Abstract
Background:
Communicating healthcare costs to patients is an important component of delivering high-quality value-based care, yet cost data is lacking. This is especially relevant for ovarian cancer, where no clinical consensus on optimal first-line treatment exists.
Objective:
The objective of this study was to generate cost estimates of different primary management strategies in ovarian cancer.
Study Design:
All women who underwent treatment for ovarian cancer from 2006–2015 were identified from the MarketScan database (n=12,761) in this observational cohort study. Total and out-of-pocket costs were calculated using all claims within 8 months from initial treatment and normalized to 2017 US dollars. The generalized linear model method was used to assess cost by strategy.
Results:
Among patients who underwent neoadjuvant chemotherapy and those who underwent primary debulking, mean adjusted total costs were $113,660 and $107,153 (p<.001), and mean out-of-pocket costs were $2519 and $2977 (p<.001), respectively. Total costs for patients who had intravenous standard, intravenous dose-dense, and intraperitoneal/intravenous chemotherapy were $105,047, $115,099, and $121,761 (p<.001); and out-of-pocket costs were $2838, $3405, and $2888 (p<.001), respectively. Total costs for regimens including bevacizumab were higher than those without it ($171,468 vs $104,482, p<.001), as were out-of-pocket costs ($3127 vs $2898, p<.001). Among patients who did not receive bevacizumab, 25% paid ≥$3875, and 10% paid ≥$6265. For patients who received bevacizumab, 25% paid ≥$4480, and 10% paid ≥$6635. Among patients enrolled in high-deductible health plans, median out-of-pocket costs were $4196, with 25% paying ≥$6680, and 10% paying ≥$9751.
Conclusions:
Costs vary across different treatment strategies and patients bear a significant out-of-pocket burden, especially those enrolled in high-deductible health plans.
Keywords: Ovarian cancer, cost, out-of-pocket costs, value-base care, high-deductible health plan, bevacizumab, chemotherapy, financial toxicity
Introduction
In 2016, healthcare costs comprised 17.9% of the gross domestic product (GDP) in the United States. Projections from the Center for Medicare and Medicaid Services suggest that medical costs will reach 20% of the GDP by 2026.1 The costs of cancer care have been disproportionately rising over the past decade, with total and out-of-pocket costs becoming a major burden for both the healthcare system and patients.2 With increasing costs, insurers have shifted more of the financial burden to patients, who often face unpredictable or uncontrollable costs, including high deductibles or co-insurance.3 Financial toxicity, or financial distress as an adverse effect of cancer treatment, has been associated with greater symptom burden, poorer quality of life and an increased risk of mortality.4, 5
Communicating healthcare costs to patients is an important component of delivering high-quality value-based care. Given the potential significant impact of costs on patients and their families, the Institute of Medicine recommends providing them with information on the cost of care.6 This is especially relevant for patients with ovarian cancer, where no clinical consensus on optimal first-line treatment exists. Patients may be treated with primary debulking surgery followed by adjuvant chemotherapy, or neoadjuvant chemotherapy followed by interval debulking surgery.7 Chemotherapy may be administered intravenously every 3 weeks, intravenously every week, or intraperitoneally.8, 9 Additionally, regimens may or may not include bevacizumab, an angiogenesis inhibitor.10 All of these treatment approaches are approved by the current National Comprehensive Cancer Network guidelines for ovarian cancer.11 Despite the recommendation to share cost information with patients, cost data is lacking. Therefore, the objective of our study was to generate total and out-of-pocket cost estimates of different primary management strategies in ovarian cancer.
Materials and Methods
This was a retrospective cohort study using the Truven Health Analytics MarketScan® database, a commercial healthcare claims database. It contains de-identified claims data on 240 million patients in the United States enrolled in commercial health insurance plans sponsored by more than 100 payers since 1995.12 The database includes monthly enrollment data, inpatient admission records, outpatient services, outpatient prescription drug claims, and costs of services, with robust longitudinal follow-up. We used a combination of International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes, Common Procedural Terminology (CPT) codes, and Healthcare Common Procedure Coding System (HCPCS) codes to identify relevant covariates, treatments, and outcomes (supplementary Table S1). Institutional Review Board approval was obtained.
We aimed to assess claims data for women who underwent primary treatment for epithelial ovarian cancer (ICD-9 codes 183.0, 183.2, 183.8). Patients were included if they underwent both surgery and chemotherapy, if they received platinum-based chemotherapy (considered standard of care), if they had two inpatient diagnoses claims for ovarian cancer or at least two outpatient diagnosis claims that were more than 30 days apart, and if they had complete healthcare coverage for 8 months after diagnosis. We considered 8 months to be an appropriate time interval for receipt of surgery, post-operative recovery, and receipt of neoadjuvant or adjuvant chemotherapy. Since our goal was to identify costs associated with primary therapy only, we chose not to extend this time limit as that would risk including the costs of treatment for disease progression or recurrence. We identified a total of 36,853 patients who underwent primary treatment from January 2006 to December 2015. The year 2006 was chosen as the starting year for data collection as that was when a landmark study reporting on the use of intraperitoneal/intravenous chemotherapy was published.9 We sequentially excluded 513 patients who received bleomycin/etoposide (considered as treatment for non-epithelial ovarian cancer), 1,203 patients with ICD-9 codes for ‘personal history of malignant neoplasm of ovary’ (V10.43), 305 patients who received chemotherapy or surgery before 2006, 13,175 patients who did not get both surgery and chemotherapy within 8 months post diagnosis, and 7,545 patients who did not have complete coverage 6 months before and 8 months after diagnosis. 12,761 patients remained, comprising our study population.
Patients were classified based on: 1) whether they underwent primary debulking surgery followed by postoperative chemotherapy, or neoadjuvant chemotherapy followed by interval debulking surgery; 2) type of chemotherapy regimen administered (intravenous every 3 weeks [standard], intravenous every week [dose-dense], or intraperitoneal/intravenous); and 3) if regimens included bevacizumab (supplementary Table S1). Patients were considered to have had neoadjuvant chemotherapy if there was at least one billing code for a chemotherapeutic agent before a surgical code. Patients were classified as having received intraperitoneal chemotherapy based on the presence of at least one billing code for the intraperitoneal delivery of a chemotherapeutic drug. Patients without a code for intraperitoneal chemotherapy were classified as having received either dose-dense or standard chemotherapy based on the administration schedule. Dose-dense chemotherapy is typically administered as a platinum agent every three weeks in combination with a weekly taxane. However, because some women may not receive a taxane every week due to toxicity, dose-dense chemotherapy was defined as a ratio of ≥1.5 of a taxane to platinum (patients receiving standard chemotherapy would have a ratio of 1:1).8, 13 Patients who did not meet the criteria for either dose-dense or intraperitoneal chemotherapy were considered to have had standard chemotherapy.
Patient-level variables collected included age at diagnosis (grouped as ≤49, 50–59, 60–69, ≥70 years), Charlson comorbidity index, year of diagnosis (2006–2008, 2009–2011, 2012–2015), region of treatment (northeast, north central, south, west), and insurance type (health maintenance organization, preferred provider organization, other). We used the Klabunde modification of the Charlson comorbidity index to assess patient comorbidity, using claims in the 6 months prior to cancer diagnosis.14 To prevent over-estimation, a patient’s comorbidity diagnosis had to appear on at least two different claims that were more than 30 days apart.
The primary outcomes of this study were total and out-of-pocket costs within 8 months from initial treatment, stratified by treatment strategy. The starting time to calculate costs was the initial treatment claim, whether it was a billing code for a chemotherapeutic agent or a surgical code. A secondary outcome was the analysis of costs of patients enrolled in high-deductible health plans. High-deductible plans were coded as an insurance type in the MarketScan® database. Costs were defined as reimbursed costs (not charges), and were calculated using all inpatient, outpatient, and prescription claims within that time period. Out-of-pocket costs were calculated as the sum of co-insurance, co-payments, and deductibles. Total costs consisted of patient out-of-pocket expenses, in addition to insurance payments made. All cost estimates were normalized to 2017 U.S. dollars using the medical care component of the consumer price index.15 Mean and median costs were calculated. Due to the skewed and non-normal distribution of cost, a generalized linear model with a gamma family and log link function was used to assess cost by strategy, adjusting for clinical and demographic factors (age, Charlson comorbidity index, region of care, insurance plan, year of diagnosis). All these covariates were significantly associated with cost variability on univariate analysis. All statistical tests were two-sided, with a p value of <.05 considered significant. Analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
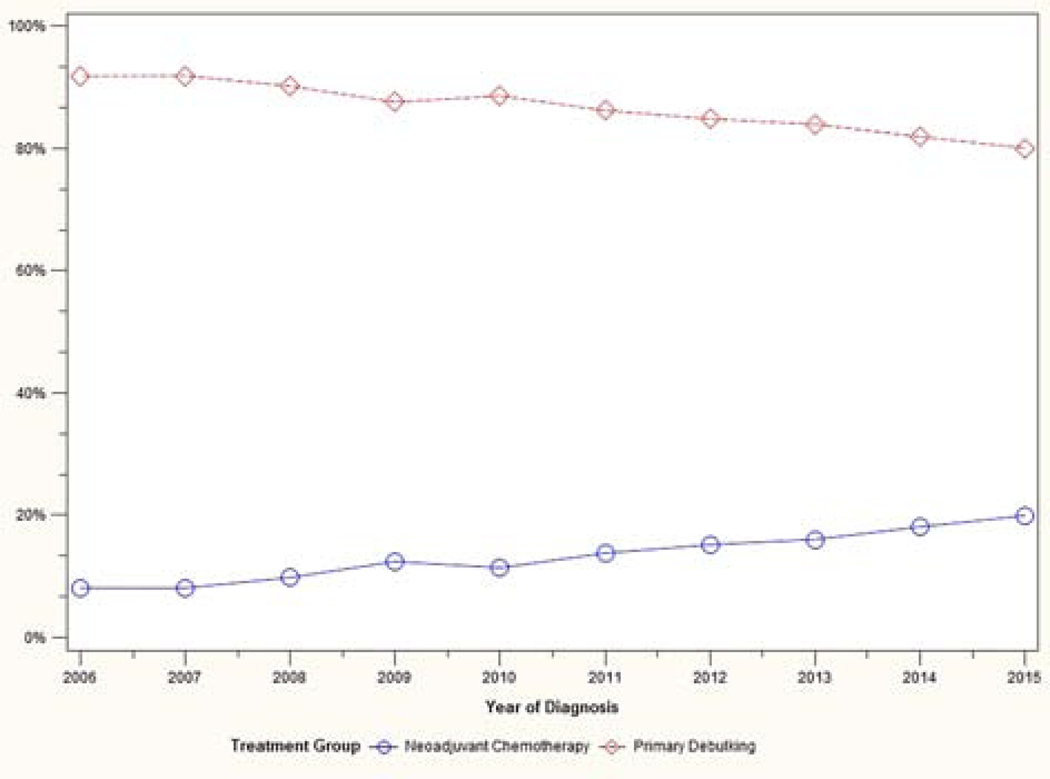
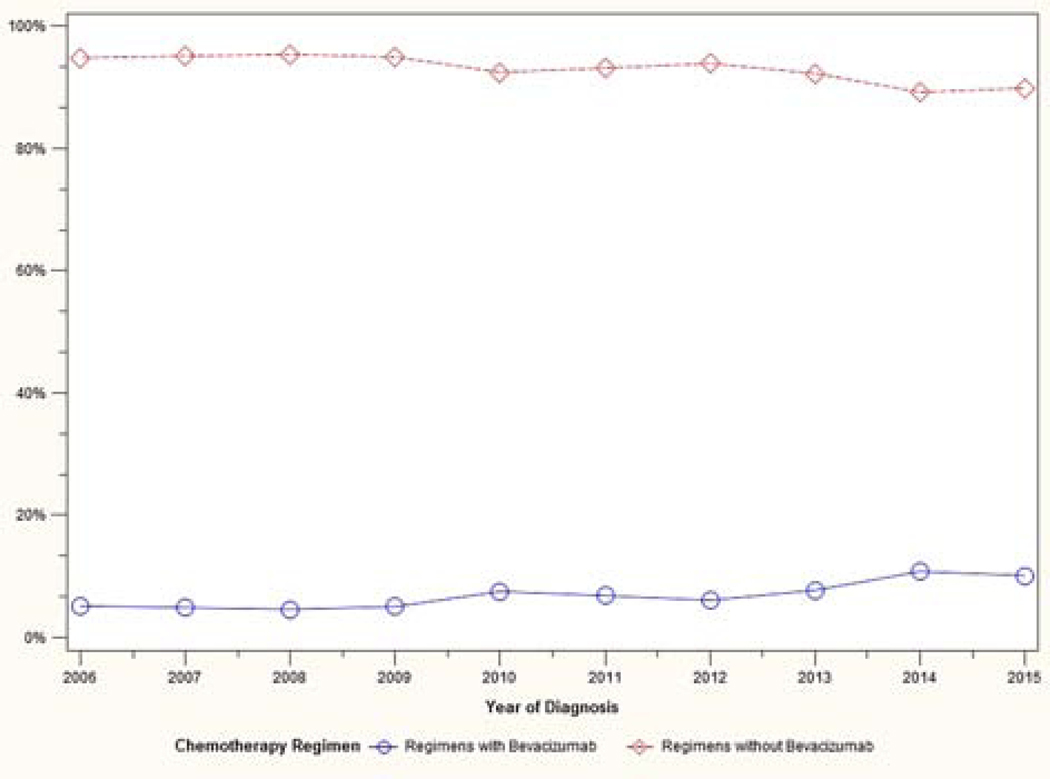
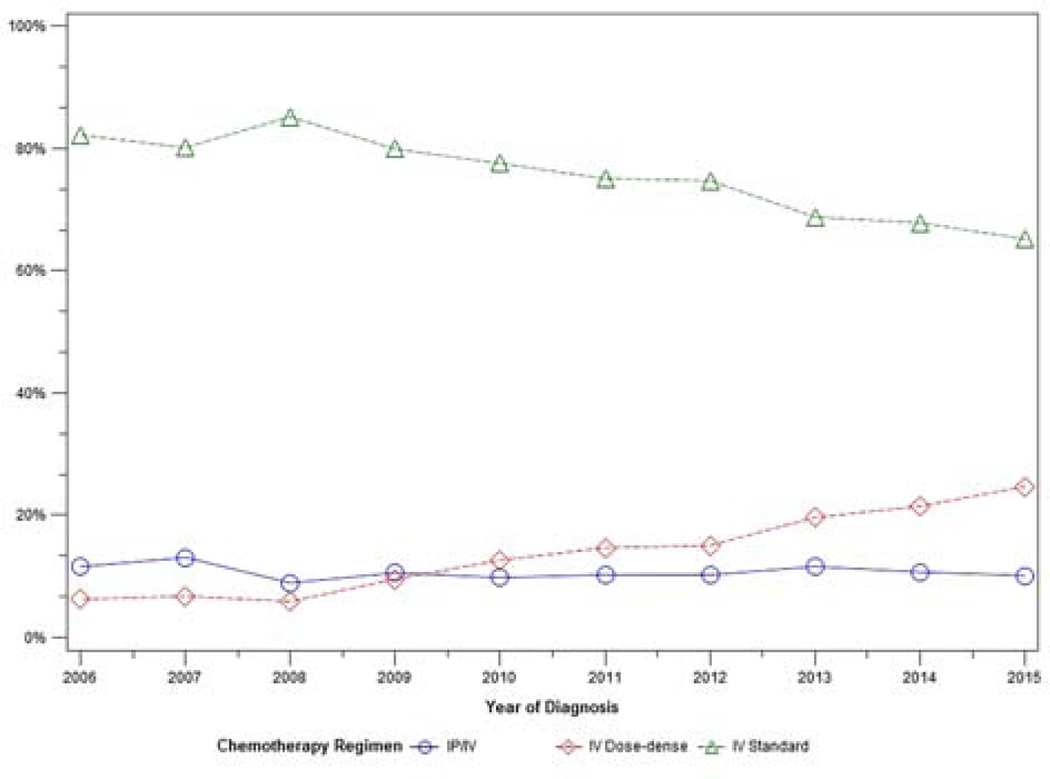
A total of 12,761 patients met eligibility criteria. Patient characteristics are shown in Table 1. Trends in treatment variability over time are shown in Figures 1–3. The use of neoadjuvant chemotherapy increased over time, from 8% in 2006 to 20% in 2015. The use of intravenous standard chemotherapy decreased from 82% to 65% during that time period, while intravenous dose-dense chemotherapy administration increased from 6% to 25%. Intraperitoneal/intravenous chemotherapy use remained stable during the study period (11% to 10%). Bevacizumab use rose gradually from 5% in 2006 to 10% in 2015.
Table 1:
Patient characteristics (n = 12,761)
| Characteristic | n | % |
|---|---|---|
| Age | ||
| ≤49 | 2404 | 19% |
| 50–59 | 4788 | 37% |
| 60–69 | 3697 | 29% |
| ≥70 | 1872 | 15% |
| Charlson comorbidity index | ||
| 0 | 11010 | 86% |
| 1 | 1425 | 11% |
| ≥2 | 326 | 3% |
| Year of diagnosis | ||
| 2006 – 2008 | 3041 | 24% |
| 2009 – 2011 | 5263 | 41% |
| 2012 – 2015 | 4457 | 35% |
| Insurance plan | ||
| HMO (Health Maintenance Organization) | 1447 | 11% |
| PPO (Preferred Provider Organization) | 7469 | 59% |
| Other | 3845 | 30% |
| Region of care | ||
| Northeast | 2595 | 20% |
| North Central | 3353 | 27% |
| South | 4367 | 34% |
| West | 2303 | 18% |
| Unknown | 148 | 1% |
Figure 1:
Trend in treatment approach over time
Figure 3:
Trend in bevacizumab use over time
Total and out-of-pocket costs by treatment approach, chemotherapy regimen, and inclusion of bevacizumab are shown in Tables 2 and 3. Among patients who underwent neoadjuvant chemotherapy and those who underwent primary debulking, mean adjusted total costs were $113,660 and $107,153 (p<.001), and mean adjusted out-of-pocket costs were $2519 and $2977 (p<.001), respectively. Mean adjusted total costs were highest for patients who had intraperitoneal/intravenous chemotherapy ($121,761), followed by intravenous dose-dense ($115,099), and intravenous standard chemotherapy ($105,047) (p<.001). Mean adjusted out-of-pocket costs were highest for the intravenous dose-dense group ($3405), followed by intraperitoneal/intravenous ($2888) and intravenous standard chemotherapy ($2838) (p<.001). Mean adjusted total costs for regimens including bevacizumab were higher than those without it ($171,468 vs $104,482, p<.001), as were out-of-pocket costs ($3127 vs $2898, p<.001).
Table 2:
Total costs ($US dollars) by treatment approach, chemotherapy regimen, and inclusion of bevacizumab (n = 12,761)
| n (%) | Unadjusted total costs | Adjust total costs* | ||||
|---|---|---|---|---|---|---|
| Median | Mean | Mean | 95% CI | p | ||
| Primary debulking | 11,091 (87%) | $89,228 | $109,745 | $107,153 | $105,962 – $108,357 | <.001 |
| Neoadjuvant chemotherapy | 1670 (13%) | $92,770 | $112,879 | $113,660 | $110,404 – $117,012 | |
| Chemotherapy regimen | ||||||
| IV standard | 9739 (76%) | $85,879 | $106,753 | $105,047 | $103,799 – $106,309 | <.001 |
| IV dose-dense | 1679 (13%) | $95,136 | $117,729 | $115,099 | $111,798 – $118,497 | |
| IP/IV | 1343 (11%) | $109,995 | $125,359 | $121,761 | $117,890 – $125,760 | |
| Regimens without bevacizumab | 11,912 (93%) | $86,859 | $105,525 | $104,482 | $103,362 – $105,613 | <.001 |
| Regimens with bevacizumab | 849 (7%) | $153,389 | $175,122 | $171,468 | $164,650 – $178,567 | |
IV: Intravenous; IP: Intraperitoneal
Costs were adjusted for age, Charlson comorbidity index, region of care, insurance plan, and year of diagnosis
Table 3:
Out-of-pocket costs ($US dollars) by treatment approach, chemotherapy regimen, and inclusion of bevacizumab (n = 12,761)
| n (%) | Unadjusted out-of-pocket costs | Adjusted out-of-pocket costs* | ||||
|---|---|---|---|---|---|---|
| Median | Mean | Mean | 95% CI | p | ||
| Primary debulking | 11,091 (87%) | $2111 | $3021 | $2977 | $2923 – $3031 | <.001 |
| Neoadjuvant chemotherapy | 1670 (13%) | $1489 | $2367 | $2519 | $2403 – $2640 | |
| Chemotherapy regimen | ||||||
| IV standard | 9739 (76%) | $1982 | $2870 | $2838 | $2784 – $2893 | <.001 |
| IV dose-dense | 1679 (13%) | $2103 | $3279 | $3405 | $3250 – $3571 | |
| IP/IV | 1343 (11%) | $2145 | $2981 | $2888 | $2742 – $3042 | |
| Regimens without bevacizumab | 11,912 (93%) | $1982 | $2917 | $2898 | $2848 – $2948 | <.001 |
| Regimens with bevacizumab | 849 (7%) | $2449 | $3197 | $3127 | $2931 – $3336 | |
IV: Intravenous; IP: Intraperitoneal
Costs were adjusted for age, Charlson comorbidity index, region of care, insurance plan, and year of diagnosis
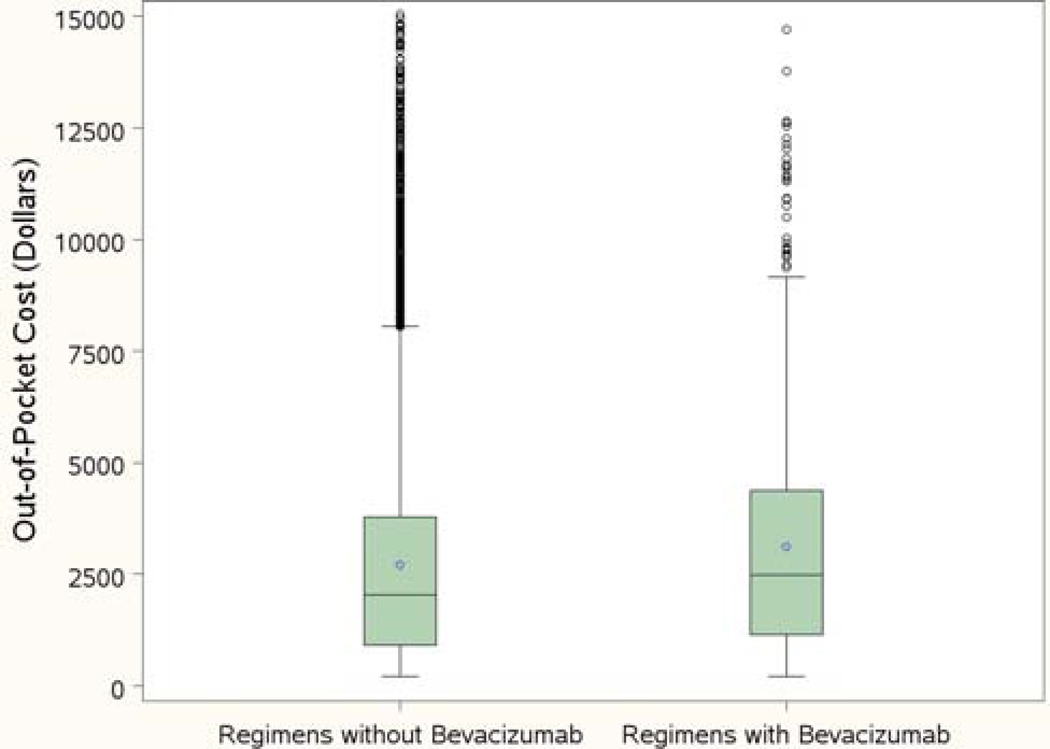
The distribution of out-of-pocket costs by receipt of bevacizumab is shown in Figure 4. Overall, 7% of the cohort received a chemotherapy regimen with bevacizumab. As seen in the figure, the range of out-of-pocket payments was quite large. Among the patients who did not receive bevacizumab, 25% paid more than $3875, and 10% paid more than $6265. For patients who received bevacizumab, 25% paid more than $4480, and 10% paid at least $6635.
Figure 4:
Out-of-pocket costs for regimens with or without bevacizumab. Box: 25% to 75% percentiles; bold horizontal line: median cost; dot: mean cost; circles: outliers.
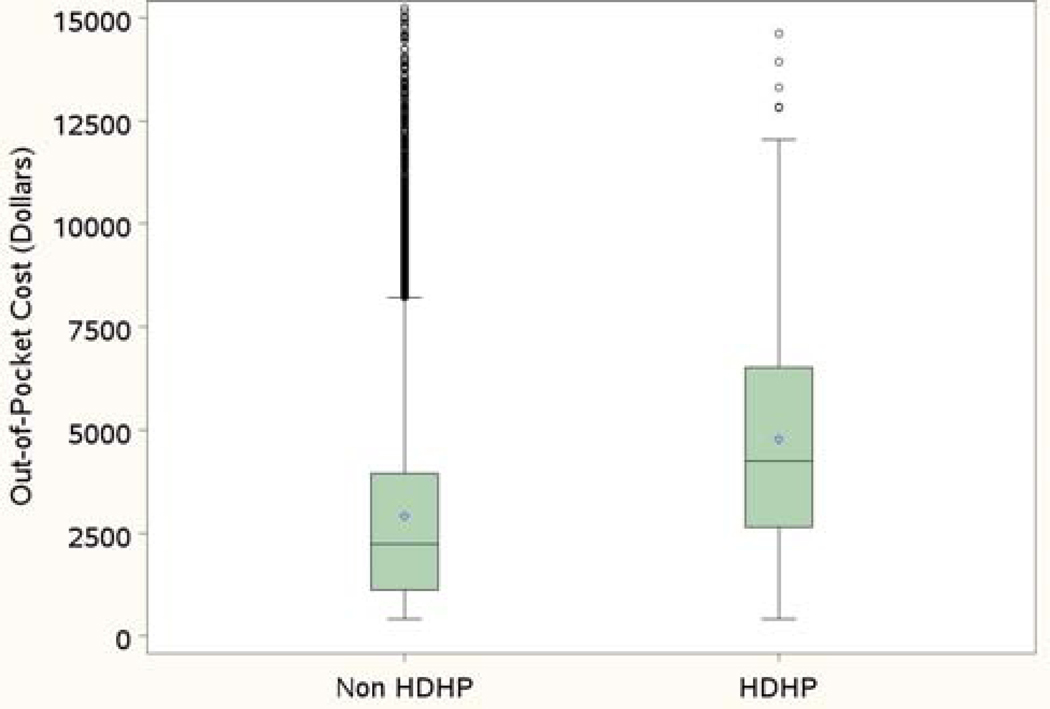
Patients enrolled in high-deductible health plans made up 2% of our cohort. Among these patients, median out-of-pocket costs were $4196, with 25% paying more than $6680, and 10% paying at least $9751 (Figure 5). In this cohort, mean adjusted out-of-pocket costs were higher for patients who underwent primary surgery compared to neoadjuvant chemotherapy ($5238 vs 2989, p=.004). Intraperitoneal/intravenous chemotherapy was associated with the highest mean adjusted out-of-pocket costs ($6156), compared to intravenous dose-dense chemotherapy ($4896), and intravenous standard ($4754). However, this difference was not significant (p=.18). In comparison, among patients not enrolled in high-deductible health plans, median out-of-pocket costs were $1983, with 25% paying more than $3852, and 10% paying at least $6219.
Figure 5:
Out-of-pocket costs for high-deductible health plans (HDHP) and non-high-deductible health plans. Box: 25% to 75% percentiles; bold horizontal line: median cost; dot: mean cost; circles: outliers.
Discussion
In this study, we described total and out-of-pocket cost estimates of different primary management strategies in ovarian cancer. The costs of cancer care have been steadily rising in the United States, and are estimated to reach $158 billion by 2020.16 In order to address these increasing costs, the American Society of Clinical Oncology has developed a framework to assess and compare the value of cancer treatment options. This framework has defined value by emphasizing three critical elements: clinical benefit, complications, and cost.17 Our data not only add to the growing literature on the costs of cancer care to patients, but can also help inform discussions regarding the relative value of different interventions.
With rising co-insurance costs and deductibles, patients have picked up an ever-increasing share of this financial burden.3 Rising out-of-pocket costs can lead to financial toxicity, which has been associated with adverse clinical outcomes and reduced quality of life.5 There is increasing awareness of the need to communicate healthcare costs to patients and their families, as a component of delivering quality care. In a survey asking 5000 patients to identify key characteristics of high-value health care, a plurality (45%) chose “my out-of-pocket costs are affordable”.18 This is particularly relevant when there is no consensus on optimal treatment strategies, as in ovarian cancer.
In our study, neoadjuvant chemotherapy followed by surgery was associated with higher mean total costs than primary debulking surgery followed by chemotherapy. These results are comparable to those published by Urban et al and Forde et al, who both used the SEER-Medicare database for their analysis.19, 20 Total costs were highest for patients who had intraperitoneal/intravenous chemotherapy, followed by intravenous dose-dense, and intravenous standard chemotherapy. This is in-line with the report by Wright and colleagues.13 On the other hand, primary debulking, intravenous dose-dense chemotherapy, and receipt of bevacizumab were all associated with higher out-of-pocket costs. While Bercow et al assessed the costs of care within the first year of diagnosis of ovarian cancer, to our knowledge, no other studies have compared out-of-pocket costs of different interventions in this population.21
Bevacizumab is a monoclonal antibody directed against vascular endothelial growth factor. It was recently approved by the Food and Drug Administration (FDA) in the United States for the front-line treatment of ovarian cancer, based on two phase-three clinical trials.10, 22 Importantly, these trials only showed a progression-free survival benefit and no overall survival benefit. The adjusted mean difference in out-of-pocket costs for regimens with bevacizumab vs those without was $229 ($3127 vs $2898). Although that may not appear to be a large difference, it is important to note that the range of out-of-pocket payments was quite large for patients who received bevacizumab, with 25% paying more than $4480, and 10% paying at least $6635. When looking at total costs which included insurance payments, the difference was substantial ($171,468 vs $104,482). This calls into question the use of an agent with such high costs but no overall survival benefit. This issue becomes even more significant when taking quality of life into account. In a cost-utility analysis of bevacizumab use in the primary treatment of ovarian cancer, regimens including bevazicumab were not cost-effective compared to regimens without it when evaluating quality-adjusted life expectancy.23 Of note, the FDA recently approved mvasi (bevacizumab-awwb) as a biosimilar to bevacizumab for the treatment of colorectal, lung, brain, kidney and cervical cancers. While it has not been approved for ovarian cancer, a future approval and use in this disease could lead to lower healthcare costs.
Patients enrolled in high-deductible health plans had significantly higher out-of-pocket expenses than the rest of the cohort. Twenty-five percent of these patients paid more than $6680, and 10% paid at least $9751. Given the unclear survival benefit of different therapeutic strategies, it may be helpful to pursue treatment options with a lower out-of-pocket burden for these patients. This also applies to some patients who were not in high-deductible health plans. Although the median out-of-pocket payment was $1983, 25% paid more than $3852, and 10% paid at least $6219. Hunter and colleagues showed that even brief conversations between oncologists and patients about cancer costs may help reduce treatment expenses.24
The strengths of this study lie in the fact that our cost data was based on actual inpatient and outpatient insurance reimbursements made to both hospitals and providers. We also evaluated the global 8 month period after diagnosis, which takes into account the cost of the entire hospital stay for surgery, the management of complications, emergency room visits, and readmissions. Additionally, the MarketScan® database includes patients who are enrolled in commercial insurance plans sponsored by more than 100 payers, which allowed us to examine costs nationally. Our study is limited by the fact that since costs were based on insurance reimbursements, they reflect the payer’s perspective, which may or may not reflect the cost of delivering care from a provider/hospital perspective. MarketScan® lacks information regarding certain demographic and tumor variables such as race, histology, grade, and stage, which precluded us from assessing the association of these factors with costs. Additionally, since the database only includes patients who are commercially insured, these results may not be generalizable to other populations.
In conclusion, the costs of initial treatment of ovarian cancer vary across different treatment strategies and patients bear a significant out-of-pocket burden, especially those enrolled in high-deductible plans. As no consensus exists on optimal first-line management, these data may help inform value-based discussions between providers and patients.
Supplementary Material
Figure 2:
Chemotherapy regimen use over time
Condensation:
Costs of initial care in ovarian cancer vary across different treatment strategies and patients bear a significant out-of-pocket burden, especially those enrolled in high-deductible plans.
AJOG at a Glance:
A. Why was this study conducted?
Although communicating healthcare costs to patients is an important component of delivering value-based care, cost data is lacking. Our objective was to generate cost estimates of different primary management strategies in ovarian cancer.
B. Key findings?
We described total and out-of-pocket cost estimates of different treatment approaches and types of chemotherapy regimens administered. The range of out-of-pocket payments was large for patients who received bevacizumab: although the mean was $3127, 25% paid ≥$4480, and 10% paid ≥$6635. Patients enrolled in high-deductible health plans had significantly higher out-of-pocket expenses, with 25% paying ≥$6680, and 10% paying ≥$9751.
C. What does this add to what is known?
Costs vary across different treatment strategies and patients bear a significant out-of-pocket burden, especially those enrolled in high-deductible plans.
Acknowledgments
Funding Sources/Conflicts of Interest Statements:
This research is supported in part by the Duncan Family Institute, a Cancer Center Support Grant (CCSG) for National Cancer Institute-designated Cancer Centers (#CA016672), and a National Cancer Institute grant (#P30 CA016672).
Rudy Suidan:
Research support: National Institutes of Health T32 grant (#5T32 CA101642).
Larissa Meyer:
Research support: National Cancer Institute K award (#K07 CA201013)
Sharon Giordano:
Research support: Cancer Prevention and Research Institute of Texas grant (#RP160674) and Komen grant (#SAC150061).
Relevant financial activities outside the supported work:
Larissa Meyer
Research support: AstraZeneca
Consultant: Clovis Oncology
Charlotte Sun:
Research support: AstraZeneca
Footnotes
The other authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Centers for Medicare and Medicaid Services. Available from URL: https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nhe-fact-sheet.html [accessed December 12, 2018]. [PubMed]
- 2.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The State of Cancer Care in America, 2017: A Report by the American Society of Clinical Oncology. J Oncol Pract. 2017;13:e353–e394. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey SD, Bansal A, Fedorenko CR, et al. Financial Insolvency as a Risk Factor for Early Mortality Among Patients With Cancer. J Clin Oncol. 2016;34:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of Financial Strain With Symptom Burden and Quality of Life for Patients With Lung or Colorectal Cancer. J Clin Oncol. 2016;34:1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levit L, Balogh E, Nass S, Ganz PA. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC; The National Academies Press; 2013. [PubMed] [Google Scholar]
- 7.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. [DOI] [PubMed] [Google Scholar]
- 8.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–1026. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. [DOI] [PubMed] [Google Scholar]
- 10.Ra Burger, Brady MF Bookman Ma, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (NCCN). NCCN guidelines for ovarian, fallopian tube, and primary peritoneal cancer treatment regimens. Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf [accessed December 18, 2018].
- 12.Truven Health Analytics MarketScan® database. Available from URL: https://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases [accessed December 3, 2018].
- 13.Wright JD, Hou JY, Burke WM, et al. Utilization and Toxicity of Alternative Delivery Methods of Adjuvant Chemotherapy for Ovarian Cancer. Obstet Gynecol. 2016;127:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44:921–928. [DOI] [PubMed] [Google Scholar]
- 15.Measuring price change for medical care in the CPI. Consumer price index. Washington, DC: Division of Consumer Prices and Price Indexes, U.S. Bureau of Labor Statistics; Available from URL: https://www.bls.gov/cpi/factsheets/home.htm [accessed December 25, 2018]. [Google Scholar]
- 16.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J Clin Oncol. 2015;33:2563–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pendleton RC. We Won’t Get Value-Based Health Care Until We Agree on What “Value” Means. Available from URL: https://hbr.org/2018/02/we-wont-get-value-based-health-care-until-we-agree-on-what-value-means [accessed December 25, 2018]. [Google Scholar]
- 19.Urban RR, He H, Alfonso-Cristancho R, Hardesty MM, Goff BA. The Cost of Initial Care for Medicare Patients With Advanced Ovarian Cancer. J Natl Compr Canc Netw. 2016;14:429–437. [DOI] [PubMed] [Google Scholar]
- 20.Forde GK, Chang J, Ziogas A, Tewari K, Bristow RE. Costs of treatment for elderly women with advanced ovarian cancer in a Medicare population. Gynecol Oncol. 2015;137:479–484. [DOI] [PubMed] [Google Scholar]
- 21.Bercow AS, Chen L, Chatterjee S, et al. Cost of Care for the Initial Management of Ovarian Cancer. Obstet Gynecol. 2017;130: 1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. [DOI] [PubMed] [Google Scholar]
- 23.Cohn DE, Barnett JC, Wenzel L, et al. A cost-utility analysis of NRG Oncology/Gynecologic Oncology Group Protocol 218: incorporating prospectively collected quality-of-life scores in an economic model of treatment of ovarian cancer. Gynecol Oncol. 2015;136:293–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter WG, Zafar SY, Hesson A, et al. Discussing Health Care Expenses in the Oncology Clinic: Analysis of Cost Conversations in Outpatient Encounters. J Oncol Pract. 2017;13:e944–e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.