lung function [9, 10]. Species of Malassezia were broadly detected in CF samples but at levels 10-fold to 50-fold lower than Candida [11]. Malassezia species have similarly been found in the lungs of asthmatic patients [12] and healthy individuals [8] and may be commonplace in the lungs. In addition, dominant fungal taxa associated with the lungs of healthy individuals include species of Cladosporium, Aspergillus, and Penicillium [12, 13]. Analyses of sputum samples from both diseased and healthy human airways have indicated a fungal community composed largely of transient species, suggesting that the majority of fungi in such samples represent recent inhalation rather than colonization [14, 15]. It is possible, however, that fungal colonization of airways is somewhat stochastic, resulting in the appearance of transient inhalation effects when colonization has in fact occurred. It is also the case that sputum samples might fail to sample fungi that are deeply invasive in lung tissue.
Several fungi deserve special attention in the context of lungs. These include species of Pneumocystis, which are obligate lung commensals detected in 20 to 60% of human lungs [16, 17], Aspergillus fumigatus, which is the primary cause of aspergillosis, and members of the order Onygenales. Medically important members of the Onygenales include species of Coccidioides, Paracoccidioides, Blastomyces, Emmonsia, and Histoplasma as well as members of a newly described genus, Emergomyces [18, 19]. While members of these groups are often associated with some type of pathology, it is now a legitimate question whether many of them are part of a normal lung mycobiome.
Into the wild: Fungi in the lungs of nonhuman mammals
While the full mycobiome of nonhuman mammals has not been studied, such studies hold substantial promise for investigating the diversity and adaptations of fungi that occur in mammalian lungs. More than one-half century before interest in the human microbiome, several scientists systematically explored the microbiology of the vertebrate lung in rodents, rabbits, and carnivores in the context of pulmonary diseases. These studies strongly influenced our understanding of lung pathogens, their distributions, and life cycles. What these early studies missed was the fact that the lung is a microenvironment rich in microorganisms, many of which appear to be adapted to persist there. Similarly, mycobiome studies of humans stand to be informed by studies of wild rodents and other nonhuman mammals, in part because of limitations of sampling and sequencing approaches available for human studies (reviewed by [20]), but also because comparative analyses can help reveal long-term coevolutionary relationships.
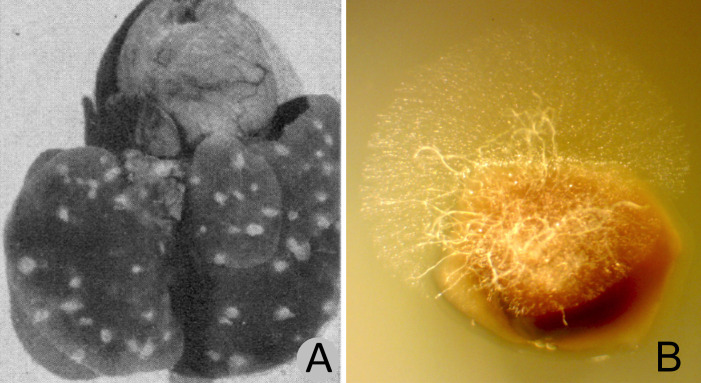
In the 1940s and 1950s, culture-based and histopathological studies led by Chester Emmons identified and characterized two fungi from the lungs of mammals in Arizona (Fig 1). These fungi were known at the time as Haplosporangium parvum and Coccidiodies immitis [21]. These studies were followed by additional studies of H. parvum across the United States and Canada [22, 23], resulting in the recovery of pulmonary fungi from a number of deer mice (Peromyscus), pocket mice (Perognathus and Chaetodipus), woodrats (Neotoma), red squirrel (Tamiasciurus), beaver (Castor canadensis), cottontail rabbit (Sylvilagus), pika (Ochotona princeps), skunk (Mephitis), marten (Martes americana), and weasels (Mustela frenata and one M. erminea) [21–23]. Haplosporangium parva was later renamed Emmonsia parva [24] and, recently, is considered as a member of the genus Blastomyces (as Blastomyces parvus) [18]. C. immitis was later divided into two species, C. immitis and C. posadasii, with C. posadasii most common in Arizona and other locations outside California [25].
Fig 1. Small-mammal lung tissues showing fungal growth.
(A) Lung from Peromyscus sp. collected in the vicinity of San Carlos, Arizona, with fungal lesions believed to be Emmonsia parva (Blastomyces parvus), reproduced from Emmons and Ashburn [21]. (B) Fungal hyphae in lung tissue from apparently healthy Dipodomys heermanni collected in Kern County, California, (MVZ:239394) from which E. parva was recovered in pure culture. The lung fragment shown was incubated for 48 hours on water agar with tetracycline (10 mg/ml) and chloramphenicol (50 mg/ml). The fungal growth shown in B is typical for small-mammal lungs we have examined from diverse species. Segments of any given lung plated on growth medium will often result in growth of multiple fungal species. The image shown in A is from Public Health Reports (volume 57) and is in the public domain.
Onygenealan fungi emerged as presumed highly adapted pathogens of animal hosts approximately 150 million years ago [26]. Most or all appear capable of saprobic growth at some stage of their life cycle, ultimately producing spores that can be inhaled by a susceptible host, then switching to pathogenic forms (yeast-like stages, spherules, and adiaspores). Recently, Taylor and Barker [27] reviewed modern studies that support Emmons’ hypothesis [28, 29] that small mammals provide an environmental reservoir for species of Coccidioides as an endozoan commensal that then becomes a saprobe taking advantage of soil disturbance for wind dispersal. This hypothesis is supported in part by the fact that Coccidioides genomes have a reduced number of genes associated with plant cell-wall degradation and an increased number of genes associated with animal pathogenesis [26]. The hypothesis is also supported by growth studies of a close Coccidioides relative, Uncinocarpus reesei, which both shares the gene family expansions and contractions seen in Coccidioides and exhibits a preference for proteinaceous growth substrates over carbohydrates [30].
Historical and recent studies suggest that this hypothesis should be expanded to include other members of the Onygenales, such as E. parva (B. parvus) and members of the genus Emergomyces as well as other fungi that appear to be adapted for the lung mycobiome, including the special case of species of Pneumocystis. Because many of these fungi are known for their ability to cause opportunistic infections, from one point of view they could be considered to be commensal organisms with the potential to become pathogenic in hosts that become immune compromised or are otherwise weakened by comorbidities. An alternative interesting possibility is that these fungi are actually part of a community of organisms that coexist with host tissues and provide defenses against other infectious agents. In this context we note that although members of the Onygenales appear not to have been extensively studied in terms of antimicrobial compounds, reports of biologically active secondary metabolites, including compounds with antifungal activities, do exist [e.g., 31].
Perhaps the most exquisitely adapted members of lung mycobiomes, species of Pneumocystis are widespread among mammals, are specifically adapted to lung tissues, and exhibit host specificity. Co-evolution between mammalian hosts and species of Pneumocystis has been shown for humans, nonhuman primates, and bats [32–34]. Studies of such Pneumocystis-host associations have the potential to provide insights for transmission, phylogenetic relationships, and cell biology.
A proposed framework for thinking about the lung mycobiome
Among the fungi observed in lung tissues, it is possible to conceive of three broad categories of fungi found in lungs of healthy mammals: (1) fungal cells that result from incidental inhalation, arguably not part of the true mycobiome; (2) fungi adapted to be part of the normal mammalian mycobiome but are not specialized for specific tissues; and 3) fungi evolved to inhabit lung tissues, whether as commensals, mutualists, or pathogens. Fungi that produce abundant quantities of airborne spores, such as the conidia of Cladosporium, Aspergillus, and Penicillium, or basidiospores of Agaricales [35, 36], are candidates for the first group. The second group might well include species of Candida and Malassezia, which are common members of the human mycobiome occurring in association with skin [37–39]. Species of Pneumocystis, long evolved symbionts that can be viewed as either commensals or opportunistic pathogens, easily belong in the third category; and multiple members of the Onygenales likely belong there as well. When the immune system of the human host is suppressed or compromised (e.g., asthmatics, CF, cancer treatment, and organ transplant), fungi that normally inhabit group one, for example A. fumigatus, can become life-threatening pathogens and constitute an additional group of opportunistic lung pathogens [40]. Teasing apart the differences and overlaps among these categories in wild animals can at least begin with molecular surveys that determine which fungal sequences are recovered repeatedly from lungs of animals. Ultimately, it will be valuable to compare the results from such surveys with those obtaining airborne fungi in the same geographic area, and with those of different host characteristics (species, age, sex, diet, genetics, and comorbidities) to understand fungal–host community interactions.
Museum collections will prove important
Natural history collections are proving extremely useful to the goal of characterizing the mammalian lung mycobiome. More generally, these collections represent essential infrastructure for research, training, and education that continue to play vital roles in long-established fields (biodiversity discovery, systematics, and evolution), while now contributing to new research areas (genomics, stable isotopes, and pathogen research [41]). Next-generation sequencing paired with culture-based approaches and modern methods in cell biology allow a holistic approach for studying the mycocosm of the lung and for discovery of a diverse array of parasites and zoonotic pathogens. Museum collections can help address mycobiome questions related to spatial or temporal changes, community composition, host specificity (e.g., species), and individual variation (e.g., age, sex, diet, genetics, and comorbidities). For example, the geographical and chronological emergence of the chytrid fungus Batrachochytrium dendrobatidis in amphibians was documented using museum collections [42]. Likewise, museum specimens of bats collected prior to the emergence of white-nose syndrome caused by the fungus Pseudogymnoascus destructans have provided insights into the history [43]. The high frequency of detection of Pneumocystis in the autopsied lungs of humans [17] supports the hypothesis that frozen lung samples from nonhuman specimens in museum collections will prove valuable to studies of Pneumocystis and other members of the lung mycobiome. In this context we note that the Museum of Southwestern Biology at the University of New Mexico and the Museum of Vertebrate Zoology at the University of California, Berkeley, hold large ultrafrozen tissue collections of wild mammals [44] that are facilitating new avenues of research in pathobiology [45–47].
Can’t we all get a lung: Conclusions and questions for the future
Recent studies point to the existence of a lung microbiome with a mycobiome component. The lung mycobiome community in small mammals appears to include, typically or often, members of the Onygenales, a conclusion where modern sequencing studies and decades-old culture studies now meet. Many questions remain: How do lung mycobiomes differ across mammalian species and larger vertebrate groups (mammals versus birds versus amniotes)? What roles do geography and climate play? Where are fungi located within diverse lung tissues? Which fungi are simply in transit, which are coevolved or mutualistic symbionts, and which cause disease? What proportion of debilitating fungal lung infections arise from fungi that were already present in previously healthy individuals? What specific adaptations allow lung-inhabiting fungi to survive in a hostile immune environment? What role do wild vertebrates play in dispersal or as zoonotic reservoirs of these fungi? And finally, do lung fungal communities provide benefits to animal hosts?
Acknowledgments
We thank Dr. James L. Patton, Museum of Vertebrate Zoology, University of California, Berkeley, for providing lung tissue from Dipodomys heermanni.
Funding Statement
PSH was supported in part by a University of New Mexico (UNM) Sevilleta LTER Summer Graduate Student Fellowship (National Science Foundation awards DEB 1655499 and DEB 1440478), with additional support from research awards from the UNM Graduate and Professional Student Association and the Department of Biology Graduate Research Allocations Committee. PSH was also supported by the Mycological Society of America John Rippon Award and by the UNM Sevilleta Field Station endowment fund. JWT acknowledges the Valley Fever Research Initiative at the University of California, VFR-19-633952 and grant R01 AI4263794 from the NIAID. JAC acknowledges National Science Foundation support (NSF1561342) for upgrades to the Division of Genomic Resources, Museum of Southwestern Biology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gehr P, Bachofen M, Weibel ER. The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respir Physiol. 1978; 32:121–140. 10.1016/0034-5687(78)90104-4 [DOI] [PubMed] [Google Scholar]
- 2.Brochu P, Ducr-Robitaille JF, Brodeur J. Physiological daily inhalation rates for free-living individuals aged 1 month to 96 years, using data from doubly labeled water measurements: A proposal for air quality criteria, standard calculations and health risk assessment. Hum Ecol Risk Assess. 2006; 12:675–701. 10.1080/10807030600801550 [DOI] [Google Scholar]
- 3.Bauer H, Schueller E, Weinke G, Berger A, Hitzenberger R, Marr IL, et al. Significant contributions of fungal spores to the organic carbon and to the aerosol mass balance of the urban atmospheric aerosol. Atmos Environ. 2008; 42:5542–5549. [Google Scholar]
- 4.Pashley CH, Fairs A, Free RC, Wardlaw AJ. DNA analysis of outdoor air reveals a high degree of fungal diversity, temporal variability, and genera not seen by spore morphology. Fungal Biol. 2012; 116:214–224. 10.1016/j.funbio.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 5.The Human Microbiome project consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger G, Wunderink RG. Lung microbiota: genuine or artifact? Isr Med Assoc J. 2013; 15:731–733. [PubMed] [Google Scholar]
- 7.Hogan DA, Willger SD, Dolben EL, Hampton TH, Stanton BA, Morrison HG, et al. Analysis of lung microbiota in bronchoalveolar lavage, protected brush and sputum samples from subjects with mild-to-moderate Cystic Fibrosis lung disease. PLoS ONE. 2016; 11(3):e0149998 10.1371/journal.pone.0149998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraczek MG, Chishimba L, Niven RM, Bromley M, Simpson A, Smyth L, et al. Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. J Allergy Clin Immunol. 2018; 142(2):407–14. 10.1016/j.jaci.2017.09.039 [DOI] [PubMed] [Google Scholar]
- 9.Chotirmall SH, O’Donoghue E, Bennett K, Gunaratnam C, O’Neill SJ, McElvaney NG. Sputum Candida albicans presages FEV1 decline and hospitalized exacerbations in cystic fibrosis. Chest. 2010; 138(5):1186–1195. [DOI] [PubMed] [Google Scholar]
- 10.Leclair LW, Hogan DA. Mixed bacterial-fungal infections in the CF respiratory tract. Med Mycol. 2010; 48:S125–32. 10.3109/13693786.2010.521522 [DOI] [PubMed] [Google Scholar]
- 11.Willger SD, Grim SL, Dolben EL, Shipunova A, Hampton TH, Morrison HG, et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome. 2014; 2:40 10.1186/2049-2618-2-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Woerden HC, Gregory C, Brown R, Marchesi JR, Hoogendoorn B, Matthews IP. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infect Dis. 2013; 13:69 10.1186/1471-2334-13-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delhaes L, Monchy S, Fréalle E, Hubans C, Salleron J, Leroy S, et al. The airway microbiota in cystic fibrosis: a complex fungal and bacterial community—implications for therapeutic management. PLoS ONE. 2012; 7:e36313 10.1371/journal.pone.0036313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer R, Sauer-Heilborn A, Welte T, Guzman CA, Abraham WR, Hofle MG. Cohort study of airway mycobiome in adult cystic fibrosis patients: differences in community structure between fungi and bacteria reveal predominance of transient fungal elements. J Clin Microbiol. 2015; 53(9):2900–7. 10.1128/JCM.01094-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botterel F, Angebault C, Cabaret O, et al. Fungal and bacterial diversity of airway microbiota in adults with cystic fibrosis: concordance between conventional methods and ultra-deep sequencing, and their practical use in the clinical laboratory. Mycopathologia. 2018; 183:171–183. 10.1007/s11046-017-0185-x [DOI] [PubMed] [Google Scholar]
- 16.Medrano FJ, Montes-Cano M, Conde M, de la Horra C, Respaldiza N, Gasch A, et al. Pneumocystis jirovecii in general population. Emerg Infect Dis. 2005; 11(2):245–250. 10.3201/eid1102.040487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponce CA, Gallo M, Bustamante R, Vargas SL. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010; 50(3): 347–353. 10.1086/649868 [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Dukik K, Muñoz JF, Sigler L, Schwartz IS, Govender NP, et al. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers. 2018; 90:245–291. [Google Scholar]
- 19.Schwartz IS, Govender NP, Sigler L, Jiang Y, Maphanga TG, Toplis B, et al. Emergomyces: The global rise of new dimorphic fungal pathogens. PLoS Pathog. 2019; 15(9):e1007977 10.1371/journal.ppat.1007977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver D, Gago S, Bromely M, Bowyer P. The human lung mycobiome in chronic respiratory disease: limitations of methods and our current understanding. Curr Fungal Infect Rep. 2019; 13:109–119. [Google Scholar]
- 21.Emmons CW, Ashburn LL. The isolation of Haplosporangium parvum n. sp. and Coccidioides immitis from wild rodents. Their relationship to coccidioidomycosis. Pub Health Rep. 1942; 57:1715–1746.19315895 [Google Scholar]
- 22.Jellison WL. Haplomycosis in Montana rabbits, rodents, and carnivores. Pub Health Rep. 1950; 65:1057–1063. [PubMed] [Google Scholar]
- 23.Bakerspigel A. Haplosporangium in Saskatchewan rodents. Mycologia. 1956; 48(4):568–572. [Google Scholar]
- 24.Ciferri R, Montemartini A. Taxonomy of Haplosporangium parvum. Mycopath Mycol Appl. 1959; 10:303–316. [DOI] [PubMed] [Google Scholar]
- 25.Fisher MC, Koenig GL, White TJ, Taylor JW. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002; 94:73–84. [PubMed] [Google Scholar]
- 26.Sharpton TJ, Stajich JE, Rounsley SD, Gardner MJ, Wortman JR, Jordar VS, et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009; 19:17221731 10.1101/gr.087551.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor JW, Barker BM. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med Mycol. 2019; 57:S16–S20. 10.1093/mmy/myy039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emmons CW. A reservoir of coccidioidomycosis in wild rodents. J Bacteriol. 1943; 45:306. [Google Scholar]
- 29.Emmons CW. Coccidioidomycosis in wild rodents. A method of determining the extent of endemic areas. Pub Health Rep. 1943; 58:1–5.19315902 [Google Scholar]
- 30.Desjardins CA, Champion MD, Holder JW, Muszewska A, Goldberg J, et al. Comparative genomic analysis of human fungal pathogens causing Paracoccidioidomycosis. PLoS Genet. 2011; 7(10):e1002345 10.1371/journal.pgen.1002345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarrocco S, Diquattro S, Baroncelli R, Cimmino A, Evidente A, Vannacci G, et al. A polyphasic contribution to the knowledge of Auxarthron (Onygenaceae). Mycol Progress. 2015; 14:112. [Google Scholar]
- 32.Demanche C, Berthelemy M, Petit T, Polack B, Wakefield AE, Dei-Cas E, et al. Phylogeny of Pneumocystis carinii from 18 primate species confirms host specificity and suggests coevolution. J Clin Microbiol. 2001; 39(6):2126–2133. 10.1128/JCM.39.6.2126-2133.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cissé OH, Hauser PM. Genomics and evolution of Pneumocystis species. Infection. 2018; 65:308–320. [DOI] [PubMed] [Google Scholar]
- 34.Akbar H, Pinçon C, Aliouat-Denis C-M, Derouiche S, Taylor M-L, Pottier M, et al. Characterizing Pneumocystis in the lungs of bats: understanding Pneumocystis evolution and the spread of pneumocystis organisms in mammal populations. Appl Environ Microbiol. 2012; 78: 8122–8136. 10.1128/AEM.01791-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flannigan B, Samson RA, Miller JD. Microorganisms in home and indoor work environments: diversity, health impacts, investigation and control. CRC Press. 2017. [Google Scholar]
- 36.Van Woerden HC, Gregory C, Brown R, Marchesi JR, Hoogendoorn B, Matthews IP, et al. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infect Dis. 2013; 13:69 10.1186/1471-2334-13-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause R, Halwachs B, Thallinger GG, Klymiuk I, Gorkiewicz G, Hoenigl M, et al. 2016. Characterisation of Candida within the mycobiome/microbiome of the lower respiratory tract of ICU patients. PloS ONE. 2016; 11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tipton L, Ghedin E, Morris A. The lung mycobiome in the next-generation sequencing era. Virulence. 2017; 8(3):334–341. 10.1080/21505594.2016.1235671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013; 498(7454):367–370. 10.1038/nature12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latgé JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clinical Microbiol Rev. 2019; 33(1): e00140–18. 10.1128/CMR.00140-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindel DE, Cook JA. The next generation of natural history collections. PLoS Biol. 2018; 16(7):e2006125 10.1371/journal.pbio.2006125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng TL, Rovito SM, Wake DB, Vredenburg VT. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen. Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2011;108:9502–9507. 10.1073/pnas.1105538108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campana MG, Kurata NP, Foster JT, Helgen LE, Reeder DM, Fleischer RC, Helgen KM. White- nose syndrome fungus in a 1918 bat specimen from France. Emerg Infect Dis 2017; 23:1611–1612. 10.3201/eid2309.170875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunnum JL, McLean BS, Dowler RC, The Systematic Collections Committee of the American Society of Mammalogists. Mammal collections of the Western Hemisphere: a survey and directly of collections. J Mammal. 2018; 99(6):1307–1322. 10.1093/jmammal/gyy151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanagihara R, Gu SH, Arai S, Kang H J, Song J-W. Expanded host diversity and global distribution of hantaviruses: Implications for identifying and investigating previously unrecognized hantaviral diseases In: Global Virology—Identifying and investigating viral diseases. New York: Springer-Verlag; 2015. pp. 161–198. [Google Scholar]
- 46.Dunnum JL, Yanagihara R, Johnson KM, Armien B, Batsaikhan N, Morgan L, et al. Biospecimen repositories and integrated databases as critical infrastructure for pathogen discovery and pathobiology research. PloS Neglect Trop Dis. 2017; 11:e0005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitt CJ, Cook JA, Zamudio K, Edwards SV. Museum specimens of terrestrial vertebrates are sensitive indicators of environmental change in the Anthropocene. Philos Trans R Soc B. 2018; 374:20170387. [DOI] [PMC free article] [PubMed] [Google Scholar]