Abstract
Biologic therapies have revolutionized the management of inflammatory bowel disease [IBD], but primary and secondary non-responses occur in a significant proportion of patients. Therapeutic drug monitoring [TDM] now has an established role in the treatment algorithm for managing secondary loss of response to anti-tumour necrosis factor [anti-TNF] agents during maintenance therapy. Data to support the use of TDM in the management of secondary loss of response to vedolizumab and ustekinumab are emerging. The potential to prevent primary non-response to biologic agents during induction is of equal, and potentially greater, clinical importance. Again, most data supporting the use of ‘proactive' TDM during induction pertains to the use of anti-TNF agents, but signals of efficacy for the use of TDM during induction with other biologic classes are now appearing. This review aims to summarize data on the use of TDM during induction to prevent pharmacokinetic primary non-response to all three classes of biologic therapy currently available for the treatment of IBD.
Keywords: therapeutic drug monitoring, induction, primary non-response
1. Introduction
The management of inflammatory bowel disease [IBD] has been transformed in recent years by the concurrent emergence of both highly effective medical therapies and better strategies of using these agents. The goals of therapy have evolved from clinical remission to deep remission, including mucosal healing, which has demonstrably better short- and long-term clinical outcomes in both Crohn’s disease [CD] and ulcerative colitis [UC].1–3 The major advance in treatment strategy has been the gradual adoption of a ‘treat-to-target' approach in which therapeutic goals have evolved beyond symptomatic control to the normalization of objective markers of inflammation, and mucosal healing in particular. This treatment strategy of tight disease monitoring, which is probably of equal or greater importance than the choice of medical therapy used, has recently been prospectively proven to improve clinical outcomes in CD.4 The major advance in medical therapeutics has been the availability of biologic therapies which arguably have the highest benefit–risk ratio of all agents used to treat moderate–severe IBD. Three classes of biologic therapy are available for the treatment of IBD: anti-tumour necrosis factor agents (anti-TNFs—infliximab [IFX], adalimumab [ADA], certolizumab pegol, golimumab), adhesion molecule inhibitors [vedolizumab] and the anti-interleukin [anti-IL] 12/23 agent ustekinumab.
Therapeutic drug monitoring [TDM] has emerged as a means of optimizing biologic therapies, and can be either ‘reactive' or ‘proactive'. Reactive TDM is performed when loss of response [LOR] to a biologic agent has occurred, usually secondary loss of response in a patient who has initially responded. Proactive TDM involves measuring and utilizing serum drug levels as an independent treatment target irrespective of a patient's disease activity and response status. Most data pertain to the use of reactive TDM of anti-TNF agents in the setting of secondary LOR during maintenance therapy. The use of reactive TDM at secondary LOR allows for rational treatment decision-making to determine whether an individual patient is most likely to respond to dose-escalation of the same anti-TNF, switching in class to another anti-TNF, or switching out of class to an agent with a different mechanism of action. Reactive TDM has demonstrable clinical benefits and is cost-effective, as shown in both retrospective and prospective studies, and has been endorsed by multiple international consensus guidelines.5–8 The role of proactive TDM of anti-TNFs remains to be determined and is the subject of considerable debate. Two randomized controlled trials utilizing proactive TDM of anti-TNFs failed to show significant benefits over clinically based decision-making, although both had inherent design limitations and some secondary end points did favour the use of proactive TDM.9,10 In contrast, observational studies, predominantly from a few centres, have demonstrated the benefits of proactive TDM with anti-TNFs, including less secondary LOF, reduced immunogenicity, and a reduction in hospitalizations and surgeries.11,12 Of equal importance to the type of TDM [reactive vs proactive] is the timing of TDM [during induction or maintenance]. Most studies of either reactive or proactive TDM have been performed during maintenance therapy. More recently, interest has emerged in the use of proactive TDM during induction with the aim of preventing primary non-response [PNR].13–15 PNR is usually defined as non-response by the end of induction and may be for pharmacokinetic or pharmacodynamic [mechanistic] reasons. Pharmacokinetic PNR is due to increased drug clearance, which may be immune mediated or non-immune mediated [e.g. due to high inflammatory burden]. Pharmacodynamic PNR occurs when active disease persists despite therapeutic biologic drug levels, implying that disease pathophysiology is driven by an alternative inflammatory pathway.14
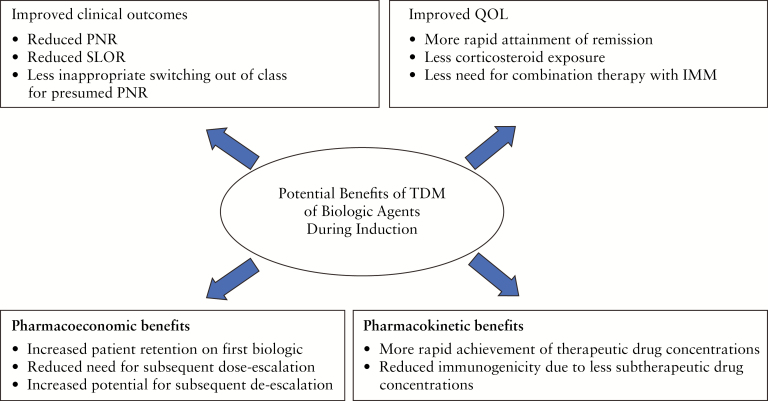
Concurrently, pharmacokinetic models are emerging which support individualization of anti-TNF dosing during induction to achieve adequate drug levels to overcome the inflammatory burden of active disease, after which de-escalation may be attempted during maintenance therapy when disease is in remission.16 Most data pertaining to proactive TDM during induction are from studies of anti-TNFs but such data are now emerging with the newer classes of biologic therapy. The potential benefits of TDM of biologic agents during induction are shown in Figure 1. In this review we aim to summarize the evidence to date on the use of proactive TDM during induction to prevent primary pharmacokinetic non-response with anti-TNFs, vedolizumab and ustekinumab.
Figure 1.
Potential benefits of TDM of biologic agents during induction. PNR, primary non-response; SLOR, secondary loss of response; QOL, quality of life.
2. Anti-TNFs
2.1. Infliximab
IFX is a chimeric IgG1 monoclonal antibody targeting soluble and membrane-bound TNF-α. It is approved for the treatment of moderate–severe adult and paediatric CD and UC. The safety and efficacy of IFX in IBD was demonstrated in the registration studies ACCENT 1 and 2 [adult luminal and fistulizing CD respectively], REACH [paediatric CD], and ACT 1 and 2 [adult UC].17–20
2.1.1. Pharmacokinetics of infliximab
IFX is administered intravenously, ensuring 100% bioavailability, after which the concentration–time profile demonstrates high peak-to-trough ratios. Due to its high molecular weight and hydrophilic distribution, IFX is restricted to the bloodstream and the volume of distribution is low at 3–6 L. Clearance is predominantly via catabolism rather than renal excretion; the half-life of IFX is approximately 9 days.21 Parameters that may influence IFX pharmacokinetics, leading to higher non-immune clearance and lower drug concentrations, are those associated with high disease activity, such as high C-reactive protein [CRP], serum and tissue TNF, and white blood cell counts, and low albumin and haemoglobin concentrations.21 In the ACT 1 and ACT 2 trials of IFX for treatment of active UC, elevated body weight, lower albumin, the presence of anti-drug antibodies and male sex independently increased drug clearance leading to lower drug concentrations.19,22 Immune mechanisms of clearance include the development of anti-drug antibodies that can neutralize the drug's effect. In a cohort of 125 consecutive CD patients, Baert et al. showed that the development of antibodies against IFX was associated with an increased risk of infusion reactions and a reduced duration of response to treatment. Concomitant immunosuppressive therapy reduced the prevalence and titre of anti-IFX antibodies.23
2.1.2. Therapeutic drug monitoring of IFX during induction therapy
Up to 30% of IBD patients do not benefit from IFX induction and are considered primary non-responders at week 14.17,24,25 For these patients, another biologic agent can be considered, although efficacy rates are low in both CD and UC, meaning that adverse disease outcomes, including surgery, are common in primary non-responders.26,27 Accordingly, there is a need to improve drug efficacy during induction; early proactive TDM targeting adequate drug concentrations during induction could be an effective strategy to achieve this aim. In a post-hoc analysis of the ACCENT 1 pivotal trial in CD, sustained clinical response at 1 year was associated with IFX levels ≥3.5 µg/mL at week 14.13 More recently, in a small retrospective paediatric cohort of 35 children receiving IFX [23 CD, 12 UC], week 14 IFX levels were higher in patients achieving, vs not achieving, clinical remission [4.6 vs 1.5 µg/mL, p ≤ 0.002] and biological remission [4.6 vs 2.6 µg/mL, p ≤ 0.002] at week 52.28 In a large retrospective case-control study of CD and UC patients, Bar-Yoseph et al. confirmed that lower IFX levels at week 2 and week 6 were associated with PNR at week 14.29 Recently, in a small, single-centre retrospective study of 83 IFX patients [76 CD, 7 UC], proactive TDM of IFX monotherapy based on dose-adjustment from week 10 IFX levels aiming for a level of 5–10 µg/mL at week 14 [proactive TDM group, n = 16] was compared to a strategy of reactive TDM at or after week 14 in patients on IFX monotherapy [monotherapy standard of care group, n = 32] or combination therapy [combination therapy standard of care group, n = 35]. During 12 months of follow-up, no patients in the proactive TDM group discontinued IFX, compared to 8/32 [25%] of patients in the monotherapy standard of care group and 1/35 [3%] of patients in the combination therapy standard of care group [p = 0.04 and p = 1.0 respectively]. At the time of first TDM, no proactive TDM patients had antibodies to IFX [ATI], compared to 41% of monotherapy standard of care patients [p = 0.002] and 6% of combination therapy standard of care patients [p = 1.0]. Median IFX concentrations throughout maintenance were 9.5 µg/mL in proactive TDM patients; this was higher than in monotherapy standard of care patients [6.4 µg/mL, p = 0.04], but not combination therapy standard of care patients [7.6 µg/mL, p = 0.1].30
These data have been reproduced in several other small prospective studies. Beltran et al. prospectively followed 35 CD patients during induction and found significantly lower IFX levels at week 6 and week 14 in primary non-responders compared to responders [7.3 vs 11.2 µg/mL, p = 0.09; 1.5 vs 4.7 µg/mL p = 0.02, respectively].31 In another small prospective cohort of 17 patients [10 CD, 7 UC] IFX levels >4.8 µg/mL at week 14 were associated with clinical response at that timepoint.32 In a prospective paediatric cohort of 72 CD patients, clinical responders at week 14 had higher IFX levels than non-responders at week 2 [27.8 vs 18.8 µg/mL, p < 0.001] and week 6 [14.0 vs 7.8 µg/mL, p < 0.01].33
The personalized anti-TNF therapy in Crohn’s Study [PANTS] is a prospective observational UK-wide study of anti-TNF use in 1610 biologic naïve patients with active luminal CD (955 IFX [753 remicade; 202 biosimilar CT-P13] and 655 ADA]. PNR at week 14 occurred in 21.9% (125/466; 95% confidence interval [CI] 19.1–25.0%) of patients treated with IFX. On multivariable analysis, only low IFX levels at week 14 were independently associated with PNR at week 14 (odds ratio [O]R 0.35, 95% CI 0.20–0.62, p = 0.00038) and subsequent non-remission at week 54 [OR 0.29, 95% CI 0.16–0.52, p < 0.0001]. The optimal week 14 IFX drug level associated with remission at weeks 14 and 54 was 7 µg/mL. Low week 14 IFX levels predicted the development of immunogenicity, which in turn predicted low drug concentrations at week 54; this relationship was also seen with ADA. Immunogenicity was reduced by concomitant immunomodulator use with both IFX [OR 0.39, 95% CI 0.32–0.46] and ADA [OR, 0.44 95% CI 0.31–0.64; p < 0.0001 for both].34 More recently, data from prospective cohorts using the IFX biosimilar CT-P13 have demonstrated associations between early threshold trough levels during induction and subsequent clinical outcomes. In a Hungarian cohort, week 2 IFX levels >20.4 µg/mL in CD [n = 184] and >15.3 µg/mL in UC [n = 107] were associated with clinical remission at week 14.35
Data supporting the use of proactive TDM during induction are also emerging from dedicated UC studies. In a post-hoc analysis from a randomized controlled trial in UC, IFX concentrations at week 2 were associated with clinical remission at week 14 and mucosal healing at week 30.36 A further post-hoc analysis of 728 patients from the ACT 1 and 2 trials demonstrated that week 6 IFX levels >22 µg/mL were associated with clinical response at week 8, and week 14 IFX levels >5.1 µg/mL predicted clinical response at week 30.22
In a small cohort of moderate–severe UC patients, Brandse et al. prospectively studied the relationship between IFX levels and endoscopic response at week 8. Median IFX level at week 6 was 8.1 µg/mL among responders vs 2.9 µg/mL in non-responders [p = 0.03].37 In another challenging clinical scenario, acute severe UC [ASUC], it has been suggested that early IFX dose optimization during induction could improve clinical outcomes. In an Irish retrospective analysis of 50 patients, colectomy rate during IFX induction was lower when patients received an accelerated IFX regimen given over a median of 24 days (6.7% [1/15] vs 40% [14/35], p = 0.04), although subsequent need for colectomy during the follow-up period was unchanged between the groups. Unfortunately, no data are available regarding IFX levels in this study.38 More recently, several observational studies and one meta-analysis could not confirm these results. In a retrospective analysis from three centres, colectomy rates during admission and at 3, 6, 12 and 24 months were no different between 81 patients receiving an accelerated IFX regimen compared to 132 patients receiving a standard IFX regimen [p > 0.20 for all comparisons]. A meta-analysis of seven studies reported concurrently showed no difference in in-hospital colectomy rates between patients receiving accelerated [n = 181] vs standard [n = 436] IFX regimens [OR 0.76; 95% CI 0.36–1.61, p = 0.47].39
Beyond control of symptoms, therapeutic goals in IBD now include endoscopic healing. Cut-offs for IFX levels depend on the desired therapeutic end point, with higher levels required to achieve endoscopic rather than clinical remission. In the ACCENT 1 trial including patients with active luminal CD, the cut-off found for sustained clinical response was as low as 3.5 µg/mL.13 Conversely, in a retrospective study of 101 UC patients, Papamichael et al. found a cut-off of 15 µg/mL at week 6 to predict mucosal healing at weeks 10–14.15 In a post-hoc analysis of 484 UC patients from the ACT 1 and 2 trials, endoscopic remission at week 8 [defined as Mayo Endoscopic Score 0 or 1] was associated with IFX levels ≥18.6 µg/mL at week 2, ≥10.6 µg/mL at week 6 and ≥34.9 µg/mL at week 8.40
In a post-hoc analysis of the induction phase of the TAILORIX study, IFX levels >23.1 µg/mL at week 2 and >10.0 µg/mL at week 6 predicted endoscopic remission at week 12.41 Evidence is emerging that patients with perianal fistulizing CD require higher anti-TNF levels to achieve fistula healing than is required to achieve mucosal healing of luminal CD. Davidov et al. identified an IFX level cut-off of 9.25 µg/mL at week 2 and 7.25 µg/mL at week 6 for predicting fistula response at week 14.42 In a post-hoc analysis of 282 perianal fistulizing CD patients from the ACCENT 2 trial, IFX levels at weeks 2, 6 and 14 were compared with clinical complete fistula response at week 14 [CFR14]. In patients with CFR14, median IFX levels were significantly higher at week 6 (18.4 [12.7–27.8] μg/mL vs 15.2 [9.1–26.0] μg/mL; p = 0.038) and week 14 (6.4 [2.3–10.8] μg/mL vs 3.7 [1.5–7.3] μg/mL; p = 0.001). On receiver operating characteristic [ROC] analysis, IFX level thresholds of 13.9 μg/mL at week 6 (area under the receiver operating characteristic [AUROC] 0.57, 95% CI 0.51–0.64, p = 0.036) and 4.8 μg/mL at week 14 [AUROC 0.61, 95% CI 0.54–0.68, p = 0.001] were associated with CFR14.43
The pathophysiology of primary non-response has not yet been elucidated. Several hypotheses have been suggested, including the development of immunogenicity to biologics, such as IFX. In the above-cited observational studies, ATI were detected as soon as before the third infusion and were more prevalent and at higher titres in non-responders.37,42 A week 2 ATI titre above 4.3 μg/mL-eq was found to be predictive of PNR in the study by Bar-Yoseph et al.29 The high inflammatory burden of active disease also increases drug clearance independent of immunogenicity, leading to low serum drug levels that themselves predispose to increased immunogenicity, which further accelerates clearance.26,37,44,45 For example, faecal drug loss in patients with acute severe colitis has been observed. Brandse et al. showed in a cohort of 30 moderate–severe UC patients that the IFX faecal concentration was high in the first days after the first infusion, and higher in non-responders than in responders.46 However, no correlation was found between IFX faecal concentration and serum IFX levels. These findings were consistent with a French cohort of ASUC patients, where IFX was detected in the faeces in 6/15 patients but there was no correlation between serum or faecal IFX levels and the severity of endoscopic lesions when measured during the first 2 days of IFX therapy.47 Despite a consistent association between IFX levels and disease outcomes, no causality has been demonstrated to date. High, or ‘therapeutic', IFX levels may rather be a marker or a consequence of decreasing inflammatory burden. The mechanism of PNR could also be independent of immunogenicity or drug clearance and could be due to a non-TNF-mediated inflammatory disease phenotype.
No interventional controlled study to date has evaluated the benefit of IFX optimization based on IFX levels during induction. In the Through Concentration Adapted Infliximab Treatment [TAXIT] trial, the first randomized controlled study to evaluate concentration-based dosing to maintain remission in IBD patients treated by IFX, patients were included in the maintenance phase.9 In the TAILORIX trial [a randomized controlled trial investigating tailored treatment with IFX for active luminal CD], all patients received a standard induction regimen with no dose-individualization.10
2.2. Adalimumab
ADA is a fully human IgG1 monoclonal antibody targeting soluble and membrane-bound TNF-α. Registration studies confirming the safety and efficacy of adalimumab in IBD include GAIN, CLASSIC I and II and CHARM [CD], and ULTRA 1 and 2 [UC].48–53
2.2.1. Pharmacokinetics of ADA
In healthy volunteers the bioavailability after a single 40-mg subcutaneous ADA dose is 64% and time to peak serum concentration [Cmax] is 5.5 days. In a population pharmacokinetic analysis from CD patients, median clearance is 14.9 mL/h and median volume of distribution is 8.7 L.54 Significant variability in bioavailability after subcutaneous injection has been demonstrated. In a recent small prospective cohort of 28 CD patients, a four-fold difference in the range of ADA concentrations was seen at 7 days on pharmacokinetic modelling; antibodies to adalimumab and higher lean body weight were associated with increased adalimumab clearance.55
2.2.2. Therapeutic drug monitoring of ADA during induction therapy
Early evidence of an exposure–response relationship [EER] for ADA during induction came from post-hoc analyses of the CLASSIC I and II clinical trials. From CLASSIC I week 4 ADA levels were higher in responders than in non-responders [8.1 vs 5.1 μg/mL, p < 0.05]. In both CLASSIC I and II week 4 ADA levels were independently associated with clinical remission, although a cut-off threshold associated with remission could not be determined due to significant inter-patient variability in drug levels.56 In a prospective study of 168 CD patients with prior IFX failure, the use of ADA resulted in a sustained clinical benefit in two-thirds of patients during a median follow-up period of almost 2 years. In total, 130 patients had TDM performed, of which 67 had week 4 TDM data during induction; median week 4 ADA levels were 5.3 μg/mL. Although no direct relationship between week 4 ADA levels and short-term clinical outcomes was demonstrated, patients who discontinued ADA by week 4 had lower trough levels than those who continued therapy [2.5 vs 5.9 μg/mL, p = 0.12]. Patients who developed anti-adalimumab antibodies [AAAs] at any time during follow-up were found to have lower week 4 ADA levels compared to AAA-negative patients [2.1 vs 6.1 μg/mL, p < 0.02].57 In a subsequent post-hoc analysis of the same cohort, 20% of patients developed AAAs by a median of 34 weeks, and the presence of AAAs correlated inversely with ADA trough levels. Week 4 ADA levels <5 μg/mL were associated with the subsequent development of AAA [Hazard Ratio (HR) 25.1; 95% CI 5.6–111.9, p = 0.002], which themselves were associated with secondary LOF [OR 3.0; 95% CI 1.04–9.09, p = 0.034]. Immunogenicity was reduced by concomitant immunomodulator use [HR 0.23; 95% CI 0.06–0.86, p = 0.03].57,58 More recent studies of ADA in CD have led to a better understanding of the different mechanisms of PNR and thus to the increased interest in proactive TDM during ADA induction. The first study was a prospective, multicentre cohort study from Israel. Ninety-eight CD patients starting ADA were prospectively followed [median follow-up 44 weeks]. Thirty-three patients [32%] developed AAA, 18/33 [55%] of them as early as week 2, and 26/33 [79%] by week 14. AAA during induction were strongly associated with PNR [OR = 5.4, 95% confidence interval 1.6–17.8, p = 0.005]. At week 2, ADA levels were significantly associated with clinical remission by the end of induction (week 2 median levels = 6.8 vs 4.85 μg/mL, interquartile range [IQR] 1.5–19.2 and 0–8.2 μg/mL, among those in clinical remission vs those clinically active at week 14, p = 0.0005). Moreover, on ROC curve analysis, week 2 ADA levels >6.7 μg/mL were significantly associated with clinical remission by the end of induction [p < 0.0001, AUC = 0.73].44 In a recent study, the pharmacokinetics of ADA during induction in 116 CD patients naive to anti-TNF were analysed retrospectively. Patients with low serum ADA levels at week 4 [median levels <8.3 μg/mL] were at significantly higher risk to be AAA-positive by week 12 [46.7% vs 13.0%, p = 0.009]. The group of patients with AAAs at week 12 [21.4%] had significant higher needs for dose escalation [p < 0.001], and experienced sustained clinical benefit less frequently due to PNR or secondary LOF [p = 0.02]. Week 4 ADA levels >12 μg/mL were associated with biological remission at week 12. Moreover, in this study, a novel lateral flow rapid test assay for quantitative determination of ADA levels was used which could allow physicians to optimize therapy immediately at the time of outpatient clinic appointments.45 In another small prospective cohort of 18 patients [CD 13, UC 5], week 4 ADA levels >3.5 μg/mL were associated with clinical response at that time point.32 From the PANTS cohort described above, PNR at week 14 occurred in 26.8% [125/466; 95% CI 22.9–31.1%] of patients treated with ADA. Univariable analyses demonstrated the strongest associations with PNR to ADA were week 14 ADA and AAA levels. Multivariable analyses confirmed that only week 14 drug level was associated independently with PNR [OR 0.13, 95% CI 0.06–0.28, p < 0.0001]. Week 14 ADA levels were also independently associated with a lack of remission at week 54 [OR 0.03, 95% CI 0.01–0.12, p < 0.0001]. As with IFX, patients with PNR who continued standard ADA dosing rarely entered remission, suggesting the need for proactive dose-optimization early in these patients. A dose–response association for week 14 ADA levels and remission was observed up to 12 µg/mL.34 Most recently, the PAILOT study was a multi-centre, non-blinded randomized trial of bio-naïve CD children [6–18 years] commencing ADA. At week 4, 80 responders to ADA induction therapy, 43% of whom were on concomitant immunomodulators, were randomized to proactive TDM [where ADA doses were regularly optimized to maintain drug levels >5 μg/mL], or to reactive TDM, where TDM use was based on symptoms or biomarkers of active disease. The primary end point, sustained corticosteroid-free remission between weeks 8 and 72, was achieved in 87% of the proactive TDM group and 49% of the reactive TDM group [p < 0.001].59
In UC, several cohort studies have shown an association between serum ADA levels during induction and early mucosal healing. In a retrospective, single-centre study including 43 consecutive UC patients treated with ADA, five patients had PNR and 12 patients achieved short-term mucosal healing [STMH]. Patients with STMH had higher ADA levels at week 4 compared to those without [10.6 vs 7.4 μg/mL, p = 0.014]. Multivariate logistic regression analysis, after excluding patients with PNR to IFX, identified ADA levels ≥7.5 μg/mL at week 4 [OR 15.7; 95% CI 1.3–185; p = 0.029] and baseline endoscopic Mayo score 3 [OR 0.13; 95% CI 0.02–0.98; p = 0.047] as factors independently associated with STMH.60 In another single-centre cohort study, 73 UC patients previously exposed to IFX were assessed for response to ADA at weeks 12 and 52. Prior response to IFX and early serum ADA concentrations correlated well with clinical response. Independent predictors for response at week 12 were primary response to IFX [OR 8.33; 95% CI 1.8–33.3; p = 0.006] and an ADA concentration ≥4.58 μg/mL at week 4 [OR 4.85; 95% CI 1.3–18.6; p = 0.009].61 It must be acknowledged that all these studies demonstrating a relationship between early ADA levels and clinical remission or early mucosal healing are non-interventional. However, these studies do confirm an association between serum ADA levels at week 2 or week 4 and PNR. The presence of AAAs occurs early and frequently with ADA use. AAAs are associated with lower serum ADA levels and LOF or PNR in both CD and UC. Current best practices, including concomitant immunomodulator use, and dose intensification in at-risk patients during induction [including the use of TDM], may reduce pharmacokinetic treatment failures. Due to the heterogeneity of study designs to date, no consistent ADA thresholds have emerged on which to base recommendations for ADA induction TDM.
2.3. Golimumab
Golimumab is a fully human IgG1 monoclonal antibody targeting TNF and is registered for the treatment of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and UC. The safety and efficacy of golimumab in UC was confirmed by the PURSUIT registration studies.62–64
2.3.1. Pharmacokinetics of golimumab
From the PURSUIT SC induction and maintenance studies, median serum golimumab concentrations peaked at week 2 and were approximately dose-proportional during induction and maintenance therapy. Steady-state drug concentrations were reached after 8 weeks of maintenance therapy [14 weeks of golimumab] and were also dose-proportional. During induction and maintenance, drug concentrations in the lower quartiles were associated with increased weight, low albumin, high faecal calprotectin and CRP, and antibodies to golimumab. Immunomodulators increased steady-state drug concentrations only in the 50-mg, but not 100-mg, golimumab maintenance group. Up to week 54, 3% of patients developed antibodies to golimumab; immunogenicity rates were lower in patients receiving immunomodulators compared to golimumab monotherapy patients [1.5% vs 3.5%].65
2.3.2. Therapeutic drug monitoring of golimumab during induction therapy
In the PURSUIT SC induction study, median week 6 golimumab concentrations were 0.8 μg/mL [1.0 ± 0.75 μg/mL], 1.9 μg/mL [2.4 ± 1.87 μg/mL] and 3.9 μg/mL [4.5 ± 2.89 μg/mL] in the golimumab 100/50-mg, 200/100-mg and 400/200-mg groups, respectively. On quartile analysis of week 6 drug levels an ERR was demonstrated for clinical response, remission and mucosal healing. Week 6 golimumab drug levels were higher in clinical responders vs non-responders [2.96 vs 1.55 μg/mL, p < 0.001], remitters vs non-remitters [3.14 vs 2.13 μg/mL, p < 0.001] and patients achieving vs not-achieving mucosal healing [3.14 vs 1.70 μg/mL, p < 0.001]. On multivariate analysis of variables during induction, only week 6 golimumab levels and female sex were predictors of all efficacy outcomes, i.e. clinical response, remission and mucosal healing. Analysis of the association between golimumab levels at earlier time points and outcomes at week 6 demonstrated that week 2 and 4 golimumab levels were predictive of clinical response and mucosal healing, but only week 4 levels were predictive of clinical remission rates at week 6. On ROC curve analysis, the optimal week 6 golimumab drug level associated with clinical response at week 6 was 2.5 μg/mL. Optimal golimumab levels at earlier time points associated with subsequent clinical response at week 6 were 8.9 μg/mL [week 2] and 7.4 μg/mL [week 4].65
The only other report of golimumab TDM during induction therapy comes from Leuven where 21 patients with moderate–severe UC received golimumab 200 mg at week 0, then 100 mg at week 2 as induction therapy. Median serum golimumab concentrations at weeks 2 and 6 were 8.0 [5.3–10.3] μg/mL and 4.3 [2.0–6.9] μg/mL, respectively.
Comparing responders and non-responders, week 2 levels were 10.0 [7.8–10.5] μg/mL vs 7.4 [4.8–8.3] μg/mL [p = 0.035], and week 6 levels were 5.1 [4.0–7.9] μg/mL vs 2.1 [1.8–4.2] μg/mL [p = 0.037], respectively. On ROC curve analysis a week 6 golimumab level of 2.6 μg/mL was associated with partial clinical response at week 14; this optimal week 6 drug level is very similar to that seen in the PURSUIT analysis.66
2.4. Certolizumab pegol
Certolizumab pegol [CZP] is a PEGylated, humanized, antigen-binding fragment [Fab′] of an anti-TNF monoclonal antibody. Unlike other available anti-TNF agents, it has no crystallizable fragment [Fc] domain. The lack of an Fc domain means it does not cause complement-dependent cytotoxicity or antibody-dependent cell-mediated cytotoxicity, nor induce apoptosis.67,68 The safety and efficacy of CZP in CD was demonstrated in the registration studies PRECISE 1 and 2.69
2.4.1. Pharmacokinetics of CZP
The pharmacokinetics of CZP are dose-proportional; peak plasma concentrations occur 54–171 h after injection.67 Clearance is linear and elimination is via proteolysis and urinary excretion. PEGylation of CZP delays renal elimination and increases the half-life to 14 days.68 The pharmacokinetics of CZP has been extensively studied using a population-based pK model which included 12 926 CZP concentrations obtained from 2157 patients with CD from nine clinical trials.70 CZP drug levels were measured using an ELISA, and anti-drug antibodies to CZP were measured using a drug-sensitive ELISA. In this study, clearance was estimated to be 0.53 L/day with an interpatient variability of 19.6%. Factors which increased CZP clearance included anti-drug antibodies, increasing patient weight, low albumin and high CRP. Female sex was associated with a small, not clinically significant increase in clearance of 7%. This study assessed the impact of covariates both at baseline and during treatment. For example, in patients who responded to CZP, an elevated CRP at baseline was associated with a subsequent decrease in CRP during treatment with a concomitant decrease in clearance over time.
2.4.2. Therapeutic drug monitoring of CZP during induction therapy
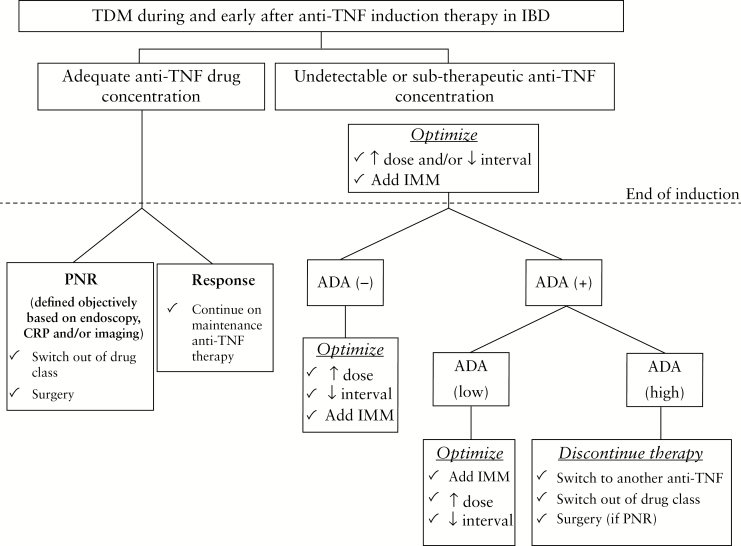
Using pooled data from nine clinical trials involving 2157 CD patients treated with CZP, the exposure–response relationship for CZP was evaluated for several clinical and biological end points assessed at weeks 6 and 26. Patients who achieved week 6 clinical response, clinical remission or biological remission [CRP ≤ 5 mg/L or faecal calprotectin ≤250 μg/g] had higher CZP levels at weeks 2, 4 and 6 for all outcomes compared to patients who did not achieve these end points [p < 0.001 for all end points]. For the composite end point of week 6 clinical remission (Crohn’s disease activity index [CDAI] < 150 points) and faecal calprotectin ≤250 μg/g, higher CZP concentrations were observed in patients who achieved this outcome compared to those who did not, at week 2 [22.1 vs 20.6 μg/mL, p = 0.003], week 4 [34.2 vs 30.3 μg/mL, p < 0.001] and week 6 [38.0 vs 32.0 μg/mL, p < 0.001]. On ROC analysis, CZP concentrations >36.1 μg/mL at week 6 and >14.8 μg/mL at week 12 were associated with improved clinical outcomes at weeks 6 and 26 respectively.71 The MUSIC [Endoscopic Mucosal Improvement in Patients with Active CD Treated with CZP] trial was a 54-week, multicentre, open-label study assessing endoscopic response to CZP in 89 patients with endoscopically active CD. Patients received 400 mg CZP at weeks 0, 2 and 4 and then 4-weekly up to week 52. Endoscopic evaluations scored by the CD Endoscopic Index of Severity [CDEIS] were performed at weeks 0, 10 and 54. CZP concentrations were drawn at weeks 8 and 54, from which quartile analyses were compared to endoscopic outcomes in 45 patients with paired results. On quartile analysis from CZP levels taken during induction at week 8, mean CZP levels were 3.6 µg/L [quartile 1], 13.3 µg/L [quartile 2], 19.7 µg/L [quartile 3] and 30.1 µg/L [quartile 4]. Compared to patients in the lowest week 8 CZP level quartile, patients in the highest three quartiles had higher rates of endoscopic response [p = 0.002] and remission [p = 0.03] at week 10.72 A summary of the evidence supporting threshold drug levels during induction and associated clinical outcomes for anti-TNF agents is shown in Table 1. A suggested algorithm for the use of induction TDM for anti-TNF agents is shown in Figure 2.
Table 1.
Biologic induction TDM thresholds and associated therapeutic outcomes for anti-TNF agents.
| IBD type | Study type and sample size | Total N | Threshold concentration [µg/mL] | Associated therapeutic outcome and time point | Reference |
|---|---|---|---|---|---|
| Infliximab | |||||
| Week 2 | |||||
| CD | Post-hoc analysis of RCT [TAILORIX] | 122 | >23.1 | Endoscopic remission at week 12 | Dreesen et al41 |
| CD | Retrospective cohort [fistulizing CD] | 36 | >9.3 | Fistula response at week 14 | Davidov et al42 |
| CD | Prospective cohort | 72 | ≥26.7 | Clinical response at week 14 | Clarkston et al33 |
| CD | Prospective cohorta | 184 | >20.4 | Clinical remission at week 14 | Gonczi et al35 |
| CD | Prospective cohorta | 184 | >16.9 | Clinical response at week 14 | Gonczi et al35 |
| UC | Prospective cohorta | 107 | >15.3 | Clinical remission at week 14 | Gonczi et al35 |
| UC | Prospective cohorta | 107 | >11.5 | Clinical response at week 14 | Gonczi et al35 |
| UC | Post hoc analysis of RCT [JAPIC] | 82 | >21.3 | Clinical remission at week 14 | Kobayashi et al36 |
| UC | Post-hoc analysis of two RCTs [ACT 1 and 2] | 484 | ≥18.6 | Mayo endoscopic score <2 at week 8 | Vande Casteele et al40 |
| UC | Retrospective cohort | 101 | ≥28.3 | Mucosal healing at weeks 10–14 | Papamichael et al15 |
| CD/UC | Retrospective case-control | 140 | <6.8 | Primary non-response at week 14 | Bar-Yoseph et al29 |
| Week 6 | |||||
| CD | Post-hoc analysis of RCT [TAILORIX] | 122 | > 10 | Endoscopic remission at week 12 | Dreesen et al41 |
| CD | Retrospective cohort [fistulizing CD] | 36 | > 7.3 | Fistula response at week 14 | Davidov et al42 |
| CD | Prospective cohort | 72 | ≥15.9 | Clinical response at week 14 | Clarkston et al33 |
| UC | Post-hoc analysis of two RCTs [ACT 1 and 2] | 484 | ≥10.6 | Mayo endoscopic score <2 at week 8 | Vande Casteele et al40 |
| UC | Post-hoc analysis of two RCTs [ACT 1 and 2] | 728 | >22 | Clinical response at week 8 | Adedokun et al22 |
| UC | Prospective cohort | 19 | > 6.6 | Endoscopic response at week 8 | Brandse et al37 |
| UC | Retrospective cohort | 101 | ≥15 | Mucosal healing at week 10–14 | Papamichael et al15 |
| Week 14 | |||||
| CD | Post-hoc analysis of RCT [ACCENT 1] | 291 | ≥3.5 | Sustained clinical response up to week 54 | Cornillie et al13 |
| CD | Prospective cohort [PANTS] | 955 | > 7.0 | Clinical remission at both week 14 and week 54 | Kennedy et al34 |
| UC | Post-hoc analysis of two RCTs [ACT 1 and 2] | 728 | > 5.1 | Clinical response at week 30 | Adedokun et al22 |
| UC | Post-hoc analysis of two RCTs [ACT 1 and 2] | 484 | ≥5.1 | Mayo endoscopic score <2 at week 8 | Vande Casteele et al40 |
| UC | Post-hoc analysis of two RCTs [ACT 1 and 2] | 484 | ≥6.7 | Mayo endoscopic score = 0 at week 8 | Vande Casteele et al40 |
| UC | Retrospective cohort | 101 | ≥2.1 | Mucosal healing at weeks 10–14 | Papamichael et al15 |
| CD/UC | Prospective cohort | 35 | >4.8 | Clinical response at week 14 | Tighe et al32 |
| Adalimumab | |||||
| Week 2 | |||||
| CD | Prospective cohort | 98 | > 6.7 | Clinical remission at week 14 | Ungar et al44 |
| Week 4 | |||||
| CD | Prospective cohort | 116 | <8.3 | AAA formation at week 12 | Verstockt et al45 |
| CD | Prospective cohort | 116 | >12 | Biological remission at week 12 | Verstockt et al45 |
| CD | Prospective cohort | 28 | > 7.3 | Clinical remission at week 12 | Vande Casteele et al55 |
| UC | Retrospective cohort | 43 | ≥7.5 | Mucosal healing at weeks 8–14 | Papamichael et al60 |
| UC | Retrospective cohort | 73 | ≥4.6 | Clinical response at week 12 | Baert et al61 |
| UC | Retrospective cohort | 73 | ≥7 | Clinical response at week 52 | Baert et al61 |
| CD/UC | Prospective cohort | 35 | >3.5 | Clinical response at week 4 | Tighe et al32 |
| Golimumab | |||||
| Week 2 | |||||
| UC | Post-hoc analysis of RCT [PURSUIT] | 1064 | > 8.9 | Clinical response at week 6 | Adedokun et al65 |
| Week 6 | |||||
| UC | Post-hoc analysis of RCT [PURSUIT] | 1064 | > 2.5 | Clinical response at week 6 | Adedokun et al65 |
| UC | Retrospective cohort | 21 | > 2.6 | Partial clinical response at week 14 | Detrez et al66 |
| Certolizumab pegol | |||||
| Week 6 | |||||
| CD | Post-hoc analysis of nine RCTs | 2157 | >31.8 | Clinical response/remission at week 6 | Vande Casteele et al71 |
| CD | Post-hoc analysis of nine RCTs | 2157 | >31.9 | CRP ≤5 mg/L at week 6 | Vande Casteele et al71 |
| CD | Post-hoc analysis of nine RCTs | 2157 | >32.7 | FC <250 mg/g at week 6 | Vande Casteele et al71 |
| CD | Post-hoc analysis of nine RCTs | 2157 | >34.5 | FC <250 mg/g and CDAI ≤150 at week 6 | Vande Casteele et al71 |
| CD | Post-hoc analysis of nine RCTs | 2157 | >36.1 | FC [<250 mg/g] and CDAI ≤150 at week 26 | Vande Casteele et al71 |
| Week 8 | |||||
| CD | Post-hoc analysis of RCT [MUSIC] | 45 | >23.3 | Endoscopic remission at week 10 | Colombel et al72 |
Abbreviations: CD, Crohn’s disease; UC, ulcerative colitis; RCT, randomized controlled trial; AAA, anti-adalimumab antibodies; FC, faecal calprotectin; CRP, C-reactive protein; CDAI, Crohn’s disease activity index.
aCT-P13.
Figure 2.
Suggested algorithm for induction TDM for anti-TNF agents. IMM, immunomodulator; PNR, primary non-response.
3. Vedolizumab
Vedolizumab is a recombinant human IgG1 monoclonal antibody that binds to the α4β7 integrin expressed on gut-selective lymphocytes; this binding prevents lymphocyte adhesion to mucosal addressin cell-adhesion molecule 1 [MAdCAM-1], which is expressed in the gut vascular endothelium. It was registered for the treatment of patients with moderate-to-severe CD and UC intolerant of, or unresponsive to, conventional immunomodulators or corticosteroids following the GEMINI I and II studies.73,74 Efficacy as induction therapy in CD anti-TNF failures was subsequently demonstrated in the GEMINI III study, albeit not until week 10.75
3.1. Pharmacokinetics of vedolizumab
In healthy volunteers given a single dose of vedolizumab at 0.2–10 mg/kg, clearance is linear until the concentration reaches 10 μg/mL; below this clearance is non-linear.76 In repeated doses across a range of 2–10 mg/kg, pharmacokinetics are dose-proportional.77 No differences in drug levels were observed between patients with UC and CD at week 6 in the GEMINI I and II studies.73,74 In these studies, responders to vedolizumab induction therapy at week 6 who were intensified to 4-weekly vedolizumab had higher mean drug levels at week 46 compared to those receiving 8-weekly vedolizumab [38 and 34 μg/mL vs 11 and 13 μg/mL, in GEMINI I and II, respectively].78 A population pharmacokinetic analysis estimated the half-life of vedolizumab to be 25.5 days.79 Factors which increase clearance are extreme weight [>120 kg], hypoalbuminaemia [<3.2 g/dL] and the presence of anti-vedolizumab antibodies. Concomitant immunomodulation does not influence vedolizumab drug levels. In GEMINI I, UC patients with higher Mayo endoscopic scores had lower VDZ concentrations at week 6. CRP and faecal calprotectin exert a slight influence on clearance, although this is not clinically significant.78 Complete α4β7 receptor saturation is achieved with standard vedolizumab dosing regimens and at serum concentrations of 1 μg/mL.79
3.2. TDM of vedolizumab during induction therapy
The GEMINI studies provide the largest prospectively collected dataset demonstrating an ERR between vedolizumab drug levels and outcomes during induction. On quartile analysis in GEMINI I, patients with UC with drug levels in the highest quartile [Q4: 33.6–65.6 μg/mL] had higher rates of clinical response and remission compared to those in the lowest quartile at week 6 [Q1: 0–16.7 μg/mL; 74.1 vs 29.6% for response and 37.0 vs 5.6% for remission].73 In GEMINI II, CD patients with drug levels in the highest quartile [Q4: 33.8–142.0 μg/mL] had higher rates of clinical response and remission compared to those in the lowest quartile [Q1: 0–15.2 μg/mL; 48.0 vs 20.4% for response and 22.0 vs 6.1% for remission] at week 6.74 In a post-hoc analysis of these studies, median vedolizumab trough concentrations at week 6 were higher in remitters vs non-remitters in GEMINI I [34.7 vs 23.7 μg/mL] and GEMINI II [26.8 vs 23.5 μg/mL], but substantially more overlap between the groups was observed in GEMINI II.80 Higher rates of mucosal healing at week 6 were observed in UC patients with trough concentrations in the highest quartile [Q4 > 35.7 μg/mL] compared to the lowest quartile [≤17.1 μg/mL; 62.9 vs 20.1%].80 In GEMINI III the magnitude of the ERR was less robust; at week 6 increasing trough concentrations from the lowest to highest quartile [≤17.1 to >32.5 μg/mL] resulted in an absolute increase in remission rates of only 5%. However, at week 10 the clinical remission rate increased 22% when comparing patients with the lowest quartile to the highest, suggesting that in CD, vedolizumab may take longer to be efficacious.80 In these studies ROC analyses were not performed to identify a target drug level threshold associated with favourable outcomes. The most recent post-hoc analysis of the vedolizumab registration trials dataset aimed to determine the ERR of vedolizumab therapy in UC after adjusting for confounding variables via a propensity-score-based case-matching analysis using data from GEMINI 1 and a previously published population pharmacokinetic study.79 Variables included in the model included age, weight, anti-TNF history, albumin and faecal calprotectin. At week 6, on quartile analysis a robust ERR was observed between vedolizumab concentration and clinical response, and a similarly robust inverse correlation was seen between vedolizumab clearance and response. Proposed target vedolizumab levels associated with clinical response at week 6, week 14 and steady-state were 37.1, 18.4 and 12.7 μg/mL, respectively. Week 6 was shown to be the earliest time point at which vedolizumab concentrations were predictive of clinical remission at weeks 14 and 52.81
There are a small number of uncontrolled studies comparing vedolizumab drug levels with outcomes during induction. A French multicentre study prospectively assessed the relationship between early vedolizumab drug levels [taken at weeks 2, 6 and 14] and subsequent rates of mucosal healing between weeks 14 and 52 amongst 82 patients with IBD. In total, 44/82 [54.4%] of patients were dose-intensified due to inadequate response. Only drug levels at week 6 differed between patients with and without mucosal healing within the first year of treatment [26.8 vs 15.1 μg/mL, p = 0.0035]. A therapeutic cut-off of >18 μg/mL was proposed with a sensitivity, specificity, positive predictive value [PPV] and negative predictive value [NPV] of 88.2%, 66.7%, 78.9% and 80.0%, respectively, with an AUROC of 0.735.82 In another prospective observational study of 47 consecutive IBD patients, the same authors found that a week 6 vedolizumab drug level <18.5 μg/mL was associated with the need for dose intensification due to inadequate clinical response. All dose-intensified patients clinically responded.83 A retrospective study from Leuven compared vedolizumab trough drug levels measured by ELISA in 179 patients with IBD [66 UC, 113 CD] and compared these to a range of outcomes [mucosal healing in UC at week 14 and CD at week 22, physician global assessment at week 14 for UC and week 22 in CD, and biochemical response/remission at weeks 6 and 22 in CD]. Higher vedolizumab trough levels at weeks 2 and 6 predicted better outcomes for both UC and CD. Considering UC, a week 2 trough level of >28.9 μg/mL was associated with week 14 mucosal healing [specificity 0.62, sensitivity 0.73, PPV 0.59 and NPV 0.75, AUROC 0.7, p = 0.16] and clinical response [specificity 0.59, sensitivity 0.75, PPV 0.4 and NPV 0.87, AUROC 0.67, p = 0.049]. A week 6 trough level of >20.8 μg/mL was associated with clinical response at week 16 [specificity 0.75, sensitivity 0.69, PPV 0.5, NPV 0.87, AUROC 0.72, p = 0.08]. Considering CD, a week 2 vedolizumab trough concentration >29.8 μg/mL was associated with biological remission at week 6 [specificity 0.67, sensitivity 0.73, PPV 0.88, NPV 0.42, AUROC 0.71, p = 0.026]. Baseline characteristics associated with higher vedolizumab concentrations on multivariate analysis included female sex, lower body mass index, non-smoking, lower CRP, higher albumin and higher haemoglobin.84 Summarizing these data across both UC and CD, the authors concluded that improved clinical and endoscopic outcomes are associated with vedolizumab trough concentrations >30.0 μg/mL at week 2, 24.0 μg/mL at week 6 and >14.0 μg/mL during maintenance therapy.84 A further single-centre retrospective analysis of 81 patients [40 CD, 41 UC] correlated vedolizumab trough concentrations during induction and subsequent clinical response during maintenance. Week 6 vedolizumab levels were higher in patients with sustained clinical response compared to patients experiencing treatment failure during maintenance therapy [33.0 vs 24.0 μg/mL, p = 0.02]. A week 6 vedolizumab level >28.0 μg/mL predicted sustained clinical response [AUC 0.723, p = 0.017]. Interestingly, week 6 vedolizumab levels were lower in anti-TNF experienced compared to anti-TNF naïve patients [22.5 vs 36.0 μg/mL, p = 0.03].85
Unlike the robust ERR seen with anti-TNF therapy, the relationship between serum levels of vedolizumab and clinical outcomes is weaker. In part this may reflect the incomplete and evolving understanding of the exact mechanism of action of vedolizumab in IBD. Although the effect of vedolizumab on circulating gut-selective lymphocytes, and the complex interplay of binding between α4β7 and MAdCAM-1, is well recognized, recent in vivo data from a single small case series suggest that some of the mechanism of action may be due to effects on the innate, rather than adaptive, immune system.86 In a prospective multicentre study of 106 patients with IBD, researchers compared vedolizumab drug levels with outcomes and with α4β7 saturation on peripheral and lamina propria T-lymphocytes during induction and maintenance. Clinical remission was observed in 45% of patients at week 6. Vedolizumab drug levels were higher in week 6 clinical remitters vs non-remitters [40.2 vs 29.7 μg/mL, p = 0.05] and between patients in quartiles 3 and 4 compared to patients in quartile 2 [p = 0.02 and 0.006, respectively] at the same time point. Complete α4β7 saturation on peripheral and lamina propria T-lymphocytes at weeks 2 and 14 was seen in most patients and was not different according to clinical outcomes.87 In a retrospective proof of concept study in 62 IBD patients responding to vedolizumab induction therapy at week 10, Paul et al. compared levels of soluble MAdCAM-1 [sMAdCAM-1], retinoic acid [RA] and vedolizumab drug levels between patients who maintained remission and those that relapsed. RA was chosen as a candidate biomarker given its effect on inducing expression of α4β7 on T cells. During maintenance, sMAdCAM-1 was significantly lower in those who maintained remission compared to those who relapsed [p < 0.001]. On multivariate analysis, undetectable sMAdCAM-1 and a VDZ drug level >19 μg/mL [measured during maintenance, not induction] were independently associated with maintenance of clinical remission [OR = 7.5, p = 0.006 and OR = 2.2, p = 0.045, respectively] with a PPV for remission of 92.5%. At baseline, only RA > 1.86 ng/mL was predictive of clinical remission during follow-up [AUROC 80.7%]. The authors propose that, pending validation in prospective studies, an algorithm incorporating vedolizumab trough levels, sMAdCAM-1 and RA could be used to predict clinical response to vedolizumab therapy.88 A summary of the evidence supporting threshold drug levels during induction and associated clinical outcomes for vedolizumab is shown in Table 2.
Table 2.
Biologic induction TDM thresholds and associated therapeutic outcomes for vedolizumab and ustekinumab.
| IBD type | Study type and sample size | Total N | Threshold concentration [µg/mL] | Associated therapeutic outcome and time point | Reference |
|---|---|---|---|---|---|
| Vedolizumab | |||||
| Week 2 | |||||
| CD/UC | Retrospective cohort | 179 | >28.9 | Clinical response in UC at week 14 | Dreesen et al.83 |
| CD/UC | Retrospective cohort | 179 | >35.2 | Biochemical remission in CD at week 6 | Dreesen et al.83 |
| Week 6 | |||||
| UC | Post-hoc analysis of RCT [GEMINI1] | 693 | >37.1 | Clinical response at week 6 | Osterman et al80 |
| CD/UC | Retrospective cohort | 179 | >20.8 | Clinical response in UC at week 14 | Dreesen et al.83 |
| CD/UC | Retrospective cohort | 81 | >28 | Sustained clinical response | Liefferinckx et al84 |
| CD/UC | Prospective cohort | 82 | >18 | Mucosal healing within 52 weeks | Yacoub et al81 |
| CD/UC | Prospective cohort | 47 | <18.5 | Need for dose-escalation within 24 weeks | Willett et al82 |
| Week 14 | |||||
| UC | Post-hoc analysis of RCT [GEMINI1] | 693 | >18.4 | Clinical response at week 14 | Osterman et al80 |
| CD/UC | Retrospective cohort | 179 | >12.6 | Clinical response in UC at week 14 | Dreesen et al.83 |
| CD/UC | Retrospective cohort | 179 | >17 | Mucosal healing in UC at week 14 | Dreesen et al.83 |
| Ustekinumab | |||||
| Week 4 | |||||
| CD | Prospective cohort | 86 | >15.9 | 50% decrease in FC at week 8 | Verstockt et al94 |
| CD | Prospective cohort | 51 | >13 | Clinical and biochemical response at week 16 | Soufflet et al.95 |
| Week 8 | |||||
| CD | Post-hoc analysis of RCTs [UNITI-1 and 2] | 701 | >3.3 | Clinical remission at week 8 | Adedokun et al.91 |
| CD | Prospective cohort | 86 | >7.2 | Biological remission at week 8 | Verstockt et al.94 |
| CD | Prospective cohort | 86 | >4.2 | 50% decrease in FC at week 8 | Verstockt et al.94 |
| CD | Prospective cohort | 51 | >2 | Clinical and biochemical response at week 16 | Soufflet et al.95 |
Abbreviations: CD, Crohn’s disease; UC, ulcerative colitis; RCT, randomized controlled trial; AAA, anti-adalimumab antibodies; FC, faecal calprotectin.
aCT-P13.
4. Ustekinumab
Ustekinumab is a fully human IgG1 monoclonal antibody targeting the shared p40 subunit of IL 12 and 23. It has regulatory approval for use in psoriasis, psoriatic arthritis and CD, and efficacy and safety in UC has recently been confirmed.89 The registration trials in CD included two induction studies [UNITI-1 and 2] and one pivotal maintenance study [IM-UNITI].90
4.1. Pharmacokinetics of ustekinumab
From population pharmacokinetic studies the median half-like of ustekinumab in CD patients is 19 days, and bioavailability after SC injection is 57%.91,92 From phase III CD trials, peak serum ustekinumab concentrations occurred 1 h after the intravenous [IV] infusion, and steady-state serum concentrations were achieved by the time of the second 8-weekly or 12-weekly SC dose.92,93 At week 8 median ustekinumab concentrations after 6 mg/kg induction dosing were 6.4 μg/mL [UNITI-1] and 6.3 μg/mL [UNITI-2]. Throughout the 44-week IM-UNITI maintenance study ustekinumab concentrations remained stable in both dosing groups [2.0–2.2 μg/mL in the 8-weekly dosing group, and 0.6–0.8 μg/mL in the 12-weekly dosing group]; trough concentrations were three-fold higher in the 8-weekly compared to 12-weekly dosing schedule.94 As with other monoclonal antibodies, clearance of ustekinumab is affected by multiple pharmacokinetic variables including body weight, albumin, CRP, prior anti-TNF status, sex and immunogenicity. Given the weight-based dosing schedule for ustekinumab IV induction therapy in CD, body weight is the biggest determinant of ustekinumab volume of distribution and clearance.93 Unlike anti-TNF agents, concomitant immunomodulators do not significantly modify ustekinumab pharmacokinetics and clearance.94
4.2. TDM during induction therapy of ustekinumab
The largest analysis to date of the role of TDM during ustekinumab induction therapy comes from the UNITI studies. At the end of induction at week 8, median ustekinumab concentrations were 6.4 and 2.1 μg/mL for the 6-mg/kg and 130-mg groups, respectively. Initially, both the 130-mg and 6-mg/kg groups were combined into ustekinumab week 8 drug concentration quartiles, which demonstrated a positive ERR between drug concentration and clinical remission. Higher clinical remission rates were observed in the two highest quartiles vs the lower quartiles; this relationship was stronger in UNITI-2 [p = 0.007] than UNITI-1 [p = 0.039]. However, analysis of just the approved 6-mg/kg group did not show a significant ERR between week 8 quartile levels and clinical response in either UNITI-1 [p = 0.225] or UNITI-2 [p = 0.101]. A combined week 8 quartile analysis of the 130-mg and 6-mg/kg groups demonstrated a positive association between drug levels and reduction and normalization of week 8 CRP levels [p < 0.001 for both analyses in both UNITI-1 and UNITI-2]. On ROC curve analysis a target week 8 ustekinumab level of 3.3 μg/mL correlated best with week 8 clinical remission, albeit with modest accuracy [AUC 0.57, sensitivity 0.63, specificity 0.52, p = 0.001]. In the UNITI studies the incidence of anti-ustekinumab antibodies was very low at 0.2%. There was no association between quartile analysis of week 8 ustekinumab drug levels and infections, serious infections or serious adverse events.94
Data from other smaller studies supporting an ERR for ustekinumab during induction therapy are less conclusive. An open-label pilot study in 19 CD patients with prior anti-TNF failure studied pharmacokinetic outcomes after subcutaneous [SC], rather than IV, ustekinumab induction therapy given as 180 mg SC at week 0, 90 mg SC at week 1 and 90 mg SC at week 2. Trough ustekinumab levels at weeks 1, 2 and 8 were 16 μg/mL [IQR 14.5–20 µg/mL], 20 μg/mL [IQR 14.5–20 µg/mL] and 6.1 μg/mL [IQR 14.5–20 µg/mL] respectively. This week 8 level of 6.1 μg/mL compares favourably with the level of 6.4 μg/mL achieved in the 6-mg/kg IV arm of the UNITI-1 trial. Although faecal calprotectin levels dropped significantly overall after ustekinumab therapy, median week 8 ustekinumab trough levels were similar in calprotectin responders (7.1 μg/mL [IQR 4.60–9.43 μg/mL]) and non-responders (6.1 μg/mL [3.9–9.7 μg/mL]) [p = 1.00].95 In a single-centre prospective study of 42 CD patients refractory to anti-TNF therapy, patients received 90 mg SC ustekinumab at weeks 0, 4 and 12, and the week 12 clinical response rate [defined as a reduction in Harvey Bradshaw Index of 3 points] was 57%. However, there was no difference in median week 12 ustekinumab trough levels between responders [1.16 μg/mL; IQR: 0.60–1.64] and non-responders [1.56 μg/mL; IQR: 0.49–2.76, p = 0.24].96 In a single-centre prospective cohort of 86 predominantly biologic-experienced CD patients receiving ustekinumab, an ERR was demonstrated during both induction and maintenance. Week 24 clinical remission rates were 39.5%, but endoscopic response and remission rates were significantly lower at 20.5% and 7.1%, respectively. During induction, median ustekinumab concentrations were 21.3 μg/mL at week 4 and 7.2 μg/mL at week 8. Higher baseline albumin, lower baseline faecal calprotectin and female sex were predictors of higher ustekinumab concentrations during induction. A 50% decrease in faecal calprotectin at week 8 was associated with ustekinumab concentrations >15.9 μg/mL at week 4 and >4.2 μg/mL at week 8. Numerically, ustekinumab concentrations were higher in endoscopic responders at all time points up to week 24.97 Recently, in a prospective multicentre open-label study of 52 CD biologic-experienced patients [100% anti-TNF-exposed, 71% vedolizumab-exposed], the ERR of ustekinumab during induction therapy was assessed. The primary end point was defined as steroid-free clinical and biochemical remission at week 16 [Harvey Bradshaw Index ≤4 points with CRP <5 mg/L and faecal calprotectin <250 μg/g]. In total, 32 patients [63%] achieved the primary end point at week 16. At week 8, median ustekinumab levels were significantly higher in responders compared to non-responders (6.0 μg/mL [IQR 3.1–8.0 μg/mL] vs 1.3 μg/mL [IQR, 0.9–5.6 μg/mL]; p = 0.03). ROC analysis showed that week 8 trough ustekinumab concentrations >2.0 μg/mL were associated with response to induction therapy [AUROC 0.75, 95% CI 0.53–0.96, p = 0.04]. A decrease in faecal calprotectin, but not CRP, correlated well with response.98 A summary of the evidence supporting threshold drug levels during induction and associated clinical outcomes for ustekinumab is shown in Table 2.
5. Discussion
PNR and secondary LOF to biological agents in IBD are important clinical problems. Secondary LOF to anti-TNF agents during maintenance therapy is common and reactive TDM is now considered standard of care in assessing and managing this scenario.7,8 Data are gradually emerging to support the use of reactive TDM in managing secondary LOF to other classes of biologic therapy.99,100 Of equal potential significance is the use of proactive TDM to prevent secondary LOF to biologic agents. The benefits of proactive TDM to prevent secondary LOF to anti-TNFs have been demonstrated only in observational studies, but not randomized controlled trials, to date.9–12 Of potentially even greater significance is whether PNR to biologic agents can be prevented by targeting threshold serum drug levels and reducing immunogenicity during the induction period, during which time higher drug levels are required to overcome the high inflammatory burden of active disease. Data from observational studies and post-hoc analyses of registration trials of all three biologic classes are evolving to support this practice, and potential threshold drug level targets at specific time points during induction are emerging. It must be acknowledged that these emerging data demonstrate an association between drug levels and various depths of remission during induction, but this does not imply causality. An alternative hypothesis is that higher drug levels are themselves secondary to reduced disease activity leading to reduced drug clearance. A summary of the evidence supporting threshold drug levels during induction and associated clinical outcomes for each biologic class is shown in Tables 1 and 2. These studies employed a variety of assays, meaning care must be taken in directly comparing thresholds between studies. At present, for IFX we recommend measuring week 14 levels in all patients, aiming for a target level of 7–10 μg/mL. In patients with a high baseline inflammatory burden [e.g. ASUC or perianal fistulizing CD] we also recommend ideally measuring IFX levels earlier in induction and targeting higher drug concentrations at these time points: week 2 [20–25 μg/mL] and week 6 [10–15 μg/mL]. Acknowledging that for ADA there is only recently emerging evidence, we suggest measuring week 4 ADA levels in all patients, aiming for a target level of 8–12 μg/mL. Higher levels will again be required for patients with a high baseline inflammatory burden, although currently there are no data on which to make specific recommendations in these patients. Currently, it is not possible to give firm recommendations for induction TDM targets for vedolizumab and ustekinumab, but emerging signals suggest that week 6 vedolizumab levels >20 μg/mL and week 8 ustekinumab levels >3 μg/mL are associated with improved clinical outcomes. We acknowledge that these potential target levels will probably undergo significant refinement as the science of proactive TDM during induction evolves. Different threshold concentrations are likely to emerge for CD and UC at the same time point, and the earliest time point at which drug levels can reliably predict subsequent clinical outcomes remains to be determined. Although randomized controlled trials to date have not confirmed the utility of proactive TDM of anti-TNF agents during either induction or maintenance, this may be due to methodological limitations. Hopefully future studies will incorporate study designs that allow for the intuitive benefits of proactive TDM to be demonstrated. For example, the SERENE studies, which incorporate TDM, are comparing higher vs standard ADA dosing regimens for induction and maintenance therapy in both CD [Clinicaltrials.gov Identifier NCT02065570] and UC [Clinicaltrials.gov Identifier NCT02065622]. It is likely that future studies will also include dashboard-guided dosing models that allow for individualization of dosing by incorporating pharmacokinetic variables that affect drug clearance during induction.16 Future studies using point-of-care rapid testing assays would be particularly suited to use during induction TDM studies when prompt availability of results would allow for rapid dose individualization that would be especially suitable during periods of high inflammatory burden. Most proactive TDM induction data to date pertain to the use of anti-TNF agents; more data are required to confirm the utility of proactive induction TDM of vedolizumab and ustekinumab in clinical practice. Hopefully future studies will prove the clinical and economic benefits of proactive TDM of biologic agents during induction, thereby validating the intuitive dictum that ‘an ounce of prevention is worth a pound of cure'.
Acknowledgments
There was no funding nor writing assistance for this review.
Conflict of Interest
M.P.S. has received educational grants or research support from Ferring and Orphan; has received speakers fees from Janssen, Abbvie, Ferring, Takeda, Pfizer, Celgene, MSD and Shire; and has served on advisory boards for Janssen, Takeda, Pfizer, Celgene, Abbvie and MSD. K.P. has received lecture fees from Mitibishi Tanabe Pharma. K.P. is supported by Ruth L. Kirschstein NRSA Institutional Research Training Grant 5T32DK007760-18. The content of this project is solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH). S.P. have no conflicts of interest.
M.G.W. has served as a speaker for Janssen, AbbVie, Ferring, Takeda, Shire and MSD and received research funding from AbbVie and Ferring. P.R. has received consulting or speakers fees or research support from Abbvie and Amgen. D.L. has received lecture fees from Abbvie, Biogaran, Biogen, Ferring, HAC-pharma, Janssen, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda, Theradiag and Tillots; and consultancy fees from Abbvie, Ferring, Janssen, MSD, Pfizer, Roche, Takeda. X.R. has received consulting or speakers fees or research support from MSD, Pfizer, Takeda, Janssen and Abbvie.
Author Contributions
M.P.S., K.P., M.G.W., X.R.: manuscript concept and design, manuscript writing, critical review of the manuscript; P.R., D.L., S.P.: critical review of manuscript.
References
- 1. Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43:317–33. [DOI] [PubMed] [Google Scholar]
- 2. Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1245–55 e8. [DOI] [PubMed] [Google Scholar]
- 3. Schnitzler F, Fidder H, Ferrante M, et al.. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- 4. Colombel JF, Panaccione R, Bossuyt P, et al.. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 5. Afif W, Loftus EV Jr, Faubion WA, et al.. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steenholdt C, Brynskov J, Thomsen OØ, et al.. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. [DOI] [PubMed] [Google Scholar]
- 7. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34. [DOI] [PubMed] [Google Scholar]
- 8. Mitrev N, Vande Casteele N, Seow CH, et al.; IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group Review article: Consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017;46:1037–53. [DOI] [PubMed] [Google Scholar]
- 9. Vande Casteele N, Ferrante M, Van Assche G, et al.. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.e3. [DOI] [PubMed] [Google Scholar]
- 10. D'Haens G, Vermeire S, Lambrecht G, et al.; GETAID Increasing infliximab dose based on symptoms, biomarkers, and serum drug concentrations does not increase clinical, endoscopic, and corticosteroid-free remission in patients with active luminal Crohn’s disease. Gastroenterology 2018;154:1343–1351.e1. [DOI] [PubMed] [Google Scholar]
- 11. Papamichael K, Chachu KA, Vajravelu RK, Vaughn BP, Ni J, Osterman MT, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cornillie F, Hanauer SB, Diamond RH, et al.. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papamichael K, Gils A, Rutgeerts P, et al.. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97. [DOI] [PubMed] [Google Scholar]
- 15. Papamichael K, Van Stappen T, Vande Casteele N, et al.. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:543–9. [DOI] [PubMed] [Google Scholar]
- 16. Strik AS, Wang YC, Ruff LE, Yashar W, Messmer BT, Mould DR. Individualized dosing of therapeutic monoclonal antibodies – a changing treatment paradigm? AAPS J 2018;20:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanauer SB, Feagan BG, Lichtenstein GR, et al.; ACCENT I Study Group Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 18. Sands BE, Anderson FH, Bernstein CN, et al.. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85. [DOI] [PubMed] [Google Scholar]
- 19. Rutgeerts P, Sandborn WJ, Feagan BG, et al.. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 20. Hyams J, Crandall W, Kugathasan S, et al.; REACH Study Group Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007;132:863–73; quiz 1165–6. [DOI] [PubMed] [Google Scholar]
- 21. Hemperly A, Vande Casteele N. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet 2018;57:929–42. [DOI] [PubMed] [Google Scholar]
- 22. Adedokun OJ, Sandborn WJ, Feagan BG, et al.. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–1307.e5. [DOI] [PubMed] [Google Scholar]
- 23. Baert F, Noman M, Vermeire S, et al.. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601–8. [DOI] [PubMed] [Google Scholar]
- 24. Colombel JF, Sandborn WJ, Reinisch W, et al.; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 25. D'Haens G, Baert F, van Assche G, et al.; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660–7. [DOI] [PubMed] [Google Scholar]
- 26. Papamichael K, Rivals-Lerebours O, Billiet T, et al.. Long-term outcome of patients with ulcerative colitis and primary non-response to infliximab. J Crohns Colitis 2016;10:1015–23. [DOI] [PubMed] [Google Scholar]
- 27. Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis. 2018;12:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Hoeve K, Dreesen E, Hoffman I, et al.. Adequate infliximab exposure during induction predicts remission in paediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2019;68:847–53. [DOI] [PubMed] [Google Scholar]
- 29. Bar-Yoseph H, Levhar N, Selinger L, et al.. Early drug and anti-infliximab antibody levels for prediction of primary nonresponse to infliximab therapy. Aliment Pharmacol Ther 2018;47:212–8. [DOI] [PubMed] [Google Scholar]
- 30. Lega S, Phan BL, Rosenthal CJ, et al.. Proactively optimized infliximab monotherapy is as effective as combination therapy in IBD. Inflamm Bowel Dis 2019;25:134–41. [DOI] [PubMed] [Google Scholar]
- 31. Beltrán B, Iborra M, Sáez-González E, et al.. Fecal calprotectin pretreatment and induction infliximab levels for prediction of primary nonresponse to infliximab therapy in Crohn’s disease. Dig Dis 2019;37:108–15. [DOI] [PubMed] [Google Scholar]
- 32. Tighe D, Smith S, O'Connor A, Breslin N, Ryan B, McNamara D. Positive relationship between infliximab and adalimumab trough levels at completion of induction therapy with clinical response rates, at a tertiary referral center. JGH Open 2017;1:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clarkston K, Tsai YT, Jackson K, Rosen MJ, Denson LA, Minar P. Development of infliximab target concentrations during induction in pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr 2019;69:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341–53. [DOI] [PubMed] [Google Scholar]
- 35. Gonczi L, Vegh Z, Golovics PA, et al.. Prediction of short- and medium-term efficacy of biosimilar infliximab therapy. do trough levels and antidrug antibody levels or clinical and biochemical markers play the more important role? J Crohns Colitis 2017;11:697–705. [DOI] [PubMed] [Google Scholar]
- 36. Kobayashi T, Suzuki Y, Motoya S, et al.. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol 2016;51:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brandse JF, Mathôt RA, van der Kleij D, et al.. Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:251–8.e1–2. [DOI] [PubMed] [Google Scholar]
- 38. Gibson DJ, Heetun ZS, Redmond CE, et al.. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:330–335.e1. [DOI] [PubMed] [Google Scholar]
- 39. Nalagatla N, Falloon K, Tran G, et al.. Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multicenter study and meta-analysis. Clin Gastroenterol Hepatol 2019;17:502–509.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vande Casteele N, Jeyarajah J, Jairath V, Feagan BG, Sandborn WJ. Infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019;17:1814–1821.e1. [DOI] [PubMed] [Google Scholar]
- 41. Dreesen E DHG, Baert F, et al. Infliximab exposure predicts superior endoscopic outcomes in patient with active Crohn’s disease: pharmacokinetic-pharmacodynamic analysis of TAILORIX. J Crohns Colitis. 2018;12:S063–S4. [Google Scholar]
- 42. Davidov Y, Ungar B, Bar-Yoseph H, et al.. Association of induction infliximab levels with clinical response in perianal Crohn’s disease. J Crohns Colitis 2017;11:549–55. [DOI] [PubMed] [Google Scholar]
- 43. Vande Casteele N PK, Jeyarajah J et al. Adequate infliximab exposure during the induction phase is associated with early complete fistula response in patients with fistulizing Crohn’s disease: a post-hoc analysis of the ACCENT-2 trial. J Crohns Colitis 2019;13:S053–S4. [Google Scholar]
- 44. Ungar B, Engel T, Yablecovitch D, et al.. Prospective observational evaluation of time-dependency of adalimumab immunogenicity and drug concentrations: The POETIC study. Am J Gastroenterol 2018;113:890–8. [DOI] [PubMed] [Google Scholar]
- 45. Verstockt B, Moors G, Bian S, et al.. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther 2018;48:731–9. [DOI] [PubMed] [Google Scholar]
- 46. Brandse JF, van den Brink GR, Wildenberg ME, van der Kleij D, Rispens T, Jansen JM, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015;149:350–5 e2. [DOI] [PubMed] [Google Scholar]
- 47. Poullenot F, Nivet D, Paul S, Riviere P, Roblin X, Laharie D. Severe endoscopic lesions are not associated with more infliximab fecal loss in acute severe ulcerative colitis. Dig Liver Dis 2018;50:1100–3. [DOI] [PubMed] [Google Scholar]
- 48. Sandborn WJ, Rutgeerts P, Enns R, et al.. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007;146:829–38. [DOI] [PubMed] [Google Scholar]
- 49. Hanauer SB, Sandborn WJ, Rutgeerts P, et al.. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006;130:323–33; quiz 591. [DOI] [PubMed] [Google Scholar]
- 50. Sandborn WJ, Hanauer SB, Rutgeerts P, et al.. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007;56:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Colombel JF, Sandborn WJ, Rutgeerts P, et al.. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 52. Reinisch W, Sandborn WJ, Hommes DW, D'Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–7. [DOI] [PubMed] [Google Scholar]
- 53. Sandborn WJ, van Assche G, Reinisch W, et al.. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65.e1–3. [DOI] [PubMed] [Google Scholar]
- 54. Plosker GL, Lyseng-Williamson KA. Adalimumab: in Crohn’s disease. BioDrugs 2007;21:125–32; discussion 133–4. [DOI] [PubMed] [Google Scholar]
- 55. Vande Casteele N, Baert F, Bian S, et al. Subcutaneous absorption contributes to observed interindividual variability in adalimumab serum concentrations in Crohn’s disease: a prospective multicentre study. J Crohns Colitis 2019;13:1248–56. [DOI] [PubMed] [Google Scholar]
- 56. Chiu YL, Rubin DT, Vermeire S, et al.. Serum adalimumab concentration and clinical remission in patients with Crohn’s disease. Inflamm Bowel Dis 2013;19:1112–22. [DOI] [PubMed] [Google Scholar]
- 57. Karmiris K, Paintaud G, Noman M, et al.. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology 2009;137:1628–40. [DOI] [PubMed] [Google Scholar]
- 58. Baert F, Kondragunta V, Lockton S, et al.. Antibodies to adalimumab are associated with future inflammation in Crohn’s patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 2016;65:1126–31. [DOI] [PubMed] [Google Scholar]
- 59. Assa A MM, Turner D, et al. Proactive adalimumab trough measurements increase corticosteroid-free clinical remission in paediatric patients with Crohn’s disease: the paediatric Crohn’s disease adalimumab-level-based optimisation treatment (PAILOT) trial. Gastroenterology 2019;157:985–96. [DOI] [PubMed] [Google Scholar]
- 60. Papamichael K, Baert F, Tops S, et al.. Post-induction adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis 2017;11:53–9. [DOI] [PubMed] [Google Scholar]
- 61. Baert F, Vande Casteele N, Tops S, et al.. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther 2014;40:1324–32. [DOI] [PubMed] [Google Scholar]
- 62. Rutgeerts P, Feagan BG, Marano CW, et al.; PURSUIT-IV study group Randomised clinical trial: a placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment Pharmacol Ther 2015;42:504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95; quiz e14-5. [DOI] [PubMed] [Google Scholar]
- 64. Sandborn WJ, Feagan BG, Marano C, et al.; PURSUIT-Maintenance Study Group Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96–109.e1. [DOI] [PubMed] [Google Scholar]
- 65. Adedokun OJ, Xu Z, Marano CW, et al.. Pharmacokinetics and exposure–response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis 2017;11:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Detrez I, Dreesen E, Van Stappen T, et al.. Variability in golimumab exposure: a ‘Real-Life' observational study in active ulcerative colitis. J Crohns Colitis 2016;10:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. BioDrugs. 2014;28:S15–23. [DOI] [PubMed] [Google Scholar]
- 68. Tun GS, Lobo AJ. Evaluation of pharmacokinetics and pharmacodynamics and clinical efficacy of certolizumab pegol for Crohn’s disease. Expert Opin Drug Metab Toxicol 2015;11:317–27. [DOI] [PubMed] [Google Scholar]
- 69. Sandborn WJ, Feagan BG, Stoinov S, et al.; PRECISE 1 Study Investigators Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007;357:228–38. [DOI] [PubMed] [Google Scholar]
- 70. Vande Casteele N, Mould DR, Coarse J, et al.. Accounting for pharmacokinetic variability of certolizumab pegol in patients with Crohn’s disease. Clin Pharmacokinet 2017;56:1513–23. [DOI] [PubMed] [Google Scholar]
- 71. Vande Casteele N, Feagan BG, Vermeire S, et al.. Exposure–response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn’s disease. Aliment Pharmacol Ther 2018;47:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Colombel JF, Sandborn WJ, Allez M, et al.. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:423–31.e1. [DOI] [PubMed] [Google Scholar]
- 73. Feagan BG, Rutgeerts P, Sands BE, et al.; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 74. Sandborn WJ, Feagan BG, Rutgeerts P, et al.; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 75. Sands BE, Feagan BG, Rutgeerts P, et al.. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–627.e3. [DOI] [PubMed] [Google Scholar]
- 76. Rosario M, Wyant T, Leach T, et al.. Vedolizumab pharmacokinetics, pharmacodynamics, safety, and tolerability following administration of a single, ascending, intravenous dose to healthy volunteers. Clin Drug Investig 2016;36:913–23. [DOI] [PubMed] [Google Scholar]
- 77. Parikh A, Leach T, Wyant T, et al.. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis 2012;18:1470–9. [DOI] [PubMed] [Google Scholar]
- 78. Rosario M, Dirks NL, Milch C, et al.. A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of vedolizumab. Clin Pharmacokinet 2017;56:1287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rosario M, Dirks NL, Gastonguay MR, et al.. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther 2015;42:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rosario M, French JL, Dirks NL, et al.. Exposure–efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis 2017;11:921–9. [DOI] [PubMed] [Google Scholar]
- 81. Osterman MT, Rosario M, Lasch K, et al.. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther 2019;49:408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yacoub W, Williet N, Pouillon L, et al.. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. [DOI] [PubMed] [Google Scholar]
- 83. Williet N, Boschetti G, Fovet M, et al.. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017;15:1750–1757.e3. [DOI] [PubMed] [Google Scholar]
- 84. Dreesen E, Verstockt B, Bian S, et al.. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1937–1946.e8. [DOI] [PubMed] [Google Scholar]
- 85. Liefferinckx C, Minsart C, Cremer A, et al.. Early vedolizumab trough levels at induction in inflammatory bowel disease patients with treatment failure during maintenance. Eur J Gastroenterol Hepatol 2019;31:478–85. [DOI] [PubMed] [Google Scholar]
- 86. Zeissig S, Rosati E, Dowds CM, et al.. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019;68:25–39. [DOI] [PubMed] [Google Scholar]
- 87. Ungar B, Kopylov U, Yavzori M, et al.. Association of vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:697–705.e7. [DOI] [PubMed] [Google Scholar]
- 88. Paul S, Williet N, Di Bernado T, Berger AE, Boschetti G, Filippi J, et al. Soluble mucosal addressin cell adhesion molecule 1 and retinoic acid are potential tools for therapeutic drug monitoring in patients with inflammatory bowel disease treated with vedolizumab: a proof of concept study. J Crohns Colitis 2018. Epub 2018/06/04. doi:10.1093/ecco-jcc/jjy077. PubMed PMID: 29860366. [DOI] [PubMed] [Google Scholar]
- 89. Sandborn WJ SB, Panaccione R, et al. Efficacy and safety of ustekinumab as maintenance therapy in ulcerative colitis: week 44 results from UNIFI. J Crohns Colitis 2019;13:S025–S6. [Google Scholar]
- 90. Feagan BG, Sandborn WJ, Gasink C, et al.; UNITI–IM-UNITI Study Group Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 91. Restellini S, Khanna R, Afif W. Therapeutic drug monitoring with ustekinumab and vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis 2018;24:2165–72. [DOI] [PubMed] [Google Scholar]
- 92. stelarainfo.com. Prescribing information for Stelara [ustekinumab] injection, for subcutaneous or intravenous use 2016. https://www.stelarainfo.com Accessed May 11, 2017.
- 93.European Medicines Agency. Stelara 130 mg for solution for infusion: EU summary of product characteristics. 2017. http://www.ema.europa.eu/. Accessed May 11, 2017
- 94. Adedokun OJ, Xu Z, Gasink C, et al.. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology 2018;154:1660–71. [DOI] [PubMed] [Google Scholar]
- 95. Rowan CR, Keegan D, Byrne K, et al.. Subcutaneous rather than intravenous ustekinumab induction is associated with comparable circulating drug levels and early clinical response: a pilot study. Aliment Pharmacol Ther 2018;48:333–9. [DOI] [PubMed] [Google Scholar]
- 96. Claire P SB, Nicolas D, et al. Trough levels and antibodies to ustekinumab are not correlated to response to ustekinumab treatment in Crohn’s disease patients. J Crohns Colitis 2017. S260–S1. [Google Scholar]
- 97. Verstockt B, Dreesen E, Noman M, Outtier A, Van den Berghe N, Aerden I, et al. Ustekinumab exposure-outcome analysis in Crohn’s disease only in part explains limited endoscopic remission rates. J Crohns Colitis 2019;13:864–72. [DOI] [PubMed] [Google Scholar]
- 98. Soufflet N BG, Roblin X, et al. Concentrations of ustekinumab during induction therapy associate with remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2019; in press. [DOI] [PubMed] [Google Scholar]
- 99. Battat R, Kopylov U, Bessissow T, et al.. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2017;15:1427–1434.e2. [DOI] [PubMed] [Google Scholar]
- 100. Ward MG, Sparrow MP, Roblin X. Therapeutic drug monitoring of vedolizumab in inflammatory bowel disease: current data and future directions. Therap Adv Gastroenterol 2018;11:1756284818772786. [DOI] [PMC free article] [PubMed] [Google Scholar]