Abstract
Because of microbicide non-compliance and lack of a durable, highly effective vaccine, a combined approach might improve HIV prophylaxis. We tested whether a vaccine-microbicide combination would enhance protection against SIV infection in rhesus macaques. Four macaque groups included: vaccine only, vaccine-microbicide, microbicide only and controls. Vaccine groups were primed twice mucosally with replicating Adenovirus type 5 host range mutant (Ad5hr) SIV env/rev, gag, and nef recombinants and boosted twice intramuscularly with SIV gp120 proteins in alum. Controls and the microbicide only group received Ad5hr empty vector and alum. The microbicide was SAMT-247, a 2-mercaptobenzamide thioester which targets the viral nucleocapsid protein NCp7, causing zinc ejection and preventing RNA encapsidation. Following vaccination, macaques were challenged intravaginally with repeated weekly low-doses of SIVmac251 administered 3 hours after application of 0.8% SAMT-247 gel (vaccine-microbicide and microbicide groups) or placebo gel (vaccine only and control groups). The microbicide only group exhibited potent protection: 10 of 12 macaques remained uninfected following 15 SIV challenges. The vaccine only group developed strong mucosal and systemic humoral and cellular immunity but did not exhibit delayed acquisition compared to adjuvant controls. However, the vaccine-microbicide group exhibited significant acquisition delay compared to both control and vaccine only groups indicating further exploration of the combination strategy is warranted. Impaired protection in the vaccine-microbicide group compared to the microbicide only group was not attributed to a vaccine-induced increase in SIV target cells. Possible antibody-dependent enhancement will be further investigated. The potent protection provided by SAMT-247 encourages its movement into human clinical trials.
Keywords: HIV/SIV vaccine, microbicide, nucleocapsid inhibitor, rhesus macaque
INTRODUCTION
Despite the great variety of anti-HIV-1 chemotherapeutic agents developed in the past decades, there remains no cure for AIDS. Thus, effective methods for preventing HIV infection are still necessary for control of the AIDS pandemic. In 2016 women made up 19% of new HIV diagnoses in the United States and each year account for almost 50% of newly infected patients worldwide (1). Moreover, young women in developing countries still bear the brunt of unacceptably high HIV-1 prevalence as a consequence of biological, behavioral, socioeconomic and cultural factors (2, 3). Women commonly acquire HIV through sex with an HIV infected male partner. As no current vaccines have shown long lasting efficacy, it is widely believed that preventing viral transmission by use of a vaginal microbicide applied topically or contained in a vaginal ring for sustained protection will be key to preventing heterosexual transmission of HIV to women in the immediate future (4).
Topical microbicide candidates include CCR5 inhibitors (5–7), non-nucleotide reverse transcriptase inhibitors (NNRTIs) (8, 9) and nucleotide reserve transcriptase inhibitors (NRTIs) (10, 11). Resistance to CCR5 inhibitors has been well documented, as envelope mutations result in the ability of the virus to use CCR5 in an inhibitor-insensitive manner or expansion of pre-existing CXCR4-using viruses (12, 13). CXCR4-using variants are not targeted by CCR5 blocking compounds (14). NNRTI and NRTI inhibitors also face difficulties in preventing infection by strains of HIV carrying drug resistance mutations (15, 16). Due to these limitations, development of alternative microbicides is important. A novel microbicide target is the viral nucleocapsid protein, NCp7. It is formed by the basic gag-polyprotein domain, which contains two copies of the retroviral zinc finger motif C-X2-C-X4-H-X4-C delimited by highly conserved residues within HIV-1 subtypes (17, 18). Anti-HIV compounds known as 2-mercaptobenzamide thioesters (SAMTs) can cause zinc ejection which inactivates the nucleocapsid and leads to production of immature viral particles (19). SAMTs have been shown to retain efficacy in the presence of cervical mucus and to lack toxicity to human cervical tissue, expanding their potential clinical applicability (20). One analogue of SAMT termed SAMT-247 showed efficacy in inhibiting several strains of HIV, including multidrug resistant isolates in vitro (21). Moreover, SAMT-247 prevented infection of 5 of 6 female rhesus macaques by a mixed R5/X4 vaginal SHIV challenge suggesting further development was warranted (22).
While prophylactic vaccines have been considered the best strategy for controlling the HIV pandemic, combined implementation of vaccination and vaginal microbicide as preexposure prophylaxis (PrEP) have suggested improved defense against SIV or SHIV exposures (7, 23). Delayed SHIV acquisition and viremia control were also obtained by combined SIVGag/Pol DNA prime/Ad5gag/pol boost immunization and vaginal administration of a 0.1% SAMT-247 gel formulation prior to vaginal SHIV challenge (24). The lack of an Env component in the vaccine regimen may have limited the overall efficacy as correlates of vaccine-induced protection observed in the modestly protective RV144 HIV vaccine trial in Thailand showed the importance of antibody responses targeting the envelope protein gp120 (25–27). We reasoned that our vaccine regimen based on mucosal priming with replication-competent adenovirus type 5 host range mutant (Ad5hr) SIV recombinants followed by systemic gp120 boosting would provide a good alternative to the SIVgag/pol DNA vaccine as it has consistently induced B cell responses and mucosal and systemic antibodies with functional activity associated with protection (28–33).
In the current study, we investigated effects of a combined vaccine-microbicide regimen consisting of mucosal Ad5hr-SIV priming and systemic gp120 protein boosting followed by repeated low-dose intravaginal SIVmac251 challenge in the presence or absence of a 0.8% gel formulation of SAMT-247. Potent protection in 10 of 12 macaques was achieved by the microbicide alone when administered 3 hours prior to the SIV challenges supporting continued development of this promising compound. The vaccine alone was not protective while the combined vaccine-microbicide regimen exhibited delayed SIV acquisition compared to the vaccine only and control groups, but impaired efficacy compared to the microbicide group alone. Factors associated with these mixed outcomes are described and highlight areas of critical importance as vaccines, microbicides, and combined regimens move toward human trials.
MATERIALS AND METHODS
Research animals and ethics statement
Details regarding animal welfare are similar or identical to those described in one of our recent publications (34). Animal housing, diet, immunization, tissue collections and viral challenges of Indian rhesus macaques (Macaca mulatta) were performed in rigorous conformity with the recommendations of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and with standards determined in the Guide for the Care and Use of Laboratory Animals of the United States (35). Animals were housed at the AAALAC-accredited NCI Animal Facility, Bethesda, Maryland. The main and pilot studies were carried out under protocols VB-027 and VB-020, respectively, which were approved by the NCI Animal Care and Use Committee. Animals were anesthetized by a standard protocol (10 to 25mg/kg of ketamine) prior to immunizations, sample collections and viral challenges. Measures to minimize animal stress were applied in compliance with the Guide and Recommendations of the Weatherall Report. Macaques were housed under temperatures of 21o and 26oC, relative humidity of 30–70% and a 12-hour light/dark cycle. Animals were fed with commercial primate diet and fresh fruits twice a day. Water was always available. Animals were also monitored for activity and overall health twice a day. Euthanasia was performed in accordance with the American Veterinary Medical Association Panel on Euthanasia with an overdose of pentobarbital (80 mg/kg) at the end of the study.
Vaccination, microbicide application and viral challenge
Sixty female Indian rhesus macaques, 4 to 5 years old (mean of 4.5) were used in the main study. All macaques were free of SIV, simian retrovirus (SRV) and simian T cell leukemia virus 1 (STLV-1) before the studies were initiated. Typing of MHC class I haplotypes revealed that 6 were Mamu-A*01 positive, 3 were Mamu-B*08 positive and 8 were Mamu-B*17 positive. These animals were distributed among 4 vaccination groups so that in each group 25 to 30% of the macaques possessed protective haplotypes. The 4 groups included vaccine plus SAMT-247 combined (n = 20), vaccine only (n = 18), SAMT-247 only (n = 12), and controls (n = 10) (Fig. 1). The vaccinated macaques were primed twice with adenovirus replicating vectors: Ad5hr-SIVsmH4env/rev, Ad5hr-SIVgag and Ad5hr-SIVnef prepared in phosphate buffered saline (PBS) at a dose of 5×108 PFU per recombinant per route of administration. The first prime (week 0) was administered both intranasally and orally. The second mucosal prime was administered intratracheally at week 13. At weeks 26 and 38, macaques received intramuscular booster immunizations consisting of a 2 ml preparation containing 200μg of SIVM766 and SIVCG7V gD-gp120 proteins in alum hydroxide as adjuvant. The boosts were administered 1mL in each inner thigh. The microbicide only group and the control group received mucosal primes of empty Ad5hr vector (1.5×109 PFU in 500uL of PBS per route) and alum adjuvant only.
Figure 1. Schematic representation of the immunization regimen, sample collection and SIV challenges.
Sixty rhesus macaques included in this study were subdivided into 4 groups: vaccine + SAMT-247 (n=20), vaccine only (n=18), SAMT-247 only (n=12) and controls (n=10) (lower box). Thirty-eight animals were mucosally primed with Ad5hr-SIV recombinants encoding Env/Rev, Gag and Nef and systemically boosted with SIV gD-gp120 proteins in alum hydroxide at the indicated timepoints. Twenty-two animals received empty-Ad5hr vector and alum hydroxide only as placebo controls at the same timepoints. Beginning at week 45, protective efficacy against SIVmac251 was assessed by subjecting all animals to up to 15 weekly intravaginal viral exposures (black arrows) in the presence or absence of SAMT-247 until infection was confirmed. Animals either received 0.8% SAMT-247 in HEC gel (n=32) or HEC gel only (n=28) 3 hours prior to each low-dose SIVmac251 challenge. The different tissue samples collected 4 weeks before the first prime (Minus 4 wks), and 3 days (3d), 14 days (14d) or 21 days (21d) following immunizations are represented by different symbols as shown in the figure. IN = intranasal; O = oral; IT = intratracheal; IM = intramuscular
Prior to the challenge phase of the study a pilot study including 24 female Indian rhesus macaques, 5 to 15 years old (mean of 9.5), was conducted to determine a time interval between microbicide application and vaginal SIV challenge which would result in minimal protection. In Group I, 8 macaques received repetitive low-dose intravaginal SIVmac251 challenges 2 hours following SAMT-247 application while 4 macaques received placebo gel 2 hours prior to each challenge. In group II, 8 macaques received the same low-dose intravaginal challenges 4 hours following SAMT-247 application, while 4 macaques received placebo gel under the same schedule. Infection was determined by analysis of plasma for viral loads ≥ 50 SIV RNA copies/ml plasma assessed weekly by the droplet digital PCR method (Chung et al., in preparation). Based on this pilot study a 3-hour window between microbicide application and SIV administration was chosen.
At week 45, SIVmac251 challenges of macaques in the main study were initiated. Microbicide only and vaccine/microbicide groups were first vaginally administered 2ml of gel containing 0.8% SAMT-247 microbicide formulated in HEC gel (2.7% Natrosol cellulose 250HX Pharma, 0.01% DMSO, 0.9% saline). The vaccine only and control macaques received placebo HEC gel without SAMT-247. Microbicide or gel only applications were carried out prior to every challenge and were followed 3 hours later by intravaginal low-dose SIVmac251 administration (800 TCID50). Up to 15 weekly challenges were administered to macaques that remained uninfected. Infection was determined weekly as described above.
Tissue collection and sample processing
For assessment of vaccine dependent cellular and humoral responses in secondary lymphoid organs, lymph node (LN) biopsies were obtained 4 weeks prior to vaccination, 3 days and 14 days following the 2nd adeno and 2nd boost immunizations. In order to minimize sequential LN sampling of all macaques, 30 animals representative of the 4 immunization groups had LN biopsies prior to and 3 days following immunizations whereas the other 30 animals representative of the 4 immunization groups had LN biopsies prior to and 14 days following immunizations.
To evaluate systemic and mucosal immune responses, blood, serum, bone marrow, rectal pinch biopsies, endocervical cytobrushes, vaginal washes and rectal swabs were obtained from all macaques four weeks prior to vaccination and 21 days following each immunization. Lymphocytes were isolated from LN biopsies as previously described (36) and stored frozen in FBS/10% DMSO solution. Blood and bone marrow samples were centrifuged over Ficoll gradients for isolation of leukocytes, treated with ACK buffer for red blood cell lysis and stored frozen in FBS/10% DMSO as previously described (31).
Rectal biopsies were processed as previously described (37) and assayed immediately. Briefly, the biopsies were rinsed and minced using a scalpel and 19G needle in pre-warmed digestive medium consisting of RPMI 1640, 1X antibiotic-antimycotic solution, 2mM L-glutamine (all Invitrogen) and 2mg/ml collagenase (Sigma-Aldrich). The minced material was transferred in 10 ml of the same medium to 50 ml conical tubes, incubated at 37oC for 30 minutes and pulse vortexed every 5 minutes. The digested mucosal tissue was transferred into 6 well plates and passed 5 times through a blunt end cannula attached to a 12 ml syringe for liberation of cells. Cells were then passed through a 70μm cell strainer and washed with 30 mL of R10 medium (RPMI 1640, 1X antibiotic-antimycotic solution, 2mM L-glutamine and 10% FBS). The freshly isolated rectal cells were stained for flow cytometry evaluation of rectal SIVgp120 specific memory B cells or used in B cell ELISpot assays for evaluation of SIVgp120 specific plasmablasts and plasma cells.
Cervicovaginal lymphocytes were collected using Cytobrush Samplers (Rovers™ Medical Devices B.V., The Netherlands) and placed in 15mL conical tubes containing 5mL of R10 medium. Tubes containing cytobrushes were vigorously vortexed 4X for 10 seconds and cytobrushes were removed from medium with scraping of bristles on the edge of the tube for full recovery of attached material. Tubes were centrifuged for 10 min at 1500 rpm, medium was carefully aspirated, and cells were resuspended in 10mL of PBS. Cells were centrifuged once again for 10 min at 1500 rpm and supernatant was removed. Freshly isolated cells were stained for flow cytometry for assessment of cervicovaginal T cell populations.
Vaginal washes were collected with 2 ml PBS and placed in 15 ml conical tubes, centrifuged to pellet vaginal epithelial cells, and the supernatant was stored frozen at −80ºC until analyzed. Rectal secretions were collected with polyester-tipped swabs (Becton-Dickson, Cockeysville, MD, USA) and transferred into cryovials containing 0.1% BSA, 0.01% thimerosal and 750 Kallikrein inhibitor units of aprotinin/ml storage solution (all from Sigma Aldrich). Both rectal swab solutions and vaginal washes were used for gp120 specific IgG and IgA detection by ELISA.
SIV-specific antibody secreting cells in bone marrow and rectal biopsies
Both total IgG and IgA and SIVM766 and SIVCG7V gp120-specific IgG and IgA antibody secreting cells (ASC) were quantified in bone marrow and rectal mucosa by ELISpot as previously described (29). Env-specific IgG and IgA ASC were standardized to the total number of IgG and IgA ASC and reported as percentage IgG and IgA Env-specific activity relative to the number of total IgG and IgA ASC.
Flow cytometry identification of LN Env-specific TFH cells, circulating and rectal memory T cells, and cervical CD4+ T cells
Env-specific T follicular helper (TFH) cells in LN germinal centers (GC) were detected by the Activation Induced Marker (AIM) assay using CD25 and CD134 for identification of antigen-specific T cells as previously reported (38, 39). GC TFH cells in rhesus macaques were defined as PD-1high CXCR5+ CD4+ CD3+ T cells as described elsewhere (40). Viably frozen LN cells were thawed, washed with R10 and separated into three groups of 2–3 ×106 cells each for no stimulation, antigen stimulation with 1ug/mL pooled SIVCG7V gp120 peptides consisting of complete sets of 15-mer peptides overlapping by 11 aa (Advanced Bioscience Laboratories Inc, Rockville, MD; ABL), or 1X PMA/Ionomycin cell stimulation cocktail (eBiosciences). Cells received a mixture of 2ug/mL anti-CD49-d and anti-CD28 as well as APC-eFluor780 anti-CCR7 (Table I) and were incubated for approximately 43 hours at 37oC and 5% CO2. Following incubation, the cells were washed with FACS buffer (DPBS containing 2%FBS) and stained for surface markers (Table I). Cells were then washed with FACS buffer, permeabilized with Foxp3/transcription factor buffer set (eBioscience) according to the manufacturer’s recommendations and incubated with intracellular staining (ICS) antibodies (Table I). After a final wash with eBiosciences Wash Buffer, cells were resuspended in 2% formaldehyde (2% PFA) fixation solution and maintained at 4°C until acquisition. LN Env-specific AIM+ (CD25+CD134+) cells were calculated by subtracting non-stimulated values from stimulated values.
Table I.
Antibodies used in flow cytometry assays for identifying LN TFHcells, memory CD4+and CD8+ T cells in PBMCs and rectal tissue, and CD4+ T cells in cervical lymphocytes.
| Assay | Marker | Clone | Fluorochrome | Company | Staining |
|---|---|---|---|---|---|
| AIM assay, LN TFH Cells | CD49d | R1-2 | unconjugated | BD Biosciences | Stimulation |
| CD28 | CD28.2 | unconjugated | BD Biosciences | Stimulation | |
| CCR7 (CD197) | 2D12 | PerCP-eFluor780 | e-biosciences | Stimulation | |
| PD-1 | EH12.2H7 | BV605 | Biolegend | Surface | |
| CD25 | BC96 | PE-Cy5 | Biolegend | Surface | |
| CXCR5 | MU5UBEE | PerCP-eFluor710 | eBioscience | Surface | |
| CD134 | L106 | PE | BD Biosciences | Surface | |
| CD4 | L200 | BV711 | BD Biosciences | Surface | |
| Live/Dead | - | Aqua Dye | Invitrogen | Surface | |
| Ki67 | B56 | BV786 | BD Biosciences | ICS* | |
| CD3 | SP34-2 | AlexaFluor-700 | BD Biosciences | ICS | |
| Bcl6 | K112-91 | BV421 | BD Biosciences | ICS | |
| Rectal and Blood Memory T Cells | CD28 | CD28.2 | PE-Texas Red | Beckman Coulter | Stimulation |
| CD49d | 9F10 | unconjugated | eBioscience | Stimulation | |
| CD4 | L200 | PerCp-Cy5.5 | eBioscience | Surface | |
| CD8 | RPA-T8 | Qdot655 | eBioscience | Surface | |
| CD95 | DX2 | PE-Cy5 | eBioscience | Surface | |
| Live/Dead | - | Aqua Dye | Invitrogen | Surface | |
| CD3 | SP34-2 | Pacific Blue | BD Pharmingen | ICS | |
| IFN-g | B27 | APC | BD Pharmingen | ICS | |
| TNF-a | Mab11 | FITC | BD Pharmingen | ICS | |
| IL-2 | MQ1-17H12 | PE | BD Pharmingen | ICS | |
| Cervical CD4 T Cells | CD45 | D058-1283 | BV786 | BD Biosciences | Surface |
| CD3 | SP34-2 | BUV395 | BD Biosciences | Surface | |
| CD4 | L200 | BV711 | BD Biosciences | Surface | |
| CCR5 | 2D5 | BV605 | BD Biosciences | Surface | |
| CCR6 | 11A9 | APC-R700 | BD Biosciences | Surface | |
| Live/Dead | - | Blue Dye | Invitrogen | Surface | |
ICS: Intracellular Staining
Memory CD4+ and CD8+ T cells in the circulation and rectal biopsies had cellular immune responses evaluated by ICS for SIV-specific IFN-γ, IL-2 and TNF-α cytokine secreting cells. Peripheral blood mononuclear cells (PBMC) were kept frozen until assay while rectal lymphocytes were assayed fresh. Thawed PBMCs and rectal lymphocytes were stimulated with SIV pooled peptides (all 15-mers overlapping by 11 amino acids) including SIV Gag (NIH AIDS Research and Reference Reagent Program) and SIVM766 gp120 and SIVCG7V gp120 pooled peptides (ABL) at 1μg/ml final concentration. Non-stimulated and Leucocyte activation Cocktail (BD Pharmingen) tubes were included as controls. Anti-CD28 PE/Texas-red and anti-CD49d (Table I) were also added during stimulation along with a protein transport inhibitor (BD Pharmingen). Following 6h incubation at 37°C, cells were washed with PBS, then stained as previously described (29) with antibodies listed in Table I. After incubation for 30 min at 4°C in the dark, cells were washed with FACS buffer, permeabilized in 250μl fix/ perm solution (BD Pharmingen) according with manufacturer’s specifications and resuspended in 100μl wash buffer plus the ICS antibodies (Table I). After 30 min at 4°C in the dark, cells were washed with BD perm/wash buffer and resuspended in 2% PFA and stored at 4oC until acquisition. CD3+ CD19− T cells were used as a gate for CD4+ and CD8+ T cells, and each population was further divided into CD28+CD95+ central memory (CM) and CD28−CD95+ effector memory (EM) cells. Frequencies of cytokine secreting cells in each memory cell subset were determined following subtraction of the values obtained with non-stimulated samples. Both subsets were summed to give the total memory (TM) T-cell population.
For assessment of cervicovaginal CD4+ T cells, freshly isolated cervical lymphocytes were washed with FACS buffer and stained for surface markers (Table I). Cells were washed with FACS buffer and fixed with 2% PFA and saved at 4°C until acquisition. Cervical CD4+ T cells were defined as CD45+ CD14− CD4+ CD3+ T cells. Stained LN, PBMCs and cervical lymphocytes were acquired using an 18 laser LSRII (BD Biosciences) and analyzed with FlowJo 10.2 (FlowJo, Ashland, OR). Gates were defined using isotype and fluorescence-minus-one controls.
Serum and mucosal binding antibodies
Heat inactivated serum samples, vaginal washes and rectal swabs were assayed for SIVM766 and SIVCG7V gD-gp120-specific IgG and IgA binding antibodies by ELISA as previously described (41). For serum ELISA assays, Nunc Maxisorb 96-well plates were coated overnight at 4ºC with 50ng/well of gp120CG7V in 100uL of Sodium Bicarbonate buffer (Sigma-Aldrich, St. Louis, MO) and wells were blocked with 200uL of SuperBlock buffer (Pierce) in PBS for 1h at room temperature (RT). 100uL of serially diluted inactivated serum samples were added and incubated for 1h at 37ºC and washed 5 times with PBS containing 0.05% tween 20 (Sigma-Aldrich). Horseradish peroxidase-labeled goat anti-human IgG (100uL at a 1:100,000 dilution; Rockland, Gibertsville, PA) was added and plates were incubated for 1h at 37ºC. Plates were washed as described above and 100uL of K-blue aqueous 3,3’, 5’,5’ -tetramethylbenzidine (TMB) substrate (Neogen) was added for 30 min at RT. Color development was stopped with 1M sulfuric acid. Plates were read at 450nm using a Molecular Devices E-max plate reader. Antibody titers were defined as the reciprocal of the serum dilution at which the absorbance of the test serum was twice the absorbance of a negative macaque serum sample at a 1:50 dilution.
Rectal secretions, processed as described above, were filtered with centrifugal filters Durapore PVDF 5.0μm (Merck Millipore, Tullagreen Carrigtwohill, CO) prior to antibody assays. Both rectal filtrates and vaginal washes were tested for blood contamination using Chemstrip® 5OB urine test strips (Roche, Indianapolis, IN) and were not assayed if blood was present. For ELISA assays of vaginal and rectal secretions, half area plates were coated with 50 μl of 2μg/ml gp120CG7V or gp120M766 for Env-specific IgG and IgA antibody, and 50μl of 1μg/ml unconjugated anti-monkey IgG or IgA (AlphaDiagnostic) for total IgG and IgA antibody in sodium-bicarbonate buffer (pH 9.6) overnight at 4°C. The plates were blocked with 185 μl of 1% BSA in D-PBS for 2 h at room temperature. Vaginal washes or rectal secretions were serially diluted in PBS containing 1% BSA. 50 μl of serial five-fold dilutions were incubated on the plates for 1 h at 37°C. The plates were then washed 4 times and 50uL of HRP-conjugated goat anti-monkey IgG or IgA (AlphaDiagnostic) at 1:10000 dilution in 1% BSA solution were added. Plates were incubated at room temperature for 1h, washed 4 times, and TMB substrate (KPL) was used in sequential steps, followed by reading the OD at 450 nm after stopping the reaction with 1M sulfuric acid. For total IgG and IgA antibody, standards were obtained from the Nonhuman Primate Reagent Resource and goat anti-monkey HRP conjugates were used as detection antibodies at a 1:10000 dilution. Env-specific IgG and IgA, derived from purified serum IgG and IgA obtained from SIVmac251-infected macaques and quantified as described (42), was used to generate a standard curve for Env-specific IgG and Env-specific IgA. Mucosal antibodies were reported as ng Env-specific IgG or IgA per ug total IgG or IgA.
Non-neutralizing antibody activities
ADCC activity was assessed as previously described using EGFP-CEM-NKr-CCR5-SNAP cells that constitutively express GFP as targets (43). Briefly, one million target cells were incubated with 50 μg of SIV gp120 protein for 2 hours at 37ºC, washed, and labeled with SNAP-Surface® Alexa Fluor® 647 (New England Biolabs, Ct S9136S) as recommended by the manufacturer for 30 min at RT. Serum samples, heat inactivated at 56ºC for 30 minutes, were serially diluted (7 ten-fold dilutions starting at 1:10) and 100 μl were added to wells of a 96-well V-bottom plate (Millipore Sigma). 5000 target cells (50 μl) and 250,000 human PBMCs (50 μl) as effectors were added to each well to give an effector/target (E/T) ratio of 50:1. The plate was incubated at 37ºC for 2 hours followed by two PBS washes. The cells were re-suspended in 200 μl of a 2% PBS–paraformaldehyde solution and acquired on a LSRII equipped with a high throughput system (BD Biosciences, San Jose, CA, USA). Specific killing was measured by loss of GFP from the SNAP-Alexa647+ target cells. Target and effector cells cultured in the presence of medium only were used as negative controls. Anti-SIVmac gp120 monoclonal antibody, KK17 (NIH AIDS Reagent Program), was used as a positive control. Normalized percent killing was calculated as: (killing in the presence of rectal secretion - background)/ (killing in the presence of KK17- background) ×100. ADCC endpoint titer is defined as the reciprocal dilution at which the percent ADCC killing was greater than the mean percent killing of the negative control wells containing medium, target and effector cells, plus three standard deviations.
ADCP activity was measured as previously described (44) with minor modifications. In short, SIVm766 gp120 was biotinylated with a Biotin-XX microscale protein labeling kit (Thermo Fisher Scientific, Waltham, MA) and incubated with a 100-fold dilution of 1 μg yellow-green streptavidin-fluorescent beads (Thermo Fisher Scientific) overnight at 4ºC in the dark. A 1:100 dilution of serum from each macaque was added to 400,000 THP-1 cells, plated in a U-bottom 96-well plate. The bead-gp120 mixture was further diluted 5-fold in R10 media, and 50 μl was added to the cells and incubated for 3 h at 37°C. After incubation, 70 μl of 2% paraformaldehyde was added for fixation. Fluorescent bead uptake by THP-1 cells was assessed using a BD Biosciences LSRII flow cytometer (BD Biosciences, San Jose, CA). The phagocytic score of each sample was calculated by multiplying the percentage of bead-positive cells (frequency) by the degree of phagocytosis measured as mean fluorescence intensity (MFI) and dividing by 106. Values were normalized to background values (cells and beads without serum) by dividing the phagocytic score of the test sample by the phagocytic score of the background sample. Phagocytic scores post-vaccination were obtained by dividing the phagocytic score of the test sample by the phagocytic score of the pre-vaccination time point.
RESULTS
Vaccine-induced mucosal and systemic Env-specific humoral immune responses
In this study we used the rhesus macaque-SIV model to investigate potential additive or synergistic protective efficacy elicited by a replicating Ad5hr vector-based mucosal prime/gp120 protein systemic boost regimen together with the microbicide SAMT-247. Ad5hr recombinants were delivered intranasally and orally at wk0 and intratracheally at wk13 to ensure efficient immune responses in genital mucosa (Fig. 1).
To monitor vaccine-induced humoral responses at the main mucosal sites of HIV/SIV transmission, vaginal washes and rectal swabs were collected 3 weeks after each immunization and evaluated for Env-specific IgG and IgA antibodies. Both Env-specific IgG and IgA present in vaginal and rectal secretions increased to the highest levels following the systemic Env protein boosts (Fig. 2A, B and D, E). To investigate the effect of immunization on mucosal B cell responses, rectal antibody secreting cells (ASC) were assessed by ELISpot. Vaginal tissues were not sampled so as not to compromise the site of future intravaginal SIV challenges. The vaccine regimen elicited ASC at the mucosal site, as rectal SIV Env-specific IgG and IgA plasmablasts (PBs) and plasma cells (PCs) were detected and exhibited peak levels following the 1st boost (wk29) (Fig. 2G, H). IgA ASC levels were maintained following the 2nd boost whereas IgG levels displayed a significant decline. Overall, Env-specific IgG levels were significantly higher than Env-specific IgA levels in both vaginal and rectal secretions at wk41, the last collection timepoint prior to initiation of SIV challenges, suggesting that the vaccine was more efficient in generating IgG rather than IgA specific antibodies (Fig. 2C, F). However, as Env-specific IgG and IgA ASC levels at wk41 in rectal tissue did not differ (Fig. 2I), the higher IgG antibody levels in mucosal secretions may have resulted in part from transudation.
Figure 2. Quantification of Env-specific humoral responses in rectal and vaginal mucosa of rhesus macaques in response to immunization.
(A) Env-specific IgG and (B) IgA levels in vaginal washes collected 3 weeks after each immunization. (C) Comparison of vaginal Env-specific IgG and IgA levels at wk41, the last time point prior to SIV challenge. (D) Env-specific IgG and (E) IgA levels in rectal secretions collected 3 weeks after each immunization. (F) Comparison of rectal Env-specific IgG and IgA levels at wk41. Vaginal and rectal secretions that tested positive for blood were excluded from the analysis. Panels A-C: Pre-vaccination n=28; 1st Adeno n=33; 2nd Adeno n =35; 1st boost n=37; 2nd boost n=36. Panels D-F: Pre-vaccination n=25; 1st Adeno n=23; 2nd Adeno n=24; 1st boost n=30; 2nd boost n= 28. Dynamics of Env-specific antibody secreting cells (ASC: PBs and PCs) assessed by ELISpot for (G) IgG secreting cells and (H) IgA secreting cells. (I). Eighteen immunized animals were selected for analysis of rectal Env-specific IgA and IgG ASC. All 18 samples were included in rectal IgA Env-specific ASC ELISpot assays for all timepoints. Some samples did not have sufficient cells for the IgG Env-specific ASC ELISpot as follows: Panel G: Pre-vaccination n=14; 1st Adeno n=18; 2nd Adeno n=14, 1st boost n=15; 2nd boost n=18. (I) Comparison of vaccinated and control animals for IgG and IgA Env-specific ASC at wk41. Data were analyzed by the Wilcoxon Signed-rank Test; Comparisons between vaccinated and control groups in (C), (F) and (I) were analyzed by the Mann-Whitney U test. Horizontal and vertical bars denote mean and SEM. *p < 0.05; **p<0.01; ***p<0.001; ****p<0.0001.
We next examined vaccine-induction of systemic Env-specific humoral responses. Serum IgG antibody titers to gp120M766 and gp120CG7V were assessed by ELISA and were only detected following the systemic protein immunizations where they exhibited high titers (Fig. 3A, B). Serum titers against the gp120M766 envelope were significantly higher compared to those against gp120CG7V after both boost immunizations (Fig. 3C). Bone marrow (BM) Env-specific IgG and IgA secreting PBs and PCs were assessed by ELISpot for a comprehensive investigation of systemic humoral responses. Both IgG and IgA gp120CG7V-specific ASC were induced over the course of immunization (Fig. 3D, E). Both IgG and IgA ASC in BM at wk41 reached similar levels (Fig. 3F), in concert with the similar levels of ASC observed in the rectal mucosa (Fig. 2I). Collectively, these data indicate that the vaccine regimen elicited robust Env-specific humoral immune responses both mucosally and systemically.
Figure 3. Quantification of systemic Env-specific humoral responses in immunized macaques.
Serum binding antibody titers to (A) SIV gp120M766 and (B) gp120CG7V after the 1st boost (wk29) and 2nd boost (wk41) were compared to pre-vaccination values. (C) Comparison between serum antibody titers against gp120CG7V and gp120M766 at post-boost timepoints. Panels A-C include all immunized animals (n=38). Evaluation of Env-specific (D) IgG and (E) IgA ASC (PBs and PCs) from bone marrow by ELISpot. (F) Frequencies of IgG and IgA Env-specific ASC at wk41. Twenty-four immunized macaques were selected for Env-specific IgG or IgA ASC ELISpot assay in bone marrow. Sufficient cells were not available for some samples at specific timepoints. Panels D-F: Pre-vaccine, 1st Adeno, 2nd Adeno, 2nd boost: n=22 for all; 1st boost n=24. Data were analyzed by the Wilcoxon Signed-rank Test; Comparisons between vaccinated and control groups in (A&B) and (F) were analyzed by the Mann-Whitney U test. Horizontal and vertical bars denote mean and SEM. *p < 0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Vaccination induces cellular responses in lymph nodes, blood, and mucosal tissues of rhesus macaques
HIV-1 vaccines that stimulate T cell immunity have been suggested to contribute to protection (45). Moreover, effector T cells in the vaginal mucosa of female rhesus macaques have been consistently associated with protection upon intravaginal inoculation of pathogenic SIVmac239 (46). To evaluate cellular responses induced by the immunization platform, CD4 and/or CD8 T cell populations were investigated in LNs, PBMCs, and rectal tissue of the rhesus macaques. In secondary lymphoid organs, TFH cells represent the CD4+ T cell population specialized in supporting GC reactions and contributing to clonal selection of antigen specific memory B cells and long-lived plasma cells (47). Recently, our group demonstrated that early elicitation of GC TFH responses in immunized rhesus macaques contributed to the generation of Env-specific humoral responses that correlated with control of SIV viremia (34). Here, LN samples were evaluated for Env-specific GC TFH responses using the Activated Induced Marker (AIM) technique (38, 39) and gp120CG7V pooled peptides for T cell stimulation. GC TFH cells, gated as shown in Fig. 4A and defined as CD4+CXCR5+PD-1hi T cells (40), specific for Env tended to increase following the 2nd Ad5hr-SIV mucosal immunization and significantly peaked at day 3 following the 2nd systemic protein boost, indicating elicitation of the cell population needed for SIV-specific antibody responses (Fig. 4B). Significant alterations of the Env-specific GC TFH cell population were not observed at day 14 post-immunizations (data not shown).
Figure 4. Analysis of SIV-specific LN GC TFH cells elicited by immunization in vaccinated macaques.
A) Characterization of CXCR5+ PD-1high GC TFH cells in LN of rhesus macaques. CD25 and OX40 were further used for identification of Env-specific GC TFH cells by the Activation Induced Marker (AIM) assay. GC TFH cells were stimulated with SIV-Env pooled peptides or unstimulated. Env-specific subsets were detected using CD25 and OX40 co-expression in SIV Env-stimulated samples followed by subtraction of non-stimulated samples. (B) Frequency of Env-specific GC TFH responses at day 3 following 2nd mucosal prime and 2nd intramuscular boost (n=14). Frequencies of AIM+ Env-specific GC TFH cells were compared by the Wilcoxon Signed-rank Test. Horizontal and vertical bars denote mean and SEM. *p < 0.05.
SIV-specific total memory CD4+ and CD8+ T cells, gated as shown in Fig. 5A, were evaluated in peripheral blood by ICS for expression of IL-2, INF-γ and TNF-α over the course of immunization using pooled Gag, gp120M766 or gp120CG7V peptides for T cell stimulation. Percentages of vaccine-induced cells expressing each of the three cytokines were combined and reported as percent cytokine+ cells. Compared to pre-immunization values the vaccine regimen induced significant increases of SIV EnvM766-specific (Fig. 5B) and close to significant increases of SIV EnvCG7V-specific total memory cytokine producing CD4+ T cells (Fig. 5C) following the first Env protein boost, but both subsequently declined after the second Env boost. CD8+ memory T cells expressing the three cytokines were not induced significantly in PBMCs above pre-vaccination levels over the course of immunization (data not shown). However, the vaccine regimen induced significant levels of SIV EnvM766-specific total memory cytokine producing CD4+ T cells in rectal tissue (Fig. 5D). In contrast to peripheral blood, rectal CD8+ T cells were strongly induced following immunization. Significant levels of cytokine+ CD8+ total memory T cells specific for SIV gp120M766 (Fig. 5E) and gp120CG7V (Fig. 5F) were generated over the course of immunization. Although vaginal biopsies were not obtained, the induction of immunity in rectal tissues suggests that the vaccine in general was able to induce mucosal cellular immune responses.
Figure 5. Analysis of SIV-specific cellular immune responses elicited by immunization in PBMCs and rectal tissue of vaccinated macaques.
(A) Gating strategy used for detection of CD4+ and CD8+ memory T cell subsets in PBMCs and rectal tissue. CD4+ or CD8+ T cells were gated on CD3+ CD19− T cells and further classified as T central memory cells (TCM: CD28+ CD95+ T cells) or T effector memory cells (TEM: CD28− CD95+ T cells). Env-specific memory CD4 or CD8 T cell subsets were determined by stimulation with SIV-Env pooled peptides and assessment of IFN-γ, TNF-α and IL-2 secretion by stimulated cells after unstimulated values were subtracted. (B) Levels of gp120M766- and (C) gp120CG7V-specific cytokine producing CD4+ T cell responses in PBMCs over the course of immunization. Frequencies of Env-specific cytokine producing CD4+ T cells in PBMCs were assessed in 18 immunized macaques. (D) Levels of gp120M766-specific total cytokine producing CD4+ T cells in rectal tissue over the course of immunization. (E) Levels of gp120M766-specific and (F) gp120CG7V-specific total cytokine producing CD8+ T cells in rectal mucosa of rhesus macaques over the course of immunization. Frequencies of Env-specific cytokine producing CD4+ T cells and CD8+ T cells in rectal tissues were investigated in 20 immunized macaques. Data were analyzed by the Wilcoxon Rank-Sum test. Horizontal and vertical bars denote mean and SEM. *p < 0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Assessment of combined vaccine plus microbicide efficacy
Having shown that the vaccine regimen was immunogenic, we next assessed vaccine efficacy. In order to evaluate a possible additive or synergistic effect of the combined vaccine plus microbicide strategy, it was critical to use the SAMT-247 microbicide under conditions in which it would only be partially protective in order not to obscure any vaccine effect. Therefore, in a pilot study, infection was monitored following application of SAMT-247 or placebo gel 2 or 4 hours prior to repeated low-dose intravaginal SIV challenges. Results indicated that the 4-hour interval between microbicide administration and SIV exposure only induced marginal protection compared to placebo gel-treated animals (p = 0.060) (Fig. 6A). In contrast, the 2-hour interval resulted in significant acquisition delay in macaques that received SAMT-247 compared to the placebo gel recipients (p = 0.031) (Fig. 6B). Therefore, we selected a 3-hour window between microbicide application and each SIV challenge of the 60 study animals in order to provide the desired suboptimal protection against SIV acquisition.
Figure 6. SIV vaginal challenge outcomes and viral load analyses of infected macaques.
Kaplan-Meier analysis from pilot study showing SIV challenge with (A) SAMT-247 gel formulation administered 4 hours prior to challenge and (B) 2 hours prior to challenge. (C) Similar acquisition rate in vaccine-only and control groups. (D) Comparison of geometric means of acute viral loads (VL) over weeks 1 to 6 post-infection for all vaccinated animals that became infected (n=29) versus all mock-vaccinated animals that became infected (n =12). (E) Viral loads of macaque groups over time including only macaques expressing protective haplotypes. (F) Comparison of acute VL (wk1-wk6 post-infection) between vaccinated (n=7) and mock-vaccinated macaques (n=5) with protective haplotypes. (G) Viral loads of all macaque groups over time excluding macaques possessing protective haplotypes. Data in (D) and (F) were analyzed by the Mann-Whitney U test. Viral load geometric means were compared between groups in (E) and (G) by the Wilcoxon Signed-rank Test. Horizontal and vertical bars denote mean and SEM. *p < 0.05; **p<0.01.
After up to 15 weekly low-dose SIVmac251 challenges, the vaccine only group showed no delayed acquisition compared to the adjuvant controls which received empty Ad5hr vectors and adjuvant only (Fig. 6C). This was surprising as we recently reported significantly delayed acquisition following intrarectal SIV challenge using a very similar vaccine regimen (29), although the challenge route differed and MF59 adjuvant was used instead of the alum adjuvant used here.
Vaccination did impact viral replication in the vaccine only and vaccine plus microbicide groups when the animals that became infected were analyzed together. Moderately reduced acute phase viremia was observed in these animals when compared to mock-vaccinated macaques in the combined microbicide only plus control groups (Fig. 6D). Further analysis showed that this outcome could be attributed to responses in the macaques with protective haplotypes, Mamu A*01, Mamu B*08, and Mamu B*17, associated with elite control of viral replication (48–51). Viral loads post-infection in vaccinated macaques that possessed these protective haplotypes (vaccine only and vaccine plus microbicide groups) were significantly lower compared to those of mock vaccinated animals (microbicide only and control groups) that also possessed these haplotypes (Fig. 5E). Acute viral loads in these vaccinated macaques were also significantly lower compared to the controls (Fig. 5F). In contrast, vaccinated macaques that lacked protective haplotypes showed no decrease in viremia (Fig. 6G) and no difference in acute viral loads compared to mock vaccinated animals. The decreased viremia in the vaccinated macaques with protective haplotypes is presumably attributable to cellular immune responses elicited by the immunodominant epitopes expressed by the Ad5hr-SIV recombinants expressing Env, Gag, and Nef.
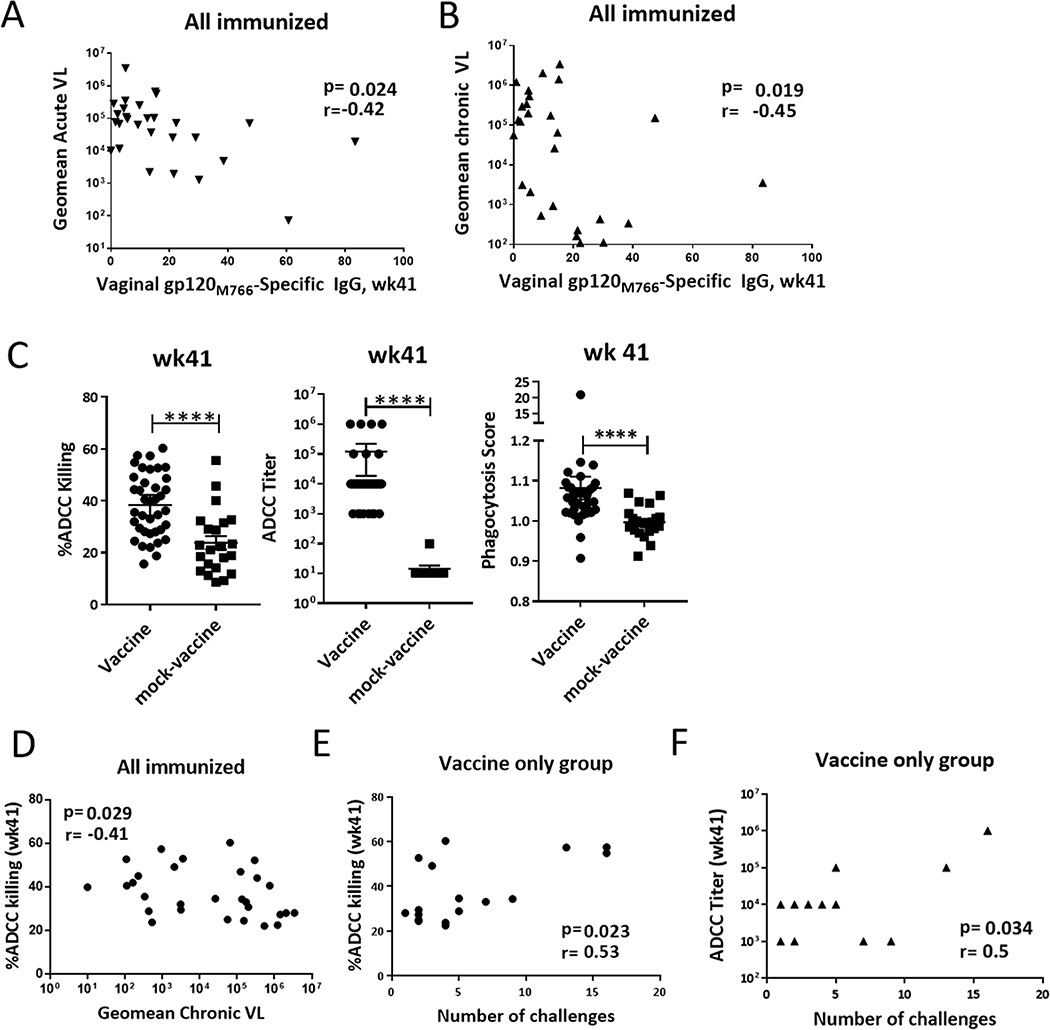
We also saw that vaccine-induced humoral responses were associated with control of viremia and delayed SIV acquisition. Env-specific IgG titers in vaginal secretions from immunized animals prior to challenge (wk41) correlated with reduced acute (r= −0.42; p=0.024) and chronic (r= −0.45; p=0.019) viremia (Fig. 6A, B). In addition, we evaluated effects of vaccine-induced non-neutralizing antibody activities: antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) (Fig. 7C). ADCC activity of serum antibodies obtained from all vaccinated macaques at the end of the vaccine regimen (wk 41) correlated significantly with reduced chronic viremia (Fig. 7D). To examine potential effects on SIV acquisition, we limited our evaluation to sera from macaques in the vaccine only group to avoid effects of the microbicide. ADCC activity (both percent killing and end point titer) correlated significantly with the number of challenges needed for infection (Fig. 7E,F), indicating a potential protective effect previously observed in other pre-clinical and clinical vaccine studies (33, 52–54). In contrast, even though higher ADCP activity was observed in the vaccine group, we did not find any association with potential vaccine-induced protection. Collectively, these data show that the vaccine regimen elicited mucosal and systemic immune responses with the potential to protect against viral acquisition and control viral replication.
Figure 7. Effects of vaccine-induced immune responses on SIV challenge outcomes.
Correlations of vaginal Env-specific IgG levels at 3 weeks post- 2nd boost (wk41) with (A) acute (weeks 1 – 6 post-infection) and (B) chronic (weeks 8 – 32 post-infection) viremia. Of the 29 immunized macaques that became infected, one was excluded from the correlation analyses due to blood detection in the vaginal wash. (C) Comparison of % ADCC killing, ADCC titer and phagocytosis score between all vaccinated (n=38). and mock-vaccinated (n=22) animals at wk41. (D) Correlation between % ADCC killing at wk41 with chronic viremia (n=29). Correlation between % ADCC killing (E) and ADCC titer (F) with number of challenges needed for infection. E and F correlation analyses include exclusively animals from the vaccine only group (n=18). Some points are overlapping, therefore not visible. Data in (C) were analyzed by the Mann-Whitney U test. Horizontal and vertical bars denote mean and SEM. Data in (A-B) and (D-F) were analyzed by the Spearman correlation test. Horizontal and vertical bars denote mean and SEM. ****p<0.0001.
In contrast to the vaccine only group, the microbicide only group exhibited strong protection with 10 of 12 macaques remaining uninfected after 15 challenges (Fig. 8A). Due to this strong protection observed with the microbicide alone, we did not see enhanced protection in the combined vaccine and microbicide group compared to the microbicide group alone. However, the microbicide application did enhance protection achieved by the vaccine alone as shown when the combined group was compared to the adjuvant controls (Fig. 8B), and when the combined group was compared to the vaccine only group (Fig. 8C). A puzzling outcome, however, was the lesser protection observed when the vaccine plus microbicide group was compared to microbicide alone (Fig. 8D). The apparent protection was less than that achieved by the microbicide only suggesting that additional factors might have affected the outcome of the combined group.
Figure 8. Outcome of SIV vaginal challenges and analysis of SIV cellular targets in vaginal mucosa.
(A) Significant protection in the microbicide only group compared to controls by Kaplan Meyer analysis. (B) Delayed SIV acquisition in the vaccine + microbicide group was seen compared to controls and the (C) vaccine only group. (D) Delayed SIV acquisition in the microbicide only group compared to the vaccine + microbicide group. (E) Gating strategy used for identification of CD3+ CD4+ T cells in cervical cytobrush samples. Frequencies of (F) total cervical CD4+ T cells, (G) CD4+CCR5+ cervical T cells and (H) CD4+CCR6+ cervical T cells over the course of immunization in vaccine + microbicide (n=20) and microbicide only groups (n=12). For pre-vaccination and 1st Adeno timepoints, only 10 samples from the vaccine + microbicide group and 6 samples from the microbicide only group were available for analysis. Data in (F-H) were analyzed by the Wilcoxon Sign-rank test for paired animals and Mann-Whitney U test for animals belonging to different groups. Horizontal and vertical bars denote mean and SEM. *p < 0.05; **p<0.01.
To investigate this issue further, we assessed CD4+ T cell populations of the vaginal mucosa, gated as shown in Fig. 8E, for changes that may have led to an enhanced presence of SIV target cells. Cervicovaginal cytobrush samples collected over the course of immunization were investigated by flow cytometry for CD4+, CCR5+CD4+, and CCR6+CD4+ T cells. CCR5, the co-receptor for HIV/SIV infection of CD4+ T cells, has been identified as a preferential target of HIV in the cervix (55). CCR6+CD4+ T cells of the Th17 lineage have also been shown to be primary SIV targets during vaginal transmission (56, 57). As shown in Figure 8F, the total frequency of CD4+ T cells increased over the course of immunization in the vaccine/microbicide group and tended to increase in the microbicide only group which had received Ad5hr empty vector and alum only, similar to the controls. However, by 41 weeks, the last time point prior to SIV challenge, there was no difference between the groups. Further, the frequencies of CCR5+CD4+ and CCR6+CD4+ T cells between the two groups did not differ (Fig. 8G, H). Therefore, vaccine induction of additional SIV target cells in the vaginal compartment did not seem be the reason for the diminished protection in the vaccine/microbicide group relative to the microbicide only group.
DISCUSSION
The selection of the SAMT-247 nucleocapsid inhibitor as a vaginal microbicide for this pre-clinical trial was based on its lack of toxicity, low-cost of production and mutational resistance (58) and the delayed SHIV acquisition and attenuated viral replication previously observed using a Gag-based DNA vaccine in combination with the microbicide in rhesus macaques (24). Here we have shown that the SAMT-247 microbicide gave exceptional results, protecting 10 of 12 macaques (83%) when administered as a 0.8% gel, 3 hours prior to intravaginal challenge. This improves upon results of a previous study in which the microbicide protected 5 of 6 macaques against SHIV infection when administered as a 1% gel, 20 minutes prior to intravaginal challenge (22) and demonstrates the protective efficiency of the microbicide persists for a longer time period prior to viral exposure The protection achieved was also superior to a macaque study in which 1% tenofovir gel protected 43% of cynomolgus macaques against SHIV162P3 challenge administered 1 hour after the microbicide (23), similar to results of the CAPRISA Trial in South Africa where 1% tenofovir gel reduced HIV infection by 39% (59). Our results indicate SAMT-247 is one of the most promising microbicide candidates to date and strongly support ongoing efforts to implement its use via a vaginal ring. Vaginal rings have a long history of providing controlled release of small-molecule therapeutics for several clinical indications in women’s healthcare such as hormonal contraception and hormone replacement therapy (60, 61). Hence, there is a growing interest in implementing vaginal rings with microbicides for sustained protection of women against HIV infection (5, 62).
While Ad5 is no longer a viable vaccine candidate due to failure in the STEP trial and suggestion of enhanced infection in Ad5 seropositive individuals (63) the mutant Ad5hr vector is provides a useful model of a replicating mucosal vector. Unlike human Ads it replicates in rhesus macaques and persists for prolonged periods (64). Numerous studies have shown mucosal priming with Ad5hr-recombinants leads to protective responses, including delayed SIV acquisition in female macaques (29, 65). Surprisingly, here the vaccine by itself did not result in significantly delayed SIV acquisition compared to the adjuvant controls, although modest viral load reduction was attributed to the immunization regimen. Moreover, humoral immune responses, including SIV Env-specific vaginal antibodies, were associated with decreased acute and chronic viremia, and serum ADCC activity correlated with an increased number of challenges needed for infection. The lack of significantly delayed SIV acquisition here in the vaccine only macaques compared to the adjuvant controls differs strikingly from a prior study in which significant delay of SIV acquisition was observed following low dose intrarectal challenges (29). Differences in the studies include the adjuvant employed, alum here and MF59 in the prior trial, and the route of challenge: intravaginal here versus intrarectal previously. Both these factors could have contributed to the challenge outcomes and should be further explored. We note that here vaccine-induced vaginal IgG antibodies were associated with viremia control while rectal IgA Env-specific antibodies were associated with the delayed SIV acquisition seen earlier (29). This suggests that the quality of vaccine-induced mucosal antibodies is one avenue for further investigation.
Previous studies in non-human primates have shown that vaccine-microbicide combinations may provide better protection against viral infection than either component alone. These include studies of an Ad35/Ad26 based SIVenv/gag/pol vaccine combined with the CCR5 inhibitor maraviroc in protection of rhesus macaques against a single high dose of SHIVSF162P3 (7); an HIV Env-based vaccine coupled with 1% tenofovir in protecting cynomolgus macaques against repeated low doses of SHIVSF162P3 (23); and a DNA/Ad5 prime boost regimen targeting SIV Gag and Pol combined with 0.1% SAMT-247 in delaying rhesus macaque acquisition of SHIVSF162P3 following repeated low dose challenges (24). Here, due to the strong protection observed in the microbicide only group, we did not see any enhancement in overall protection in the combined vaccine/microbicide group. We did see, however, that although the vaccine only group showed no protection, significant acquisition delay was seen in the vaccine/microbicide group both when compared to the vaccine only group (Fig. 8C) and when compared to controls (Fig. 8B), supporting the vaccine plus microbicide concept.
An unexpected result upon further evaluation of the various macaque groups was the decreased protection observed in the vaccine/microbicide group compared to the microbicide only group (Fig. 8D). Investigation of CCR5+ and CCR6+ CD4+ cells in the cervicovaginal compartment did not attribute this to any enhancement of potential SIV targets as a result of the vaccine regimen. Another factor which may have led to this outcome is based on the vaccine-induction of vaginal IgG and IgA antibodies (Fig. 2A, B). The IgG antibodies were shown to have protective effects, being negatively correlated with acute and chronic viremia (Fig. 7A, B). However, antibodies that do not neutralize virus can lead to antibody-dependent enhancement (ADE) of infection by complexing with virus and leading to its infection via Fc receptors, thereby targeting cells not normally susceptible to the virus (66, 67). It is difficult to determine if this occurred in vivo in this experiment. To prevent possible ADE, the SAMT-247 microbicide would need to be present in all cells in the local environment. SAMT-247 is a small molecule and should readily enter all cells by passive diffusion, although this has not been experimentally determined. Analysis of SAMT-247 uptake in vivo by cells in the vaginal compartment should be explored in the future to address this question. If a gradient of uptake exists among cell types, it might be addressed by simply increasing the SAMT-247 concentration which was decreased in this experiment to explore the combined effect with vaccine.
In order to conduct this study we needed to use the SAMT-247 microbicide under suboptimal conditions so that we would be able to see a vaccine effect. To this end, we performed a pilot study which indicated that a time interval of between 2 and 4 hours after microbicide application and prior to intravaginal challenge would result in partial protection using the microbicide alone (Fig. 6A, B). Surprisingly, using a 3-hour window, we observed much better protection in the microbicide only group (Fig. 8A) than that seen in the pilot study following a 2-hour delay (Fig. 6B). As the same batch and dose of SAMT-247 was used for both experiments, the age of the macaques may have influenced the outcomes. Macaques available for the pilot study had a mean age of 9.5 years. In contrast, macaques used in the main study had a mean age of 4.5 years. It is known that in humans, the aging of the immune system is associated with inefficient adaptive responses and decreased vaccine efficacy (68). In rhesus macaques, antibody responses to influenza vaccination were diminished in aged rhesus macaques (>19 years old) compared to young adults (4–7 years old) (69). A recent study in rhesus macaques vaccinated with various heterologous viral vectors and HIV envelope reported that younger animals (< 8 years old) exhibited enhanced protection against SHIV infection compared to older animals (70). Another reported that vaccination with a DNA/MVA regimen elicited better protective efficacy against SIVsmE660 in younger macaques (71). In addition, a pre-clinical study using a DNA/ALVAC/Env vaccine regimen has shown better protection against SIV challenge in younger compared to older macaques (Bissa et al. manuscript in preparation). Overall, in addition to differences in induced immunity, it may be that older macaques are simply more susceptible to SIV infection than younger animals, a possibility perhaps related to impaired natural immunity in older animals, and one that will require further investigation.
Here the strong protection obtained using SAMT-247 administered 3 hours prior to intravaginal low-dose SIV exposure, identifies this agent as highly promising microbicide that should move toward implementation in slow-release vaginal rings in future clinical trials. While the vaccine here did not result in protection, the combination of microbicide and vaccine led to enhanced protection in comparison to the controls and animals receiving vaccine only. Thus, the concept of a combination approach perhaps with a different vaccine regimen remains a promising option for future preventive strategies, as microbicide efficacy is hampered by non-compliance. Our study also identifies several areas for additional rigorous investigation. As the risk of SIV acquisition may be associated with immunization effects on the vaginal mucosa, future studies should investigate vaccine effects on local mucosal cells and designs capable of inducing mucosal immunity with adequate quality so as to avoid potential ADE. With regard to SAMT-247 itself, determining its in vivo distribution should facilitate use leading to protective synergism and improved protection against HIV-1 infection in women.
Key points.
SAMT-247 microbicide induces robust SIV protection in female rhesus macaques
Strong vaccine-induced mucosal and systemic immunity did not delay SIV acquisition
SAMT-247/vaccine combination enhanced protection compared to vaccine-only regimen
ACKNOWLEDGMENTS
We gratefully acknowledge the veterinarians Drs. Josh Kramer and Matthew Breed, and William Magnanelli, Michelle Metrinko and their staff for expert care of the rhesus macaques, implementation of the research protocol and collection of all tissues at the NCI animal facility. We thank H.K. Chung, R. Pal, and Maria Ferrari from Advanced Bio- Science Laboratories, Inc., Rockville, MD, for the viral load and CD4 assays. We thank Nancy Miller (NIAID) for provision of the SIVmac251 titered challenge stock originally obtained from Dr. Ronald Desrosiers. Rhesus macaque IgG and IgA standards were obtained from the NIH Non-human Primate Reagent Resource. The NIH AIDS Reagent Program, DAIDS, NIAID, provided the complete set of SIVmac239 Gag peptides and the KK17 anti-SIV gp120 monoclonal antibody. We thank Katherine McKinnon and Sophia Brown (Vaccine Branch, NCI) for flow cytometry support.
This study was supported by the Intramural Research Program of the NIH, National Cancer Institute.
REFERENCES
- 1.Estimated HIV Incidence and Prevalence in the United States, 2010–2016. Available at: https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-incidence-prevalence-2010-2016.pdf. Date of access: August 19, 2019
- 2.Ramjee G, and Daniels B. 2013. Women and HIV in Sub-Saharan Africa. AIDS Res. Ther. 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn TC, and Overbaugh J. 2005. HIV/AIDS in women: an expanding epidemic. Science 308: 1582–1583. [DOI] [PubMed] [Google Scholar]
- 4.Tintori C, Brai A, Dasso Lang MC, Deodato D, Greco AM, Bizzarri BM, Cascone L, Casian A, Zamperini C, Dreassi E, Crespan E, Maga G, Vanham G, Ceresola E, Canducci F, Arien KK and Botta M. 2016. Development and in vitro evaluation of a microbicide gel formulation for a novel non-nucleoside reverse transcriptase inhibitor belonging to the N-dihydroalkyloxybenzyloxopyrimidines (N-DABOs) family. J. Med. Chem. 59: 2747–2759. [DOI] [PubMed] [Google Scholar]
- 5.McBride JW, Boyd P, Dias N, Cameron D, Offord RE, Hartley O, Kett VL and Malcolm RK. 2019. Vaginal rings with exposed cores for sustained delivery of the HIV CCR5 inhibitor 5P12-RANTES. J. Control Release 298: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veazey RS, Ling B, Green LC, Ribka EP, Lifson JD, Piatak M Jr, Lederman MM, Mosier D, Offord R, and Hartley O. 2009. Topically applied recombinant chemokine analogues fully protect macaques from vaginal simian-human immunodeficiency virus challenge. J. Infect. Dis. 199: 1525–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch DH, Klasse PJ, Dufour J, Veazey RS, and Moore JP. 2012. Macaque studies of vaccine and microbicide combinations for preventing HIV-1 sexual transmission. Proc. Natl. Acad. Sci. U S A 109: 8694–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan P, Chen W, Li Z, Li X, Chen X, Tian Y, Pannecouque C, Clercq ED, and Liu X. 2012. Discovery of novel 2-(3-(2-chlorophenyl)pyrazin-2-ylthio)-N-arylacetamides as potent HIV-1 inhibitors using a structure-based bioisosterism approach. Bioorg. Med. Chem. 20: 6795–6802. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Cao Y, Zhan P, Pannecouque C, Balzarini J, Clercq ED, Shen Y, and Liu X. 2013. Arylazolylthioacetanilide. Part 11: design, synthesis and biological evaluation of 1,2,4-triazole thioacetanilide derivatives as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Med. Chem. 9: 968–973. [DOI] [PubMed] [Google Scholar]
- 10.Ozdener AE, Park TE, Kalabalik J, and Gupta R. 2017. The future of pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) infection. Expert. Rev. Anti. Infect. Ther. 15: 467–481. [DOI] [PubMed] [Google Scholar]
- 11.Delany-Moretlwe S, Lombard C, Baron D, Bekker LG, Nkala B, Ahmed K, Sebe M, Brumskine W, Nchabeleng M, Palanee-Philips T, Ntshangase J, Sibiya S, Smith E, Panchia R, Myer L, Schwartz JL, Marzinke M, Morris L, Brown ER, Doncel GF, Gray G, and Rees H. 2018. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 18: 1241–1250. [DOI] [PubMed] [Google Scholar]
- 12.Moore JP, and Kuritzkes DR. 2009. A piece de resistance: how HIV-1 escapes small molecule CCR5 inhibitors. Curr. Opin. HIV AIDS 4: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche M, Salimi H, Duncan R, Wilkinson BL, Chikere K, Moore MS, Webb NE, Zappi H, Sterjovski J, Flynn JK, Ellett A, Gray LR, Lee B, Jubb B, Westby M, Ramsland PA, Lewin SR, Payne RJ, Churchill MJ, and Gorry PR. 2013. A common mechanism of clinical HIV-1 resistance to the CCR5 antagonist maraviroc despite divergent resistance levels and lack of common gp120 resistance mutations. Retrovirology 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedellec R, Coetzer M, Lederman MM, Offord RE, Hartley O, and Mosier DE. 2011. Resistance to the CCR5 inhibitor 5P12-RANTES requires a difficult evolution from CCR5 to CXCR4 coreceptor use. PLoS One 6: e22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cihlar T, and Ray AS. 2010. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 85: 39–58. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RK, Gregson J, Parkin N, Haile-Selassie H, Tanuri A, Andrade Forero L, Kaleebu P, Watera C, Aghokeng A, Mutenda N, Dzangare J, Hone S, Hang ZZ, Garcia J, Garcia Z, Marchorro P, Beteta E, Giron A, Hamers R, Inzaule S, Frenkel LM, Chung MH, de Oliveira T, Pillay D, Naidoo K, Kharsany A, Kugathasan R, Cutino T, Hunt G, Avila Rios S, Doherty M, Jordan MR, and Bertagnolio S. 2018. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect. Dis. 18: 346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundquist WI, and Krausslich HG. 2012. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2: a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller Jenkins LM, Ott DE, Hayashi R, Coren LV, Wang D, Xu Q, Schito ML, Inman JK, Appella DH, and Appella E. 2010. Small-molecule inactivation of HIV-1 NCp7 by repetitive intracellular acyl transfer. Nat. Chem. Biol. 6: 887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller Jenkins LM, Paine EL, Deshmukh L, Nikolayevskiy H, Lyons GC, Scerba MT, George Rosenker K, Luecke HF, Louis JM, Chertova E, Gorelick RJ, Ott DE, Clore GM, and Appella DH. 2019. Inhibition of HIV maturation via selective unfolding and cross-linking of Gag polyprotein by a mercaptobenzamide acetylator. J. Am. Chem. Soc. 141: 8327–8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schito ML, Soloff AC, Slovitz D, Trichel A, Inman JK, Appella E, Turpin JA, and Barratt-Boyes SM. 2006. Preclinical evaluation of a zinc finger inhibitor targeting lentivirus nucleocapsid protein in SIV-infected monkeys. Curr. HIV. Res. 4: 379–386. [DOI] [PubMed] [Google Scholar]
- 21.Hartman TL, Yang L, Helfrick AN, Hassink M, Shank NI, George Rosenker K, Scerba MT, Saha M, Hughes E, Wang AQ, Xu X, Gupta P, Buckheit RW Jr, and Appella DH. 2016. Preclinical evaluation of a mercaptobenzamide and its prodrug for NCp7-targeted inhibition of human immunodeficiency virus. Antiviral Res. 134: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace GS, Cheng-Mayer C, Schito ML, Fletcher P, Miller Jenkins LM, Hayashi R, Neurath AR, Appella E, and Shattock RJ. 2009. Human immunodeficiency virus type 1 nucleocapsid inhibitors impede trans infection in cellular and explant models and protect nonhuman primates from infection. J. Virol. 83: 9175–9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Grand R, Dereuddre-Bosquet N, Dispinseri S, Gosse L, Desjardins D, Shen X, Tolazzi M, Ochsenbauer C, Saidi H, Tomaras G, Prague M, Barnett SW, Thiebaut R, Cope A, Scarlatti G, and Shattock RJ. 2016. Superior efficacy of a human immunodeficiency virus vaccine combined with antiretroviral prevention in simian-human immunodeficiency virus-challenged nonhuman primates. J. Virol. 90: 5315–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng-Mayer C, Huang Y, Gettie A, Tsai L, Ren W, Shakirzyanova M, Sina ST, Trunova N, Blanchard J, Jenkins LM, Lo Y, Schito ML, and Appella E. 2011. Delay of simian human immunodeficiency virus infection and control of viral replication in vaccinated macaques challenged in the presence of a topical microbicide. Aids 25: 1833–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, and Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366: 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, Morris DE, Tomaras G, Rao M, Billings E, Berman P, Shen X, Andrews C, O’Connell RJ, Ngauy V, Nitayaphan S, de Souza M, Korber B, Koup R, Bailer RT, Mascola JR, Pinter A, Montefiori D, Haynes BF, Robb ML, Rerks-Ngarm S, Michael NL, Gilbert PB, and Kim JH. 2013. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One 8: e53629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Arworn D, Shen X, Tomaras GD, Currier JR, Jiang M, Magaret C, Andrews C, Gottardo R, Gilbert P, Cardozo TJ, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Paris R, Greene K, Gao H, Gurunathan S, Tartaglia J, Sinangil F, Korber BT, Montefiori DC, Mascola JR, Robb ML, Haynes BF, Ngauy V, Michael NL, Kim JH, and de Souza MS. 2012. The Thai phase III HIV type 1 vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res. Hum. Retroviruses 28: 1444–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vargas-Inchaustegui DA, Tuero I, Mohanram V, Musich T, Pegu P, Valentin A, Sui Y, Rosati M, Bear J, Venzon DJ, Kulkarni V, Alicea C, Pilkington GR, Liyanage NP, Demberg T, Gordon SN, Wang Y, Hogg AE, Frey B, Patterson LJ, DiPasquale J, Montefiori DC, Sardesai NY, Reed SG, Berzofsky JA, Franchini G, Felber BK, Pavlakis GN, and Robert-Guroff M. 2014. Humoral immunity induced by mucosal and/or systemic SIV-specific vaccine platforms suggests novel combinatorial approaches for enhancing responses. Clin. Immunol. 153: 308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuero I, Mohanram V, Musich T, Miller L, Vargas-Inchaustegui DA, Demberg T, Venzon D, Kalisz I, Kalyanaraman VS, Pal R, Ferrari MG, LaBranche C, Montefiori DC, Rao M, Vaccari M, Franchini G, Barnett SW, and Robert-Guroff M. 2015. Mucosal B cells are associated with delayed SIV acquisition in vaccinated female but not male rhesus macaques following SIVmac251 rectal challenge. PLoS Pathog. 11: e1005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF,and Robert-Guroff M. 2012. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J. Virol. 86: 4644–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohanram V, Demberg T, Musich T, Tuero I, Vargas-Inchaustegui DA, Miller-Novak L, Venzon D, and Robert-Guroff M. 2016. B cell responses associated with vaccine-induced delayed SIVmac251 acquisition in female rhesus macaques. J. Immunol. 197: 2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D,Kozlowski PA, Hidajat R, Demberg T, and Robert-Guroff M. 2010. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 84: 7161–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, Montefiori DC, and Robert-Guroff M. 2009. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J. Virol. 83: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helmold Hait S, Vargas-Inchaustegui DA, Musich T, Mohanram V, Tuero I, Venzon DJ, Bear J, Rosati M, Vaccari M, Franchini G, Felber BK, Pavlakis GN, and Robert-Guroff M. 2019. Early T follicular helper cell responses and germinal center reactions are associated with viremia control in immunized rhesus macaques. J. Virol. 93: e01687–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guide for the Care and Use of Laboratory Animals, 8th edition ed. National Academies Press (US), Washington (DC). [Google Scholar]
- 36.Demberg T, Boyer JD, Malkevich N, Patterson LJ, Venzon D, Summers EL, Kalisz I, Kalyanaraman VS, Lee EM, Weiner DB, and Robert-Guroff M. 2008. Sequential priming with simian immunodeficiency virus (SIV) DNA vaccines, with or without encoded cytokines, and a replicating adenovirus-SIV recombinant followed by protein boosting does not control a pathogenic SIVmac251 mucosal challenge. J. Virol. 82: 10911–10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demberg T, Mohanram V, Venzon D, and Robert-Guroff M. 2014. Phenotypes and distribution of mucosal memory B-cell populations in the SIV/SHIV rhesus macaque model. Clin. Immunol. 153: 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Pena A, Kasturi SP, Dan JM, Bothwell M, Sanders RW, Pulendran B, Silvestri G, and Crotty S. 2016. Cytokine-independent detection of antigen-specific germinal center T follicular helper cells in immunized nonhuman primates using a live cell activation-induced marker technique. J. Immunol. 197: 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiss S, Baxter AE, Cirelli KM, Dan JM, Morou A, Daigneault A, Brassard N, Silvestri G, P Routy J, Havenar-Daughton C, Crotty S, and Kaufmann DE. 2017. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS One 12: e0186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velu V, Mylvaganam GH, Gangadhara S, Hong JJ, Iyer SS, Gumber S, Ibegbu CC, Villinger F, and Amara RR. 2016. Induction of Th1-biased T follicular helper (Tfh) cells in lymphoid tissues during chronic simian immunodeficiency virus infection defines functionally distinct germinal center Tfh cells. J. Immunol. 197: 1832–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demberg T, Brocca-Cofano E, Xiao P, Venzon D, Vargas-Inchaustegui D, Lee EM, Kalisz I, Kalyanaraman VS, Dipasquale J, McKinnon K, and Robert-Guroff M. 2012. Dynamics of memory B-cell populations in blood, lymph nodes, and bone marrow during antiretroviral therapy and envelope boosting in simian immunodeficiency virus SIVmac251-infected rhesus macaques. J. Virol. 86: 12591–12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manrique M, Kozlowski PA, Wang SW, Wilson RL, Micewicz E, Montefiori DC, Mansfield KG, Carville A, and Aldovini A. 2009. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunol. 2: 536–550. [DOI] [PubMed] [Google Scholar]
- 43.Orlandi C, Flinko R, and Lewis GK. 2016. A new cell line for high throughput HIV-specific antibody-dependent cellular cytotoxicity (ADCC) and cell-to-cell virus transmission studies. J. Immunol. Methods 433: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, and Alter G. 2011. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J. Immunol. Methods 366: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMichael AJ, and Koff WC. 2014. Vaccines that stimulate T cell immunity to HIV-1: the next step. Nat. Immunol. 15: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genesca M, Skinner PJ, Hong JJ, Li J, Lu D, McChesney MB, and Miller CJ. 2008. With minimal systemic T-cell expansion, CD8+ T Cells mediate protection of rhesus macaques immunized with attenuated simian-human immunodeficiency virus SHIV89.6 from vaginal challenge with simian immunodeficiency virus. J. Virol. 82: 11181–11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crotty S 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM, Lifson JD, O’Connor DH, Carrington M, and Watkins DI. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80: 5074–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang ZQ, Fu TM, Casimiro DR, Davies ME, Liang X, Schleif WA, Handt L, Tussey L, Chen M, Tang A, Wilson KA, Trigona WL, C Freed D, Tan CY, Horton M, Emini EA, and Shiver JW. 2002. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J. Virol. 76: 12845–12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, Lifson JD, Carrington M, and Watkins DI. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 81: 8827–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martins MA, Tully DC, Pedreno-Lopez N, von Bredow B, Pauthner MG, Shin YC, Yuan M, Lima NS, Bean DJ, Gonzalez-Nieto L, Domingues A, Gutman MJ, Maxwell HS, Magnani DM, Ricciardi MJ, Bailey VK, Altman JD, Burton DR, Ejima K, Allison DB, Evans DT, Rakasz EG, Parks CL, Bonaldo MC, Capuano S 3rd, Lifson JD, Desrosiers RC, Allen TM, and Watkins DI. 2018. Mamu-B*17(+) rhesus macaques vaccinated with env, vif, and nef manifest early control of SIVmac239 replication. J. Virol. 92: e00690–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, and Robert-Guroff M. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 174: 2185–2189. [DOI] [PubMed] [Google Scholar]
- 53.Robinson HL 2013. Non-neutralizing antibodies in prevention of HIV infection. Expert Opin. Biol. Ther. 13: 197–207. [DOI] [PubMed] [Google Scholar]
- 54.Forthal DN, and Finzi A. 2018. Antibody-dependent cellular cytotoxicity in HIV infection. Aids 32: 2439–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joag VR, McKinnon LR, Liu J, Kidane ST, Yudin MH, Nyanga B, Kimwaki S, Besel KE, Obila JO, Huibner S, Oyugi JO, Arthos J, Anzala O, Kimani J, Ostrowski MA, and Kaul R. 2016. Identification of preferential CD4+ T-cell targets for HIV infection in the cervix. Mucosal Immunol. 9: 1–12. [DOI] [PubMed] [Google Scholar]
- 56.Monteiro P, Gosselin A, Wacleche VS, El-Far M, Said EA, Kared H, Grandvaux N, Boulassel MR, Routy JP, and Ancuta P. 2011. Memory CCR6+CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin beta7. J. Immunol. 186: 4618–4630. [DOI] [PubMed] [Google Scholar]
- 57.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, Gelmon L, Block KE, Cicala C, Anzala AO, Arthos J, Kimani J, and Kaul R. 2011. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J. Immunol. 187: 6032–6042. [DOI] [PubMed] [Google Scholar]
- 58.Nikolayevskiy H, Scerba MT, Deschamps JR, and Appella DH. 2018. Reaction kinetics direct a rational synthesis of an HIV-1 inactivator of nucleocapsid protein 7 and provide mechanistic insight into cellular metabolism and antiviral activity. Chemistry 24: 9485–9489. [DOI] [PubMed] [Google Scholar]
- 59.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, and Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329: 1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malcolm RK, Boyd PJ, McCoy CF, and Murphy DJ. 2016. Microbicide vaginal rings: Technological challenges and clinical development. Adv. Drug. Deliv. Rev. 103:33–56. [DOI] [PubMed] [Google Scholar]
- 61.Murphy DJ, Boyd P, McCoy CF, Kumar S, Holt JD, Blanda W, Brimer AN, and Malcolm RK. 2016. Controlling levonorgestrel binding and release in a multi-purpose prevention technology vaginal ring device. J. Control Release 226: 138–147. [DOI] [PubMed] [Google Scholar]
- 62.Thurman AR, Schwartz JL, Brache V, Clark MR, McCormick T, Chandra N, Marzinke MA, Stanczyk FZ, Dezzutti CS, Hillier SL, Herold BC, Fichorova R, Asin SN, Rollenhagen C, Weiner D, Kiser P, and Doncel GF. 2018. Randomized, placebo controlled phase I trial of safety, pharmacokinetics, pharmacodynamics and acceptability of tenofovir and tenofovir plus levonorgestrel vaginal rings in women. PLoS One 13: e0199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, and Robertson MN. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patterson LJ, Kuate S, Daltabuit-Test M, Li Q, Xiao P, McKinnon K, DiPasquale J, Cristillo A, Venzon D, Haase A, and Robert-Guroff M. 2012. Replicating adenovirus-simian immunodeficiency virus (SIV) vectors efficiently prime SIV-specific systemic and mucosal immune responses by targeting myeloid dendritic cells and persisting in rectal macrophages, regardless of immunization route. Clin. Vaccine Immunol. 19: 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patterson LJ, and Robert-Guroff M. 2008. Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development. Expert Opin. Biol. Ther. 8:1347–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, and Mahalingam S. 2015. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 268: 340–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gorlani A, and Forthal DN. 2013. Antibody-dependent enhancement and the risk of HIV infection. Curr. HIV Res. 11: 421–426. [DOI] [PubMed] [Google Scholar]
- 68.Lord JM 2013. The effect of ageing of the immune system on vaccination responses. Hum. Vaccin. Immunother. 9: 1364–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coe CL, Lubach GR, and Kinnard J. 2012. Immune senescence in old and very old rhesus monkeys: reduced antibody response to influenza vaccination. Age (Dordr) 34: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petitdemange C, Kasturi SP, Kozlowski PA, Nabi R, Quarnstrom CF, Reddy PBJ, Derdeyn CA, Spicer LM, Patel P, Legere T, Kovalenkov YO, Labranche CC, Villinger F, Tomai M, Vasilakos J, Haynes B, Kang CY, Gibbs JS, Yewdell JW, Barouch D, Wrammert J, Montefiori D, Hunter E, Amara RR, Masopust D, and Pulendran B. 2019. Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques. JCI Insight 4: e126047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chamcha V, Kannanganat S, Gangadhara S, Nabi R, Kozlowski PA, Montefiori DC, LaBranche CC,Wrammert J, Keele BF, Balachandran H, Sahu S, Lifton M, Santra S, Basu R, Moss B, Robinson HL, and Amara RR. 2016. Strong, but age-dependent, protection elicited by a deoxyribonucleic acid/modified Vaccinia Ankara simian immunodeficiency virus vaccine. Open Forum Infect. Dis. 3: ofw034. [DOI] [PMC free article] [PubMed] [Google Scholar]