Abstract
Macrophage immunometabolism, the changes in intracellular metabolic pathways that alter the function of these highly plastic cells, has been the subject of intense interest in the past few years, in part because macrophage immunometabolism plays important roles in atherosclerosis and other inflammatory diseases. In this review article, part of the Compendium on Atherosclerosis, we introduce the concepts of i) intracellular immunometabolism – the canonical concept of intrinsic cell activation leading to changes in intracellular metabolism, which in turn alter cellular function; and ii) intercellular immunometabolism – conditions in which intermediates of cellular metabolism are transferred from one cell to another, thereby altering the function of the recipient cell. The recent discovery that the metabolite cargo of dead and dying cells ingested through efferocytosis by macrophages can alter metabolic pathways and downstream function of the efferocyte is markedly changing the way we think about macrophage immunometabolism. Metabolic transitions of macrophages contribute to their functions in all stages of atherosclerosis, from lesion initiation to formation of advanced lesions characterized by necrotic cores, to lesion regression following aggressive lipid lowering. This review article discusses recent advances in our understanding of these different aspects of macrophage immunometabolism in atherosclerosis. With the increasing understanding of the roles of macrophage immunometabolism in atherosclerosis, new exciting concepts and potential targets for intervention are emerging.
Keywords: Atherosclerosis, Efferocytosis, Glucose glycolysis, Immunometabolism, Macrophage, Oxygen consumption
Subject terms: Atherosclerosis, Inflammation, Metabolism
Brief introduction to immunometabolism
The area of immunometabolism has attracted an immense increase in interest and research efforts in the recent few years. This research has demonstrated that metabolic alterations in immune cells, often referred to as metabolic flexibility or reprogramming, not only occur in response to inflammatory mediators and other environmental cues, but are critical for allowing immune cells both to induce inflammation and to terminate the inflammatory response and initiate tissue healing.
The term immunometabolism was introduced in 2011 by Mathis and Shoelson,1 who defined it as the interplay between inflammation and metabolic disease, although this link had been studied for decades prior to coining the term.2 More recently, immunometabolism has taken on a different meaning with several components: contributions of key metabolic pathways to immune cell development, fate, and behavior;3 changes in intracellular metabolic pathways in immune cells that alter their function;4 and metabolic reprogramming of immune cells.5
For the purpose of this review we define immunometabolism as changes in intracellular metabolic pathways that alter immune cell function. We review the concepts of i) intracellular immunometabolism – the canonical concept of cellular activation leading to changes in intracellular metabolism, which in turn alter cellular function; and ii) intercellular immunometabolism – conditions in which intermediates of cellular metabolism are transferred from one cell to another, thereby altering the function of the recipient cell. Examples of the first concept are the metabolic changes that occur when macrophages change phenotypes to become inflammatory macrophages or alternatively activated macrophages, which are based primarily on in vitro studies. Examples of the latter concept include metabolic intermediates derived from the cargo of efferocytosed dead or dying cells that alter the metabolism of the efferocyte, as well as released metabolites acting on cellular receptors through autocrine or paracrine mechanisms.
This review will cover these two concepts in immunometabolism in macrophages, as it relates to different processes and functions in atherosclerosis. We primarily focus on metabolism of glucose, fatty acids and amino acids rather than sterols, which have been covered by other recent reviews.6, 7
Macrophage intracellular immunometabolism
Inflammatory activation of macrophages by lipopolysaccharide leads to metabolic rewiring to increase glycolysis and block certain steps in the TCA cycle
The now classical observations of Otto Warburg in the 1920’s brought understanding on how cells alter metabolic pathways to adjust to environmental cues and cellular needs. Warburg observed that cancer cells “fermented” glucose to lactate even in the presence of adequate oxygen levels (aerobic glycolysis), a process different from that used by non-cancerous cells, which relied primarily on oxidative phosphorylation under aerobic conditions.8 This propensity of some cells to increase glycolysis and downstream lactate production, rather than metabolizing substrates through pyruvate fueling the tricarboxylic acid (TCA) cycle and subsequent oxidative phosphorylation, is termed the Warburg effect or aerobic glycolysis.9
Soon after Warburg’s studies, similar studies were pursued in leukocytes, which were shown to have a high capacity of aerobic glycolysis.10 Zanvil Cohn later conducted pioneering studies on endotoxin-stimulated polymorphonuclear leukocytes from rabbits, and observed a large increase in glycolysis following exposure of these cells to endotoxin without changes in oxygen consumption.11 Numerous later studies confirmed the finding that the Gram-negative bacteria cell wall component lipopolysaccharide (LPS; endotoxin) stimulates aerobic glycolysis and the Warburg effect in macrophages.12, 13 LPS, which activates its receptor toll-like receptor 4 (TLR4) in macrophages and other cells, has since been used as a model inflammatory molecule in many pioneering studies on macrophage immunometabolism. Interestingly, the use of aerobic glycolysis to aid in the protection against bacteria has been conserved from insects to humans,14 indicating a critical role of this mechanism.
The biological reason behind the use of glycolysis rather than oxidative phosphorylation for energy production remained a mystery for decades, given that oxidative phosphorylation is a much more efficient process in terms of ATP generation (2 ATP molecules/glucose molecule are generated by glycolysis versus approximately 30 ATP molecules/glucose molecule generated during oxidative phosphorylation when a glucose molecule is completely oxidized to CO2 and H2O). One hypothesis was that glycolysis can result in faster generation of ATP than can oxidative phosphorylation, allowing immune cells to quickly respond to an encountered pathogen; another hypothesis was that glycolysis avoids overaccumulation of deleterious reactive oxygen species (ROS) generated during oxidative phosphorylation. While these explanations are valid, recent research provides evidence that glycolysis confers additional important benefits. For example, lactate, the end-product of glycolysis has recently been shown to be involved in terminating the inflammatory response at later time-points, as discussed below. Furthermore, the pentose phosphate pathway, a glycolysis side-pathway often increased in glycolytically activated cells because the first step is conversion of glucose 6-phosphate by glucose 6-phosphate dehydrogenase, provides ribose sugars and NADPH that is required for biosynthetic reactions, such fatty acid and sterol synthesis to be used for membrane structural organization and for redox control.15 These generated molecules are needed for activated cells e.g., to initiate programs of membrane reorganization and expansion. In addition, increased glycolysis in inflammatory macrophages (macrophages that produce and release primarily pro-inflammatory mediators, such as IL-1β, TNF-α, CCL2, IL-12, and nitric oxide through inducible nitric oxide synthase) is linked to inhibition of specific steps in the TCA cycle, which supports inflammatory processes as discussed below. Therefore, aerobic glycolysis turns out to be critical for inflammatory cell activation and later for termination of the inflammatory program.15, 16
It is now known that inflammatory activation of macrophages, for example when encountering LPS, results in increased glycolysis through a number of activation steps. Inflammatory activation is also linked to suppression of the oxygen consumption rate (OCR), indicating decreased mitochondrial oxidative phosphorylation6, 17 (Figure 1A). The suppressed OCR in LPS-stimulated macrophages has been linked to the flux discontinuity within the TCA cycle, which accompanies the increased glycolysis in these cells (see below).18 This mechanism is distinct from the increase in mitochondrial ROS formation in macrophages stimulated with TLR ligands that has been described to increase bactericidal activity through translocation of the TLR adaptor TRAF6 to mitochondria, and subsequent mitochondrial and cellular ROS generation through a process mediated by ubiquitination of the protein ECSIT, which stabilizes complex I in the respiration chain.19
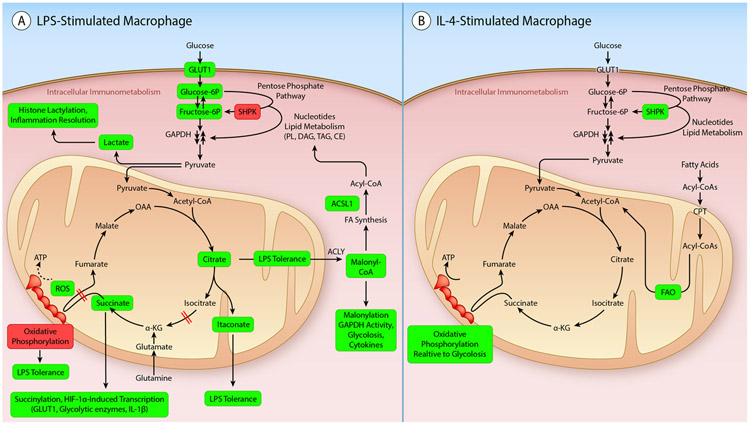
Figure 1. Canonical intracellular immunometabolism in macrophages.
A. Changes in metabolic pathways in macrophages stimulated by LPS, as an example of the changes described in a classically activated inflammatory macrophage. The enhanced glycolysis and TCA cycle blocks in LPS-stimulated macrophages serve both to promote a maximal inflammatory response as well as to terminate the inflammatory response at later times. B. Changes in metabolic pathways in macrophages stimulated by IL-4, as an example of an alternatively activated tissue healing macrophage. These macrophages rely more heavily on fatty acid oxidation (FAO) as compared with glycolysis. Elevated metabolites and processes are highlighted in light green. Suppressed processes are highlighted in dark red. TCA cycle blocks are indicated by red intersecting double lines. (Illustration Credit: Ben Smith)
With regard to glycolysis, LPS increases expression of glucose transporter 1 (GLUT1; gene name SLC2A1), a non-insulin-dependent glucose transporter expressed in macrophages, to allow for increased glucose influx.20 LPS also increases macrophage expression of several key and rate-limiting enzymes in glycolysis, including hexokinase 3,21 one of the hexokinase isoforms in macrophages that catalyze the first and irreversible step in glycolysis by phosphorylating glucose to form glucose-6-phosphate. Furthermore, LPS stimulation results in upregulation of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3),21, 22 an important positive regulator of glycolysis, and the pyruvate kinase isoform PKM2.23 Compared to the PKM1 isoform, which generates pyruvate from phosphoenolpyruvate (PEP), PKM2 is less active as a pyruvate kinase, but is required for reprogramming to aerobic glycolysis.24 This effect of PKM2 is mediated, at least in part, through actions in the nucleus to promote glycolysis by induction of GLUT1 and glycolytic genes by serving as a co-activator of hypoxia-inducible factor 1α (HIF1α), a subunit of the transcription factor HIF1, which binds to hypoxia response elements in target genes.25 Pyruvate, the last metabolite in glycolysis, can be converted to lactate by lactate dehydrogenase or transported from the cytosol, where glycolysis takes place, to the mitochondrial matrix where it is converted to acetyl-CoA and enters the TCA cycle (Figure 1A). This step is also regulated by LPS, diverting pyruvate from entering mitochondria to fuel the TCA cycle, thus hampering oxidative phosphorylation.
The glycolytic genes discussed above are under control of HIF1α. HIF1α is upregulated under hypoxic conditions, allowing cells to switch to glycolysis and generate ATP under conditions in which oxygen is scarce, such as can be the case in injured tissues in which blood flow is impaired. However, HIF1α can also be induced following NF-κB activation by multiple inflammatory stimuli, including LPS, under normoxic conditions in macrophages.26 The end result is an increase in glycolysis and a limitation of pyruvate via acetyl-CoA to enter the TCA cycle, limiting subsequent oxidative phosphorylation (Figure 1A).
Furthermore, LPS increases flux through the pentose phosphate pathway paralleling the increased glycolysis.17 Interestingly, LPS markedly inhibits expression of the sedoheptulose kinase SHPK (formerly known as CARKL), an enzyme that generates sedoheptulose 7-phosphate and controls the non-oxidative phase of the pentose phosphate pathway. SHPK appears to refocus cellular metabolism to a high reduction state.17 The non-oxidative phase of the pentose phosphate pathway operates when a cell needs much more NADPH as a reducing agent than it needs pentose; it can act to convert 5-carbon sugars back to glucose 6-phosphate through a series of reactions, which can then be used by the oxidative phase to generate more NADPH to be used for biosynthesis of fatty acids and sterols and for redox control. SHPK has been shown to link the pentose phosphate pathway with glycolysis and it partly co-localizes with the first enzyme of the pentose phosphate pathway, glucose 6-phosphate dehydrogenase. Interestingly, knocking down SHPK in macrophage cell lines is sufficient to mimic the stimulatory effect of LPS on glycolysis, suggesting that downregulation of SHPK is critical in supporting glycolysis in these cells.17 The exact mechanism whereby SHPK works and its physiological role are still uncertain.27
In addition to promoting aerobic glycolysis, thereby preventing flow through the TCA cycle and oxidative phosphorylation, LPS has recently been shown to disrupt the TCA cycle at specific points.28 In activated inflammatory macrophages, the TCA cycle is disrupted in two places: after citrate and after succinate, leading to accumulation of these metabolites.15, 29 The accumulation of citrate is due to transcriptional repression of isocitrate dehydrogenase, which catalyzes the conversion of isocitrate to α-ketoglutarate.18 Citrate can be used for fatty acid synthesis through exit from the TCA cycle to the cytoplasm, where it is converted by ATP citrate lyase (ACLY) to acetyl-CoA and then malonyl-CoA used for fatty acid synthesis (Figure 1A). Citrate accumulation is also linked to itaconate production (Figure 1A), as discussed below. The accumulation of succinate in LPS-stimulated macrophages is due to inhibition of the enzyme succinate dehydrogenase, which catalyzes the oxidation of succinate to fumarate as part of the TCA cycle and thereby also reduces ubiquinone in the electron transport chain (complex II). Succinate dehydrogenase therefore acts both in the TCA cycle and the electron transport chain, and oxidation of succinate has been shown to increase HIF1α stability.30 Succinate promotes HIF1α stability through both direct and indirect mechanisms.28, 30 Itaconate serves as an endogenous inhibitor of succinate dehydrogenase,31 linking the two blocks of the TCA cycle. The accumulation of succinate has also been ascribed to increased glutamine anapleurosis (the process of replenishing TCA cycle intermediates used for biosynthesis) and increased oxidation in the TCA cycle.13 Thus, these blocks in the TCA cycle further contribute to the effects of LPS on metabolism.
Together, these findings show that LPS and at least some other pathogen-derived molecules cause a marked shift towards aerobic glycolysis and away from oxidative metabolism. However, different cytokines and pathogens have different effects on metabolism and should therefore not be assumed to act like LPS.5, 6 Furthermore, almost all of the evidence summarized above is based on in vitro studies, in which a largely homogenous population of cells is stimulated with an inflammatory mediator, such as LPS, at one point in time and then followed over a limited amount of time. Little is known about how these mechanisms translate to different in vivo situations, in which many different cell types interact and the macrophage is exposed to multiple inflammatory and anti-inflammatory cues regulated in different temporal fashions.
Increased glycolysis and TCA cycle blocks contribute to inflammatory activation and then resolution of the inflammatory response in macrophages in vitro
In vitro studies have provided critical insights into the role of glycolysis and TCA cycle blocks in macrophages and have shown that these processes govern both the surge in inflammatory activation and the resolution of inflammation. For example, inhibition of glucose uptake achieved through downregulation of GLUT1 or inhibition of different steps of glycolysis prevents the full inflammatory activation repertoire induced by LPS in macrophages.17, 21 As would be expected, deletion of SHPK has the opposite effect, leading to stimulation of glycolysis and increased production of cytokines (Table 1).17 Also consistent with the concept that increased glycolysis promotes inflammatory processes in macrophages; insulin enhances glycolysis in macrophages following feeding in mouse models, an effect mediated by the insulin receptor-Akt pathway and increased expression of GLUT1 and hexokinase 2.32 Interestingly, this effect of insulin on glycolysis is dependent on macrophage activation status and was shown to enhance IL-1β secretion through inflammasome activation, leading, in turn, to enhanced IL-1β-mediated insulin secretion from pancreatic islets during feeding.32 In this manner, insulin and IL-1β act in concert to regulate systemic glucose metabolism in response to food intake, and the macrophage plays a critical role in this process.
Table 1.
Macrophage immunometabolism and atherosclerosis
| Metabolite/ regulating step |
Cellular source |
Possible stimuli in lesion |
Effect on macrophages | Effect on atherosclerosis |
|---|---|---|---|---|
| Intracellular | ||||
| Glucose/SLC2A1 | MΦ | Apoptotic cell, TLR4 ligands hypoxia | Altered inflammation and increased efferocytosis21, 50, 124 | Prevention of necrotic cores* (mouse)50, 124 |
| Lactate | MΦ | TLR4 ligands, hypoxia, oxLDL | Resolution of inflammation16 | Unknown |
| Non-oxPPP/SHPK | MΦ | IL-4 | Suppression of inflammatory activation17 | Unknown |
| Succinate | MΦ | TLR4 ligands | IL-1β production | Unknown |
| Citrate | MΦ | TLR4 ligands | TNF-α translation37 | Unknown |
| α-ketoglutarate | MΦ | TLR4 ligands | LPS tolerance39 | Unknown |
| Itaconate/ACOD1 | MΦ | TLR4 ligands | Resolution of inflammation and LPS tolerance41 | Unknown |
| Oxaloacetate/citrate | MΦ | oxPAPC | IL-1β production64 | Promotes atherosclerosis^ (mouse)64 |
| Arginine/Arg1 | MΦ | IL-4 | Promotes foam cell formation143 | No effect on atherosclerosis§ (mouse)143 |
| Arginine/iNOS | MΦ | TLR4 ligands | Suppresses IL-12 production144 | Promotes lesion area§ (mouse)145 |
| Serine | MΦ | TLR4 ligands | IL-1β production67, 68 | Unknown |
| Acyl-CoA/ACSL1 | MΦ | TLR4 ligands, oxLDL | Promotes inflammatory activation86, 89 | Promotes lesion initiation* (diabetic mouse)86 |
| Fatty acids/FAS | MΦ | oxysterols | Promotes lipid loading146 | Promotes atherosclerosis* (mouse)146 |
| Fatty acids/FABP4 | MΦ | oxLDL | Promotes inflammatory activation and CE accumulation84 | Promotes lesion area§ (mouse)84 |
| Mitochondrial ROS | MΦ | oxLDL | Promotes CCL2, IL-1β, and IL-6 production55, 78 | Promotes lesion initiation* (mouse)78 |
| Associated with coronary artery disease (humans)55 | ||||
| Intracellular | ||||
| Lactate/SLC16A1 | MΦ cargo | Apoptotic cell | Resolution of inflammation50 | Unknown |
| Lesional cells | Inflammation, hypoxia | |||
| Succinate/ SUCNR1 | Lesional cells | TLR4 ligands | IL-1β28 | Unknown |
| Arginine/putrescine | MΦ cargo | Apoptotic cell | Continual efferocytosis125 | Promotes lesion regression (mouse)125 |
CE, cholesteryl ester; HK1, hexokinase 1; MΦ, macrophage; non-oxPPP, non-oxidative phase of pentose phosphate pathway; SLC2A1, GLUT1
Myeloid cell targeted deletion or overexpression
Hematopoietic deletion (bone marrow transplant)
Indirect evidence
The dependence of macrophages on glycolysis for optimal inflammatory activation has been ascribed not primarily to altered ATP generation but to increased expression of glycolytic enzymes and accumulation of TCA cycle intermediates. Interestingly, glycolytic enzymes have been shown to directly bind signaling molecules. For example, increased expression of hexokinase 1 is required for inflammasome activation and IL-1β release in macrophages, perhaps through direct interaction between hexokinase 1 and the mitochondrial voltage-dependent ion channel VDAC.33, 34 As another example, PKM2 forms a complex with HIF1α, which binds the Il1b promoter and induces IL-1β expression.23 PKM2 has also been reported to enhance inflammasome activation through inhibition of autophagy.35
Furthermore, TCA cycle intermediates may have important functions that can alter macrophage function.28, 36 LPS-induced succinate accumulation promotes IL-1β production through the stabilization of HIF1α,28 and increased mitochondrial oxidation of succinate and an elevation of mitochondrial membrane potential combine to drive a pro-inflammatory phenotype in macrophages.30 Malonylation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as a result of increased citrate levels in LPS-stimulated macrophages, increases GAPDH activity, glycolysis, and TNF-α translation (Table 1).37
Whereas glycolysis is required for induction of the inflammatory response in macrophages, an increasing number of studies have shown that glycolysis and TCA cycle intermediates also act to terminate the inflammatory response (Figure 1A). For example, the effect of LPS on citrate accumulation is mediated by autocrine interferon type I production,38 and this pathway contributes to development of LPS tolerance in macrophages.39 Furthermore, itaconate, which is significantly increased in LPS-activated macrophages40 has been proposed to drive LPS tolerance.41 A range of inflammatory stimuli increase the expression of the enzyme aconitate decarboxylase 1 (ACOD1; also known as IRG1), resulting in diversion of cis-aconitate away from the TCA cycle to itaconate production (Figure 1A). This response, initiated 12-24 h after LPS stimulation, results in resolution of the inflammatory response in part through the transcription factor ATF3.41 In addition, itaconate modifies proteins by alkylation of cysteine residues; one of its targets is the protein KEAP1 (Kelch-like ECH-associated protein 1).40 Alkylation of KEAP1 disrupts its binding and degradation of the anti-inflammatory transcription factor Nrf2, which in turn leads to nuclear accumulation of Nrf2, and increased expression of genes involved in cellular redox control, including Hmox1, Gclm and Nqo1. Accordingly, a cell-permeable itaconate derivative resulted in suppressed levels of IL-1β, inducible nitric oxide synthase (iNOS) and HIF-1α in macrophages stimulated with LPS.40
The increase in lactate production as a result of increased aerobic glycolysis in LPS-stimulated macrophages has recently been shown to result in histone lactylation, a process that leads to arginase 1 (Arg1) expression and resolution of inflammation.16 These findings are consistent with earlier studies demonstrating that lactate induces Arg1 and other markers of an alternatively activated macrophage phenotype.42
Together, these in vitro studies have demonstrated that LPS-induced glycolysis and inhibition of the TCA cycle affect both the induction and resolution of the inflammatory response in cultured macrophages. The temporal changes of glycolysis and TCA cycle blocks in vivo are largely unknown.
Interfering with macrophage glycolysis has multiple downstream effects on lesions of atherosclerosis
In vitro models of classical and alternative activation of macrophages (Figure 1A-B) are highly simplified as compared with the environment a macrophage encounters in an atherosclerotic lesion, where complex combinations of cytokines are present and change in temporal fashions, and other environmental factors (such as hypoxia) together with influences of other cell types and extracellular matrix impact macrophage phenotype. Furthermore, macrophage populations are heterogeneous and respond differently to stimulation. However, the fact that macrophages readily take up glucose through GLUT1 and other glucose transporters has allowed the use of positron emission tomography (PET) to detect the accumulation of the glucose analog 18F-fluoro-2-deoxy-D-glucose (18F-FDG) in lesions and to identify actively progressing inflamed lesions in humans.43 Such PET imaging has demonstrated that increased aortic 18F-FDG uptake is associated with an increased risk of incident cardiovascular disease (CVD) and that the 18F-FDG signal appears to correspond most closely to macrophage abundance and/or inflammatory activation in atherosclerotic plaques.43, 44 However, macrophages are not the only lesional cell type to take up 18F-FDG, and it is not known if an increased 18F-FDG signal signifies an increase in abundance of lesional macrophages, an increase in their inflammatory activity, or both. In rabbits, increased 18F-FDG uptake correlated with macrophage-rich lesions, which also exhibited increased levels of glycolysis, pentose phosphate pathway metabolites, and TCA cycle metabolites, as compared with smooth muscle cell-rich lesions.45 The 18F-FDG PET studies are therefore suggestive that human lesional macrophages exhibit increased glycolytic flux, but the evidence is not conclusive.
Given the requirement of glycolysis for a macrophage to respond to inflammatory stimuli and to mount a fully activated inflammatory response ex vivo, several mouse studies have been conducted to investigate the causative role of myeloid cell glycolysis in atherosclerosis. Such studies show that unobstructed glycolysis is required for macrophage functions in atherogenesis through several mechanisms (Figure 2). Consistent with the role of HIF1α in promoting glycolysis, myeloid cell HIF1α-deficiency resulted in reduced GLUT1 gene expression in macrophages and reduced atherosclerosis in Ldlr−/− mice transplanted with bone marrow from myeloid cell-targeted HIF1α-deficient mice.46 The lesion morphology indicated that myeloid cell HIF1α promotes necrotic core formation without affecting the abundance of lesional macrophages. Furthermore, HIF1α-deficient macrophages exhibited reduced expression of chemokines and other markers of the classically activated phenotype and demonstrated reduced apoptosis under normoxic conditions.46 Conversely, reversal of lesional hypoxia (measured by the marker pimonidazole) in Ldlr−/− mice via exposing the mice to high oxygen air reduced plaque necrosis and improved efferocytosis by lesional macrophages.47 Moreover, exposing macrophages to hypoxia in vitro impaired MerTK-mediated efferocytosis, which may be related to decreased glycolysis, as mentioned below. These studies showing atherogenic roles of hypoxia are interesting, as a recent report showed that in other settings hypoxia can promote alternative activation of macrophages, production of resolution mediators, and enhancement of efferocytosis.48 Thus, in the setting atherosclerosis, this protective hypoxia-resolution pathway may somehow become disabled.
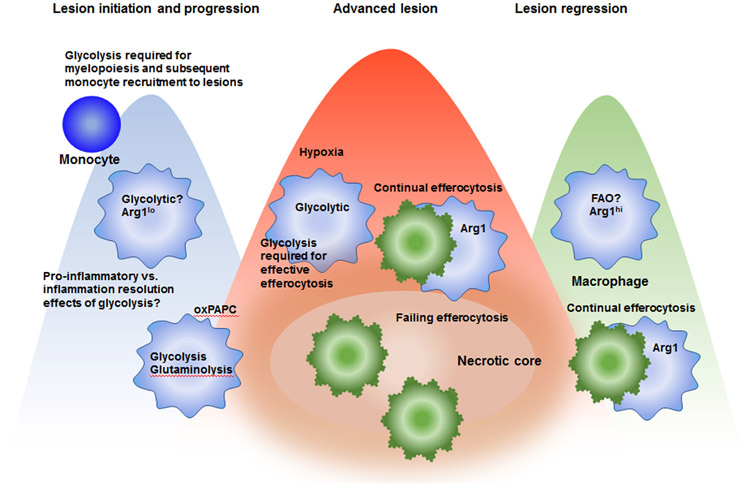
Figure 2. Schematic representation of some of the likely immunometabolic phenotypes of lesional macrophages in different phases of atherosclerosis.
In early lesions and progressing lesions, macrophages are likely activated to exhibit a pro-inflammatory phenotype, characterized by aerobic glycolysis and low Arg1 expression. This glycolytic phenotype is required for optimal production of cytokines and chemokines and also for termination of the inflammatory response. The relative importance of proinflammatory versus inflammation resolution effects of increased glycolysis in lesions is unknown. Glycolysis is also required for myelopoiesis in the bone marrow, which indirectly affects the growing lesion as it allows an increased accumulation of blood monocytes. Lesional macrophages are exposed to increased levels of oxidized phospholipids (oxPAPC), which result in a metabolic shift characterized by simultaneous glycolysis and mitochondrial respiration, and glutaminolysis and oxaloacetate accumulation, leading to IL-1β production. As an advanced lesion is formed, hypoxic regions are likely present at a greater extent, which further enhances macrophage aerobic glycolysis. At the same time, continual efferocytosis becomes overwhelmed and macrophage death predominates over clearance of dead cells. In regressing lesions, macrophages take on an alternative activated phenotype, characterized by increased Arg1 expression and which is likely also characterized by increased fatty acid oxidation (FAO) vis-à-vis glycolysis. Together, these processes would be predicted to increase efferocytosis efficiency and reduce inflammatory processes.
HIF1α has many target genes in addition to those involved in glycolysis. Therefore, studies in which the glucose transporter GLUT1 or other proteins directly involved in glycolysis are modulated are more informative as to the role of glycolysis and glucose metabolism. One such study used a bone marrow transplant approach to introduce GLUT1-deficient hematopoietic cells into Apoe−/− mice.49 These studies demonstrated that deletion of GLUT1 in hematopoietic cells prevented myelopoiesis, monocyte recruitment to lesions and atherosclerosis progression in the Apoe−/− mice, and suggested that the primary role of GLUT1 in this model was to promote both the proliferation of bone marrow hematopoietic stem cells and multi-potential progenitors and the commitment of these cells to the myeloid lineage.49 Finally, there is a converse scenario that has recently come to light and will be discussed in a following section, namely, that expression of GLUT1 and glycolysis in macrophages exposed to apoptotic cells are required for efficient efferocytosis and thereby help protect atherosclerotic lesions from becoming necrotic50 (Figure 2 and Table 1).
To investigate if increased glucose flux through GLUT1 is sufficient to affect atherosclerosis, one study overexpressed GLUT1 under control of the CD68 promoter in hematopoietic chimeras, which results in expression primarily in myeloid cells.21 Although the approach worked well to increase 18F-FDG uptake in macrophages in vivo and to increase glycolysis, forced GLUT1 overexpression did not induce inflammatory changes in primary macrophages and did not induce monocytosis or accelerate atherosclerosis or necrotic core expansion in Ldlr−/− mice fed a low-fat or high-fat diet.21, 51 Thus, increased glucose flux through GLUT1 in myeloid cells is not sufficient to promote lesion progression or necrotic core expansion in mice. These results could potentially be explained by the lack of TCA cycle blocks that normally accompany increased glycolysis in LPS-stimulated macrophages or to the possibility that the pro-inflammatory and resolution phases of glycolysis resulted in a zero-sum effect.
In this context, it is interesting that the carbohydrate-responsive element binding protein (ChREBP), a glucose-sensitive transcription factor, is downregulated in macrophages stimulated with LPS.52 Knockdown of ChREBP in bone marrow cells resulted in accelerated atherosclerosis in Ldlr−/− mice – a response associated with lesional macrophage accumulation and necrotic core expansion. The protective effect of ChREBP was ascribed to maintenance of glutathione levels through NADPH generation via the pentose phosphate pathway. Consistent with this idea, loss of ChREBP was shown to result in increased ROS levels and macrophage death.52 The reason LPS suppresses ChREBP while increasing glycolysis remains unknown, but glycolysis measured as lactate production was further increased in LPS-stimulated ChREBP-deficient macrophages, suggesting that ChREBP acts to suppress glycolysis under these conditions. Other studies have reported that suppression of the pentose phosphate pathway by hypercholesterolemia in vivo diminishes LPS-induced cytokine secretion.53 The roles of the non-oxidative and oxidative phases of the pentose phosphate pathway in inflammation and atherosclerosis in vivo need further study.
Even less is known about the metabolic state of macrophages in relation to atherosclerosis in humans, as only a few studies in this area have been conducted. One of these studies used targeted metabolomics to demonstrate that high-risk carotid artery plaques obtained by endarterectomy exhibit increased glycolysis, elevated amino acid utilization and decreased fatty acid oxidation.54 However, it should be noted that different cell types were included in these plaque samples. An interesting study on monocytes and macrophages from patients with atherosclerotic coronary artery disease demonstrated that increased glucose uptake and glycolytic flux fuel the generation of mitochondrial ROS, which in turn promotes dimerization of PKM2 and its translocation to the nucleus (Table 1). Nuclear PKM2 functions as a protein kinase that phosphorylates the transcription factor STAT3, thus boosting IL-6 and IL-1β production.55
Further research is needed to clarify metabolic phenotypes of lesional macrophages and to understand how these macrophages are altered in different stages of atherosclerosis.6, 56 So far, lack of methods for cell isolation and subsequent analysis of macrophage metabolomes have hampered this research area.
Oxidative phosphorylation and fatty acid oxidation in pro-resolving/repair macrophage phenotypes
In cultured macrophages, the cytokine IL-4 induces an alternatively activated phenotype, which has been implicated in tissue healing and macrophage proliferation. These alternatively activated macrophages are known to have very different metabolic requirements than macrophages activated by LPS or inflammatory mediators.57 Thus, whereas LPS increases glycolysis and decreases OCR, IL-4-stimulated macrophages show no increase in overall OCR and have only a modest and delayed increase in glycolysis as compared with LPS-stimulated macrophages.17 The extent to which IL-4-induced proliferation relies on increased glycolysis or the pentose phosphate pathway, which generates 5-carbon sugars for nucleotide synthesis, is unclear. The delayed increase in glycolysis in response to IL-4 has been shown to be due to Akt activation, which in turn promotes histone acetylation through ACLY activation and genes involved in proliferation.58 However, others have cautioned that the use of 2-deoxyglucose to block glycolysis leads to ATP depletion, which can introduce off-target effects,59 and might have confounded prior data interpretation. One interesting possibility is that the delayed glycolysis induced by IL-4 is involved in suppression of inflammatory activation through the mechanisms discussed above.
LPS-stimulated macrophages exhibit reduced OCR as a measure of mitochondrial oxidative phosphorylation, in part because of the reduced utilization of glucose for oxidation, but also due to a reduction in fatty acid oxidation.15 The reduced oxidative metabolism in LPS-stimulated macrophages has been linked to development of LPS tolerance at later time-points after LPS stimulation60 (Figure 1A). In LPS-tolerant macrophages, decreased oxidative metabolism limits the ability of glucose oxidation to support production of acetyl-CoA, which in turn limits histone acetylation at genes encoding inflammatory mediators, contributing to suppression of inflammatory responses.60
IL-4 induces fatty acid oxidation and mitochondrial biogenesis through the transcription factors STAT-6 and PGC1β,57 and IL-4 also induces a modest increase in SHPK expression in macrophages17 (Figure 1B). Early studies revealed that fatty acid oxidation mediated by PGC1β was required for the anti-inflammatory effects of IL-4.57 More recent studies have shown that fatty acid oxidation is dispensable for IL-4-mediated macrophage polarization.61, 62 The latter studies used lower and more specific concentrations (3 μM) of etomoxir (a carnitine palmitoyltransferase-1 inhibitor) as well as CPT1α knockout mice to reach the conclusion that fatty acid oxidation is not required for expression of many of the known markers of the IL-4 phenotype, including Arg1. Other studies showed that IL-10, another anti-inflammatory and pro-resolving cytokine, suppresses LPS-induced glycolysis, promotes oxidative phosphorylation, and eliminates damaged mitochondria through mitophagy, resulting in reduced inflammasome-mediated IL-1β release.63 The roles of fatty acid oxidation and oxidative phosphorylation in pro-resolving macrophage phenotypes in vitro and in vivo therefore need further research.
Amino acid metabolism is altered with inflammatory state in macrophages
In addition to glucose and fatty acids, amino acids provide important substrates for oxidative phosphorylation in macrophages. Glutamine is particularly important in this respect, as glutaminolysis fuels the TCA cycle when glutamine is converted to glutamate and then α-ketoglutarate (Figure 1A). Glutamate can also be used as a source of citrate for fatty acid synthesis catalyzed by fatty acid synthase (FAS). A recent study demonstrated interesting links among glutaminolysis and IL-1β production in macrophages exposed to oxidized phospholipids derived from 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine (oxPAPC) and atherosclerosis.64 This study demonstrated that exposure of macrophages to oxPAPC in combination with LPS resulted in a shift in metabolism, as compared with that of macrophages stimulated with LPS alone, characterized by increased mitochondrial respiration, glutaminolysis and accumulation of oxaloacetate, leading to stabilization of HIF1α and increased IL-1β production (Figure 2). Systemic inhibition of this pathway reduced early atherosclerosis in mice concomitant with reduced IL-1β immunoreactivity in CD68+ lesional cells64 (Table 1). This pathway might be of human relevance because peripheral blood cells from human subjects with a pro-atherogenic lipid profile exhibited enriched signatures of glutamine utilization, citrate and oxaloacetate metabolism and mitochondrial respiration in relation to the pro-atherosclerotic lipid profile.64 Furthermore, a role for this pathway in atherosclerotic lesions is supported by the finding that 14C-glutamine significantly accumulates in atherosclerotic lesions from Ldlr−/− mice, although the values did not approach those of 18F-FDG accumulation.65 In addition, there is now causative evidence in humans that IL-1β promotes atherosclerotic heart disease.66
A recent study showed that LPS stimulation of macrophages increases their uptake of serine. Together with glucose and methionine, this increased serine fuels generation of S-adenosylmethionine, which is required for IL-1β production through histone methylation.67 Another study demonstrated that serine is required for IL-1β production through glycine, which is needed for glutathione synthesis.68
In addition, arginine can be used for synthesis of polyamines through the action of Arg1, which catalyzes the conversion of arginine to ornithine and is induced by IL-4. Ornithine is used for generation of the polyamines putrescine, spermidine and spermine. A recent study demonstrated that spermidine is critical in determining the toggling between glycolysis and oxidative phosphorylation in macrophages by hypusinating the translation factor eukaryotic initiation factor 5A (eIF5A).69 Hypusine is an unusual amino acid found in eIF5A. Inhibition of this pathway blunted oxidative phosphorylation-dependent alternative activation of the cells, while leaving aerobic glycolysis-dependent classical activation intact.69
Also of relevance in this context is that arginine can be diverted away from polyamine synthesis to citrulline via nitric oxide synthase. Inducible nitric oxide synthase (iNOS) is abundantly expressed primarily in classically activated macrophages and in macrophages in progressing lesions. Nitric oxide (NO) production from arginine is a likely contributor to metabolic switch. Thus, nitric oxide downstream of iNOS has been shown to suppress oxidative phosphorylation in inflammatory macrophages and activated dendritic cells.70, 71 Conversely, arginine is converted by Arg1 to putrescine in alternatively activated macrophages and in macrophages in regressing lesions of atherosclerosis72-74 (Figure 2). These two pathways of arginine metabolism can suppress each other under certain circumstances. Hence, NO suppresses the activity of ornithine decarboxylase, which catalyzes the conversion of ornithine to putrescine, by the S-nitrosylation of a cysteine critical for enzymatic activity.75 Conversely, ornithine decarboxylase hampers the inflammatory activation of macrophages.76 This rewiring of arginine metabolism contributes to pro-inflammatory and resolution processes, as discussed below. Therefore, arginine metabolism directly regulates core metabolic features of inflammatory and pro-resolving macrophages, and also determines their inability to reverse metabolic and phenotypic profiles.
Together, these findings demonstrate that different amino acids alter macrophage functions in distinct manners. Further studies are needed to better understand the interaction and integration of these pathways and their roles in atherosclerosis.
Macrophage oxidative phosphorylation, fatty acid metabolism, and atherosclerosis
Compared with the role of glycolysis, even less is known about the role of oxidative phosphorylation in macrophages in atherosclerosis. One study demonstrated reduced OCR in fibrous cap and core areas of human atherosclerotic plaques.77 This effect was ascribed to mitochondrial dysfunction rather than to a shift in the metabolic flux in lesional cells. Mice overexpressing the mitochondrial helicase Twinkle, which protects mitochondrial integrity, when crossed with Apoe−/− mice exhibited no difference in overall plaque burden, but exhibited increased fibrous caps and decreased necrotic cores. There were also increased levels of proliferation and reduced levels of apoptosis in these lesions.77 Bone marrow transplant studies suggested that Twinkle expression in macrophages contributes to the necrotic core phenotype but not to the fibrous cap phenotype. In line with these studies, macrophage mitochondrial oxidative stress has been shown to promote atherosclerosis and mediate inflammation through the NF-κB pathway in fat-fed Ldlr−/− mice.78
Lesional macrophages most likely rely increasingly on fatty acid oxidation relative to glycolysis under conditions in which the cells take on a more alternatively activated phenotype (in some aspects similar to that of an IL-4-stimulated cell), such as in regressing lesions,79 as shown in Figure 2. Interestingly, the hampered regression in diabetic hyperglycemic mice was recently shown to be prevented by elevated HDL/apolipoprotein A1 levels in mice, suggesting that metabolic perturbations of macrophages in the setting of diabetes can be prevented by increasing cholesterol export from the cells.80 Recent studies have further highlighted the links among fatty acids, cholesterol, and inflammation. One such study suggested that acetoacetyl-CoA arising from FAS activity promotes cholesterol synthesis, and that this process is required for TLR4 to enter lipid rafts and facilitate TLR4 signaling in macrophages.81 Another study found that myeloid cell FAS-deficiency alters membrane order and composition, impairing the retention of plasma membrane cholesterol and disrupting Rho GTPase trafficking, which is required for cell adhesion, migration and inflammatory activation. Importantly, this phenotype could be rescued by exogenous cholesterol.82
In cells, fatty acids are bound to intracellular binding proteins, and in macrophages, deletion of fatty acid binding protein 4 (FABP4) has been shown to reduce inflammatory responses, presumably due to changes in fatty acid fates in the cell resulting in increased peroxisome proliferator-activated receptor-γ (PPARγ) activation and reduced NF-κB activation.83 Deletion of FABP4 in hematopoietic cells also prevented atherosclerosis in Apoe−/− mice.84 In order for fatty acids to be used for oxidation, they must first be converted into their acyl-CoA derivatives by enzymes with acyl-CoA synthetase activity. Acyl-CoA species can be used for many cellular functions, one of which is fatty acid oxidation after conversion of the acyl-CoA to acyl-carnitine and transport into mitochondria. Only one of the five acyl-CoA synthetases, ACSL1, has been studied in relation to macrophage immunometabolism and atherosclerosis. Such studies have shown that ACSL1 is induced by LPS, oxidized LDL, and a number of other inflammatory mediators in macrophages.85-89 Interestingly, macrophages deficient in ACSL1 do not exhibit defective OCR after stimulation with LPS in the presence of palmitate, but these cells appear to use less fatty acids for oxidation.88 These studies suggest that ACSL1 might induce a metabolic switch in macrophages, favoring fatty acid oxidation over other substrates used for oxidative phosphorylation. Silencing of ACSL1 has recently been shown to protect cells against the membrane rigidifying effects of palmitate.90 Deletion of ACSL1 in myeloid cells did not alter atherosclerosis in non-diabetic Ldlr−/− mice, but it prevented the increase in atherosclerosis and inflammation in diabetic mice, suggesting increased roles for myeloid cell ACSL1 and fatty acids in the setting of diabetes-accelerated atherosclerosis86 (Table 1). The role of macrophage ACSL1 in metabolic flexibility and inflammation needs further studies in vivo.
Together, these studies indicate that OCR may be reduced in certain areas of lesions of atherosclerosis owing to mitochondrial dysfunction, and that fatty acids have important effects in macrophages that alter atherosclerosis by additional processes distinct from oxidative phosphorylation. The studies discussed below showing fatty acid oxidation during efferocytosis91 suggest that fatty acid oxidation may facilitate a tissue repair program.
Macrophage intercellular immunometabolism
Paracrine and autocrine effects of metabolites mediated by membrane receptors
Metabolites of glycolysis and the TCA cycle can be released from cells and exert autocrine or paracrine effects (Figure 3). For example, lactate, the end product of aerobic glycolysis, is released from (and taken up by) cells through monocarboxylic acid transporters and can act on macrophages to promote an alternatively activated phenotype when added extracellularly.42 Interestingly, lactate has been shown to promote a synthetic smooth muscle cell phenotype and to increase proliferation and migration of these cells,92 which could be relevant to atherosclerosis. Part of the effects of lactate were mediated by the monocarboxylic acid transporter MCT4 (Slc16a3), and accordingly, knockdown of MCT4 reduced smooth muscle cell proliferation and collagen production. Increased smooth muscle proliferation, migration and collagen production could contribute to a more stable lesion by enhancing fibrous cap thickness. These results are in agreement with experiments in which the glucose transporter GLUT1 was overexpressed in smooth muscle cells, which resulted in increased lactate production and increased smooth muscle cells in lesions of atherosclerosis.51 However, there was also an increased recruitment of monocytes to these lesions. Furthermore, increased lactate levels in inflamed tissues are responsible for T cell entrapment at sites of inflammation.93
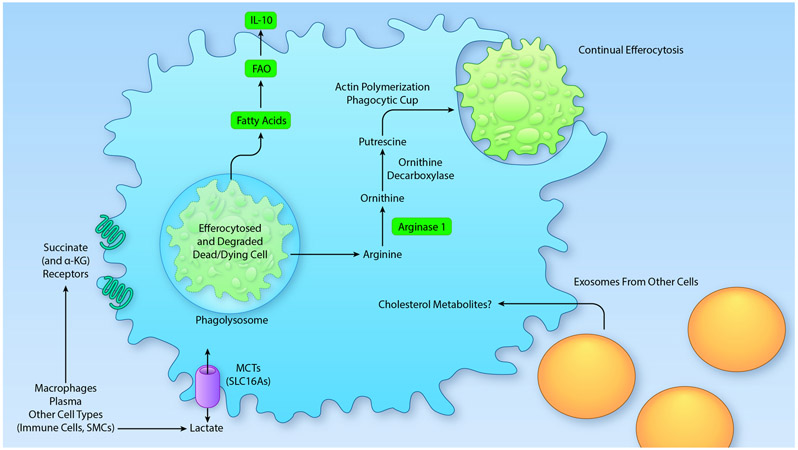
Figure 3. Examples of intercellular immunometabolism.
Recent research shows that macrophage metabolism and function are altered by metabolites derived from other cells, a process we term intercellular immunometabolism. For example, fatty acid and amino acid metabolism can be affected by the cargo of an efferocytosed and degraded dead or dying cell. The metabolite cargo from the efferocytosed cell thus alters the metabolic pathways and downstream functions of the efferocytosing macrophage. Fatty acids derived from ingested cells are likely to fuel fatty acid oxidation (FAO) and subsequent production of the anti-inflammatory cytokine IL-10. Arginine from efferocytosed cells undergo conversion to putrescine, which is critically important for continual efferocytosis. Extracellular metabolites can also act on macrophages through receptors or transporters, or might be delivered by exosomes, which are ingested by the macrophage. The latter process has been shown to deliver cholesterol to macrophages, but it is possible that metabolites are also delivered through this route. . (Illustration Credit: Ben Smith)
Some TCA cycle metabolites are also released from cells. Released succinate has been shown to act through the G-protein coupled receptor succinate receptor 1 (SUCNR1; formerly known as GPR91).28 SUCNR1 has been suggested to be a sensor of immunological danger.94 In this manner, extracellular succinate released from macrophages can increase antigen presentation by dendritic cells and enhance IL-1β production.28 Likewise, extracellular α-ketoglutarate has been shown to act on the receptor OXRG1 (formerly known as GPR99).95 This receptor, which is not specific to α-ketoglutarate does not appear to be abundantly expressed in macrophages.
Taken together, these studies suggest that metabolites released from macrophages might alter atherosclerotic lesion phenotypes by autocrine or paracrine effects. Conversely, macrophage immunometabolism will likely be influenced by metabolites from other vascular and stromal cells, such as by the lactate produced by smooth muscle cells discussed above, as well as by other immune cells and endothelial cells.
Cellular effects of metabolites derived from the cargo of efferocytosed cells
The metabolites that drive the intracellular reactions in macrophages described in the previous sections can be obtained from endogenous biosynthesis or from cellular uptake through plasma membrane transport systems. However, there is a third mechanism of metabolite delivery that is functionally integrated with the fundamental process of efferocytosis. This overall topic is highly relevant to atherosclerosis, as impairment of efferocytosis during atherosclerosis is thought to be a major mechanism of advanced plaque progression.96-98 Through the process of phagosomal maturation, engulfed apoptotic cell (AC) cargo is delivered to phagolysosomes, which are formed through the fusion of phagosomes with acid hydrolase-containing lysosomes.99, 100 The AC cargo includes both metabolic building blocks, e.g. cholesterol and certain fatty acids, and macromolecules, e.g., proteins, complex lipids, and nucleic acids. These macromolecules are degraded by the lysosomal hydrolases into basic metabolites, notably amino acids, fatty acids and other lipid-derived molecules, and nucleosides.99 Some of these molecules can be exported from the macrophage by transport of cargo-containing vesicles from the phagolysosome to the plasma membrane, resulting in discharge of the cargo to the extracellular space via exocytosis.101, 102 This cargo might in turn affect neighboring cells. Other AC cargo is transferred into the macrophage cytoplasm through lysosomal transport systems, and this process can also lead to cargo removal via specific efflux pathways, as is the case with AC-derived cholesterol. As will be reviewed below, the cholesterol example illustrates that these export/efflux processes can maintain macrophage health by ridding the cell of certain molecules that can be cytotoxic when in excess. Most interestingly, however, some of the AC-derived cargo that is transported from the phagolysosome to the cytoplasm can be metabolized by the macrophage. As will be reviewed below, these metabolic pathways can be critical for proper macrophage function, including macrophage function in atherosclerotic lesions, particularly in the ability of macrophages to carry out continual efferocytosis and resolution/repair pathways. As with the efferocytosis, defective inflammation resolution has been linked to the progression of atherosclerosis.103, 104
All cells have a high cholesterol content to support membrane structure and function. Thus, when a macrophage engulfs an AC, it is confronted with a large load of cholesterol, which can be toxic to cells when there is excessive accumulation of unesterified (“free”) cholesterol.105 In the case of atherosclerosis, many of the dead cells are apoptotic cholesterol-loaded macrophages, or foam cells, and in this case the living efferocytic macrophages in the lesions are confronted with huge amounts of excess cholesterol.106 When this scenario was modeled in vitro, i.e., by incubating macrophages with apoptotic foam cells, detoxification was carried out by two mechanisms: an esterification reaction in which acyl-CoA:cholesterol acyltransferase (ACAT) in the ER converts membrane-active free cholesterol to “inert” cholesterol fatty acid esters, which are sequestered in neutral lipid droplets in the cytoplasm; and free cholesterol efflux from the macrophages.107 Enhanced cholesterol efflux has been linked to efferocytosis through several pathways, all involving up-regulation of the cholesterol efflux transport protein, ABCA1. For example, oxysterols resulting from efferocytosis activate the nuclear receptors liver x receptor (LXR)-α and -β, which induce ABCA1.108 The origin of these oxysterols is an interesting topic, with recent evidence suggesting that lysosomal acid lipase (LIPA)-mediated hydrolysis of AC-derived cholesteryl esters provides substrate for the metabolism of cholesterol into certain oxysterols in macrophages.108 As another example, AC-mediated activation of the efferocytosis receptor lipoprotein receptor-related protein 1 (LRP1) activates LXR/ABCA1-mediated efflux by first activating the LXR inducer, PPARγ.109 A third pathway of enhanced ABCA1-mediated cholesterol efflux involves AC internalization by the efferocytosis receptor brain-specific angiogenesis inhibitor 1 (BAI1), but in this case the mechanism remains to be elucidated, as it was not blocked by inhibiting or deleting LXR.110 While these studies did not include atherosclerosis data per se, many of the molecules discussed here are highly relevant to atherosclerosis, including LXR, ABCA1, PPARγ, and LRP1.111, 112 In terms of LIPA, its effects on atherosclerosis are not straightforward,113, 114 and this area requires further investigation, particularly with regard to the LIPA-LXR pathway.
A key question in the area of efferocyte metabolism is how the mitochondria of macrophages respond to the influx of AC-derived metabolic cargo. Not unexpectedly, this influx of cargo was found to increase the mitochondrial membrane potential.115 Prolongation of this process could lead to adverse cellular effects, but macrophages have evolved a compensatory mechanism whereby upregulation of the mitochondrial uncoupling protein, UCP2, renders the mitochondrial membrane potential increase transient. As evidence, prevention of the UCP2 response during efferocytosis both in vitro and in vivo caused defects in continual efferocytosis, which refers to the ability of any given macrophage to clear multiple ACs within a relative short period of time.115 Although this study did not examine atherosclerosis, a previous study showed an atheroprotective effect of UCP2.116 Further work is needed to elucidate (a) the mechanism through which UCP2 is upregulated during efferocytosis, although the aforementioned study108 suggests a link to LIPA-enabled metabolism of cholesterol to 25-hydroxycholesterol, perhaps through stimulating cholesterol efflux; and (b) how the lowering of transiently elevated mitochondrial membrane potential allows continual efferocytosis. However, one of the most important conclusions to come from this study is that continual efferocytosis is critical to avoid accumulation of uncleared dead cells in vivo.
Key consequences of efferocytosis include dampening of inflammation, enhancement of inflammation resolution, and repair of tissue damage,117-120 and these consequences are particularly important in atherosclerosis.97 While dampening of inflammation has been linked mostly to activation of cell-surface efferocytosis receptors, notably those of the Tyro-Axl-MerTK (TAM) family, by ACs, AC metabolism pathways may also be involved. For example, when phagolysosomal hydrolysis of AC lipids by LIPA was prevented in cultured macrophages, the inflammasome was activated, which was linked to defective metabolism of cholesterol to 25-hydroxycholesterol and subsequent excessive mitochondrial oxidative stress.108 Both inflammasome activation and mitochondrial oxidative stress have been shown to play roles in the progression of atherosclerosis, with the former pathway linked to coronary artery disease in humans.66, 78, 121 In another report, metabolism of AC-derived fatty acids was implicated in the ability of macrophages in the heart to repair myocardial damage following myocardial infarction.91 The authors showed that mitochondrial fatty acid oxidation during efferocytosis produced NAD+, which promoted a Sirtuin1 pathway leading to induction of IL-10 (Figure 3). Although the authors did not show directly that fatty acids fueling this pathway came from ingested ACs, the study discussed above108 showed that inhibition of LIPA during efferocytosis lowered OCR.
IL-10 is a key mediator of resolution and repair, and it also promotes efferocytosis by a positive-feedback pathway.122, 123 In this context, when mitochondrial electron transport, and thus NAD+ production, was prevented in macrophages by genetic targeting in a mouse model of myocardial infarction, there was a defect in IL-10 production and cardiac repair.91 The mechanism involved a pathway in which NAD+ promoted Sirtuin1-mediated activation of a Il10 gene inducer called pre-B cell leukemia transcription factor-1 (PBX-1). Future in vivo studies will be needed to link IL-10 and tissue repair to the full NAD+/Sirtuin1/PBX-1 pathway and to determine if failure of this pathway plays a role in atherosclerosis progression.
As briefly mentioned in a previous section, there are also links between intact glycolysis and efficient efferocytosis (Figure 2). A recent study showed that the addition of ACs to macrophages increased glycolysis, which was mediated by induction of both GLUT1 (SLC2A1) and a kinase that activates GLUT1 called SGK1. When these molecules were targeted both in vitro and in vivo, including deletion of GLUT1 in fat-fed Ldlr−/− mice, lesional efferocytosis was impaired and plaque necrosis was worsened (Table 1). The atherosclerosis data were confirmed by a subsequent study, which also showed that bone marrow-derived macrophages from the GLUT1-deficient mice had severely diminished glucose uptake and exhibited an increased reliance on other metabolic fuels, such as fatty acids.124 There is an increased realization that glycolysis is needed for both proinflammatory effects and pro-resolving processes in macrophages, as discussed above. Temporal changes in glycolysis, perhaps in concert with other metabolic changes, might govern its different roles in inflammation and resolution. Furthermore, because resolution is a natural response to inflammation, an early surge in glycolysis in response to inflammatory stimuli might promote a later resolution response. Another possibility is that increased glycolysis is necessary to allow an efferocyte to process the increased metabolic load following ingestion of an apoptotic cell. One of the byproducts of glycolysis is lactate, and the authors of the first study discussed above showed that blocking lactate export by silencing its transporter SLC16A1 prevented efferocytosing bone marrow-derived macrophages to induce IL-10 in neighboring macrophages.50 Thus, lactate, perhaps in concert with other components in the media of efferocytes, stimulates IL-10 production in macrophages. Both the mechanism of this process and its relevance to IL-10 production in efferocytosis settings in vivo remain to be elucidated.
The metabolism of amino acids arising from the phagolysosomal degradation of AC proteins in efferocytosing macrophages represents another area of interest. A recent study has examined one of these amino acids, arginine.125 In vitro and in vivo studies showed that the metabolism of AC-derived arginine to ornithine by Arg1 and then to putrescine by ornithine decarboxylase enhanced continual efferocytosis. Human macrophages were able to directly metabolize AC-derived ornithine to putrescine, i.e., by relying on ornithine decarboxylase rather than Arg1. The increase in putrescine in macrophages engulfing a first AC maintains uptake of the next AC, i.e., continual efferocytosis, by ensuring adequate activation of actin-regulating Rac1-GTPase, which is needed for AC internalization (Figure 3). Arg1 is increased in macrophages in regressing atherosclerotic lesions,73 and inhibition of this pathway in that setting, where efferocytosis is high, prevented the regression-induced decrease in plaque size and increase in efferocytosis. Conversely, feeding mice putrescine during atherosclerosis progression, where lesional macrophages have low Arg1 expression, enhanced efferocytosis and decreased plaque progression125 (Table 1). As discussed above, iNOS-dependent NO synthesis and Arg1-dependent ornithine generation compete for arginine, and in some cases activation of one pathway leads to suppression of the other. Atherosclerosis progression is dominated by the presence iNOShigh Arg1low inflammatory macrophages, while the opposite is the case during atherosclerosis regression.72-74, 79 These observations and the findings that Arg1 is a critical enzyme in continual efferocytosis suggest that the resolution program is “reawakened” during atherosclerosis regression, including an increase in continual efferocytosis driven by Arg1, ornithine and putrescine. Future studies will likely elucidate additional roles of putrescine derived from AC arginine/ornithine as well as effects resulting from the metabolism of other amino acids and other types of metabolites resulting from the engulfment and phagolysosomal degradation of ACs.
How do changes in systemic metabolism affect macrophage immunometabolism?
A dyslipidemic environment has been shown to alter macrophage immunometabolism through several mechanisms. Oxidized phospholipids, which are present at increased levels in hyperlipidemic states, result in increased glutaminolysis and oxaloacetate accumulation in macrophages, and subsequently to increased IL-1β production,64 as discussed above. Oxidized LDL was recently shown to suppress oxidative phosphorylation in macrophages, resulting in increased superoxide levels and a compensatory increase in glycolysis, which resulted in an inflammatory response.89 This pathway depends on increased fatty acid uptake by CD36 and was associated with increased expression of FABP4 and ACSL1 (discussed above) and CPT1 and CPT2 (carnitine palmitoyltransferase 1 and 2). As such, oxidized LDL drives CD36 signaling in macrophages and links dysregulated fatty acid metabolism to mitochondrial oxidative stress and chronic inflammation. Oxidized LDL also enhances the effects of hypoxia on increasing glycolysis.126 One can speculate that the increased glycolytic capacity of lesional macrophages in progressing lesions (Figure 2) is in part explained by these mechanisms, and that diabetes, which results in upregulation of ACSL1 and a proinflammatory macrophage phenotype,86 also acts in part through this mechanism.
Diabetes is associated with systemically elevated levels of glucose, but also often with dyslipidemia. The role of hyperglycemia in altering macrophage immunometabolism is unclear, but increased glycolysis due to forced overexpression of GLUT1 in myeloid cells had no effect on atherosclerosis in Ldlr−/− mice.21 It is might be more likely that the inflammatory environment, rather than hyperglycemia per se, increases GLUT1 expression, glycolysis, and downstream changes in macrophage immunometabolism. This question, however, will require further research. It is also likely that changes in lipids or apolipoproteins that accompany diabetes are more important than hyperglycemia in accelerating atherosclerosis and coronary artery disease events.127, 128
Furthermore, high insulin levels associated with insulin resistance or reduced insulin signaling in macrophages affect macrophages directly to promote atherosclerosis lesion progression.32, 129
Summary
Interest in the area of immunometabolism in relation to atherosclerosis is rapidly growing. In this review we have summarized the field to date and highlighted the new concept of intercellular immunometabolism. Four topics we consider to be particularly interesting and critical areas for future research are: 1) how the in vitro data gathered on isolated macrophages activated by defined stimuli translate to different in vivo situations and macrophage populations in tissues; 2) the pro-resolving versus inflammatory effects of glycolysis and how they relate to atherosclerosis; 3) mechanisms whereby immunometabolism and atherosclerosis are affected by the transfer of metabolites from efferocytosed cells to the efferocyte and by metabolites released from cells; and 4) the effects of disease states such as dyslipidemia and diabetes on macrophage immunometabolism and atherosclerosis, and whether targeting immunometabolism can result in novel treatment and prevention strategies.
Future directions
Despite the recent escalation in research on macrophage immunometabolism discussed in this review, there are many unanswered questions related to immunometabolism in macrophages in atherosclerosis. Further mechanistic mouse studies using myeloid cell-targeted approaches are needed to provide evidence of causal roles of myeloid cell metabolites in different states of atherosclerosis. Importantly, new methodology is needed in order to assess metabolic flux in lesional macrophages in different stages of atherosclerosis. Singe-cell RNA-sequencing data have revealed the complexity of lesional macrophage populations both in mice and humans,130-135 but so far, gene expression studies have not revealed a clear picture of the metabolic flexibility of lesional macrophages. Several significant hurdles remain before we can get similar data on metabolomes in lesional macrophages. One such hurdle is that many metabolites are very short-lived (seconds or fractions of seconds), which makes isolation of lesional macrophages with a preserved metabolome challenging. Laser capture microdissection of macrophage-rich lesion areas followed by metabolomics could provide a new option. Another interesting approach based on in situ analysis of up to five different dehydrogenases representing glycolysis, the pentose phosphate pathway and TCA cycle in tissue sections, combined with analysis of cell markers, was recently described by Miller and colleagues.130 Furthermore, spatially resolved metabolomics methods take advantage of mass spectrometry imaging-based in situ metabolomics and immunohistochemistry to validate findings.136 Histo-cytometry has also been used to visualize and quantify phenotypically complex immune cell populations in tissue sections,137 and might be informative in the context of metabolic phenotyping of lesional macrophages. Moreover, single cell metabolomics is on the horizon, but is still insensitive. Such an approach would also still have the problem of metabolites changing during cell isolation. These methods are mostly in their infancy, but will begin to give a picture of immunometabolism in cells in tissues, such as lesions of atherosclerosis. The realization that metabolites can be transferred from one cell to another through efferocytosis, cellular uptake through transport proteins, or even perhaps through exosomes138 further complicates definition of the lesional macrophage metabolic phenotype. It is quite possible that we will see an increase in studies showing intercellular effects of metabolites carried through exosomes in the future.
Another interesting challenge is to clarify if metabolic changes occur in myeloid cells or their precursors prior to entering the lesion. Bone marrow progenitor cells might be altered by the metabolic environment in the bone marrow and might transfer those traits, through a process called trained immunity139 mediated by epigenetic changes, to recruited monocytes. This epigenetic memory of metabolic changes might affect lesional macrophages indirectly. Epigenetic memory may be particularly relevant to the topic of clonal hematopoiesis, in which aging-related loss-of-function somatic mutations in epigenetic modifier genes in hematopoietic cells convey risk of atherothrombotic disease in humans, at least in part through inflammatory changes in monocytes and macrophages.140
Will the immunometabolism area reveal new potential targets for intervention and prevention of incident CVD? Lipid lowering has been successful, but beneficial effects are primarily mediated by the liver. An ACLY inhibitor, bempedoic acid, has been used to lower LDL-cholesterol. This inhibitor attenuates atherosclerosis in several animal models and has been tested in clinical trials for LDL-cholesterol lowering.141 The beneficial effect of this inhibitor is likely primarily due to increased hepatic expression of LDL receptors, leading to more efficient LDL clearance, although it is likely that some effects are due to suppression of macrophage inflammatory activation.58 In fact, the recent study on oxidized phospholipids and macrophage metabolic changes discussed above64 showed that ACLY inhibition in Ldlr−/− mice resulted in reduced lesional IL-1β immunoreactivity and reduced early atherosclerosis without changes in plasma lipid levels. Another interesting topic is whether work in this area will inform our efforts to stem the epidemic of obesity-mediated cardiometabolic disease, as effects of diabetes and insulin on lesional macrophage metabolism may have important roles in accelerated atherosclerosis in these settings.129 Lastly, macrophage metabolism in both progressing and regressing atherosclerotic lesions is strongly linked to efferocytosis and inflammation resolution. In view of the potential for resolution mediator therapy to improve efferocytosis and resolution in atherosclerosis and block plaque progression with less compromise of host defense,142 further knowledge of the mechanisms linking macrophage metabolism, efferocytosis, and resolution in the setting of atherosclerosis may lead to new strategies to suppress plaque progression and promote plaque regression.
Acknowledgments:
We thank Arif Yurdagul Jr. and Amanda C. Doran for helpful discussions. We apologize to our many colleagues whose important work we were unable to cite due to space limitations.
Sources of Funding: The authors are supported by the National Institutes of Health, under awards R01HL127694, R21AI135447, P01HL092969, R01HL126028, DP3DK108209 (KEB); R35HL145228, R01HL127464, P01HL087123 (IT). Research in KEB’s laboratory is also supported in part by the Diabetes Research Center at the University of Washington (P30DK017047) and research in IT's laboratory is supported in part by a grant from the Leducq Foundation.
Nonstandard Abbreviations and Acronyms
- ACLY
ATP citrate lyase
- AC
apoptotic cell
- ACAT
acyl-CoA:cholesterol acyltransferase
- Arg1
arginase 1
- BAI1
brain-specific angiogenesis inhibitor 1
- ChREBP
carbohydrate-responsive element binding protein
- CVD
cardiovascular disease
- eIF5A
eukaryotic initiation factor 5A
- 18F-FDG
18F-fluoro-2-deoxy-D-glucose
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GLUT1 (gene name SLC2A1)
glucose transporter 1
- HIF1α
hypoxia-inducible factor 1α
- iNOS (gene name NOS2)
inducible nitric oxide synthase
- LIPA
lysosomal acid lipase
- LRP1
lipoprotein receptor-related protein 1
- LXR
liver x receptor
- OCR
oxygen consumption rate
- PEP
pyruvate from phosphoenolpyruvate
- PET
positron emission tomography
- PFKFB3
phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- PPARγ
peroxisome proliferator-activated receptor-γ
- TAM family
Tyro-Axl-MerTK family
- TCA cycle
tricarboxylic acid cycle (also known as the Krebs cycle and citric acid cycle)
Footnotes
Disclosures: None
References
- 1.Mathis D, Shoelson SE. Immunometabolism: An emerging frontier. Nat Rev Immunol. 2011;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PJ, Rathmell J, Pearce E. Snapshot: Immunometabolism. Cell Metab. 2015;22:190–190 e191. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell DG, Huang L, VanderVen BC. Immunometabolism at the interface between macrophages and pathogens. Nat Rev Immunol. 2019;19:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warburg O On the origin of cancer cells. Science. 1956;123:309–314. [DOI] [PubMed] [Google Scholar]
- 9.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempner W The nature of leukemic blood cells as determined by their metabolism. J Clin Invest. 1939;18:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn ZA, Morse SI. Functional and metabolic properties of polymorphonuclear leucocytes. Ii. The influence of a lipopolysaccharide endotoxin. J Exp Med. 1960;111:689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces il-1beta through hif-1alpha. Nature. 2013;496:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krejcova G, Danielova A, Nedbalova P, Kazek M, Strych L, Chawla G, Tennessen JM, Lieskovska J, Jindra M, Dolezal T, Bajgar A. Drosophila macrophages switch to aerobic glycolysis to mount effective antibacterial defense. Elife. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van den Bossche J, O'Neill LA, Menon D. Macrophage immunometabolism: Where are we (going)? Trends Immunol. 2017;38:395–406. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haschemi A, Kosma P, Gille L, et al. The sedoheptulose kinase carkl directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. [DOI] [PubMed] [Google Scholar]
- 19.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. Tlr signalling augments macrophage bactericidal activity through mitochondrial ros. Nature. 2011;472:476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuzumi M, Shinomiya H, Shimizu Y, Ohishi K, Utsumi S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via glut1. Infect Immun. 1996;64:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishizawa T, Kanter JE, Kramer F, et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 2014;7:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Garcia A, Monsalve E, Novellasdemunt L, Navarro-Sabate A, Manzano A, Rivero S, Castrillo A, Casado M, Laborda J, Bartrons R, Diaz-Guerra MJ. Cooperation of adenosine with macrophage toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of pfkfb3 gene. J Biol Chem. 2011;286:19247–19258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase m2 regulates hif-1alpha activity and il-1beta induction and is a critical determinant of the warburg effect in lps-activated macrophages. Cell Metab. 2015;21:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The m2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. [DOI] [PubMed] [Google Scholar]
- 25.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase m2 is a phd3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stincone A, Prigione A, Cramer T, et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015;90:927–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan DG, Murphy MP, Frezza C, Prag HA, Chouchani ET, O'Neill LA, Mills EL. Coupling krebs cycle metabolites to signalling in immunity and cancer. Nat Metab. 2019;1:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill LA. A broken krebs cycle in macrophages. Immunity. 2015;42:393–394. [DOI] [PubMed] [Google Scholar]
- 30.Mills EL, Kelly B, Logan A, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, Koseki H, Cabrales P, Murphy AN, Hiller K, Metallo CM. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem. 2016;291:14274–14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dror E, Dalmas E, Meier DT, et al. Postprandial macrophage-derived il-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol. 2017;18:283–292. [DOI] [PubMed] [Google Scholar]
- 33.Moon JS, Hisata S, Park MA, DeNicola GM, Ryter SW, Nakahira K, Choi AMK. Mtorc1-induced hk1-dependent glycolysis regulates nlrp3 inflammasome activation. Cell Rep. 2015;12:102–115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Hughes MM, O'Neill LAJ. Metabolic regulation of nlrp3. Immunol Rev. 2018;281:88–98. [DOI] [PubMed] [Google Scholar]
- 35.Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H, Cao L, Jiang J, Tang D. Pkm2-dependent glycolysis promotes nlrp3 and aim2 inflammasome activation. Nat Commun. 2016;7:13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills E, O'Neill LA. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014;24:313–320. [DOI] [PubMed] [Google Scholar]
- 37.Galvan-Pena S, Carroll RG, Newman C, et al. Malonylation of gapdh is an inflammatory signal in macrophages. Nat Commun. 2019;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Souza DP, Achuthan A, Lee MK, Binger KJ, Lee MC, Davidson S, Tull DL, McConville MJ, Cook AD, Murphy AJ, Hamilton JA, Fleetwood AJ. Autocrine ifn-i inhibits isocitrate dehydrogenase in the tca cycle of lps-stimulated macrophages. J Clin Invest. 2019;129:4239–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu PS, Wang H, Li X, et al. Alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18:985–994. [DOI] [PubMed] [Google Scholar]
- 40.Mills EL, Ryan DG, Prag HA, et al. Itaconate is an anti-inflammatory metabolite that activates nrf2 via alkylation of keap1. Nature. 2018;556:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Neill LAJ, Artyomov MN. Itaconate: The poster child of metabolic reprogramming in macrophage function. Nat Rev Immunol. 2019;19:273–281. [DOI] [PubMed] [Google Scholar]
- 42.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sriranjan RS, Tarkin JM, Evans NR, Le EPV, Chowdhury MM, Rudd JHF. Atherosclerosis imaging using positron emission tomography (pet): Insights and applications. Br J Pharmacol. 2019 [DOI] [PubMed] [Google Scholar]
- 44.Verweij SL, Duivenvoorden R, Stiekema LCA, Nurmohamed NS, van der Valk FM, Versloot M, Verberne HJ, Stroes ESG, Nahrendorf M, Bekkering S, Bernelot Moens SJ. Ccr2 expression on circulating monocytes is associated with arterial wall inflammation assessed by 18f-fdg pet/ct in patients at risk for cardiovascular disease. Cardiovasc Res. 2018;114:468–475. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita A, Zhao Y, Matsuura Y, Yamasaki K, Moriguchi-Goto S, Sugita C, Iwakiri T, Okuyama N, Koshimoto C, Kawai K, Tamaki N, Zhao S, Kuge Y, Asada Y. Increased metabolite levels of glycolysis and pentose phosphate pathway in rabbit atherosclerotic arteries and hypoxic macrophage. PLoS One. 2014;9:e86426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aarup A, Pedersen TX, Junker N, Christoffersen C, Bartels ED, Madsen M, Nielsen CH, Nielsen LB. Hypoxia-inducible factor-1alpha expression in macrophages promotes development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1782–1790. [DOI] [PubMed] [Google Scholar]
- 47.Marsch E, Theelen TL, Demandt JA, et al. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34:2545–2553. [DOI] [PubMed] [Google Scholar]
- 48.Norris PC, Libreros S, Serhan CN. Resolution metabolomes activated by hypoxic environment. Sci Adv. 2019;5:eaax4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarrazy V, Viaud M, Westerterp M, Ivanov S, Giorgetti-Peraldi S, Guinamard R, Gautier EL, Thorp EB, De Vivo DC, Yvan-Charvet L. Disruption of glut1 in hematopoietic stem cells prevents myelopoiesis and enhanced glucose flux in atheromatous plaques of apoe(−/−) mice. Circ Res. 2016;118:1062–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morioka S, Perry JSA, Raymond MH, Medina CB, Zhu Y, Zhao L, Serbulea V, Onengut-Gumuscu S, Leitinger N, Kucenas S, Rathmell JC, Makowski L, Ravichandran KS. Efferocytosis induces a novel slc program to promote glucose uptake and lactate release. Nature. 2018;563:714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wall VZ, Barnhart S, Kanter JE, Kramer F, Shimizu-Albergine M, Adhikari N, Wight TN, Hall JL, Bornfeldt KE. Smooth muscle glucose metabolism promotes monocyte recruitment and atherosclerosis in a mouse model of metabolic syndrome. JCI Insight. 2018;3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarrazy V, Sore S, Viaud M, Rignol G, Westerterp M, Ceppo F, Tanti JF, Guinamard R, Gautier EL, Yvan-Charvet L. Maintenance of macrophage redox status by chrebp limits inflammation and apoptosis and protects against advanced atherosclerotic lesion formation. Cell Rep. 2015;13:132–144. [DOI] [PubMed] [Google Scholar]
- 53.Baardman J, Verberk SGS, Prange KHM, et al. A defective pentose phosphate pathway reduces inflammatory macrophage responses during hypercholesterolemia. Cell Rep. 2018;25:2044–2052 e2045. [DOI] [PubMed] [Google Scholar]
- 54.Tomas L, Edsfeldt A, Mollet IG, Perisic Matic L, Prehn C, Adamski J, Paulsson-Berne G, Hedin U, Nilsson J, Bengtsson E, Goncalves I, Bjorkbacka H. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur Heart J. 2018;39:2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, Weyand CM. The glycolytic enzyme pkm2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ketelhuth DFJ, Lutgens E, Back M, Binder CJ, Van den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O'Neill L, Weber C, Evans PC. Immunometabolism and atherosclerosis: Perspectives and clinical significance: A position paper from the working group on atherosclerosis and vascular biology of the european society of cardiology. Cardiovasc Res. 2019;115:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and pgc-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Covarrubias AJ, Aksoylar HI, Yu J, et al. Akt-mtorc1 signaling regulates acly to integrate metabolic input to control of macrophage activation. Elife. 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, Dzeja PP, Herrmann J. Glycolytic stimulation is not a requirement for m2 macrophage differentiation. Cell Metab. 2018;28:463–475 e464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langston PK, Nambu A, Jung J, et al. Glycerol phosphate shuttle enzyme gpd2 regulates macrophage inflammatory responses. Nat Immunol. 2019;20:1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Divakaruni AS, Hsieh WY, Minarrieta L, et al. Etomoxir inhibits macrophage polarization by disrupting coa homeostasis. Cell Metab. 2018;28:490–503 e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van den Bossche J, van der Windt GJW. Fatty acid oxidation in macrophages and t cells: Time for reassessment? Cell Metab. 2018;28:538–540. [DOI] [PubMed] [Google Scholar]
- 63.Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of il-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Gioia M, Spreafico R, Springstead JR, Mendelson MM, Joehanes R, Levy D, Zanoni I. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat Immunol. 2020;21:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tavakoli S, Downs K, Short JD, Nguyen HN, Lai Y, Jerabek PA, Goins B, Toczek J, Sadeghi MM, Asmis R. Characterization of macrophage polarization states using combined measurement of 2-deoxyglucose and glutamine accumulation: Implications for imaging of atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 67.Yu W, Wang Z, Zhang K, Chi Z, Xu T, Jiang D, Chen S, Li W, Yang X, Zhang X, Wu Y, Wang D. One-carbon metabolism supports s-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol Cell. 2019;75:1147–1160 e1145. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez AE, Ducker GS, Billingham LK, Martinez CA, Mainolfi N, Suri V, Friedman A, Manfredi MG, Weinberg SE, Rabinowitz JD, Chandel NS. Serine metabolism supports macrophage il-1beta production. Cell Metab. 2019;29:1003–1011 e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puleston DJ, Buck MD, Klein Geltink RI, et al. Polyamines and eif5a hypusination modulate mitochondrial respiration and macrophage activation. Cell Metab. 2019;30:352–363 e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen HJ, Boshuizen MC, Ahmed M, Hoeksema MA, de Vos AF, de Winther MP. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–696. [DOI] [PubMed] [Google Scholar]
- 71.Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, Pearce EL, Pearce EJ. Commitment to glycolysis sustains survival of no-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pourcet B, Feig JE, Vengrenyuk Y, Hobbs AJ, Kepka-Lenhart D, Garabedian MJ, Morris SM Jr., Fisher EA, Pineda-Torra I. Lxralpha regulates macrophage arginase 1 through pu.1 and interferon regulatory factor 8. Circ Res. 2011;109:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willecke F, Yuan C, Oka K, Chan L, Hu Y, Barnhart S, Bornfeldt KE, Goldberg IJ, Fisher EA. Effects of high fat feeding and diabetes on regression of atherosclerosis induced by low-density lipoprotein receptor gene therapy in ldl receptor-deficient mice. PLoS One. 2015;10:e0128996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bauer PM, Buga GM, Fukuto JM, Pegg AE, Ignarro LJ. Nitric oxide inhibits ornithine decarboxylase via s-nitrosylation of cysteine 360 in the active site of the enzyme. J Biol Chem. 2001;276:34458–34464. [DOI] [PubMed] [Google Scholar]
- 76.Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C, Steeves MA, Cleveland JL, Schneider C, Piazuelo MB, Gobert AP, Wilson KT. Ornithine decarboxylase regulates m1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci U S A. 2017;114:E751–E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu EPK, Reinhold J, Yu H, Starks L, Uryga AK, Foote K, Finigan A, Figg N, Pung YF, Logan A, Murphy MP, Bennett M. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler Thromb Vasc Biol. 2017;37:2322–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappab-mediated inflammation in macrophages. Circ Res. 2014;114:421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, Gusarova V, Gromada J, Weinstock A, Moore KJ, Loke P, Fisher EA. Inflammatory ly6chi monocytes and their conversion to m2 macrophages drive atherosclerosis regression. J Clin Invest. 2017;127:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barrett TJ, Distel E, Murphy AJ, Hu J, Garshick MS, Ogando Y, Liu J, Vaisar T, Heinecke JW, Berger JS, Goldberg IJ, Fisher EA. Apolipoprotein ai) promotes atherosclerosis regression in diabetic mice by suppressing myelopoiesis and plaque inflammation. Circulation. 2019;140:1170–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carroll RG, Zaslona Z, Galvan-Pena S, Koppe EL, Sevin DC, Angiari S, Triantafilou M, Triantafilou K, Modis LK, O'Neill LA. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J Biol Chem. 2018;293:5509–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, Ory DS, Semenkovich CF. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, ap2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of ap2 impacts peroxisome proliferator-activated receptor gamma and ikappab kinase activities. J Biol Chem. 2005;280:12888–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein ap2 protects mice deficient in apolipoprotein e against atherosclerosis. Nat Med. 2001;7:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubinow KB, Wall VZ, Nelson J, et al. Acyl-coa synthetase 1 is induced by gram-negative bacteria and lipopolysaccharide and is required for phospholipid turnover in stimulated macrophages. J Biol Chem. 2013;288:9957–9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-coa synthetase 1. Proc Natl Acad Sci U S A. 2012;109:E715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang YL, Morales-Rosado J, Ray J, Myers TG, Kho T, Lu M, Munford RS. Toll-like receptor agonists promote prolonged triglyceride storage in macrophages. J Biol Chem. 2014;289:3001–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalugotla G, He L, Weber KJ, Daemen S, Reller A, Razani B, Schilling JD. Frontline science: Acyl-coa synthetase 1 exacerbates lipotoxic inflammasome activation in primary macrophages. J Leukoc Biol. 2019;106:803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y, Yang M, Huang W, et al. Mitochondrial metabolic reprogramming by cd36 signaling drives macrophage inflammatory responses. Circ Res. 2019;125:1087–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruiz M, Bodhicharla R, Stahlman M, Svensk E, Busayavalasa K, Palmgren H, Ruhanen H, Boren J, Pilon M. Evolutionarily conserved long-chain acyl-coa synthetases regulate membrane composition and fluidity. Elife. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, Ben-Sahra I, Gius DR, Yvan-Charvet L, Chandel NS, Schumacker PT, Thorp EB. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019;29:443–456 e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang L, Gao L, Nickel T, Yang J, Zhou J, Gilbertsen A, Geng Z, Johnson C, Young B, Henke C, Gourley GR, Zhang J. Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ Res. 2017;121:1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haas R, Cucchi D, Smith J, Pucino V, Macdougall CE, Mauro C. Intermediates of metabolism: From bystanders to signalling molecules. Trends Biochem Sci. 2016;41:460–471. [DOI] [PubMed] [Google Scholar]
- 94.Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, Schwarzler C, Junt T, Voshol H, Meingassner JG, Mao X, Werner G, Rot A, Carballido JM. Triggering the succinate receptor gpr91 on dendritic cells enhances immunity. Nat Immunol. 2008;9:1261–1269. [DOI] [PubMed] [Google Scholar]
- 95.He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan g-protein-coupled receptors. Nature. 2004;429:188–193. [DOI] [PubMed] [Google Scholar]
- 96.Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. [DOI] [PubMed] [Google Scholar]
- 97.Yurdagul A Jr., Doran AC, Cai B, Fredman G, Tabas IA. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front Cardiovasc Med. 2017;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Linton MF, Babaev VR, Huang J, Linton EF, Tao H, Yancey PG. Macrophage apoptosis and efferocytosis in the pathogenesis of atherosclerosis. Circ J. 2016;80:2259–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kinchen JM, Doukoumetzidis K, Almendinger J, Stergiou L, Tosello-Trampont A, Sifri CD, Hengartner MO, Ravichandran KS. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat Cell Biol. 2008;10:556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. Microtubule-associated protein 1 light chain 3 alpha (lc3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A. 2011;108:17396–17401. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Appelqvist H, Waster P, Kagedal K, Ollinger K. The lysosome: From waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5:214–226. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, Subramanian M, Yurdagul A Jr., Barbosa-Lorenzi VC, Cai B, de Juan-Sanz J, Ryan TA, Nomura M, Maxfield FR, Tabas I. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. 2017;171:331–345 e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kasikara C, Doran AC, Cai B, Tabas I. The role of non-resolving inflammation in atherosclerosis. J Clin Invest. 2018;128:2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tabas I Cholesterol in health and disease. J Clin Invest. 2002;110:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cui D, Thorp E, Li Y, Wang N, Yvan-Charvet L, Tall AR, Tabas I. Pivotal advance: Macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol. 2007;82:1040–1050. [DOI] [PubMed] [Google Scholar]
- 108.Viaud M, Ivanov S, Vujic N, et al. Lysosomal cholesterol hydrolysis couples efferocytosis to anti-inflammatory oxysterol production. Circ Res. 2018;122:1369–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xian X, Ding Y, Dieckmann M, Zhou L, Plattner F, Liu M, Parks JS, Hammer RE, Boucher P, Tsai S, Herz J. Lrp1 integrates murine macrophage cholesterol homeostasis and inflammatory responses in atherosclerosis. Elife. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fond AM, Lee CS, Schulman IG, Kiss RS, Ravichandran KS. Apoptotic cells trigger a membrane-initiated pathway to increase abca1. J Clin Invest. 2015;125:2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li AC, Glass CK. Ppar- and lxr-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J Lipid Res. 2004;45:2161–2173. [DOI] [PubMed] [Google Scholar]
- 112.Mueller PA, Zhu L, Tavori H, Huynh K, Giunzioni I, Stafford JM, Linton MF, Fazio S. Deletion of macrophage low-density lipoprotein receptor-related protein 1 (lrp1) accelerates atherosclerosis regression and increases c-c chemokine receptor type 7 (ccr7) expression in plaque macrophages. Circulation. 2018;138:1850–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Du H, Grabowski GA. Lysosomal acid lipase and atherosclerosis. Curr Opin Lipidol. 2004;15:539–544. [DOI] [PubMed] [Google Scholar]
- 114.Evans TD, Zhang X, Clark RE, Alisio A, Song E, Zhang H, Reilly MP, Stitziel NO, Razani B. Functional characterization of lipa (lysosomal acid lipase) variants associated with coronary artery disease. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119313443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park D, Han CZ, Elliott MR, Kinchen JM, Trampont PC, Das S, Collins S, Lysiak JJ, Hoehn KL, Ravichandran KS. Continued clearance of apoptotic cells critically depends on the phagocyte ucp2 protein. Nature. 2011;477:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. [DOI] [PubMed] [Google Scholar]
- 117.Lemke G, Rothlin CV. Immunobiology of the tam receptors. Nat Rev Immunol. 2008;8:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving tgf-beta, pge2, and paf. J Clin Invest. 1998;101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin CS, Spite M, Fredman G, Tabas I. Mertk cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A. 2016;113:6526–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grebe A, Hoss F, Latz E. Nlrp3 inflammasome and the il-1 pathway in atherosclerosis. Circ Res. 2018;122:1722–1740. [DOI] [PubMed] [Google Scholar]
- 122.Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ogden CA, Pound JD, Batth BK, Owens S, Johannessen I, Wood K, Gregory CD. Enhanced apoptotic cell clearance capacity and b cell survival factor production by il-10-activated macrophages: Implications for burkitt's lymphoma. J Immunol. 2005;174:3015–3023. [DOI] [PubMed] [Google Scholar]
- 124.Freemerman AJ, Zhao L, Pingili AK, et al. Myeloid slc2a1-deficient murine model revealed macrophage activation and metabolic phenotype are fueled by glut1. J Immunol. 2019;202:1265–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yurdagul A Jr., Subramanian M, Wang X, Crown SB, Ilkayeva O, Darville L, Kolluru GK, Rymond CC, Zheng Z, Kuriakose G, Kevil CG, Koomen JM, Cleveland JL, Muoio DM, Tabas I. Metabolism of apoptotic cell-derived arginine and ornithine into putrescine by macrophages promotes continual efferocytosis. Cell Metab. 2020;In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tawakol A, Singh P, Mojena M, et al. Hif-1alpha and pfkfb3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler Thromb Vasc Biol. 2015;35:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kanter JE, Shao B, Kramer F, et al. Increased apolipoprotein c3 drives cardiovascular risk in type 1 diabetes. J Clin Invest. 2019;130:4165–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Basu A, Bebu I, Jenkins AJ, Stoner JA, Zhang Y, Klein RL, Lopes-Virella MF, Garvey WT, Budoff MJ, Alaupovic P, Lyons TJ, Diabetes Control Complications Trial/Epidemiology of Diabetes Interventions Complications Research G. Serum apolipoproteins and apolipoprotein-defined lipoprotein subclasses: A hypothesis-generating prospective study of cardiovascular events in t1d. J Lipid Res. 2019;60:1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller A, Nagy C, Knapp B, Laengle J, Ponweiser E, Groeger M, Starkl P, Bergmann M, Wagner O, Haschemi A. Exploring metabolic configurations of single cells within complex tissue microenvironments. Cell Metab. 2017;26:788–800 e786. [DOI] [PubMed] [Google Scholar]
- 131.Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, Ramsey SA, Fisher EA, Loke P. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Winkels H, Ehinger E, Vassallo M, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell rna-sequencing and mass cytometry. Circ Res. 2018;122:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim K, Shim D, Lee JS, et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ Res. 2018;123:1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A. Single-cell rna-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122:1661–1674. [DOI] [PubMed] [Google Scholar]
- 135.Fernandez DM, Rahman AH, Fernandez NF, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25:1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun C, Li T, Song X, et al. Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proc Natl Acad Sci U S A. 2019;116:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: A method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37:364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blaser MC, Aikawa E. Roles and regulation of extracellular vesicles in cardiovascular mineral metabolism. Front Cardiovasc Med. 2018;5:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burke AC, Telford DE, Huff MW. Bempedoic acid: Effects on lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol. 2019;30:1–9. [DOI] [PubMed] [Google Scholar]
- 142.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science. 2013;339:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren B, Van Kampen E, Van Berkel TJ, Cruickshank SM, Van Eck M. Hematopoietic arginase 1 deficiency results in decreased leukocytosis and increased foam cell formation but does not affect atherosclerosis. Atherosclerosis. 2017;256:35–46. [DOI] [PubMed] [Google Scholar]
- 144.Xiong H, Zhu C, Li F, Hegazi R, He K, Babyatsky M, Bauer AJ, Plevy SE. Inhibition of interleukin-12 p40 transcription and nf-kappab activation by nitric oxide in murine macrophages and dendritic cells. J Biol Chem. 2004;279:10776–10783. [DOI] [PubMed] [Google Scholar]
- 145.Ponnuswamy P, Ostermeier E, Schrottle A, Chen J, Huang PL, Ertl G, Nieswandt B, Kuhlencordt PJ. Oxidative stress and compartment of gene expression determine proatherosclerotic effects of inducible nitric oxide synthase. Am J Pathol. 2009;174:2400–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schneider JG, Yang Z, Chakravarthy MV, Lodhi IJ, Wei X, Turk J, Semenkovich CF. Macrophage fatty-acid synthase deficiency decreases diet-induced atherosclerosis. J Biol Chem. 2010;285:23398–23409. [DOI] [PMC free article] [PubMed] [Google Scholar]