Abstract
Background/Aim:
Ulcerative colitis (UC) has been implicated to imbalanced enteric flora and reduced microbial diversity. Stachyose is a kind of natural prebiotic which favorably modulate the composition of the gut microbiota. The present study aims to investigate the effects of stachyose on inflammatory levels and gut microbiota of acute colitis mice.
Materials and Methods:
In this study, the mice were randomly divided into four groups: (1) control group; (2) stachyose group; (3) dextran sulfate sodium (DSS) group; (4) stachyose + DSS group. Hemotoxylin and Eosin (H and E) staining was performed for the distal colon to examine the inflammation and tissue damage. The inflammatory cytokines including IL-6, IL-10, IL-17a, and TNF-α in serum were determined by ELISA assay. The differences in the gut microbiota were analyzed by 16S rDNA gene sequencing.
Results:
Histological assay showed that the stachyose treatment significantly reduced the lesions of the colon in DSS-induced colitis. And the upregulated inflammatory cytokines induced by DSS were significantly inhibited by stachyose treatment. Additionally, the sequencing analysis showed that the stachyose changed the gut microbiota composition with a higher level of Akkermansia, as well as selectively increasing some probiotics, including Lactobacillus.
Conclusions:
Our results suggested that stachyose increased beneficial microbiota and bacterial diversity to alleviate acute colitis in mice, which might be a new promising option to UC patients.
Keywords: Dextran sulfate sodium, gut microbiota, probiotics, stachyose, ulcerative colitis
INTRODUCTION
Gut micro-ecosystem is a large and complex microbial community in the human body, including bacteria, fungi, viruses, parasites, and other eukaryotes.[1] Under normal conditions, trillions of bacteria form a microbial barrier to maintain the intestinal immune homeostasis, resist the invasion of pathogenic bacteria and assist in digestion and absorption of nutrients.[2] In general, there is a dynamic balance between host and gut microbiota. It has been reported that microbiota imbalance is associated with various diseases, such as inflammatory bowel disease, diabetes, and depression. Several studies demonstrated that patients with ulcerative colitis (UC) who received fecal microbiota transplantation showed significant remission.[3,4,5] It was suggested that the recovery and reconstruction of the gut microbiota might be an alternative therapeutic option for UC.
Stachyose is a tetrasaccharide present in the raffinose family of oligosaccharides with few side effects.[6] It is a kind of natural prebiotic and its consumption is able to favorably modulate the composition of the gut microbiota in humans and animals.[7,8] It is not decomposed by digestive enzymes and it will be changed in specific conditions when in direct contact with the intestinal tract.[9] Stachyose can increase probiotic activity such as bifidobacteria and lactobacilli and regulate the balance of microecological flora in the human gastrointestinal tract. It can promote the formation of the dominant bacteria in the digestive tract and inhibit the production of spoilage bacteria such as Clostridium. Stachyose also promotes intestinal peristalsis and accelerates the excretion of pathogens and toxins.[10]
Many studies showed that UC results from imbalanced enteric flora and reduced microbial diversity.[11,12] Compared with healthy individuals, UC patients were observed to have decreased Firmicutes while bacteroidetes and proteobacteria increased.[13,14] In addition, the performance of UC patients in different stages of intestinal flora is not the same. It is found that compared with patients in remission, active UC patients had a decreased number of lactobacilli, bifidobacteria, Bacteroides and increased number of Escherichia coli, Enterococcus, and Clostridium spp.[15,16,17] Since the pathogenesis of UC involves an imbalance of bacteria, the strategies for regulating gut microbiota has gained increasing prominence.[18]
Acute colitis mice model induced by dextran sulfate sodium (DSS) was similar to UC in symptoms and pathologic manifestations.[19] In the present study, we explore the effects of stachyose on inflammatory levels and gut microbiota of acute colitis mice, to have a deeper understanding of microbiota and colitis.
MATERIALS AND METHODS
Animals
This study was ethically approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University on September 30, 2018 (SYSU-IACUC-2018-000189) and was conducted in conformity with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Twenty-eight male, 6–8 weeks old, C57BL/6J mice weighing 18–20 g (purchased from Beijing Vital River Laboratory Animal Technology Company) were housed in specific pathogen-free conditions. The mice were maintained in a temperature-controlled (temperature, 25 ± 2°C; relative humidity, 50 ± 5%) facility with a 12-h light/dark cycle and free access to a standard mouse chow diet.
Experimental design
The mice were randomly divided into four groups: (1) a blank group which was neither given DSS (W/V) (36000–50000 kDa; MP Biomedicals, Solon, OH, USA) nor stachyose (YZ-4283, Extrasynthese, France); (2) stachyose group, which was only given stachyose (1.5 g/kg/day) for 28 days; (3) DSS group, which was only given 3% DSS drinking water for 7 days; (4) stachyose + DSS group, which was given stachyose (1.5 g/kg/day) for 28 days during the pretreatment (21 days prior) and period of acute colitis induced by 3% DSS in drinking water (ad libitum 7 days).
Histological studies
The distal colon segments from each animal were fixed in 4% paraformaldehyde (PFA), embedded in paraffin and cut into sections of 4 μm in thickness. The sections were then stained with hematoxylin and eosin (H and E). HE-stained sections were examined for inflammation and tissue damage.
Analysis of inflammation-related cytokines in serum by enzyme-linked immunosorbent assay (ELISA)
For the serum cytokine assay, blood from the eyeball was collected in a tube and centrifuged at 1100 × g, 4°C for 15 min. The serum was aspirated and assayed as described below. Concentrations of inflammatory-related cytokines IL-6, IL-10, IL-17a, and TNF-α in serum were measured by ELISA according to the manufacturer's instructions (Biolegend, San Diego, CA, USA). Briefly, biotinylated antibody reagent was added to 96-well plates, then supernatants of homogenized serum were added and the plates were incubated at 37°C in CO2for 2 h. After washing with phosphate-buffered saline (PBS), streptavidin-horseradish peroxidase (HRP) solution was added and the plate was incubated for 30 min at room temperature. The absorbance was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Analysis of microbiota composition by 16S rDNA gene sequencing
Total microbial DNA was extracted from the feces using the QIAamp DNA Stool Minikit (Qiagen Ltd, Strasse, Germany). The extracted DNA was used as the template to amplify the V3–V4 region of 16S rDNA genes by polymerase chain reaction (PCR), and the DNA libraries were constructed by the Ion Plus Fragment Library Kit (Thermo Scientific, USA). The next-generation sequencing of 16S rDNA was performed by the Ion S5TM (Thermo Scientific, USA). Quality control was carried out through Fast-QC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Cluster reads into operational taxonomic units (OTUs) using Quantitative Insights into Microbial Ecology (QIIME, version 1.17) at the 97% sequence similarity. The α-diversity analysis was carried out to determine the species richness and species diversity, including the Chao1 index and Shannon index.
Statistical analysis
Continuous variables were described as mean ± standard deviation, while categorical variables were presented as numbers and proportions. Difference between two groups was statistically examined through unpaired student t-test, and analysis of variance (ANOVA) was used for the statistical comparisons between multiple groups. P values were two-tailed, and P < 0.05 was considered statistically significant. All analyses were performed with Statistical Package for Social Sciences (SPSS; version 19, SPSS Inc., Chicago, IL, USA).
RESULTS
Effects of stachyose on histological damage in the colon tissue of mice with DSS-induced colitis
The colon length of four groups was presented in Figure 1. We found that the stachyose group had approximately 16.7% of the shortening rate of the colon while the DSS group had approximately 38.9%. After collecting colonic tissue samples, the severity of colitis was characterized by macroscopic examination of the colon and histological analysis of HE stained colonic sections. The inhibitory effects of the stachyose on the DSS-induced damage of the colon tissue are shown in Figure 2. HE staining analysis indicated that the administration of DSS markedly increased the severity of the colitis compared with that in the normal mice [Figure 2a and b]. A typical lesion of the colon in the DSS administration group manifested multifocal areas, mucosal erosion, loss of epithelial and goblet cells, shortening and collapse of crypts, and submucosal edema. Stachyose administration reduced the lesions of the colon in DSS-induced colitis, as shown in Figure 2c and d. The protective and healing effects of stachyose on colon damage were more prominent.
Figure 1.
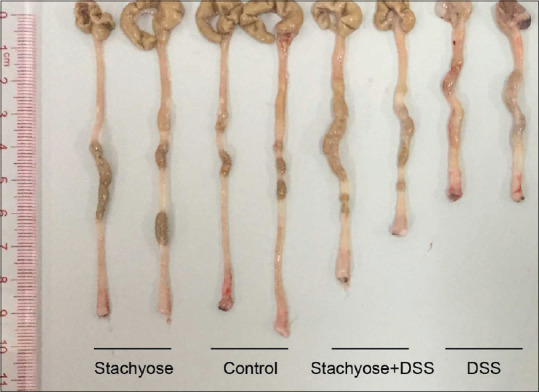
The colon length of four groups
Figure 2.
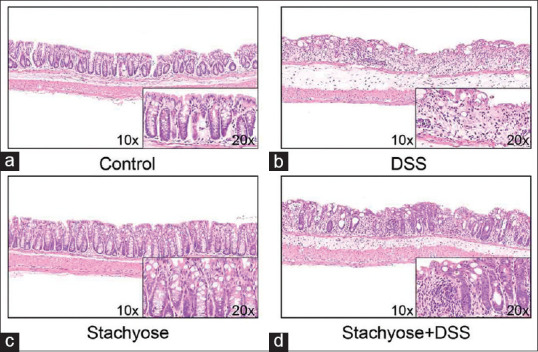
Effects of stachyose on histological damage in the colon tissue of mice with dextran sulfate sodium (DSS)-induced colitis. Histologic analysis of colon tissue for (a) control group, (b) DSS group, (c) Stachyose group and (d) stachyose + DSS group
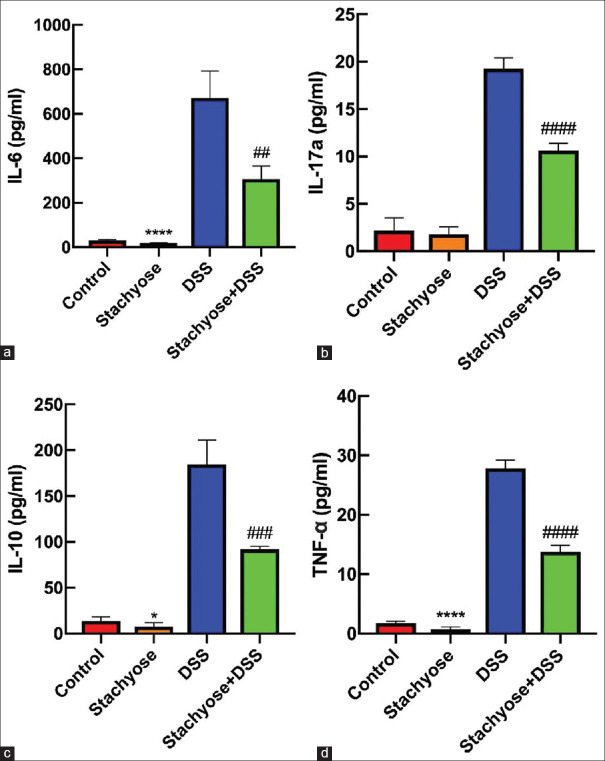
Effect of stachyose on serum levels of IL-6, IL-10, IL-17a, and TNF-α
The IL-6 levels of control group, stachyose group, DSS group, and stachyose + DSS group were 30.58 ± 3.59 pg/mL, 17.75 ± 2.32 pg/mL, 672.45 ± 120.05 pg/mL, and 306.36 ± 59.39 pg/mL, respectively. The IL-6 level was significant between the DSS group and the stachyose + DSS group (P < 0.01). The IL-10 levels of control group, stachyose group, DSS group, and stachyose + DSS group were 13.68 ± 4.64 pg/mL, 7.61 ± 4.40 pg/mL, 184.67 ± 26.56 pg/mL, and 91.73 ± 3.62 pg/mL, respectively. The IL-10 level was significant between the DSS group and the stachyose + DSS group (P < 0.001). The IL-17a levels in control group, stachyose group, DSS group, and stachyose + DSS group were 2.15 ± 1.36 pg/mL, 1.78 ± 0.80 pg/mL, 19.27 ± 1.14 pg/mL, and 10.62 ± 0.79 pg/mL, respectively. The IL-17a level was significant between the DSS group and the stachyose + DSS group (P < 0.0001). The TNF-α levels in control group, stachyose group, DSS group, and stachyose + DSS group were 1.76 ± 0.33 pg/mL, 0.70 ± 0.43 pg/mL, 27.81 ± 1.41 pg/mL, and 13.78 ± 1.07 pg/mL, respectively. The TNF-α level was significant between the DSS group and the stachyose + DSS group (P < 0.0001). The results are presented in Figure 3.
Figure 3.
Effect of stachyose on serum levels of IL-6, IL-10, IL-17a, and TNF- and #945; comparison of the serum IL-6 (a), IL-17a (b), IL-10 (c), and TNF- and #945; (d) levels among the groups. The data are expressed as the means ± SD. *P< 0.05; ****P< 0.0001 vs control. ##P< 0.01; ###P< 0.001; ####P< 0.0001 vs DSS
Stachyose changed the gut microflora community composition in mice
The results of the taxonomic analysis are shown in Figure 4. At the genus level, Escherichia-Shigella was only detected in the DSS model and the DSS group was higher than the stachyose + DSS group (relative abundance: 0.175 vs 0.036). Stachyose group displayed higher Akkermansia than the control group (relative abundance: 0.102 vs 0.004). Besides, Lactobacillus was decreased in the control group compared with the stachyose group (relative abundance: 0.175 vs 0.355).
Figure 4.
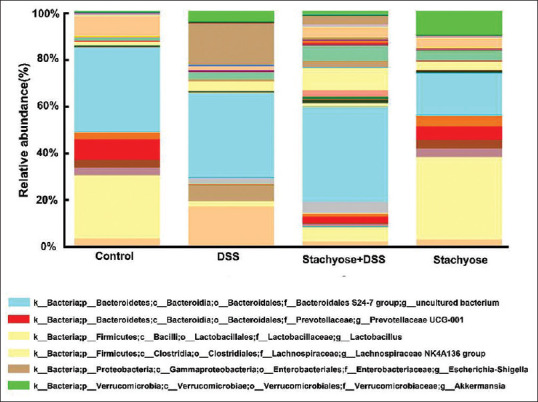
Composition of gut microbiota at the genus level in groups of mice
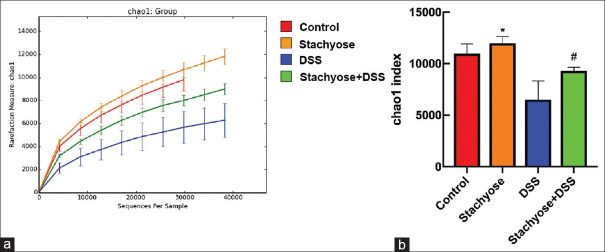
Alpha diversity in the DSS administration group was reduced. In addition, alpha diversity was more abundant in the stachyose group than the control group, and alpha diversity was more abundant in the stachyose + DSS group than the DSS group. The chao1 index is shown in Figure 5.
Figure 5.
Stachyose modulated the microbial diversity in mice. (a) The chao1 curves, (b) Comparison of chao1 index among the groups. The data are expressed as the means ± SD. *P< 0.05 vs Control. #P< 0.05 vs. DSS
DISCUSSION
The balance of gut microbiota plays a critical role in maintaining the mucosal barrier and immune function, which is involved with the production of antibacterial substances and the regulation of host immunity.[11] Sellon et al. demonstrated that there was no intestinal inflammation in germ-free mice, and the mice underwent colitis after the recovery of gut microbiota.[20] The incidence of UC has gradually increased in the past 30 years, and studies have found that there exists a close relationship between the disease and intestinal flora.[21] It was suggested that imbalanced intestinal microbiota was involved in the occurrence and development of UC. The mechanisms underlying the effects of imbalanced intestinal microbiota on UC may involve the stimulation of immune response and production of inflammatory cytokines. Manichanh et al. suggested that the levels of beneficial bacteria such as Lactobacillus and Bifidobacterium were significantly lower in patients with intestinal inflammation than those in healthy people.[22] This study was conducted to investigate whether stachyose could alleviate the inflammatory levels and regulate the gut microbiota of acute colitis mice.
Prebiotics could promote the growth of probiotics such as Lactobacillus and Bifidobacterium to improve intestinal microecology and fight intestinal inflammation. There exists a large amount of research on probiotics while the impact of prebiotics on intestinal microbiota is rarely studied. Qian et al. demonstrated that stachyose administration significantly alleviated DSS-induced colitis in mice,[23] which was consistent with our result. Our study further investigated the role of stachyose in the intestinal microbiota of colitis mice through the 16s rDNA sequence.
Our results show that stachyose has protective and healing effects on colon damage. The addition of stachyose could reduce the level of serum IL-6, IL-10, IL-17a, and TNF-α. According to the results of the 16s rDNA sequence, we could find the increase and decrease of many genera. Among them, changes of Lactobacillus and Akkermansia were most significant. Our results indicate that Lactobacillus is obviously increased in the stachyose group compared with the control group, which is beneficial to the intestinal flora.
Stachyose group showed a higher level of Akkermansia than the control group, and the stachyose + DSS group had more abundant Akkermansia than DSS group, which suggested the promoting role of stachyose on the proliferation of Akkermansia. It was reported that Akkermansia participated in mucin degradation, production of short-chain fatty acids, and providing energy for the host.[24] A recent study demonstrated that the abundance of Akkermansia showed a negative correlation with the level of IL-6 in serum,[25] which was consistent with our result. Furthermore, studies showed that Akkermansia could improve inflammatory response in diabetic mice,[26] protect intestinal epithelial cells, and maintain the mucosal barrier.[27] Routy et al. demonstrated that the Akkermansia could ameliorate the antitumor effects of programmed death-1 (PD-1) blockade.[28] It has been proved that Akkermansia could encode various secretory proteins such as sulfates, proteases, and glycohydrolase.[29] In our study, Escherichia-Shigella was only detected in the DSS model and the DSS group was higher than the stachyose + DSS group. Hence, we speculated that Akkermansia might decompose stachyose and facilitate its own proliferation and inhibit harmful bacteria. We also suggest that stachyose could increase the diversity of intestinal flora, which might be beneficial in reducing intestinal inflammation.
The effect of prebiotics in clinical patients has also been reported. For example, de Cossío et al. found that fructooligosaccharide could modulate microbiota in patients with metabolic syndrome,[30] lactitol could increase the number of Lactobacillus and Bifidobacterium, and reduce the level of endotoxin in chronic hepatitis patients. However, the role of stachyose in patients with UC has not been previously reported. The analysis was carried out at the genus level because of inadequacy in species level. The effect of stachyose on mucous membrane flora is not observed, thus, further validation was still needed. Our results indicate that animal models could provide direction for clinical research to some extent, but could not completely simulate the physiologic and pathologic state of humans. In our study, the effects of stachyose on intestinal microbiota in acute colitis mice are preliminarily explored. The influence of different preparations on clinical patients and its mechanism still needs to be further studied to be better applied to patients.
In conclusion, this study found that stachyose increased beneficial microbiota and bacterial diversity to alleviate acute colitis in mice. It might be a new promising option for UC patients.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (81470795) and the Science and Technology Planning Project of Guangdong Province, China (2013B022000035).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–93. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65:330–9. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–9.e6. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Ding X, Li Q, Li P, Zhang T, Cui B, Ji G, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Safety. 2019;42:869–80. doi: 10.1007/s40264-019-00809-2. [DOI] [PubMed] [Google Scholar]
- 5.Jacob V, Crawford C, Cohen-Mekelburg S, Viladomiu M, Putzel GG, Schneider Y, et al. Single delivery of high-diversity fecal microbiota preparation by colonoscopy is safe and effective in increasing microbial diversity in active ulcerative colitis. Inflamm Bowel Dis. 2017;23:903–11. doi: 10.1097/MIB.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin J, Yang G, Wang S, Chen Y. Purification and determination of stachyose in Chinese artichoke (Stachys Sieboldii Miq) by high-performance liquid chromatography with evaporative light scattering detection. Talanta. 2006;70:208–12. doi: 10.1016/j.talanta.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Macfarlane GT, Cummings JH. Probiotics and prebiotics: Can regulating the activities of intestinal bacteria benefit health? West J Med. 1999;171:187–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Lu X, Yang X. Stachyose-enriched α-galacto-oligosaccharides regulate gut microbiota and relieve constipation in mice. J Agric Food Chem. 2013;61:11825–31. doi: 10.1021/jf404160e. [DOI] [PubMed] [Google Scholar]
- 9.Liying Z, Li D, Qiao S, Johnson EW, Li B, Thacker PA, et al. Effects of stachyose on performance, diarrhoea incidence and intestinal bacteria in weanling pigs. Archiv Tierernahr. 2003;57:1–10. [PubMed] [Google Scholar]
- 10.Xu Q, Chao YL, Wan QB. Health benefit application of functional oligosaccharides. Carbohydr Polym. 2009;77:435–41. [Google Scholar]
- 11.Batista D, Raffals L. Role of intestinal bacteria in the pathogenesis of pouchitis. Inflamm Bowel Dis. 2014;20:1481–6. doi: 10.1097/MIB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 12.McLean MH, Dieguez D, Miller LM, Young HA. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut. 2015;64:332–41. doi: 10.1136/gutjnl-2014-308514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyler AD, Knox N, Kabakchiev B, Milgrom R, Kirsch R, Cohen Z, et al. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PLoS One. 2013;8:e66934. doi: 10.1371/journal.pone.0066934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michail S, Durbin M, Turner D, Griffiths AM, Mack DR, Hyams J, et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis. 2012;18:1799–808. doi: 10.1002/ibd.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajilić-Stojanović M, Shanahan F, Guarner F, de Vos WM. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis. 2013;19:481–8. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 16.Yukawa T, Ohkusa T, Shibuya T, Tsukinaga S, Mitobe J, Takakura K, et al. Nested culture method improves detection of Fusobacterium from stool in patients with ulcerative colitis. Jpn J Infect Dis. 2013;66:109–14. doi: 10.7883/yoken.66.109. [DOI] [PubMed] [Google Scholar]
- 17.Day AS. Inflammatory Bowel Disease and the Intestinal Microbiota. J Pediatr Biochem. 2015;5:60–4. [Google Scholar]
- 18.Derikx LA, Dieleman LA, Hoentjen F. Probiotics and prebiotics in ulcerative colitis. Best Pract Res Clin Gastroenterol. 2016;30:55–71. doi: 10.1016/j.bpg.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Chassaing B, Aitken JA, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104 doi: 10.1002/0471142735.im1525s104. Unit 15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Wang W, Zhou R, Ng SC, Li J, Huang M, et al. Characteristics of fecal and mucosa-associated microbiota in Chinese patients with inflammatory bowel disease. Medicine. 2014;93:e51. doi: 10.1097/MD.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–11. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Y, Zhao X, Song JL, Zhu K, Sun P, Li GJ, et al. Inhibitory effects of resistant starch (RS3) as a carrier for stachyose on dextran sulfate sodium-induced ulcerative colitis in C57BL/6 mice. Exp Ther Med. 2013;6:1312–6. doi: 10.3892/etm.2013.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73:7767–70. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Carrio J, Salazar N, Margolles A, González S, Gueimonde M, de Los Reyes-Gavilán CG, et al. Free fatty acids profiles are related to gut microbiota signatures and short-chain fatty acids. Front Immunol. 2017;8:823. doi: 10.3389/fimmu.2017.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285–94. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 27.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81:3655–62. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (New York, N.Y.) 2018;359:91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 29.Singla V, Chakkaravarthi S. Applications of prebiotics in food industry: A review. Food Sci Technol Int. 2017;23:649–67. doi: 10.1177/1082013217721769. [DOI] [PubMed] [Google Scholar]
- 30.de Cossío LF, Fourrier C, Sauvant J, Everard A, Capuron L Cani PD, et al. Impact of prebiotics on metabolic and behavioral alterations in a mouse model of metabolic syndrome. Brain Behav Immun. 2017;64:33–49. doi: 10.1016/j.bbi.2016.12.022. [DOI] [PubMed] [Google Scholar]