Abstract
Background
Few studies report the effects of tamoxifen intake and the occurrence of de novo fatty liver and the deterioration of existing fatty liver. The aim of this study was to investigate the effects of tamoxifen on fatty change of liver over time and also the impact of fatty liver on the prognosis of patients with breast cancer.
Methods
This was a single-center, retrospective study of patients who were diagnosed with primary breast cancer from January 2007 to July 2017. 911 consecutive patients were classified into three groups according to treatment method: tamoxifen group, aromatase inhibitor (AI) group, and control group.
Results
Median treatment duration was 49 months (interquartile range, IQR; 32–58) and median observational period was 85 months (IQR; 50–118). Long-term use of tamoxifen significantly aggravated fatty liver status compared to AI or control groups [hazard ratio (HR): 1.598, 95% confidence interval (CI): 1.173–2.177, P = 0.003] after adjusting other factors. When analyzed separately depending on pre-existing fatty liver at baseline, tamoxifen was involved in the development of de novo fatty liver [HR: 1.519, 95% CI: 1.100–2.098, P = 0.011) and had greater effect on fatty liver worsening (HR: 2.103, 95% CI: 1.156–3.826, P = 0.015). However, the progression of fatty liver did not significantly affect the mortality of breast cancer patients.
Conclusions
Tamoxifen had a significant effect on the fatty liver status compared to other treatment modalities in breast cancer patients. Although fatty liver did not affect the prognosis of breast cancer, meticulous attention to cardiovascular disease or other metabolic disease should be paid when used for a long time.
Introduction
Breast cancer is the most common cancer in women, with an annual incidence of two million cases worldwide. Also, about 70% of breast cancer patients are hormone receptor positive.[1] Tamoxifen, a selective estrogen receptor modulator (SERM), is a well-known adjuvant endocrine treatment for estrogen receptor (ER)-positive breast cancer patients. Since tamoxifen can increase the rates of disease-free survival and overall survival of patients at all stages, and reduce the local recurrence rate, its usage in ER-positive patients is recommended for at least five years.[2, 3] However, side effects due to long-term use of tamoxifen such as vaginal bleeding, endometrial cancer, deep vein thrombosis, pulmonary embolism, and fatty liver have been reported.[4] Among these side effects, fatty liver is a serious complication because it can reduce drug compliance and increase the incidence of other metabolic diseases.[5, 6] In addition, it was reported that 43% of breast cancer patients treated with tamoxifen developed hepatic steatosis within the first two years.[7]
Aromatase inhibitor (AI) has the same mechanism as tamoxifen by inhibiting estrogen action. However, unlike tamoxifen, AI does not significantly increase the incidence of fatty liver. Recently, studies have reported the benefits of using tamoxifen for up to 10 years.[8] Thus, more attention should be paid to side effects of long-term use of tamoxifen. However, existing studies usually have short-term follow-up with the diagnosis of fatty liver based on elevated liver enzymes rather than imaging diagnosis.[9] In addition, reports on the use of tamoxifen in high risk groups of fatty liver are limited. Furthermore, the effect of fatty liver on the prognosis of breast cancer patients is not well known yet.
Thus, the purpose of this study was to investigate the effects of tamoxifen on the risk of fatty liver in comparison with other treatment modalities practicing long-term follow-ups and identify high risk groups. In addition, we investigated the effect of fatty liver caused by tamoxifen on the mortality of breast cancer patients.
Materials and methods
Patients and study design
This was a single-center, retrospective cohort study. We retrospectively reviewed data of patients who were first pathologically diagnosed with primary breast cancer and treated at a tertiary referral hospital from January 2007 to July 2017 (S1 Fig). Exclusion criteria were: (1) treatment period of less than two years, (2) evidence of viral hepatitis (e.g., hepatitis B surface antigen positive or hepatitis C antibody positive) or liver cirrhosis, (3) significant alcohol consumption, (4) no imaging study during the follow-up period, and (5) evidence of double primary cancer. Ongoing or recent alcohol consumption >21 standard drinks on average per week in men and >14 standard drinks on average per week in women was the criteria for significant alcohol consumption.[10]
911 patients were enrolled and classified into three groups according to treatment method: 1) tamoxifen group, patients taking tamoxifen for more than two years; 2) aromatase inhibitor group, patients taking anastrozole or letrozole for more than 2 years; and 3) control group, patients who had received treatment other than tamoxifen or AI (e.g., conventional chemotherapy, radiotherapy, or surgery alone). The chemotherapeutic agents of anticancer drugs were diverse, however, anthracycline and taxane-based chemotherapy was most widely used. Clinical, histological, imaging, and laboratory records of these patients were retrospectively reviewed. Patients were prescribed tamoxifen or AI every three months with laboratory tests at each regular visit. To monitor possible recurrences of breast cancer, follow-up computed tomography (CT) or ultrasonography (USG) were performed every 6months for patients with high risk of recurrence, and every 12months for others as recommended in the NCCN guideline.[11] If recurrence was not observed for five years, monitoring was carried out on a yearly basis.
Informed consent was waived because of the retrospective nature of the study and the analysis used anonymous clinical data. This study was approved by the Institutional Review Board of Soonchunhyang University Hospital, Bucheon, Korea (SCHBC-2018-12-002-001). The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki.
Diagnosis of fatty liver
The incidence or severity of fatty liver was assessed by non-contrast CT or abdominal USG. We used only one technique (among CT or USG) as a diagnostic tool to determine fatty liver throughout the follow-up period. All the images were evaluated by two experienced radiologists (LMH and LJE) who were blinded to the study aims and the administered drug. In USG examination, the severity of fatty liver was graded as normal, mild, moderate, or severe according to echogenicity of the liver parenchyma.[12] In non-contrast CT examination, hepatic steatosis was assessed based on Hounsfield unit (HU) hepatic attenuation using published method.[13, 14] Hepatic attenuation was measured as mean of three circular regions of interest (ROIs) on three transverse sections at different hepatic levels: confluence of right hepatic vein, umbilical portion of left portal vein, and posterior branch of the right portal vein. Mean splenic attenuation was also calculated for three random area ROI values of attenuation measurement at three different splenic levels. Fatty liver was defined when the ratio of mean hepatic attenuation to mean splenic attenuation was lower than 0.9.[13, 14]
Definition
Baseline was regarded as just before receiving hormonal therapy after breast cancer surgery and follow-up CT scan. The follow-up period was considered the period either during hormonal drug intake or until the time of loss or death. Patients were classified into the following four groups according to existence of fatty liver at baseline and the change of fatty liver status during follow-up period. No fatty liver group was defined as having a normal liver at baseline and remaining normal during follow-up period. De novo fatty liver group was defined as having a normal liver at baseline, but developing a new fatty liver. Stable fatty liver group was defined as having a fatty liver at baseline and no change of fatty liver severity during follow-up period. Worsened fatty liver group was defined as having a fatty liver at baseline and worsening of fatty liver status at any point during follow-up period (e.g., increased grade of fatty liver on abdominal USG or decreasing liver attenuation index value on non-contrast CT).[15, 16] These four groups mentioned above were also categorized based on aggravation of fatty liver. De novo fatty liver group and worsened fatty liver group were categorized as progression group while no fatty liver group and stable fatty liver group categorized as non-progression group.
Statistical analysis
Frequencies and percentages were used for descriptive statistics. Statistical differences between groups were investigated using one-way analysis of variance (ANOVA) or Student’s t-test for continuous variables and chi-squares test or Fisher’s exact test for categorical variables as appropriate. Kaplan-Meier survival analysis was used for survival rate and incidence of fatty liver. To identify predictive factors associated with development of fatty liver according to age, Cox proportional hazards (PH) regression analysis was used. Any variable showing a significance at 0.1 in the univariate model was selected as a candidate for the multivariable model.[17] The final multiple Cox PH regression model was chosen by the stepwise selection based on the Akaike information criterion (AIC).
Propensity score (PS) matching analysis was conducted to minimize the probability of selection bias by pairing tamoxifen group and control group based on propensity scores. PS matching was generated by multiple logistic regressions. This model included all variables such as age, body mass index (BMI), breast cancer stage, diabetes, and hypertension that are likely to affect the process of fatty deposition in the liver. We used the nearest available matching (1:1) method for PS matching with the caliper of 0.05. For validation, we generated additional matched datasets using the caliper of 0.03 and 0.01.
All statistical analyses were performed using R (version 3.6.1, The R Foundation for Statistical Computing, Vienna, Austria) and SPSS software (version 21.0; SPSS Inc., Chicago, IL, USA). Statistical significance was defined at P < 0.05.
Results
Patients’ demographics at baseline
Baseline characteristics of 911 patients enrolled in this study are described in Table 1. Tamoxifen, prescribed to 416 (45.7%) patients, accounted for the highest proportion followed by a group of patients (296, 32.5%) who did not take any anti-estrogen hormonal treatment, and next by a group of patients (199, 21.8%) who were prescribed AI. Mean age and BMI were 50.13 ± 10.32 years and 24.17 ± 3.62, respectively. Median treatment duration was 49 months [interquartile range (IQR) 32–58)], and median observational period was 85 months (IQR 50–118). From the outset 260 (28.5%) of patients already had fatty liver. Seventy (7.7%) patients had diabetes and 176 (19.3%) had hypertension. Mean follicle stimulating hormone (FSH) level was 37.38 ± 30.15 IU/L. Breast cancer of stage I was most common (42.4%), followed by stage II (40.8%) and stage III (8.1%). Lymph node metastasis was found in 284 (31.2%) patients.
Table 1. Baseline characteristics of patients.
| Variable | All | Control | Tamoxifen | Aromatase inhibitor | P-value |
|---|---|---|---|---|---|
| (N = 911) | (N = 296) | (N = 416) | (N = 199) | ||
| Age (year) | 50.13 ± 10.32 | 49.78 ± 10.60 | 46.87 ± 9.43 | 57.46 ± 7.68 | <0.001 |
| Body mass index kg/m2) | 24.17 ± 3.62 | 24.18 ± 3.78 | 23.67 ± 3.32 | 25.22 ± 3.76 | <0.001 |
| Diabetes mellitus | 70 (7.68%) | 21 (7.09%) | 24 (5.77%) | 25 (12.56%) | 0.011 |
| Hypertension | 176 (19.32%) | 49 (16.55%) | 72 (17.31%) | 55 (27.64%) | 0.003 |
| Treatment duration (month) | 49.04 ± 21.85 | 50.88 ± 30.87 | 50.00 ± 17.07 | 44.30 ± 11.55 | 0.005 |
| Cancer-related factor | |||||
| Stage | <0.001 | ||||
| 0 | 71 (7.79%) | 19 (6.42%) | 49 (11.78%) | 3 (1.51%) | |
| 1 | 386 (42.37%) | 129 (43.58%) | 160 (38.46%) | 97 (48.74%) | |
| 2 | 372 (40.83%) | 120 (40.54%) | 167 (40.14%) | 85 (42.71%) | |
| 3 | 74 (8.12%) | 22 (7.43%) | 38 (9.13%) | 14 (7.04%) | |
| 4 | 8 (0.88%) | 6 (2.03%) | 2 (0.48%) | 0 (0%) | |
| Pathology | 0.007 | ||||
| Invasive ductal carcinoma | 757 (83.10%) | 248 (83.78%) | 332 (79.81%) | 177 (88.94%) | |
| Ductal carcinoma in situ | 73 (8.01%) | 22 (7.43%) | 48 (11.54%) | 3 (1.51%) | |
| Mucinous carcinoma | 24 (2.63%) | 4 (1.35%) | 12 (2.88%) | 8 (4.02%) | |
| Infiltrating lobular carcinoma | 27 (2.96%) | 9 (3.04%) | 12 (2.88%) | 6 (3.02%) | |
| Intraductal papilloma | 13 (1.43%) | 2 (0.68%) | 6 (1.44%) | 5 (2.51%) | |
| Tubular carcinoma | 2 (0.22%) | 1 (0.34%) | 1 (0.24%) | 0 (0%) | |
| Apocrine carcinoma | 2 (0.22%) | 1 (0.34%) | 1 (0.24%) | 0 (0%) | |
| Squamous carcinoma | 2 (0.22%) | 1 (0.34%) | 1 (0.24%) | 0 (0%) | |
| Medullary carcinoma | 5 (0.55%) | 3 (1.01%) | 2 (0.48%) | 0 (0%) | |
| Others | 6 (0.66%) | 5 (1.69%) | 1 (0.24%) | 0 (0%) | |
| Lymph node metastasis | 284 (31.17%) | 107 (36.15%) | 115 (27.64%) | 62 (31.16%) | 0.054 |
| ER (Intermediate or High) | 614 (67.70%) | 108 (36.49%) | 328 (79.61%) | 178 (89.45%) | <0.001 |
| PR (Intermediate or High) | 555 (61.19%) | 102 (34.46%) | 313 (75.97%) | 140 (70.35%) | <0.001 |
| HER2 (Intermediate or High) | 340 (37.32%) | 83 (28.04%) | 167 (40.14%) | 90 (45.23%) | <0.001 |
| p53 | 328 (41.41%) | 121 (49.79%) | 152 (41.30%) | 55 (30.39%) | <0.001 |
| Ki67 (≥ 40%) | 124 (15.31%) | 77 (29.96%) | 31 (8.36%) | 16 (8.79%) | <0.001 |
| Laboratory test | |||||
| FSH (IU/L) | 37.38 ± 30.15 | 30.37 ± 29.13 | 34.34 ± 29.78 | 55.55 ± 25.18 | <0.001 |
| Platelet (103 mm3) | 238.66 ± 72.70 | 247.55 ± 66.89 | 231.54 ± 74.33 | 240.35 ± 76.27 | 0.007 |
| AST (U/L) | 23.85 ± 13.53 | 21.28 ± 9.07 | 25.60 ± 15.42 | 24.03 ± 14.28 | <0.001 |
| ALT (U/L) | 22.17 ± 16.86 | 19.35 ± 15.97 | 23.93 ± 18.43 | 22.67 ± 13.98 | <0.001 |
| Serum albumin (mg/dL) | 4.20 ± 0.45 | 4.36 ± 0.45 | 4.11 ± 0.43 | 4.14 ± 0.43 | <0.001 |
| Total bilirubin (mg/dL) | 0.59 ± 0.33 | 0.65 ± 0.42 | 0.55 ± 0.25 | 0.58 ± 0.31 | <0.001 |
| Total cholesterol (mg/dL) | 185.16 ± 35.97 | 185.90 ± 36.59 | 181.12 ± 33.83 | 192.48 ± 38.28 | 0.118 |
| Triglyceride (mg/dL) | 132.95 ± 96.18 | 146.31 ± 110.62 | 124.34 ± 91.42 | 133.36 ± 82.19 | 0.060 |
| HDL-cholesterol (mg/dL) | 53.40 ± 13.41 | 52.29 ± 13.02 | 55.45 ± 13.92 | 51.18 ± 12.51 | 0.002 |
| LDL-cholesterol (mg/dL) | 107.32 ± 32.39 | 107.98 ± 31.99 | 104.32 ± 30.80 | 112.13 ± 35.42 | 0.096 |
| Fasting blood glucose (mg/dL) | 110.07 ± 28.42 | 106.83 ± 24.72 | 109.74 ± 28.28 | 115.60 ± 32.83 | 0.001 |
computed by one-way ANOVA for continuous variables and chi-squares test for categorical variables.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal growth factor receptor 2; FSH, follicle stimulating hormone; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NFS, nonalcoholic fatty liver disease fibrosis score; FIB-4, fibrosis-4; CT, computed tomography.
Changes in fatty liver status over time
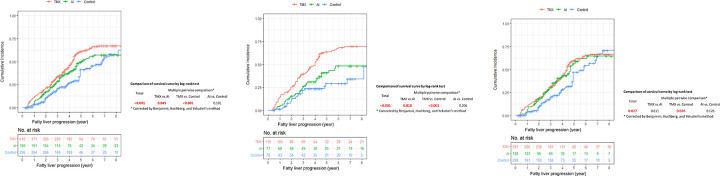
There was no change of fatty liver status in 499 (54.8%) patients (non-progression group) while there was a deterioration of fatty liver status in 412 (45.2%) patients (progression group). Table 2 shows the baseline data according to the change of fatty liver status. BMI, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and fasting blood glucose of patients in the progression group were significantly higher than those in the non-progression group. The worsened fatty liver group had the highest mean BMI, followed by stable fatty liver, de novo fatty liver, and no fatty liver groups. Levels of liver enzymes including AST, ALT, and other lipid profiles showed the same pattern. Of note, tamoxifen significantly increased fatty liver progression compared to AI or control over time (P < 0.001, Fig 1A). These differences were more pronounced in patients who had previously fatty liver (Fig 1B). In patients without basal fatty liver, tamoxifen also increased the incidence of de novo fatty liver more than control (Fig 1C).
Table 2. Clinical characteristics depending on the status of fatty liver.
| Variable | All | No fatty liver | De novo fatty liver | Stable fatty liver | Worsened fatty liver | P | Fatty liver progression* (-) | Fatty liver progression* (+) | P |
|---|---|---|---|---|---|---|---|---|---|
| (N = 911) | (N = 364) | (N = 287) | (N = 135) | (N = 125) | |||||
| (N = 499) | (N = 412) | ||||||||
| Treatment modality | <0.001 | <0.001 | |||||||
| Control | 296 | 159 (53.72%) | 67 (22.64%) | 52 (17.57%) | 18 (6.07%) | 211 (71.29%) | 85 (28.71%) | ||
| Aromatase inhibitor | 199 | 73 (36.68%) | 55 (27.64%) | 41 (20.60%) | 30 (15.08%) | 114 (57.28%) | 85 (42.72%) | ||
| Tamoxifen | 416 | 132 (31.73%) | 165 (39.66%) | 42 (10.10%) | 77 (18.51%) | 174 (41.83%) | 242 (58.17%) | ||
| Age (year) | 50.13 ± 10.32 | 49.65 ± 10.69 | 49.70 ± 10.40 | 52.58 ± 10.30 | 49.84 ± 8.64 | 0.018 | 50.44 ± 10.66 | 49.75 ± 9.89 | 0.306 |
| Body mass index (kg/m2) | 24.17 ± 3.62 | 23.03 ± 3.15 | 24.06 ± 3.25 | 25.38 ± 3.92 | 26.47 ± 3.93 | <0.001 | 23.67 ± 3.53 | 24.79 ± 3.64 | <0.001 |
| Diabetes mellitus | 70 (7.68%) | 18 (4.95%) | 18 (6.27%) | 18 (13.33%) | 16 (12.80%) | 0.002 | 36 (7.21%) | 34 (8.25%) | 0.645 |
| Hypertension | 176 (19.32%) | 55 (15.11%) | 52 (18.12%) | 33 (24.44%) | 36 (28.80%) | 0.003 | 88 (17.64%) | 88 (21.36%) | 0.183 |
| Treatment duration (month) | 49.04 ± 21.85 | 44.48 ± 20.83 | 48.57 ± 18.57 | 60.01 ± 28.09 | 51.55 ± 19.64 | <0.001 | 48.68 ± 24.01 | 49.48 ± 18.92 | 0.075 |
| Cancer-related factor | |||||||||
| Stage | 0.460 | 0.062a | |||||||
| 0 | 71 (7.79%) | 26 (7.14%) | 27 (9.41%) | 5 (3.70%) | 13 (10.40%) | 31 (6.21%) | 40 (9.71%) | ||
| 1 | 386 (42.37%) | 171 (46.98%) | 110 (38.33%) | 60 (44.44%) | 45 (36.00%) | 231 (46.29%) | 155 (37.62%) | ||
| 2 | 372 (40.83%) | 138 (37.91%) | 121 (42.16%) | 57 (42.22%) | 56 (44.80%) | 195 (39.08%) | 177 (42.96%) | ||
| 3 | 74 (8.12%) | 26 (7.14%) | 26 (9.06%) | 12 (8.89%) | 10 (8.00%) | 38 (7.62%) | 36 (8.74%) | ||
| 4 | 8 (0.88%) | 3 (0.82%) | 3 (1.05%) | 1 (0.74%) | 1 (0.80%) | 4 (0.80%) | 4 (0.97%) | ||
| Pathology | 0.099 | ||||||||
| Invasive ductal carcinoma | 757 (83.10%) | 312 (85.71%) | 229 (79.79%) | 117 (86.67%) | 99 (79.20%) | 0.101 | |||
| Ductal carcinoma in situ | 73 (8.01%) | 27 (7.42%) | 26 (9.06%) | 6 (4.44%) | 14 (11.20%) | 429 (85.97%) | 328 (79.61%) | ||
| Mucinous carcinoma | 24 (2.63%) | 6 (1.65%) | 11 (3.83%) | 4 (2.96%) | 3 (2.40%) | 33 (6.61%) | 40 (9.71%) | ||
| Infiltrating lobular carcinoma | 27 (2.96%) | 13 (3.57%) | 11 (3.83%) | 3 (2.22%) | 0 (0%) | 10 (2.00%) | 14 (3.40%) | ||
| Intraductal papilloma | 13 (1.43%) | 1 (0.27%) | 7 (2.44%) | 1 (0.74%) | 4 (3.20%) | 16 (3.21%) | 11 (2.67%) | ||
| Tubular carcinoma | 2 (0.22%) | 1 (0.27%) | 0 (0%) | 0 (0%) | 1 (0.80%) | 2 (0.40%) | 11 (2.67%) | ||
| Apocrine carcinoma | 2 (0.22%) | 1 (0.27%) | 0 (0%) | 0 (0%) | 1 (0.80%) | 1 (0.20%) | 1 (0.24%) | ||
| Squamous carcinoma | 2 (0.22%) | 0 (0%) | 1 (0.35%) | 1 (0.74%) | 0 (0%) | 1 (0.20%) | 1 (0.24%) | ||
| Medullary carcinoma | 5 (0.55%) | 1 (0.27%) | 2 (0.70%) | 1 (0.74%) | 1 (0.80%) | 1 (0.20%) | 1 (0.24%) | ||
| Others | 6 (0.66%) | 2 (0.55%) | 0 (0%) | 2 (1.48%) | 2 (1.60%) | 2 (0.40%) | 3 (0.73%) | ||
| Lymph node metastasis | 284 (31.17%) | 104 (28.57%) | 96 (33.45%) | 47 (34.81%) | 37 (29.60%) | 0.420 | 151 (30.26%) | 133 (32.28%) | 0.559 |
| ER (Intermediate or High) | 614 (67.70%) | 230 (63.19%) | 208 (73.24%) | 82 (61.19%) | 94 (75.20%) | 0.004 | 312 (62.65%) | 302 (73.84%) | <0.001 |
| PR (Intermediate or High) | 555 (61.19%) | 200 (54.95%) | 192 (67.61%) | 70 (52.24%) | 93 (74.40%) | <0.001 | 270 (54.22%) | 285 (69.68%) | <0.001 |
| HER2 (Intermediate or High) | 340 (37.32%) | 134 (36.81%) | 111 (38.68%) | 39 (28.89%) | 56 (44.80%) | 0.061 | 173 (34.67%) | 167 (40.53%) | 0.080 |
| p53 | 328 (41.41%) | 135 (42.86%) | 97 (39.75%) | 55 (45.08%) | 41 (36.94%) | 0.544 | 190 (43.48%) | 138 (38.87%) | 0.216 |
| Ki67 (≥ 40%) | 124 (15.31%) | 58 (17.85%) | 33 (13.10%) | 22 (18.03%) | 11 (9.91%) | 0.124 | 80 (17.90%) | 44 (12.12%) | 0.030 |
| Laboratory test | |||||||||
| Initial | |||||||||
| FSH (IU/L) | 37.38 ± 30.15 | 37.01 ± 31.61 | 33.93 ± 28.73 | 43.40 ± 29.05 | 39.98 ± 28.98 | 0.048 | 38.65 ± 31.07 | 35.74 ± 28.89 | 0.416 |
| Platelet (103 mm3) | 238.66 ± 72.70 | 237.47 ± 73.38 | 241.26 ± 74.23 | 233.10 ± 71.58 | 242.18 ± 68.66 | 0.562 | 236.29 ± 72.85 | 241.54 ± 72.51 | 0.216 |
| AST (U/L) | 23.85 ± 13.53 | 21.79 ± 9.42 | 22.43 ± 9.37 | 26.08 ± 17.47 | 30.73 ± 21.92 | <0.001 | 22.95 ± 12.27 | 24.95 ± 14.86 | 0.033 |
| ALT (U/L) | 22.17 ± 16.86 | 18.39 ± 13.70 | 20.43 ± 12.38 | 27.13 ± 21.14 | 31.80 ± 23.05 | <0.001 | 20.75 ± 16.49 | 23.88 ± 17.16 | <0.001 |
| Serum albumin (mg/dL) | 4.20 ± 0.45 | 4.22 ± 0.46 | 4.14 ± 0.47 | 4.23 ± 0.44 | 4.23 ± 0.38 | 0.092 | 4.22 ± 0.45 | 4.17 ± 0.45 | 0.040 |
| Total bilirubin (mg/dL) | 0.59 ± 0.33 | 0.60 ± 0.27 | 0.56 ± 0.27 | 0.64 ± 0.57 | 0.58 ± 0.25 | 0.081 | 0.61 ± 0.38 | 0.56 ± 0.26 | 0.024 |
| Total cholesterol (mg/dL) | 185.16 ± 35.97 | 182.03 ± 37.16 | 183.82 ± 34.51 | 193.00 ± 36.18 | 188.87 ± 34.33 | 0.006 | 185.00 ± 37.18 | 185.35 ± 34.50 | 0.884 |
| Triglyceride (mg/dL) | 132.95 ± 96.18 | 109.55 ± 64.12 | 132.42 ± 90.74 | 144.71 ± 82.52 | 185.90 ± 156.16 | <0.001 | 119.58 ± 71.52 | 148.85 ± 117.20 | <0.001 |
| HDL-cholesterol (mg/dL) | 53.40 ± 13.41 | 54.86 ± 13.91 | 54.02 ± 13.85 | 52.64 ± 12.87 | 48.47 ± 9.98 | <0.001 | 54.30 ± 13.67 | 52.36 ± 13.05 | 0.059 |
| LDL-cholesterol (mg/dL) | 107.32 ± 32.39 | 105.79 ± 31.31 | 104.31 ± 30.29 | 113.44 ± 34.13 | 113.38 ± 37.54 | 0.039 | 107.68 ± 32.16 | 106.89 ± 32.72 | 0.743 |
| Fasting blood glucose (mg/dL) | 110.07 ± 28.42 | 107.40 ± 25.31 | 109.61 ± 30.42 | 112.27 ± 31.95 | 116.58 ± 27.28 | 0.001 | 108.72 ± 27.32 | 111.72 ± 29.64 | 0.043 |
| BARD | 1.95 ± 0.77 | 1.96 ± 0.62 | 1.95 ± 0.74 | 1.93 ± 0.94 | 1.95 ± 1.01 | 0.930 | 1.95 ± 0.72 | 1.95 ± 0.83 | 0.984 |
| NFS | -1.04 ± 1.31 | -1.05 ± 1.30 | -1.06 ± 1.35 | -0.93 ± 1.30 | -1.10 ± 1.25 | 0.589 | -1.01 ± 1.30 | -1.07 ± 1.32 | 0.393 |
| FIB-4 | 1.27 ± 1.03 | 1.26 ± 0.72 | 1.24 ± 1.23 | 1.38 ± 1.43 | 1.26 ± 0.82 | 0.332 | 1.29 ± 0.96 | 1.25 ± 1.12 | 0.083 |
| ALT elevation | <0.001 | 0.461 | |||||||
| No | 524 (57.52%) | 224 (61.54%) | 187 (65.16%) | 69 (51.11%) | 44 (35.20%) | 293 (58.72%) | 231 (56.07%) | ||
| Yes | 387 (42.48%) | 140 (38.46%) | 100 (34.84%) | 66 (48.89%) | 81 (64.80%) | 206 (41.28%) | 181 (43.93%) |
Data are reported as means and standard deviation (SD) (mean ± SD) for continuous variables and frequency (percentage) for categorical variables. Proportions are presented as percentages for categorical variables. P-values were computed by one-way ANOVA or Student’s t-test for continuous variables and chi-squares test or Fisher’s exact test for categorical variables as appropriate (a, computed by Fisher’s exact test).
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal growth factor receptor 2; FSH, follicle stimulating hormone; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NFS, nonalcoholic fatty liver disease fibrosis score; FIB-4, fibrosis-4.
* Definition of fatty liver progression is increased grade of fatty liver according to abdominal ultrasound or decreasing liver attenuation index value on non-contrast computed tomography.
Fig 1. Cumulative incidence of fatty liver progression before propensity score matching.
(A) All patients (N = 911), (B) Patients with initial fatty liver (N = 260), (C) Patients without initial fatty liver (N = 651).
Next, we investigated factors affecting fatty liver progression by Cox proportional hazard regression analyses. Table 3 shows that tamoxifen significantly contributed to the progression of fatty liver [hazard ratio (HR): 1.598, 95% confidence interval (CI): 1.173–2.177, P = 0.003] as compared with other treatments modality after adjusting for BMI, progesterone receptor (PR), and FSH. This effect of tamoxifen on fatty liver was consistently meaningful whether or not fatty liver was present at baseline (S1 Table).
Table 3. Multivariable analysis for the risk factors associated with fatty liver progression.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Treatment modality | ||||
| Control | 1 (Reference) | 1 (Reference) | ||
| Aromatase inhibitor | 1.331 (0.985–1.798) | 0.063 | 1.230 (0.839–1.803) | 0.289 |
| Tamoxifen | 1.744 (1.361–2.234) | <0.001 | 1.598 (1.173–2.177) | 0.003 |
| Age (year) | 1.002 (0.993–1.012) | 0.618 | ||
| BMI (kg/m2) | 1.061 (1.036–1.087) | <0.001 | 1.083 (1.052–1.114) | <0.001 |
| Diabetes | 1.197 (0.843–1.701) | 0.315 | ||
| Hypertension | 1.214 (0.959–1.537) | 0.107 | ||
| Cancer stage | ||||
| ≤1 | 1 (Reference) | |||
| 2 | 1.230 (1.003–1.507) | 0.047 | ||
| ≥3 | 1.204 (0.857–1.693) | 0.284 | ||
| Pathology | ||||
| Invasive ductal carcinoma | 1 (Reference) | |||
| Ductal carcinoma in situ | 1.271 (0.915–1.764) | 0.153 | ||
| Mucinous carcinoma | 1.300 (0.761–2.22) | 0.337 | ||
| Infiltrating lobular carcinoma | 1.051 (0.576–1.917) | 0.871 | ||
| Intraductal papilloma | 2.187 (1.199–3.991) | 0.011 | ||
| Tubular carcinoma | 0.878 (0.123–6.252) | 0.896 | ||
| Apocrine carcinoma | 0.923 (0.13–6.574) | 0.936 | ||
| Squamous carcinoma | 1.005 (0.141–7.16) | 0.996 | ||
| Medullary carcinoma | 0.900 (0.289–2.807) | 0.856 | ||
| Others | 0.820 (0.204–3.294) | 0.780 | ||
| Lymph node metastasis | 1.115 (0.907–1.37) | 0.303 | ||
| ER (Intermediate or High) | 1.459 (1.17–1.82) | 0.001 | ||
| PR (Intermediate or High) | 1.621 (1.312–2.002) | <0.001 | 1.395 (1.049–1.857) | 0.022 |
| HER2 (Intermediate or High) | 1.215 (0.998–1.479) | 0.052 | ||
| Chemotherapy | 0.827 (0.664–1.029) | 0.089 | ||
| Radiotherapy | 1.092 (0.9–1.326) | 0.372 | ||
| FSH | 0.995 (0.991–0.999) | 0.012 | 0.995 (0.991–0.999) | 0.024 |
| Platelet | 1.002 (1.001–1.003) | 0.004 | 1.002 (1.001–1.004) | 0.004 |
| AST | 1.004 (0.998–1.011) | 0.207 | ||
| ALT | 1.004 (0.999–1.009) | 0.115 | ||
| Serum albumin | 0.902 (0.729–1.115) | 0.339 | ||
| Total bilirubin | 0.620 (0.419–0.917) | 0.017 | ||
| Total cholesterol | 0.999 (0.997–1.002) | 0.668 | ||
| Triglyceride | 1.001 (1–1.002) | 0.002 | ||
| HDL-cholesterol | 0.983 (0.975–0.992) | <0.001 | ||
| LDL-cholesterol | 1 (0.996–1.003) | 0.972 | ||
| Fasting blood glucose | 1.003 (1–1.006) | 0.088 | ||
| BARD | 1.044 (0.921–1.184) | 0.503 | ||
| NFS | 0.947 (0.876–1.024) | 0.170 | ||
| FIB-4 | 0.937 (0.83–1.056) | 0.287 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal growth factor receptor 2; FSH, follicle stimulating hormone; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NFS, nonalcoholic fatty liver disease fibrosis score; FIB-4, fibrosis-4.
Propensity score matching analysis
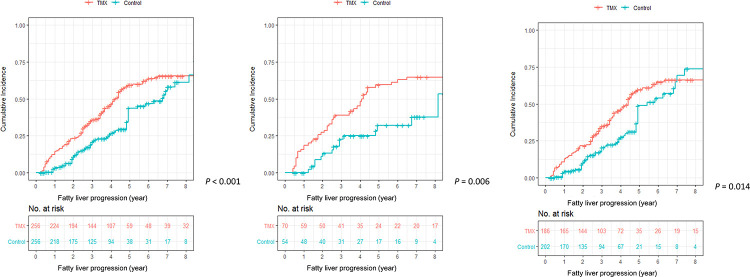
We performed PS matching in these patients (S2 Table). After PS matching, factors that might affect the development of fatty liver such as age, BMI, cancer stage, diabetes, and hypertension were well-balanced (S2 Fig). In the matched cohort, tamoxifen significantly increased the incidence of fatty liver progression compared with the control group (P<0.001, Fig 2A). And this phenomenon was observed in the same manner regardless of fatty liver status at baseline (Fig 2B and 2C). Univariate (S3 Table) and multivariate analyses (Table 4) according to baseline fatty liver status were also performed for the matched cohort, showing similar results with non-matched cohort. Overall, tamoxifen increased the risk of fatty liver by 1.385 times (95% CI: 1.019–1.883, P = 0.037) compared to control after adjusting for BMI and PR status. The effect of tamoxifen on fatty liver progression was also confirmed to the same degree and significance in other matched datasets (HR: 1.4, 95% CI: 1.018–1.927 in dataset with caliper 0.3; HR: 1.452, 95% CI: 1.021–2.065 in dataset with caliper 0.1) (S5 and S6 Tables).
Fig 2. Cumulative incidence of fatty liver progression after propensity score matching.
(A) All patients (N = 512), (B) Patients with initial fatty liver (N = 124), (C) Patients without initial fatty liver (N = 388).
Table 4. Propensity score matching analysis for risk factors associated with fatty liver progression.
| Variable | Multivariable | |
|---|---|---|
| HR (95% CI) | P-value | |
| All (N = 512) | ||
| Treatment modality | ||
| Control | 1 (Reference) | |
| Tamoxifen | 1.385 (1.019–1.883) | 0.037 |
| Body mass index (kg/m2) | 1.069 (1.032–1.107) | <0.001 |
| PR (Intermediate or High) | 1.607 (1.186–2.178) | 0.002 |
| Fatty liver (-) at baseline (N = 388) | ||
| Treatment modality | ||
| Control | 1 (Reference) | |
| Tamoxifen | 2.214 (1.416–3.464) | <0.001 |
| Body mass index (kg/m2) | 1.067 (1.014–1.122) | 0.013 |
| Triglyceride | 1.005 (1.002–1.007) | <0.001 |
| Fatty liver (+) at baseline (N = 124) | ||
| Treatment modality | ||
| Control | 1 (Reference) | |
| Tamoxifen | 2.103 (1.156–3.826) | 0.015 |
| Body mass index (kg/m2) | 1.049 (1.005–1.096) | 0.029 |
| HER2 (Intermediate + High) | 1.804 (1.06–3.071) | 0.030 |
| Radiotherapy | 2.081 (1.217–3.56) | 0.007 |
| Total cholesterol | 0.988 (0.981–0.996) | 0.002 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Prediction model associated with fatty liver progression
Apart from multivariate analysis, we have made prediction models 1, 2, 3, and 4, incorporating various confounding factors to re-confirm the causal relationship between tamoxifen use and fatty liver progression. As shown in Table 5, tamoxifen was significantly associated with progression of fatty liver regardless of all these factors. After adjusting for age, BMI, diabetes, hypertension, menopausal status, cancer-related factors and other laboratory findings, tamoxifen was independently associated with increased risk of fatty liver (before matching, HR: 2.998, 95% CI: 1.646–5.462, P < 0.001; after matching, HR: 2.907, 95% CI: 1.451–5.821, P = 0.003).
Table 5. Prediction model related to fatty liver progression.
| Variable | N | Model 1 * | Model 2 † | Model 3 § | Model 4 ¶ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| Before matching | All | |||||||||
| Control | 296 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| Aromatase inhibitor | 199 | 1.331 (0.985–1.798) | 0.063 | 1.232 (0.902–1.682) | 0.190 | 1.191 (0.858–1.654) | 0.295 | 1.381 (0.806–2.367) | 0.239 | |
| Tamoxifen | 416 | 1.744 (1.361–2.234) | <0.001 | 1.882 (1.461–2.424) | <0.001 | 1.860 (1.416–2.444) | <0.001 | 1.859 (1.167–2.96) | 0.009 | |
| Fatty liver (-) at baseline | ||||||||||
| Control | 226 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| Aromatase inhibitor | 128 | 1.389 (0.971–1.985) | 0.072 | 1.231 (0.848–1.788) | 0.274 | 1.100 (0.742–1.631) | 0.634 | 1.135 (0.597–2.156) | 0.699 | |
| Tamoxifen | 297 | 1.467 (1.103–1.952) | 0.009 | 1.665 (1.244–2.227) | 0.001 | 1.612 (1.172–2.217) | 0.003 | 1.511 (0.843–2.708) | 0.165 | |
| Fatty liver (+) at baseline | ||||||||||
| Control | 70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| Aromatase inhibitor | 71 | 1.514 (0.844–2.718) | 0.164 | 1.581 (0.87–2.871) | 0.133 | 1.749 (0.898–3.405) | 0.100 | 2.410 (0.666–8.722) | 0.180 | |
| Tamoxifen | 119 | 2.691 (1.61–4.497) | <0.001 | 2.672 (1.575–4.534) | <0.001 | 2.998 (1.646–5.462) | <0.001 | 3.668 (1.232–10.925) | 0.020 | |
| After matching | All | |||||||||
| Control | 256 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| Tamoxifen | 256 | 1.636 (1.237–2.165) | 0.001 | 1.714 (1.293–2.271) | <0.001 | 1.773 (1.294–2.43) | <0.001 | 1.716 (0.98–3.005) | 0.059 | |
| Fatty liver (-) at baseline | ||||||||||
| Control | 202 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| Tamoxifen | 186 | 1.481 (1.075–2.042) | 0.016 | 1.612 (1.166–2.23) | 0.004 | 1.590 (1.092–2.314) | 0.016 | 1.497 (0.745–3.007) | 0.257 | |
| Fatty liver (+) at baseline | ||||||||||
| Control | 54 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| Tamoxifen | 70 | 2.241 (1.244–4.038) | 0.007 | 2.219 (1.226–4.015) | 0.008 | 2.907 (1.451–5.821) | 0.003 | 279.154 (2.887–26992.851) | 0.016 | |
Abbreviations: HR, hazard ratio; CI, confidence interval.
* Model 1: unadjusted.
† Model 2: adjusted for age, BMI, and underlying disease (diabetes, hypertension).
§ Model 3: Model 2 plus cancer-related factors (stage, pathology, lymph node metastasis).
¶ Model 4: Model 3 plus initial laboratory factors (FSH, platelet, AST, ALT, total bilirubin, total cholesterol, triglyceride, fasting blood glucose).
Next, we performed a subgroup analysis in the tamoxifen group to find any correlations between the degree of hepatic steatosis with drug duration or changes in liver enzymes. However, we discovered no significant or clinically meaningful correlations when using the spearman’s correlation coefficient (data not shown).
Effect of fatty liver progression on mortality
In addition, we analyzed the effect of fatty liver on the prognosis of breast cancer patients. When patients were classified into two groups according to progression of fatty liver, there was no significant difference in long-term mortality between the two groups (before matching, P = 0.928, S3A Fig; after matching, P = 0.471, S3B Fig). Given the follow-up period with our cohort, cardiovascular risk possibly caused by tamoxifen was not fully assessed. In univariate (S4 Table) and multivariate analyses (Table 6), progression of fatty liver did not significantly affect mortality of breast cancer patients (HR: 0.753, 95% CI: 0.380–1.493, P = 0.417), although cancer stage and age were associated with mortality.
Table 6. Multivariable Cox proportional hazards regression for death.
| Variable | Multivariable | |
|---|---|---|
| HR (95% CI) | P-value | |
| All (N = 911) | ||
| Fatty liver progression | ||
| No | 1 (Reference) | |
| Yes | 0.753 (0.380–1.493) | 0.417 |
| Age (year) | 1.041 (1.014–1.068) | 0.003 |
| ER (Intermediate or High) | 0.314 (0.164–0.599) | <0.001 |
| Cancer stage | ||
| ≤1 | 1 (Reference) | |
| 2 | 1.647 (0.795–3.412) | 0.180 |
| ≥3 | 3.789 (1.587–9.045) | 0.003 |
Abbreviations: ER, estrogen receptor; HR, hazard ratio; CI, confidence interval.
Discussion
Recent studies have shown that 10 years of adjuvant treatment of tamoxifen is superior to 5 years of tamoxifen use in reducing the risk of breast cancer recurrence or death.[8, 18] Since then, extended adjuvant tamoxifen (10 years) is recommended for selected high risk groups. However, there are still few studies considering the effect of tamoxifen on the incidence of fatty liver from a large population. Since the use of tamoxifen has been extended to be 10 years, its side effect such as fatty liver is expected to increase even more.
The largest study of tamoxifen-induced fatty liver was conducted in 2005 enrolling 5,408 patients in Italy.[9] However, the major disadvantage of that study was that the occurrence of fatty liver was not evaluated by imaging study, but by an increase in liver enzyme. In addition, patients included in that study were not breast cancer patients, but healthy women who underwent hysterectomy. Thereafter, although several studies were reported, the patient group was so heterogeneous as to come to a solid conclusion, and factors that could affect fatty liver development were not adjusted.[19, 20] In this study, we presented the risk of tamoxifen versus control or AI accurately using PS matching and multiple Cox regression analysis with a large cohort of patients. Tamoxifen had a great effect both on the development of de novo fatty liver and fatty liver worsening than other treatment modalities. In addition, there was a steady increase in fatty liver incidence in proportion to the cumulative dose as seen on the Kaplan-Meier curve.
The mechanism of how tamoxifen causes fatty liver is not well understood yet. The most representative hypothesis is the “multiple hit” hypothesis which suggests that fat accumulation would make liver vulnerable to oxidants, and then second hits (e.g., tamoxifen) could promote progress to steatohepatitis.[9] In this process, production of large amounts of reactive oxygen species, decreased mitochondrial β-oxidation, and increased TNF-α is known to play a major role in drug-induced steatohepatitis.[21] Our study supported this hypothesis with the fact that tamoxifen was more associated with deterioration of fatty liver in patients with pre-existing fatty liver than in those without.
The second hypothesis is that tamoxifen decreases lipid metabolism by inhibiting estrogen. Estrogen can regulate hepatic lipid metabolism through ERα, ERβ or GPER with regard to the extent of liver gene expression.[22] Indeed, the incidence of fatty liver gradually increases after menopause. It can be interpreted that with greater estrogen inhibition, more fatty liver will occur. Tamoxifen is known to cause higher levels of estrogen deprivation than AI. While AI blocks estrogen production in menopausal women who have already shown decreased estrogen production, tamoxifen blocks estrogen by binding to its receptor in the premenopausal status.[23, 24] Our study also demonstrated that the incidence of fatty liver was proportional to the degree of estrogen deprivation, which was highest in the tamoxifen group followed by the aromatase inhibitor and control group.
Because tamoxifen can induce or aggravate fatty liver mainly in susceptible patients, it is important to do close surveillance in advance, especially in high risk groups such as those with high BMI or presence of basal fatty liver. However, whether tamoxifen should be discontinued after the development of fatty liver is currently unclear. In our study, the occurrence of fatty liver did not affect the prognosis of breast cancer. Thus, we are not certain of the benefits and harms of suspending tamoxifen intake because it is difficult to adequately reflect the risk of cardiovascular disease during the five-year follow-up period. The guideline recommends weight reduction by 10%, rather than stopping the drug immediately.[21] Also, there was a report showing that moderate-intensity exercise of more than 150-minutes a week could reduce the risk of fatty liver development.[25] If fatty liver does not improve using this method, suspending tamoxifen or changing it to AI may be considered.
Our study has several limitations. First, the main limitation is predominant from its retrospective nature. The patients were not randomly assigned to the modalities which meant that the choice of modalities might have been biased. Also, there were some subjects who had no imaging study during the follow-up period which resulted in missing data. Along with the above drawbacks, this study has been designed for bias-reduction by propensity score matching. This result can be used to implement further prospective cohort study. Second, the quantification of steatosis was evaluated by either USG or CT. For the diagnostic ability of non-enhanced CT for fatty liver, sensitivity of macrovesicular steatosis of 30% or greater was 0.991 (95% CI: 0.960–0.999), and specificity was 1.0 (95% CI: 0.97, 1.00).[26, 27] In general, diagnostic performance of non-enhanced CT is comparable to USG and MR-PDFF.[28] Considering accessibility and price, it has slight advantages over MRI, and the inter-reader and intra-reader accordance is superior to ultrasound. In addition, recent studies by Artz et al.[29] and Luca et al.[15] suggested that simple ROI-based mean attenuation measurement with CT has an inverse linear relationship with fat content measured by MR spectroscopy. These studies imply that CT may be used as a quantitative imaging biomarker for both detection and quantification of liver fat content. Third, we did not evaluate whether fatty liver was improved after discontinuation of tamoxifen because of the relatively short follow–up period. It would ascertain the causal relationship between tamoxifen use and fatty liver progression. Lastly, studies on Asians may not be applicable to all other races.
In conclusion, tamoxifen may be an important risk factor in the incidence and progression of fatty liver. Close follow-up and screening are necessary for high risk patients. Also, multidisciplinary approach with hepatologists should be considered for these populations. In the future, studies using accurate steatosis quantification (e.g., transient elastography, MR-based technique) may be needed with a large, prospective cohort.
Supporting information
(DOCX)
(DOCX)
(A) Before matching, (B) After matching.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Eun-Ae Jung (Librarian, Medical Library, Soonchunhyang University Bucheon Hospital) for carefully proofreading the manuscript.
List of abbreviations
- AI
aromatase inhibitor
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- CT
computed tomography
- ER
estrogen receptor
- HR
hazard ratio
- HU
hounsfield unit
- MR
magnetic resonance
- PR
progesterone receptor
- PS
propensity score
- ROI
regions of interest
- SERM
selective estrogen receptor modulator
- USG
ultrasonography
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology. 2012;26(8):688–94, 96. . [PubMed] [Google Scholar]
- 2.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351(9114):1451–67. . [PubMed] [Google Scholar]
- 3.Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. The New England journal of medicine. 1989;320(8):479–84. 10.1056/NEJM198902233200802 . [DOI] [PubMed] [Google Scholar]
- 4.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2011;103(17):1299–309. 10.1093/jnci/djr242 . [DOI] [PubMed] [Google Scholar]
- 5.Lee B, Jung EA, Yoo JJ, Kim SG, Lee CB, Kim YS, et al. Prevalence, incidence and risk factors of tamoxifen-related non-alcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020;40(6):1344–55. Epub 2020/03/15. 10.1111/liv.14434 . [DOI] [PubMed] [Google Scholar]
- 6.Hong N, Yoon HG, Seo DH, Park S, Kim SI, Sohn JH, et al. Different patterns in the risk of newly developed fatty liver and lipid changes with tamoxifen versus aromatase inhibitors in postmenopausal women with early breast cancer: A propensity score-matched cohort study. Eur J Cancer. 2017;82:103–14. Epub 2017/06/27. 10.1016/j.ejca.2017.05.002 . [DOI] [PubMed] [Google Scholar]
- 7.Nishino M, Hayakawa K, Nakamura Y, Morimoto T, Mukaihara S. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. AJR American journal of roentgenology. 2003;180(1):129–34. Epub 2002/12/20. 10.2214/ajr.180.1.1800129 . [DOI] [PubMed] [Google Scholar]
- 8.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–16. 10.1016/S0140-6736(12)61963-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, et al. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. Bmj. 2005;330(7497):932 10.1136/bmj.38391.663287.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. Epub 2017/07/18. 10.1002/hep.29367 . [DOI] [PubMed] [Google Scholar]
- 11.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(3):310–20. Epub 2018/03/11. 10.6004/jnccn.2018.0012 . [DOI] [PubMed] [Google Scholar]
- 12.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–50. 10.1053/gast.2002.35354 . [DOI] [PubMed] [Google Scholar]
- 13.Lupsor M, Badea R. Imaging diagnosis and quantification of hepatic steatosis: is it an accepted alternative to needle biopsy? Romanian journal of gastroenterology. 2005;14(4):419–25. . [PubMed] [Google Scholar]
- 14.Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230(1):276–80. 10.1148/radiol.2301021176 . [DOI] [PubMed] [Google Scholar]
- 15.Saba L, di Martino M, Bosco S, Del Monte M, de Cecco CN, Lombardo V, et al. MDCT classification of steatotic liver: a multicentric analysis. Eur J Gastroenterol Hepatol. 2015;27(3):290–7. Epub 2015/01/30. 10.1097/MEG.0000000000000277 . [DOI] [PubMed] [Google Scholar]
- 16.Vohra S, Goyal N, Gupta S. Preoperative CT evaluation of potential donors in living donor liver transplantation. The Indian journal of radiology & imaging. 2014;24(4):350–9. 10.4103/0971-3026.143897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David W. Hosmer SL Jr., Susanne May. Applied survival analysis: regression modeling of time-to-event data. John Wiley & Sons. 2011;618. [Google Scholar]
- 18.Al-Mubarak M, Tibau A, Templeton AJ, Cescon DW, Ocana A, Seruga B, et al. Extended adjuvant tamoxifen for early breast cancer: a meta-analysis. PloS one. 2014;9(2):e88238 10.1371/journal.pone.0088238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan HJ, Chang HT, Lee CH. Association between tamoxifen treatment and the development of different stages of nonalcoholic fatty liver disease among breast cancer patients. Journal of the Formosan Medical Association = Taiwan yi zhi. 2016;115(6):411–7. Epub 2015/06/15. 10.1016/j.jfma.2015.05.006 . [DOI] [PubMed] [Google Scholar]
- 20.Yang YJ, Kim KM, An JH, Lee DB, Shim JH, Lim YS, et al. Clinical significance of fatty liver disease induced by tamoxifen and toremifene in breast cancer patients. Breast. 2016;28:67–72. Epub 2016/05/31. 10.1016/j.breast.2016.04.017 . [DOI] [PubMed] [Google Scholar]
- 21.Osman KA, Osman MM, Ahmed MH. Tamoxifen-induced non-alcoholic steatohepatitis: where are we now and where are we going? Expert opinion on drug safety. 2007;6(1):1–4. 10.1517/14740338.6.1.1 . [DOI] [PubMed] [Google Scholar]
- 22.Palmisano BT, Zhu L, Stafford JM. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv Exp Med Biol. 2017;1043:227–56. Epub 2017/12/11. 10.1007/978-3-319-70178-3_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque R, Ahmed SA, Fisher A, Avila CC, Shi J, Guo A, et al. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer medicine. 2012;1(3):318–27. 10.1002/cam4.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez EA. Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2007;18 Suppl 8:viii26–35. 10.1093/annonc/mdm263 . [DOI] [PubMed] [Google Scholar]
- 25.Chang HT, Pan HJ, Lee CH. Prevention of Tamoxifen-related Nonalcoholic Fatty Liver Disease in Breast Cancer Patients. Clinical breast cancer. 2018;18(4):e677–e85. 10.1016/j.clbc.2017.11.010 . [DOI] [PubMed] [Google Scholar]
- 26.Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol. 2012;19(7):811–8. Epub 2012/04/24. 10.1016/j.acra.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239(1):105–12. Epub 2006/02/18. 10.1148/radiol.2391050361 . [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10(8):530–42. Epub 2018/09/08. 10.4254/wjh.v10.i8.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artz NS, Hines CD, Brunner ST, Agni RM, Kuhn JP, Roldan-Alzate A, et al. Quantification of hepatic steatosis with dual-energy computed tomography: comparison with tissue reference standards and quantitative magnetic resonance imaging in the ob/ob mouse. Invest Radiol. 2012;47(10):603–10. Epub 2012/07/28. 10.1097/RLI.0b013e318261fad0 [DOI] [PMC free article] [PubMed] [Google Scholar]