Abstract
Expanded RNA repeats cause >30 incurable diseases. One approach to mitigate their toxicity is by using small molecules that assemble into potent, oligomeric species upon binding to the disease-causing RNA in cells. Herein, we show that the expanded repeat [r(CUG)exp] that causes myotonic dystrophy type 1 (DM1) catalyzes the in situ synthesis of its own inhibitor using an RNA-templated tetrazine ligation in DM1 patient-derived cells. The compound synthesized on-site improved DM1-associated defects at picomolar concentrations, enhancing potency by 10,000-fold, compared to its parent compounds that cannot undergo oligomerization. A fluorogenic reaction is also described where r(CUG)exp templates the synthesis of its own imaging probe to enable visualization of the repeat in its native context in live cells and muscle tissue.
Graphical Abstract

RNA is an important target of small molecules, causing or contributing to disease by diverse pathomechanisms. Myotonic dystrophy type 1 (DM1), the most common form of adult onset muscular dystrophy, is caused by a CTG repeat expansion ranging from 75 to several thousand repeats in the 3’ untranslated region (UTR) of the dystrophia myotonica protein kinase (DMPK) gene.1 This CTG repeat is transcribed into r(CUG)exp and forms a structure containing repeating 1×1 nucleotide U/U internal loops (Fig. 1A). These loops binds and sequester the pre-mRNA splicing regulator muscleblind-like 1 (MBNL1) in nuclear foci,2 causing mis-regulation of alternative splicing events (Figs. 1A & B).3, 4 Small molecules that improve DM1-associated defects have various modes of action, including binding to r(CUG)exp,5-7 cleaving r(CUG)exp,8, 9 inhibiting transcription of r(CUG)exp,10 and mixed modes of inhibition.11
Figure 1.
A r(CUG)exp-templated tetrazine ligation. (A) DM1 is caused by r(CUG)exp in the 3’ UTR of DMPK mRNA, which sequesters MBNL1, resulting in nuclear foci and alternative pre-mRNA splicing defects. (B) MBNL1 protein regulates the splicing of its own pre-mRNA. In DM1-affected cells, MBNL1 exon 5 is included too frequently due to its sequestration of MBNL1 by r(CUG)exp. (C) Scheme of r(CUG)exp-catalyzed tetrazine ligation, where multivalent compounds are synthesized upon binding to the RNA to improve DM1-associated defects.
Due to the repeating nature of r(CUG)exp, compounds that occupy multiple U/U loops simultaneously bind with higher affinity and improve DM1-associated defects more potently than compounds that only bind one site (monomeric).12 As the valency and hence molecular weight of modularly assembled compounds increases, cellular permeability can be negatively affected13 and limit bioactivity.14 One way to overcome this liability is to assemble multivalent compounds in situ.5 In situ click chemistry using azide and alkyne reactive handles has been used to synthesize in vitro inhibitors of acetylcholine esterase15 and DNA-binding polyamides16 and cellular inhibitors of r(CUG)exp and r(CCUG)exp, where an oligomeric therapeutic is only formed in disease-affected cells that express the RNA repeat expansion.5, 17 Herein, we have developed a superior r(CUG)exp-templated bioorthogonal reaction using a tetrazine ligation (Fig. 1C). Not only did the r(CUG)exp-templated tetrazine ligation afford more rapid formation of product resulting in a more rapid improvement of DM1-associated defects in cells, but it also enabled an approach to monitor reaction progress and image r(CUG)exp in its native context through the creation of a fluorogenic product.
Results and Discussion:
Bioorthogonal reactions are key to the development of in situ synthesis and can be tuned to control the rate and extent of biomolecule-catalyzed reactions. The tetrazine ligation is a rapid bioorthogonal reaction between 1,2,4,5-tetrazines and various dienophiles18 including cyclopropenes 19, 20 that have been used for oligonucleotide-templated reactions.21 To investigate the ability of tetrazine and cyclopropene to undergo a r(CUG)exp-templated reaction, we employed a small molecule that selectively recognizes r(CUG)exp’s 3D structure with nanomolar affinity and improves DM1-associated defects.5 The compound consists of a peptoid backbone spacer with two RNA-binding modules that each bind a 1×1 U/U internal loop.5 This dimer was appended with: (i) methyl tetrazine; and/or (ii) cyclopropene containing C-1 and C-2 methyl groups and a C-3 amide, as these substituents slow reaction rate and can eliminate reaction in the absence of r(CUG)exp (Fig. 2).19
Figure 2.
Structures of compounds that bind r(CUG)exp and undergo a r(CUG)exp-templated tetrazine ligation; R (purple sphere) indicates the RNA-binding module (top structure).
To determine the optimal length between reactive handles for an efficient and selective r(CUG)exp-templated reaction, compounds 1-4 (tetrazine-containing) were screened for their ability to react with 5-6 (cyclopropene-containing) in the presence of r(CUG)12 in vitro, but not in its absence, using mass spectrometry. [Note: in vitro r(CUG)12 forms the same structure as r(CUG)exp.] Mass spectrometry analysis was performed at various timepoints to identify the combination of linkers that began to form product most rapidly in the presence of r(CUG)12. The optimal combination, 2 and 6 (Fig. 2), formed product in 1 h, while product formation with shorter or longer linkers was detectable at 4 – 8 h (Fig. 3A). In the presence of r(CUG)12, reaction of 2 and 6 proceeded such that ~25% was converted to tetramer after 24 h (Figs. 3B and S1). In contrast, no product was formed when incubated with GC-paired RNA [r(GC)8], and a negligible amount (< 2%) was observed in the absence of RNA (Figs. 3B and S1). Thus, r(CUG)12 can selectively template the reaction of binding modules containing tetrazine and cyclopropene in vitro. Notably, for all combinations of 1-4 and 5-6, a significant improvement in reaction rate was observed as compared to our previously studied azide and alkyne reaction that formed detectable r(CUG)-templated product after 24 h.5
Figure 3.
The r(CUG)exp-templated reaction in vitro. (A) Monitoring product formation in the presence of r(CUG)12 via mass spectrometry revealed that 2 & 6 formed a tetramer most rapidly. The y-axis indicates the time-point at which product was first observed. (B) Quantification of reaction of 2 & 6 in the presence of r(CUG)12, r(GC)8, and buffer (n = 3). Error bars represent SD; *** P < 0.001, one-way ANOVA relative to samples in buffer. (C) Schematic of r(CUG)exp-templated fluorogenic tetrazine ligation where fluorescein (F) in compound 8 is quenched by the neighboring tetrazine. Upon reaction with 6, in the presence of r(CUG)exp, FAM fluorescence increased. (D) Enhancement of fluorescence was observed when 6 and 8 were co-incubated with r(CUG)12 but not r(GC)8 (n = 5). Error bars represent SD; * P < 0.05, ** P < 0.01, *** P < 0.001, Student t-test relative to r(GC)8 samples.
As an alternate way to monitor r(CUG)12-templated reactions in vitro, we developed a fluorogenic RNA-templated tetrazine ligation. Tetrazine quenches the fluorescence of green-emitting dyes in close proximity.18, 22 Thus, we synthesized compound 8 where fluorescein (FAM) is quenched until reaction with 6, as templated by r(CUG)exp (Figs. 3C & S2). Indeed, a significant increase in fluorescence was observed upon reaction with 6 in the presence of r(CUG)12 but not r(GC)8 (Fig. 3D & S3).
Importantly, the RNA-templated fluorogenic click reaction occurred in situ, enabling reaction monitoring and imaging of r(CUG)exp in living cells. DM1-patient derived fibroblasts expressing r(CUG)500 or wild-type fibroblasts expressing r(CUG)20 (non-pathological number of repeats) were treated with 6 and 8, and fluorescence was monitored using live cell microscopy. After 6 h, an increase in fluorescence was observed in the nucleus and cytoplasm of r(CUG)500 cells (Figs. 4A & S4). Fluorescence continued to increase over 24 h, however the tetramer that formed was trafficked to the lysosome over time, as determined by co-staining with Lysotracker (Figs. S2 & S5). Importantly, no significant increase in fluorescence was observed in wild-type fibroblasts treated with 6 and 8 or in fibroblasts treated with 8 alone (Figs. 4A & S4). Thus, reaction between 6 and 8 was specific for the mutant, disease-driving DMPK allele containing r(CUG)exp.
Figure 4.
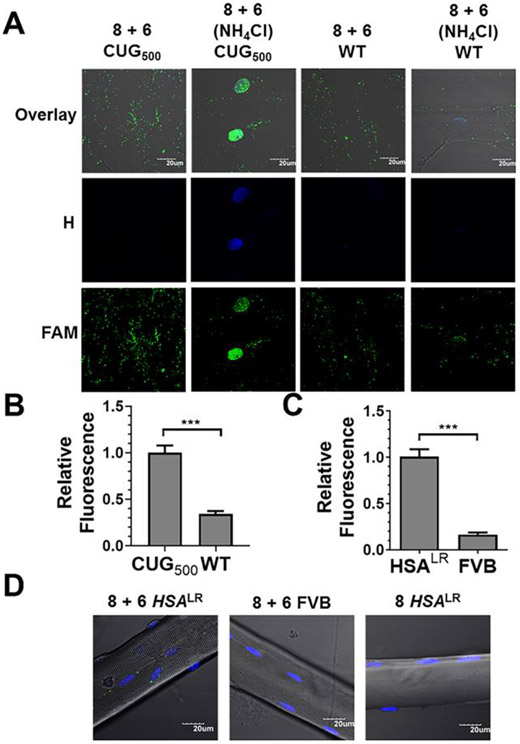
The r(CUG)exp-templated fluorogenic tetrazine ligation to image the RNA. (A) Representative images of r(CUG)500 and wild-type cells treated with 8 + 6 in the presence or absence of NH4Cl to prevent lysosomal trafficking. (B) Quantification of relative fluorescence intensity in r(CUG)500 and wild-type cells treated with 8 + 6. (C) Quantification of relative fluorescence intensity in HSALR and FVB (wild-type) myofibers. (D) Representative images of HSALR and FVB myofibers treated with 8 + 6 or 8 alone. For all panels, n = 5; 8 images quantified/replicate. Error bars represent SEM; *** P < 0.001, as determined by a Student t-test.
To confirm that the compound synthesized on-site was trafficked to the lysosome, we used trans-cyclooctene (TCO) and a dimer containing TCO (9; Fig. S6), both of which react rapidly with tetrazine in the absence of r(CUG)exp (reaction products provided in Fig. S5). DM1 fibroblasts were treated with 8 for 12 h followed by washing and treatment with TCO or 9 for 4 h. Cells treated with 8 and TCO showed fluorescence in the nucleus, whereas cells that formed tetramer by treating with 8 and 9 showed fluorescence in the lysosome, supporting that only the tetrameric compound is trafficked to the lysosome, not 8 itself (Fig. S6). In order to prevent lysosomal trafficking and to quantify the increase in fluorescence, fibroblasts were treated with NH4Cl for 1 h prior to imaging to raise the pH of the lysosome, thereby inhibiting lysosomal accumulation of small molecules.23, 24 After 6 h, a robust increase in fluorescence was visible in the nuclei of r(CUG)500 cells where the RNA is sequestered in foci (Fig. 4A & B). Importantly, fluorescence was significantly less in wild-type cells (Fig. 4A & B). Thus, fluorescence turn-on from the reaction of 8 with 6 was only seen in the presence of r(CUG)exp and can be used to monitor reaction progress and to image r(CUG)exp in live, patient-derived cells.
To image r(CUG)exp in a more complex system, muscle fibers from the extensor digitoris longus (EDL) muscle of DM1 (HSALR transgenic mice with 250 CTG repeats25) and FVB (wild-type) mice were isolated and cultured. Myofibers were treated with 6 and 8 for 48 h followed by live fiber imaging. In DM1 myofibers, a significant increase in fluorescence was observed, indicating templated reaction by r(CUG)250 (Fig. 4C & D). In contrast, no fluorescence was produced when DM1 myofibers were treated with 8 alone or in wild-type myofibers treated with 6 and 8 (Figs. 4C & D).
The r(CUG)exp-templated fluorogenic tetrazine ligation enabled imaging in live cells and myofibers, a significant advantage over traditional methods such as fluorescence in situ hybridization (FISH)26 that is completed in fixed samples. Furthermore, this reaction allows for RNA imaging in its native context without exogenous tags that aptamer-based RNA imaging systems require.27 Imaging r(CUG)exp with a freely cell permeable small molecules also offers improvement over Cas9-based imaging methods which require transfection of the Cas9-imaging probe and appendage of a protein.28 Indeed, the fluorogenic reaction afforded an imaging probe with minimal background fluorescence, as turn-on was only observed upon templating by r(CUG)exp.
We next assessed if an r(CUG)exp-templated tetrazine ligation improved DM1-associated defects, including the presence of r(CUG)exp-MBNL1 nuclear foci,2 reduced nucleocytoplasmic transport of DMPK mRNA,29 and mis-splicing of a pre-mRNA that is regulated by MBNL1 (Figs. 1A & B).4,30 In situ synthesis of multivalent compounds from cell permeable fragments, templated by r(CUG)exp, overcomes limitations in potency caused by decreased permeability of larger multivalent compounds.13, 14 Here, 6 localized in the nucleus of cells, however, the tetramer formed between 6 and 2 was less cell permeable and localized in the cytoplasm (Fig. S7).
To evaluate bioactivity of a compound capable of oligomerizing upon binding to r(CUG)exp, we synthesized and tested compound 7, which has both tetrazine and cyclopropene functionalities (Fig. 2). In vitro, 7 reacted once when incubated with r(CUG)12, as expected since r(CUG)12 displays five consecutive U/U loops and thus only contains two binding sites for 7 (Figs. 5A & S8). In the presence of r(CUG)24, which displays 11 U/U loops, 7 reacted twice, suggesting that oligomerization can occur with longer r(CUG) repeats (Figs. 5A & S9). In DM1 fibroblasts, 7 significantly reduced r(CUG)exp-MBNL1-containing nuclear foci and did so to a greater extent than 2 or 6 (Figs. 5B & C). Reduction in nuclear foci liberates MBNL1 to resume its normal function, suggesting that 7 can improve DM1-associated splicing defects. Indeed, treatment with as little as 100 pM of 7 rescued the MBNL1 exon 5 splicing defect, as determined by RT-PCR and gel electrophoresis analysis (Figs. 5D and S10). Notably, 2 and 6 only improved splicing defects at 1 μM. Therefore, on-site synthesis enhanced potency by 10,000-fold (Fig. 5D). This improvement was confirmed by RT-qPCR (Fig. S10). Importantly, total MBNL1 mRNA levels were not affected, thus the decrease of MBNL1 exon 5 abundance upon treatment with 7 was due to improvement of pre-mRNA splicing defects (Fig. S10). None of the compounds affected MBNL1 exon 5 splicing in wild-type cells (Fig. S11) nor a non-MBNL1 regulated splicing event, MAP4K4 exon 22a inclusion (Fig. S12), indicating that the compounds specifically improve DM1-associated defects. The levels of DMPK mRNA were also not affected by compound treatment (Fig. S13). Collectively, 7 is one of the most potent compounds known to target RNA to date.
Figure 5.
Activity of 7 in vitro and in DM1 patient-derived cells. (A) Compound 7 reacted once in the presence of r(CUG)12 (left) and twice in the presence of r(CUG)24 (right). Full mass spectra are shown in Figs. S8 & S9. (B) Representative images from RNA-FISH and MBNL1 immunostaining in DM1 fibroblasts to image nuclear foci. (C) Quantification of nuclear foci in cells treated with 2, 6, and 7. n = 3; 40 cells counted/replicate. (D) Ability of 2, 6, and 7 to rescue MBNL1 exon 5 splicing defect in DM1 fibroblasts (n = 6). (E) Ability of 2, 6, and 7 to rescue reduced nucleocytoplasmic transport of a luciferase gene harboring r(CUG)800 in its 3’ UTR (n = 5). For all panels, error bars represent SD; * P < 0.05, ** P < 0.01, *** P < 0.001, as determined by one-way ANOVA relative to untreated samples.
Nucleocytoplasmic transport of DMPK is also de-regulated in DM1, as r(CUG)exp is retained in nuclear foci.29 In C2C12 cells stably expressing luciferase harboring r(CUG)800 in its 3’ UTR,14 7 increased luciferase translation at concentrations as low as 100 pM, 100 times more potently than 2 or 6 (Fig. 5E). This increase was specific to r(CUG)exp as luminescence was not affected in cells without r(CUG)exp (Fig. 5E). Thus, through a r(CUG)exp-templated oligomerization, 7 specifically improved multiple DM1-associated defects 100-10,000 times more potently than 2 and 6. When compared to previously used r(CUG)exp-templated azide-alkyne chemistry5 (Compound 10, Fig. S14), 7 more rapidly improved splicing defects, with ~30% improvement observed at 100 pM after 24 h, while 10 which undergoes RNA-templated reaction more slowly, had no effect at this time-point (Fig. S14). After 48 h, the cellular activity of both r(CUG)exp-templated chemistry approaches was comparable, with improvement observed at 100 pM for both 7 and 10 (Fig. S15).5
In conclusion, we have shown that r(CUG)exp templates the synthesis of its own inhibitor in vitro and in cellulis using a rapid tetrazine ligation. The templated reaction forms product in only 1 h in vitro, which is a significant rate enhancement over previous RNA-templated reactions with small molecules.5 Furthermore, this reaction was developed into a fluorogenic variant that allows for detection of click reaction and imaging of r(CUG)exp in live, patient-derived cells and DM1 mouse myofibers. A compound capable of oligomerizing upon binding to r(CUG)exp resulted in the in cellulis synthesis of a therapeutic that is 100-10,000 times more potent than compounds that cannot undergo a r(CUG)exp-templated reaction. These studies lay a foundation to image RNA in its native context broadly and to deliver precision therapeutics for RNA, which is considered very challenging to target with small molecules.
Methods:
A detailed description of methods can be found in Supporting Information. HSALR and WT (FVB) mice used for harvesting myofibers were housed in the Scripps Florida vivarium, and all experiments using live animals were approved by the Scripps Florida Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgment.
This work was funded by the US National Institutes of Health (grant DP1 NS096898 to M.D.D. and grant F31 NS110269 to A.J.A.). We thank J. Childs-Disney for editing the manuscript and S. Rzuczek for experimental advice.
Footnotes
Supporting Information. The supporting information contains experimental procedures, synthetic methods, and all supporting figures.
M.D.D. is a founder of Expansion Therapeutics.
References:
- (1).Brook JD; McCurrach ME; Harley HG; Buckler AJ; Church D; Aburatani H; Hunter K; Stanton VP; Thirion JP; Hudson T; et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 1992, 68, 799–808. [DOI] [PubMed] [Google Scholar]
- (2).Taneja KL; McCurrach M; Schalling M; Housman D; Singer RH Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell. Biol 1995, 128, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jiang H; Mankodi A; Swanson MS; Moxley RT; Thornton CA Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum. Mol. Genet 2004, 13, 3079–88.. [DOI] [PubMed] [Google Scholar]
- (4).Nakamori M; Sobczak K; Puwanant A; Welle S; Eichinger K; Pandya S; Dekdebrun J; Heatwole CR; McDermott MP; Chen T; Cline M; Tawil R; Osborne RJ; Wheeler TM; Swanson M; Moxley RT; Thornton CA Splicing biomarkers of disease severity in myotonic dystrophy. Ann. Neurol 2013, 74, 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Rzuczek SG; Colgan LA; Nakai Y; Cameron MD; Furling D; Yasuda R; Disney MD Precise small-molecule recognition of a toxic CUG RNA repeat expansion. Nat. Chem. Biol 2017, 13, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Nakamori M; Taylor K; Mochizuki H; Sobczak K; Takahashi MP Oral administration of erythromycin decreases RNA toxicity in myotonic dystrophy. Ann. Clin. Transl. Neurol 2016, 3, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Li J; Nakamori M; Matsumoto J; Murata A; Dohno C; Kiliszek A; Taylor K; Sobczak K; Nakatani K A Dimeric 2,9-Diamino-1,10-phenanthroline derivative improves alternative splicing in myotonic dystrophy type 1 cell and mouse models. Chemistry 2018, 24, 18115–18122. [DOI] [PubMed] [Google Scholar]
- (8).Angelbello AJ; Rzuczek SG; Mckee KK; Chen JL; Olafson H; Cameron MD; Moss WN; Wang ET; Disney MD Precise small-molecule cleavage of an r(CUG) repeat expansion in a myotonic dystrophy mouse model. Proc. Natl. Acad. Sci. U.S.A 2019, 116, 7799–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Guan L; Disney MD Small-molecule-mediated cleavage of RNA in living cells. Angew. Chem. Int. Ed. Engl 2013, 52, 1462–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Siboni RB; Nakamori M; Wagner SD; Struck AJ; Coonrod LA; Harriott SA; Cass DM; Tanner MK; Berglund JA Actinomycin D specifically reduces expanded CUG repeat RNA in myotonic dystrophy models. Cell Rep. 2015, 13, 2386–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lee J; Bai Y; Chembazhi UV; Peng S; Yum K; Luu LM; Hagler LD; Serrano JF; Chan HYE; Kalsotra A; Zimmerman SC Intrinsically cell-penetrating multivalent and multitargeting ligands for myotonic dystrophy type 1. Proc. Natl. Acad. Sci. U.S.A 2019, 116, 8709–8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lee MM; Childs-Disney JL; Pushechnikov A; French JM; Sobczak K; Thornton CA; Disney MD Controlling the specificity of modularly assembled small molecules for RNA via ligand module spacing: targeting the RNAs that cause myotonic muscular dystrophy. J. Am. Chem. Soc 2009, 131, 17464–17472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Childs-Disney JL; Hoskins J; Rzuczek SG; Thornton CA; Disney MD Rationally designed small molecules targeting the RNA that causes myotonic dystrophy type 1 are potently bioactive. ACS Chem. Biol 2012, 7, 856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Childs-Disney JL; Parkesh R; Nakamori M; Thornton CA; Disney MD Rational design of bioactive, modularly assembled aminoglycosides targeting the RNA that causes myotonic dystrophy type 1. ACS Chem. Biol 2012, 7, 1984–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lewis WG; Green LG; Grynszpan F; Radic Z; Carlier PR; Taylor P; Finn MG; Sharpless KB Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew. Chem. Int. Ed. Engl 2002, 41, 1053–7. [DOI] [PubMed] [Google Scholar]
- (16).Poulin-Kerstien AT; Dervan PB DNA-templated dimerization of hairpin polyamides. J. Am. Chem. Soc 2003, 125, 15811–15821. [DOI] [PubMed] [Google Scholar]
- (17).Rzuczek SG; Park H; Disney MD A Toxic RNA catalyzes the in cellulo synthesis of its own inhibitor. Angew. Chem. Int. Ed. Engl 2014, 53, 10956–10959. [DOI] [PubMed] [Google Scholar]
- (18).Oliveira BL; Guo Z; Bernardes GJL Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev 2017, 46, 4895–4950. [DOI] [PubMed] [Google Scholar]
- (19).Patterson DM; Nazarova LA; Xie B; Kamber DN; Prescher JA Functionalized cyclopropenes as bioorthogonal chemical reporters. J. Am. Chem. Soc 2012, 134, 18638–18643. [DOI] [PubMed] [Google Scholar]
- (20).Yang J; Šečkutė J; Cole CM; Devaraj NK Live-Cell imaging of cyclopropene tags with fluorogenic tetrazine cycloadditions. Angew. Chem. Int. Ed. Engl 2012, 51, 7476–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Seckute J; Yang J; Devaraj NK Rapid oligonucleotide-templated fluorogenic tetrazine ligations. Nucleic Acids Res. 2013, 41, e148–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Devaraj NK; Hilderbrand S; Upadhyay R; Mazitschek R; Weissleder R Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew. Chem. Int. Ed. Engl 2010, 49, 2869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ohkuma S; Poole B Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci 1978, 75, 3327–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Daniel WA; Wójcikowski J Lysosomal trapping as an important mechanism involved in the cellular distribution of perazine and in pharmacokinetic interaction with antidepressants. Eur. Neuropsychopharmacol 1999, 9, 483–491. [DOI] [PubMed] [Google Scholar]
- (25).Mankodi A; Logigian E; Callahan L; McClain C; White R; Henderson D; Krym M; Thornton CA Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 2000, 289, 1769–73. [DOI] [PubMed] [Google Scholar]
- (26).Femino AM; Fay FS; Fogarty K; Singer RH Visualization of single RNA transcripts in situ. Science 1998, 280, 585–90. [DOI] [PubMed] [Google Scholar]
- (27).Paige JS; Wu KY; Jaffrey SR RNA mimics of green fluorescent protein. Science 2011, 333, 642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Nelles DA; Fang MY; O'Connell MR; Xu JL; Markmiller SJ; Doudna JA; Yeo GW Programmable RNA tracking in live cells with CRISPR/Cas9. Cell 2016, 165, 488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Mastroyiannopoulos NP; Feldman ML; Uney JB; Mahadevan MS; Phylactou LA Woodchuck post-transcriptional element induces nuclear export of myotonic dystrophy 3' untranslated region transcripts. EMBO Rep. 2005, 6, 458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Gates DP; Coonrod LA; Berglund JA Autoregulated splicing of muscleblind-like 1 (MBNL1) pre-mRNA. J. Biol. Chem 2011, 286, 34224–34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.