Abstract
Chlamydiosis is the most significant infectious disease of koalas (Phascolarctos cinereus). It is primarily a systemic sexually transmitted disease caused by Chlamydia pecorum and was responsible for 46% of the 2348 koala admissions to Australia Zoo Wildlife Hospital between 2013 and 2017. Treatment of chlamydiosis in koalas is complicated by three major factors. Firstly, koalas rely on bacterial fermentation of their high fibre diet making antibiotic therapy a risk. Secondly, they possess efficient metabolic pathways for the excretion of plant toxins and potentially of therapeutic agents. Thirdly, wild koalas, often present to rehabilitation facilities with chronic and severe disease. Traditional anti-chlamydial antibiotics used in other species may cause fatal dysbiosis in koalas or be excreted before they can be effective. We compared five anti-chlamydial antibiotics, azithromycin, chloramphenicol, doxycycline, enrofloxacin and florfenicol, which were used to treat 86 wild koalas with chlamydiosis presented to Australia Zoo Wildlife Hospital under consistent conditions of nutrition, housing, husbandry and climate. Response to treatment was assessed by recovery from clinical signs, and clearance of detectable Chlamydia via quantitative PCR. Doxycycline was the most effective anti-chlamydial antibiotic of the five, producing a 97% cure rate, followed by chloramphenicol (81%), enrofloxacin (75%), florfenicol (66%) and azithromycin (25%). The long-acting injectable preparation of doxycycline was well tolerated by koalas when administered via the subcutaneous route, and the weekly dosing requirement is a major advantage when treating wild animals. These findings indicate that doxycycline is the current drug of choice for the treatment of chlamydiosis in koalas, with chloramphenicol being the best alternative.
Introduction
Chlamydiosis is the most significant infectious disease of koalas (Phascolarctos cinereus), with a prevalence of up to 100% in some wild populations [1–3]. The major clinical signs are conjunctivitis and a brown-stained wet rump caused by chronic cystitis (Fig 1). Epidemics of ocular disease in koalas have been reported since the early 1900s [4]. In 1974 Chlamydia psittaci was first linked to koala keratoconjunctivitis [5] and soon after as the cause of urogenital disease in koalas [6]. In 1995 the causative organism of chlamydiosis in koalas was reclassified as two species: Chlamydia pecorum and Chlamydia pneumoniae [7].
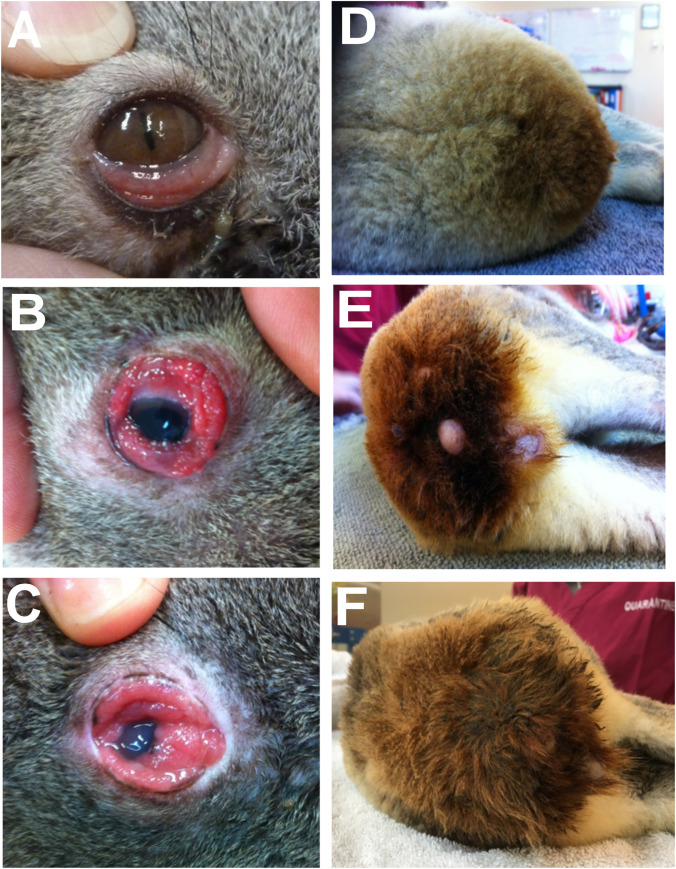
Fig 1. Primary clinical signs of chlamydiosis in koalas.
(A-C) Mild, moderate and severe chlamydial conjunctivitis and (D-F) mild, moderate and severe urine staining of the rump fur caused by chronic dribbling of urine due to chlamydial cystitis. Equivalent to Grades 1–3.
Since the aetiology was confirmed, thousands of koalas have been treated for chlamydiosis with a range of antibiotics. As in other species, antibiotic choice is an evidence-based decision based on knowledge of pathogen sensitivity, generic and species-specific adverse effects, metabolism and excretion pathways, and the required route of administration, frequency and duration of therapy. In addition, knowledge of the pharmacokinetics of various antibiotics in koalas provides information on drug metabolism that supports decisions on dose, route, and frequency of administration. Species-specific metabolic pathways in wildlife can alter treatment regimens from those developed for humans or domestic species. Response to treatment is assessed by improvement in clinical signs, and a lack of detectable infectious organism post-treatment.
Although described as easy to treat in humans [8], treatment of chlamydiosis in wild koalas is complicated by the chronic nature of their disease at presentation and by their hindgut fermentative digestion. Some antibiotics such as oxytetracycline, which are effective against chlamydia in other species, have caused fatal dysbiosis (alteration in the caecal microbiome) due to their effect on vital tannin-protein complex-degrading enterobacteria (T-PCDE) [9]. Since the late 1980s, antibiotics such as enrofloxacin and chloramphenicol have been used with clinical success to treat chlamydiosis in koalas, while avoiding fatal impacts on gut flora. Since 2010, pharmacokinetic studies in koalas of some commonly used anti-chlamydial antibiotics have been undertaken [10–12], resulting in widespread use of chloramphenicol to treat chlamydiosis in koalas, however this drug is no longer commercially available. The reduced availability of chloramphenicol, and the evidence of occasional bone marrow hypoplasia in koalas treated with prolonged courses of this antibiotic led to this evaluation of alternative anti-chlamydial antibiotics in koalas.
The aim of this study was to compare the efficacy of five anti-chlamydial antibiotics to find the ideal therapeutic agent for wild koalas with the intention to: 1) produce a high microbial cure rate (˃90%); 2) be readily available and inexpensive; 3) cause minimal adverse effects; 4) not cause pain on injection; 5) be available in concentrations that allow small injection volumes; 6) be effective following subcutaneous or intramuscular injection; and 7) require infrequent dosing.
Materials and methods
Study location
The study was conducted at Australia Zoo Wildlife Hospital (AZWH), Beerwah, Queensland. The purpose of the hospital is to treat sick, injured and orphaned wildlife with the goal of returning animals to the wild fit, healthy and capable of reproducing. The study was undertaken in a clinical setting where animal welfare takes precedence over other considerations.
Animals and allocation to treatment
All koalas presented to AZWH with ocular or urogenital signs of chlamydiosis from June 2016 to June 2017 were assessed for inclusion in this study. A total of 86 koalas, 48 males and 38 females, were suitable for inclusion based on high probability of recovery. Koalas being treated ranged in age between 1 and 10 years, as determined by tooth wear class [13]. Eligible animals were assigned to a treatment group on the basis of temperament, disease severity, body condition and history of previous antibiotic treatment. Of the 86 koalas treated, 6 required re-treatment, bringing the total number of overall antibiotic treatments to 92 (Table 1). Anxious or aggressive koalas were treated with antibiotics requiring less frequent administration. Koalas which had been treated and released previously and returned with clinical signs of chlamydiosis were not treated again with chloramphenicol. All koalas were treated and assessed at AZWH under consistent conditions of husbandry and nutrition. Koala which recovered were released within their prescribed habitat (within 5 km of point of rescue) (Table 1).
Table 1. Antibiotic, sex, age, date of admission and outcome of treated koalas.
| Antibiotic | Koala Accession # | Male/Female | Koala Age in years | Admission Date | Outcome |
|---|---|---|---|---|---|
| Azithromycin | 65306 | Male | 6 | 4/18/2016 | Released |
| Azithromycin | 65647 | Female | 6 | 5/13/2016 | Deceased |
| Azithromycin | 65707 | Male | 10 | 6/25/2016 | Deceased |
| Azithromycin | 66116 | Male | 6 | 6/18/2016 | Deceased |
| Chloramphenicol | 69720 | Male | 4 | 1/2/2017 | Released |
| Chloramphenicol | 67376 | Female | 3 | 9/23/2016 | Released |
| Chloramphenicol | 63513 | Female | 1 | 1/17/2016 | Deceased |
| Chloramphenicol | 65391 | Female | 5 | 4/26/2016 | Re-treated |
| Chloramphenicol | 65704 | Female | 10 | 5/20/2016 | Deceased |
| Chloramphenicol | 66239 | Female | 5 | 7/1/2016 | Released |
| Chloramphenicol | 67538 | Male | 4 | 9/30/2016 | Released |
| Chloramphenicol | 68349 | Male | 10 | 11/10/2016 | Released |
| Chloramphenicol | 68350 | Female | 7 | 11/10/2016 | Deceased |
| Chloramphenicol | 68352 | Male | 3 | 11/10/2016 | Released |
| Chloramphenicol | 68381 | Male | 5 | 11/11/2016 | Released |
| Chloramphenicol | 68856 | Female | 4 | 11/24/2016 | Released |
| Chloramphenicol | 68857 | Male | 7 | 11/24/2016 | Released |
| Chloramphenicol | 68858 | Male | 3 | 11/24/2016 | Released |
| Chloramphenicol | 68865 | Female | 3 | 11/24/2016 | Released |
| Chloramphenicol | 69013 | Female | 1 | 12/1/2016 | Released |
| Chloramphenicol | 69030 | Female | 7 | 12/1/2016 | Deceased |
| Chloramphenicol | 69480 | Male | 3 | 12/20/2016 | Released |
| Chloramphenicol | 69505 | Female | 10 | 12/22/2016 | Released |
| Chloramphenicol | 69716 | Female | 12 | 1/2/2017 | Released |
| Chloramphenicol | 69718 | Female | 5 | 1/2/2017 | Released |
| Chloramphenicol | 69719 | Female | 4 | 1/2/2017 | Released |
| Chloramphenicol | 69722 | Female | 4 | 1/2/2017 | Released |
| Chloramphenicol | 69798 | Male | 8 | 1/6/2017 | Released |
| Chloramphenicol | 69958 | Female | 7 | 1/9/2017 | Released |
| Chloramphenicol | 70139 | Male | 10 | 1/17/2017 | Deceased |
| Chloramphenicol | 70468 | Female | 5 | 2/3/2017 | Released |
| Chloramphenicol | 70678 | Male | 3 | 2/17/2017 | Deceased |
| Chloramphenicol | 71766 | Male | 3 | 4/23/2017 | Deceased |
| Chloramphenicol | 70908 | Female | 1 | 3/2/2017 | Released |
| Chloramphenicol | 71632 | Male | 4 | 4/21/2017 | Released |
| Doxycycline | 65391 | Female | 5 | 4/26/2016 | Recovered |
| Doxycycline | 65741 | Female | 7 | 5/24/2016 | Released |
| Doxycycline | 66415 | Male | 6 | 7/15/2016 | Deceased |
| Doxycycline | 66517 | Male | 3 | 7/27/2016 | Released |
| Doxycycline | 66991 | Female | 2 | 8/14/2016 | Released |
| Doxycycline | 67684 | Male | 7 | 10/6/2016 | Deceased |
| Doxycycline | 67696 | Male | 5 | 10/6/2016 | Released |
| Doxycycline | 67784 | Male | 4 | 10/13/2016 | Released |
| Doxycycline | 67967 | Female | 3 | 10/22/2016 | Deceased |
| Doxycycline | 68006 | Male | 7 | 10/24/2016 | Released |
| Doxycycline | 68234 | Female | 3 | 11/4/2016 | Released |
| Doxycycline | 68250 | Male | 7 | 11/5/2016 | Recovered |
| Doxycycline | 68299 | Male | 5 | 11/7/2016 | Deceased |
| Doxycycline | 68333 | Male | 4 | 11/9/2016 | Released |
| Doxycycline | 68334 | Female | 3 | 11/9/2016 | Released |
| Doxycycline | 68335 | Male | 3 | 11/9/2016 | Released |
| Doxycycline | 68757 | Male | 4 | 11/21/2016 | Recovered |
| Doxycycline | 68788 | Male | 5 | 11/22/2016 | Recovered |
| Doxycycline | 69293 | Male | 12 | 12/12/2016 | Recovered |
| Doxycycline | 69505 | Female | 10 | 12/22/2016 | Released |
| Doxycycline | 69908 | Female | 4 | 1/6/2017 | Released |
| Doxycycline | 70024 | Female | 9 | 1/13/2017 | Released |
| Doxycycline | 70195 | Female | 3 | 1/20/2017 | Released |
| Doxycycline | 70718 | Female | 4 | 2/21/2017 | Recovered |
| Doxycycline | 71123 | Male | 5 | 3/14/2017 | Released |
| Doxycycline | 71126 | Male | 7 | 3/14/2017 | Released |
| Doxycycline | 71232 | Male | 1.5 | 3/18/2017 | Released |
| Doxycycline | 71988 | Female | 3 | 5/7/2017 | Deceased |
| Doxycycline | 72021 | Male | 5 | 5/9/2017 | Released |
| Doxycycline | 72246 | Male | 3 | 5/27/2017 | Released |
| Doxycycline | 72296 | Female | 3 | 5/31/2017 | Deceased |
| Doxycycline | 69720 | Male | 4 | 1/2/2017 | Released |
| Enrofloxacin | 63513 | Female | 1 | 1/17/2016 | Re-treated |
| Enrofloxacin | 64241 | Male | 4 | 2/7/2016 | Released |
| Enrofloxacin | 64653 | Female | 2 | 2/29/2016 | Released |
| Enrofloxacin | 65147 | Male | 8 | 4/7/2016 | Released |
| Enrofloxacin | 65306 | Male | 6 | 4/18/2016 | Re-treated |
| Enrofloxacin | 65321 | Male | 7 | 4/19/2016 | Released |
| Enrofloxacin | 65375 | Male | 3 | 4/24/2016 | Released |
| Enrofloxacin | 65491 | Male | 9 | 4/30/2016 | Released |
| Enrofloxacin | 65651 | Male | 3 | 5/13/2016 | Released |
| Enrofloxacin | 65704 | Female | 10 | 5/20/2016 | Re-treated |
| Enrofloxacin | 66008 | Male | 12 | 4/20/2016 | Released |
| Enrofloxacin | 70048 | Male | 5 | 1/14/2017 | Euthanised |
| Enrofloxacin | 70195 | Female | 3 | 1/20/2017 | Released |
| Enrofloxacin | 70508 | Female | 8 | 2/6/2017 | Released |
| Enrofloxacin | 70961 | Female | 4 | 3/5/2017 | Recovered |
| Enrofloxacin | 71123 | Male | 5 | 3/14/2017 | Re-treated |
| Florfenicol | 66801 | Female | 5 | 8/20/2016 | Deceased |
| Florfenicol | 67156 | Male | 5 | 9/11/2016 | Deceased |
| Florfenicol | 67227 | Male | 3 | 9/12/2016 | Deceased |
| Florfenicol | 67285 | Female | 4 | 9/17/2016 | Deceased |
| Florfenicol | 67384 | Male | 6 | 9/24/2016 | Deceased |
| Florfenicol | 67572 | Male | 6 | 10/2/2016 | Released |
| Florfenicol | 67703 | Female | 4 | 10/7/2016 | Deceased |
| Florfenicol | 67784 | Male | 4 | 10/13/2016 | Released |
| Florfenicol | 67376 | Female | 3 | 9/23/2016 | Re-treated |
Re-treated animals had a positive PCR at the end of treatment with antibiotic one and required treatment with a second antibiotic to clear their chlamydial infections.
Recovered animals that were not released had insufficient vision at the end of treatment due to chronic corneal scarring caused by the Chlamydial infection.
Deceased animals were not necessarily deceased due to antibiotic side effects, as chronic Chlamydial disease is extremely debilitating.
Animals which exhibited pain on urination during the treatment trial were additionally treated with analgesics and/or anti-inflammatory drugs as indicated. This included Temgesic® (Indivior: buprenorphine 300ug/kg @ 0.01 mg/kg I/M every 8 hours as required), Rimadyl® (Zoetis: Carprofen 50 mg/ml @ 4 mg/kg loading dose followed by 2 mg/kg S/C SID for 5–7 days), or Pentosan® (Ceva: Pentosan Polysulphate 100 mg/ml @ 3 mg/kg S/C every 7 days x 4).
Koalas which did not respond to treatment, had life threatening side effects, or developed life threatening issues unrelated to chlamydiosis treatment were euthanised by sedating with intramuscular Alfaxan® (Jurox: alfaxalone 10 mg/ml) and then euthanased with intravenous Lethabarb® (Virbac: pentobarbitone 325 mg/ml) (Table 1).
Wild animals are cared for under a Department of Environment and Heritage Protection Rehabilitation permit WIRP18601117 and Department of Agriculture and Fisheries Scientific Purposes permit SUR000234. All samples were collected during normal clinical protocols.
Selection of antibiotics and treatment regimens
The antibiotics and treatment regimens used in this study were based on recommendations for treating chlamydiosis in koalas, or domestic species and humans, if no precedent existed (Table 2).
Table 2. Treatment regimens for assessed anti-chlamydial antibiotics in koalas.
| Antibiotic (Manufacturer) | Concentration | Dose rate | Frequency | Route | Duration | Comments | Ref |
|---|---|---|---|---|---|---|---|
| Azithromycin (Pfizer) | 100 mg/mL | 20 mg/kg | SID | IV | 3 days | Diluted to a 2 mg/mL solution in sterile 0.9% saline and infused over 30 minutes in sedated koalas. Child dose rate. | [14] |
| Chloramphenicol (Ceva) | 150 mg/mL | 60 mg/kg | SID | S/C | 28 days | Ready to use; rotated between sites; dose rate previously used to successfully treat chlamydiosis in koalas. | [15] |
| Doxycycline LA (Vetafarm) | 50 mg/mL | 5 mg/kg | Every 7 days | IM or S/C | 28 days | Diluted 50:50 in saline; less tissue reaction if given s/c, rotated between sites; small animal dose rates. | [16] |
| Enrofloxacin (Bayer) | 50 mg/mL | 10 mg/kg loading dose; then 5 mg/kg | SID | S/C | 28 days | Diluted 50:50 in saline; rotated between sites; small animal dose rates. | [17] |
| Florfenicol (Merck) | 300 mg/mL | 20 mg/kg | Every 2 days | S/C | 28 days | Ready to use, domestic animal dose rate. | [18] |
LA = long-acting formulation; SID = once daily, S/C = Subcutaneous, IV = Intravenous, IM = Intramuscular.
Topical treatment of conjunctivitis
Chlamydiosis is a systemic infection and conjunctivitis responds to systemic antibiotic treatment with or without topical treatment. Particularly during the first week of treatment, ocular therapeutic support is recommended. During this trial ocufloxacin eye drops (Ocuflox®) and chloramphenicol eye ointment were used in conjunction with systemic antibiotic therapy. Anti-inflammatory therapy with dexamethasone (Maxidex®) or Chloroptosone ointment (provided corneal ulceration had been excluded) or with ketorolac tromethamine (Acular®) is also beneficial when inflammation is severe. Subconjunctival injection with a long-acting corticosteriod (Depo-Medrol®) proved useful to reduce severe hyperplastic conjunctivitis in chronic cases, and judicious surgical debridement of excessive proliferative tissue at the end of systemic treatment is occasionally indicated.
Clinical assessment and sample collection
Each koala received a full clinical examination under isoflurane anaesthesia on admission to AZWH. This examination included monitoring temperature, heart rate and respiration; measuring packed cell volume and total protein of a blood sample; ultrasound evaluation of the reproductive and urinary tracts; microscopic evaluation of an abdominal paracentesis sample; and other diagnostic tests as indicated during the examination. Clinical signs of chlamydiosis were graded as mild, moderate or severe on the basis of the examination and clinical progress was then evaluated daily. Copan® 160C aluminium shaft minitip dry swabs were used to collect epithelial cells from the conjunctival, urogenital tract and rectum of koalas for quantification of the chlamydial load via qPCR. Prior to June 2016, Clearview® tests were used to confirm Chlamydial infections. Swab samples were collected at time of admission (time–point 0), and at the end of treatment. Swab samples were stored frozen at -20°C until analysed. All koalas received an end of treatment visual check and a full clinical examination under anaesthesia 3 weeks after the end of treatment to collect post treatment PCR swabs and carry out pre-release procedures (tagging and microchipping). End of treatment sample collection at 3 weeks post treatment was selected as this is the optimal time in humans to avoid false positives from residual nucleic acid fragments [14]. Koalas received an additional evaluation under anaesthetic whenever there was a clinical concern during treatment.
Bodyweight
All koalas were weighed during their initial examination and at regular intervals throughout treatment. In uncomplicated cases the frequency of weight measurements was every 7 days, or up to daily if there were clinical concerns during treatment. Body weight for each individual was charted to monitor trends during treatment.
DNA extraction and Chlamydia pecorum quantification
Swab samples collected from the ocular, urogenital and rectal sites of koalas, were thawed at room temperature and added to 1.5 mL sucrose phosphate glutamate at pH 7.4 (0.2 M sucrose, 3.8 mM potassium phosphate monobasic, 8.6 mM disodium phosphate, 4.9 mM glutamic acid). Swabs were vortexed for 3 mins then 1 mL solution was removed and centrifuged at 18 000 rpm for 20 mins. The pellet was re-suspended in 50 μL TE buffer and heated at 95ºC for 10 mins. DNA extraction was then performed using QIAmp DNA mini kit (Qiagen), as per manufactures instructions, with the exception that the proteinase K digestion was incubated at 56ºC for 12 hours. Extracted DNA was then screened for the presence of C. pecorum and subsequent chlamydial load using quantitative real-time PCR (qPCR). The forward primer: 5’ AGTCGAACGGAATAATGGCT 3’, and the reverse primer: 5’ CCAACAAGCTGATATCCCAC 3’ targeted a 204 bp fragment of the C. pecorum 16S rRNA gene. Each PCR reaction contained 5 μL of DNA template added to a mastermix containing, 1x Quantitect SYBR Green (Qiagen), 0.5 μM of each forward and reverse primer and molecular grade water making a final volume of 20 μL per reaction. For PCR amplification, there was an initial denaturation at 95ºC for 15 mins, followed by 35 cycles of 94ºC for 15 secs, 57ºC for 15 secs and 72ºC for 30 secs. All reactions were performed in duplicate and samples of ˃ 100 copies/μL were considered positive. All reactions were carried out on a Rotor-Gene Q 5-plex HRM platform (Qiagen).
Clinical pathology
For a subset of the treated koalas, a haematological screen (Procyte) and a 13-parameter biochemistry panel (Idexx) were evaluated at admission, during the treatment period if any clinical concerns were noted, and at the end of treatment.
Effect on gut flora
The impact of the antibiotics on gut flora was evaluated via faecal smears if abnormalities in faecal pellets, body weight decreases or inappetence were observed during treatment. Koalas with faecal candida or changes in normal faecal flora were treated with additional therapeutic agents, as indicated.
Detection of persistent infection
Successful treatment of infection was assessed via qPCR results on ocular, urogenital and rectal swabs taken at 3 weeks after treatment. Quantitative-PCRs were considered positive at a threshold of >100 copies/μl. Treatment success was defined as negative PCR (<100 copies/μl) at all three sites and recovery from clinical signs of disease. Treatment failure was defined as persistent positive PCR (>100 copies/μl) at any of the 3 sites, or persistent clinical signs of disease. Successfully treated koalas were released back into the wild. Those animals which tested positive underwent a further 28 day course of treatment with a different antibiotic (usually doxycycline as more evidence became available).
Improvement in clinical signs
In cases of conjunctivitis, improvement was assessed by resolution of erythema, swelling, epiphora, corneal inflammation and pain (evidenced by squinting). In cases of cystitis, improvement was assessed by absence of pain on urination (evidenced by not vocalising when urinating), resolution of incontinence (dry fur around the cloaca and rump), reduction in bladder wall thickness at ultrasound and the absence of blood or excess sediment in the urine. Orange brown staining of the fur of the rump persists for many weeks after the resolution of cystitis as stained fur is gradually replaced by new white hairs.
Results
Clearance of infection
C. pecorum loads, greater than a million copies/μL, were detected at admission. After the initial 28 day treatment, 15 koalas continued to have a positive qPCR result from at least one of the three sample sites, requiring further treatment. Urogenital and rectal swabs were more frequently positive after treatment than were conjunctival swabs (Table 3). None of the antibiotics were 100% effective (Table 4). Mortality occurred in all treatment groups, except enrofloxacin. Mortality was not necessarily the consequence of antibiotic therapy, as chronic chlamydiosis is an extremely debilitating disease in koalas and co-infection with other pathogens can also alter the outcome.
Table 3. Koalas with persistent positive qPCR results after antibiotic treatment for chlamydiosis.
| Antibiotic | Koala ID | Pre-Treatment | 3 weeks post treatment end | ||||
|---|---|---|---|---|---|---|---|
| Conjunctiva | UGS | Rectal | Conjunctiva | UGS | Rectal | ||
| Azithromycin | 65707 | Clearview +ve | 0 | 0 | 20 000 | ||
| Chloramphenicol | 65391 | Clearview +ve | 0 | 2 000 | 4 000 | ||
| Chloramphenicol | 69505 | 1 325 | 18 300 | 180 000 | 0 | 0 | 1 503 |
| Chloramphenicol | 69720 | 3 300 | 0 | 0 | 0 | 5 780 | 0 |
| Chloramphenicol | 68433 | 10 000 | 2 926 | NR | 0 | 518 | 0 |
| Chloramphenicol | 69718 | 242 | 0 | NR | 300 | 0 | 0 |
| Chloramphenicol | 63513 | Clearview +ve | 0 | 900 | 900 | ||
| Doxycycline | 65391 | 0 | 2 000 | 4 000 | 0 | 240 | 0 |
| Enrofloxacin | 70195 | 0 | 21 000 | 63 000 | 0 | 44 457 | 20 920 |
| Enrofloxacin | 65683 | Clearview +ve | 400 | 2 200 | 1 000 | ||
| Enrofloxacin | 71123 | 516 | 0 | 0 | 585 | 0 | 0 |
| Enrofloxacin | 70718 | 0 | 8 677 | 16 487 | 0 | 190 | 16 620 |
| Florfenicol | 67376 | 0 | 350 | 700 | 0 | 125 | 120 |
| Florfenicol | 67227 | 15 | 5 500 | 2 500 | 0 | 165 000 | 550 |
| Florfenicol | 67285 | Clearview +ve | 100 | 250 | 200 | ||
qPCR = quantitative polymerase chain reaction; Conj = conjunctival swab; UGS = urogenital system swab; Rectal = rectal swab, NR–no result.
Table 4. Efficacy of antibiotic treatment in koalas assessed 3 weeks after the end of treatment.
| Antibiotic | Number treated | Treatment failures (%)a | Treatment Success (%)b |
|---|---|---|---|
| Azithromycin | 4 | 3 (75) | 1 (25) |
| Doxycycline | 32 | 1 (3) | 31 (97) |
| Chloramphenicol | 31 | 6 (19) | 25 (81) |
| Enrofloxacin | 16 | 4 (25) | 12 (75) |
| Florfenicol | 9 | 3 (33) | 6 (66) |
| Total | 92 | 17 (18) | 75 (82) |
aTreatment failure was defined as persistent positive conjunctival and/or urogenital and/or rectal quantitative polymerase chain reaction tests (qPCRs), and/or persistence of clinical signs
btreatment success was defined as negative conjunctival and urogenital and rectal qPCRs 3 weeks after treatment, and recovery from clinical signs. Negative PCR was defined as less than 100 copies/μl.
Clinical response
The five antibiotics produced varying degrees of improvement in the clinical signs of chlamydiosis. Doxycycline was the most effective anti-chlamydial antibiotic tested, achieving both clinical recovery and infection control in 97% of cases. Side effects included those typical of broad-spectrum antibiotic therapy in koalas: occasional weight loss, depression, candidiasis, dysbiosis and typhlocolitis. In addition, marked pain on intramuscular injection was overcome by diluting the long acting preparation of doxycycline 50:50 with sterile saline prior to subcutaneous injection.
Chloramphenicol was also effective with 81% of treated individuals being qPCR negative at the end of treatment (Table 4). Side effects also included weight loss, depression, candidiasis, dysbiosis and typhlocolitis but also bone marrow hypoplasia. Florfenicol, an analogue of chloramphenicol known to cause less bone marrow suppression in domestic animals, produced poor results in koalas on the treatment regimen used in this study, being effective in 66% of cases treated (Table 4). It was also associated with pronounced weight loss, inappetence and depression. On this basis, use of this drug was discontinued after treating 8 koalas. Enrofloxacin achieved both clinical improvement and elimination of chlamydial DNA in 75% of the koalas in this treatment group (Table 4).
Azithromycin in koalas was trialled using the regime developed for children, with the advantage of a 3-day course of treatment. The azithromycin treatment group experienced the most severe gastrointestinal side effects, depression and body weight loss, and only one of 4 koalas treated survived to release. However, all koalas selected for treatment with azithromycin had very severe clinical signs of chlamydiosis and all showed a rapid and dramatic improvement in their clinical signs during the first 14 days of therapy. Another disadvantage of azithromycin is that it is painful even on intravenous injection; therefore, it was diluted and given intravenously, over 30 minutes, to sedated or anaesthetised koalas (Table 2). These factors made azithromycin the least effective (Table 4) and least convenient of the five antibiotics used. Its effectiveness as an anti-chlamydial antibiotic suggests that with advances in supportive care, in particular maintaining the caecal microbiome, there may be a role for this antibiotic in specific cases.
The efficacy of the five antibiotics were evaluated on their ability to meet seven criteria developed to represent an ideal therapeutic antibiotic (Table 5). Doxycycline performed the best meeting six of the seven criteria.
Table 5. Efficacy of five antibiotics against seven criteria.
| Azithromycin | Chloramphenicol | Doxycycline | Enrofloxacin | Florfenicol | |
|---|---|---|---|---|---|
| Effective >90% | No | Almost | Yes | No | No |
| Available and affordable | Yes | No | Yes | Yes | Yes |
| Minimal Side effects | No | Yes though worse than D and E | Yes | Yes | No |
| No pain on injection | No, intravenous injection painful even when diluted and given over 30 minutes. Done under GA. | Yes | No | Yes | Yes |
| Painful if given i/m as manufactured | |||||
| Less painful if diluted 1:1 in saline and given s/c | |||||
| Small injection volume | No | Yes | Yes | Yes | Yes |
| Absorbed s/c or i/m | No | Yes | Yes | Yes | Yes |
| Infrequent Dosing | Yes | No | Yes | No | No |
i/m = intramuscular, s/c = subcutaneous, GA = general anaesthetic.
Bodyweight
In the majority of cases, treatment for chlamydiosis was associated with loss of bodyweight, particularly during the first week when wild animals are adapting to temporary captivity. Many of the treated koalas experienced a less than 5% drop in bodyweight during week 1 of treatment. However, koalas treated with azithromycin lost an average of 9% body weight (range 4.4 to 12.4%) during the first week of treatment.
Clinical pathology
In the subset of koalas in which blood was analysed, the haematological and biochemical parameters measured were within the normal range for koalas in all treatment groups. Abnormalities detected were primarily in electrolytes, PCV/TP or white cell differentials, but were not consistently associated with any treatment group.
Effect on gut flora
During treatment with any broad-spectrum antibiotic, koalas may develop gastrointestinal pathology, primarily candidiasis, diarrhoea, caeco-colic dysbiosis (altered gut flora) or typhlocolitis (inflammation of the caecum and colon). The use of chloramphenicol was frequently associated with gastrointestinal candidiasis, usually at about 2 weeks, into the 28-day treatment. Diarrhoea is a frequent intermittent occurrence in koalas and may be associated with leaf species ingested or as a side effect of antibiotic therapy. Dysbiosis, as assessed by faecal smear, was observed in koalas in all treatment groups. Typhlocolitis can be a life-threatening condition in koalas and was detected occasionally in the koalas in this study and likely to be associated with the impact of broad spectrum antibiotics on essential microbe populations in the caecum and colon.
Discussion
In this hospital-based study of the treatment of chlamydiosis in koalas, doxycycline and chloramphenicol were the most effective antibiotics of the five tested and produced good clinical outcomes. The microbiological cure rates for doxycycline and chloramphenicol were 97% and 81%, respectively. All antibiotics used were effective in some of the koalas treated, however none were effective in all animals.
Side effects from the use of broad-spectrum antibiotics are common in koalas, which depend on hindgut fermentative digestion of their high-fibre diet. Hence, management of dysbiosis is an important component of treatment for chlamydiosis in koalas. In this study, oral and intestinal yeast infections from about day 14 of treatment were common. Gastro-intestinal candidiasis can be managed in koalas with nystatin or amphotericin B. Caeco-colic dysbiosis can be managed with the oral administration of fresh caecal contents harvested from recently deceased koalas (stored at 4⁰C for up to 14 days). More detailed analysis of the gut associated side effects for doxycycline and chloramphenicol in koalas are in process.
Doxycycline was the only antibiotic tested that met most (6 of 7) of the criteria for an ‘ideal’ anti-chlamydial agent for koalas. The only criterion not satisfied was that of non-painful injection. Intramuscular injection of the long-acting 50 mg/ml solution is painful, which produces a vigorous patient reaction and can cause transient lameness and, occasionally, swelling. Hence, we modified the treatment regimen by diluting the oily preparation (50:50 in sterile saline) immediately before subcutaneous injection. The subcutaneous route for Doxycycline proved clinically effective in this study and pharmacokinetics for this route in this species is underway.
Chloramphenicol is a highly effective and well-tolerated broad-spectrum antibiotic, but it can cause blood dyscrasias [19] and is no longer commercially manufactured in the injectable form because of human susceptibility to this side effect. Chloramphenicol has been used to successfully treat chlamydial conjunctivitis and cystitis in koalas at the dose and regimen used in this study [20]. Pharmacokinetic studies support that the current dosage regimen is probably effective in koalas [10]. Gastro-intestinal side effects are common in koalas and can be managed as discussed above. Bone marrow hypoplasia is also a common side effect of chloramphenicol in other species [21] and has been identified in koalas at the Australia Zoo Wildlife Hospital, but could also be associated with exogenous koala retrovirus (KoRV) infection.
Compared with chloramphenicol, florfenicol, a thiamphenicol derivative, is significantly more active in vitro against many pathogenic strains of bacteria. Molecular structural differences improve efficacy, reduce toxicity and reduce bacterial resistance; Chlamydia species are susceptible [22]. In koalas, pharmacokinetic studies suggest that florfenicol does not persist beyond 24 hours when given by intravenous injection at 10 mg/kg and does not reach the mean inhibitory concentration (MIC) for Chlamydia [12]; the dose regimen used in this study was 20 mg/kg given subcutaneously every second day for 28 days based on the dose rate used in livestock. On this treatment regimen, florfenicol produced a low cure rate compared to the other antibiotics trialled, but did nevertheless eliminate detectable chlamydial DNA from 6 koalas.
When indicated by culture and sensitivity, enrofloxacin has been used in koalas to successfully treat a range of bacterial infections, including skin and pulmonary infections, and has been given by the intravenous, subcutaneous, oral and inhalation routes. However, poor oral bioavailability at dose rates up to 20 mg/kg was reported by Griffith et al. (2010) [10]. The authors speculated that oral enrofloxacin is unlikely to be effective against Chlamydia in koalas. The recommended dose rate used in this study was extrapolated from small animal dose rates: 10 mg/kg subcutaneous loading dose followed by 5 mg/kg subcutaneously daily for 28 days. Enrofloxacin was only moderately effective in this study (75% success rate) but caused no major side effects or mortality. Despite pharmacokinetic evidence, enrofloxacin has produced good clinical results in koalas with one hospital releasing more than 828 koalas over a 10 year period that responded to treatment, particularly those with conjunctivitis. This was prior to the availability of qPCR to confirm therapeutic effectiveness. Enrofloxacin has a good uptake into infected cells and this clinical improvement may be associated with a reduction of the chlamydial pathogen load, and the supportive care that the koalas receive once admitted to hospital [23]. However, the effectiveness of enrofloxacin in the treatment of Chlamydia-infected koalas has also been shown to fail, with koalas remaining positive after treatment of up to six-months [24].
The US Centers for Disease Control and Prevention (CDC) recommend azithromycin or doxycycline to treat human chlamydial infections; both drugs have a 95% microbial cure rate in humans. Azithromycin has been used to treat chlamydial infertility in women and conjunctivitis in babies [25–27]. Azithromycin was only used in koalas with very severe disease because there was an unpublished report of mortality in a koala treated with it. Severe chronic chlamydiosis contributed to the high mortality in this group. Daily monitoring of body weight in this group suggested the most severe gut associated side effects. Despite this, one koala which had not been cleared of his clinical chlamydiosis after a 28 day course of chloramphenicol and a 28 day course of enrofloxacin, made a full recovery and was released after treatment with 3 intravenous injections of azithromycin.
Limitations
Successful treatment of chlamydial infection is more likely if initiated early in the disease process. Wild koalas are admitted to wildlife hospitals when they have either become so sick as to be on the ground, or when their clinical signs are easily detected while they are in a tree, and they are rescued for treatment. The duration of their infection is not known at the time of admission. There is variation in the susceptibility to the disease, with some koalas in populations with a high prevalence of chlamydiosis living and breeding without contracting the disease. Co-infection with KoRV may also modulate immune responses to chlamydial infection and contribute to the severity of the disease within individuals.
Attempts to match animals in the treatment groups in terms of age, sex and severity of disease were abandoned due to the random presentation of cases from the wild. For practical reasons, koalas were allocated into treatment groups according to temperament, severity of disease, body condition and history of previous antibiotic treatment failure. For example, koalas which were considered likely to die from their chlamydial disease without rapid intervention were treated with azithromycin. Thus, the evaluation of azithromycin was compromised by its use in koalas with a lower-than-average chance of recovery. Also, animals allocated to the doxycycline treatment group included the more aggressive or anxious koalas because this drug had the lowest dosing frequency; these animals experienced higher levels of stress from their confinement in captivity, but this drug performed the best in this trial. Further, koalas which had not responded to 28 days of therapy with either chloramphenicol (n = 6) or enrofloxacin (n = 4) were then treated with doxycycline, which may have made the residual infection easier to treat although some of the qPCRs were still very high at the commencement of doxycycline therapy.
The impact of antibiotics on gut flora could be more thoroughly assessed by faecal culture and gram stain before treatment and weekly during therapy. Further studies will include more detail in this area.
Conclusion
This hospital-based study found that, of 5 antibiotics compared, doxycycline was the most effective antibiotic in treating chlamydiosis in wild koalas and that chloramphenicol was the next best alternative. Although antibiotic sensitivity testing before treatment is desirable, for Chlamydia, cell culture is required. An important additional consideration in antibiotic choice is the gastrointestinal side effects related to the koala’s unique biology, which must be managed during any broad-spectrum antibiotic therapy. The long-term and widespread use of antibiotics in rehabilitated wild animals may lead to antibiotic resistance and may be responsible for changes in observed clinical success of antibiotics over the last three decades where koalas have been treated and released to the wild.
Neither natural infection nor treatment of clinical disease with antibiotics produces any protection from repeat infection. Chlamydiosis remains a major threatening process for koalas, particularly when populations also face ongoing habitat fragmentation and destruction and loss of genetic diversity. Vaccination of young koalas prior to infection may improve the prospects for this species. Only habitat protection, restoration and connection will allow the survival of the existing koala population, and lead to an improvement in genetic diversity and potentially a reduction in disease susceptibility to conserve koalas in the long term.
Acknowledgments
We would like to thank the veterinarians, veterinary nurses and keepers at Australia Zoo Wildlife Hospital who assisted in the care and treatment of the koalas in this study. We would also like to thank Professor Peter Timms for his advice and assistance with editing and Dr Leigh Findlay for editorial assistance with the manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by an Australia Research Council Linkage program received by PT. SN received a USC PhD scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nyari S, Waugh CA, Dong J, Quigley BL, Hanger J, Loader J, et al. Epidemiology of chlamydial infection and disease in a free-ranging koala (Phascolarctos cinereus) population. PloS one. 2017;12(12):e0190114 10.1371/journal.pone.0190114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson JL, Lynch M, Anderson GA, Noormohammadi AH, Legione A, Gilkerson JR, et al. The prevalence and clinical significance of Chlamydia infection in island and mainland populations of Victorian koalas (Phascolarctos cinereus). Journal of Wildlife Diseases. 2015;51(2):309–17 10.7589/2014-07-176 [DOI] [PubMed] [Google Scholar]
- 3.Martin R, Handasyde KA. The koala: natural history, conservation and management 2nd ed ed: Kensington, NSW: UNSW Press; 1999. [Google Scholar]
- 4.Troughton ELeG. The Koala. In a Treasure of Australian Wildlife. Sydney, Australia: Ure Smith; 1967. [Google Scholar]
- 5.Cockram F, Jackson A. Keratoconjunctivitis of the koala, Phascolarctos cinereus, caused by Chlamydia psittaci. Journal of Wildlife Diseases. 1981;17(4):497–504. 10.7589/0090-3558-17.4.497 [DOI] [PubMed] [Google Scholar]
- 6.Brown A, Grice R. Experimental transmission of Chlamydia psittaci in the koala In Chlamydial Infections, Proceedings of the 6th International Symposium on Human Chlamydial Infections, Sanderstead, Surry, 15–21 June 1986. Oriel J, Ridgway G, Schachter J, Taylor-Robinson D, Ward M, editors. Cambridge: Cambridge University Press; 1986. 349–52 p. [Google Scholar]
- 7.Glassicki T, Giffard P, Timms P. Outer Membrane Protein 2 Gene Sequences Indicate that Chlamydia pecorum and Chlamydia pneumoniae Cause Infections in Koalas. Systemic and Applied Microbiology. 1996;19(3):457–64. [Google Scholar]
- 8.Chlamydia treatment and care. Available from: https://www.cdc.gov/std/chlamydia/treatment.htm.
- 9.Osawa R, Bird PS, Harbrow DJ, Ogimoto K, Seymore GJ. Microbiological Studies of the Intestinal Microflora of the Koala, Phascolarctos cinereus. I. Colonisation of the Caecal Wall by Tannin-protein-complexdegrading Enterobacteria. Australian Journal of Zoology. 1993;41(6):599–609. [Google Scholar]
- 10.Griffith JE, Higgins DP, Li KM, Krockenberger MB, Govendir M. Absorption of enrofloxacin and marbofloxacin after oral and subcutaneous administration in diseased koalas (Phascolarctos cinereus). Journal of Veterinary Pharmacology and Therapeutics. 2010;33(6):595–604. 10.1111/j.1365-2885.2010.01169.x [DOI] [PubMed] [Google Scholar]
- 11.Black LA, Higgins DP, Govendir M. In vitro activity of chloramphenicol, florfenicol and enrofloxacin against Chlamydia pecorum isolated from koalas (Phascolarctos cinereus). Australian veterinary journal. 2015;93(11):420–3. 10.1111/avj.12364 [DOI] [PubMed] [Google Scholar]
- 12.Budd C, Flanagan C, Gillett A, Hanger J, Loader JJ, Govendir M. Assessment of florfenicol as a possible treatment for chlamydiosis in koalas (Phascolarctos cinereus). Australian Veterinary Journal. 2017;95(9):343–9. 10.1111/avj.12617 [DOI] [PubMed] [Google Scholar]
- 13.Gordon G. Estimation of the age of the koala, Phascolarctos cinereus (Marsupialia: Phascolarctidae) from tooth wear and growth. Australian Mammalogy. 1991;14:5–12. [Google Scholar]
- 14.Sexually Transmitted Diseases Treatment Guidelines. Available from: https://www.cdc.gov/std/tg2015/chlamydia.htm.
- 15.Markey B, Wan C, Hanger J, Phillips C, Timms P. Use of quantitative real-time PCR to monitor the shedding and treatment of chlamydiae in the koala (Phascolarctos cinereus). Veterinary Microbiology. 2007;120(3–4):334–42. 10.1016/j.vetmic.2006.11.022 [DOI] [PubMed] [Google Scholar]
- 16.Plumb DC. Plumb's Veterinary Drug Handbook. 9th Edition, editor: Wiley-Blackwell; 2018. [Google Scholar]
- 17.Blanshard W, Bodley K. Koalas, in Medicine of Australian Mammals In: Vogelnest L, Woods R, editors. Medicine of Australian Mammals: An Australian Perspective: CSIRO Publishing; 2008. p. 334–480. [Google Scholar]
- 18.Plumb DC. Plumb's Veterinary Drug Handbook. 9th Edition, editor: Wiley-Blackwell; 2018. [Google Scholar]
- 19.Hill CG. Blood dyscrasias associated with chloramphenicol. Canadian Medical Association Journal. 1972;106:147–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins A, Loader J, Timms P, Hanger J. Optimising the short and long-term clinical outcomes for koalas (Phascolarctos cinereus) during treatment for chlamydial infection and disease. PloS one. 2018;13(12):e0209679 10.1371/journal.pone.0209679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson AD. Chloramphenical toxicity in dogs. Research in Veterinary Science. 1977;23:66–9. [PubMed] [Google Scholar]
- 22.Merck Veterinary Manual 2017. Available from: https://www.merckvetmanual.com/news/editorial/2018/01/12/15/33/most-popular-pet-names-of-2017-revealed.
- 23.Govendir M. Review of some pharmacokinetic and pharmacodynamic properties of anti-infective medicines administered to the koala (Phascolarctos cinereus). Journal of Veterinary Pharmacology and Therapeutics. 2018;41(1):1–10. Epub 2017/07/14. 10.1111/jvp.12435 [DOI] [PubMed] [Google Scholar]
- 24.Bodetti TJ, Johnston S, Pospischil A, Knox C, Timms P. Screening semen from koalas (Phascolarctos cinereus) for Chlamydia species by PCR. The Veterinary record. 2002;151:147–9. 10.1136/vr.151.5.147 [DOI] [PubMed] [Google Scholar]
- 25.Srivastava P, Vardhan H, Bhengraj AR, Jha R, Singh LC, Salhan S, et al. Azithromycin treatment modulates the extracellular signal-regulated kinase mediated pathway and inhibits inflammatory cytokines and chemokines in epithelial cells from infertile women with recurrent Chlamydia trachomatis infection. DNA Cell Biol. 2011;30(8):545–54. 10.1089/dna.2010.1167 [DOI] [PubMed] [Google Scholar]
- 26.Oldenburg CE, Arzika AM, Maliki R, Kane MS, Lebas E, Ray KJ, et al. Safety of azithromycin in infants under six months of age in Niger: A community randomized trial. PLoS Neglected Tropical Diseases. 2018;12(11):e0006950 10.1371/journal.pntd.0006950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Review of Anti-infective Therapy. 2011;9(1):61–70. 10.1586/eri.10.156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.