Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Drugs, Vaccines, Clinical trials
Abbreviations: COVID-19, Coronavirus Disease 2019; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; WHO, World Health Organization; ICTV, International Committee on Taxonomy of Viruses; ARDS, acute respiratory distress syndrome; SARS, Severe Acute Respiratory Syndrome; MERS, Middle East Respiratory Syndrome; R0, reproductive ratio; SI, Serial Interval; PPE, Personal Protective Equipment; ACE2, angiotensin I converting enzyme 2; RAS, Renin-Angiotensin System; ATII, alveolar Type II cells; RdRp, RNA-dependent RNA polymerase; TMPRSS2, type-2 transmembrane serine protease; IL-6, interleukin 6; TNFα, tumor necrosis factor α; MCP-1, monocyte chemoattractant protein-1; NK, natural killer cells; S protein, viral spike protein; aAPC, artificial antigen-presenting cells; CP, Convalescent Plasma therapy; CRS, Cytokine-release syndromes; ADR, Adverse Drug Reactions
Abstract
COVID-19, the greatest public health emergency of the 21st century, has affected 215 countries and territories around the world resulting in 15,151,738 confirmed cases and 621,121 deaths. The outbreak has continued at breakneck pace despite stringent public health measures, ravaging the global economy and causing profound human casualties. Vaccination is currently the best bet for the prevention of COVID-19. Still, in its absence, there has been considerable interest in repurposing existing therapeutic agents to reduce the severity of the illness and ease the burden on the already strained healthcare systems. This review outlines the current evidence regarding proposed treatments- experimental or repurposed, for COVID-19, and gives an insight into the clinical trial landscape for drugs as well as vaccines.
1. Introduction
In late 2019, there was a sudden surge in the number of individuals being admitted to local hospitals of Wuhan, in the Hubei Province of China, with a pneumonia-like illness of unknown etiology [1]. Although results from preliminary epidemiological investigations pointed towards a zoonotic origin from a local seafood market in Huanan, the exponential increase in the number of cases suggested the possibility of human-to-human transmission [2], [3]. The World Health Organization (WHO) was notified of the outbreak by the Chinese authorities on December 31, 2019. By January 7, 2020, scientists had isolated a novel coronavirus from the lower respiratory tract samples of patients who were admitted to a hospital in Wuhan [4]. This pathogen was later termed as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) [5]. The WHO declared this novel viral disease a Public Health Emergency of International Concern on 30 January 2020, and subsequently termed it as COVID-19 (Coronavirus Disease 2019) [6] on February 11, 2020. The first fatality was reported on Jan 11, 2020. The mass migration of people during the Chinese New Year fuelled the rapid spread of the disease to all parts of the globe, which led to the official declaration of COVID-19 as a pandemic [7] by the WHO on March 11, 2020. COVID-19 has wreaked havoc worldwide, resulting in 15,151,738 confirmed cases and 621,121 deaths across 215 countries and territories [8] as of July 22, 2020. Despite stringent public health measures, the outbreak has continued at a breakneck pace and has laid bare the glaring inadequacies of the present healthcare systems around the world.
2. Virology
Coronaviruses (derived from the Latin word corona, meaning crown) are positive sense, single-stranded enveloped RNA viruses with a diameter of 60 nm to 140 nm, and are widely dispersed in nature [4]. Four (hCoV-229E, OC43, NL63 and HKU1) out of the seven species of beta-coronaviruses found in humans cause mild illnesses of the upper respiratory tract such as common cold (also caused by rhinoviruses). Two other kinds, Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV), are considerably more dangerous (mortality rates of 9.6% and 35% respectively [9], [10]). SARS-CoV first emerged in late 2002 in China and spread rapidly to other parts of the world from Hong Kong via air travel. The virus was believed to have originated from a bat (primary host) from where it mutated to infect the intermediate host- Civet cats (a nocturnal mammal sold for meat in China) and subsequently infected humans [11]. It turned into an epidemic with over 8000 cases and 744 deaths [12]. Almost a decade later, MERS emerged in the Middle East with Dromedary Camel as the intermediate host from which it mutated enough to affect humans, resulting in 2494 cases and 858 deaths [13]. SARS-CoV-2, the pathogenic factor for COVID-19, is a novel zoonotic RNA betacoronavirus. Several studies have conducted phylogenetic analyses of SARS-CoV-2 to determine its sequence homology with other viruses of the family Coronaviridae [4], [14], [15], [16], [17], [18]. It has been reported that SARS-CoV-2 shares a 79.6% sequence homology with SARS-CoV, and 96.2% resemblance with bat coronavirus (HKU4) [14]. Fig. 1 depicts the structure of SARS-CoV-2. While the exact source of the virus remains a mystery, it is widely reported to have originated from a bat [4], [14] and made its way to humans via an intermediate animal host, the pangolin [11], [15], a nocturnal mammal that is highly trafficked around the world for the medicinal properties of its scales and its meat. The ecological origin and subsequent transmission of the three aforementioned Coronavirus species are illustrated in Fig. 2 .
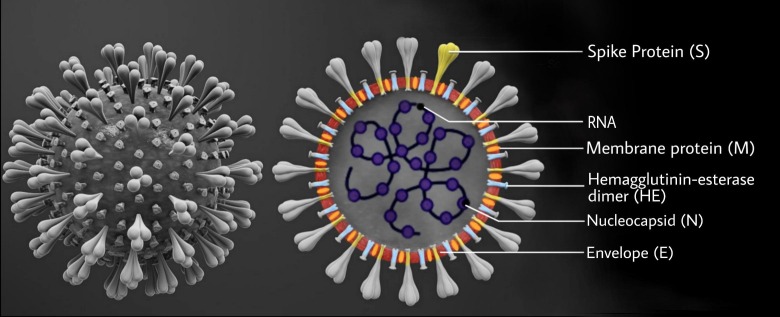
Fig. 1.
Diagrammatic representation of the structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The figure represents the viral structure of SARS-CoV-2. The spike glycoprotein (S protein) confers a crown like appearance to the virus, hence the name ‘Coronavirus’. The S protein mediates the binding of the virus to cellular receptors. The role of hemagglutinin-esterase (HE) in Coronaviruses is poorly understood but it is reported to influence virion attachment in other viruses.
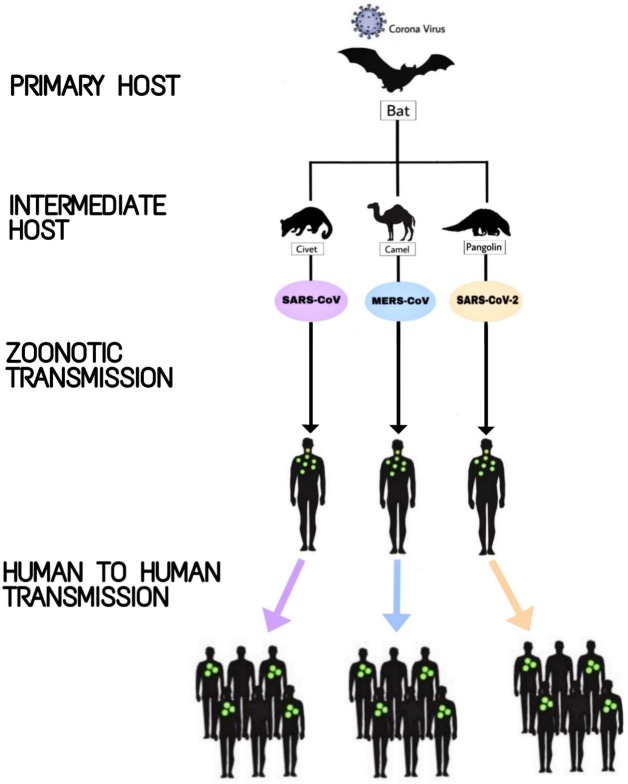
Fig. 2.
Ecological origin and transmission of different species of Coronaviruses. This schematic representation illustrates the ecological origin, different animal hosts, and the subsequent transmission of SARS-CoV, MERS-CoV, and SARS-CoV-2 to the human population, eventually resulting in the three major epidemics/pandemic of the 21st century.
3. Clinical features and susceptibility
People of all age groups are susceptible to COVID-19. The symptoms, which have been found to resemble those of the seasonal flu (Influenza), include fever, cough, sore throat, headache, fatigue, myalgia, loss of smell and taste, and dyspnea. Up to 80% of cases are asymptomatic or have mild disease [19]. In some patients, underlying co-morbidities can further exacerbate the diseased condition, leading to pneumonia, acute respiratory distress syndrome (ARDS), and multi-organ dysfunction by the end of the first week, eventually proving fatal.
4. Transmission
Inhalation of respiratory droplets generated by symptomatic patients’ cough and sneeze is the main mode of transmission of COVID-19. Infected droplets can spread to distances of up to 2 m and deposit on surfaces, which can act as potential fomites for transmission of the virus to seemingly healthy individuals who touch their mucosa (mouth, nose) and conjunctiva (eyes) without proper sanitization. Asymptomatic or pre-symptomatic people can also spread the infection. The virus is also believed to be present in the stool, and transmission via the feco-oral route cannot be ruled out [20]. There is currently no evidence of transmission of the virus from a pregnant mother to her fetus via transplacental route.
5. Physicochemical properties
SARS-CoV-2 can remain viable on surfaces like plastic and stainless steel for up to 72 h in favourable atmospheric conditions [21] but is susceptible to common disinfectants like sodium hypochlorite, hydrogen peroxide, diethyl ether, 75% ethanol, chloroform etc [22]. Soap is found to be equally effective since it readily dissolves the lipid bilayer of the virus. SARS-CoV-2 can also be inactivated by UV or when heated at 60 °C for 30 min [23], [24].
6. Epidemiology
Despite being relatively more benign than its two ancestors, SARS-CoV-2 has crossed the 15 million cases mark worldwide, aided in part by inadequate risk assessment regarding the urgency of the situation. However, the overriding reasons for its exponential growth are the high reproductive ratio (R0) – ranging from 2 to 6.47 [25], the long incubation period of 2 to 14 days (median 5 days) and a Serial Interval (SI) of 5–7.5 days [26], which are indicative of an infectious disease that has the potential of rapidly turning into a pandemic. Sanche et al. recently suggested that the disease had a median R0 of 5.7 (95% CI of 3.8–8.9) and SI of 6–9 days in China during the initial period of the outbreak [27]. In comparison, the R0 of SARS was 2 and 1.3 for the 2009 H1N1 Influenza [26].The mortality rate for COVID-19 ranges between 2 and 5%, with ARDS being the leading cause of death [4].
7. Current management
In the absence of any established pharmacological agents, supportive care remains the cornerstone of clinical management for COVID-19. Prevention involves isolation of suspected cases, home quarantine for the healthy, and the use of effective Personal Protective Equipment (PPE) to reduce the risk of interpersonal transmission. The conspicuous absence of any proven interventions for COVID-19 [28], coupled with the alarmingly high infectivity of SARS-CoV-2 has rendered this pandemic an unmitigated disaster. Swiftly expanding knowledge of the virology of SARS-CoV-2 has laid the groundwork for the identification of effective therapeutic agents for the prevention and treatment of the disease by providing potential drug targets. This review outlines the measures currently in place to curb its spread and gives a glimpse into the roadmap for the development of a vaccine.
8. Methodology
A literature search was conducted using PubMed to identify relevant English-language articles published through July 21, 2020. Search terms included Coronavirus, SARS-CoV-2 and COVID-19. Active clinical trials were identified using the search terms COVID-19 and SARS-CoV-2 on ClinicalTrials.gov till July 21, 2020. The search yielded 2713 trials, with 1393 actively recruiting trials specific to COVID-19. Of these, 1174 were observational trials while 1515 were intervention trials for the treatment of COVID-19. Among these, 75 were Phase 4, 350 were Phase 3, 598 were Phase 2, 171 were Phase 1, and 26 were Early Phase 1 trials. 493 trials were not categorized by any particular phase or were not applicable. Further, 228 studies had been listed completed at the time of writing. Results are still awaited for all completed studies.
9. Pathophysiology
SARS-CoV-2 enters human cells through its Spike (S) protein- a type I surface glycoprotein, and binds to the angiotensin I converting enzyme 2 (ACE2) receptor [29]. ACE2 is a type 1 transmembrane aminopeptidase expressed in the heart and lungs. It negatively regulates the Renin-Angiotensin System (RAS) and plays a significant role in neurohumoral regulation of the cardiovascular system. When SARS-CoV-2 binds to ACE2, it alters the ACE2 signaling pathways, potentially causing acute myocardial and lung injury [30], [31]. ACE2 receptors are abundantly distributed in the epithelia of the lung- especially the alveolar Type II (ATII) cells. Once the virus binds to the receptor, it spreads widely on entering the blood circulation. Fig. 3 shows a schematic representation of the viral replication of SARS-CoV-2 inside a host ATII cell. ACE2 receptors are also said to be present in the liver, digestive organs and kidneys. These tissues and organs could thus be potential targets for SARS-CoV-2 invasion [32]. This explains why many patients with COVID-19 present with extra-pulmonary symptoms [2]. When the over activated immune system is engaged in killing the virus, there is a sharp spike in the production of inflammatory factors, such as IL-2, IL-6, GCSF, MCP-1, TNFα, etc. This, in turn, may pave the way for a cytokine storm [2] and eventually lead to ARDS, secondary infections and multi-organ damage, resulting in death.
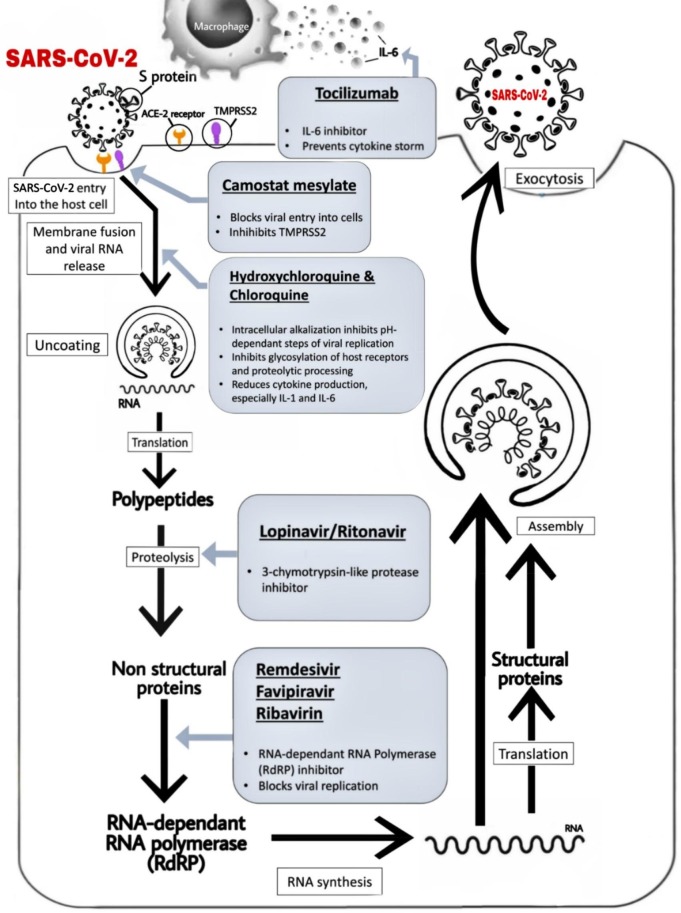
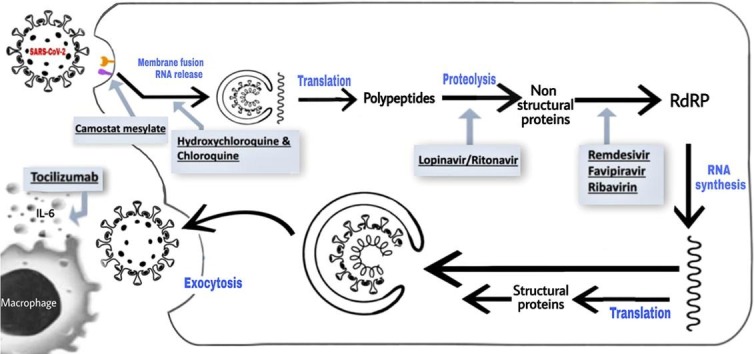
Fig. 3.
Schematic representation of the entry of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) into host cell, its viral lifecycle and potential drug targets. The above-mentioned figure depicts the viral processing of SARS-CoV-2 within host cells. S protein has a major role in binding of the virus to the host receptor cells. It attaches itself to the ACE2 receptor of the human host cell, leading to the release of the viral RNA into the host cell which triggers a cascade that ultimately results in respiratory infection. Select repurposed drugs and their target sites of action are also represented in this schematic. ACE2: angiotensin-converting enzyme 2; S protein: spike protein; TMPRSS2: type 2 trans-membrane serine protease and IL-6: interleukin 6.
10. Pharmacological treatments with potential clinical benefits
In the months since the novel coronavirus burst onto the scene, drug makers small and large have scrambled to put their best foot forward in an attempt to thwart the pandemic. Some are taking cues from older antivirals, while others are aiming to develop novel therapeutic agents to treat COVID-19. Vaccine development, convalescent plasma therapy, cell-based therapies and monoclonal antibodies are some of the potential approaches being targeted by researchers worldwide. However, drug development is an expensive and time-consuming endeavor with a high attrition rate [33]. The enormity of the current global health emergency means that time is of the essence. Thus, there has been considerable interest in repurposing existing drugs and expediting the development of vaccines, to allow rapid identification of suitable drug candidates. The dearth of proven clinical data on therapeutic agents for COVID-19 has led to a reliance on existing therapies for other viruses such as SARS-CoV-1, MERS-CoV, and HIV etc. It is unclear whether the data from repurposed antiviral drugs can be extrapolated to SARS-CoV-2, as unlike antibiotics, antivirals are specific for a particular virus in most cases. Table 1 lists some of the potential therapeutic agents that have been used as repurposed drugs for COVID-19 treatment. Their sites and mechanisms of action against SARS-CoV-2 are illustrated in Fig. 3.
Table 1.
Potential therapeutic agents as repurposed drugs for the treatment of COVID-19.
| Drug (Year of first usage) | Class & Type | Rationale for Use | Target Disease | Dosage in Current Trials | Supportive Evidence | Caveats | References |
|---|---|---|---|---|---|---|---|
| Remdesivir(2014) | Antiviral; Adenosine nucleotide analogue | Acts as an RNA-chain terminator by binding to RNA dependent RNA polymerase (RdRP). | Ebola | 200 mg intravenously (IV) on day 1 followed by 100 mg IV daily for up to 10 days (NCT04292899) | Effective against SARS and MERS in-vitro | Not currently FDA-approved. Can only be obtained via compassionate use. | [34], [35], [36], [37] |
| Lopinavir/Ritonavir (LPV/r)(2000) | Antiviral; Protease inhibitors | HIV type 1 aspartate protease inhibitor with inhibitory activity against SARS-CoV in-vitro. | HIV | 400/100 mg twice daily for 14 days. (NCT04321174) | Effective against SARS-CoV-1 both in vitro and human studies | Current data suggest a limited role in treatment of COVID-19. | [45], [46], [47] |
| Oseltamivir(1999) | Antiviral; Neuraminidase inhibitor | Inhibits viral replication. | Influenza | 75 mg orally twice a day for 5 days (NCT04338698) | No in-vitro activity against SARS-CoV | Has no role in the management of COVID-19 once influenza has been ruled out. No data against SARS. | [50] |
| Favipiravir(2014) | Antiviral; RNA polymerase inhibitor | RNA-dependent RNA polymerase inhibitor. Also involved in blocking viral replication. | Influenza, arenavirus, filovirus | 1600 mg twice daily on day 1, then 600 mg twice daily thereafter for 7 days (NCT04310228) | In vitro activity seen against SARS-CoV-2 | No available data on its efficacy and safety for the treatment of COVID-19 | [34], [52], [53] |
| Ribavirin (1986) | Antiviral; Nucleoside analogue | Inhibits viral RNA synthesis and mRNA capping. | Syncytial virus, viral hemorrhagic fever, | 400 mg twice daily for 14 days (NCT04276688) | No evidence in SARS and MERS | Risks of ADR (hematologic toxicity, teratogenicity & contraindications in pregnancy) outweigh potential clinical benefit. | [34], [50] |
| Camostatmesylate (2006) | Protease inhibitors | Blocks viral maturation and entry to cells. Inhibits TMPRSS2. | Pancreatitis | 2 × 100 mg pills 3 times daily for 5 days (NCT04321096) | Effectively blocked SARS-CoV-2 in lung cells in vitro. Also showed antiviral activity in an animal model for SARS-CoV infection | Limited data available. | [57], [58] |
| Hydroxychloroquine / chloroquine (HCQ/CQ) (1955/1934) | Antimalarial | Block viral entry into cells by inhibiting glycosylation of host receptors, proteolytic processing, and endosomal acidification. | Malaria | Chloroquine: 500 mg twice daily for 10 days Hydroxychloroquine: 400 mg twice daily × 1 day then 200 mg twice daily × 5 days (NCT04345692) |
In vitro activity against SARS-CoV-2 | Paucity of adequate data to validate their use in COVID-19.Concerns of cardiac arrhythmias | [52], [59], [61] |
| Tocilizumab (2005) | Monoclonal Antibody | IL-6 inhibitor. May block cytokine storm in COVID-19 patients. | Rheumatoid arthritis | 8 mg/kg IV (up to a maximum of 800 mg per dose), with an interval of 12 h(NCT04317092) | No data on SARS or MERS. | Limited data to support current use. | [67] |
| Methylpred-nisolone (1957) | Corticosteroids | Potent anti-inflammatory and antifibrotic properties; May prevent “cytokine storm”; reduce pulmonary and systemic inflammation in pneumonia. | Arthritis, psoriasis, etc. | 120 mg/day IV infusion for 3 days (NCT04345445) | No impact on clinical outcomes in SARS | No proven benefit for their use in COVID-19. Risks of ADRs outweigh the benefits | [73], [74] |
This table represents a list of repurposed therapeutic agents that are being used for the treatment of COVID-19, their class, mechanism of action, dosage, original target disease, and evidence supporting their use in COVID-19 patients.
10.1. Review of selected repurposed drugs
10.1.1. Antivirals
10.1.1.1. Remdesivir
Remdesivir, a monophosphate prodrug of an adenosine analog, was originally developed to combat the Ebola outbreak in 2014. It has been highly touted as a potential antiviral drug for COVID-19. It has been shown to have potent in-vitro activity against SARS-CoV-2 (EC50 of 0.77 μM [34]), SARS-CoV-1 and MERS-CoV [35]. Because of its high selectivity for viral polymerases, Remdesivir is less likely to cause toxicity in humans. The drug also exhibits a high genetic barrier to resistance in coronaviruses and its long intracellular half-life is conducive for once-a-day dosing [36]. There are currently 38 registered trials listed on ClinicalTrials.gov for investigating the efficacy of this drug. Preliminary results from a small cohort show clinical improvement in 68% of hospitalized patients [37] on administration of Remdesivir. However, the small sample size and the lack of a control group limit its significance. Wang et al. reported that in a randomized, double-blind, placebo-controlled, multicentre trial of 237 participants (NCT04257656), Remdesivir did not exhibit significant clinical benefits [38]. On April 29, the US NIH reported that in a high powered RCT of 1063 participants, patients in the Remdesivir group had shown a 31% faster time to recovery (median 11 days) [39] than those in the placebo group (15 days) (p < 0.001). Even though the findings do not definitively reveal whether the drug is curative, Remdesivir is slated to be the new standard of care [40] for COVID-19 patients in the United States. It has also been approved for therapeutic use in COVID-19 patients by the regulatory bodies of Japan, Australia, Singapore, and Europe [41]. The use of Remdesivir might prove to have a measurable impact on healthcare capacity by minimizing the length of hospital stay and reducing the overall severity of the illness. In a comparative analysis of the Phase 3 SIMPLE-severe trial and a retrospective cohort study of severe COVID-19 patients, Remdesivir has recently shown a 62% reduction in the risk of mortality as opposed to the standard of care and an improvement in clinical recovery [42]. The drug is also being tested on outpatients as an inhaled, nebulised form in a Phase 1a trial [43].
10.1.1.2. Lopinavir/Ritonavir (LPV/r)
It is an antiviral combination of protease inhibitors that are used to treat HIV infections. Eighty trials are listed on clinical trial registries around the world for testing the drug as a COVID-19 treatment. As Lopinavir has insufficient oral bioavailability (it gets rapidly catabolized by the Cytochrome P450 enzyme system) [44], ritonavir (a CYP3A4 inhibitor) is added to increase its plasma half-life. Even though LPV/r was found to be effective in retrospective studies on SARS-CoV [45], it was far less potent than Remdesivir and Chloroquine in-vitro [46]. In a randomized open-label study of 199 patients with COVID-19 (ChiCTR2000029308), LPV/r failed to reduce overall mortality as well as viral load [47]. Thus it is difficult to ascertain whether LPV/r may have an effective role for the treatment of COVID-19 based on the available data. LPV/r has also been discontinued from randomization in the treatment arms of the RECOVERY trial, and the SOLIDARITY trial as it did not demonstrate any beneficial effect in either clinical recovery or mortality [48], [49].
10.1.1.3. Oseltamivir
Oseltamivir, a neuraminidase inhibitor, is frequently prescribed for the treatment of Influenza. It was prescribed to treat flu-like symptoms in COVID-19 patients at the onset of the outbreak in China before SARS-CoV-2 was identified as the etiologic factor. It has no scientific basis for the treatment of COVID-19 as viruses of the Coronaviridae family do not utilize the enzyme neuraminidase. Moreover, Oseltamivir does not exhibit in-vitro activity against SARS-CoV [50]. Eighteen studies are currently listed for testing the drug on COVID-19 patients. Oseltamivir was shown to be ineffective in the treatment of COVID-19 by an in silico assessment, which also included an in vitro and retrospective study [51].
10.1.1.4. Favipiravir
Favipiravir is an RNA-dependent RNA polymerase (RdRp) inhibitor. It is used in China for the treatment of Influenza and is capable of blocking the replication of other RNA viruses [52] such as arenavirus, filovirus, bunyavirus etc. Favipiravir is activated into its phosphoribosylated form (favipiravir-RTP) in cells, which then inhibits viral RNA polymerase activity [53]. It has shown potential in-vitro activity against SARS-CoV-2 [34]. There are currently 32 studies underway which are aimed at investigating the efficacy of this drug in COVID-19. Favipiravir has been approved for experimental use in COVID-19 patients in Italy, China and Russia [54], [55]. In a recent multicenter trial in Japan, Favipiravir did not show a significant benefit in mild and moderate COVID-19 cases [56].
10.1.1.5. Ribavirin
Ribavirin, a guanosine analog prescribed for viral hemorrhagic fever and respiratory syncytial virus, inhibits viral RNA polymerase and mRNA capping. It has no in-vitro activity against SARS [50], and is far less potent against SARS-CoV-2 in-vitro than antivirals like Remdesivir [34]. It is associated with hemolytic anemia which could lead to exacerbation of cardiac disease in co-morbid patients. Further, it is also known to be teratogenic.
10.1.2. Protease inhibitors
10.1.2.1. Camostat mesylate
It is a protease inhibitor that is used for the treatment of chronic pancreatitis. Camostat inhibits the host cell serine protease TMPRSS2 [57], which primes the viral S protein for entry into human cells. In mice, camostat mesylate showed a 60% survival rate following SARS-CoV infection, at dose concentrations similar to that in humans [58]. It also was found to block viral maturation and entry of SARS-Cov-2 in vitro. Eight trials on COVID-19 are currently underway around the globe.
10.1.3. Antimalarial
10.1.3.1. Hydroxychloroquine / Chloroquine (HCQ/CQ)
These are antimalarial drugs which are also believed to have antiviral activity [52]. Both HCQ/CQ demonstrated potent in vitro activity against SARS-CoV-2 with an EC50 of 6.14 μM and 23.90 μM, respectively [59]. The drugs have recently gained notoriety due to much sociopolitical hype that has led to a spate of newly launched clinical trials- more than 240 studies are currently underway around the world. There is very limited data to advocate the use of HCQ/CQ as therapeutic options in COVID-19. In a small open-label non-RCT of 36 patients (20 treated/ 16 controls), significantly improved virologic clearance was observed in the HCQ (N = 14) group. All six patients in the HCQ plus Azithromycin group had significantly better viral clearance [60]. However, six patients in the HCQ group were removed from the study due to adverse effects or intolerance of the medication. A study in Brazil (NCT04323527) was forced to prematurely halt patient recruitment due to the high fatality rate in the CQ group [61]. The authors of the study opined that treatment providers should refrain from administering high doses of CQ to critically ill patients with COVID-19 because of its potential safety hazards. There was also no reported evidence of a decrease in viral load or improvement in other clinical outcomes. A recent multinational observational study on more than 96,000 patients across six continents reported an increased occurrence of ventricular arrhythmias in patients who received hydroxychloroquine or chloroquine, when used alone or in conjunction with macrolides [62]. The authors reported that the drug regimen did not have any beneficial effect on treatment outcomes in COVID-19 patients, and exhibited an increased risk of mortality in the treatment group as compared to the control. HCQ/CQ has several potential Adverse Drug Reactions (ADR), such as the risk of cardiac arrhythmias (e.g., QT prolongation), GI Disturbances, ECG abnormalities, hypoglycemia, and retinal damage upon long-term/high dose use. In a recent single-center study on 2541 patients, treatment with HCQ alone and HCQ + Azithromycin demonstrated a significant reduction in mortality among hospitalized COVID-19 patients [63]. However, a randomized double-blind placebo-controlled trial which tested HCQ as postexposure prophylaxis did not find the drug to be effective in preventing COVID-19 after a high-risk exposure [64]. Hydroxychloroquine was discontinued from the RECOVERY trial owing to no evidence of beneficial effect on either mortality or clinical improvement outcomes [65]. The WHO also discontinued the Hydroxychloroquine trial arm of the SOLIDARITY trial as it did not produce any reduction in mortality of COVID-19 patients when compared to the standard of care [49]. Similarly, the FDA has warned against the use of HCQ/CQ as therapeutic interventions against COVID-19 due to the risk of potential ADRs, and has revoked its status as an emergency-use drug for hospitalized patients who are not enrolled in registered clinical trials [66].
10.2. Adjunctive pharmacological therapies
10.2.1. Immunomodulatory agents
10.2.1.1. Tocilizumab
Tocilizumab is a recombinant monoclonal antibody that inhibits IL-6 receptors and is used for the treatment of rheumatoid arthritis and the “cytokine storm” immune overresponse in cancer patients. IL-6 is implicated in immunologic response in patients with Cytokine-release syndromes (CRS). Elevated levels of IL-6 have been associated with hyperinflammatory states and CRS in severe COVID-19 cases and can potentially lead to increased rates of mortality [67]. Patients with thrombocytopenia and neutropenia are at a greater risk of possible ADRs of this drug, which include GI perforation and hepatotoxicity. Sixety two registered trials are currently testing the safety and efficacy of Tocilizumab on COVID-19 patients. A recent study reported that Tocilizumab improved survival, as well as clinical markers in patients with CRS [68]. The drug was also reported to be associated with lower mortality in a cohort of mechanically ventilated COVID-19 patients [69]. However, a retrospective cohort study reported that Tocilizumab did not show a significant difference versus standard care in either clinical improvement or mortality [70].
10.2.1.2. Sarilumab
IL-6 Receptor-Inhibiting Monoclonal Antibody that has been previously used in the treatment of Rheumatoid arthritis. It is currently being tested in more than fifteen registered trials targeted at managing the “cytokine storm” immune response in severely ill COVID-19 patients. Results from a recent Phase 3 trial showed that Sarilumab did not meet any of the primary or secondary endpoints of the study and is thus ineffective in COVID-19 patients requiring mechanical ventilation [71].
10.2.1.3. Ruxolitinib
Ruxolitinib has been found to be effective against inflammatory and autoimmune diseases, and is in late-stage development as a topical ointment for atopic dermatitis. Twenty trials are currently testing the efficacy of the drug in COVID-19 patients.
10.2.1.4. Ifx-1
IFX-1 is a monoclonal antibody designed to induce an anti-inflammatory response by blocking the biological activity of human complement factor C5a. One trial (NCT04333420) is currently being held in the Netherlands to test its efficacy in patients with severe COVID-19, with preliminary results expected by December 31, 2020. It will soon be studied in a Phase 3 trial on patients with severe COVID-19 induced pneumonia after promising results in the earlier phases [72].
10.3. Corticosteroids
10.3.1. Methylprednisolone
It is a corticosteroid with potent anti-inflammatory properties which is prescribed for the treatment of arthritis, and has potential benefit in the management of pneumonia. However, data for its use in severe coronavirus diseases is inconclusive and controversial as it shows no impact on clinical outcomes in SARS [73]. Moreover, the benefits are often outweighed by the risks [74] of secondary infection and delayed viral clearance.
10.3.2. Dexamethasone
It is a glucocorticoid that has been used for several years in the treatment of various inflammatory and autoimmune diseases. Preliminary results from the RECOVERY trial have shown that Dexamethasone reduces mortality by one-third in mechanically ventilated patients hospitalized with severe COVID-19, and by one-fifth in patients requiring oxygen without mechanical ventilation [75]. The drug did not improve survival in patients not requiring respiratory support. Dexamethasone has been approved for use as a COVID-19 treatment in the UK [76]. As is the case with other corticosteroids, Dexamethasone suppresses the immune system and could thus potentially exacerbate viral infections. However, its immunosuppressant effects might benefit COVID-19 patients who experience CRS.
10.4. Miscellaneous
10.4.1. NKG2D-ACE2 CAR-NK cells
NKG2D is an activating receptor for the immune system's natural killer (NK) cells. SARS-CoV-2 binds to the ACE-2 receptor using its S protein. By targeting the S protein of the virus and NKG2DL on the surface of infected cells with ACE-2 and NKG2D respectively, it is expected that SARS-CoV-2 can be eliminated from the infected cells. Moreover, ACE2 CAR-NK cells can competitively inhibit infection of ATII cells by SARS-CoV-2 through ACE2 receptors, thereby preventing the production of infectious virus particles. A multicenter, randomized Phase 1/2 trial (NCT04324996) is currently testing this cell therapy.
10.4.2. RhACE2 APN01
RhACE- recombinant human angiotensin-converting enzyme 2 is currently undergoing a Phase-2 clinical trial (NCT04335136) in ALI (Acute Lung Injury) and PAH (Pulmonal arterial hypertension). This synthetic version of the ACE2 is being studied on 200 participants in Austria to determine if it can prevent viral entry and reduce viral replication in COVID-19 patients. Results from the study are expected in November 2020.
10.4.3. Aspirin, Clopidogrel, Rivaroxaban, Atorvastatin, omeprazole
These drugs are being trialled to study their cardioprotective effect and prevent direct damage to the heart muscle that appears to exacerbate the severity of COVID-19 in certain patients. The Randomized, open label trial (NCT04333407) will include 3170 patients and will be conducted in the United Kingdom, with a completion date of March 30, 2021.
11. Non-Pharmacological therapies for Covid-19
11.1. Immunoglobulin therapy
11.1.1. Convalescent plasma therapy (CP)
Inducing passive immunization by infusion of CP is a potential therapeutic option in the absence of a proven anti-viral agent or vaccine. In CP therapy, blood plasma of patients who have recovered from COVID-19 is transfused into patients who are currently ill, in the hope that the freshly-made antibodies will help overcome the virus. Convalescent plasma has previously been used in the treatment of viral diseases such as poliomyelitis, influenza, SARS, and MERS. A case series of five critically ill COVID-19 patients with ARDS reported clinical improvements after the administration of convalescent plasma [77]. Another study on ten severely ill patients in China showed that the clinical symptoms improved significantly within three days, and that the viral load was undetectable after seven days of transfusion [78]. A recent study on more than 20,000 COVID-19 patients who received CP therapy reported that transfusion of CP is safe and does not heighten the risk of adverse events [79]. The study also indicated a reduction in mortality after transfusion of CP. Ianevski et al. reported on the differential nature of immune reactions to COVID-19 in different patients and suggested that the ability of CP to elicit a neutralizing immune response might wane with time [80]. More than 130 listed trials are in progress across the world to gather stronger evidence that would warrant the use of plasma therapy. Plasma banks for the transfusion of CP from freshly recovered COVID-19 patients have been set up in several countries, including India, to counter the rapidly increasing number of cases [81]. Adverse effects associated with plasma transfusion include pathogen transmission and allergic transfusion reactions. The greatest obstacle for a large scale rollout of plasma therapy is to find prospective donors with high levels of neutralizing antibody titer (>1:640) [78]. Studies on SARS have reported that the level of specific neutralizing antibodies titer decreased rapidly within four months of recovery [82], suggesting that immune protection may wane over time. Such short-lasting humoral immune response suggests that plasma from recently recovered patients is more effective for plasma therapy. The presence of antibodies points to a past infection. However, further research is needed to know more about the type and concentration of virus-neutralizing antibodies that protect against a new infection, and the duration for which the immunity might last. Current data is still insufficient on whether mild or asymptomatic infections generate adequate antibody responses or protection to COVID-19.
12. Vaccines
Vaccination is the best bet for COVID-19 control. DNA plasmids, epitopes, mRNA, and artificial antigen-presenting cells (aAPCs) are some of the biotechnological platforms on which the hunt for a global vaccine is based [83]. Currently, there are no FDA-approved vaccines for COVID-19. There is limited information on the specific antigens used in the development of vaccines against SARS-CoV-2. Most candidate vaccines are aimed at inducing neutralizing antibodies against the viral spike (S) protein, which prevents its uptake via the ACE2 receptor. Table 2 provides an overview of the global landscape of COVID-19 vaccine development activity for active clinical trials [84]. The chain of events from conception to market availability of a vaccine usually takes over ten years, and has a mere 6% probability of successful market entry [85]. If a commercially-available SARS-CoV-2 vaccine were to be made available for use in 12–18 months, assuming its path from the lab to the market is unhindered, it would represent a seismic change from the traditional vaccine development pathway. This would require a multipronged strategy involving novel vaccine development paradigms, flexible development phases, ramping up existing manufacturing capacity, unprecedented scale and speed of global R&D, and radical changes in regulatory processes. It will also require careful evaluation of safety and efficacy every step of the way.
Table 2.
COVID-19 vaccine candidates currently enrolled in active clinical trials.
| Name | Platform | Allocation & Masking | Developer | Clinical Trial Status | Strength | Trial Location | Estimated Date of Completion | References |
|---|---|---|---|---|---|---|---|---|
| mRNA 1273 | Lipid nanoparticle encapsulated mRNA vaccine encoding S protein. | Randomized; Double blind | Moderna/National Institute Of Allergy And Infectious Diseases (NIAID) | Phase II trial (NCT04405076) | 600 | United States | August 2021 | [83], [84] |
| Lentiviral Minigene Vaccines (LV-SMENP-DC) | Lentiviral vector system (NHP/TYF) used to modify dendritic cells (DCs) and to activate T cells. | Non-Randomized; Open Label | Shenzhen Geno-Immune Medical Institute | Phase I (NCT04276896) | 100 | Shenzen, China | December 31, 2024 | |
| Bacillus Calmette-Guerin (BCG) live-attenuated vaccine* | Live-attenuated vaccine that induces a broad immune-system response. | Randomized; Open Label AND Randomized; Quadruple |
Murdoch Children's Research Institute; And UMC Utrecht |
Phase III BRACE trial - (NCT04327206) AND Phase III BCG-CORONA trial - (NCT04328441) |
4,170 (Australia) AND 1500 (Netherlands) |
Australia AND The Netherlands |
March 30, 2022 AND December 25, 2020 |
|
| INO-4800 | DNA plasmid vaccine. | Non-Randomized; Open Label | Inovio Pharmaceuticals AND Coalition For Epidemic Preparedness Innovations (CEPI) |
Phase I (NCT04336410) | 40 | Philadelphia and Kansas City, USA | April 2021 | |
| AD5-nCov | Recombinant non-replicating viral vector vaccine. (adenovirus type 5 vector) | Non-Randomized; Open Label | Cansino Biological Inc./Beijing Institute of Biotechnology | Phase I trial (NCT04313127) AND Randomized controlled phase II trial (ChiCTR2000031781) |
108 AND 500 |
Wuhan, Hubei, China | December 20, 2022 (Phase 1) AND January 2021 (Phase 2) |
|
| AZD1222 (PreviouslyChAdOx1 nCoV-19) | Non-replicating chimpanzee adenovirus vector. | Randomized; Single blinded | University Of Oxford | Phase II/III trial (NCT04400838) | 10,260 | United Kingdom | August 2021 | |
| Pathogen-specific aAPC | aAPCs modified with lentiviral vector expressing synthetic minigenes. | Non-Randomized; Open Label | Shenzhen Geno-Immune Medical Institute | Phase I (NCT04299724) | 100 | Shenzen, China | December 31, 2024 | |
| CoronaVac (Previously PiCoVacc) | Formalin-Inactivated + alum adjuvant | Randomized; Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) | Sinovac | Phase I (NCT04352608) AND Phase II (NCT04383574). |
143 AND 600 |
Jiangsu, China | December 13, 2020 | |
| BNT162 | Four mRNA vaccines: 2 nucleoside modified mRNA-based (modRNA), 1 uridine containing mRNA-based (uRNA), and 1 self-amplifying mRNA-based (saRNA). | Non-Randomized; Open Label | Pfizer and BioNtech | Phase I/II (NCT04380701) | 200 | Berlin, Germany | August 2020 | |
| Inactivated Vaccine | Inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) | Randomized; Double blind | Wuhan Institute of Biological Products AND China National Pharmaceutical Group (Sinopharm) |
Phase I/II (ChiCTR2000031809) | 1456 | Wuhan, China | November 2021 | |
| BBIBP-CorV | Inactivated novel coronavirus (2019-CoV) vaccine (Vero cells) | Randomized; Double blind | Beijing Institute of Biological Products AND China National Pharmaceutical Group (Sinopharm) |
Phase II (ChiCTR2000032459) | 2128 | China | November 2021 | |
| GX-19 | COVID-19 Preventive DNA Vaccine | Randomized; Double blind | Genexine | Phase I/IIa (NCT04445389) | 190 | Seoul, Republic of Korea | March 2021 | |
| Gam-COVID-Vac | Non-replicating viral vector COVID-19 vaccine candidate | Non-Randomized; Open Label | Gamaleya Research Institute, AND Acellena Contract Drug Research and Development |
Phase I (NCT04436471) | 38 | Moscow, Russia | August 2020 | |
| mRNA-based vaccine SCB-2019 | Non-chemically modified nucleotides within mRNA for activation of the immune system | TBD** | CureVac AND German federal government |
Phase I (Yet to commence) | 168 | Germany and Belgium | TBD** | |
| SCB-2019 | COVID-19 vaccine candidate that uses Clover's S-Trimer platform, GSK's AS03 adjuvant, and Dynavax's CpG 1018 adjuvant with potassium aluminum sulfate (Alum) | Randomized; Triple blind | GlaxoSmithKline, Sanofi, Clover Biopharmaceuticals, Dynavax and Xiamen Innovax | Phase I (NCT04405908) | 150 | Australia | March 2021 | |
| COVAX-19 | Monovalent recombinant protein vaccine | Randomized; Triple blind | Vaxine Pty Ltd. | Phase I (NCT04453852) | 40 | Adelaide, Australia | July 2021 | |
| bacTRL-Spike | Bifidobacteria monovalent SARS-CoV-2 DNA oral vaccine candidate | Randomized; Triple blind | Symvivo | Phase I (NCT04334980) | 112 | Canada and United States | December 2021 | |
| Covaxin (BBV152) | Inactivated whole-virion vaccine | Randomized; Triple blind | Bharat Biotech AND National Institute of Virology |
Phase I/II (NCT04471519) | 1125 | India | June 2021 | |
| ZyCoV-D | DNA-plasmid based vaccine | TBD** | Zydus Cadila | Phase I/II (Yet to commence) | 1048 | India | 2021 |
*- Repurposed Vaccine; Original indication: Tuberculosis (TB) pediatric vaccine **- To-be-declared.
This table gives an overview of the global COVID-19 vaccine landscape.
13. Clinical trials
The sheer volume of clinical trials that are investigating prospective treatment options for COVID-19 emphasizes the need to expedite the production of effective measures to curb its spread and provide much-needed relief to patients and healthcare providers the world over. Table 3 summarizes the interventional clinical trials on COVID-19 that have been listed as completed on ClinicalTrials.gov [86].
Table 3.
Interventional trials on COVID-19 listed as completed on ClinicalTrials.gov.
| NCT Number | Title | Interventions | Phase | Allocation & masking | Enrollment no. (age) | Primary outcome measures | References |
|---|---|---|---|---|---|---|---|
| NCT04343768 | An Investigation Into Beneficial Effects of Interferon β-1a, Compared to Interferon β-1b And The Base Therapeutic Regiment in Moderate to Severe COVID-19: A Randomized Clinical Trial | Hydroxychloroquine + Lopinavir / Ritonavir + Interferon-β-1a VS HCQ + LPV /r + IF-β-1b VS Control group: HCQ + LPV/r |
Phase 4 | Randomized; Open Label | 60 (≥18) | Time to clinical improvement | [86] |
| NCT04244591 | Glucocorticoid Therapy for Critically Ill Patients With Severe Acute Respiratory Infections Caused by COVID-19: a Prospective, Randomized Controlled Trial | Experimental: standard care + Methylprednisolone 40 mg q12h for 5 days VS Placebo Comparator: standard care |
Phase 2 Phase 3 |
Randomized; Open Label | 80 (≥18) | Lower Murray lung injury score | |
| NCT04291729 | Evaluation of Ganovo (Danoprevir) Combined With Ritonavir in the Treatment of SARS-CoV-2 Infection | Experimental: Ganovo + ritonavir with or without interferon nebulization | Phase 4 | Single Group Assignment;Open Label | 11 (18 to 75 y/o) | Rate of composite adverse outcomes [ Time Frame: 14 days ] | |
| NCT04261517 | Efficacy and Safety of Hydroxychloroquine for Treatment of COVID-19 | Experimental: HCQ (400 mg per day for 5 days) and conventional treatments VS No Intervention: Conventional treatments |
Phase 3 | Randomized; Open Label | 30(≥18) | The virological clearance rate of throat swabs, sputum, or lower respiratory tract secretions at day 3, 5, and 7. The mortality rate of subjects at week 2 |
|
| NCT04359251 | Avoiding High PEEP in COVID-19 Induced ARDS: a Multi-center Study | Experimental: Optimizing oxygenation Best oxygenation during PEEP titration VS Experimental: Optimizing compliance Best compliance during PEEP titration VS Experimental: ARDSnet PEEP settings according to ARDSnet table |
Not Applicable | Non-Randomized; Open Label | 20 (18 to 80 y/o) | Respiratory system compliance improvement [ Time Frame: 20 min ] | |
| NCT04358614 | Baricitinib Therapy in COVID-19: A Pilot Study on Safety and Clinical Impact | Cases: Baricitinib Oral Tablet 4 MG /day VS Controls: Standard therapy. |
Phase 2 Phase 3 |
Non-Randomized; Open Label | 12 (>18 and <75) | To assess the safety of baricitinib combined with antiviral (lopinavir-ritonavir) in terms of serious or non-serious adverse events incidence rate. [ Time Frame: 2 weeks ] | |
| NCT04276688 | Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | Study group: LPV/r 400 mg/100 mg twice daily for 14 days + Ribavirin 400 mg twice daily for 14 days + IF β-1b 0.25 mg subcutaneous injection alternate day for 3 days VS Control group: LPV/r 400 mg/100 mg twice daily for 14 days |
Phase 2 | Randomized; Open Label | 127(≥18) | Time to negative nasopharyngeal swab (NPS) 2019-n-CoV coronavirus viral RT-PCR | |
| NCT04368377 | Platelet Inhibition With GP IIb/IIIa Inhibitor in Critically Ill Patients With Coronavirus Disease 2019 (COVID-19). A Compassionate Use Protocol | Experimental: 25 µg/kg of body weight tirofiban as bolus IV injection (3 min) followed by continuous infusion at a rate of 0.15 µg /kg/min for 48 h. Acetylsalicylic acid 250 mg IV before starting tirofiban, and this will be continued at a dose of 75 mg daily for 30 days. A loading dose of clopidogrel 300 mg PO, followed by 75 mg daily for 30 days. Concurrent fondaparinux 2.5 mg s/c per day for the duration of the hospital stay |
Phase 2 | Single Group Assignment;Open Label | 5(≥18) | P/F ratio; PaO2 difference; AND A-a O2 difference at baseline, 24, 48 and 168 h after treatment initiation |
|
| NCT04324489 | DAS181 for Severe COVID-19: Compassionate Use | Experimental: DAS181 Treatment Patient receives nebulized DAS181 (4.5 mg BID/day, a total 9 mg/day) for 10 days. |
Not Applicable | Single Group Assignment;Open Label | 4 (18 to 70 y/o) | Improved clinical status [ Time Frame: Day 14 ] AND Return to room air [ Time Frame: Day 14 ] |
|
| NCT04321421 | Hyperimmune Plasma for Critical Patients With COVID-19 (COV19-PLASMA) | Experimental: administration of hyperimmune plasma at day 1 and based on clinical response on day 3 and 5 | Not Applicable | Single Group Assignment;Open Label | 49(≥18) | Death [ Time Frame: within 7 days ] | |
| NCT04273321 | Efficacy and Safety of Corticosteroids in COVID-19 | Experimental: Methylprednisolone 1 mg/kg/day ivgtt for 7 days | Not Applicable | Single Group Assignment;Open Label | 86(≥18) | The incidence of treatment failure in 14 days [ Time Frame: 14 days ] | |
| NCT04378712 | Hydrogen/Oxygen Mixed Gas Inhalation for Coronavirus Disease 2019 (COVID-19) | Experimental: Intervention Group Patients in treatment group inhaled H2-O2 (66% hydrogen; 33% oxygen) at 3 L/min via nasal cannula by using the Hydrogen/Oxygen Generator (model AMS-H-03, Shanghai Asclepius Meditech Co., Ltd., China) until discharge. Control Group: Standard-of-care consisted of the supportive therapies (including oxygen therapy) recommended by the Chinese National Health Commission |
Not Applicable | Non-Randomized; Open Label | 90 (18 to 75 y/o) | Proportion of patients with improved disease severity at day 2 [ Time Frame: from baseline to day 2 ]; Proportion of patients with improved disease severity at day 3 [ Time Frame: from baseline to day 3 ]; AND Proportion of patients with improved disease severity at the day before hospital discharge [ Time Frame: up to 14 days (from baseline to the day before hospital discharge) ] |
|
| NCT04280705 | Adaptive COVID-19 Treatment Trial (ACTT) | Experimental: Remdesivir 200 mg of Remdesivir administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of Remdesivir while hospitalized for up to a 10 days total course. n = 286. VS Placebo Comparator: Placebo |
Phase 3 | Randomized; Double-blind; Placebo-controlled | 1062 (18 to 99 y/o) | Time to recovery [ Time Frame: Day 1 through Day 29 ] | |
| NCT04304053 | Treatment of COVID-19 Cases and Chemoprophylaxis of Contacts as Prevention (HCQ4COV19) | Experimental: Testing, treatment and prophylaxis of SARS-CoV-2 Study 1 – Contacts receive Hydroxychloroquine prophylaxis. Contacts will complete a survey collecting demographic, epidemiological and clinical and provides a swab for RT-PCR testing at baseline and day 14. Contacts will be offered a prophylactic regimen of hydroxychloroquine (200 mg tablets) 800 mg on day 1, and 400 mg on days 2–7. Study 2 – Index case receives Hydroxychloroquine. Index case completes a survey collecting demographic, epidemiological and clinical data and provides a swab for RT-PCR testing at baseline and on days 3, and 7. Cases will be offered a therapeutic regimen hydroxychloroquine (200 mg tablets) 800 mg on day 1, and 400 mg on days 2–7 VS Active Comparator: No Intervention- SARS-CoV-2 surveillance Isolation of patient and contact tracing as per national guidelines. |
Phase 3 | Randomized; Open Label | 2300 (≥18) | Study 1 – Clinical and virological outcome in exposed contacts [ Time Frame: Up to 14 days after start of treatment ] Study 1 – Transmission of SARS-CoV-2 in exposed contacts [ Time Frame: Up to 14 days after start of treatment ] Study 2 – Virological outcome in index cases [ Time Frame: Up to 7 days after start of treatment ] Study 2 – Clinical outcome in index cases [ Time Frame: Up to 28 days after start of treatment ] |
|
| NCT04331795 | Tocilizumab to Prevent Clinical Decompensation in Hospitalized, Non-critically Ill Patients With COVID-19 Pneumonitis (COVIDOSE) | Experimental: Group A: Tocilizumab (beginning dose 200 mg) Single dose is provisioned, patient is eligible to receive up to two doses, with re-evaluation of clinical and biochemical responses performed every 24 h. VS Experimental: Group B: Low-dose tocilizumab (beginning dose 80 mg) Single dose is provisioned, patient is eligible to receive up to two doses, with re-evaluation of clinical and biochemical responses performed every 24 h. |
Phase 2 | Non-Randomized; Open Label | 32(≥18) | Clinical response [ Time Frame: Assessed for the 24 h period after tocilizumab administration ] Biochemical response [ Time Frame: Assessed every 24 h during patient's hospitalization, up to 4 weeks after tocilizumab administration ] |
|
| NCT04473170 | Study Evaluating the Safety and Efficacy of Autologous Non-Hematopoietic Peripheral Blood Stem Cells in COVID-19 (SENTAD-COVID) | Experimental: Group A Autologous Non-Hematopoietic Peripheral Blood Stem Cells (NHPBSC) therapy as add-on COVID-19 standard care. The NHPBSC were characterized as CD90+, CD133+, Oct-4+ (pluripotent markers), and CD45-, CD71-, based on multiparameter flow cytometry. VS Active Comparator: Group B COVID-19 Standard care. |
Phase 1/2 | Randomized; Open Label | 146(≥18) | Adverse reactions incidence. [ Time Frame: Day 0–28 ] Rate of mortality within 28-days. [ Time Frame: Day 0–28 ] Time to clinical improvement on a seven-category ordinal scale. [ Time Frame: Day 0–28 ] |
|
| NCT04349241 | Efficacy and Safety of Favipiravir in Management of COVID-19 (FAV-001) | Experimental: Favipiravir in a regimen of 3200 mg (1600 mg 12 hourly) loading dose on day-1 followed by 1200 mg maintenance dose (600 mg 12 hourly daily) on day-2 to day-10 VS Active Comparator: Standard of care therapy Oseltamivir 75 mg 12 hourly for 5–10 days and Hydroxychloroquine 400 mg 12 hourly day −1 followed by 200 mg 12 hourly daily on day-2 to day-5–10. |
Phase 3 | Randomized; Open Label | 100 (18 to 80 y/o) | Viral clearance [ Time Frame: 14 days ] Clinical improvement [ Time Frame: 14 days ] |
|
| NCT04475120 | Efficacy and Safety of Liposomal Lactoferrin in COVID-19 Patients With Mild-to-Moderate Disease and in COVID-19 Asymptomatic Patients | Experimental: Group 1a (COVID-19 mild to moderate patients) received liposomal lactoferrin in 200 mg cps (equal to 100 mg of lactoferrin), 10 capsules per day for patients weighing less than or equal to 70 kg divided into 5 capsules in the morning and 5 capsules in the evening for 30 days for a total of 1 g of lactoferrin / day; patients with body weight over 70 kg, 15 capsules per day divided into 3 administrations / day for 30 days for a total of 1.5 g of lactoferrin per day; intra-nasal spray: 2 sprays per nostril 3 times a day, inhaling deeply during administration. Group 2a (COVID-19 asymptomatic patients) received liposomal lactoferrin in 200 mg tablets (equal to 100 mg of lactoferrin), 5 capsules per day, 3 of which in the morning and 2 in the evening for 30 days (total dosage 500 mg of apo-lactoferrin per day); intra-nasal spray: 2 sprays per nostril 3 times a day, inhaling deeply during administration. Before administration, it was recommended to carefully clean the nasal cavity. VS No Intervention: Group 1b 15 mild-to-moderate symptomatic patients in hospitalization regimen were enrolled in the control group 1b to be paired by age group and gender to the aforementioned experimental group (1a) Group 2b 15 asymptomatic patients were enrolled as a control group (group 2b) to be paired by age group and gender to the aforementioned experimental group (2a) |
Phase 2/3 | Randomized; Open Label | 60(≥20) | Rate of viral clearance Time to viral clearance [ Time Frame: 30 days ] time to naso-oro-pharingeal swab negativization | |
| NCT04345276 | Efficacy and Safety of Ganovo (Danoprevir) Combined With Ritonavir in the Treatment of SARS-CoV-2 Infection | Experimental: Danoprevir + Ritonavir groupDanoprevir 100 mg, one tablet each time, twice per day, up to 10 days. Ritonavir 100 mg, one tablet each time, twice per day, up to 10 days. | Phase 4 | Single Group Assignment;Open Label | 10 (18 to 75 y/o) | Rate of composite adverse outcomes [ Time Frame: Within 10 days after administration ] Defined as SPO2 ≤ 93% without oxygen supplementation, PaO2/FiO2 ≤ 300 mmHg or a respiratory rate ≥ 30 breaths per min without supplemental oxygen min without supplemental oxygen |
|
| NCT04346446 | Efficacy of Convalescent Plasma Therapy in Severely Sick COVID-19 Patients | Experimental: Convalescent Plasma + Supportive Care Convalescent plasma from recovered COVID-19 patients will be transfused to severely sick COVID-19 infected patients VS Active Comparator: Random Donor Plasma + Supportive Care |
Phase 2 | Randomized; Open Label | 29(≥18) | Proportion of patients remaining free of mechanical ventilation in both groups [ Time Frame: Day 7 ] | |
| NCT04343092 | Efficacy of Ivermectin as Add on Therapy in COVID19 Patients | Experimental: Ivermectin (IVM) 12 mg /weekly + Hydroxychloroquin (HCQ) 400 mg/daily + Azithromycin (AZT) 500 mg daily VS No Intervention: Hydroxychloroquine 400 mg/daily + azithromycin 500 mg daily |
Phase 1 | Randomized; Double blind | 100(≥18) | Number of cured patients [ Time Frame: 4 weeks ] | |
| NCT04475588 | Efficacy and Safety of Itolizumab in COVID-19 Complications | Experimental: Best supportive care with Itolizumab Start at 1.6 mg/kg dose IV infusion, if well tolerated and improvement in patient observed, investigator has the discretion to continue with 1.6 mg/kg dose every 2 weeks or 0.8 mg/kg weekly regimen. VS Active Comparator: Best supportive care |
Phase 2 | Randomized; Open Label | 30(18 to 99 y/o) | One-month mortality rate between the two arms [ Time Frame: One-month ] | |
| NCT04376814 | Favipiravir Plus Hydroxychloroquine and Lopinavir/Ritonavir Plus Hydroxychloroquine in COVID-19 | Experimental: Test Group In this group, Patients will be given a stat dose of 1600 mg Favipiravir tablets for the first time, and for next time they will be given 600 mg of favipiravir tablets three times per day for 7 days, plus 200 mg of Hydroxychloroquine two times per day will be given to patients for 7 days. VS Active Comparator: Control Group In this group, Patients will be given a stat dose of 400 mg Hydroxychloroquine tablets plus 200/50 mg of Lopinavir/Ritonavirtwo times per day for seven days. |
Not Applicable | Non-Randomized; Open Label | 40 (16 to 100y/o) | Mortality [ Time Frame: Up to 28 days ] long of hospitalization [ Time Frame: Up to 28 days ] Laboratory Treatment Response (Blood cell count) [ Time Frame: Up to 28 days ] Laboratory Treatment Response (CRP) [ Time Frame: Up to 28 days ] Dyspnea [ Time Frame: Up to 28 days ] Oxygen saturation without supplemental oxygen. [ Time Frame: Up to 28 days ] Oxygen therapy [ Time Frame: Up to 28 days ] |
|
| NCT04407208 | Convalescent Plasma Therapy in Patients With COVID-19 | Experimental: Convalescent plasma recipient Recipients receive 3 times of each 100 ml convalescent plasma on day 0, 3, and 6 |
Phase 1 | Single Group Assignment;Open Label | 10(≥18) | Plaque reduction neutralization test (PNRT) [ Time Frame: day 7 after first transfusion ] D-dimer [ Time Frame: day 1, 4, 7, 14 after first transfusion ] C-Reactive Protein (CRP) [ Time Frame: day 1, 4, 7, 14 after first transfusion ] International Normalized Ratio (INR) [ Time Frame: day 1, 4, 7, 14 after first transfusion ] Oxygenation Index [ Time Frame: day 1, 4, 7, 14 after first transfusion ] Chest X-ray [ Time Frame: day 1, 4, 7, 28 after first transfusion ] |
|
| NCT04342650 | Chloroquine Diphosphate in the Prevention of SARS in Covid-19 Infection (CloroCOVID19II) | Active Comparator: Intervention CQ 450 mg twice daily (3 tablets of 150 mg, every 12 h) on day 1, followed by CQ 450 mg once daily (3 tablets of 150 mg) from D2 to D5. Oral administration. VS Placebo Comparator: Placebo |
Phase 2 | Randomized; Quadruple blind | 152(≥18) | Proportion of patients with onset of severe acute respiratory syndrome (SARS) [ Time Frame: 7 days after randomization ] | |
| NCT04441424 | Convalescent Plasma Therapy on Critically-ill Novel Coronavirus (COVID-19) Patients | Experimental: Convalescent plasma group 400 ml of convalescent plasma (plasma taken 2 weeks from the recovered COVID-19 patients) and was transfused over 1–2 h to the recipients by blood donation set. VS Control group The control group of COVID-19 patients were given Hydroxychloroquine 400 mg PO twice per day for 5 days and Azithromycin once PO 500 mg per day for 5 days. |
Not Applicable | Randomized; Open Label | 49(≥18) | Death versus survival of treated patients [ Time Frame: Up to 8 weeks ] | |
| NCT04321278 | Safety and Efficacy of Hydroxychloroquine Associated With Azithromycin in SARS-CoV2 Virus (Coalition Covid-19 Brasil II) | Experimental: Hydroxychloroquine + azithromycin Hydroxychloroquine [400 mg 2×/day, 12/12 h] + azithromycin [500 mg 1×/day] VS Active Comparator: Hydroxychloroquine Hydroxychloroquine [400 mg 2×/day, 12/12 h] |
Phase 3 | Randomized; Open Label | 440(≥18) | Evaluation of the clinical status [ Time Frame: 15 days after randomization ] | |
| NCT04323527 | Chloroquine Diphosphate for the Treatment of Severe Acute Respiratory Syndrome Secondary to SARS-CoV2 (CloroCOVID19) | Active Comparator: Low Dose Chloroquine Diphosphate (5 days) Low dose chloroquine group consists of 450 mg bid (3 tablets of 150 mg + 1 placebo tablet, every 12 h) on D1, 3 × 150 mg tablets + 1 placebo followed by 4 placebo tablets 12 h later from D2 to D5, and 4 placebo tablets every 12 h, D6-D10. Oral administration or via nasogastric tube in case of orotracheal intubation. VS Active Comparator: High Dose Chloroquine Diphosphate (10 days) consists of 600 mg bid (4 tablets of 150 mg, every 12 h) for 10 days. Oral administration or via nasogastric tube in case of orotracheal intubation. |
Phase 2 | Randomized; Quadruple blind |
278(≥18) | Mortality rate reduction of 50% by day 28 [ Time Frame: 28 days after randomization ] | |
| NCT04442958 | Effectiveness of Convalescent Immune Plasma Therapy | Experimental: Convalescent Plasma Therapy Group One dose of 200 ml of convalescent ımmune plasma derived from recently recovered donors with the neutralizing antibody titers above 1:640 was transfused to the patients as an addition to standart critical care treatment. VS No Intervention: Non-Plasma Therapy Group Standart critical care treatment group |
Not Applicable | Randomized; Double blind | 60(18 to 90 y/o) | Plasma ferritin level [ Time Frame: 7. day ] Lymphocyte count [ Time Frame: 7. day ] D-Dimer level [ Time Frame: 7. day ] C-Reactive protein level [ Time Frame: 7. day ] Plasma procalcitonin level [ Time Frame: 7. day ] Plasma fibrinogen level [ Time Frame: 7. day ] |
|
| NCT04308668 | Post-exposure Prophylaxis / Preemptive Therapy for SARS-Coronavirus-2 (COVID-19 PEP) | Experimental: Hydroxychloroquine 200 mg tablet; 800 mg orally once, followed in 6 to 8 h by 600 mg, then 600 mg once a day for 4 consecutive days VS Placebo Comparator: Placebo |
Phase 3 | Randomized; Quadruple blind | 1309(≥18) | Incidence of COVID19 Disease among those who are asymptomatic at baseline [ Time Frame: 14 days ] Overall change in disease severity over 14 days among those who are symptomatic at baseline [ Time Frame: 14 days ] |
|
| NCT04444986 | Bioequivalence Study of Favir 200 mg Film Tablet Kocak Under Fasting Conditions | Experimental: Participants first received Favicovir 200 mg FT in a fasting state. After a washout period of 48 h, they then received Avigan FT200 mg in a fasting state. VS Experimental: Participants first received Avigan 200 mg FT in a fasting state. After a washout period of 48 h, they then received Favicovir FT200 mg in a fasting state. |
Phase 1 | Randomized; Open Label | 30(18 to 40 y/o) | AUC0-tlast of favipiravir [ Time Frame: 0 to 24 h post-dose ] Cmax of favipiravir [ Time Frame: 0 to 24 h post-dose ] |
This table looks at the interventional trials that have been completed and listed on ClinicalTrials.gov.
13.1. Other notable trials
The SOLIDARITY trial is an international collaborative clinical trial initiated by the WHO to help find an effective treatment for COVID-19 in over 100 countries. The trial will assess the efficacy of four treatment options against COVID-19, namely: Remdesivir; LPV/r; LPV/r with Interferon β-1a; and HCQ/CQ. Trial arms in multiple countries will help expedite the results to rapidly discover whether any of the drugs are effective against SARS-COV-2 [87]. The WHO recently brought the HCQ arm of the SOLIDARITY trial to a halt [88] after reports of a higher mortality rate and an increased risk of adverse effects in patients who received the drug [62] came to light.
DISCOVERY (NCT04315948) – a multi-centre, adaptive, randomized, open label clinical trial to assess the safety and efficacy of treatments of COVID-19 in hospitalized adults, was initiated by the French Institut National de la Santé Et de la Recherche Médicale (INSERM) as the European counterpart of the SOLIDARITY trial, with the same treatment regimen. The study will be conducted on 3100 patients in seven countries across Europe [89].
The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial is a multi-centre, randomised controlled trial of over 11,000 patients across the UK. The treatment arms include LPV/r (discontinued due to lack of efficacy), Dexamethasone, HCQ (discontinued), Azithromycin, Tocilizumab, and Convalescent plasma [48].
14. Future perspectives
In order to understand the long-term impact of COVID-19, vigilant follow-up of those recovering from the disease is needed. Previous respiratory diseases brought about by coronaviruses have resulted in deranged lipid and glucose metabolism as well as cardiovascular issues in recovered individuals for up to 12 years [90]. Widescale serology testing of recovered individuals for the identification of antibodies is key to developing a broader understanding of the disease dynamics of COVID-19. It will also shed light on the neutralizing antibodies, which could potentially act as templates for monoclonal antibody therapies and vaccine candidates in the near future. New diagnostic modalities, serological tests, contact-tracing technologies and disease surveillance tools are essential to reduce the impact of the COVID-19 pandemic, and help prepare for future outbreaks with better preparation and preventive measures.
15. Limitations
It is important to acknowledge the limitations of the current study. First, the review only included studies conducted on individuals aged 18 or above. Thus, findings from the studies might not always be generalizable to the pediatric population. Second, the search was limited to only English-language articles which might have been a bottleneck because much of the early data on COVID-19 came from studies which were based in China.
16. Conclusions
In summary, SARS-CoV-2 is a highly contagious novel coronavirus that has rapidly brought about a global pandemic, destroying lives and livelihood at an alarming pace. Even though much is known about its genetic sequence and virology due to our knowledge of its predecessors, several questions regarding the epidemiological properties, transmission and pathogenesis remain unanswered. Animal studies and clinical trials on therapeutics directed against immunopathologic host responses are needed to elucidate the full spectrum of damage caused by this pathogen. Repurposing of existing drugs and the use of non-pharmacological therapies such as Convalescent Plasma are currently the best treatment avenues until a safe and efficacious vaccine is discovered. Although certain prospective agents listed in this review are promising, definitive evidence regarding their effectiveness remains inconclusive. COVID-19 has emerged to be a formidable adversary to the very existence of life as we know it. Overcoming these tough times requires the concerted efforts of people from all walks of life. It necessitates extensive international collaboration between academia, healthcare professionals, pharmaceutical companies, regulators, policymakers, public health organizations and philanthropic associations. The onus on coordinated technical and financial resource mobilization has never been higher, and is indispensable if we are to get the better of this pandemic.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Mr. Rohan Chakraborty is a recipient of Junior Research Fellowship from University Grants Commission, India (UGC JRF Ref No: 190510369725). The Grant no. [SR/FST/LS-I/2017/05(C)] and [SR/PURSE Phase 2/39 (C)] received from the Ministry of Science & Technology, Government of India, to Jamia Hamdard is also thankfully acknowledged.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A.E. Gorbalenya, et al., Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group, Microbiology (2020). doi:10.1101/2020.02.07.937862.
- 6.Guarner J. Three emerging coronaviruses in two decades. Am. J. Clin. Pathol. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020 https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. (2020). Accessed 28 April 2020.
- 8.WHO Health Emergency Dashboard COVID-19 https://covid19.who.int/. (2020). Accessed 22 July 2020.
- 9.Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003 https://www.who.int/csr/sars/country/table2004_04_21/en/. (2020). Accessed 25 April 2020.
- 10.Middle East respiratory syndrome coronavirus (MERS-CoV) https://www.who.int/emergencies/mers-cov/en/. (2020). Accessed 25 April 2020.
- 11.van Staden C. COVID-19 and the crisis of national development. Nat. Hum. Behav. 2020 doi: 10.1038/s41562-020-0852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan-Yeung M., Xu R.-H. SARS: epidemiology. Respirology. 2003;8:S9–S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killerby M.E., Biggs H.M., Midgley C.M., Gerber S.I., Watson J.T. Middle east respiratory syndrome coronavirus transmission. Emerg. Infect. Dis. 2020;26:191–198. doi: 10.3201/eid2602.190697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.T.T.-Y. Lam, et al., Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China, Microbiology (2020). doi:10.1101/2020.02.13.945485.
- 16.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. USA. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes L.R., de Mattos Cardillo G., Paiva P.B. Molecular evolution and phylogenetic analysis of SARS-CoV-2 and hosts ACE2 protein suggest Malayan pangolin as intermediary host. Braz. J. Microbiol. 2020 doi: 10.1007/s42770-020-00321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaimes J.A., André N.M., Chappie J.S., Millet J.K., Whittaker G.R. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. J. Mol. Biol. 2020;432:3309–3325. doi: 10.1016/j.jmb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Q&A: Similarities and differences – COVID-19 and influenza https://www.who.int/news-room/q-a-detail/q-a-similarities-and-differences-covid-19-and-influenza. (2020). Accessed 24 April 2020.
- 20.Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment, 7th ed. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. (2020). Accessed 25 April 2020.
- 21.van Doremalen N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Y., et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.J. Kowalski Wladyslaw, T.J. Walsh, V. Petraitis, COVID-19 Coronavirus ultraviolet susceptibility, 2020. doi:10.13140/RG.2.2.22803.22566.
- 24.G. Kampf, A. Voss, S. Scheithauer, Inactivation of coronaviruses by heat, J. Hospital Infect. S0195670120301249 (2020). doi:10.1016/j.jhin.2020.03.025. [DOI] [PMC free article] [PubMed]
- 25.Cheng Z.J., Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48:155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.M. Faiq, et al., COVID-19: A review on molecular basis, pathogenic mechanisms, therapeutic aspects and future projections, Life Sci. (2020). doi:10.20944/preprints202004.0091.v1.
- 27.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. Jul 2020;2020 doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therapeutic Options for COVID-19 Currently Under Investigation https://covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/. (2020). Accessed 22 April 2020.
- 29.M. Hoffmann, et al., The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells, Mol. Biol. (2020). doi:10.1101/2020.01.31.929042.
- 30.T.-Y. Xiong, S. Redwood, B. Prendergast, M. Chen, Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. ehaa231 (2020). doi:10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed]
- 31.Li B., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.C. Fan, K. Li, Y. Ding, W.L. Lu, J. Wang, ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection, Urology (2020). doi:10.1101/2020.02.12.20022418. [DOI] [PMC free article] [PubMed]
- 33.Moreno L., Pearson A.D. How can attrition rates be reduced in cancer drug discovery? Expert Opin. Drug Discov. 2013;8:363–368. doi: 10.1517/17460441.2013.768984. [DOI] [PubMed] [Google Scholar]
- 34.Wang M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med. Infect. Dis. 2020;101615 doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.M.L. Agostini, et al., Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, mBio 9 (2018) e00221–18. /mbio/9/2/mBio.00221-18.atom. [DOI] [PMC free article] [PubMed]
- 37.J. Grein, et al., Compassionate Use of Remdesivir for Patients with Severe Covid-19, N. Engl. J. Med. NEJMoa2007016 (2020). doi:10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed]
- 38.Wang Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;S0140673620310229 doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NIH clinical trial shows Remdesivir accelerates recovery from advanced COVID-19. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. (2020). Accessed 29 April 2020.
- 40.Hopes rise on coronavirus drug remdesivir, https://www.nature.com/articles/d41586-020-01295-8. (2020). Accessed 29 April 2020. [DOI] [PubMed]
- 41.COVID-19 therapeutics tracker, https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-therapeutics-tracker. Accessed 14 July 2020.
- 42.Gilead reports reduced mortality risk with remdesivir for Covid-19, https://www.clinicaltrialsarena.com/news/remdesivir-covid-19-analysis-data/. Accessed 21 July 2020.
- 43.Gilead begins testing of inhaled version of remdesivir for Covid-19, https://www.clinicaltrialsarena.com/news/gilead-inhaled-remdesivir-phasei/. Accessed 21 July 2020.
- 44.Sham H.L., et al. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 1998;42:3218–3224. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Wilde A.H., et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of middle east respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.B. Cao, et al., A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19, N. Engl. J. Med. NEJMoa2001282 (2020). doi:10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed]
- 48.No clinical benefit from use of lopinavir-ritonavir in hospitalised COVID-19 patients studied in RECOVERY, https://www.recoverytrial.net/news/no-clinical-benefit-from-use-of-lopinavir-ritonavir-in-hospitalised-covid-19-patients-studied-in-recovery. Accessed 19 July 2020.
- 49.WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19, https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19. Accessed 19 July 2020.
- 50.Tan E.L.C., et al. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Q. Tan, Y. Jin, Ostavimir is ineffective against COVID-19: in silico assessment, in vitro and retrospective study, Infect. Diseases, except HIV/AIDS (2020). doi:10.1101/2020.05.15.20102392.
- 52.L. Dong, S. Hu, J. Gao, Discovering drugs to treat coronavirus disease 2019 (COVID-19), 3 (2020). [DOI] [PubMed]
- 53.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Japan Acad. Ser. B: Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russian Ministry of Health approves the first COVID-19 drug Avifavir produced by JV of RDIF and ChemRar, https://rdif.ru/Eng_fullNews/5220/. Accessed 14 July 2020.
- 55.China Approves Favipiravir (Avigan) As An Experimental Drug To Treat Coronavirus, https://www.thailandmedical.news/news/china-approves-favipiravir-avigan--as-an-experimental-drug-to-treat-coronavirus. Accessed 14 July 2020.
- 56.Results from Trial of Antiviral Favipiravir in Patients with Asymptomatic or Mild COVID-19 conducted at Fujita Health University, https://www.fujita-hu.ac.jp/en/news/kka9ar0000000gmz.html. Accessed 14 July 2020.
- 57.K. Sonawane, et al., Homology modeling and docking studies of TMPRSS2 with experimentally known inhibitors camostat mesylate, nafamostat and bromhexine hydrochloride to control SARS-Coronavirus-2. (2020). doi:10.26434/chemrxiv.12162360.v1. [DOI] [PMC free article] [PubMed]
- 58.Zhou Y., et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao X., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.M.G.S. Borba, et al., Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study), Infect. Diseases (except HIV/AIDS), (2020). doi:10.1101/2020.04.07.20056424.
- 62.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;S0140673620311806 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Arshad S., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Diseases. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D.R. Boulware, et al., A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19, N. Engl. J. Med. NEJMoa2016638 (2020). doi:10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed]
- 65.No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19, https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19. Accessed 18 July 2020.
- 66.FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems, https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. Accessed 18 July 2020.
- 67.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Price C.C., et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients. Chest. 2020;S0012369220316706 doi: 10.1016/j.chest.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.E.C. Somers, et al., Tocilizumab for treatment of mechanically ventilated patients with COVID-19. 27. [DOI] [PMC free article] [PubMed]
- 70.Campochiaro C., et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Internal Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Regeneron and Sanofi Provide Update on Kevzara® (sarilumab) Phase 3 U.S. Trial in COVID-19 Patients https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-kevzarar-sarilumab-phase-3. Accessed 18 July 2020.
- 72.InflaRx Announces Decision to Enter Phase III Development of IFX-1 in Severe COVID-19 Induced Pneumonia, https://www.globenewswire.com/news-release/2020/07/21/2064803/0/en/InflaRx-Announces-Decision-to-Enter-Phase-III-Development-of-IFX-1-in-Severe-COVID-19-Induced-Pneumonia.html. Accessed 21 July 2020.
- 73.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.P. Horby, et al., Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report, Infect. Diseases (except HIV/AIDS), (2020). doi:10.1101/2020.06.22.20137273.
- 76.World first coronavirus treatment approved for NHS use by government, https://www.gov.uk/government/news/world-first-coronavirus-treatment-approved-for-nhs-use-by-government. Accessed 21 July 2020.
- 77.Shen C., et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan K., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA. 2020;202004168 doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joyner M.J., et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 2020;S0025619620306510 doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ianevski A., et al. Potential antiviral options against SARS-CoV-2 infection. Viruses. 2020;12:642. doi: 10.3390/v12060642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coronavirus India: Rush for plasma therapy as Covid-19 cases rise, https://www.bbc.com/news/world-asia-india-53387607. Accessed 21 July 2020.
- 82.Cao W.-C., Liu W., Zhang P.-H., Zhang F., Richardus J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 2007;357:1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 83.Chen W.-H., Strych U., Hotez P.J., Bottazzi M.E. The SARS-CoV-2 vaccine pipeline: an overview. Curr. Trop. Med. Rep. 2020 doi: 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DRAFT landscape of COVID-19 candidate vaccines – 20 April 2020, https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf. (2020). Accessed 01 May 2020.
- 85.Pronker E.S., Weenen T.C., Commandeur H., Claassen E.H.J.H.M., Osterhaus A.D.M.E. Risk in vaccine research and development quantified. PLoS ONE. 2013;8:e57755. doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.ClinicalTrials.gov: Federally-funded clinical studies related to COVID-19, https://clinicaltrials.gov/ct2/results?cond=COVID-19. (2020). Accessed 21 July 2020.
- 87.“Solidarity” clinical trial for COVID-19 treatments, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments. (2020). Accessed 26 April 2020.
- 88.WHO Director-General’s opening remarks at the media briefing on COVID-19 - 25 May 2020, https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---25-may-2020. Accessed 31 May 2020.
- 89.Vanden Eynde J.J. COVID-19: a brief overview of the discovery clinical trial. Pharmaceuticals. 2020;13:65. doi: 10.3390/ph13040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Q. Wu, Jing, et al., Altered lipid metabolism in recovered SARS patients twelve years after infection, Sci. Rep. 7 (2017) 9110. [DOI] [PMC free article] [PubMed]