SUMMARY
BACKGROUND
Treatment outcomes among survivors of cancer diagnosed during adolescence and early young adulthood (early-AYA) have not been independently characterized. We aimed to describe the chronic health conditions and all-cause and cause-specific mortality among survivors of early-AYA cancer.
METHODS
The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort with longitudinal follow-up of five-year survivors diagnosed with cancer before the age of 21 years at 27 academic institutions in North America between 1970-1999. We evaluated outcomes among survivors of early-AYA cancer (15-20 years at diagnosis) and survivors diagnosed <15 years (matched on primary cancer diagnosis, including leukemia, lymphoma, CNS tumors, neuroblastoma, Wilms tumor, soft tissue sarcomas, and bone cancer) by comparing both to same age siblings. Mortality was ascertained using the National Death Index. Chronic health conditions were classified using the Common Terminology Criteria for Adverse Events. Standardized mortality ratios (SMRs) were estimated using age-, sex- and calendar year-specific U.S. rates. Cox proportional hazard models estimated hazard ratios (HR) and 95% confidence intervals (CIs).
FINDINGS
Among 5,804 early-AYA survivors (median age 42 years, IQR 34-50) and 5,804 childhood cancer survivors (median age 34 years, IQR 27-42), SMRs compared to the general population for all-cause mortality were 5·9 (95% CI, 5·5-6·2) and 6·2 (5·8-6·6), respectively. Early-AYAs had lower SMR for death from health-related causes (i.e. conditions that exclude recurrence or progression of the primary cancer and external causes, but include the late effects of cancer therapy) than childhood cancer survivors (SMR=4·8; 95% CI, 4·4-5·1 vs. SMR=6·8; 6·2-7·4), primarily evident more than 20 years after cancer diagnosis. Early-AYAs and childhood cancer survivors were both at greater risk of developing severe/disabling, life-threatening, or fatal (grade 3-5) health condition compared to same age siblings (HR=4·2; 95% CI, 3·7-4·8 and 5·6; 4·9-6·3), though early-AYA survivors’ HR was lower, as were HRs for grade 3-5 cardiac (4·3 vs. 5·6), endocrine (3·9 vs. 6·4), and musculoskeletal conditions (6·5 vs. 8·0).
INTERPRETATION
Early-AYA cancer survivors had elevated risks of mortality and severe/life threatening chronic health conditions in comparison to the general population. However, early-AYAs had lower nonrecurrent, health-related SMRs and relative risks for grade 3-5 chronic health conditions in comparison to same age siblings, than childhood cancer survivors, most notably more than 20 years after their original cancer. These results highlight the need for long-term screening of both childhood and early-AYA cancer survivors.
FUNDING
National Cancer Institute, Cancer Center Support Grants, American Lebanese-Syrian Associated Charities.
INTRODUCTION
Nearly 70,000 adolescent and young adults (AYAs) between the ages of 15 and 39 years are diagnosed with cancer annually in the United States (U.S.).1 Almost 80% of these AYAs will survive more than five years after their cancer diagnosis.2 As such, survivors of AYA cancer represent a population with a significant number of potential life-years saved who remain at risk of developing long-term morbidity or dying prematurely due to their prior cancer treatments.
In 2006, the Adolescent and Young Adult Oncology Progress Review Group (PRG), supported by the National Cancer Institute (NCI) and the LIVESTRONG Foundation, identified research priorities to improve outcomes for survivors of AYA cancer.3 One priority called for research to understand long-term health outcomes associated with AYA cancer and its treatment, an essential step toward providing appropriate risk-based care. Yet, a decade after the PRG report, there is a paucity of data regarding the long-term morbidity and late mortality in this population. While studies have examined the morbidity and premature mortality of survivors of both pediatric and adult cancers,4–7 few have focused on those treated for their cancer as adolescents and early young adults (between the ages of 15-20 years). Thus, the objective of the current analysis was to describe chronic health conditions and all-cause and cause-specific late mortality among survivors of cancer diagnosed during adolescence and early young adulthood within the Childhood Cancer Survivor Study (CCSS), compared to survivors diagnosed as children with the same primary cancers and non-cancer populations.
METHODS
Study design and participants
The CCSS is a retrospective cohort with longitudinal follow-up of 24,363 five-year survivors diagnosed with cancer <21 years at 27 academic institutions in North America between 1970-1999. Details of the CCSS have been reported previously.8 Briefly, eligible survivors were identified and initially recruited through their original cancer treatment institutions. Survivors who died after their five year anniversary of cancer diagnosis were eligible and a proxy (parent, sibling, or spouse) was asked to answer a baseline survey on their behalf. A sibling control group of 5,059 was enrolled by randomly selecting 30% of the participating survivors, determining whether they had a full sibling and inviting that sibling to participate through their survivor sibling. The study was approved by the IRB at each institution, and informed consent was obtained. Continued contact with participating survivors and siblings is carried out through the coordinating center at St. Jude Children’s Hospital (study details available at https://ccss.stjude.org).
In this analysis, CCSS participants diagnosed with cancer between the ages ≥15 to <21 years were defined as early-AYAs. Early-AYA cancer diagnoses included leukemia, central nervous system (CNS) malignancy, Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), Wilms tumor, neuroblastoma, soft-tissue sarcoma, and bone cancer. Since the youngest survivor of an early-AYA cancer would be at least 20 years old at cohort entry, only siblings 20 years of age at the time of baseline completion were included in comparisons to early-AYAs. Additionally, to eliminate potential sources of bias, a cohort of survivors diagnosed with cancer during childhood (<15 years of age) were randomly selected from frequency-matched (1:1) strata based on primary cancer diagnosis, with siblings age ≥5 years of age as a comparison group for them.
Procedures
Primary cancer characteristics and detailed treatment information were abstracted from medical records.8 Cumulative alkylating agent dose was reported as cyclophosphamide-equivalent dose (CED),9 while cumulative anthracycline dose was reported as doxorubicin-equivalent.10 Radiation records were used to estimate region and organ-based dosimetry.11
Cause of death was determined among all subjects eligible for the CCSS through a search of the U.S. National Death Index (NDI) through 2013. The NDI identified the underlying and multiple causes of death for deceased subjects using the International Classification of Disease (ICD)-9th and 10th Revision. For deaths that predated the NDI, death certificates were requested from states where deaths occurred. Deaths were reviewed by two or more clinicians and grouped into three mutually exclusive categories using ICD-9 and ICD-10 coding: 1) recurrence/progression of the primary cancer; 2) external causes (accidents, suicides, and poisonings); and 3) nonrecurrent, health-related causes including subsequent malignant neoplasm (SMN), cardiovascular causes, pulmonary causes, and all other medical causes. Canadian participants were excluded from mortality analyses since the NDI does not ascertain death of subjects outside the U.S.
Chronic health conditions were reported by participating survivors and siblings on one baseline and up to three follow-up surveys administered at 2-4 year intervals. The severity of each health condition was graded based on the Common Terminology Criteria for Adverse Events (CTCAE version 4·03) system developed by the NCI. Conditions were coded as mild (grade 1), moderate (grade 2), severe/disabling (grade 3), life-threatening (grade 4), or fatal (grade 5), as previously described,12 based on questions regarding age at onset of specific chronic health conditions (appendix pp1-3).4 The self-reported health conditions were reviewed and CTCAE grading was adjudicated by an expert panel (appendix pp4-6). If information available was insufficient to assign between two grades, the lower severity grade was assigned. Grade 5 conditions were obtained from the cause of death information ascertained from the NDI. Questionnaires were completed by a proxy if the survivor was deceased, <18 years of age, or unable to complete it themselves. The self-report of a SMN was validated by review of a pathology report, medical record, or death certificate.
Outcomes
The study’s objective was to describe the chronic health conditions and late mortality of survivors diagnosed with cancer as early-AYAs, compared to a matched population of survivors diagnosed as children and a non-cancer population.
Statistical analysis
Among the U.S. based eligible survivors for the CCSS, Kaplan-Meier estimates of overall survival probabilities were computed, first, as a function time since cancer diagnosis and second, with age as the time scale, using left-truncation to account for staggered age of entry to the cohort.13 Greenwood formula was used to calculate 95% confidence intervals (CI). To compare to the U.S. population, expected survival was computed based on the expected number of deaths each year since diagnosis using sex-, age-, and calendar year-specific U.S. mortality rates from the National Center for Health Statistics14 and plotted as comparative survival curves. Cumulative mortality was conditioned on survival at five years since diagnosis. Cumulative incidence was also estimated for each cause-specific mortality, treating other causes of death as competing risks.
Standardized mortality ratios (SMRs) were estimated as the number of observed deaths divided by the expected number of deaths based on the same U.S. mortality rates used for the Kaplan-Meier curves. Cause-specific SMRs were generated for deaths attributable to SMNs, cardiac causes, pulmonary causes, external (accidents/suicide/homicide), and all other medical causes, stratified by both attained age and time since diagnosis. CIs and p-values for SMRs were calculated on the basis of Poisson probability models for deaths, splitting each person’s data record into person-year intervals with unique combinations of age, calendar year and sex, with relevant covariate and death data for each and using the number of expected events in the U.S. in that time interval as the offset term.
Cumulative incidence rates were estimated for grades 1-5 and 3-5 conditions, overall and by organ system, based on time from diagnosis to the first condition after cohort entry and also calculated with age as the time scale.13 Death from chronic conditions was treated as an event, but death from other causes was treated as a competing risk. Age-matched groups of siblings were used to calculate comparative incidence estimates.
To evaluate risks of chronic health conditions in comparison to same age non-cancer survivors, Cox proportional hazard models evaluated hazard ratios (HR) comparing grade 1-5 and grade 3-5 chronic health conditions for early-AYAs relative to same age siblings, and childhood cancer survivors compared to same age siblings, adjusted for sex, race/ethnicity and censored at the earliest age of death or last follow-up. Age was used as the scale to adjust for the increasing risk of severe health conditions with age, using siblings of similar ages as the reference group. Rather than modeling the time to first condition, the models used a counting process approach to account for all reported unique conditions until death or last follow-up.15 Sandwich standard-error estimates adjusted for intra-participant correlations and survivor-sibling pairs.16 Among early-AYAs, treatment-related risk factors for grade 3-5 conditions in specific organ systems were assessed in additional multivariable Cox models, adjusted for sex and race/ethnicity. Survivors with missing treatment data were excluded from these models. Initial choice of treatment covariates was based on previous CCSS publications, and final models retained factors significant at the 0·05 level in addition to a priori selected factors of interest.17 Models included any surgery, any bleomycin, any platinum, any methotrexate, CED dose (none, <4000, 4000-<8000, 8000+), anthracycline dose (none, <300, 300+), and radiation location in hierarchy: total body irradiation, chest/neck, abdomen/pelvis, brain, other, none. Supplemental Table 4 lists organ specific treatments used for organ-specific models. Smoking status was also included in pulmonary, cardiovascular and musculoskeletal models. All models were assessed for violations of proportional hazards. Data were analyzed with SAS software, version 9·4 (SAS Institute) and Stata/SE 14·1 (StataCorp).
Standardized incidence ratios (SIRs) were calculated for SMNs, with the number observed divided by the expected number based on sex-, age-, and calendar year-specific U.S. incidence rates from the Surveillance, Epidemiology, and End Results program,1 stratified by time since diagnosis and attained age. CI and p-values for SIRs were calculated using similar Poisson probability models as described for SMRs. Comparisons of SIRs and SMRs between groups were based on tests of coefficients for group membership in these Poisson models.
ROLE OF FUNDING
The funders had no role in the study design, data collection, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
A total of 5,979 survivors were diagnosed with cancer as early-AYAs in the CCSS (Table 1). The mortality analysis excluded 175 Canadian participants or those not in the NDI (appendix p7), resulting in 5,804 survivors of early-AYA cancer along with 5,804 childhood cancer survivors, followed for a median of 21 years (IQR 13-28) and 21 years (14-28), respectively to death or date of NDI search. The chronic health condition analysis included 4,082 early-AYAs with a median age at last follow-up of 38 years (IQR 32-43), 36 years (30-42) for 3,806 siblings, and 30 years (24-36) for 4,082 childhood cancer survivors. Among living participants, the median years of follow-up since cohort entry for early-AYA survivors was 16·9 years (IQR 11·5-22·1), 16·6 years (10·6-22·6) for siblings, and 16·7 years (11·6-21·7) for childhood cancer survivors. At their most recent survey, 3504 (86%) of 4082 early-AYA survivors and 3304 (81%) of 4082 childhood cancer survivors completed their own survey, as opposed to a proxy responder. The majority of early-AYAs were diagnosed with lymphoma: 1438 (35·2%) of 4082 with HL and 409 (10·0%) of 4082 with NHL.
Table 1.
Demographic and treatment characteristics of early-AYA cancer survivors, childhood cancer survivors matched on primary diagnosis and siblings available for analysis of chronic health conditions and late mortality.
| Chronic Health Condition Analyses | Late Mortality Analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early-AYA Survivors (n=4082) | Childhood Survivors (n=4082) | Siblings (n=3806) | Early-AYA Survivors (n=5804) | Childhood Survivors (n=5804) | |||||||
| N | % | N | % | N | % | N | % | N | % | ||
| Vital Status | Alive | - | - | - | - | - | - | 4452 | 76·7 | 4867 | 83·9 |
| Dead | - | - | - | - | - | - | 1352 | 23·3 | 937 | 16·1 | |
| Sex | Female | 1909 | 46·8 | 1847 | 45·2 | 2043 | 53·7 | 2556 | 44·0 | 2413 | 41·6 |
| Male | 2173 | 53·2 | 2235 | 54·8 | 1763 | 46·3 | 3248 | 56·0 | 3391 | 58·4 | |
| Race/Ethnicity1 | Non-Hispanic White | 3501 | 86·0 | 3311 | 81·5 | 3340 | 90·7 | 3356 | 85·8 | 3080 | 80·1 |
| Non-Hispanic Black | 196 | 4·8 | 286 | 7·0 | 101 | 2·7 | 192 | 4·9 | 277 | 7·2 | |
| Hispanic | 253 | 6·2 | 324 | 8·0 | 152 | 4·1 | 249 | 6·4 | 349 | 9·1 | |
| Non-Hispanic Other | 120 | 2·9 | 143 | 3·5 | 90 | 2·4 | 114 | 2·9 | 140 | 3·6 | |
| Missing | 12 | NA | 18 | NA | 123 | NA | 1893 | NA | 1958 | NA | |
| Treatment era | 1970–79 | 1230 | 30·1 | 1222 | 29·9 | NA | 0·0 | 1751 | 30·2 | 1718 | 29·6 |
| 1980–89 | 1561 | 38·2 | 1514 | 37·1 | NA | 0·0 | 2158 | 37·2 | 2060 | 35·5 | |
| 1990–99 | 1291 | 31·6 | 1346 | 33·0 | NA | 0·0 | 1895 | 32·6 | 2026 | 34·9 | |
| Diagnosis | Acute lymphoblastic leukemia | 440 | 10·8 | 440 | 10·8 | NA | 0·0 | 588 | 10·1 | 588 | 10·1 |
| Acute myeloid leukemia | 142 | 3·5 | 142 | 3·5 | NA | 0·0 | 193 | 3·3 | 193 | 3·3 | |
| Other leukemia | 61 | 1·5 | 61 | 1·5 | NA | 0·0 | 91 | 1·6 | 91 | 6·1 | |
| Astrocytomas | 293 | 7·2 | 293 | 7·2 | NA | 0·0 | 434 | 7·5 | 434 | 7·5 | |
| Medulloblastoma/PNET | 58 | 1·4 | 58 | 1·4 | NA | 0·0 | 81 | 1·4 | 81 | 1·4 | |
| Other Central Nervous System tumors | 110 | 2·7 | 110 | 2·7 | NA | 0·0 | 156 | 2·7 | 156 | 2·7 | |
| Hodgkin lymphoma | 1438 | 35·2 | 1438 | 35·2 | NA | 0·0 | 2023 | 34·9 | 2023 | 34·9 | |
| Non-Hodgkin lymphoma | 409 | 10·0 | 409 | 10·0 | N/A | 0·0 | 577 | 9·9 | 577 | 9·9 | |
| Kidney tumors | 23 | 0·6 | 23 | 0·6 | NA | 0·0 | 24 | 0·4 | 24 | 0·4 | |
| Neuroblastoma | 18 | 0·4 | 18 | 0·4 | NA | 0·0 | 31 | 0·5 | 31 | 0·5 | |
| Non-rhabdomyosarcoma, soft tissue sarcoma | 201 | 4·9 | 201 | 4·9 | NA | 0·0 | 372 | 6·4 | 372 | 6·4 | |
| Rhabdomysarcoma | 126 | 3·1 | 126 | 3·1 | NA | 0·0 | 130 | 2·2 | 130 | 2·2 | |
| Ewings sarcoma | 209 | 5·1 | 209 | 5·1 | NA | 0·0 | 289 | 5·0 | 289 | 5·0 | |
| Osteosarcoma | 513 | 12·6 | 513 | 12·6 | NA | 0·0 | 742 | 12·8 | 742 | 12·8 | |
| Other bone tumors | 41 | 1·0 | 41 | 1·0 | NA | 0·0 | 73 | 1·3 | 73 | 1·3 | |
| Treatment | No chemotherapy or radiation | 312 | 8·3 | 259 | 7·0 | NA | 0·0 | 305 | 7·9 | 210 | 5·6 |
| Missing | 34 | 19 | NA | 37 | 20 | ||||||
| Any chemotherapy | 2817 | 75·5 | 3017 | 82·1 | NA | 0·0 | 2939 | 76·4 | 3230 | 84·8 | |
| Missing | 47 | 31 | NA | 52 | 28 | ||||||
| Alkylating agent | 2245 | 60·4 | 2427 | 66·1 | NA | 0·0 | 2342 | 61·2 | 2598 | 68·8 | |
| Missing | 63 | 34 | NA | 69 | 31 | ||||||
| Platinum based | 389 | 10·4 | 365 | 12·6 | NA | 0·0 | 420 | 10·9 | 521 | 13·6 | |
| Missing | 33 | 18 | NA | 37 | 19 | ||||||
| Anti-metabolites | 1463 | 39·0 | 1558 | 42·2 | NA | 0·0 | 1529 | 39·3 | 1625 | 42·5 | |
| Missing | 26 | 17 | NA | 27 | 14 | ||||||
| Anthracyclines | 2014 | 53·9 | 2117 | 57·5 | NA | 0·0 | 2121 | 55·1 | 2263 | 59·0 | |
| Missing | 42 | 24 | NA | 45 | 27 | ||||||
| Plant alkaloids | 2390 | 63·7 | 2593 | 70·3 | NA | 0·0 | 2489 | 64·9 | 2789 | 72·9 | |
| Missing | 26 | 17 | NA | 27 | 14 | ||||||
| Bleomycin | 655 | 17·5 | 653 | 17·7 | NA | 0·0 | 707 | 18·3 | 709 | 18·6 | |
| Missing | 36 | 24 | NA | 38 | 22 | ||||||
| Any irradiation | 2383 | 63·3 | 2172 | 58·4 | NA | 0·0 | 2452 | 63·1 | 2250 | 58·5 | |
| Missing | 50 | 25 | NA | 54 | 26 | ||||||
| Brain irradiation | 572 | 15·6 | 582 | 16·1 | NA | 0·0 | 574 | 15·9 | 647 | 17·1 | |
| Missing | 159 | 133 | NA | 173 | 135 | ||||||
| Chest irradiation | 1436 | 39·3 | 1182 | 32·7 | NA | 0·0 | 1487 | 39·6 | 1179 | 31·1 | |
| Missing | 158 | 132 | NA | 172 | 135 | ||||||
| Spine irradiation | 109 | 3·0 | 103 | 2·9 | NA | 0·0 | 114 | 3·0 | 121 | 3·2 | |
| Missing | 161 | 133 | NA | 175 | 136 | ||||||
| Abdominal irradiation | 952 | 26·0 | 726 | 20·1 | NA | 0·0 | 980 | 26·0 | 731 | 19·6 | |
| Missing | 158 | 134 | NA | 172 | 137 | ||||||
| Pelvic irradiation | 733 | 20·0 | 555 | 15·4 | NA | 0·0 | 758 | 20·1 | 588 | 15·7 | |
| Missing | 158 | 133 | NA | 172 | 136 | ||||||
| Total body irradiation | 104 | 2·8 | 67 | 1·9 | NA | 0·0 | 109 | 2·9 | 81 | 2·2 | |
| Missing | 161 | 133 | NA | 175 | 136 | ||||||
| Any surgery | 3258 | 87·2 | 3181 | 86·5 | NA | 0·0 | 3346 | 86·8 | 3279 | 86·5 | |
| Missing | 42 | 27 | NA | 43 | 28 | ||||||
| Follow-uptime2 (years) | <=10 years | 1035 | 25·4 | 903 | 22·1 | 869 | 22·8 | 852 | 14·7 | 616 | 10·6 |
| 11–20 years | 1831 | 44·9 | 1993 | 48·8 | 1634 | 42·9 | 1952 | 33·6 | 2070 | 35·7 | |
| 21–30 years | 1089 | 26·7 | 1023 | 25·1 | 1047 | 27·5 | 2002 | 34·5 | 1981 | 34·1 | |
| >30 years | 127 | 3·1 | 163 | 4·0 | 256 | 6·7 | 998 | 17·2 | 1137 | 19·6 | |
| All: Median(IQR) | 16·0 (9·9-21·2) | 16·0 (10·6-21·1) | 16·6 (10·6-22·6) | 20.6 (12·5-27.8) | 21.1 (13·5-28.4) | ||||||
| Alive: Median(IQR) | 16·9 (11·5-22·1) | 16·7 (11·6-21·7) | 16·6 (10.6-22.6) | 22.9 (15·2-28.8) | 22.4 (15·1-29.1) | ||||||
| Dead: Median(IQR) | 4·2 (1·7-8·0) | 4·8 (1·6-9.6) | 16·8 (12.8-20.8) | 10.2 (3·3-20·8) | 11.9 (4·3-22.4) | ||||||
| Age at cancer diagnosis (years) | Median(IQR) | 17 (15-18) | 10 (5-13) | NA | 17 (15-18) | 10 (5-12) | |||||
| Age at last follow-up2 (years) | All: Median(IQR) | 38 (32-43) | 30 (24-36) | 36 (30-42) | 42 (34-50) | 34 (27-42) | |||||
| Alive: Median(IQR) | 39 (33-44) | 30 (25-37) | 36 (30-42) | 45 (37-51) | 36 (29-43) | ||||||
| Dead: Median(IQR) | 26 (24-31) | 20 (16-25) | 36 (32-40) | 33 (26-43·5) | 26 (19-37) | ||||||
Race known for those who completed baseline questionnaire.
Age at last follow-up represents date of last questionnaire or death for morbidity; date of death or December 31, 2013 for mortality. Follow-up time represents years since study entry.
Percentages shown among those with known values.
Treatment data is shown among those with medical record abstraction: (Chronic health conditions: n=3779 early-AYA, 3706 childhood; Mortality: n=3897 early-AYA, 3838 childhood).
Among 5,804 early-AYA 5-year survivors, there were 1,352 deaths (Table 1). Health-related causes of late mortality (other than recurrence/progression of the primary cancer or external causes) including SMN, cardiovascular disease, pulmonary disease, and other medical causes accounted for 711 (52·4%) of 1357 (weighted) deaths among the early-AYAs, followed by recurrence/progression of the primary cancer (492 (36·3%) of 1357) and external causes (91 (6·7%) of 1357) (Table 2).
Table 2.
Standardized mortality ratios and frequency of death among survivors of early-AYA and matched childhood cancer, overall and by diagnosis
| early-AYA | Childhood | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cause of death | Group | Level | # deaths | # expected | Rate/1000 PY | SMR (95% CI) | # deaths | # expected | Rate/1000 PY | SMR (95% CI) | p-value |
| All causes | Among all patients | 1357 | 231·9 | 11·5 | 5·9(5·5-6·2) | 963 | 155·7 | 7·5 | 6·2(5·8-6·6) | 0·22 | |
| Primary diagnosis | Leukemia | 166 | 30·4 | 9·9 | 5·5(4·6-6·5) | 128 | 19·6 | 5·5 | 6·5(5·2·8·2) | 0·23 | |
| CNS | 170 | 21·8 | 14·1 | 7·8(6·6·9·2) | 113 | 12·2 | 9·0 | 9·3(7·6-11·3) | 01·8 | ||
| HL | 561 | 82·6 | 134 | 6·8(6·2·7·4) | 395 | 65·2 | 88 | 6·1(5·5·6·7) | 0·083 | ||
| NHL | 91 | 24·0 | 7·7 | 3·8(3·1-4·7) | 52 | 16·0 | 4·3 | 3·3(2·5-4·3) | 0·38 | ||
| Soft tissue sarcoma | 109 | 25·7 | 9·2 | 4·2(3·5-5·2) | 77 | 12·2 | 7·0 | 6·3(4·9-8·0) | 0·014 | ||
| Bone cancer | 240 | 45·8 | 10·7 | 5·2(4·6-6·0) | 195 | 29·6 | 8·6 | 6-6(5·7-7·7) | 0·027 | ||
| Nonrecurrent, Health-related cause | Among all patients | 711 | 149·0 | 6·0 | 4·8(4·4-5·1) | 506 | 74·4 | 4·0 | 6·8(6·2-7·4) | <·0001 | |
| Primary diagnosis | Leukemia | 57 | 17·8 | 3·4 | 3·2(2·5-4·2) | 41 | 8·0 | 1·8 | 5·1(3·5-7·4) | 0·056 | |
| CNS | 75 | 13·4 | 6·2 | 5·6(4·4-7·1) | 49 | 5·2 | 3·9 | 9·4(7·0-12·7) | 0·0066 | ||
| HL | 359 | 55·2 | 8·6 | 6·5(5·9-7·2) | 271 | 32·7 | 6·0 | 8·3(7·4-9·3) | 0·0026 | ||
| NHL | 66 | 14·7 | 5·6 | 4·5(3·5-5·8) | 32 | 7·0 | 2·6 | 4·6(3·2-6·5) | 0·92 | ||
| Soft tissue sarcoma | 49 | 17·6 | 4·2 | 2·8(2·1-3·7) | 37 | 5·8 | 3·4 | 6·4(4·6-9·0) | <·001 | ||
| Bone cancer | 101 | 29·2 | 4·5 | 3·5(2·8-4·2) | 75 | 15·4 | 3·3 | 4·9(3·9-6·1) | 0·029 | ||
| Subsequent malignant neoplasm | Among all patients | 323 | 41·5 | 2·7 | 7·8(7·0-8·7) | 221 | 18·6 | 1·7 | 11·9(10·4-13·6) | <·0001 | |
| Primary diagnosis | Leukemia | 20 | 4·6 | 1·2 | 4·4(2·8-6·9) | 17 | 2·0 | 0·7 | 8·8(5·3-14·5) | 0·042 | |
| CNS | 26 | 3·7 | 2·2 | 7·1(4·8-10·6) | 17 | 1·2 | 1·4 | 13·6(8·4-22·1) | 0·043 | ||
| HL | 172 | 16·1 | 4·1 | 10·7(9·2-12·4) | 120 | 8·2 | 2·7 | 14·7(12·3-17·6) | 0·0072 | ||
| NHL | 28 | 3·8 | 2·4 | 7·4(5·0-10·8) | 8 | 1·6 | 0·7 | 5·0(2·5-10·0) | 0·34 | ||
| Soft tissue sarcoma | 27 | 5·1 | 2·3 | 5·3(3·6-7·8) | 23 | 1·5 | 2·1 | 15·6(10·2-23·8) | <·001 | ||
| Bone cancer | 48 | 7·9 | 2·1 | 6·1(4·6-8·1) | 36 | 4·1 | 1·6 | 8·8(6·3-12·3) | 0·097 | ||
| Cardiac causes | Among all patients | 141 | 32·3 | 1·2 | 4·4(3·7-5·2) | 101 | 14·8 | 0·8 | 6·8(5·6-8·3) | <·001 | |
| Primary diagnosis | Leukemia | 11 | 3·9 | 0·7 | 2·9(1·6-5·2) | 2 | 1·4 | 0·1 | 1·4(0·4-5·6) | 03·6 | |
| CNS | 3 | 2·9 | 0·2 | 1·0(0·3-3·3) | 5 | 1·0 | 0·4 | 5·1(2·1-12·4) | 0·031 | ||
| HL | 95 | 11·7 | 2·3 | 8·1(6·6-9·9) | 70 | 6·7 | 1·6 | 10·4(8·2-13·2) | 0·11 | ||
| NHL | 11 | 3·3 | 0·9 | 3·3(1·8-6·0) | 7 | 1·5 | 0·6 | 4·8(2·3-10·1) | 0·44 | ||
| Soft tissue sarcoma | 3 | 3·8 | 0·3 | 0·8(0·3-2·4) | 3 | 1·1 | 0·3 | 2·6(0·8-8·2) | 0·14 | ||
| Bone cancer | 18 | 6·4 | 0·8 | 2·8(1·8-4·4) | 13 | 3·0 | 0·6 | 4·3(2·5-7·4) | 0·24 | ||
| Pulmonary causes | Among all patients | 58 | 7·9 | 0·5 | 7·4(5·7-9·5) | 42 | 4·1 | 0·3 | 10·3(7·6-14·0) | 0·095 | |
| Primary diagnosis | Leukemia | 7 | 0·9 | 0·4 | 8·1(3·8-17·0) | 2 | 0·5 | 0·1 | 4·2(1·0-16·7) | 0·41 | |
| CNS | 11 | 0·7 | 0·9 | 15·4(8·5-28·0) | 4 | 0·3 | 0·3 | 13·4(5·0-35·8) | 0·81 | ||
| HL | 26 | 3·0 | 0·6 | 8·6(5·9-12·7) | 23 | 1·7 | 0·5 | 13·4(8·9-20·1) | 0·13 | ||
| NHL | 6 | 0·8 | 0·5 | 8·0(3·6-17·7) | 5 | 0·4 | 0·4 | 13·5(5·6-32·3) | 0·39 | ||
| Soft tissue sarcoma | 2 | 1·0 | 0·2 | 2·1(0·5-8·4) | 3 | 0·3 | 0·3 | 9·2(3·0-28·8) | 0·11 | ||
| Bone cancer | 6 | 1·5 | 0·3 | 3·9(1·8-8·8) | 5 | 0·8 | 0·2 | 5·9(2·5-14·2) | 0·50 | ||
| Other medical causes$ | Among all patients | 188 | 67·2 | 1·6 | 2·8(2·4-3·2) | 141 | 36·9 | 1·1 | 3·8(3·2-4·6) | 0·0073 | |
| Primary diagnosis | Leukemia | 18 | 8·4 | 1·1 | 2·2(1·4-3·5) | 19 | 4·1 | 0·8 | 4·7(2·5-8·8) | 0·060 | |
| CNS | 35 | 6·2 | 2·9 | 5·7(4·1-7·9) | 23 | 2·7 | 1·8 | 8·6(5·7-13·1) | 0·12 | ||
| HL | 66 | 24·3 | 1·6 | 2·7(2·1-3·5) | 58 | 16·1 | 1·3 | 3·6(2·8-4·7) | 0·11 | ||
| NHL | 21 | 6·8 | 1·8 | 3·1(2·0-4·7) | 12 | 3·5 | 1·0 | 3·4(1·9-6·0) | 0·79 | ||
| Soft tissue sarcoma | 17 | 7·7 | 1·4 | 2·2(1·4-3·6) | 8 | 2·9 | 0·7 | 2·8(1·4-5·6) | 0·58 | ||
| Bone cancer | 29 | 13·4 | 1·3 | 2·2(1·5-3·1) | 21 | 7·5 | 0·9 | 2·8(1·8-4·3) | 0·37 | ||
| External causes | Among all patients | 91 | 82·9 | 0·8 | 1·1(0·9-1·3) | 95 | 81·3 | 0·7 | 1·2(0·9-1·5) | 0·65 | |
| Primary diagnosis | Leukemia | 10 | 12·6 | 0·6 | 0·8(0·4-1·5) | 17 | 11·6 | 0·7 | 1·5(0·8-3·0) | 0·17 | |
| CNS | 14 | 8·4 | 1·2 | 1·7(1·0-2·8) | 9 | 7·0 | 0·7 | 1·3(0·7-2·5) | 0·54 | ||
| HL | 27 | 27·4 | 0·6 | 1·0(0·7-1·4) | 37 | 32·6 | 0·8 | 1·1(0·8-1·6) | 0·57 | ||
| NHL | 10 | 9·3 | 0·8 | 1·1(0·6-2·0) | 12 | 9·0 | 1·0 | 1·3(0·8-2·3) | 0·62 | ||
| Soft tissue sarcoma | 8 | 8·1 | 0·7 | 1·0(0·5-2·0) | 3 | 6·4 | 0·3 | 0·5(0·1-1·4) | 0·27 | ||
| Bone cancer | 22 | 16·6 | 1·0 | 1·3(0·9-2·0) | 16 | 14·1 | 0·7 | 1·1(0·7-1·9) | 0·63 | ||
| Recurrence/progression of primary cancer | Among all patients | 492 | NA | NA | NA | 325 | NA | NA | NA | NA | |
| Primary diagnosis | Bone cancer | 109 | NA | NA | NA | 100 | NA | NA | NA | NA | |
| CNS | 72 | NA | NA | NA | 51 | NA | NA | NA | NA | ||
| HL | 152 | NA | NA | NA | 67 | NA | NA | NA | NA | ||
| Leukemia | 86 | NA | NA | NA | 64 | NA | NA | NA | NA | ||
| NHL | 11 | NA | NA | NA | 7 | NA | NA | NA | NA | ||
| Soft tissue sarcoma | 46 | NA | NA | NA | 35 | NA | NA | NA | NA | ||
Abbreviations: y, year; PY, person-year; SMR, standardized mortality ratio; CI, confidence interval; CNS, central nervous system; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; NA, not applicable.
Kidney and Neuroblastoma not included due to low # of early-AYA survivors with these diagnoses.
and % include weighting, e.g. 1357 deaths after weighting.
Other medical causes: Nonrecurrent, health-related causes of death other than subsequent malignant neoplasms, cardiac causes, or pulmonary causes.
Cumulative mortality is summarized in Figure 1 by both time since diagnosis and attained age. At 30 years post-diagnosis, cumulative mortality was 23·0% (95% CI, 21·8-24·2) for early-AYAs at which time they were 45-50 years old. This corresponds to Figure 1c with a cumulative mortality of 24% (22·4-25·4) at 45 years of age. Cumulative mortality at 30 years post-diagnosis for childhood cancer survivors was 15·6% (14·6-16·7), when they ranged from 30-44 years of age. This also corresponds to Figure 1c with cumulative mortality of 16·0% (14·0-18·1) at age 30 and 26·5% (24·4-28·7) at age 45.
Figure 1. All-cause cumulative mortality >5 years from cancer diagnosis for survivors of A) early-AYA cancers compared to U.S. population by time since diagnosis; B) childhood cancers compared to U.S. population by time since diagnosis; C) early-AYA and childhood cancers by attained age.
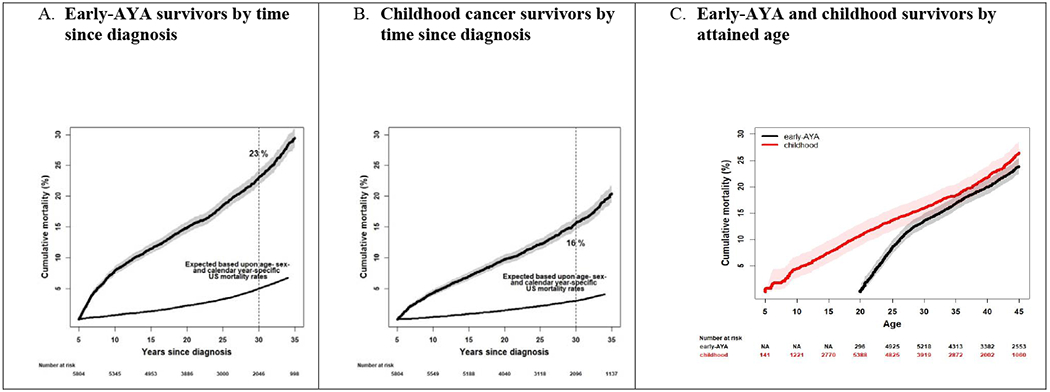
*Numbers at risk are unweighted
Compared to expected rates at the same ages in the general population, the SMR for all-cause mortality among early-AYAs was 5·9 (95% CI, 5·5-6·2) (Table 2). Early-AYAs diagnosed with HL and CNS malignancies had the highest SMRs of 6·8 (6·2-7·4) and 7·8 (6·6-9·2), respectively. Compared to the general population, early-AYAs had a statistically significant higher risk of death due to any nonrecurrent, health-related cause (SMR=4·8; 95% CI, 4·4-5·1), SMN (7·8; 7·0-8·7), cardiovascular event (4·4; 3·7-5·2), pulmonary disease (7·4; 5·7-9·5), or from some other medical cause (2·8; 2·4-3·2). Risk of death due to external causes was similar to the general population (1·1; 0·9-1·3).
SMR for all-cause mortality among childhood cancer survivors was 6·2 (95% CI, 5·8-6·6) compared to the deaths expected in the same age general population (Table 2). Children diagnosed with bone cancers (SMR=6·6; 5·7-7·7) and CNS malignancies (9·3; 7·6-11·3) had the highest risk of death. Compared to the general population, childhood cancer survivors had a SMR of 6·8 (6·2-7·4) for any nonrecurrent, health-related cause, 11·9 (10·4-13·6) for SMN deaths, 6·8 (5·6-8·3) for cardiac deaths, and 10·3 (7·6-14·0) for pulmonary deaths. All SMRs except that for pulmonary deaths were significantly higher than SMRs for the same causes among early-AYAs (all p-values <0·001). Similar to early-AYAs, childhood cancer survivors had similar SMR for death due to external causes.
Evaluation of SMRs for nonrecurrent, health-related causes of death demonstrated decreasing SMRs with increasing number of years from diagnosis for both childhood and early-AYA cancer survivors (Figure 2b; appendix pp9-14). However, starting at 20 years post-diagnosis, SMR for nonrecurrent health-related deaths was greater for childhood cancer survivors compared to early-AYAs. Similarly, childhood cancer survivors had greater SMRs for cardiac deaths also beginning 20 years after diagnosis compared to early-AYAs (Figure 2d). This late cardiac mortality risk was seen at both 20-39 year and 40+ year attained ages. SMRs for SMN deaths demonstrated a decreasing risk with increasing age, however, childhood cancer survivors had greater SMRs starting five years post-diagnosis and peaking 10-19 years post-diagnosis compared to early-AYAs (Figure 2c).
Figure 2. Standardized mortality ratios (SMR) for all-cause; nonrecurrent, health-related cause; subsequent malignant neoplasm; and cardiac mortality among early-AYAs and matched childhood cancer survivors stratified by attained age and time since diagnosis.
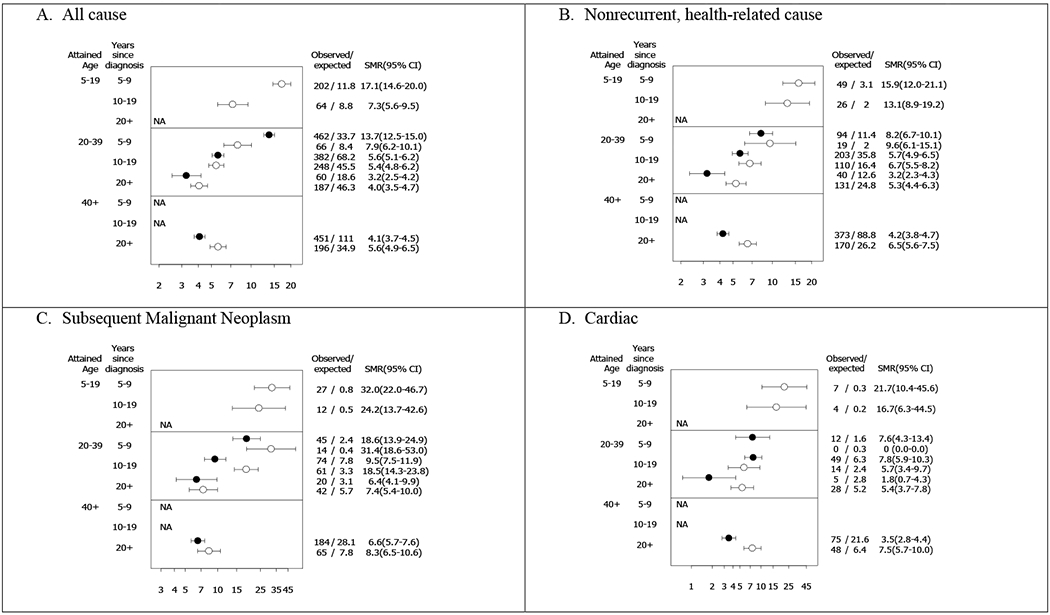
Open circles represent childhood cancer survivors; Filled circles represent early-AYA cancer survivors.
NA indicates not possible based on age at diagnosis and follow-up. No cardiac deaths at 5-9 years post diagnosis among childhood cancer survivors age 20-39.
SMRs for all-cause of death stratified by time since diagnosis and attained age (Figure 2a) likely reflects the impact of recurrence/progression of the primary cancer on mortality with elevated SMRs in the immediate years (5-9 years) after diagnosis among childhood cancer survivors (5-19 attained age panel) and early-AYAs (20-39 attained age panel). Since recurrence/progression of the primary cancer could not be specifically examined by SMRs (no recurrences in the general population) and since recurrence/progression is more dependent on time since diagnosis rather than attained age, the risk of death due to recurrence/progression of the primary cancer was examined via Cox models with time since diagnosis as the time scale. Early-AYAs had nearly two-times higher risk of death due to recurrence/progression of the primary cancer compared to childhood cancer survivors, at similar times since diagnosis, adjusted for sex (HR=1·6; 95% CI, 1·4-1·9; appendix p16).
By 45 years of age, the cumulative incidence of a grade 3-5 health condition was 39·4% (95% CI, 36·9-42·0%) among early-AYAs, 56·3% (52·0-60·3%) among childhood cancer survivors, and 12·1% (10·5-13·8%) among siblings. At 30 years post-diagnosis, the cumulative incidence of a grade 3-5 health condition was 45·6% (95% CI, 42·8-48·5%) among early-AYAs and 39·6% (36·6-42·5%) among childhood cancer survivors (Table 3). Compared to same age siblings, early-AYAs were more likely to develop any chronic health condition (HR=2·2; 95% CI, 2·0-2·3; Table 3) and any grade 3-5 health condition (4·2; 3·7-4·8). Hazard ratios were highest for grade 3-5 musculoskeletal conditions (6·5; 3·9-11·1), pulmonary conditions (6·3; 3·2-12·7), and cardiac conditions (4·3; 3·5-5·4) for early-AYAs compared to siblings.
Table 3.
Chronic health conditions among early-AYAs, childhood cancer survivors, and siblings according to CTCAE severity grade.
| Organ System | Early-AYA Survivors (n=4082) | Childhood Survivors (n=4082) | Siblings (n=3806) | HR for Early-AYA Survivors (Siblings as referent group) | HR for Childhood Survivors (Siblings as referent group) |
|---|---|---|---|---|---|
| Any Grade 1-5 conditions | |||||
| N (%) | 2637 (64·6%) | 2419 (59·3%) | 1562 (41·0%) | ||
| Cumulative Incidence by age 45 years (95% CI) | 73·0% (70·1 to 75·6) | 87·1% (84·0 to 90·0) | 56·7% (53·6 to 59·6) | ||
| Cumulative Incidence by 30 years post diagnosis (95% CI) | 80·8% (77·8 to 83·9) | 74·5% (71·2 to 77·8) | NA | ||
| HR (95% CI) | 2·2 (2·0 to 2·3) | 2.7 (2.5 to 2.9) | |||
| Any Grade 3-5 conditions | |||||
| N (%) | 1254 (30·7%) | 1044 (25·6%) | 320 (8·4%) | ||
| Cumulative Incidence by age 45 years (95% CI) | 39·4% (36·9 to 42·0) | 56·3% (52·0 to 60·3) | 12·1% (10·5 to 13·8) | ||
| Cumulative Incidence by 30 years post diagnosis (95% CI) | 45·6% (42·8 to 48·5) | 39·6% (36·6 to 42·5) | NA | ||
| HR (95% CI) | 4·2 (3·7 to 4·8) | 5.6 (4.9 to 6.3) | |||
| Grade 3-5 Cardiac | |||||
| N (%) | 464 (11·4%) | 334 (8·2%) | 107 (2·8%) | ||
| Cumulative Incidence by age 45 years (95% CI) | 15·4% (13·8 to 17·0) | 19·9% (17·3 to 22·7) | 4·1% (3·2 to 5·1) | ||
| Cumulative Incidence by 30 years post diagnosis (95% CI) | 19.7% (17.6-21.7) | 13.6% (11.7-15.4) | NA | ||
| HR (95% CI) | 4·3 (3·5 to 5·4) | 5.6 (4.5 to 7.1) | |||
| Grade 3-5 Endocrine | |||||
| N (%) | 244 (6·0%) | 324 (7·9%) | 65 (1·7%) | ||
| Cumulative Incidence by age 45 years (95% CI) | 8·1% (7·0 to 9·3) | 15·3% (13·3 to 17·4) | 2·9% (2·1 to 3·8) | ||
| Cumulative Incidence by 30 years post diagnosis (95% CI) | 8·8% (7·6 to 10·1) | 11·4% (9·9 to 12·9) | NA | ||
| HR (95% CI) | 3·9 (2·9 to 5·1) | 6.4 (5.1-8.0) | |||
| Grade 3-5 Pulmonary | |||||
| N (%) | 66 (1·6%) | 44 (1·1%) | 11 (0·3%) | ||
| Cumulative Incidence by age 45 years (95% CI) | 2·0% (1·5 to 2·6) | 2·8% (1·8 to 4·0) | 0·5% (0·3 to 0·9) | ||
| Cumulative Incidence by 30 years post diagnosis (95% CI) | 2·5% (1·7 to 3·2) | 1·7% (1·0 to 2·3) | NA | ||
| HR (95% CI) | 6·3(3·2 to 12·7) | 7.3(3.6 to 14.9) | |||
| Grade 3-5 Musculoskeletal | |||||
| N (%) | 110 (2·7%) | 80 (2·0%) | 17 (0·4%) | ||
| Cumulative Incidence by age 45 years (95% CI) | 3·4% (2·7 to 4·3) | 3·7% (2·6 to 5·0) | 0·5% (0·3 to 1·0) | ||
| Cumulative Incidence by 30 years post diagnosis (95% CI) | 3·6 % (2·7 to 4·5) | 2·7% (2·0 to 3·4) | NA | ||
| HR (95% CI) | 6·5 (3·9 to 11·1) | 8.0(4.6 to 14.0) | |||
| Grade 3-5 Neurological | |||||
| N (%) | 83 (2·0%) | 77 (1.9%) | 33 (0·9%) | ||
| Cumulative Incidence by age 45 years (95% CI) | 2·4% (1·8 to 3·0) | 3·7% (2·5 to 5·3) | 1·4% (0·9 to 2·0) | ||
| Cumulative Incidence by 30 years post diagnosis (95% CI) | 2·7% (2·0 to 3·4) | 2·4% (1·7 to 3·1) | NA | ||
| HR (95% CI) | 2·2 (1·4 to 3·3) | 3.2 (2.1 to 5.0) |
Also includes in situ breast cancers and benign meningioma requiring surgery
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; HR, hazard ratio; CI, confidence interval; SMN, subsequent malignant neoplasm.
Elazard ratios adjusted for sex, race/ethnicity. Comparisons between survivors and siblings used age as the time scale and late entry, with survivor entering at an age equivalent to 5 years post diagnosis and siblings age 5 or older.
Childhood cancer survivors had higher HRs in comparison to similar age siblings than did early-AYA for developing any chronic health condition (childhood HR=2·7; 95% CI, 2·5-2·9) and any grade 3-5 health condition (5·6; 4·9-6·3). Hazard ratios were also higher for childhood cancer survivors for developing grade 3-5 cardiac (HR=5·6 vs. 4·3), endocrine (6·4 vs. 3·9), and musculoskeletal (8·0 vs. 6·5) conditions than those of early-AYAs, with same age siblings as the reference group for both. The HRs (vs. same age siblings) of grade 3-5 pulmonary or neurologic conditions were similar for the childhood and early-AYA cancer survivors.
Multivariable analyses describing the risk factors associated with grade 3-5 chronic health conditions is described in appendix ppl7-18. Among early-AYAs, exposure to radiation fields that included the chest/neck (HR=3·9; 95% CI, 2·6-5·7) was associated with an increased risk for grade 3-5 endocrine conditions compared to those not exposed to any radiotherapy. Further, early-AYAs exposed to ≥35 Gy chest radiation had a greater risk for cardiac conditions than early-AYAs not exposed to chest radiotherapy (3·3; 2·4-4·6); and anthracycline exposure was not associated with increased risk of cardiac conditions in this population. Of note, in additional analyses among childhood cancer survivors alone, the HR for ≥300 mg/m2 vs. no anthracycline exposure was 1·8 (95% CI, 1·2-2·7, p=0·0023). Exposure to any radiation (2·2; 1·6-2·9), as well as platinum chemotherapy (2·1; 1·3-3·3) or high cumulative doses of alkylator chemotherapy (CED >8000mg/m2 vs. none; 1·6; 1·1-2·3), increased the risk for SMNs among early-AYAs. Among all diagnoses, HL was associated with the greatest risk for developing a grade 3-5 endocrine, cardiovascular condition, or SMN (appendix p19).
SIRs for SMNs were evaluated for early-AYAs and childhood cancer survivors, providing risks relative to age, calendar-year and sex-matched members of the general population. While SIRs were elevated across all time points relative to diagnosis for both groups, childhood cancer survivors had a significantly higher SIR for SMNs compared to early-AYAs (6·9 vs. 4·7, p=<0·0001; appendix pp20). A higher SIR for SMNs among childhood cancer survivors was evident between 10-19 years from diagnosis at 20-39 years of age compared to early-AYAs (Figure 3a). Specifically, higher SIRs for thyroid cancer and soft tissue sarcomas were observed among childhood cancer survivors compared to early-AYAs during this time period (Figure 3c–d). Although SIRs for breast cancer were high for both groups, there were no statistical differences in SIRs between childhood cancer survivors and early-AYAs (Figure 3b, appendix pp22-23). Still a greater SIR for breast cancer among childhood cancers were seen 10-19 years post-diagnosis at an attained age of 20-39 years.
Figure 3. Standardized incidence ratios (SIRs) and frequency for subsequent malignant neoplasm in survivors of early-AYA and matched childhood cancer survivors by attained age and time since diagnosis.
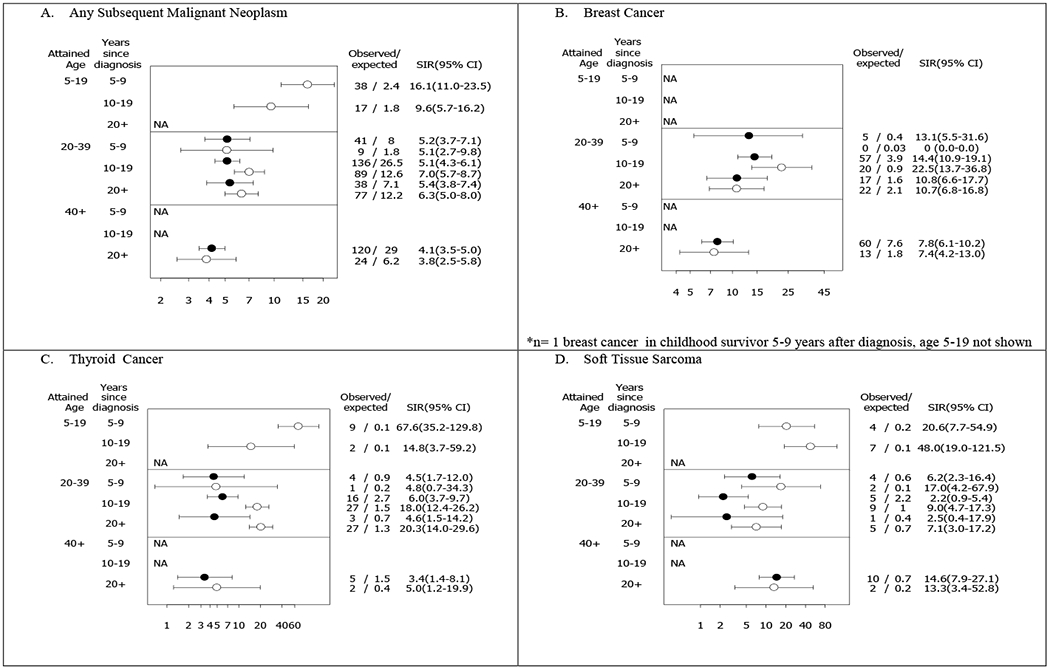
Open circles represent childhood cancer survivors; Filled circles represent early-AYA cancer survivors.
NA indicates not possible based on age at diagnosis and follow-up or # expected too low to reasonably estimate SIR
No breast cancers for childhood cancer survivors at attained age of 20-39 years, 5-9 years since diagnosis.
DISCUSSION
To our knowledge, our study is the first to comprehensively characterize long-term health outcomes in a large cohort of cancer survivors diagnosed and treated in adolescence and early adulthood (≥15 to <21 years of age) and to describe their outcomes relative to survivors of similar diagnoses diagnosed in childhood (<15 years of age) as well as the general population. Our cohort of over 4000 survivors of early-AYA cancer was at greater risk for chronic health conditions and late mortality than the general population. Both early-AYA and childhood cancer survivors had significantly higher risk of chronic health conditions compared to siblings. However, early-AYAs had a lower magnitude of risk than childhood cancer survivors. Moreover, the SMRs for nonrecurrent, health-related deaths were also lower among early-AYA than for childhood cancer survivors, a difference most evident two decades after cancer diagnosis.
When considering the composite of influence of attained age and follow-up time, all-cause cumulative mortality appears to be consistent between childhood cancer survivors and early-AYAs. Similarly, there was no difference in the risk of all-cause death compared to the general population between early-AYA and childhood cancer survivors. Interestingly, our study showed that early-AYAs were over 1·5 times more likely to die from a recurrence/progression of the primary cancer than childhood cancer survivors. When examining these differences by primary cancer diagnosis (appendix p16), this finding extends to leukemia and HL survivors. Inferior outcomes for patients diagnosed with HL between the ages of 17 to 21 years compared to patients diagnosed before the age of 17 years or over the age 21 years have been described in analyses of recent North American clinical trials.18 The greater risk of death due to late recurrence/progression of the primary cancer observed in our study may also be a reflection of known factors that contribute to poorer outcomes for AYAs with their primary cancer – these include delays in diagnosis, lack of health insurance, inferior compliance of treatment, follow-up and transition to long-term follow-up care, and sub-optimal primary care provider (PCP) familiarity with AYA cancer and cancer survivorship recommendations.19 This finding may be further explained by differences in disease biology. In acute lymphoblastic leukemia (ALL), patients over the age of 10 years are considered high-risk on pediatric trials and young adults have been shown to have inferior outcomes to pediatric patients, which appears to be in part due to biology in addition to other AYA factors with diagnosis delays and barriers with compliance described above.20
The rate of developing SMNs compared to the general population was lower among early-AYAs as compared to childhood cancer survivors while they were 10-19 years after diagnosis and 20-39 years of attained age. Childhood cancer survivors had higher SIRs for both thyroid cancer and soft tissue sarcomas during this time and age interval. The higher rate of soft tissue sarcomas may reflect a higher rate of familial or genetic cancer predisposition. Indeed, when examining SMNs by primary cancer diagnosis, childhood soft tissue sarcoma survivors had 7·6-times risk compared to 2·2-times risk for early-AYA soft tissue sarcoma survivors, supporting this hypothesis. Future studies examining the genome in survivors with SMNs in the CCSS may shed greater clarity on these differences. Reflective of these SMNs, the SMRs for death due to SMN were higher in childhood survivors relative to early-AYAs, though in both groups death due to SMNs were significantly elevated as compared to the general population. When stratified by years from diagnosis and current age, only childhood survivors 10-19 years after diagnosis with a current age of 20-39 years had statistically significant higher SMN-associated risk of death compared to early-AYAs, though similar relationship were noted for other time by age intervals. Importantly, there was elevated risk in the development of subsequent breast cancer in both early-AYAs and childhood cancer survivors. Taken together these findings support focused efforts in maximizing surveillance efforts for SMNs in both early-AYA and childhood cancer survivors to reduce mortality from SMNs (particularly breast cancer and colorectal cancer, which in both cases, early surveillance results in reduced morbidity).
Our analyses confirm previous studies that suggest that younger children may be more vulnerable to the effects of cancer treatment, such as the impact of radiation and anthracycline exposure on the development of cardiovascular conditions.17 Interestingly, when compared to same aged siblings, survivors of early-AYA cancers had lower relative risks than childhood cancer survivors for developing any grade 3-5 chronic condition, as well as grade 3-5 endocrine and musculoskeletal conditions. Consistent with these morbidities, early-AYAs had lower standardized risk of mortality due to cardiac causes and nonrecurrent, health-related causes. Younger age at cancer treatment has been shown previously to be associated with an increased risk for certain endocrinopathies, including obesity21 and diabetes mellitus.22 For other endocrine outcomes, older age at exposure is associated with greater risk. Females treated in young adolescence, when the number of primordial follicles is reduced compared to prior to puberty, have an increased risk of primary ovarian insufficiency.23 Likewise, Sklar and colleagues reported an increased risk of radiotherapy-induced hypothyroidism with older age.24 In contrast to previous studies, we observed a lower risk of musculoskeletal morbidity in early-AYAs as compared to childhood cancer survivors. In a previous CCSS analysis of osteonecrosis in survivors, older age increased risk of this outcome consistent with studies of avascular necrosis in patients with ALL.25 However, musculoskeletal morbidity due to cancer treatment includes multiple other conditions, such as osteoporosis, scoliosis, joint dysfunction, etc.26 Examination of the individual contributions of these outcomes were outside the scope of this analysis.
Several limitations should be considered when interpreting our findings. Our report is limited by the lack of inclusion of the full spectrum of malignant diagnoses seen in early-AYAs. Notably, survivors of gonadal tumors, melanomas, and thyroid cancer, which account for almost 40% of cancers diagnosed between the ages of 15 and 20 years,1 were not enrolled in the CCSS cohort. Furthermore, the early-AYAs within the CCSS may not be representative of all early-AYAs treated for cancer in the U.S., as many early-AY As in the U.S. are treated by community providers outside of tertiary academic centers or pediatric hospitals.27 Also, due to the fact that age at diagnosis defines the groups we are comparing (early-AYA and childhood cancer survivors), and with equal follow-up there are therefore different attained ages for groups (and vice versa), we cannot easily make direct comparisons between these groups. We have therefore presented results for each cohort in comparison to age-matched general population groups, and have attempted to illustrate the impact of advancing age and time since diagnosis, but these factors must be carefully considered in interpreting these finding as it is not possible to perfectly account for the intrinsic differences between the groups. The response rates across CCSS follow-up surveys range from 64% (6603 of 10,375) to 84% (1170 of 2116), declining over time, but with only moderate differences between the slightly higher responses for early-AYAs than those of childhood cancer survivors. There is some evidence that survivors who are sicker (have had at least one severe chronic health condition) are more likely to respond to a subsequent survey (among early-AYAs: 529 (79%) of 668 vs. 1001 (69%) of 1448; data not shown), which could bias our survey-based results towards worse outcomes. Chronic health conditions are determined by self-report in our study and thus, there may be some misclassification with regard to these outcomes, though SMN are validated and mortality is ascertained via NDI. Further, though we utilize reported causes of death for the grade 5 morbidities, we are dependent upon death certificates, which may have missing information. Also, modern cancer treatments have evolved and thus the results of this study may not be generalizable to those treated today. However, chemotherapy and radiation treatments still remain largely utilized for many cancers affecting early-AYAs. Lastly, we recognize that risky health behaviors, such as smoking, are associated with adverse health outcomes such as early cardiovascular disease. Our data are limited to “ever smoker” that may impact our ability to detect a significant effect. Assessment of the number of pack-years would provide a better evaluation of the potential contribution of smoking.
As highlighted by the Adolescent and Young Adult Oncology PRG,3 it is imperative to collect data specific to the AYA age group in order to develop and support recommendations and policy for health care for AYA cancer survivors. Our results suggest that guidelines for risk-based long-term follow-up care, such as the Children’s Oncology Group Long-Term Follow-Up (COG LTFU) Guidelines and National Comprehensive Cancer Network Guidelines are appropriate for survivors of early-AYA cancer. While some of the risk of morbidities (e.g. cardiac and musculoskeletal outcomes) appears lower in early-AYAs as compared to childhood cancer survivors, early AYAs still have significant elevated risk compared to siblings. In fact, our findings suggest that the recent changes to cardiac surveillance in version 5.0 of the COG LTFU Guidelines, that no longer stratifies by age of exposure to chest radiotherapy and/or anthracyclines, is appropriate. Whether these guidelines are also applicable to young adult treated after the age of 21 years will require study in other cohorts. Our data does underscore that focused efforts to ensure early-AYA survivors are receiving recommended risk-based care, with a focus on high-risk cancer screening, are needed to reduce morbidity and premature mortality. Studies to date indicate that adherence to such high-risk screening is poor.28 Over 85% of long term survivors of AYA cancer receive their care in the community from a PCP.28 These providers, while willing to care for survivors, are often unfamiliar with available guidelines and would prefer to care for survivors in communication with providers at the cancer center.29 New models for delivering risk-based survivorship care that incorporate both cancer specialists and PCPs should be studied, such as the Patient Centered Medical Home,30 and the classic chronic disease models of care utilized for diabetes mellitus and cardiovascular diseases.31 It should be ensured that electronic medical records and treatment summaries are accessible to cancer survivors and their health-care providers, and that PCPs can communicate readily with cancer specialists. Lastly, it is important that information and recommendations about AYA survivors are widely disseminated and accessible, beyond academic journals. A PCP’s search engine investigation should readily take them to the appropriate information source. In summary, these data highlight the need for multi-faceted efforts to improve the health outcomes of this vulnerable population.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
There is limited understanding of long-term outcomes in survivors of cancer diagnosed during adolescence or young adulthood. We searched PubMed from database inception to May 18, 2019 using the terms “adolescent and young adult or AYA cancer survivors” and “health conditions or morbidity” and “death or mortality” for English language publications describing the health consequences of cancer treatment in this population. Many studies have described the important psychosocial consequences of being treated for cancer as an AYA, including decrements in health-related quality of life. Several studies have examined the morbidity and mortality of AYAs based on large cancer registries (Danish, British), however, detailed treatment exposure data or severity of illness were not considered.
Added value of the study
To our knowledge, this is the first study to provide a comprehensive assessment of the long-term health outcomes in young AYAs (early-AYAs). We used detailed outcome and treatment data to summarize the experience of early-AYA survivors compared to that of survivors of childhood cancer (age <15 at diagnosis), a cohort of siblings, and the general population.
Implications of all the available evidence
Our previous understanding of the long-term consequences of cancer treatment on AYAs was largely extrapolated from data describing survivors of childhood cancer. In general, the patterns of chronic health conditions in early-AYA survivors reflect those of childhood cancer survivors, though differences in risk for nonrecurrent, health-related cause of deaths and chronic cardiac, endocrine, and musculoskeletal conditions between survivors of childhood and early-AYA cancer (despite similar treatment exposures) were identified. Importantly, this analysis confirms the significant burden of long-term health complications in the youngest subset of AYAs, will inform current therapies for this population, and underscores the need for targeted interventions to ensure life-long, risk-based follow-up care for this population.
ACKNOWLEDGMENTS
This work was supported by a grant (U24 CA-55727) to GTA from the US National Institute of Health, Bethesda, MD, Cancer Center Support (CORE) Grant (CA-21765) to St Jude Children’s Research Hospital; and the American Lebanese Syrian Associated Charities, Memphis, TN. No authors are employees of the US National Institute of Health. The Childhood Cancer Survivor Study is a publicly available data resource. Investigators can apply for specific analyses through a proposal process available on the website. The dataset specific to these analyses is not publicly available.
DECLARATION OF INTERESTS
KRK and CAS declare funding from the National Cancer Institute. KLS and WML declare funding from the National Institutes of Health. CAS declares funding from Novo Nordisk, outside the submitted work. All other authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in abstract format at the 7th Biennial Cancer Survivorship Research Conference, Atlanta, GA, June 2014.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; April 2015. https://seer.cancer.gov/archive/csr/1975_2012/ (accessed April 4, 2019). [Google Scholar]
- 2.Bleyer A Latest estimates of survival rates of the 24 most common cancers in adolescent and young adult Americans. J Adolesc Young Adult Oncol 2011; 1(1): 37–42 [DOI] [PubMed] [Google Scholar]
- 3.U. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, LIVESTRONG Young Adult Alliance. Closing the gap: research and care imperatives for adolescents and young adults with cancer. Report of the Adolescent and Young Adult Oncology Progress Review Group. Bethesda, MD: National Institutes of Health, 2006. NIH Pub. No. 06-6067 https://www.cancer.gov/types/aya/research/ayao-august-2006.pdf. (accessed on January 10, 2019). [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355(15): 1572–82. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-Year survivors of childhood cancer. N Engl J of Med 2016; 374(9): 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidler MM, Reulen RC, Winter DL, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ 2016; 354: i4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rugbjerg K, Olsen JH. Long-term risk of hospitalization for somatic diseases in survivors of adolescent or young adult cancer. JAMA Oncol 2016; 2(2): 193–200. [DOI] [PubMed] [Google Scholar]
- 8.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009; 27(14): 2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014; 61(1): 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feijen EA, Leisenring WM, Stratton KL, et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol 2015; 33(32): 3774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res 2006; 166(1 Pt 2): 141–57. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol 2014; 32(12): 1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geskus R Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics 2011; 67(1): 39–49. [DOI] [PubMed] [Google Scholar]
- 14.United States Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Health Statistics Compressed Mortality File on CDC Wonder Online Database; CMF 1999-2013, Series 20, No 2s, 2014; CMF 1968-1988, Series 20, No. 2A, 2000; CMF 1989-1998, Series 20, No. 2E. 2003 http://wonder.cdc.gov/wonder/help/cmf.html. (accessed August 2, 2019) [Google Scholar]
- 15.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat 1982; 10(4): 1100–20. [Google Scholar]
- 16.Lin DY, Wei LJ. The robust inference for the cox proportional hazards model. J Am Stat Assoc 1989; 84(408): 1074–8. [Google Scholar]
- 17.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009; 339: b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson TO, Parsons SK, Wroblewski KE, et al. Outcomes in adolescents and young adults with Hodgkin lymphoma treated on US cooperative group protocols: An adult intergroup (E2496) and Children’s Oncology Group (COG AHOD0031) comparative analysis. Cancer 2018; 124(1): 136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleyer A Young adult oncology: the patients and their survival challenges. CA Cancer J Clin 2007; 57(4): 242–55. [DOI] [PubMed] [Google Scholar]
- 20.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood 2008; 112(5): 1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2003; 21(7): 1359–65. [DOI] [PubMed] [Google Scholar]
- 22.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med 2009; 169(15): 1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 2006; 91(5): 1723–8. [DOI] [PubMed] [Google Scholar]
- 24.Sklar C, Whitton J, Mertens A, et al. Abnormalities of the thyroid in survivors of Hodgkin’s disease: data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab 2000; 85(9): 3227–32. [DOI] [PubMed] [Google Scholar]
- 25.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 2008; 26(18): 3038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gawade PL, Hudson MM, Kaste SC, et al. A systematic review of selected musculoskeletal late effects in survivors of childhood cancer. Curr Pediatr Rev 2014; 10(4): 249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfson J, Sun CL, Wyatt L, Stock W, Bhatia S. Adolescents and young adults with acute lymphoblastic leukemia and acute myeloid leukemia: impact of care at specialized cancer centers on survival outcome. Cancer Epidemiol Biomarkers Prev 2017; 26(3): 312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 2008; 26(27): 4401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh E, Daugherty CK, Wroblewski K, et al. General internists’ preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med 2014; 160(1): 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AHRQ Patient Centered Medical Home Resource Center. https://pcmh.ahrq.gov/page/defining-pcmh (accessed July 30 2018).
- 31.Grover A, Joshi A. An overview of chronic disease models: a systematic literature review. Glob J Health Sci 2014; 7(2): 210–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


