Introduction
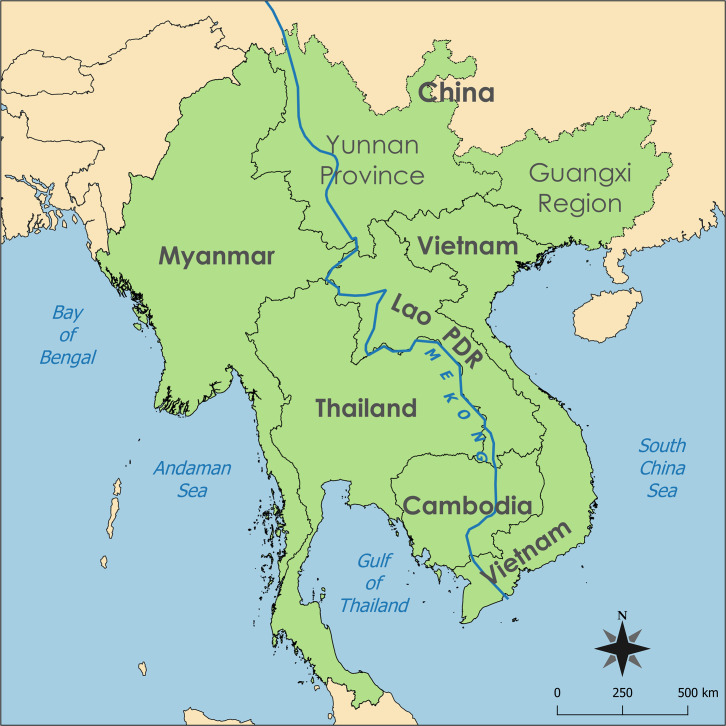
Vector-borne diseases (VBDs) are a significant and growing threat to the health of the 326 million people living in the Greater Mekong Subregion (GMS) (Fig 1). The GMS is a diverse landscape of cities, rural agricultural communities, forests, deltas, wooded hills, and mountains in the six countries along the Mekong River basin. As the GMS transforms into an increasingly important hub in the global economy, heterogeneous development, rapid urbanization, and socioeconomic risk factors result in increased migration (especially rural-to-urban migration), rapid land-use change, and urban poverty—all factors that can exacerbate transmission of VBDs [1].
Fig 1. GMS.
The map was made using QGIS version 3.4 (https://qgis.org). All map layers were created by one of the coauthors.
Historically, Plasmodium falciparum malaria has been a primary focus of public health efforts in the region, and, since the discovery of chloroquine-resistant P. falciparum malaria in Cambodia in the 1950s, containment of multidrug resistant P. falciparum malaria has been one of the most pressing public health challenges in the GMS [2]. In response to artemisinin resistance, intense commitments from governmental, nongovernmental, and multilateral agencies have increased access to antimalarials and insecticide-treated bed nets. Additionally, socio-economic development has been associated with dramatic decreases in P. falciparum cases across the region [3, 4]. As many GMS countries now strive for malaria elimination by 2030 [4], they are simultaneously confronted with other complex health problems, including, but not limited to, (1) arboviral epidemics that are superimposed on decades-long dengue (DENV) transmission and threaten the region’s economy and health [5] and (2) emerging threats such as uncharacterized tick-borne viruses or cutaneous leishmaniasis transmitted by sand flies in rapidly changing landscapes due to land-use change and/or urbanization [6, 7]. Despite a preponderance of research focused on multidrug resistant malaria, DENV, and more recently chikungunya virus (CHIKV) and Zika virus (ZIKV), many challenges remain with regard to the control of these VBDs of public health importance.
In view of these challenges, the United States National Institute of Allergy and Infectious Diseases (NIAID), in conjunction with the Cambodian Ministry of Health, hosted a workshop of 80 experts and government stakeholders in March 2019 from 14 countries (workshop, speakers, and presentation titles are listed in S1 Text) with expertise spanning clinical tropical medicine, ecology, epidemiology, infectious diseases, immunology, vaccinology, vector biology, and virology. The primary goal of this workshop was to prioritize vector research challenges in the GMS in order to better understand transmission and long-term control of VBDs.
Of particular interest was the increasing DENV burden in the region, as it continues to represent a major public health issue for the GMS, particularly in Thailand where cases exceeded 100,000 in 2019 [8, 9]. While cases of DENV continue to rise in the GMS, the overall epidemiology of the virus remains unclear in some GMS countries such as Cambodia where surveillance is limited to clinicosyndromic surveillance in pediatric populations [10]. This constitutes a gap in data necessary to coordinate control efforts within and among the affected countries [11]. Also of interest was the changing landscape of malaria epidemiology, given a 75% drop in case incidence in the GMS since 2018 [4, 12]. With the current goal of elimination by 2030 [4, 13], the overall decline in malaria cases and deaths is attributed to transmission that is increasingly limited to specific geographic locations, strong commitment from policy makers, effective partnerships, crossborder collaborations, and improved access to hard-to-reach group [8]. Understanding the dynamics of malaria transmission across the region will be critical for control programs targeting the remaining geographic and demographic clusters of this disease.
During this March 2019 workshop in Cambodia, it was noted that a group of experts similarly gathered in Singapore in 1977 to discuss the many factors contributing to increases in severe cases of DENV infection (DENV hemorrhagic fever and DENV shock syndrome) in Southeast Asia as well as the current state of knowledge regarding malaria [5]. At that meeting, experts reviewed the recent 1950s malaria elimination campaigns where DDT and chloroquine had failed, and the general consensus was that malaria control would depend upon socioeconomic “progress” in Asia as it had in other locations. Participants correctly anticipated the expansion of both Aedes distributions and at-risk, DENV-susceptible populations and discussed approaches that encompassed three major themes: vaccine developments, vector control, and transmission ecoepidemiology.
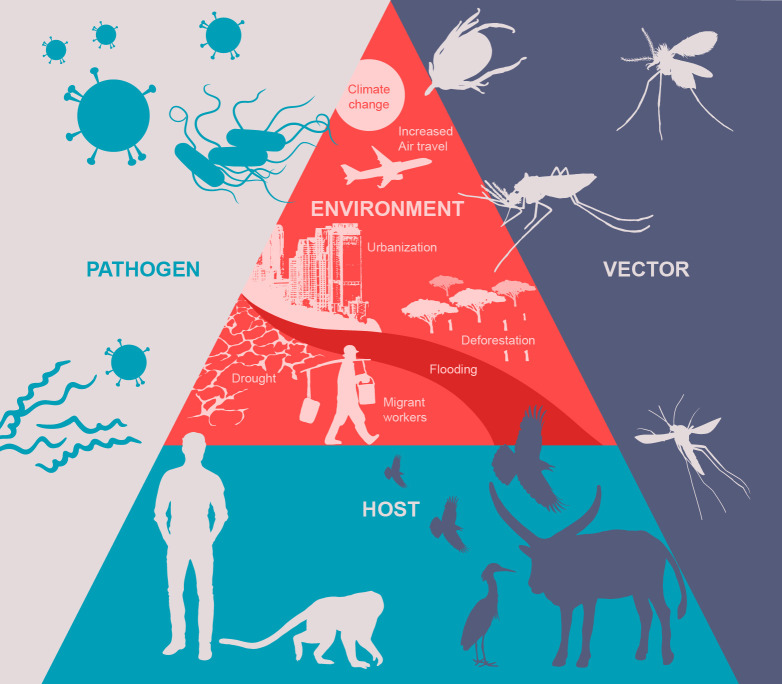
Discussion of vector-borne disease research often invokes the vector–host–pathogen triad. However, advances in the field have highlighted the importance of considering multifactorial interactions, including the contribution of the environment, which can be defined by ecological, socioeconomic, and/or climatic factors (Fig 2). An important goal is to understand how these interactions define transmission patterns in order to identify factors that can be utilized in regionally specific ways appropriate to the GMS yet can still be globally adopted into frameworks where these issues are investigated and translated into actionable items. Thus, in light of progress over the last 42 years, herein we summarize the 2019 workshop presentations, discussions, and newly identified priority outcomes with particular focus on the importance of interdisciplinary basic and translational research of the pathogen–human–vector–environment interface that may lead to the ultimate goal of vector-borne disease reduction, especially for DENV and malaria.
Fig 2. The pathogen–host–vector–environment interface representing the many factors that define transmission.
Vaccine developments
Interventions targeting the pathogen–human interface focus on prevention of infection by vaccination or by antipathogenic medications that can either be used prophylactically or postinfection. Vaccine development for VBDs has been challenging. This is evidenced by the fact that only two vector-borne disease vaccines, the highly effective yellow fever (YFV), and Japanese encephalitis virus (JEV) vaccines, are on the World Health Organization approval list for use without caveat as of 2019 [14]. The recently licensed vaccine for P. falciparum malaria requires four doses and yields 36% efficacy that wanes with time [15–17]. Efforts at creating an effective and safe DENV vaccine have been frustrated by the cross-reactivity of antibodies among serotypes and unequal immune responses against the different serotypes [18]. In 1977, there was much discussion regarding the potential for monovalent vaccines given simultaneously to produce a tetravalent immune response [5]. At that time, there was still a need to characterize DENV-4 and the unknown genetic diversity of circulating wild-type and future epidemic variants [5, 18, 19].
Prescient 1977 panel members raised concerns around the concept of DENV infection enhancement and serotype-equitable immune responses, which is the required nuance that a DENV-vaccine candidate produce robust yet equivalent levels of neutralizing antibodies to all four serotypes of DENV or else the recipient may have a worsened clinical outcome upon DENV infection. This continues to plague DENV vaccine development even with today’s only currently licensed vaccine [20, 21]. As summarized in S1 Fig from the 2019 presentations, NIAID vaccine development for DENV has taken more than 20 years to carefully address antibody-dependent enhancement (ADE) issues and advance a suitable tetravalent vaccine to Phase III trials [22]. Characterizations of these vaccine candidates—much like those of attenuated viruses presented in 1977—included hundreds of viral constructs. However, current vaccine developers have had the cutting-edge laboratory and clinical tools for high throughput screening of vaccine candidate strains, including derivation of mutants, and improved early-stage clinical evaluation using controlled human infection models [23].
Despite the advances in DENV-vaccine research, critical concerns remain regarding emergence of related mosquito-transmitted flaviviruses, such as ZIKV. The potential for crossreactivity and/or confounding of vaccine immunological indicators highlights the need for tools to elucidate these possibilities [21, 24, 25]. In addition, there is heightened concern about the development of vaccine hesitancy and antivaccination movements leading to a lack of take-up regardless of proven efficacy [26, 27]. Thus, community engagement and education regarding the efficacy and safety of future vaccine candidates will be paramount to the successful uptake of future vaccines for DENV and other VBDs. In 1977, participant experts lauded the novel power of the television for public health educational campaigns to reduce VBDs like DENV and malaria [5]. In 2019, public health agencies must acknowledge the equally powerful role of social media that can both promote, and undermine, vaccine information campaigns [27].
Novel vector-targeted vaccinology
Advances in vector-based vaccines, such as those targeting mosquito salivary peptides, have also occurred with the progress in pathogen-targeting vaccines. Several studies demonstrate that mosquito saliva coinoculated with the pathogen at the bite site can enhance the likelihood of successful transmission leading to infection as well as the course of arbovirus infection through alteration of the immune responses at the bite site [14, 19, 28–33]. In theory, vaccinated individuals would mount a rapid, Th1-skewed response to pathogens codeposited with mosquito saliva and thus interrupt the pathogen’s ability to establish infection in the dermis [34]. Vector saliva-based vaccines are in the nascent stages of development, with a single Phase I candidate composed of Anopheles gambiae salivary peptides [35], but there is growing interest in alternative utilities of vector saliva such as (1) its use as an adjuvant in a pathogen-targeted vaccine as seen in leishmaniasis [36]; (2) its use to differentiate risk of exposure to the pathogen and/or subsequent development of disease [37–46]; or (3) its role in the context of travel medicine. In terms of protection for the traveler, most saliva-specific total immunoglobulin G (IgG) has a duration of less than four months whereby saliva-vaccine induced protection could be appropriately transient, but possibly broad against infections by a vector such as Aedes aegypti, which transmits several pathogens of public health relevance (DENV, ZIKV, CHIKV, and YFV) [47].
Transmission reduction is realized when there is a partial or complete interruption of pathogen transfer to the mosquito from the infectious host. There is still a need to understand the within-host dynamics of infection not only with regard to pathogenesis but to better our understanding of transmission. Controlled human infection models, particularly if using vector delivery of pathogens as is done with malaria [48], may support new insights into pathogen dose-dependence of human-to-vector transmission and will help define parameters for effective interventions by vaccines, antimalarials, or vector saliva-based therapeutics for the goals of transmission reduction [49]. In the case of DENV and P. falciparum, mosquitoes become differentially infected depending on the concentration of pathogen found in the blood meal [50–54]. Symptomatic, asymptomatic, and subclinical disease reservoirs contribute to the transmission cycle [55–57], and laboratory, field, and modeling efforts have shown that the infectiousness of the host can be an important determinant of transmission of several VBD [51–54]. These are important considerations for vector-oriented research in the near future.
Exposure risk and transmission
Exposure to the vector is the first and necessary process leading to transmission to the human or infection of the vector. Interruption of this contact is also the cornerstone of control, either by directly blocking the contact event (i.e., bed nets) or by decreasing the risk of contact with infectious vectors (through vector population control or reduction of vector longevity). Understanding the processes that drive increases or decreases in human–vector contact are discussed here.
Even with the progress made on vaccines and drug treatments, maintaining long-term control and/or achieving disease elimination will also depend on the interruption of human–vector contact that is the hallmark of vector control programs [58]. Interruption of vector–human contact is primarily achieved via the use of bed nets (insecticide-treated or not); personal protection (clothing, chemical repellents, etc.); and the use of insecticides aimed at the reduction of the vectorial capacity of the local vector populations below the critical threshold needed to maintain transmission [59, 60]. Sprays or fogs that target adult mosquitoes have various efficacy depending on whether it is applied inside or outside the home [61, 62]. Though there is a need for future studies to definitively determine efficacy of outdoor spraying; when supported by real-time patterns of transmission, spatial and temporal analyses can provide evidence of the impact of focally applied measures [63]. Approaches of growing interest include the use of spatial repellents, biological control methods such as Wolbachia, and the release of biologically modified arthropods [64].
Insecticide resistance has been and continues to be a major concern in areas that have relied upon the use of insecticides, especially regarding DENV and some malaria vectors in which in many cases there is resistance to multiple commonly used pesticide classes [65–71]. Insecticide resistance in malaria vectors in the GMS is not as well characterized, mainly because it has been difficult to catch or rear sufficient numbers of the primary vectors and perform the necessary resistance bioassays [71–75]. Larviciding for malaria control is a recommended method for larval source management in communities where aquatic anopheline habitats are “few, fixed, and findable” but is not typically used in Southeast Asia for anopheline habitats, although, when utilized, have had success [76–78]. In contrast, larviciding is a key tenet of A. aegypti control in most urban habitats, although there is widespread resistance to temephos, an organophosphate larvicide commonly used in the GMS, thereby making Bacillus thuringiensis israelensis (Bti) or diflubenzuron more promising alternatives [13, 79]. The myriad of accessible and cryptic habitats suitable for A. aegypti development make larviciding a labor intensive and expensive undertaking that struggles to achieve high levels of coverage. Program efficiency is further reduced by a lack of a consistent, well-trained workforce in the community although there are examples of intersectoral success in Southeast Asia reliant upon nonhealth sectors such as the military, police, education, and construction industry to help control breeding sites [80–84]. Some countries in the GMS rely on community volunteers, especially for larval-based vector control; but often, trained pesticide application workers are hired during peak epidemics and, when the epidemic wanes, so does their workload and compensation. This leads to high turnover and a dearth of trained personnel, causing significant lag time at the height of epidemics. Both community engagement and workforce consistency rooted in the local community are necessary for a continuously efficacious vector control program, and partnering with nonhealth agencies and private industries should be prioritized as governments expand their vector control portfolios [85].
Insecticide resistance is a complex problem, potentially involving a large and population-specific range of physiological, behavioral, and molecular mechanisms. There are no molecular or biochemical diagnostics that can quickly and accurately characterize insecticide-resistant phenotypes or determine the impact of that phenotype on the operational efficacy of insecticides in the field [86–90].
Although peridomestic space spraying, the most commonly applied vector control method during DENV outbreaks, may reduce mosquito numbers for shorter time periods, there is no evidence that it has an effect on DENV transmission [91]. On the other hand, it is peculiar that indoor residual spraying (IRS), a successful malaria vector control intervention, is not more frequently used in DENV vector control. Both IRS, and more specifically targeted IRS, as well as indoor space spraying (ISS) may be effective against both mosquitoes and DENV transmission at high coverage in transmission settings, as they can potentially reduce parasite and pathogen transmission because of the vulnerability of largely endophagic and endophilic mosquitoes [92, 93]. However, such specific methods are only appropriate where there is a high community acceptability and Aedes vectors are predominantly endophilic, which may not be the case for A. albopictus [94]. Although A. albopictus is considered a secondary vector of DENV, its exophilic nature represents a unique regional challenge for control of other arboviruses such as CHIKV and ZIKV, in addition to DENV [91, 95].
Nonchemical-based alternatives for vector control are also being developed (e.g, biological control [copepods, guppy fish, etc.], genetically modified mosquitoes and improved community engagement strategies). In Vietnam, copepods of the genus Mesocyclops were an effective and sustainable biological control agent in the northern and central areas of Vietnam [96], but that has been less consistently adopted in areas of southern Vietnam [97]. Given the context-specific rationales for these biological control tools, current studies of pyriproxyfen and guppy fish in Cambodia suggest the potential for high acceptance and perceived effectiveness of interventions [98, 99]. However, some warn against release of nonnative species such as guppy fish as the overall efficacy of this approach may not outweigh the negative ecological impacts and monitoring for such impacts could be logistically problematic [100]. Community engagement is necessary to carry out any interventional program for vector-borne disease, but Southeast Asia lacks evidence-based community mobilization data, such as that in Central America, but studies are underway in Myanmar and Cambodia ([99, 101], personal communication).
Sterile male techniques aimed at reductions in mosquito populations include irradiation and genetic modifications [102, 103]. Further use of transgenic mosquitoes—both Aedes and Anopheles—has been put forward as a means of controlling VBDs, although consideration of fitness costs in field settings is critical [104]. However, there are promising strategies, including targeting genes, that result in a reduction of transmission of a pathogen either from the human to the mosquito or vice versa. Additionally, other approaches involve promoting or activating mosquito immune responses against infecting arboviruses that would alter transmission capabilities [105–108]. Biological control methods, such as Wolbachia infection of A. aegypti, are aimed at reducing the competence of vectors and have reduced dengue incidence in the same areas as field trials in Australia and Indonesia, with documented success in arbovirus control in Colombian releases; and controlled release of Wolbachia-induced sterile males reduced the A. aegypti populations in targeted areas in Florida [109–111]. Further, there is interest in the use of endectocides for tackling residual malaria transmission (RMT). This entails treatment of humans or domestic animals with ivermectin in order to kill mosquitoes that feed on either host [112]. This is a suitable approach for the GMS, with its specific problems of RMT, outdoor biting mosquitoes, and zoophagic mosquitoes that are poorly targeted by LLINs [113].
Targeted control methods have been successful in the past and may be successful on very specific spatial scales, as seen in Cairns, Australia [109, 114]. The question of “what happens if the ecological balance is disturbed?” creates two possible deleterious scenarios: (a) further invasions or expansions of secondary vector populations and/or (b) enhanced role of secondary vectors in areas with established transmission. Prevention of reintroduction of vector-borne pathogens is essential, particularly in the populous GMS, and must address the characterization of potential secondary vectors, possible introduction of exotic vectors, or importation of pathogens by visitors and migrants [115, 116].
Additionally, entomological indices to estimate mosquito presence and density rarely correlate with Aedes-transmitted arbovirus disease patterns, and thus the primary indicator of successful vector control—a reduction in vector populations—may also not reflect reduced Aedes-transmitted arbovirus disease burden patterns [5, 117, 118]. Newer strategies using gravid traps and rapid NS1 testing of mosquitos may be more accurate in assessing disease burden [119, 120]. Methods such as entomological incoculation rates (EIR) and human biting rate (HBR) have had better success with malaria risk assessments in high-transmission settings but are still considered time-consuming, expensive, and imprecise [121–124]. Better methods of determining the nuances of contact rates between infectious mosquito and the affected human population would provide more precision to understand transmission, especially in the context of tracking expanding vector populations. Vector salivary proteins have the potential to become powerful biomarker tools to qualify and quantify mosquito exposure and the risk to mosquito-borne pathogen exposure. Studies have determined that reactivity to mosquito salivary gland extract (SGE) can differentiate mosquito exposure risk due to environmental factors, social factors, and seasonal dynamics [38, 47]. Other studies have shown that serosurveys to SGE and/or particular mosquito salivary peptides can be used to describe differences in vector exposure in space and time due to sociodemographic, environmental, and operational (vector control) factors [37, 39–44]. For example, people living along the Thai-Myanmar border with a high antibody response to Anopheles salivary peptide gSG6-P1 had six times higher odds of also being positive to P. falciparum circumsporozoite antigens and two times higher odds of P. falciparum infection, compared to low responders to mosquito saliva antigens [45]. For Aedes mosquitos, the N-terminus 34 kDa salivary protein was recently used to track the efficacy of vector control interventions in Burkina Faso [125]. The utility of salivary biomarkers may aid targeting Aedes spp. vector interventions in places where seroprevalence studies are difficult due to multiple circulating flaviviruses or if disease incidence is underestimated due to the a high proportion of clinically inapparent disease or poor surveillance. Taken together, these data suggest that vector salivary proteins may differentiate mosquito exposure histories in space and time in order to gauge the effectiveness of an intervention and/or the expansion of vector populations (seasonally or permanently).
With regard to other vectors, such as ticks, chiggers, sand flies, and fleas, vector control is not routinely performed in the GMS. Given limited studies and funding available for these vectors and their pathogens, a general consensus concluded that the next step would be better field surveys to characterize the landscape of these vectors and the pathogens that they may carry and transmit. For example, the detection of rickettsial diseases has increased in some areas [126, 127]. Whether these diseases are on the rise or whether this is merely indicative of an increase in surveillance efforts remains to be determined. However, the continued and expanded success of vector control programs and the implementation of novel control strategies requires community buy-in [128]. And while education programs exist across the GMS, consistent compliance is often hard to determine [129].
Understanding the Eco-Environmental Drivers of Transmission
A common theme across the vector-specific research presentations at the 2019 workshop in Cambodia was a lack of detailed characterization of the ecoepidemiology of VBD systems or how the study of ecological systems of infectious diseases at the population and community levels can inform our understanding of transmission. Environmental conditions, human behavior, and population movement patterns, changes in land use, the microbiome of the vector and habitat, climate change, and intra- and interspecific variability (microbial and vector) all impact observed transmission patterns [130, 131]. The meeting discussion determined that there was a general dearth of understanding of the interrelations of the VBD triad and the further interaction with the environment in the GMS (Fig 2) [132–141]. While there were a series of discussion involving mosquito-transmitted infections, tick-borne diseases were also highlighted as emerging health concerns where the epidemiology is tightly intertwined with the ecosystem.
The actual transmission patterns of DENV and malaria, whereas most tick-borne pathogens are in general not understood in the GMS, owing to a lack of disease incidence and prevalence. This is due, in part, to high rates of clinically inapparent infections, where patients do not seek medical services or do not experience any overt illness at all. For example, experts estimate that asymptomatic DENV cases make up one-half to three-quarters of all cases worldwide, while models suggest that these inapparent infections could contribute upwards of 80% to DENV transmission [55, 142–145]. Inapparent infections in other diseases, such as malaria and leishmaniasis, are likely also contributing to a significant proportion of transmission [57, 146–150]. However, patterns of inapparent infection rates are heterogenous, further confounding efforts to estimate transmission intensity [151–155]. While cost-effective and easy-to-use rapid diagnostics exist for DENV, a lack of specific and consistently performing diagnostics contributes to this problem, especially in regions where genetically related viruses cocirculate, leading to misclassification of positive results [156–169]. Diagnostic tools for tick-borne diseases relevant to the region are nonexistent, and this has hampered confirmation of infection, identification of pathogen, and conduct of surveillance. Further, across all VBDs and discussed both in 1977 and 2019, the role of pathogen diversity in transmission intensity, pathogenesis, immune responses/diagnostics, and vaccine development is understudied [133, 156, 169].
An important example of the impact of changing landscapes is RMT. In several areas in Southeast Asia, RMT is more apparent now in forests than in villages [124, 170, 171]. In some study areas, an increase in forest fragmentation (especially encroachment of agricultural fields on forests) led to a reduction in malaria vector diversity and density [140, 141]. In other areas, this pattern has been less clear [172, 173]. In addition to the public health use of chemicals for direct vector control, frequent and widespread use of synthetic pyrethroids and organophosphates in agriculture and livestock industries also contribute to insecticide resistance [174, 175]. Further, land-use change—particularly clearing of primary forests—means that there is an increase in the overall availability of suitable habitats for the establishment and maintenance of Aedes-human contact. While more study has been devoted to the impact of rubber plantations on anopheline vectors, the presence of mature plantations can indeed provide increased habitats for Aedes vectors [176]. On the other hand, the intensification of agricultural practices (e.g., increased pesticide and fertilizer application, market-driven cropping practices, and areal extension of intensively cropped areas) alters landscapes by reducing the natural forest cover. This landscape change, albeit without urbanization, can still lead to an increase in transmission, as has been observed in the case of DENV and CHIKV in Thailand and Cambodia by providing more suitable breeding habitats for Aedes spp. [176–182]. Thus, while urbanization is often the primary land-use change associated with an increase in the risk of exposure to some vectors and their associated pathogens, other changes in land-use (such as agricultural or industrial) should also be considered when assessing risk and subsequently developing plans for mitigation [179, 183, 184].
Changing the environment may also alter the range of not only primary urban vectors, but also those of sylvatic and potential bridge vectors [132, 185]. Human settlement and primary forest clearance patterns; the development of dam, drainage, and agricultural irrigation schemes; and, generally, greater exploitation of natural resources are all drivers influencing patterns of disease distribution and incidence that are often transmission system-specific. All these create new pressures on ecosystems that, in turn, have profound impacts on vector habitats, the carrying capacity of the environment for vector populations, and infectious disease transmission [186, 187].
Conclusion
Participants at this 2019 meeting recognized a continued need for prioritization into (1) vaccines and therapeutics to prevent and control disease in the human population, (2) understanding the heterogeneity in the exposure risk and factors that define transmission in time and space, and (3) defining the ecoenvironmental drivers that support continued and/or emergent transmission of VBD (Table 1). In many ways, these questions and prioritized vector research echo those raised in 1977 and no doubt in other meetings since (See S2 Fig). However, rather than reflecting a stagnation in science, the 2019 proceedings herein demonstrate that VBD research has evolved to encompass a more in-depth understanding of the factors that govern VBD transmission and control.
Table 1. Priority discussion and research questions.
| Prioritized Discussions | Priority Research Questions |
|---|---|
| Vaccines and novel vector-targeted vaccinology | How does pathogen diversity contribute to the success of vaccine and/or therapeutic candidates? Is there a role for vector saliva in risk assessments, prophylactics against vector exposure, or diagnostics? How can we leverage continued understanding of the bite-site microenvironmental changes due to vector saliva to aid in development of therapeutics, likely using controlled human infection models? What are the diagnostic needs to accurately surveil and detect outbreaks of VBD in endemic versus epidemic and/or emergent situations? |
| Exposure risk and transmission | What is the contribution of asymptomatic and subclinical infections to the overall transmission? What factors govern the heterogeneity in asymptomatic and/or subclinical rates? How can vector control programs best engage the community as partners for control success as well as to educate for exposure awareness and mitigation? What extrinsic or intrinsic factors govern exposure risk and how do those factors themselves change in the face of anthropogenic pressures? What is the current and future roles of alternative vectors? What is the potential for secondary vectors to adapt and thus increase their public health relevance? |
| Eco-environmental drivers of transmission | How will continued urbanization and land-use changes affect the distribution and relative importance of vector populations? What are the microenvironmental effects of climate change with respect to vector–pathogen interactions that define transmission potential? What is the role of biodiversity in shaping transmission both in endemic and epidemic scenarios? How can basic VBD and vector research be integrated into future development plans to meet the needs of growing populations but still mitigate disease risk? |
As our characterizations of specific aspects of these VBD systems have grown more detailed, new questions emerge from these discoveries. This suggests that we have been dealing with a “dangling carrot” model of knowledge. That is, despite ongoing and substantial progress, we have not quite reached our objective of sufficient and necessary understanding of these pathogen–vector–host systems to achieve the desired goal of control, prerequisite for elimination, and, ultimately, eradication of these diseases.
Specifically, 2019 experts felt that pathogen diversity should be considered when developing vaccine, therapeutic, or biocontrol programs to account for the nuances of immune response [188, 189]. In addition, there was agreement that the use of vector saliva has the potential to be used for exposure prophylactic or a determinant of vector exposure risk levels. Given the immunogenicity of vector saliva in the context of pathogen transmission and infection, there was also agreement that development of controlled human infection models could provide important immunological and mechanistic insights into the success of transmission of VBD. Finally, there was consensus that continued surveillance, bolstered by more specific and sensitive diagnosticsm are needed to differentiate etiologies of disease in areas where multiple VBD cocirculate.
Regarding transmission and exposure, the role of asymptomatic and subclinical infections is an important topic as these infectious but undetected individuals are likely reservoirs for continued transmission and subsequent transmission and clinical cases. Education of communities and the engagement in at-risk communities to understand transmission and thus assist in interrupting transmission in culturally sensitive ways is paramount to long-term success of strategies. Additionally, understanding the nuances of spatial and temporality of transmission—including cryptic transmission and zoonotic transmission—is necessary for accurate risk assessment as well as overall realization of successful vector control. Specifically, understanding these nuances would only augment current understanding and study of insecticide resistance and how adjacent vector populations affect the treated vector populations. Finally, the role of alternative vectors, the importation of nonnative competent vectors, and the reestablishment of populations upon the cessation or failure (partial or complete) of current control regimens should be proactively considered.
Finally, continued development through urbanization and varied and changing agricultural practices will likely influence the vector range, vector life-traits, as well as the within-vector kinetics of VBD and should be monitored. Microclimate changes should be taken into account when performing risk assessments, especially as these changes will affect biodiversity of both the vector populations and the pathogen populations. The role of basic biodiversity was thought to be underappreciated in how it shapes the overall transmission landscape, which we know to change in time and space at focal levels, which could lead to fine-scale adaptations that could undermine efforts [64, 190]. The dramatic anthropogenic and environmental changes occurring in the GMS and elsewhere require a paradigm shift with a stronger emphasis on innovative integrated vector management including a synthesis of (1) proven effective methods, (2) new delivery approaches based on proven social theories in the local context, and (3) partnerships with sectors outside of health (e.g., water works, agriculture, forestry, defense, etc.) [191, 192]. The main challenges, however, might be the required change in human behavior and mindsets that such a vector control paradigm requires to be truly effective. How to frame VBD research to incorporate these dimensions arguably is itself a research priority.
Human driven changes, often to meet the needs of growing populations, should be met with basic intersectoral communication whereby VBD biologists and control researchers partner with government, community, urban planners, and other infrastructure experts to meet these needs while mitigating the risk of continued or intensified transmission. Thus, intersectoral collaboration is necessary for future vector research. One widely considered framework is structured around social and ecological determinants of VBD resurgence and the principle modes of environmental change—namely agricultural intensification, urbanization and natural habitat alteration [185, 193, 194]. This theoretical framework emerged from an National Institutes of Health Roadmap to the Future project and emphasizes the complexity of “human-natural systems” in which transmission dynamics are effectively embedded [195]. Understanding such dynamics are critical to further elucidation of the vector–pathogen–host triad, given its complexities spanning the population level (for humans, vectors, and pathogens), community level (human and natural), landscape level, and, ultimately, globally. Cumulatively, the collection of topics raised in this workshop represent current priorities towards achieving the ultimate goals of lessened morbidity and mortality from vector-borne disease in the GMS.
Supporting information
(DOCX)
This schematic demonstrates the process and current state of dengue vaccine candidates that have gone from preclinical development through to Phase III trials as of early 2019.
(DOCX)
Specific topics (right) were formulated by reading through the notes collected, talk slides, and discussion points raised at the 2019 Phnom Penh meeting and additionally through careful read of the proceedings, papers and discussions of the 1977 Singapore meeting. Materials were again read carefully and the cumulative frequency of mentions (relative size of ribbons) for each discussion point tallied. Ribbon colors correspond to dengue (red), malaria (green), or general VBD (blue) discussion. Ribbon connections to the left panel describe the meeting (1977 and/or 2019) at which topics and disease were discussed. Relative frequency of each topic is proportional to the size of the grey boxes surrounding the topic text.
(DOCX)
Acknowledgments
We would like to thank all workshop participants for contributing to the important discussions and presentations that provided the content of this manuscript. We would like to thank Mr. Ryan Kissinger for the manuscript artwork and Dr. Kevin Koblinski for valuable comments on the manuscript. This workshop was funded by the Office of Global Research at the NIAID in Bethesda, Maryland. The views expressed herein do not represent the official views of the NIH, US Department of Defense, or other participants not listed as author byline above.
Funding Statement
The author(s) received no specific funding for this work. The costs of the workshop were supported by the Office of Global Research at the National Institute of Allergy and Infectious Diseases.
References
- 1.McMichael C, Healy J. Health equity and migrants in the Greater Mekong Subregion. Global Health Action. 2017;10(1). 10.1080/16549716.2017.1271594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellems TE, Plowe CV. Chloroquine-Resistant Malaria. The Journal of Infectious Diseases. 2001;184(6):770–6. 10.1086/322858 [DOI] [PubMed] [Google Scholar]
- 3.Wang SQ, Li YC, Zhang ZM, Wang GZ, Hu XM, Qualls WA, et al. Prevention measures and socio-economic development result in a decrease in malaria in Hainan, China. Malar J. 2014;13:362 Epub 2014/09/17. 10.1186/1475-2875-13-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Countries of the Greater Mekong are stepping up to end malaria. World Health Organization, 2018. Contract No.: WHO/CDS/GMP/MME/2018.03. [Google Scholar]
- 5.Papers presented at the Conference on Dengue Haemorrhagic Fever: New Developments and Future Research (Part II). Dengue Haemorrhagic Fever: New Developments and Future Research; 1978. October 24–28, 1977; Singapore: Asian Journal of Infectious Diseases. [Google Scholar]
- 6.Mohd Shukri M, Ling Kho K, Ghane Kisomi M, Lani R, Marlina S, Muhd Radzi SF, et al. Seroprevalence report on tick-borne encephalitis virus and Crimean-Congo hemorrhagic fever virus among Malaysian's farm workers. BMC public health. 2015;15:704 10.1186/s12889-015-1901-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leelayoova S, Siripattanapipong S, Manomat J, Piyaraj P, Tan-Ariya P, Bualert L, et al. Leishmaniasis in Thailand: A Review of Causative Agents and Situations. The American Journal of Tropical Medicine and Hygiene. 2017;96(3):534–42. 10.4269/ajtmh.16-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbel V, Nosten F, Thanispong K, Luxemburger C, Kongmee M, Chareonviriyaphap T. Challenges and prospects for dengue and malaria control in Thailand, Southeast Asia. Trends Parasitol. 2013;29(12):623–33. Epub 2013/11/13. 10.1016/j.pt.2013.09.007 . [DOI] [PubMed] [Google Scholar]
- 9.Thailand: Dengue cases top 100K, 250 additional Chikungunya cases reported: Outbreak News Today; 2019 [cited 2019 December 9, 2019]. Available from: http://outbreaknewstoday.com/thailand-dengue-cases-top-100k-250-additional-chikungunya-cases-reported-21520/.
- 10.Manning JE, Oliveira F, Parker DM, Amaratunga C, Kong D, Man S, et al. The PAGODAS protocol: pediatric assessment group of dengue and Aedes saliva protocol to investigate vector-borne determinants of Aedes-transmitted arboviral infections in Cambodia. Parasit Vectors. 2018;11(1):664 Epub 2018/12/24. 10.1186/s13071-018-3224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawpoolsri S, Kaewkungwal J, Khamsiriwatchara A, Sovann L, Sreng B, Phommasack B, et al. Data quality and timeliness of outbreak reporting system among countries in Greater Mekong subregion: Challenges for international data sharing. PLoS Negl Trop Dis. 2018;12(4):e0006425 Epub 2018/04/26. 10.1371/journal.pntd.0006425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Eliminating malaria in the Greater Mekong Subregion: United to end a deadly disease. World Health Organization, 2016. Contract No.: WHO/HTM/GMP/2016.12. [Google Scholar]
- 13.World Health Organization. World malaria report 2019. World Health Organization, 2019. [Google Scholar]
- 14.Manning JE, Cantaert T. Time to Micromanage the Pathogen-Host-Vector Interface: Considerations for Vaccine Development. Vaccines (Basel). 2019;7(1). Epub 2019/01/24. 10.3390/vaccines7010010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, et al. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N Engl J Med. 2016;374(26):2519–29. Epub 2016/06/30. 10.1056/NEJMoa1515257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White MT, Bejon P, Olotu A, Griffin JT, Bojang K, Lusingu J, et al. A combined analysis of immunogenicity, antibody kinetics and vaccine efficacy from phase 2 trials of the RTS,S malaria vaccine. BMC Med. 2014;12:117 Epub 2014/07/12. 10.1186/s12916-014-0117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuerman L. RTS,S malaria vaccine could provide major public health benefits. Lancet. 2019. Epub 2019/07/14. 10.1016/S0140-6736(19)31567-3 . [DOI] [PubMed] [Google Scholar]
- 18.Halstead SB. Studies on the Attenuation of Dengue 4. Asian Journal of Infectious Diseases. 1978;2:112–7. [Google Scholar]
- 19.Russell PK. Progress Toward Dengue Vaccines. Asian Journal of Infectious Diseases. 1978;2:118–20. [Google Scholar]
- 20.Panel and General Discussion I. Asian Journal of Infectious Diseases. 1978;2:121–7. [Google Scholar]
- 21.Whitehead SS, Pierson TC. Effects of dengue immunity on Zika virus infection. Nature. 2019;567(7749):467–8. Epub 2019/03/27. 10.1038/d41586-019-00868-6 . [DOI] [PubMed] [Google Scholar]
- 22.NIH begins study of vaccine to protect against mosquito-borne diseases 2017 [updated 2017-02-21T10:53:22–05:00].
- 23.Whitehead SS, Durbin AP, Pierce KK, Elwood D, McElvany BD, Fraser EA, et al. In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl Trop Dis. 2017;11(5):e0005584 10.1371/journal.pntd.0005584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durbin AP, Whitehead SS. Zika Vaccines: Role for Controlled Human Infection. J Infect Dis. 2017;216(suppl_10):S971–S5. Epub 2017/12/22. 10.1093/infdis/jix491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masel J, McCracken MK, Gleeson T, Morrison B, Rutherford G, Imrie A, et al. Does prior dengue virus exposure worsen clinical outcomes of Zika virus infection? A systematic review, pooled analysis and lessons learned. PLoS Negl Trop Dis. 2019;13(1):e0007060 Epub 2019/01/27. 10.1371/journal.pntd.0007060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatima K, Syed NI. Dengvaxia controversy: impact on vaccine hesitancy. Journal of Global Health. 8(2). 10.7189/jogh.08-020312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson HJ, Hartigan-Go K, de Figueiredo A. Vaccine confidence plummets in the Philippines following dengue vaccine scare: why it matters to pandemic preparedness. Human Vaccines & Immunotherapeutics. 2019;15(3):625–7. 10.1080/21645515.2018.1522468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCracken MK, Christofferson RC, Chisenhall DM, Mores CN. Analysis of early dengue virus infection in mice as modulated by Aedes aegypti probing. J Virol. 2014;88(4):1881–9. Epub 2013/11/08. 10.1128/JVI.01218-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCracken MK, Christofferson RC, Grasperge BJ, Calvo E, Chisenhall DM, Mores CN. Aedes aegypti salivary protein "aegyptin" co-inoculation modulates dengue virus infection in the vertebrate host. Virology. 2014;468–470:133–9. Epub 2014/09/01. 10.1016/j.virol.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCracken MK, Gromowski GD, Friberg HL, Lin X, Abbink P, De La Barrera R, et al. Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog. 2017;13(8):e1006487 Epub 2017/08/05. 10.1371/journal.ppat.1006487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A, Joshi G, Nagar DP, Sharma AK, Sukumaran D, Pant SC, et al. Mosquito saliva induced cutaneous events augment Chikungunya virus replication and disease progression. Infect Genet Evol. 2016;40:126–35. Epub 2016/03/02. 10.1016/j.meegid.2016.02.033 . [DOI] [PubMed] [Google Scholar]
- 32.Conway MJ, Watson AM, Colpitts TM, Dragovic SM, Li Z, Wang P, et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol. 2014;88(1):164–75. Epub 2013/10/18. 10.1128/JVI.02235-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fong SW, Kini RM, Ng LFP. Mosquito Saliva Reshapes Alphavirus Infection and Immunopathogenesis. J Virol. 2018;92(12). Epub 2018/03/30. 10.1128/JVI.01004-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning JE, Morens DM, Kamhawi S, Valenzuela JG, Memoli M. Mosquito Saliva: The Hope for a Universal Arbovirus Vaccine? J Infect Dis. 2018;218(1):7–15. Epub 2018/04/05. 10.1093/infdis/jiy179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Study in Healthy Volunteers to Evaluate the Safety and Immunogenicity of AGS-v, a Universal Mosquito-Borne Disease Vaccine—Full Text View—ClinicalTrials.gov.
- 36.Asojo OA, Kelleher A, Liu Z, Pollet J, Hudspeth EM, Rezende WC, et al. Structure of SALO, a leishmaniasis vaccine candidate from the sand fly Lutzomyia longipalpis. PLoS Negl Trop Dis. 2017;11(3):e0005374 10.1371/journal.pntd.0005374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Londono-Renteria B, Cardenas JC, Cardenas LD, Christofferson RC, Chisenhall DM, Wesson DM, et al. Use of anti-Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PLoS ONE. 2013;8(12):e81211 10.1371/journal.pone.0081211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Londono-Renteria B, Cardenas JC, Giovanni JE, Cardenas L, Villamizar P, Rolon J, et al. Aedes aegypti anti-salivary gland antibody concentration and dengue virus exposure history in healthy individuals living in an endemic area in Colombia. Biomedica. 2015;35(4):572–81. 10.7705/biomedica.v35i4.2530 . [DOI] [PubMed] [Google Scholar]
- 39.Londono-Renteria BL, Shakeri H, Rozo-Lopez P, Conway MJ, Duggan N, Jaberi-Douraki M, et al. Serosurvey of Human Antibodies Recognizing Aedes aegypti D7 Salivary Proteins in Colombia. Front Public Health. 2018;6:111 10.3389/fpubh.2018.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doucoure S, Mouchet F, Cournil A, Le Goff G, Cornelie S, Roca Y, et al. Human antibody response to Aedes aegypti saliva in an urban population in Bolivia: a new biomarker of exposure to Dengue vector bites. Am J Trop Med Hyg. 2012;87(3):504–10. 10.4269/ajtmh.2012.11-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elanga Ndille E, Doucoure S, Damien G, Mouchet F, Drame PM, Cornelie S, et al. First attempt to validate human IgG antibody response to Nterm-34kDa salivary peptide as biomarker for evaluating exposure to Aedes aegypti bites. PLoS Negl Trop Dis. 2012;6(11):e1905 10.1371/journal.pntd.0001905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elanga Ndille E, Doucoure S, Poinsignon A, Mouchet F, Cornelie S, D'Ortenzio E, et al. Human IgG Antibody Response to Aedes Nterm-34kDa Salivary Peptide, an Epidemiological Tool to Assess Vector Control in Chikungunya and Dengue Transmission Area. PLoS Negl Trop Dis. 2016;10(12):e0005109 10.1371/journal.pntd.0005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathieu-Daude F, Claverie A, Plichart C, Boulanger D, Mphande FA, Bossin HC. Specific human antibody responses to Aedes aegypti and Aedes polynesiensis saliva: A new epidemiological tool to assess human exposure to disease vectors in the Pacific. PLoS Negl Trop Dis. 2018;12(7):e0006660 10.1371/journal.pntd.0006660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ndille EE, Dubot-Peres A, Doucoure S, Mouchet F, Cornelie S, Sidavong B, et al. Human IgG antibody response to Aedes aegypti Nterm-34 kDa salivary peptide as an indicator to identify areas at high risk for dengue transmission: a retrospective study in urban settings of Vientiane city, Lao PDR. Trop Med Int Health. 2014;19(5):576–80. 10.1111/tmi.12280 . [DOI] [PubMed] [Google Scholar]
- 45.Ya-Umphan P, Cerqueira D, Cottrell G, Parker DM, Fowkes FJI, Nosten F, et al. Anopheles Salivary Biomarker as a Proxy for Estimating Plasmodium falciparum Malaria Exposure on the Thailand-Myanmar Border. Am J Trop Med Hyg. 2018;99(2):350–6. Epub 2018/06/06. 10.4269/ajtmh.18-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sagna AB, Yobo MC, Elanga Ndille E, Remoue F. New Immuno-Epidemiological Biomarker of Human Exposure to Aedes Vector Bites: From Concept to Applications. Trop Med Infect Dis. 2018;3(3). Epub 2018/10/03. 10.3390/tropicalmed3030080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fontaine A, Pascual A, Orlandi-Pradines E, Diouf I, Remoue F, Pages F, et al. Relationship between exposure to vector bites and antibody responses to mosquito salivary gland extracts. PLoS One. 2011;6(12):e29107 Epub 2011/12/24. 10.1371/journal.pone.0029107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanisic DI, McCarthy JS, Good MF. Controlled Human Malaria Infection: Applications, Advances, and Challenges. Infection and Immunity. 2018;86(1). 10.1128/IAI.00479-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mores CN, Christofferson RC, Davidson SA. The role of the mosquito in a dengue human infection model. J Infect Dis. 2014;209 Suppl 2:S71–8. Epub 2014/05/30. 10.1093/infdis/jiu110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitehorn J, Kien DT, Nguyen NM, Nguyen HL, Kyrylos PP, Carrington LB, et al. Comparative Susceptibility of Aedes albopictus and Aedes aegypti to Dengue Virus Infection After Feeding on Blood of Viremic Humans: Implications for Public Health. J Infect Dis. 2015;212(8):1182–90. Epub 2015/03/19. 10.1093/infdis/jiv173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goncalves BP, Kapulu MC, Sawa P, Guelbeogo WM, Tiono AB, Grignard L, et al. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun. 2017;8(1):1133 Epub 2017/10/28. 10.1038/s41467-017-01270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Killeen GF, Ross A, Smith T. Infectiousness of malaria-endemic human populations to vectors. Am J Trop Med Hyg. 2006;75(2 Suppl):38–45. Epub 2006/08/26. 10.4269/ajtmh.2006.75.2_suppl.0750038 . [DOI] [PubMed] [Google Scholar]
- 53.Christofferson RC, Mores CN, Wearing HJ. Characterizing the likelihood of dengue emergence and detection in naive populations. Parasit Vectors. 2014;7:282 Epub 2014/06/25. 10.1186/1756-3305-7-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vantaux A, Samreth R, Piv E, Khim N, Kim S, Berne L, et al. Contribution to Malaria Transmission of Symptomatic and Asymptomatic Parasite Carriers in Cambodia. J Infect Dis. 2018;217(10):1561–8. Epub 2018/02/03. 10.1093/infdis/jiy060 . [DOI] [PubMed] [Google Scholar]
- 55.Ten Bosch QA, Clapham HE, Lambrechts L, Duong V, Buchy P, Althouse BM, et al. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018;14(5):e1006965 Epub 2018/05/04. 10.1371/journal.ppat.1006965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, Long KC, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A. 2015;112(47):14688–93. Epub 2015/11/11. 10.1073/pnas.1508114112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shannon KL, Khan WA, Sack DA, Alam MS, Ahmed S, Prue CS, et al. Subclinical Plasmodium falciparum infections act as year-round reservoir for malaria in the hypoendemic Chittagong Hill districts of Bangladesh. Int J Infect Dis. 2016;49:161–9. Epub 2016/06/29. 10.1016/j.ijid.2016.06.019 . [DOI] [PubMed] [Google Scholar]
- 58.Christofferson RC, Mores CN. A role for vector control in dengue vaccine programs. Vaccine. 2015;33(50):7069–74. Epub 2015/10/20. 10.1016/j.vaccine.2015.09.114 . [DOI] [PubMed] [Google Scholar]
- 59.Brady OJ, Godfray HC, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, et al. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans R Soc Trop Med Hyg. 2016;110(2):107–17. Epub 2016/01/30. 10.1093/trstmh/trv113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith Gueye C, Newby G, Gosling RD, Whittaker MA, Chandramohan D, Slutsker L, et al. Strategies and approaches to vector control in nine malaria-eliminating countries: a cross-case study analysis. Malar J. 2016;15:2 Epub 2016/01/06. 10.1186/s12936-015-1054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reiter P, Gubler DJ. Surveillance and Control of Urban Dengue Vectors Dengue and Dengue Hemorrhagic fever. London: United Kingdom: CAB International; 1997. p. 425–62. [Google Scholar]
- 62.Vazquez-Prokopec GM, Kitron U, Montgomery B, Horne P, Ritchie SA. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl Trop Dis. 2010;4(12):e920 Epub 2011/01/05. 10.1371/journal.pntd.0000920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowman LR, Donegan S, McCall PJ. Is Dengue Vector Control Deficient in Effectiveness or Evidence?: Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2016;10(3):e0004551 Epub 2016/03/18. 10.1371/journal.pntd.0004551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aldersley A, Pongsiri A, Bunmee K, Kijchalao U, Chittham W, Fansiri T, et al. Too "sexy" for the field? Paired measures of laboratory and semi-field performance highlight variability in the apparent mating fitness of Aedes aegypti transgenic strains. Parasit Vectors. 2019;12(1):357 Epub 2019/07/22. 10.1186/s13071-019-3617-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcombe S, Chonephetsarath S, Thammavong P, Brey PT. Alternative insecticides for larval control of the dengue vector Aedes aegypti in Lao PDR: insecticide resistance and semi-field trial study. Parasit Vectors. 2018;11(1):616 Epub 2018/12/05. 10.1186/s13071-018-3187-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyer S, Lopes S, Prasetyo D, Hustedt J, Sarady AS, Doum D, et al. Resistance of Aedes aegypti (Diptera: Culicidae) Populations to Deltamethrin, Permethrin, and Temephos in Cambodia. Asia Pac J Public Health. 2018;30(2):158–66. Epub 2018/03/06. 10.1177/1010539517753876 . [DOI] [PubMed] [Google Scholar]
- 67.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11(7):e0005625 Epub 2017/07/21. 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jirakanjanakit N, Rongnoparut P, Saengtharatip S, Chareonviriyaphap T, Duchon S, Bellec C, et al. Insecticide susceptible/resistance status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand during 2003–2005. J Econ Entomol. 2007;100(2):545–50. Epub 2007/04/28. 10.1603/0022-0493(2007)100[545:irsias]2.0.co;2 . [DOI] [PubMed] [Google Scholar]
- 69.Huber K, Le Loan L, Hoang TH, Tien TK, Rodhain F, Failloux AB. Aedes aegypti in south Vietnam: ecology, genetic structure, vectorial competence and resistance to insecticides. Southeast Asian J Trop Med Public Health. 2003;34(1):81–6. Epub 2003/09/16. . [PubMed] [Google Scholar]
- 70.Chareonviriyaphap T, Bangs MJ, Suwonkerd W, Kongmee M, Corbel V, Ngoen-Klan R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit Vectors. 2013;6:280 Epub 2013/12/04. 10.1186/1756-3305-6-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.World Health Organization. Malaria Threats Map: Tracking biological challenges to malaria control and elimination: World Health Organization; 2019. [cited 2019 June 10, 2019]. Available from: http://apps.who.int/malaria/maps/threats/. [Google Scholar]
- 72.Chaumeau V, Cerqueira D, Zadrozny J, Kittiphanakun P, Andolina C, Chareonviriyaphap T, et al. Insecticide resistance in malaria vectors along the Thailand-Myanmar border. Parasit Vectors. 2017;10(1):165 Epub 2017/04/01. 10.1186/s13071-017-2102-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Bortel W, Trung HD, Thuan le K, Sochantha T, Socheat D, Sumrandee C, et al. The insecticide resistance status of malaria vectors in the Mekong region. Malar J. 2008;7:102 Epub 2008/06/07. 10.1186/1475-2875-7-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marcombe S, Bobichon J, Somphong B, Phommavan N, Maithaviphet S, Nambanya S, et al. Insecticide resistance status of malaria vectors in Lao PDR. PLoS ONE. 2017;12(4):e0175984 Epub 2017/04/25. 10.1371/journal.pone.0175984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sumarnrote A, Overgaard HJ, Marasri N, Fustec B, Thanispong K, Chareonviriyaphap T, et al. Status of insecticide resistance in Anopheles mosquitoes in Ubon Ratchathani province, Northeastern Thailand. Malar J. 2017;16(1):299 Epub 2017/07/27. 10.1186/s12936-017-1948-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larval source management–a supplementary measure for malaria vector control An operational manual. World Health Organization; 2013. [Google Scholar]
- 77.Premaratne R, Wickremasinghe R, Ranaweera D, Gunasekera W, Hevawitharana M, Pieris L, et al. Technical and operational underpinnings of malaria elimination from Sri Lanka. Malar J. 2019;18(1):256 Epub 2019/07/31. 10.1186/s12936-019-2886-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee VJ, Ow S, Heah H, Tan MY, Lam P, Ng LC, et al. Elimination of malaria risk through integrated combination strategies in a tropical military training island. Am J Trop Med Hyg. 2010;82(6):1024–9. Epub 2010/06/04. 10.4269/ajtmh.2010.09-0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Utzinger J, Tanner M. Socioeconomic development to fight malaria, and beyond. Lancet. 2013;382(9896):920–2. Epub 2013/06/25. 10.1016/S0140-6736(13)61211-8 . [DOI] [PubMed] [Google Scholar]
- 80.van den Berg H, Velayudhan R, Ebol A, Catbagan BH Jr., Turingan R, Tuso M, et al. Operational efficiency and sustainability of vector control of malaria and dengue: descriptive case studies from the Philippines. Malar J. 2012;11:269 Epub 2012/08/10. 10.1186/1475-2875-11-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khun S, Manderson L. Community and school-based health education for dengue control in rural Cambodia: a process evaluation. PLoS Negl Trop Dis. 2007;1(3):e143 Epub 2007/12/28. 10.1371/journal.pntd.0000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liyanage P, Rocklov J, Tissera H, Palihawadana P, Wilder-Smith A, Tozan Y. Evaluation of intensified dengue control measures with interrupted time series analysis in the Panadura Medical Officer of Health division in Sri Lanka: a case study and cost-effectiveness analysis. Lancet Planet Health. 2019;3(5):e211–e8. Epub 2019/05/28. 10.1016/S2542-5196(19)30057-9 . [DOI] [PubMed] [Google Scholar]
- 83.Authority HKH. Safety & Health Circular No. 31/2015: Stay Alert on Prevention and Control of Dengue Fever Vector—Strengthening of Control Measures—Construction Industry Council. In: Health SfFa, editor. 2015. [Google Scholar]
- 84.M C-A, Cook ADB, Amul GGH, Sharma A. Health Governance and Dengue in Southeast Asia. Singapore: Centre for Non-Traditional Security Studies, 2015. [Google Scholar]
- 85.Paes de Andrade P, Aragao FJ, Colli W, Dellagostin OA, Finardi-Filho F, Hirata MH, et al. Use of transgenic Aedes aegypti in Brazil: risk perception and assessment. Bull World Health Organ. 2016;94(10):766–71. Epub 2016/11/16. 10.2471/BLT.16.173377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith LB, Kasai S, Scott JG. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic Biochem Physiol. 2016;133:1–12. Epub 2016/10/16. 10.1016/j.pestbp.2016.03.005 . [DOI] [PubMed] [Google Scholar]
- 87.Li CX, Guo XX, Zhang YM, Dong YD, Xing D, Yan T, et al. Identification of genes involved in pyrethroid-, propoxur-, and dichlorvos- insecticides resistance in the mosquitoes, Culex pipiens complex (Diptera: Culicidae). Acta Trop. 2016;157:84–95. Epub 2016/01/24. 10.1016/j.actatropica.2016.01.019 . [DOI] [PubMed] [Google Scholar]
- 88.Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol. 2007;44(2):175–8. Epub 2007/04/13. 10.1603/0022-2585(2007)44[175:IRITBB]2.0.CO;2;2 . [DOI] [PubMed] [Google Scholar]
- 89.Liu N, Liu H, Zhu F, Zhang L. Differential expression of genes in pyrethroid resistant and susceptible mosquitoes, Culex quinquefasciatus (S.). Gene. 2007;394(1–2):61–8. Epub 2007/03/27. 10.1016/j.gene.2007.01.032 . [DOI] [PubMed] [Google Scholar]
- 90.Ranson H, Paton MG, Jensen B, McCarroll L, Vaughan A, Hogan JR, et al. Genetic mapping of genes conferring permethrin resistance in the malaria vector, Anopheles gambiae. Insect Mol Biol. 2004;13(4):379–86. Epub 2004/07/24. 10.1111/j.0962-1075.2004.00495.x . [DOI] [PubMed] [Google Scholar]
- 91.Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010;15(5):619–31. Epub 2010/03/11. 10.1111/j.1365-3156.2010.02489.x . [DOI] [PubMed] [Google Scholar]
- 92.Samuel M, Maoz D, Manrique P, Ward T, Runge-Ranzinger S, Toledo J, et al. Community effectiveness of indoor spraying as a dengue vector control method: A systematic review. PLoS Negl Trop Dis. 2017;11(8):e0005837 Epub 2017/09/01. 10.1371/journal.pntd.0005837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hladish TJ, Pearson CAB, Patricia Rojas D, Gomez-Dantes H, Halloran ME, Vazquez-Prokopec GM, et al. Forecasting the effectiveness of indoor residual spraying for reducing dengue burden. PLoS Negl Trop Dis. 2018;12(6):e0006570 Epub 2018/06/26. 10.1371/journal.pntd.0006570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Delatte H, Desvars A, Bouetard A, Bord S, Gimonneau G, Vourc'h G, et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector Borne Zoonotic Dis. 2010;10(3):249–58. Epub 2009/07/11. 10.1089/vbz.2009.0026 . [DOI] [PubMed] [Google Scholar]
- 95.Kek R, Hapuarachchi HC, Chung CY, Humaidi MB, Razak MA, Chiang S, et al. Feeding host range of Aedes albopictus (Diptera: Culicidae) demonstrates its opportunistic host-seeking behavior in rural Singapore. J Med Entomol. 2014;51(4):880–4. Epub 2014/08/15. 10.1603/me13213 . [DOI] [PubMed] [Google Scholar]
- 96.Kay B, Vu SN. New strategy against Aedes aegypti in Vietnam. Lancet. 2005;365(9459):613–7. Epub 2005/02/15. 10.1016/S0140-6736(05)17913-6 . [DOI] [PubMed] [Google Scholar]
- 97.Tran TT, Olsen A, Viennet E, Sleigh A. Social sustainability of Mesocyclops biological control for dengue in South Vietnam. Acta Trop. 2015;141(Pt A):54–9. Epub 2014/10/15. 10.1016/j.actatropica.2014.10.006 . [DOI] [PubMed] [Google Scholar]
- 98.Shafique M, Lopes S, Doum D, Keo V, Sokha L, Sam B, et al. Implementation of guppy fish (Poecilia reticulata), and a novel larvicide (Pyriproxyfen) product (Sumilarv 2MR) for dengue control in Cambodia: A qualitative study of acceptability, sustainability and community engagement. PLoS Negl Trop Dis. 2019;13(11):e0007907 Epub 2019/11/19. 10.1371/journal.pntd.0007907 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hustedt J, Doum D, Keo V, Ly S, Sam B, Chan V, et al. Determining the efficacy of guppies and pyriproxyfen (Sumilarv(R) 2MR) combined with community engagement on dengue vectors in Cambodia: study protocol for a randomized controlled trial. Trials. 2017;18(1):367 Epub 2017/08/06. 10.1186/s13063-017-2105-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.El-Sabaawi RW, Frauendorf TC, Marques PS, Mackenzie RA, Manna LR, Mazzoni R, et al. Biodiversity and ecosystem risks arising from using guppies to control mosquitoes. Biology Letters. 2016;12(10):20160590 10.1098/rsbl.2016.0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andersson N, Nava-Aguilera E, Arostegui J, Morales-Perez A, Suazo-Laguna H, Legorreta-Soberanis J, et al. Evidence based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): cluster randomized controlled trial. BMJ. 2015;351:h3267 Epub 2015/07/15. 10.1136/bmj.h3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taracena ML, Hunt CM, Benedict MQ, Pennington PM, Dotson EM. Downregulation of female doublesex expression by oral-mediated RNA interference reduces number and fitness of Anopheles gambiae adult females. Parasit Vectors. 2019;12(1):170 Epub 2019/04/18. 10.1186/s13071-019-3437-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Culbert NJ, Maiga H, Somda NSB, Gilles JRL, Bouyer J, Mamai W. Longevity of mass-reared, irradiated and packed male Anopheles arabiensis and Aedes aegypti under simulated environmental field conditions. Parasit Vectors. 2018;11(1):603 Epub 2018/11/23. 10.1186/s13071-018-3191-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong Y, Simoes ML, Marois E, Dimopoulos G. CRISPR/Cas9 -mediated gene knockout of Anopheles gambiae FREP1 suppresses malaria parasite infection. PLoS Pathog. 2018;14(3):e1006898 Epub 2018/03/09. 10.1371/journal.ppat.1006898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto DS, Sumitani M, Kasashima K, Sezutsu H, Matsuoka H. Inhibition of Malaria Infection in Transgenic Anopheline Mosquitoes Lacking Salivary Gland Cells. PLoS Pathog. 2016;12(9):e1005872 Epub 2016/09/07. 10.1371/journal.ppat.1005872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buchman A, Gamez S, Li M, Antoshechkin I, Li HH, Wang HW, et al. Engineered resistance to Zika virus in transgenic Aedes aegypti expressing a polycistronic cluster of synthetic small RNAs. Proc Natl Acad Sci U S A. 2019;116(9):3656–61. Epub 2019/02/07. 10.1073/pnas.1810771116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Mendonca Amarante A, Jupatanakul N, de Abreu da Silva IC, Carneiro VC, Vicentino ARR, Dimopolous G, et al. The DNA chaperone HMGB1 potentiates the transcriptional activity of Rel1A in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2017;80:32–41. Epub 2016/11/22. 10.1016/j.ibmb.2016.11.006 . [DOI] [PubMed] [Google Scholar]
- 108.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4(7):e1000098 Epub 2008/07/08. 10.1371/journal.ppat.1000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Neill SL. The Use of Wolbachia by the World Mosquito Program to Interrupt Transmission of Aedes aegypti Transmitted Viruses. Adv Exp Med Biol. 2018;1062:355–60. Epub 2018/05/31. 10.1007/978-981-10-8727-1_24 . [DOI] [PubMed] [Google Scholar]
- 110.Mains JW, Kelly PH, Dobson KL, Petrie WD, Dobson SL. Localized Control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via Inundative Releases of Wolbachia-Infected Male Mosquitoes. J Med Entomol. 2019. Epub 2019/04/23. 10.1093/jme/tjz051 . [DOI] [PubMed] [Google Scholar]
- 111.Velez I, Santacruz E, Kutcher S, Duque S, Uribe A, Barajas J, et al. The impact of city-wide deployment of Wolbachia-carrying mosquitoes on arboviral disease incidence in MedellÌn and Bello, Colombia: study protocol for an interrupted time-series analysis and a test-negative design study [version 1; peer review: 1 approved, 1 approved with reservations]. F1000Research. 2019;8(1327). 10.12688/f1000research.19858.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kobylinski KC, Jittamala P, Hanboonkunupakarn B, Pukrittayakamee S, Pantuwatana K, Phasomkusolsil S, et al. Safety, pharmacokinetics, and mosquito-lethal effects of ivermectin in combination with dihydroartemisinin-piperaquine and primaquine in healthy adult Thai subjects. Clin Pharmacol Ther. 2019. Epub 2019/11/08. 10.1002/cpt.1716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pasay CJ, Yakob L, Meredith HR, Stewart R, Mills PC, Dekkers MH, et al. Treatment of pigs with endectocides as a complementary tool for combating malaria transmission by Anopheles farauti (s.s.) in Papua New Guinea. Parasites & Vectors. 2019;12(1):124 10.1186/s13071-019-3392-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soper FL. The elimination of urban yellow fever in the Americas through the eradication of Aedes aegypti. Am J Public Health Nations Health. 1963;53:7–16. Epub 1963/01/01. 10.2105/ajph.53.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gayan Dharmasiri AG, Perera AY, Harishchandra J, Herath H, Aravindan K, Jayasooriya HTR, et al. First record of Anopheles stephensi in Sri Lanka: a potential challenge for prevention of malaria reintroduction. Malar J. 2017;16(1):326 Epub 2017/08/12. 10.1186/s12936-017-1977-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018;188:180–6. Epub 2018/09/07. 10.1016/j.actatropica.2018.09.001 . [DOI] [PubMed] [Google Scholar]
- 117.Bowman LR, Runge-Ranzinger S, McCall PJ. Assessing the relationship between vector indices and dengue transmission: a systematic review of the evidence. PLoS Negl Trop Dis. 2014;8(5):e2848 10.1371/journal.pntd.0002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yalwala S, Clark J, Oullo D, Ngonga D, Abuom D, Wanja E, et al. Comparative efficacy of existing surveillance tools for Aedes aegypti in Western Kenya. J Vector Ecol. 2015;40(2):301–7. 10.1111/jvec.12168 . [DOI] [PubMed] [Google Scholar]
- 119.World Health Organization. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever. World Health Organization, 2011. http://apps.searo.who.int/pds_docs/B4751.pdf. [Google Scholar]
- 120.Lau SM, Chua TH, Sulaiman WY, Joanne S, Lim YA, Sekaran SD, et al. A new paradigm for Aedes spp. surveillance using gravid ovipositing sticky trap and NS1 antigen test kit. Parasit Vectors. 2017;10(1):151 Epub 2017/03/23. 10.1186/s13071-017-2091-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rumisha SF, Smith TA, Masanja H, Abdulla S, Vounatsou P. Relationship between child survival and malaria transmission: an analysis of the malaria transmission intensity and mortality burden across Africa (MTIMBA) project data in Rufiji demographic surveillance system, Tanzania. Malar J. 2014;13:124 Epub 2014/04/01. 10.1186/1475-2875-13-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tusting LS, Bousema T, Smith DL, Drakeley C. Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Adv Parasitol. 2014;84:151–208. Epub 2014/02/01. 10.1016/B978-0-12-800099-1.00003-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sturrock HJ, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, et al. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med. 2013;10(6):e1001467 Epub 2013/07/16. 10.1371/journal.pmed.1001467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parker DM, Tun STT, White LJ, Kajeechiwa L, Thwin MM, Landier J, et al. Potential herd protection against Plasmodium falciparum infections conferred by mass antimalarial drug administrations. Elife. 2019;8 Epub 2019/04/17. 10.7554/eLife.41023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ouedraogo S, Benmarhnia T, Bonnet E, Some PA, Barro AS, Kafando Y, et al. Evaluation of Effectiveness of a Community-Based Intervention for Control of Dengue Virus Vector, Ouagadougou, Burkina Faso. Emerg Infect Dis. 2018;24(10):1859–67. 10.3201/eid2410.180069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fox-Lewis A, Hopkins J, Sar P, Sao S, Pheaktra N, Day NPJ, et al. Seroprevalence of Dengue Virus and Rickettsial Infections in Cambodian Children. Am J Trop Med Hyg. 2019;100(3):635–8. Epub 2019/01/25. 10.4269/ajtmh.18-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mayxay M, Sengvilaipaseuth O, Chanthongthip A, Dubot-Peres A, Rolain JM, Parola P, et al. Causes of Fever in Rural Southern Laos. Am J Trop Med Hyg. 2015;93(3):517–20. Epub 2015/07/08. 10.4269/ajtmh.14-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5(3):e68 Epub 2008/03/21. 10.1371/journal.pmed.0050068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumaran E, Doum D, Keo V, Sokha L, Sam B, Chan V, et al. Dengue knowledge, attitudes and practices and their impact on community-based vector control in rural Cambodia. PLoS Negl Trop Dis. 2018;12(2):e0006268 10.1371/journal.pntd.0006268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Edwards HM, Chinh VD, Le Duy B, Thanh PV, Thang ND, Trang DM, et al. Characterising residual malaria transmission in forested areas with low coverage of core vector control in central Viet Nam. Parasit Vectors. 2019;12(1):454 Epub 2019/09/20. 10.1186/s13071-019-3695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gryseels C, Durnez L, Gerrets R, Uk S, Suon S, Set S, et al. Re-imagining malaria: heterogeneity of human and mosquito behaviour in relation to residual malaria transmission in Cambodia. Malar J. 2015;14:165 Epub 2015/04/25. 10.1186/s12936-015-0689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Young KI, Mundis S, Widen SG, Wood TG, Tesh RB, Cardosa J, et al. Abundance and distribution of sylvatic dengue virus vectors in three different land cover types in Sarawak, Malaysian Borneo. Parasit Vectors. 2017;10(1):406 Epub 2017/09/02. 10.1186/s13071-017-2341-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Andrade CC, Young KI, Johnson WL, Villa ME, Buraczyk CA, Messer WB, et al. Rise and fall of vector infectivity during sequential strain displacements by mosquito-borne dengue virus. J Evol Biol. 2016;29(11):2205–18. Epub 2016/11/02. 10.1111/jeb.12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Althouse BM, Hanley KA, Diallo M, Sall AA, Ba Y, Faye O, et al. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in Senegal. Am J Trop Med Hyg. 2015;92(1):88–97. Epub 2014/11/19. 10.4269/ajtmh.13-0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rossi SL, Nasar F, Cardosa J, Mayer SV, Tesh RB, Hanley KA, et al. Genetic and phenotypic characterization of sylvatic dengue virus type 4 strains. Virology. 2012;423(1):58–67. Epub 2011/12/20. 10.1016/j.virol.2011.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vasilakis N, Fokam EB, Hanson CT, Weinberg E, Sall AA, Whitehead SS, et al. Genetic and phenotypic characterization of sylvatic dengue virus type 2 strains. Virology. 2008;377(2):296–307. Epub 2008/06/24. 10.1016/j.virol.2008.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guo Y, Song Z, Luo L, Wang Q, Zhou G, Yang D, et al. Molecular evidence for new sympatric cryptic species of Aedes albopictus (Diptera: Culicidae) in China: A new threat from Aedes albopictus subgroup? Parasit Vectors. 2018;11(1):228 Epub 2018/04/06. 10.1186/s13071-018-2814-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garcia GA, Sylvestre G, Aguiar R, da Costa GB, Martins AJ, Lima JBP, et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl Trop Dis. 2019;13(1):e0007023 Epub 2019/01/09. 10.1371/journal.pntd.0007023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Servadio JL, Rosenthal SR, Carlson L, Bauer C. Climate patterns and mosquito-borne disease outbreaks in South and Southeast Asia. J Infect Public Health. 2018;11(4):566–71. Epub 2017/12/25. 10.1016/j.jiph.2017.12.006 . [DOI] [PubMed] [Google Scholar]
- 140.Overgaard HJ, Ekbom B, Suwonkerd W, Takagi M. Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: Implications for malaria transmission and control. Landscape Ecology. 2003;18(6):605 10.1023/a:1026074910038 [DOI] [Google Scholar]
- 141.Overgaard HJ, Suwonkerd W, Hii J. The Malaria Landscape: Mosquitoes, Transmission, Landscape, Insecticide Resistance, and Integrated Control in Thailand In: Morand S, Dujardin J, Lefait-Robin R, Apiwathnasorn C, editors. Socio-Ecological Dimensions of Infectious Diseases in Southeast Asia. Singapore: Springer Science+Business Media; 2015. p. 123–53. [Google Scholar]
- 142.Clinical Guidance: Centers for Disease Control and Prevention; January 15, 2019 [updated January 15, 2019; cited 2019 April 11, 2019]. Available from: https://www.cdc.gov/dengue/clinicallab/clinical.html.
- 143.Subissi L, Daudens-Vaysse E, Cassadou S, Ledrans M, Bompard P, Gustave J, et al. Revising rates of asymptomatic Zika virus infection based on sentinel surveillance data from French Overseas Territories. Int J Infect Dis. 2017;65:116–8. Epub 2017/10/31. 10.1016/j.ijid.2017.10.009 . [DOI] [PubMed] [Google Scholar]
- 144.Bustos Carrillo F, Collado D, Sanchez N, Ojeda S, Lopez Mercado B, Burger-Calderon R, et al. Epidemiological Evidence for Lineage-Specific Differences in the Risk of Inapparent Chikungunya Virus Infection. J Virol. 2019;93(4). Epub 2018/11/23. 10.1128/JVI.01622-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. Epub 2013/04/09. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12(12):833–40. Epub 2014/10/21. 10.1038/nrmicro3364 . [DOI] [PubMed] [Google Scholar]
- 147.Costa CH, Stewart JM, Gomes RB, Garcez LM, Ramos PK, Bozza M, et al. Asymptomatic human carriers of Leishmania chagasi. Am J Trop Med Hyg. 2002;66(4):334–7. Epub 2002/08/08. 10.4269/ajtmh.2002.66.334 . [DOI] [PubMed] [Google Scholar]
- 148.Zaw MT, Thant M, Hlaing TM, Aung NZ, Thu M, Phumchuea K, et al. Asymptomatic and sub-microscopic malaria infection in Kayah State, eastern Myanmar. Malar J. 2017;16(1):138 Epub 2017/04/06. 10.1186/s12936-017-1789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Michel G, Pomares C, Ferrua B, Marty P. Importance of worldwide asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Trop. 2011;119(2–3):69–75. Epub 2011/06/18. 10.1016/j.actatropica.2011.05.012 . [DOI] [PubMed] [Google Scholar]
- 150.Elhassan IM, Hviid L, Jakobsen PH, Giha H, Satti GM, Arnot DE, et al. High proportion of subclinical Plasmodium falciparum infections in an area of seasonal and unstable malaria in Sudan. Am J Trop Med Hyg. 1995;53(1):78–83. Epub 1995/07/01. . [PubMed] [Google Scholar]
- 151.Yoon IK, Srikiatkhachorn A, Hermann L, Buddhari D, Scott TW, Jarman RG, et al. Characteristics of mild dengue virus infection in Thai children. Am J Trop Med Hyg. 2013;89(6):1081–7. Epub 2013/10/16. 10.4269/ajtmh.13-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yoon IK, Rothman AL, Tannitisupawong D, Srikiatkhachorn A, Jarman RG, Aldstadt J, et al. Underrecognized mildly symptomatic viremic dengue virus infections in rural Thai schools and villages. J Infect Dis. 2012;206(3):389–98. Epub 2012/05/23. 10.1093/infdis/jis357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Endy TP, Anderson KB, Nisalak A, Yoon IK, Green S, Rothman AL, et al. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2011;5(3):e975 Epub 2011/03/11. 10.1371/journal.pntd.0000975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu J, Deng Y, Jing Q, Chen X, Du Z, Liang T, et al. Dengue Infection Spectrum in Guangzhou: A Cross-Sectional Seroepidemiology Study among Community Residents between 2013 and 2015. Int J Environ Res Public Health. 2018;15(6). Epub 2018/06/13. 10.3390/ijerph15061227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu Z, Soe TN, Zhao Y, Than A, Cho C, Aung PL, et al. Geographical heterogeneity in prevalence of subclinical malaria infections at sentinel endemic sites of Myanmar. Parasit Vectors. 2019;12(1):83 Epub 2019/02/20. 10.1186/s13071-019-3330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fritzell C, Rousset D, Adde A, Kazanji M, Van Kerkhove MD, Flamand C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: A scoping review. PLoS Negl Trop Dis. 2018;12(7):e0006533 Epub 2018/07/17. 10.1371/journal.pntd.0006533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A. 2016;113(28):7852–7. Epub 2016/06/30. 10.1073/pnas.1607931113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bosch I, de Puig H, Hiley M, Carre-Camps M, Perdomo-Celis F, Narvaez CF, et al. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci Transl Med. 2017;9(409). Epub 2017/09/29. 10.1126/scitranslmed.aan1589 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liles VR, Pangilinan LS, Daroy MLG, Dimamay MTA, Reyes RS, Bulusan MK, et al. Evaluation of a rapid diagnostic test for detection of dengue infection using a single-tag hybridization chromatographic-printed array strip format. Eur J Clin Microbiol Infect Dis. 2019;38(3):515–21. Epub 2019/01/27. 10.1007/s10096-018-03453-3 . [DOI] [PubMed] [Google Scholar]
- 160.Rockstroh A, Barzon L, Kumbukgolla W, Su HX, Lizarazo E, Vincenti-Gonzalez MF, et al. Dengue Virus IgM Serotyping by ELISA with Recombinant Mutant Envelope Proteins. Emerg Infect Dis. 2019;25(1):1111–5. Epub 2018/11/06. 10.3201/eid2501.180605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salje H, Lessler J, Maljkovic Berry I, Melendrez MC, Endy T, Kalayanarooj S, et al. Dengue diversity across spatial and temporal scales: Local structure and the effect of host population size. Science. 2017;355(6331):1302–6. Epub 2017/03/25. 10.1126/science.aaj9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Megawati D, Masyeni S, Yohan B, Lestarini A, Hayati RF, Meutiawati F, et al. Dengue in Bali: Clinical characteristics and genetic diversity of circulating dengue viruses. PLoS Negl Trop Dis. 2017;11(5):e0005483 Epub 2017/05/23. 10.1371/journal.pntd.0005483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bai Z, Liu LC, Jiang L, Luo L, Feng H, Lin P, et al. Evolutionary and phylodynamic analyses of Dengue virus serotype I in Guangdong Province, China, between 1985 and 2015. Virus Res. 2018;256:201–8. Epub 2018/07/11. 10.1016/j.virusres.2018.07.005 . [DOI] [PubMed] [Google Scholar]
- 164.Tan KK, Zulkifle NI, Sulaiman S, Pang SP, NorAmdan N, MatRahim N, et al. Emergence of the Asian lineage dengue virus type 3 genotype III in Malaysia. BMC Evol Biol. 2018;18(1):58 Epub 2018/04/28. 10.1186/s12862-018-1175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vu TT, Holmes EC, Duong V, Nguyen TQ, Tran TH, Quail M, et al. Emergence of the Asian 1 genotype of dengue virus serotype 2 in viet nam: in vivo fitness advantage and lineage replacement in South-East Asia. PLoS Negl Trop Dis. 2010;4(7):e757 Epub 2010/07/24. 10.1371/journal.pntd.0000757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ko HY, Li YT, Chao DY, Chang YC, Li ZT, Wang M, et al. Inter- and intrahost sequence diversity reveal the emergence of viral variants during an overwintering epidemic caused by dengue virus serotype 2 in southern Taiwan. PLoS Negl Trop Dis. 2018;12(10):e0006827 Epub 2018/10/05. 10.1371/journal.pntd.0006827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Phadungsombat J, Lin MY, Srimark N, Yamanaka A, Nakayama EE, Moolasart V, et al. Emergence of genotype Cosmopolitan of dengue virus type 2 and genotype III of dengue virus type 3 in Thailand. PLoS ONE. 2018;13(11):e0207220 Epub 2018/11/13. 10.1371/journal.pone.0207220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang CF, Chang SF, Hsu TC, Su CL, Wang TC, Lin SH, et al. Molecular characterization and phylogenetic analysis of dengue viruses imported into Taiwan during 2011–2016. PLoS Negl Trop Dis. 2018;12(9):e0006773 Epub 2018/09/21. 10.1371/journal.pntd.0006773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Keasey SL, Smith JL, Fernandez S, Durbin AP, Zhao BM, Ulrich RG. Impact of Dengue Virus Serotype 2 Strain Diversity on Serological Immune Responses to Dengue. ACS Infect Dis. 2018. Epub 2018/10/23. 10.1021/acsinfecdis.8b00185 . [DOI] [PubMed] [Google Scholar]
- 170.Schultz GW. Cemetery vase breeding of dengue vectors in Manila, Republic of the Philippines. J Am Mosq Control Assoc. 1989;5(4):508–13. Epub 1989/12/01. . [PubMed] [Google Scholar]
- 171.Marasri N, Overgaard HJ, Sumarnrote A, Thanispong K, Corbel V, Chareonviriyaphap T. Abundance and distribution of Anopheles mosquitoes in a malaria endemic area along the Thai-Lao border. J Vector Ecol. 2017;42(2):325–34. Epub 2017/11/11. 10.1111/jvec.12273 . [DOI] [PubMed] [Google Scholar]
- 172.Suwonkerd W, Overgaard HJ, Prajakwong S, Takagi M. Malaria vector densities in transmission and non-transmission areas during 23 years and land use in Chiang Mai province, Northern Thailand. Basic and Applied Ecology. 2002;3(3):197–207. [Google Scholar]
- 173.Suwonkerd W, Tsuda Y, Overgaard HJ, Chawprom S, Tuno N, Prajakwong S, et al. Changes in malaria vector densities over a twenty-three year period in Mae Hong Son Province, northern Thailand. Southeast Asian J Trop Med Public Health. 2004;35(2):316–24. Epub 2005/02/05. . [PubMed] [Google Scholar]
- 174.Hien AS, Soma DD, Hema O, Bayili B, Namountougou M, Gnankine O, et al. Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles gambiae s.l. populations from cotton growing areas in Burkina Faso, West Africa. PLoS ONE. 2017;12(3):e0173098 Epub 2017/03/03. 10.1371/journal.pone.0173098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Overgaard HJ, Sandve SR, Suwonkerd W. Evidence of anopheline mosquito resistance to agrochemicals in northern Thailand. Southeast Asian J Trop Med Public Health. 2005;36 Suppl 4:152–7. Epub 2006/01/28. . [PubMed] [Google Scholar]
- 176.Tangena JA, Thammavong P, Wilson AL, Brey PT, Lindsay SW. Risk and Control of Mosquito-Borne Diseases in Southeast Asian Rubber Plantations. Trends Parasitol. 2016;32(5):402–15. Epub 2016/02/26. 10.1016/j.pt.2016.01.009 . [DOI] [PubMed] [Google Scholar]
- 177.Vanwambeke SO, Lambin EF, Eichhorn MP, Flasse SP, Harbach RE, Oskam L, et al. Impact of Land-use Change on Dengue and Malaria in Northern Thailand. EcoHealth. 2007;4(1):37–51. 10.1007/s10393-007-0085-5. [DOI] [Google Scholar]
- 178.Sarfraz MS, Tripathi NK, Tipdecho T, Thongbu T, Kerdthong P, Souris M. Analyzing the spatio-temporal relationship between dengue vector larval density and land-use using factor analysis and spatial ring mapping. BMC Public Health. 2012;12(1):853 10.1186/1471-2458-12-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chusri S, Siripaitoon P, Silpapojakul K, Hortiwakul T, Charernmak B, Chinnawirotpisan P, et al. Kinetics of chikungunya infections during an outbreak in Southern Thailand, 2008–2009. Am J Trop Med Hyg. 2014;90(3):410–7. Epub 2014/02/05. 10.4269/ajtmh.12-0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vong S, Khieu V, Glass O, Ly S, Duong V, Huy R, et al. Dengue incidence in urban and rural Cambodia: results from population-based active fever surveillance, 2006–2008. PLoS Negl Trop Dis. 2010;4(11):e903 Epub 2010/12/15. 10.1371/journal.pntd.0000903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheong YL, Leitão PJ, Lakes T. Assessment of land use factors associated with dengue cases in Malaysia using Boosted Regression Trees. Spatial and Spatio-temporal Epidemiology. 2014;10:75–84. 10.1016/j.sste.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 182.Robinson M, Conan A, Duong V, Ly S, Ngan C, Buchy P, et al. A model for a chikungunya outbreak in a rural Cambodian setting: implications for disease control in uninfected areas. PLoS Negl Trop Dis. 2014;8(9):e3120 Epub 2014/09/12. 10.1371/journal.pntd.0003120 PubMed Central PMCID: PMC4161325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thongsripong P, Green A, Kittayapong P, Kapan D, Wilcox B, Bennett S. Mosquito vector diversity across habitats in central Thailand endemic for dengue and other arthropod-borne diseases. PLoS Negl Trop Dis. 2013;7(10):e2507 Epub 2013/11/10. 10.1371/journal.pntd.0002507 PubMed Central PMCID: PMC3814347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Overgaard HJ. Malaria mosquito resistance to agricultural insecticides: risk area mapping in Thailand. Colombo, Sri Lanka: International Water Management Institute (IWMI), 2006. [Google Scholar]
- 185.Wilcox BA, Gubler DJ, Pizer HF. 4—Urbanization and the social ecology of emerging infectious diseases In: Mayer KH, Pizer HF, editors. The Social Ecology of Infectious Diseases. San Diego: Academic Press; 2008. p. 113–37. [Google Scholar]
- 186.Molyneux DH. Patterns of change in vector-borne diseases. Ann Trop Med Parasitol. 1997;91(7):827–39. Epub 1998/06/17. 10.1080/00034989760581 . [DOI] [PubMed] [Google Scholar]
- 187.Molyneux DH. Common themes in changing vector-borne disease scenarios. Trans R Soc Trop Med Hyg. 2003;97(2):129–32. Epub 2003/10/31. 10.1016/s0035-9203(03)90097-6 . [DOI] [PubMed] [Google Scholar]
- 188.Su XZ, Lane KD, Xia L, Sa JM, Wellems TE. Plasmodium Genomics and Genetics: New Insights into Malaria Pathogenesis, Drug Resistance, Epidemiology, and Evolution. Clin Microbiol Rev. 2019;32(4). Epub 2019/08/02. 10.1128/CMR.00019-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Juraska M, Magaret CA, Shao J, Carpp LN, Fiore-Gartland AJ, Benkeser D, et al. Viral genetic diversity and protective efficacy of a tetravalent dengue vaccine in two phase 3 trials. Proc Natl Acad Sci U S A. 2018;115(36):E8378–E87. Epub 2018/08/22. 10.1073/pnas.1714250115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Evans BR, Kotsakiozi P, Costa-da-Silva AL, Ioshino RS, Garziera L, Pedrosa MC, et al. Transgenic Aedes aegypti Mosquitoes Transfer Genes into a Natural Population. Sci Rep. 2019;9(1):13047 Epub 2019/09/12. 10.1038/s41598-019-49660-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Global Strategic Framework for Integrated Vector Management. Geneva: World Health Organization; 2004. [Google Scholar]
- 192.Organization WH. Global Vector Control Response 2017–2030. Geneva: World Health Organization; 2017. [Google Scholar]
- 193.Urban Development in the Greater Mekong Subregion: Asian Development Bank; 2016. [Google Scholar]
- 194.NIH. Vector-Borne Diseases: Understanding the Environmental, Human Health, and Ecological Connections, Workshop Summary. Washington (DC): 2008 9780309108973 0309108977. [PubMed]
- 195.Wilcox BA, Colwell RR. Emerging and Reemerging Infectious Diseases: Biocomplexity as an Interdisciplinary Paradigm. EcoHealth. 2005;2(4):244 10.1007/s10393-005-8961-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
This schematic demonstrates the process and current state of dengue vaccine candidates that have gone from preclinical development through to Phase III trials as of early 2019.
(DOCX)
Specific topics (right) were formulated by reading through the notes collected, talk slides, and discussion points raised at the 2019 Phnom Penh meeting and additionally through careful read of the proceedings, papers and discussions of the 1977 Singapore meeting. Materials were again read carefully and the cumulative frequency of mentions (relative size of ribbons) for each discussion point tallied. Ribbon colors correspond to dengue (red), malaria (green), or general VBD (blue) discussion. Ribbon connections to the left panel describe the meeting (1977 and/or 2019) at which topics and disease were discussed. Relative frequency of each topic is proportional to the size of the grey boxes surrounding the topic text.
(DOCX)