Abstract
Background
Adequate water intake is critical to physiologic and cognitive functioning. Although water requirements increase with body size, it remains unclear whether weight status modifies the relation between water intake and hydration status.
Objective
We examined how the association between water intake and urine osmolality, which is a hydration biomarker, varied by weight status.
Design
NHANES cross-sectional data (2009–2012) were analyzed in 9601 nonpregnant adults aged ≥20 y who did not have kidney failure. Weight status was categorized with the use of body mass index on the basis of measured height and weight (underweight or normal weight, overweight, and obesity). Urine osmolality was determined with the use of freezing-point depression osmometry. Hypohydration was classified according to the following age-dependent formula: ≥831 mOsm/kg – [3.4 × (age − 20 y)]. Total water intake was determined with the use of a 24-h dietary recall and was dichotomized as adequate or low on the basis of the Institute of Medicine’s adequate intake recommendations for men and women (men: ≥3.7 or <3.7 L; nonlactating women: ≥2.7 or <2.7 L; lactating women: ≥3.8 or <3.8 L for adequate or low intakes, respectively). We tested interactions and conducted linear and log-binomial regressions.
Results
Total water intake (P = 0.002), urine osmolality (P < 0.001), and hypohydration prevalence (P < 0.001) all increased with higher weight status. Interactions between weight status and water intake status were significant in linear (P = 0.005) and log-binomial (P = 0.015) models, which were then stratified. The prevalence ratio of hypohydration between subjects with adequate water intake and those with low water intake was 0.56 (95% CI: 0.43, 0.73) in adults who were underweight or normal weight, 0.67 (95% CI: 0.57, 0.79) in adults who were overweight, and 0.78 (95% CI: 0.70, 0.88) in adults who were obese.
Conclusion
On a population level, obesity modifies the association between water intake and hydration status.
Keywords: effect modification, hydration status, NHANES, obesity, water intake
INTRODUCTION
Water is an essential nutrient, and meeting water needs is critical to cognitive and physiologic functioning (1–3). The consequences of even mild dehydration have been associated with increased risk of kidney stones, exercise-induced asthma, poorer cognitive function (e.g., poorer mood scores, increased fatigue, and increased pain perception) (2–6), and impaired aerobic exercise tasks because of increasing heat storage and decreasing sweat rates (7), and drinking water helps reduce these problems (8).
There is increasing interest in assessing the hydration status of populations and determining the long-term impact of inadequate hydration (9, 10). However, a population-level assessment of hydration status faces the challenge of a lack of a gold-standard laboratory measurement of adequate hydration, especially in older adults (11, 12). The clinical assessment of acute dehydration relies on the estimation of the loss of body water on the basis of the percentage of body weight and clinical signs and symptoms (e.g., skin turgor, absence of sweating and urine production, and cognitive function). However, epidemiologic studies have defined hydration status on the basis of urine osmolality (hypohydration, euhydration, and hyperhydration) and have compared the prevalence of hypohydration between populations (9, 13–15).
Human water needs vary because of physical activity, ambient temperature, sex, cultural dietary patterns, and body size (16, 17). Nevertheless, a gap in the literature exists concerning how obesity is associated with hydration status (18). Currently, the prevalence of obesity in US adults is 37.7% and comprises a growing proportion of the population (19). Obesity is associated with a variety of downstream ill-health effects that may change water needs, hydration, and metabolic processes (e.g., hypertension, kidney function, type 2 diabetes mellitus, and cardiovascular disease) (20–22). Although previous research has treated weight status as a potential confounder, it remains unclear how obesity affects the relation between water intake and hydration status (18, 23–25).
Therefore, the purpose of this study was to examine how weight status affects the association between daily water intake from all foods and liquids and hydration status as determined with the use of urine osmolality in US adults.
METHODS
Sample design
The current study used data from the NHANES, which uses a stratified, multistage probability design to provide a representative sample of the noninstitutionalized, civilian US population. Since 1999, the NHANES has been collected continuously by the National Center for Health Statistics, CDC, with cross-sectional study waves released in 2-y cycles. NHANES combines in-person interviews with health and laboratory examinations that are conducted in Mobile Examination Centers (MECs). In-depth details of the survey and sampling procedures have been described elsewhere (26, 27). The National Center for Health Statistics Research Ethics Review Board approved the continuous NHANES with an initial recruitment date of 1999 (continuation of protocol 2005–06), and all adult participants gave written informed consent.
We used data from the 2009–2010 and 2011–2012 survey cycles of the NHANES, which oversampled non-Hispanic black and Hispanic persons, as well as other groups. The combination of 4 y of data increased the stability of estimates. The examination response rate for adults aged ≥20 y was 68.3% in the NHANES 2009–2012 (28).
Urine osmolality
Adults provided spot urine samples in the MECs. Urine osmolality, which is the total concentration of dissolved particles per kilogram of water in urine, was determined by freezing-point depression osmometry in the MEC laboratory, which was calculated as milliosmoles per kilogram (mOsm/kg). Urine osmolality has been shown to be a reliable urinary biomarker of daily hydration status and is highly correlated with other biomarkers of hydration (10, 14, 29, 30). The maximal urine-concentration ability decreases with age at a mean rate of 3.4 mOsm · kg−1 · y−1 for adults >20 y of age (13, 15, 31). Therefore, Manz and Wentz (15) recommended an age-dependent cutoff for euhydration that takes this decrease into account. If an individual’s 24-h urine osmolality is
| (1) |
he or she should be classified as hypohydrated. For example, a 20-y-old’s hypohydration cutoff would be
| (2) |
whereas a 50-y-old’s hypohydration cutoff would be
| (3) |
Other authors have used a uniform cutoff of >831 mOsm/kg to classify hypohydration (32, 33) or >800 mOsm/kg to classify inadequate water intake (9), but because these cutoffs do not account for a decreasing urine concentration with age, we defined hypohydration according to the age-dependent formula with the use of spot samples.
Total water intake
At the MECs, trained interviewers used a computer-assisted, multiple-pass dietary recall interview to assess the type and quantity of all foods and liquids consumed in the previous 24 h (from midnight to midnight) (26). From the 24-h dietary recall, total moisture was calculated from all foods and liquids in grams, the equivalent of milliliters of water (reported here).
The Institute of Medicine recommends an adequate intake (AI) for total water consumption of 3.7 L for men, 2.7 L for nonlactating women, and 3.8 L for lactating women (34). We used these cutoffs to establish a dichotomous variable to classify water intake status as an indicator of whether intake was sufficient on a given day. Men who consumed ≥3.7 L, nonlactating women who consumed ≥2.7 L, and lactating women who consumed ≥3.8 L were classified as having adequate water intake, whereas subjects who consumed less than these respective amounts were classified as having low water intake.
Weight status
Height and weight were measured in the MECs by trained professionals according to standard protocols (26).BMI (in kg/m2) was used to classify weight status and was calculated as weight divided by the square of height (and rounded to one decimal place).Weight-status categories were determined as follows: under-or normal weight (BMI <25), overweight (BMI 25 to <30), and obesity (BMI ≥30) (35).
Covariates and variable definitions
Sex (male or female), age (20–39, 40–59, or ≥60), race and Hispanic origin, alcohol intake (grams), caffeine intake (milligrams), energy intake (kilocalories), physical activity (minutes), the time of examination (morning, afternoon, or evening), and diabetes status were included as covariates in the analysis to control for confounding of the relation between water intake, weight status, and urine osmolality on the basis of physiologic relations and what has been reported in the literature (13, 36–40). Participants self-reported their race and Hispanic origin and were categorized as non-Hispanic white, non-Hispanic black, Hispanic, or other (including all non-Hispanic individuals who reported >1 race group). Alcohol intake, caffeine intake, and energy intake were obtained from the 24-h dietary recall. Caffeine intake was categorized dichotomously with the use of the consumption value that increases risk of hypohydration (<400 or ≥400 mg) (36, 37).
The physical activity variable was constructed from the Global Physical Activity Questionnaire (41). The total time spent in the previous week in moderate and vigorous activities from biking or walking, work, and leisure activities were assessed in the home interview with the use of the computer-assisted personal interviewing system. Physical activity was coded as a binary variable according to whether the person met national guidelines of ≥150 min moderate physical activity/wk because higher physical activity increases water needs (16, 42).
To test for diabetes, blood specimens were collected for all participants and samples were shipped to the Fairview Medical Center Laboratory at the University of Minnesota for analysis of glycated hemoglobin. Persons with values of glycated hemoglobin ≥6.5% (43) or who reported that a doctor told them they that they ever had diabetes were categorized as having diabetes.
Statistical analysis
Linear trends across weight-status categories were tested with an F-test statistic that was adjusted for the survey design and sampling weights with the use of polynomial orthogonal contrasts, and we used Wald tests that were adjusted for the survey design with significance set at 0.05 to test for mean differences in urine osmolality, the prevalence of hypohydration, and total water intake by weight status. Multiple linear and log-binomial regression models were used to estimate associations and prevalence ratios with confounders controlled for and testing for interactions between weight status and water intake status. We first estimated the regressions including only weight status, water intake status, and the interaction between these 2 variables. We then specified the regression models that were adjusted for the potential confounders and compared the results. We repeated this process once the regressions were stratified by weight status. The results from the adjusted and unadjusted models were highly similar; therefore, we only present the adjusted models in this article because they were more precise.
We used log-binomial regression because the outcome (i.e., hypohydration) is not a rare event (<10%) (44). Log-binomial regression provides a more conservative estimate than a logistic regression does because the OR diverges away from the null as the outcome becomes more common. Because the interaction between weight-status categories and water intake status was jointly significant for both the linear (P = 0.005, F = 6.27) and log-binomial (P = 0.015, F = 4.78) models by postestimation-adjusted Wald statistics, we stratified according to weight status in the models presented. We tested for multicollinearity between the covariates included in the regression models by calculating the variance inflation factor and the tolerance. All of the independent variables had a variance inflation factor <10, which showed that collinearity was not present (45). In conjunction with the regression models, we used marginal standardization to generate predicted probabilities of hypohydration controlling for the distribution of covariates and accounting for the interactions included in the models (46, 47).
Because of the complex 4-stage sample design of the NHANES, analyses were conducted in Stata 13.1 software (StataCorp LP) and in SAS software (version 9.1; SAS Institute Inc.) with the use of survey commands and SAS-callable SUDAAN software (version 9; RTI International) with SEs estimated with the use of Taylor-series linearization. Day 1 dietary sample weights were used to adjust for oversampling, nonresponses, noncoverage, and the day of the week.
Missing and excluded data
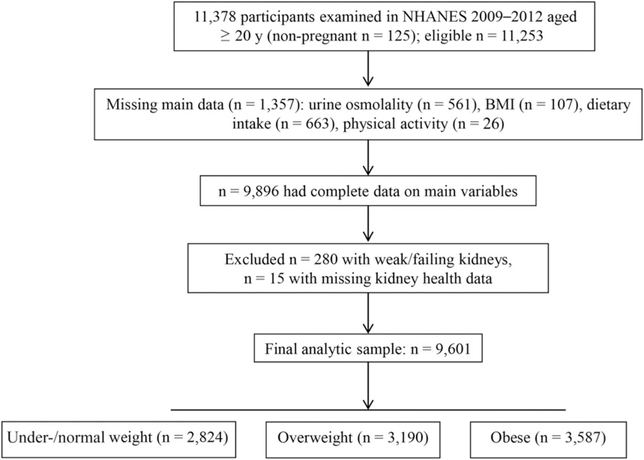
The following exclusions were made sequentially to create the main analytic sample (Figure 1) from the 11,253 examined adults aged ≥20 y who were nonpregnant in 2009–2012; 561 individuals were excluded because of missing data on urine osmolality, 107 individuals were excluded because of missing BMI data, 663 individuals were excluded because of missing dietary intake data, and 26 individuals were excluded because of missing physical activity data [total exclusion: n = 1357 (12.1%)]. These exclusions yielded 9896 respondents with non-missing data.
FIGURE 1.
NHANES 2009–2012 analytic sample flow diagram.
We also excluded an additional 280 adults who reported weak or failing kidneys in the past 12 mo, and 15 adults were excluded because of missing kidney health data to avoid confounding because of an inability to concentrate urine properly. These additional exclusions yielded the final analytic sample of 9601 subjects. Table 1 provides the analytic sample sizes by sex, age, and race and Hispanic origin to show that the cell sizes were sufficient to analyze the data.
TABLE 1.
Analytic sample breakdown of nonpregnant adults aged ≥20 y in the United States, 2009–2012, by age categories and race and Hispanic origin1
| All race and Hispanic origin | Non-Hispanic white | Non-Hispanic black | Hispanic | |
|---|---|---|---|---|
| All, n | 9601 | 4238 | 2049 | 2344 |
| 20–39 y | 3293 | 1339 | 653 | 866 |
| 40–59 y | 3287 | 1353 | 746 | 850 |
| ≥60 y | 3021 | 1546 | 650 | 628 |
| Men, n | ||||
| 20–39 y | 1717 | 684 | 343 | 455 |
| 40–59 y | 1631 | 679 | 355 | 433 |
| ≥60 y | 1506 | 780 | 327 | 289 |
| Women, n | ||||
| 20–39 y | 1576 | 655 | 310 | 411 |
| 40–59 y | 1656 | 674 | 391 | 417 |
| ≥60 y | 1515 | 766 | 323 | 339 |
Sample sizes were unweighted. “Other” category was included in all race and Hispanic origin totals but is not shown. Data source: NHANES (26).
Because 12.1% of the examined sample of adults from the NHANES 2009–2012 had missing data, we conducted nonresponse bias analyses. Adults with missing data were not significantly older than the analytic sample (mean: 47.5 compared with 47.1 y, respectively) but were more likely to be female (61.0% compared with 50.1%), less likely to be white (57.6% compared with 68.2%), and more likely to be black (15.2% compared with 11.1%). To further examine the potential for nonresponse bias, we reweighted the data with the use of the PROC WTADJUST in the SUDAAN program. The adjusted sample weights produced similar results to those that were estimated when the publicly available day 1 dietary sample weights were used. Therefore, we present results that were obtained with the use of the publicly available day 1 dietary sample weights.
Sensitivity analyses
We further tested the association between water intake, urine osmolality, and weight status by re-estimating the analyses with a uniform cutoff of hypohydration (≥831 mOsm/kg) instead of the age-dependent cutoff (32). Second, we stratified the analysis by examination time to further evaluate how the relation was affected by the use of spot urine samples from different times during the day because recent research has shown that late-afternoon spot samples of urine osmolality reflected 24-h urine osmolality (48). Third, we excluded participants with self-reported or undiagnosed diabetes (n = 1404) to evaluate whether the inclusion of adults with diabetes affected estimates, and we re-estimated the analyses with a limited sample (n = 8197). Finally, we re-estimated the regression models and interactions with the use of total water intake as a continuous variable to illustrate the predicted probabilities of hypohydration and predicted urine osmolality by weight status across the range of total water intake.
RESULTS
Table 2 presents the descriptive characteristics of the analytic sample and illustrates how the characteristics varied by weight status. Mean ± SE BMI was 28.7 ± 0.1, and 35.6% ± 0.1% of adults had BMI ≥30. Mean total water intake was 3128.8 ± 38.4 mL, and adults who were obese consumed 238.5 mL (P = 0.002, F = 10.87) more water, on average, or ~1 cup, than was consumed by adults who were underweight or normal weight. Mean urine osmolality from spot samples was 613.8 ± 5.9 mOsm/kg, and 37.6% ± 0.9% of US adults had urine osmolality values that were consistent with hypohydration. Total water intake (P = 0.002, F = 10.87), urine osmolality (P < 0.001, F = 90.25), and the prevalence of hypohydration (P < 0.001, F = 61.06) all increased with higher weight status. The percentage of adults with urine osmolality values that were consistent with hypohydration increased from 28.5% in underweight and normal weight adults to 45.7% in adults who were obese.
TABLE 2.
Descriptive characteristics by weight status in adults aged ≥20 y in the United States, 2009–20121
| Total | Underweight or normal weight (BMI <25) | Overweight (BMI 25 to <30) | Obese (BMI ≥30) | P-trend2 | |
|---|---|---|---|---|---|
| n | 9601 | 2824 | 3190 | 3587 | |
| Age, y | 47.1 ± 0.5 | 44.1 ± 0.8 | 48.6 ± 0.4 | 48.4 ± 0.4 | <0.001 |
| Sex, M, % | 49.9 ± 0.6 | 56.4 ± 1.3 | 43.1 ± 1.0 | 50.7 ± 1.3 | 0.003 |
| Race and Hispanic origin3 | |||||
| Non-Hispanic white | 68.2 ± 2.6 | 70.5 ± 2.3 | 69.4 ± 2.8 | 65.1 ± 3.1 | 0.009 |
| Non-Hispanic black | 11.1 ± 1.2 | 8.7 ± 1.1 | 9.3 ± 1.2 | 15.0 ± 1.8 | <0.001 |
| Hispanic | 13.8 ± 1.9 | 9.8 ± 1.3 | 15.6 ± 2.3 | 15.6 ± 2.4 | <0.001 |
| Height, m | 169.1 ± 0.2 | 168.7 ± 0.3 | 169.9 ± 0.3 | 168.6 ± 0.3 | 0.85 |
| BMI, kg/m2 | 28.7 ± 0.1 | 22.2 ± 0.05 | 27.4 ± 0.04 | 35.6 ± 0.1 | <0.001 |
| Weight, kg | 82.3 ± 0.3 | 63.4 ± 0.3 | 79.3 ± 0.3 | 101.4 ± 0.4 | <0.001 |
| Total water intake, mL | 3128.8 ± 38.4 | 2973.1 ± 55.8 | 3184.8 ± 48.3 | 3211.6 ± 57.9 | 0.002 |
| Urine osmolality, mOsm/kg | 613.8 ± 5.9 | 557.9 ± 9.5 | 611.2 ± 10.1 | 664.7 ± 5.9 | <0.001 |
| Hypohydration,4 % | 37.6 ± 0.9 | 28.5 ± 1.4 | 37.3 ± 1.9 | 45.7 ± 1.2 | <0.001 |
| Physical activity ≥150 min/wk, % | 63.8 ± 1.1 | 70.5 ± 1.6 | 65.9 ± 1.1 | 56.2 ± 1.3 | <0.001 |
| Caffeine intake ≥400 mg, % | 11.3 ± 0.9 | 9.5 ± 0.9 | 11.8 ± 1.1 | 12.5 ± 0.8 | 0.01 |
| Alcohol intake, g | 12.0 ± 0.6 | 13.6 ± 1.1 | 13.3 ± 1.0 | 9.5 ± 0.8 | 0.002 |
| Energy intake, kcal | 2182.7 ± 13.6 | 2152.8 ± 27.0 | 2242.1 ± 23.6 | 2153.2 ± 21.6 | 0.99 |
| Diabetes, % | 10.5 ± 0.4 | 4.2 ± 0.5 | 7.4 ± 0.4 | 18.9 ± 0.9 | <0.001 |
All values are means ± SEs or percentages ± SEs. Data source: NHANES (26).
Linear trend across BMI categories was tested with the F-test statistic and adjusted for the survey design with the use of polynomial orthogonal contrasts.
“Other” category was included in the total sample but is not shown.
mOsm/kg ≥831 − [3.4 × (age − 20 y)].
Models 1–3 in Table 3 present the linear regression models for the assessment of the association between water intake status and urine osmolality stratified by weight status because of a significant interaction (for joint significance between weight status and water intake status: P = 0.005, F = 6.27; results not shown) and when controlling for the following variables: age category (20–39, 40–59, or ≥60 y); sex; race and Hispanic origin; the time of examination; whether subjects met physical activity guidelines; high caffeine intake, alcohol intake, and energy intake; and diabetes. In adults who were underweight or normal weight, the difference in urine osmolality between those with adequate water intake and those with low water intake was β ± SE = −122.4 ± 19.7 mOsm/kg (model 1; P < 0.001). However, in adults who were obese, the difference in mean urine osmolality between those with adequate intake and those with low intake was β ± SE = −65.2 ± 9.1 mOsm/kg (model 3; P < 0.001). These models suggest that, although urine osmolality is significantly lower in adults with adequate water intake (as defined by Institute of Medicine recommendations) than in adults with low water intake, the difference in urine osmolality between adults with adequate water intake and those with low water intake decreases with higher weight status.
TABLE 3.
Multiple linear and log-binomial regression models relating the association of adequate intake of water stratified by weight status to urine osmolality and the PR of hypohydration in adults aged ≥20 y in the United States, 2009–20121
| Urine osmolality,
mOsm/kg |
Hypohydration2 |
|||||
|---|---|---|---|---|---|---|
| Underweight or normal weight |
Overweight |
Obese |
Underweight or normal weight |
Overweight |
Obese |
|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| Low water intake | Reference | Reference | Reference | Reference | Reference | Reference |
| Adequate water intake | −122.4 ± 19.73 | −99.5 ± 16.4 | −65.2 ± 9.1 | 0.56 (0.43, 0.73)4 | 0.67 (0.57, 0.79) | 0.78 (0.70, 0.88) |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| n | 2824 | 3190 | 3587 | 2824 | 3190 | 3587 |
Models were stratified by weight status because the interaction between weight status and water intake status was jointly significant in both linear (F = 6.27, P = 0.005) and log-binomial (F = 4.78, P = 0.015) regressions. All models were adjusted for the following variables: age category (20–39, 40–59, and ≥60 y); sex; race and Hispanic origin; time of examination; meeting physical activity guidelines; high caffeine intake, alcohol intake, and caloric intake; and diabetes. BMI (in kg/m2) categories were as follows: underweight or normal weight, <25; overweight, 25 to <30; and obese, ≥30. Low water intake was defined as follows: men, <3.7 L; nonlactating women, <2.7 L, and lactating women, <3.8 L; and adequate water intake was defined as follows: men, ≥3.7 L; nonlactating women, ≥2.7 L; and lactating women, ≥3.8 L. Data source: NHANES (26). PR, prevalence ratio.
mOsm/kg ≥831 − [3.4 × (age − 20 y)].
Beta ± SE (all such values).
PR; 95% CI in parentheses (all such values).
Because of the significant interaction (P = 0.015, F = 4.78) between weight status and water intake status on the prevalence ratio of hypohydration, log binomial regression models were also stratified by weight status (Table 3, models 4–6). Similar to the linear regression models, the prevalence ratio of hypohydration between subjects with adequate water intake and those with low water intake was 0.56 (95% CI: 0.43, 0.73) in adults who were underweight or normal weight compared with 0.67 (95% CI: 0.57, 0.79) in adults who were overweight and 0.78 (95% CI: 0.70, 0.88) in adults who were obese.
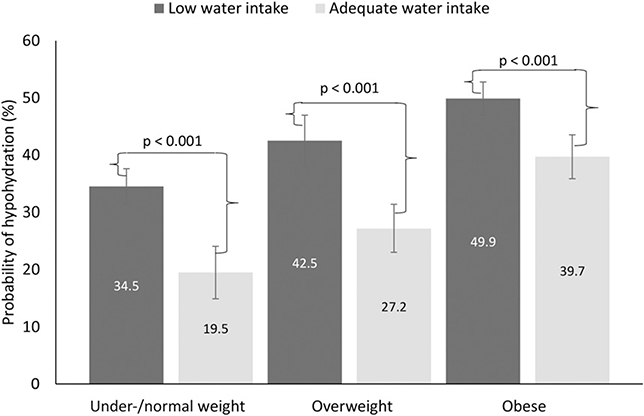
Figure 2 shows the predicted probability of hypohydration for adults by water intake status across weight-status categories with adjustment for the distribution of the covariates and the interaction between water intake and weight status. Three primary results are worth noting from this figure. First, adults who had adequate water intake had a significantly lower prevalence of hypohydration than that of their peers in the same weight-status category with low water intake (all P < 0.001). Second, the prevalence of hypohydration increases by weight-status category for both adults who had adequate water intake and adults who had low water intake. Third, the absolute (and proportional) difference in the probability of hypohydration between adults with adequate water intake and those with low water intake on a given day was smaller in adults who were obese (10.2 percentage points) than in overweight adults (15.3 percentage points) or normal or underweight adults (15.0 percentage points). That is, adequate water intake appeared to have a smaller impact on lowering the probability of hypohydration in adults who were obese than in adults in other weight-status categories.
FIGURE 2.
Predicted probabilities (95% CIs) of hypohydration by water intake status and weight status in adults aged ≥20 y in the United States, 2009–2012. n = 9601. Data were generated with the use of marginal standardization from the log-binomial regression model that included an interaction between weight status and water intake status (F = 4.78, P = 0.015). BMI (in kg/m2) categories were as follows: underweight or normal weight, <25; overweight, 25 to <30; and obese, ≥30. Low water intake (dark gray bars) was defined as follows: men, <3.7 L; women, <2.7 L; and adequate water intake (light gray bars) was defined as follows: men, ≥3.7 L; women, ≥2.7 L. Data source: CDC/National Center for Health Statistics, NHANES (26).
Sensitivity analyses supported the main analysis. When a uniform cutoff for hypohydration ≥831 mOsm/kg for all adults was applied, the prevalence ± SE of hypohydration decreased to 24.5% ± 0.7% for the population, 19.4% ± 1.2% for adults who were underweight or normal weight, 23.9% ± 1.5% for adults who were overweight, and 29.5% ± 1.0% for adults who were obese. Nevertheless, when re-estimating the analyses presented in Table 3 (models 4–6) with the use of the uniform hypohydration definition, the log-binomial regression results were consistent with the primary analyses (results not shown).
When we examined urine osmolality and the prevalence of hypohydration by the time of examination (morning, afternoon, or evening) to see whether the time of examination influenced the results, the same trend of increasing urine osmolality and the prevalence of hypohydration by weight status was observed (Supplemental Table 1). Moreover, urine osmolality did not differ significantly by the time of examination for each weight-status category. When adults with diabetes (n = 8197) were excluded, instead of controlling for this condition in the regression models, all results were consistent with the primary analyses (results not shown).
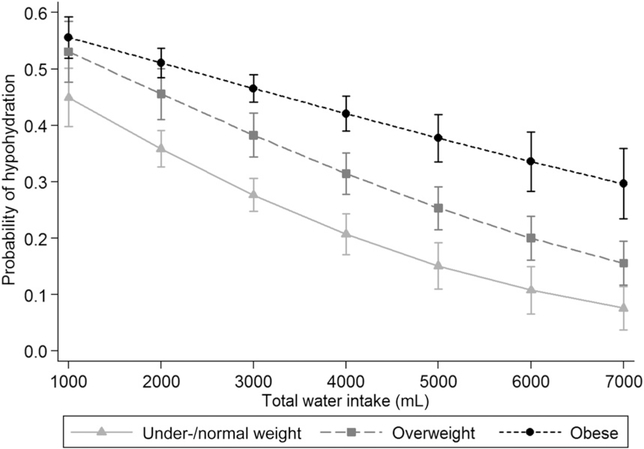
Finally, we re-estimated the models with total water intake as a continuous variable and generated predicted margins to illustrate the differences across a range of 1000–7000 mL total water intake (which represented 95% of the range of water intake reported by adults). Figure 3 and Supplemental Figure 1 illustrate that, as total water intake increased, the difference in the probability of hypohydration and urine osmolality values between adults who were underweight or normal weight and adults who were obese also increased. At 1000 mL water intake, we showed a difference of 10.7 percentage points between adults who were underweight or normal weight and adults who were obese with predicted probabilities of 44.9% and 55.6%, respectively (Figure 3). The difference in the probability of hypohydration between adults who were underweight or normal weight and adults who were obese increased to 22.8 percentage points (10.8% compared with 33.6%) at 6000 mL.
FIGURE 3.
Predicted probabilities (95% CIs) of hypohydration by weight status across 95% of the range of water intake that was reported in adults aged ≥20 y in the United States, 2009–2012. n = 9601. Data were generated with the use of marginal standardization from the sensitivity analysis (results not shown) that included an interaction between weight status and total water intake (F = 5.37, P = 0.01). BMI (in kg/m2) categories were as follows: underweight or normal weight, <25; overweight, 25 to <30; and obese, ≥30. Data source: CDC/National Center for Health Statistics, NHANES (26).
DISCUSSION
In this observational study of US adults in 2009–2012, the relation between water intake and hydration status was modified by weight status. When stratified by weight status, the association was attenuated in obese individuals whereby higher water intake was less protective against hypohydration in adults who were obese than in adults who were underweight or normal weight.
Overall, the prevalence of hypohydration was 37.6% in US adults and 45.7% in adults who were obese compared with 28.5% in adults who were underweight or normal weight. These estimates were all lower when we applied a uniform(not age-adjusted) cutoff for hypohydration. Although the cutoff of ≥831 mOsm/kg to classify hypohydration was shown to have a sensitivity and specificity of 91% from a prospective receiver operating curve experiment, the subjects who were enrolled in the study were all young adult soldiers and first morning urine samples were used, and thus, the issue of age did not arise (32). Although our estimates of the prevalence of hypohydration on the basis of urine osmolality appeared to be high, they were lower than estimates from the NHANES III, which showed that 60% of adults had borderline elevated or high plasma tonicity (49). The estimates of mean urine osmolality (613.8 mOsm/kg) from the current study were similar to the NHANES III estimates and those of a large survey of German adults (13, 50).
Previous studies have shown an association between obesity and hydration status in adults with the use of various indicators of hydration, but the studies did not examine whether an interaction existed with total water intake (23, 24, 51). Stookey et al. (24)., with the use of NHANES III (1988–1994) data, showed that US adults who were overweight and obese had significantly higher plasma tonicity as well as an altered body fluid distribution compared with adults who were normal weight. Adults who were obese were observed to have a higher extracellular fluid–to–intracellular fluid ratio than that of lean adults (24). Weight-status categories have also been associated with urine osmolality but were treated as a confounder (52). Another study showed higher plasma osmolality values in adults who were obese as well as higher body temperatures and reduced sweat rates, thereby suggesting that osmoregulation (the maintenance of water balance) and thermoregulation (the maintenance of a steady body temperature) may be competing homeostatic processes (23).
The current study extends the previous findings by illustrating that the relation between water intake and urine osmolality and hypohydration varies by weight status. A recent study showed that significant differences in urine osmolality and the likelihood of dehydration between children who were obese and normal-weight children were due to differences in the amount of fluid consumed (53).However, our results suggest that obese adults, on average, may need to consume relatively more water to experience hydration gains that are equivalent to those of adults who are underweight or normal weight. For example, at a water intake of 3 L, the probability of hypohydration for an underweight or normal-weight adult was 27.6%. At the same water intake, the probability of hypohydration was 46.6% for an obese adult. However, at a water intake of 4 L, which is greater than the AI for men and women, the probability of hypohydration for an obese adult decreased only to 42.1%.
This study is subject to a few limitations. As a cross-sectional study, causality could not be inferred, and all relations should be viewed as associations. For logistic purposes, NHANES samples in Northern regions of the US more frequently during the summer and in Southern regions during the winter. This seasonal sampling pattern may have increased the exposure to warmer temperatures overall in the sample, which is associated with increased risk of dehydration (54). The analysis used a single spot urine sample, which may not have been indicative of 24-h urine osmolality because urine osmolality changes throughout the day as food and water are consumed (40).We attempted to control for this possibility by adjusting for the time of the participant’s examination, but we acknowledge that spot urine samples are not representative of 24-h urine samples. Previous research has shown that late-afternoon spot samples can approximate 24-h urine osmolality (48). Sensitivity analyses (Supplemental Table 1) showed only minimal differences in the mean urine osmolality for morning, noon, and early evening sessions overall (620 compared with 600 compared with 620 mOsm/kg, respectively) or within each weight-status category by examination time, thereby suggesting that time of examination did not influence the observed relation. However, we did show the same significant trend of higher urine osmolality and prevalence of hypohydration with increasing weight-status categories for each examination time period. The use of a single 24-h dietary recall does not provide an estimate of usual water intake but, instead, provides an estimate of water intake on a given day. Nevertheless, with the use of the 24-h dietary recall for the day that preceded the urine sample was appropriate because the foods and drinks consumed would have directly influenced hydration status. Finally, physical activity was assessed for a typical week, which may not have accurately reflected activity patterns in the previous 24 h.
This study has several strengths. The current study contributes to the literature as one of few nationally representative studies in US adults that have examined the association between weight status, total water intake, and urine osmolality. Previous research has shown that older adults are more vulnerable to dehydration (6, 49); however, they also lose some ability to concentrate urine as they age, which means that their mean urine osmolality will be lower than that of younger adults (31). Therefore, the use of any uniform cutoff could result in the misclassification of hydration status of older adults. For this reason, we used an age-dependent cutoff for hypohydration. Finally, the results were robust to many alternate specifications of the models, including restricting the sample to adults without diabetes and estimating the association with the use of urine osmolality and total water intake as continuous and dichotomous variables.
In conclusion, dehydration is associated with increased risk of kidney stones as well as decreases in physiologic, emotional, and cognitive functioning (2, 3, 6, 7). The current study extends this important aspect of human nutrition by showing that the relation between water intake and urine osmolality is modified by weight status in US adults. Adults who are obese are more likely to be in a hypohydrated state, and the association between increased water intake and hypohydration is weaker in adults who are obese than in adults who are underweight or normal weight.
Supplementary Material
Footnotes
The authors reported no funding received for this study
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Center for Health Statistics, CDC
Supplemental Table 1 and Supplemental Figure 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://ajcn.nutrition.org
The authors’ responsibilities were as follows—AYR: drafted the manuscript; AYR, HGL, and LJA: analyzed the data; AYR, LJA, and CLO: designed the research; and all authors: edited and revised the manuscript, had responsibility for the final content of the manuscript, and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
REFERENCES
- 1.Murray B Hydration and physical performance. J Am Coll Nutr 2007; 26(5 Suppl):542S–8S. [DOI] [PubMed] [Google Scholar]
- 2.Ganio MS, Armstrong LE, Casa DJ, McDermott BP, Lee EC, Yamamoto LM, Marzano S, Lopez RM, Jimenez L, Le Bellego L, et al. Mild dehydration impairs cognitive performance and mood of men. Br J Nutr 2011;106:1535–43. [DOI] [PubMed] [Google Scholar]
- 3.Grandjean AC, Grandjean NR. Dehydration and cognitive performance. J Am Coll Nutr 2007;26(5 Suppl):549S–54S. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong LE, Ganio MS, Casa DJ, Lee EC, McDermott BP, Klau JF, Jimenez L, Le Bellego L, Chevillotte E, Lieberman HR. Mild dehydration affects mood in healthy young women. J Nutr 2012;142:382–8. [DOI] [PubMed] [Google Scholar]
- 5.Bear T, Philipp M, Hill S, Mündel T. A preliminary study on how hypohydration affects pain perception. Psychophysiology 2016;53:605–10. [DOI] [PubMed] [Google Scholar]
- 6.Popkin BM, D’Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev 2010;68:439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawka MN, Montain SJ, Latzka WA. Hydration effects on thermoregulation and performance in the heat. Comp Biochem Physiol A Mol Integr Physiol 2001;128:679–90. [DOI] [PubMed] [Google Scholar]
- 8.Benton D, Jenkins KT, Watkins HT, Young HA. Minor degree of hypohydration adversely influences cognition: a mediator analysis. Am J Clin Nutr 2016;104:603–12. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong L, Johnson E, McKenzie A, Muñoz C. An empirical method to determine inadequacy of dietary water. Nutrition 2016;32:79–82. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong LE, Pumerantz AC, Fiala KA, Roti MW, Kavouras SA, Casa DJ, Maresh CM. Human hydration indices: acute and longitudinal reference values. Int J Sport Nutr Exerc Metab 2010;20:145–53. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong LE. Assessing hydration status: the elusive gold standard. J Am Coll Nutr 2007;26(5 Suppl):575S–84S. [DOI] [PubMed] [Google Scholar]
- 12.Hooper L, Bunn DK, Abdelhamid A, Gillings R, Jennings A, Maas K, Millar S, Twomlow E, Hunter PR, Shepstone L, et al. Water-loss (intracellular) dehydration assessed using urinary tests: how well do they work? Diagnostic accuracy in older people. Am J Clin Nutr 2016;104:121–31. [DOI] [PubMed] [Google Scholar]
- 13.Manz F, Wentz A. Hydration status in the United States and Germany. Nutr Rev 2005;63(Suppl 1):S55–62. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong LE, Johnson EC, Munoz CX, Swokla B, Le Bellego L, Jimenez L, Casa DJ, Maresh CM. Hydration biomarkers and dietary fluid consumption of women. J Acad Nutr Diet 2012;112:1056–61. [DOI] [PubMed] [Google Scholar]
- 15.Manz F, Wentz A. 24-h hydration status: parameters, epidemiology and recommendations. Eur J Clin Nutr 2003;57(Suppl 2):S10–8. [DOI] [PubMed] [Google Scholar]
- 16.Sawka MN, Cheuvront SN, Carter R. Human water needs. Nutr Rev 2005;63(Suppl 1):S30–9. [DOI] [PubMed] [Google Scholar]
- 17.Rosinger A, Tanner S. Water from fruit or the river? Examining hydration strategies and gastrointestinal illness among Tsimane’ adults in the Bolivian Amazon. Public Health Nutr 2015;18:1098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ventura Marra M, Warren AL, Hollingsworth EK, Long E, Shotwell MS, Simmons SF, Silver HJ. High body mass index does not protect long term care residents from impaired hydration status, inadequate fluid intake or dehydration risk. J Acad Nutr Diet 2015;115(9 Suppl):A16. [Google Scholar]
- 19.Ogden C, Carroll M, Fryar C, Flegal K. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data brief. Hyattsville (MD): National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 20.Eckel RH, Krauss RM. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. Circulation 1998;97:2099–100. [DOI] [PubMed] [Google Scholar]
- 21.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289:76–9. [DOI] [PubMed] [Google Scholar]
- 22.Wilson PW D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002;162:1867–72. [DOI] [PubMed] [Google Scholar]
- 23.Kanikowska D, Sato M, Sugenoya J, Shimizu Y, Nishimura N, Inukai Y, Iwase S. Attenuated thermoregulatory responses with increased plasma osmolality in obese subjects during two seasons. Int J Biometeorol 2013;57:663–7. [DOI] [PubMed] [Google Scholar]
- 24.Stookey JD, Barclay D, Arieff A, Popkin BM. The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur J Clin Nutr 2007;61:190–9. [DOI] [PubMed] [Google Scholar]
- 25.Lafontan M H4H - hydration for health. Obes Facts 2014;7(Suppl 2):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics CDC. NHANES - questionnaires, datasets, and related documentation [Internet]. [cited 2015 Oct 30]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 27.Zipf G, Chiappa M, Porter K, et al. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 2013;56:1–37. [PubMed] [Google Scholar]
- 28.National Center for Health Statistics CDC. NHANES Response Rates and Population Totals [Internet]. [cited 2015 Oct 30]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm.
- 29.Baron S, Courbebaisse M, Lepicard EM, Friedlander G. Assessment of hydration status in a large population. Br J Nutr 2015;113:147–58. [DOI] [PubMed] [Google Scholar]
- 30.Perrier E, Vergne S, Klein A, Poupin M, Rondeau P, Le Bellego L, Armstrong LE, Lang F, Stookey J, Tack I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br J Nutr 2013;109:1678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindeman RD, Van Buren HC, Raisz LG. Osmolar renal concentrating ability in healthy young men and hospitalized patients without renal disease. N Engl J Med 1960;262:1306–9. [DOI] [PubMed] [Google Scholar]
- 32.Cheuvront SN, Ely BR, Kenefick RW, Sawka MN. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr 2010;92:565–73. [DOI] [PubMed] [Google Scholar]
- 33.Cheuvront SN, Kenefick RW, Charkoudian N, Sawka MN. Physiologic basis for understanding quantitative dehydration assessment. Am J Clin Nutr 2013;97:455–62. [DOI] [PubMed] [Google Scholar]
- 34.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington (DC): National Academies Press; 2004. [Google Scholar]
- 35.Expert Panel on the Identification Evaluation and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr 1998;68:899–917. [DOI] [PubMed] [Google Scholar]
- 36.Killer SC, Blannin AK, Jeukendrup AE. No evidence of dehydration with moderate daily coffee intake: A counterbalanced cross-over study in a free-living population. PLoS One 2014;9:e84154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva AM, Júdice PB, Matias CN, Santos DA, Magalhães JP, St-Onge M-P, Gonçalves EM, Armada-da-Silva P, Sardinha LB. Total body water and its compartments are not affected by ingesting a moderate dose of caffeine in healthy young adult males. Appl Physiol Nutr Metab 2013;38:626–32. [DOI] [PubMed] [Google Scholar]
- 38.Stookey JD. The diuretic effects of alcohol and caffeine and total water intake misclassification. Eur J Epidemiol 1999;15:181–8. [DOI] [PubMed] [Google Scholar]
- 39.Eisenhofer G, Johnson RH. Effect of ethanol ingestion on plasma vasopressin and water balance in humans. Am J Physiol 1982;242:R522–7. [DOI] [PubMed] [Google Scholar]
- 40.Perrier E, Demazières A, Girard N, Pross N, Osbild D, Metzger D, Guelinckx I, Klein A. Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur J Appl Physiol 2013;113:2143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health 2006;14:66–70. [Google Scholar]
- 42.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee report, 2008. Washington (DC): US Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 43.The International Expert Committee. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk? Int J Public Health 2008;53:165–7. [DOI] [PubMed] [Google Scholar]
- 45.Wooldridge JM. Introductory econometrics: a modern approach. Mason (OH): Nelson Education; 2015. [Google Scholar]
- 46.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014;43:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korn EL, Graubard BI. Predictive margins (direct standardization) In: Analysis of health surveys. New York: John Wiley & Sons, Inc.; 1999. p. 126–40. [Google Scholar]
- 48.Bottin JH, Lemetais G, Poupin M, Jimenez L, Perrier ET. Equivalence of afternoon spot and 24-h urinary hydration biomarkers in free-living healthy adults. Eur J Clin Nutr 2016;70:904–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stookey JD. High prevalence of plasma hypertonicity among community-dwelling older adults: results from NHANES III. J Am Diet Assoc 2005;105:1231–9. [DOI] [PubMed] [Google Scholar]
- 50.Manz F, Johner SA, Wentz A, Boeing H, Remer T. Water balance throughout the adult life span in a German population. Br J Nutr 2012; 107:1673–81. [DOI] [PubMed] [Google Scholar]
- 51.Chang T, Ravi N, Plegue MA, Sonneville KR, Davis MM. Inadequate hydration, BMI, and obesity among US Adults: NHANES 2009–2012. Ann Fam Med 2016;14:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeh H-C, Lin Y-S, Kuo C-C, Weidemann D, Weaver V, Fadrowski J, Neu A, Navas-Acien A. Urine osmolality in the US population: implications for environmental biomonitoring. Environ Res 2015;136:482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maffeis C, Tommasi M, Tomasselli F, Spinelli J, Fornari E, Scattolo N, Marigliano M, Morandi A. Fluid intake and hydration status in obese vs normal weight children. Eur J Clin Nutr 2016;70:560–5. [DOI] [PubMed] [Google Scholar]
- 54.Rosinger A Heat and hydration status: predictors of repeated measures of urine specific gravity among Tsimane’ adults in the Bolivian Amazon. Am J Phys Anthropol 2015;158:696–707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.