Abstract
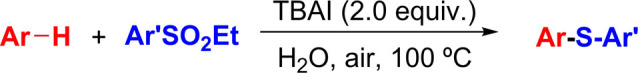
A tetrabutylammonium iodide-mediated direct sulfenylation of arenes with ethyl arylsulfinates in water was developed. Various electron-rich arenes and ethyl arylsulfinates were investigated in the reaction, and a series of aryl sulfides were obtained in excellent yields. The advantages of this green protocol were simple reaction conditions (metal-free, water as the solvent, and under air), odorless and easily available sulfur reagent, broad substrate scope, and gram-scale synthesis. Moreover, the potential application of aryl sulfides was exemplified by further transformations.
Introduction
The sulfide nucleus is a key constituent of many nature products and biological systems.1−3 The arylsulfides have been widely used in the field of total synthesis, medicinal chemistry, and materials science because of their unique biological activities and physical properties.4−10 Therefore, the development of efficient strategies for aryl sulfides has received considerable attention.11,12 Most of them construct C–S bonds by employing transition metal-catalyzed cross-coupling of thiols and their derivatives with organic halides/organic boronic acid.13−17 However, these methodologies generally suffer from a prefunctionalized starting material, harsh reaction conditions, and the formation of toxic waste. Recently, metal-free and transition metal-catalyzed direct C–H sulfenylation of arenes and various sulfur sources are particularly attractive because direct functionalization of C–H bonds for the synthesis of aryl sulfides can shorten the reaction steps and minimize the amount of waste formed.18−23 However, most of the reported protocols were established by using an organic solvent as the reaction media. From the perspective of green chemistry, water as a safe, cheap, and environmentally friendly solvent has been used in organic reactions.24−37 In 2016, Deng et al.38 developed a copper-catalyzed three-component reaction of arenes, iodohydrocarbon, and sulfur powder for the synthesis of aryl sulfides in water (Scheme 1a). Lu et al.39 reported an I2/PPh3-mediated regioselective synthesis of diaryl sulfides from sodium arylsulfinates via a metal-free radical strategy in water (Scheme 1b). Huang et al.40 described a phenyliodine(III) bis(trifluoroacetate) (PIFA)-mediated sulfenylation of arenes and allyl sulfides in water in the presence of TPGS-750-M as the surfactant (Scheme 1c). Sinha et al.41 also reported a bovine serum albumin–iodine cooperative catalyzed oxidative coupling-C(sp2)–H sulfenylation of indoles/hydroxyaryls with thiophenols on water (Scheme 1d). Yuan et al.42 developed a cobalt-catalyzed aerobic cross-dehydrogenative coupling of C–H and thiols in water for C–S formation (Scheme 1e). Although water as a green solvent has made some progress in direct C–H sulfenylation, these reported methods suffer from several limitations, such as the uses of metal catalysts, unstable biological reagents, toxic organic phosphine, sulfur agent with unpleasant odor, and complex reaction conditions. Therefore, a simple and efficient metal-free protocol using odorless sulfur reagents in combination with water as the solvent is still needed.
Scheme 1. Direct C–H Sulfenylation of Arenes in Water; (a) Deng’s Work, (b) Lu and Yi’s Work, (c) Huang’s Work, (d) Sinha’s Work, (e) Yuan’s Work, (f) This Work.
Sulfinic esters are odorless, easy-to-make, air-stable, and moisture-stable chemicals.43−52 Because of their unique electrophilic and nucleophilic reactivities, sulfinic esters have emerged as important building blocks for the synthesis of organic sulfur compounds.53−61 However, this sulfenylation of arene with sulfinic ester in water is rarely reported. As part of our continuing efforts using unconventional media in organic reactions,62−75 herein we disclose a simple and environmentally friendly method for the synthesis of aryl sulfides via direct C–H sulfenylation of arenes with ethyl arylsulfinates in water (Scheme 1f).
Results and Discussion
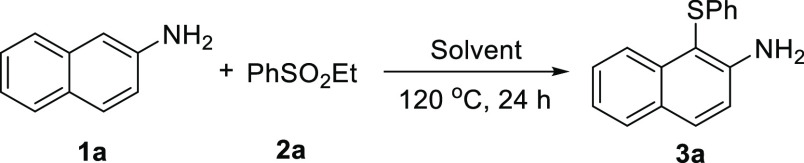
Our study began by performing the reaction of naphthalen-2-amine 1a and ethyl benzenesulfinate 2a in water at 120 °C for 24 h. The results are summarized in Table 1. Initially, when the ratio of 1a and 2a was 1:1, the sulfenylation reaction provided the desired product 3a in only 19% yield (entry 1). By increasing the dosage of 2a, the yield could be significantly increased. When 2 equiv of 2a was used, the yield of 3a reached 70% (entries 2 and 3). However, this yield could hardly be improved by only increasing the amount of 2a (entry 4). Further, we found that organic solvents are detrimental to the reaction (entries 5–8). In addition, shortening the reaction time led to a significant decrease in the yield of model reactions (entry 9).
Table 1. Optimization of the Reaction Conditionsa.
| entry | 1a/2a | solvent | yield (%) |
|---|---|---|---|
| 1 | 1.0/1.0 | H2O | 19 |
| 2 | 1.0/1.5 | H2O | 67 |
| 3 | 1.0/2.0 | H2O | 70 |
| 4 | 1.0/3.0 | H2O | 73 |
| 5 | 1.0/2.0 | nBuOAc | 12 |
| 6 | 1.0/2.0 | DMF | 22 |
| 7 | 1.0/2.0 | toluene | N.R. |
| 8 | 1.0/2.0 | PhCl | trace |
| 9b | 1.0/2.0 | H2O | 32 |
Reaction conditions: 1a (0.3 mmol), solvent (3 mL), 120 °C (oil bath), 24 h, under air.
9 h.
To increase the reaction yield and reduce the reaction temperature, a phase-transfer agent (PTA) tetrabutylammonium iodide (TBAI) was added into the reaction system. A catalytic amount of TBAI (20 mol %) had no obvious promotion effect on the reaction (Table 2, entry 1). However, with the increase in the amount of TBAI, the yield of 3a could be increased from 75 to 88% (entries 2–4). When TBAB or tetrabutylammonium chloride (TBAC) was used as a PTA, this promoting effect was not significant (entries 5 and 6). The combination of TBAC and KI seems to play the same role as TBAI, and 3a can be obtained in 83% yield (entry 7). The ionic surfactant sodium 1-dodecanesulfonate (SDS) could also promote this reaction, but the yield was slightly inferior (entry 8). Nonionic surfactants such as PEG 2000 and Tween 80 were also examined. We only used 10 mol % of amount to perform this reaction because of their higher molecular weights. The results showed that the promotion effect of PEG 2000 is quite significant compared with Tween 80, and the product 3a was obtained in 82% yield (entries 9 and 10). Further investigation revealed that the reaction yield decreased at 80 °C (entry 11). Under an argon atmosphere, the yield of 3a did not increase significantly (entry 12). Finally, the optimal reaction conditions were determined as follows: the ratio of 1a/2a = 1/2, 3 mL of water as the solvent, 2.0 equiv of TBAI, 100 °C (oil bath), reaction time 24 h, and under air.
Table 2. Effect of PTAs on the Aqueous-Phase Reaction of 1a and 2aa.
| entry | PTA | amount of PTA | yield (%) |
|---|---|---|---|
| 1 | TBAI | 20 mol % | 67 |
| 2 | TBAI | 1.0 equiv | 75 |
| 3 | TBAI | 2.0 equiv | 85 |
| 4 | TBAI | 3.0 equiv | 88 |
| 5 | TBAB | 2.0 equiv | 74 |
| 6 | TBAC | 2.0 equiv | 68 |
| 7 | TBAC/KI | 2.0 equiv | 83 |
| 8 | SDS | 2.0 equiv | 79 |
| 9 | PEG 2000 | 10 mol % | 82 |
| 10 | Tween 80 | 10 mol % | 66 |
| 11b | TBAI | 2.0 equiv | 76 |
| 12c | TBAI | 2.0 equiv | 80 |
Reaction conditions: 1a (0.3 mmol), 2a (0.6 mmol), H2O (3 mL) 100 °C (oil bath), 24 h, under air. TBAI: tetrabutylammonium iodide, TBAB: tetrabutylammonium bromide, TBAC: tetrabutylammonium chloride, SDS: sodium dodecane-1-sulfonate.
80 °C.
Under Ar.
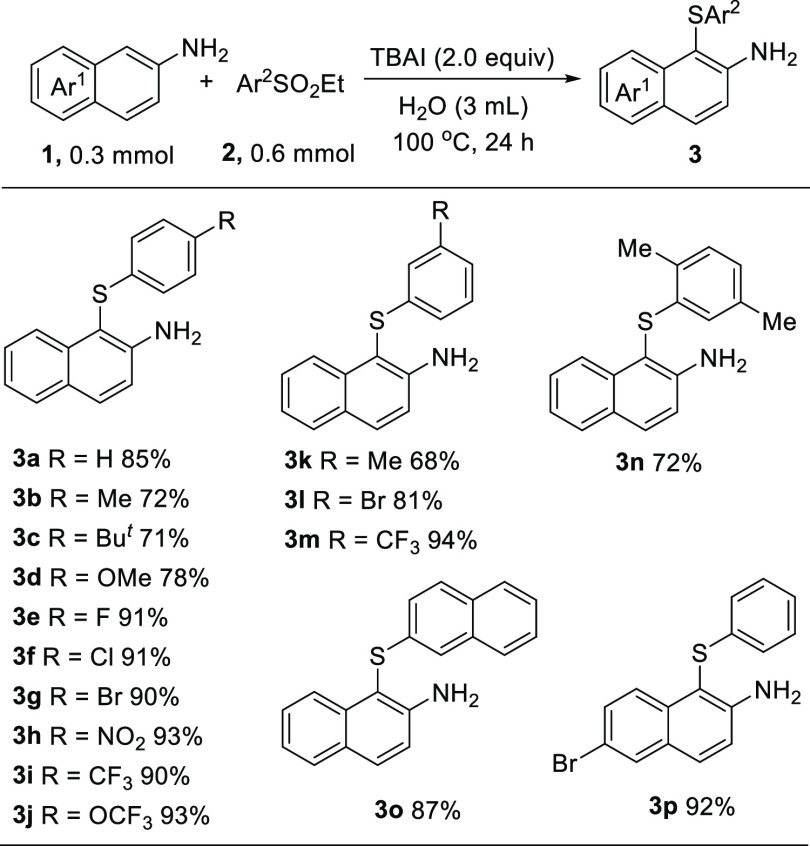
Next, we examined the scope of ethyl arylsulfinates for this direct C–H sulfenylation, and the results are shown in Scheme 2. Various ethyl aryl sulfinates were suitable to implement the 1-sulfenylation of naphthalen-2-amine, providing the desired products 3b–3m in good to excellent yields. The ethyl arylsulfinates with a variety of substituents such as methyl, tert-butyl, methoxy, fluoro, chloro, bromo, nitro, trifluoromethyl, and trifluoromethoxy groups on 3- or 4-substituted positions were tolerated. The reaction with the sterically congested substrate proceeded very well, affording the desired product 3n in 72% yield. The optimal conditions were also amenable to the reaction of 2-(ethylsulfonyl)naphthalene, and the desired product 3o was obtained in 87% yield. 6-Bromonaphthalen-2-amine was also quite well as a reactant in the transformation, and the product 3p was obtained in 92% yield. It is conceivable that the presence of a bromo substituent group in the structure of 3p should be helpful for its derivatization.
Scheme 2. Reactions of Naphthalen-2-amines with Ethyl Arylsulfinates.
It should be noted that aniline could conduct this reaction to give the product 4-(phenylthio)aniline 3q in 55% yield. The reaction of 2-phenylaniline with 2a also proceeded smoothly, affording the product 5-(phenylthio)-[1,1′-biphenyl]-2-amine 3r in 67% yield (Scheme 3).
Scheme 3. Reactions of Anilines with 2a.
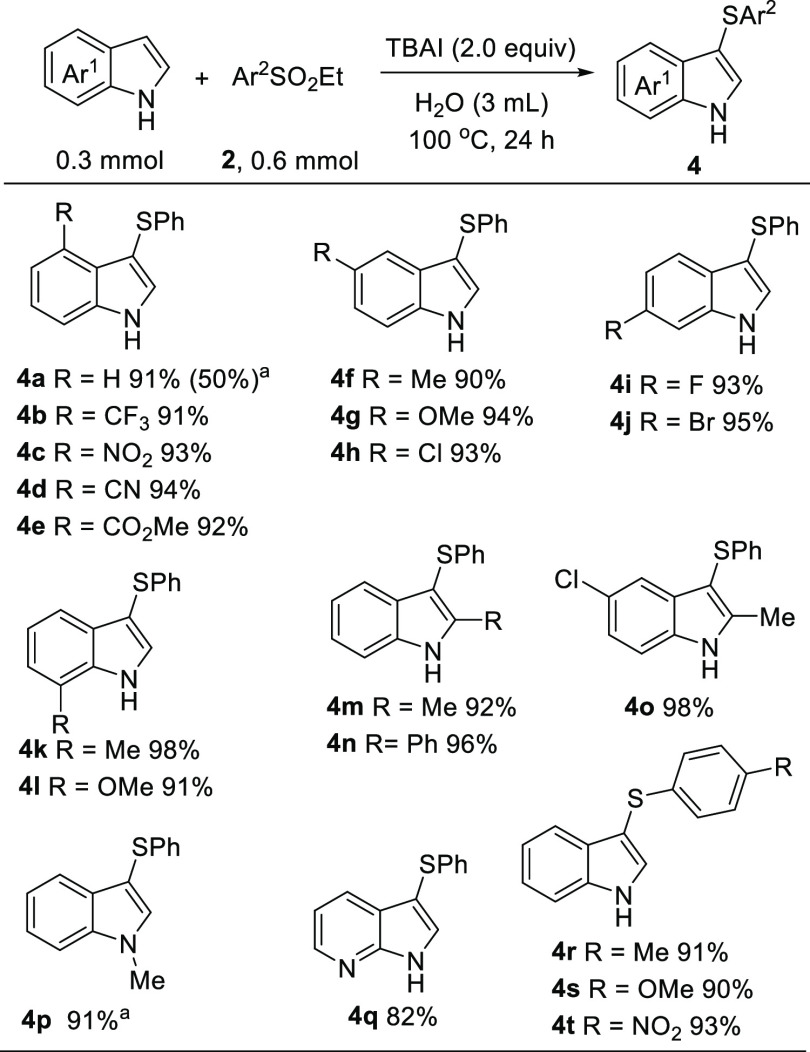
The high electrophilicity of 2a inspired us to investigate the possibility of indoles. As shown in Scheme 4, the reaction of indole with 2a proceeded very well to give the product 3-(phenylthio)-1H-indole 4a in 91% yield. In the absence of TBAI, the yield of 4a dropped to 50%. Upon repeating the reaction with 2a, indoles with different substituent groups on the arene ring all work well, efficiently providing the products C3-(phenylthio)indoles 4b–4p with excellent yields. The electron-withdrawing groups such as nitro, cyano, and methoxycarbonyl in the indole ring all tolerated well in this transformation. 7-Azaindole was also applicable in this reaction, and the desired product 4q was isolated in 82% yield. The chloro- and bromo-substituents in the indole ring could be delivered uneventfully into the products 4h and 4j. Although some methods proved to be effective for the C3-sulfenylation of indoles,76−81 the synthesis of products 4 in water has been rarely reported. In 2016, Wang et al. used sulfonyl hydrazides to implement the thiolation of indoles, but the reaction was performed at 140 °C.82 In contrast, our current system was carried out at 100 °C, and TBAI was a cheap, easily available, and recyclable reagent.
Scheme 4. Reactions of Indoles with Ethyl Aryl Sulfinates.
In the absence of TBAI.
Trimethoxybenzene proved to be a good nucleophile to react with ethyl aryl sulfinates under the standard conditions, and the electrophilic nature of the arene ring in the sulfinate component imposed a significant effect on the reaction (Scheme 5). The reaction of ethyl 4-nitrobenzenesulfinate with 1,3,5-trimethoxybenzene proceeded very well, providing the sulfenylation product 5c in 95% yield. Ethyl 4-methoxybenzenesulfonate participated into the reaction gently, and the desired product 5b was isolated in 65% yield. In addition, β-naphthol was also a suitable substrate for the sulfenylation, affording the desired product 5d in 45% yield.
Scheme 5. Reactions of 1,3,5-Trimethoxybenzene with Ethyl Aryl Sulfinates.
Intriguingly, pyrrole and 1-methylpyrrole underwent a double C–H sulfenylation in the presence of 4.0 equiv of ethyl aryl sulfinates, affording symmetrical bisthioethers 6a–6e in moderate to good yields (Scheme 6). 2,5-Dimethyl-1H-pyrrole can also be converted in a similar manner, providing the product 6f in 81% yield. It should be noted that although the synthesis of 6a- and 6f-type bisthioethers through double C–H sulfenylation of pyrroles has been observed, the reported system usually used an organic solvent as the reaction medium.84,85 To the best of our knowledge, our system is probably the first example to carry out the synthesis of bisthioethers in water. 3-Phenyl-1-tosyl-1H-pyrazol-5-amine as a multifunctional substrate also could react with ethyl phenyl sulfinate, and the product 6g was obtained in 65% yield.
Scheme 6. Synthesis of Bisthioethers Through Double Sulfenylation of Pyrroles.
To demonstrate the practicality of this method, gram-scale reactions were conducted under standard conditions. As shown in Scheme 7, the reactions could be easily scaled up with 10 mmol of 6-bromonaphthalen-2-amine or 6-bromo-1H-indole, affording the products 3p and 4j in 86% (2.83 g) and 89% (2.71 g) yields, respectively. Importantly, the products were isolated by simple extraction and filtration, avoiding the use of silica column chromatography.
Scheme 7. Gram-Scale Synthesis of 3p and 4j.
Next, the synthetic versatilities of 3p as the substrate were demonstrated by palladium-catalyzed cross-coupling reactions (Scheme 8). The compound 3p and p-tolylboronic acid underwent the Suzuki–Miyaura reaction in the presence of 10% Pd(PPh3)4, affording the corresponding product 7a in 84% yield. In addition, the compound 3p also reacted with phenylacetylene via the Sonogashira cross-coupling in the presence of PdCl2/PPh3 (10%) and CuI (20%) as the catalyst, furnishing the desired product 7b in 75% yield.
Scheme 8. Synthetic Transformations of 3p.
To elucidate the reaction mechanism, some control experiments were performed. As shown in Scheme 9, when 2.0 equiv of 2,2,6,6-tetramethylpiperidinooxy was added, 3a can be hardly detected. However, the addition of 2,6-di-tert-butyl-4-methylphenol did not significantly inhibit the reaction, and 3a can be isolated in 58% yield (Scheme 9a). We speculated that the reaction was more likely to be an electrophilic substitution reaction than a free-radical reaction. In the absence of 1a, ethyl benzenesulfinate 2a was decomposed to provide S-phenylbenzenesulfonic acid thioester 8a product. This result indicated that 8a may be an important intermediate in the reaction process. Furthermore, this reaction also proceeded well in the absence of TBAI (Scheme 9b). This fact implied that TBAI mainly acted as a PTA. Under the standard conditions, the reaction of 8a and 1a proceeded smoothly to afford the product 3a in 84% yield, and 62% yield of 3a was obtained in the absence of TBAI (Scheme 9c). These two experimental results further supported our previous point. When benzenesulfinic acid was treated under the standard conditions, 8a can be generated in 24% yield. However, this transformation failed without TBAI. This showed that TBAI plays a role as a catalyst in the formation of intermediate 8a (Scheme 9d).
Scheme 9. Control Experiments; (a) Effect of free radical scavenger on the model reaction, (b) Effect of TBAI on the conversion of ethyl benzenesulfinate, (c) Effect of TBAI on the reaction of 1a and 8a, (d) Effect of TBAI on the conversion of benzenesulfinic acid.
Based on all these observations, a plausible mechanism was proposed. As shown in Scheme 10, the initial event should be a decomposition of 2a, which gives 8a as an intermediate. A similar disproportionate reaction of sodium benzenesulfinate to a thiosulfonate has just been observed by Wang et al.86 Then, this intermediate 8a as an electrophile reacted with 1a to form the desired product 3a.87,88 Subsequently, the benzenesulfinic acid generated can be converted into 8a in the presence of TBAI.89,90 Finally, a sulfenylation cycle was established.
Scheme 10. Proposed Mechanism.
Conclusions
In summary, we have developed a TBAI-mediated direct sulfenylation of arenes with ethyl arylsulfinates in water. This reaction provided a green and efficient approach to produce diversified aryl sulfides in excellent yields. The merits of this method were simple reaction condition, odorless and easily available sulfur reagent, broad substrate scope, gram-scale synthesis, and further transformations.
Experimental Section
Unless otherwise noted, all synthetic steps were performed under an air atmosphere using Schlenk tubes. The materials obtained from commercial sources were used without further purification. Melting points were determined with a fusiometer and are not corrected. 1H NMR and 13C NMR spectra were recorded on a Bruker ADVANCE III HD 400 MHz spectrometer in CDCl3 or DMSO-d6 solution. All chemical shifts were reported in ppm (δ) relative to the internal standard tetramethylsilane (0 ppm). High-resolution mass spectra (HRMS) were acquired in electrospray ionization (ESI) mode using a time-of-flight mass analyzer.
General Procedure for TBAI-Mediated Reactions of Arenes with Ethyl Aryl Sulfinates to Aryl Sulfides
A mixture of arene (0.3 mmol), ethyl aryl sulfinate (0.6 mmol), TBAI (0.6 mmol), and H2O (3 mL) was added into a Schlenk tube. The solution was stirred and heated to 100 °C for 24 h in air. After completion of the reaction, the mixture was quenched with the saturated solution of NaCl (5 mL) and extracted with CH2Cl2 (10 mL × 3). The combined CH2Cl2 extracts were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude residue was purified by flash column chromatography on silica gel using PE/EtOAc as the eluent.
1-(Phenylthio)naphthalen-2-amine (3a)38
The crude mixture was purified via column chromatography using ethyl acetate/petroleum ether (PE) (1:30) as the eluent to give the product as a red solid (64.1 mg, 85%); mp 96.5–99.1 °C; 1H NMR (400 MHz, DMSO): δ 8.06 (d, J = 8.4 Hz, 1H), 7.79 (d, J = 8.8 Hz, 1H), 7.73 (d, J = 7.6 Hz, 1H), 7.37 (t, J = 7.8 Hz, 1H), 7.25–7.14 (m, 2H), 7.06 (t, J = 7.2 Hz, 1H), 6.99 (d, J = 8.0 Hz, 1H), 6.04 (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 150.41, 136.91, 136.31, 131.54, 128.99, 128.42, 127.48, 127.22, 125.56, 124.94, 122.87, 121.45, 118.18, 100.59.
1-(p-Tolylthio)naphthalen-2-amine (3b)42
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (52.3 mg, 72%); mp 112.4–117.0 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.06 (d, J = 8.5 Hz, 1H), 7.77 (d, J = 8.8 Hz, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.37 (td, J = 7.5, 1.3 Hz, 1H), 7.22–7.14 (m, 2H), 7.00 (d, J = 8.1 Hz, 2H), 6.90 (d, J = 8.2 Hz, 2H), 5.99 (s, 2H), 2.18 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δ 150.24, 136.30, 134.32, 133.33, 131.37, 129.63, 128.38, 127.40, 127.23, 125.92, 122.98, 121.41, 118.16, 101.38, 20.39.
1-((4-(tert-Butyl)phenyl)thio)naphthalen-2-amine (3c)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (64.6 mg, 71%); mp 120.2–122.4 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.08 (d, J = 8.4 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.37 (td, J = 7.7, 1.2 Hz, 1H), 7.24–7.14 (m, 4H), 6.91 (d, J = 8.5 Hz, 2H), 6.00 (s, 2H), 1.18 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δ 150.28, 147.56, 136.41, 133.43, 131.38, 128.37, 127.43, 127.20, 125.89, 125.50, 122.98, 121.39, 118.14, 101.18, 33.98, 31.00. HRMS (ESI): m/z calcd for C20H21NS+ (M + Na)+, 330.1287; found, 330.1281.
1-((4-Methoxyphenyl)thio)naphthalen-2-amine (3d)42
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (65.4 mg, 78%); mp 101.8–105.5 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.11 (d, J = 8.5 Hz, 1H), 7.75 (d, J = 8.9 Hz, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.41–7.36 (m, 1H), 7.16 (d, J = 8.9 Hz, 1H), 6.99 (d, J = 8.9 Hz, 1H), 6.80 (d, J = 8.9 Hz, 1H), 6.01 (s, 1H), 3.65 (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 157.48, 150.05, 136.25, 131.20, 128.36, 128.05, 127.37, 127.33, 127.21, 123.03, 121.37, 118.14, 114.80, 102.60, 55.11.
1-((4-Fluorophenyl)thio)naphthalen-2-amine (3e)19
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (73.2 mg, 91%); mp 90.6–92.8 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.05 (d, J = 8.5 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.39 (td, J = 8.0 Hz, 1.3 Hz, 1H), 7.21–7.14 (m, 2H), 7.09–6.99 (m, 4H), 6.06 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 161.47, 159.07, 150.39, 136.21, 132.40, 131.63, 128.46, 127.75, 127.67, 127.58, 127.23, 122.77, 121.50, 118.23, 116.14, 115.92, 101.07.
1-((4-Chlorophenyl)thio)naphthalen-2-amine (3f)42
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (77.8 mg, 91%); mp 123.1–125.8 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.01 (d, J = 8.4 Hz, 1H), 7.79 (d, J = 8.9 Hz, 1H), 7.72 (d, J = 7.9 Hz, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.28–7.11 (m, 4H), 6.97 (d, J = 8.6 Hz, 2H), 6.07 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 150.51, 131.79, 129.51, 128.90, 128.49, 127.63, 127.23, 127.21, 122.66, 121.54, 118.25, 100.01.
1-((4-Bromophenyl)thio)naphthalen-2-amine (3g)94
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (89.8 mg, 90%); mp 107.5–109.5 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.00 (d, J = 8.3 Hz, 1H), 7.80 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.40 (td, J = 3.5, 1.5 Hz, 2H), 7.37 (d, J = 2.0 Hz, 1H), 7.23–7.17 (m, 2H), 6.92 (d, J = 2.0 Hz, 1H), 6.90 (d, J = 2.0 Hz, 1H), 6.07 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 150.51, 136.69, 136.13, 131.80, 131.75, 128.48, 128.48, 127.64, 127.52, 127.21, 122.63, 121.53, 118.24, 117.65, 99.83.
1-((4-Nitrophenyl)thio)naphthalen-2-amine (3h)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a bright yellow solid (81.9 mg, 93%); mp 160–160.5 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.06 (d, J = 9.0 Hz, 2H), 7.91 (d, J = 8.5 Hz, 1H), 7.84 (d, J = 8.9 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.44–7.33 (td, J = 7.7, 1.2 Hz, 1H), 7.27–7.17 (m, 2H), 7.13 (d, J = 9.0 Hz, 2H), 6.15 (s, 2H). 13C NMR (101 MHz, DMSO-d6): δ 150.87, 147.55, 144.61, 135.97, 132.35, 128.63, 127.89, 127.23, 125.48, 124.11, 122.23, 121.78, 118.38, 97.70. HRMS (ESI): m/z calcd for C20H21NS+ (M + Na)+, 297.0692; found, 297.0691.
1-((4-(Trifluoromethyl)phenyl)thio)naphthalen-2-amine (3i)94
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (85.7 mg, 90%); mp 120.6–121.9 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.00 (d, J = 8.4 Hz, 1H), 7.82 (d, J = 8.9 Hz, 1H), 7.74 (d, J = 7.7 Hz, 1H), 7.46–7.36 (m, 3H), 7.31 (s, 1H), 7.25–7.11 (m, 3H), 6.12 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 150.75, 139.11, 136.16, 132.08, 130.00, 129.15, 128.56, 127.77, 127.19, 125.22, 122.46, 121.60 (t, J = 6.5 Hz), 118.27, 98.92.
1-((4-(Trifluoromethoxy)phenyl)thio)naphthalen-2-amine (3j)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red liquid (92.8 mg, 93%); 1H NMR (400 MHz, DMSO-d6): δ 8.04 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 7.9 Hz, 1H), 7.39 (t, J = 7.6 Hz, 1H), 7.25–7.20 (m, 2H), 7.20–7.15 (m, 2H), 7.06 (d, J = 8.8 Hz, 2H), 6.10 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 150.61, 145.84, 136.61, 136.25, 131.87, 128.50, 127.68, 127.27, 127.00, 122.65, 121.88, 121.56, 118.29, 99.95; 19F NMR (376 MHz, DMSO-d6): δ −57.14. HRMS (ESI): m/z calcd for C17H12F3NOS+ (M + H)+, 336.0664; found, 336.0667.
1-(m-Tolylthio)naphthalen-2-amine (3k)42
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (49.9 mg, 68%); mp 88.8–89.9 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.04 (d, J = 8.5 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.37 (td, J = 7.7, 1.3 Hz, 1H), 7.22–7.13 (m, 2H), 7.05 (t, J = 7.9 Hz, 1H), 6.92–6.84 (m, 2H), 6.69 (d, J = 7.9 Hz, 1H), 5.99 (s, 2H), 2.16 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δ 150.35, 138.23, 136.72, 136.35, 131.48, 128.86, 128.40, 127.44, 127.20, 126.10, 125.82, 122.93, 122.62, 121.43, 118.15, 118.15, 118.15, 100.75, 20.95.
1-((3-Bromophenyl)thio)naphthalen-2-amine (3l)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red liquid (79.5 mg, 81%); 1H NMR (400 MHz, DMSO-d6): δ 8.00 (d, J = 8.6 Hz, 1H), 7.81 (d, J = 8.8 Hz, 1H), 7.73 (d, J = 7.7 Hz, 1H), 7.39 (td, J = 7.9, 1.3 Hz, 1H), 7.26 (dq, J = 7.9, 0.9 Hz, 1H), 7.23–7.12 (m, 3H), 7.07 (t, J = 1.8 Hz, 1H), 6.98 (dq, J = 7.9, 1.0 Hz, 1H), 6.10 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 150.63, 139.96, 136.15, 131.97, 130.93, 128.52, 127.74, 127.72, 127.38, 127.17, 124.53, 122.55, 122.23, 121.59, 118.24, 99.33. HRMS (ESI): m/z calcd for C16H12BrNS+ (M + Na)+, 351.9766; found, 351.9772.
1-((3-(Trifluoromethyl)phenyl)thio)naphthalen-2-amine (3m)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red liquid (89.4 mg, 94%); 1H NMR (400 MHz, DMSO-d6): δ 8.02 (d, J = 8.5 Hz, 1H), 7.82 (d, J = 8.9 Hz, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.39 (dd, J = 10.0, 7.1 Hz, 2H), 7.33 (s, 1H), 7.27–7.14 (m, 1H), 6.14 (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 150.75, 139.12, 136.19, 132.08, 129.96, 129.14, 128.55, 127.22, 125.22, 122.49, 121.93–121.01 (m), 118.28, 98.99. HRMS (ESI): m/z calcd for C17H21F3NS+ (M + H)+, 320.0715; found, 320.0718.
1-((2,5-Dimethylphenyl)thio)naphthalen-2-amine (3n)94
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (60.3 mg, 72%); mp 62.7–65.1 °C; 1H NMR (400 MHz, DMSO-d6): δ 7.99 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 7.7 Hz, 1H), 7.37 (td, J = 7.7, 1.3 Hz, 1H), 7.22 (d, J = 8.9 Hz, 1H), 7.18 (td, J = 7.4, 1.1 Hz, 1H), 7.08 (d, J = 7.6 Hz, 1H), 6.78 (d, J = 8.3 Hz, 1H), 6.18 (s, 1H), 5.96 (s, 2H), 2.45 (s, 3H), 1.91 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δ 150.47, 136.44, 135.38, 135.18, 131.47, 130.03, 128.44, 127.44, 127.29, 125.32, 123.96, 122.96, 121.47, 118.16, 100.02.
1-(Naphthalen-2-ylthio)naphthalen-2-amine (3o)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a red solid (78.1 mg, 87%); mp 120.7–122.3 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.10 (d, J = 8.4 Hz, 1H), 7.83 (d, J = 8.9 Hz, 1H), 7.76 (t, J = 8.2 Hz, 3H), 7.66–7.57 (d, J = 7.9 Hz, 1H), 7.46 (s, 1H), 7.43–7.33 (m, 3H), 7.26 (d, J = 8.9 Hz, 1H), 7.17 (td, J = 8.8, 4.2 Hz, 2H), 6.09 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 150.53, 136.35, 134.66, 133.30, 131.68, 130.95, 128.53, 128.48, 127.58, 127.52, 127.27, 126.61, 126.57, 125.22, 124.54, 123.00, 122.86, 121.49, 118.27, 100.48. HRMS (ESI): m/z calcd for C20H15NS+ (M + H)+, 302.0998; found, 302.0995.
6-Bromo-1-(phenylthio)naphthalen-2-amine (3p)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a reddish solid (91.1 mg, 92%); mp 122.4–123.0 °C; 1H NMR (400 MHz, DMSO-d6): δ 7.96 (dd, J = 12.0, 5.5 Hz, 1H), 7.77 (d, J = 8.9 Hz, 1H), 7.48 (dd, J = 9.0, 2.1 Hz, 1H), 7.28–7.15 (m, dd, J = 17.5, 8.4 Hz, 3H), 7.08 (tt, J = 7.7, 1.1 Hz, 1H), 7.02–6.86 (m, 2H), 6.15 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 150.86, 136.54, 135.13, 130.71, 130.19, 130.08, 129.07, 128.44, 125.58, 125.29, 125.10, 119.38, 114.02, 100.56. HRMS (ESI): m/z calcd for C16H12BrNS+ (M + H)+, 329.9974; found, 329.995. HRMS (ESI): m/z calcd for C16H12BrNS+ (M + H)+, 329.9947; found, 329.9951.
4-(Phenylthio)aniline (3q)95
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a white solid (60.4 mg, 55%), mp 83.4–84.5 °C; 1H NMR (400 MHz, DMSO-d6): δ 7.27–7.21 (t, J = 7.7 Hz, 2H), 7.18 (d, J = 2.0 Hz, 1H), 7.17 (d, J = 2.1 Hz, 1H), 7.12–7.06 (tt, J = 7.7, 1.3 Hz, 1H), 7.01 (dd, J = 8.0, 1.6 Hz, 2H), 6.64–6.59 (dt, J = 8.6, 2.4 Hz, 2H), 5.52 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 150.03, 140.15, 136.38, 128.93, 125.92, 124.93, 114.79, 114.55.
5-(Phenylthio)-[1,1′-biphenyl]-2-amine (3r)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a reddish solid (54.5 mg, 67%), mp 136.0–137.9 °C; 1H NMR (400 MHz, DMSO-d6): δ 7.48–7.41 (m, 4H), 7.38–7.32 (m, 1H), 7.29–7.21 (m, 3H), 7.17–7.08 (m, 4H), 6.86 (d, J = 8.3 Hz, 1H), 5.23 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 146.28, 139.53, 138.51, 136.71, 135.09, 129.02, 128.87, 128.54, 127.15, 126.47, 125.22, 116.42, 116.30. HRMS (ESI): m/z calcd for C18H15NS+ (M + H)+, 278.0998; found, 278.0995.
3-(Phenylthio)-1H-indole (4a)87
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (67.6 mg, 91%), mp 121.3–122.6 °C; 1H NMR (400 MHz, CDCl3): δ 8.33 (s, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.46–7.38 (m, 2H), 7.29–7.22 (m, 1H), 7.15 (td, J = 7.8, 3.2 Hz, 3H), 7.10 (d, J = 6.8 Hz, 2H), 7.04 (t, J = 7.2 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 139.34, 136.61, 130.81, 129.22, 128.82, 125.97, 124.90, 123.18, 121.04, 119.80, 111.71, 102.95.
3-(Phenylthio)-4-(trifluoromethyl)-1H-indole (4b)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (72.9 mg, 92%), mp 190.2–190.6 °C; 1H NMR (400 MHz, CDCl3): δ 8.70 (s, 1H), 7.58 (d, J = 8.2 Hz, 1H), 7.55–7.51 (m, 2H), 7.28 (t, J = 7.9 Hz, 1H), 7.14 (tt, J = 8.5, 2.3 Hz, 2H), 7.07–6.99 (m, 3H); 13C NMR (101 MHz, CDCl3): δ 140.59, 137.92, 135.19, 128.63, 125.71, 124.72, 121.98, 119.72 (q, J = 6.3 Hz), 116.01, 102.13. 19F NMR (376 MHz, CDCl3): δ −57.58. HRMS (ESI): m/z calcd for C15H10F3NS+ (M + Na)+, 316.0378; found, 316.0383.
4-Nitro-3-(phenylthio)-1H-indole (4c)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a yellow solid (75.3 mg, 93%), mp 148.7–149.5 °C; 1H NMR (400 MHz, CDCl3): δ 9.14 (s, 1H), 7.73 (d, J = 7.6 Hz, 1H), 7.68 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.34–7.26 (m, 1H), 7.20–7.13 (m, 2H), 7.13–7.02 (m, 3H); 13C NMR (101 MHz, CDCl3): δ 143.47, 139.15, 138.98, 135.07, 128.90, 127.01, 125.51, 122.09, 120.29, 117.97, 117.07, 103.63. HRMS (ESI): m/z calcd for C14H10N2O2S+ (M + Na)+, 293.0355; found, 293.0359.
3-(Phenylthio)-1H-indole-4-carbonitrile (4d)78
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (70.5 mg, 94%), mp 156.3–158.2 °C; 1H NMR (400 MHz, DMSO-d6): δ 12.33 (s, 1H), 8.05 (d, J = 2.8 Hz, 1H), 7.86 (dd, J = 8.4, 0.8 Hz, 1H), 7.58 (dd, J = 7.6, 0.8 Hz, 1H), 7.39–7.31 (m, 1H), 7.23 (t, J = 7.6 Hz, 2H), 7.13–7.01 (m, 3H); 13C NMR (101 MHz, DMSO-d6): δ 139.52, 137.13, 136.51, 128.90, 127.70, 127.47, 125.55, 125.01, 122.16, 117.76, 117.74, 101.08, 99.36.
Methyl 3-(Phenylthio)-1H-indole-4-carboxylate (4e)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (73.9 mg, 92%), mp 188.2–188.6 °C; 1H NMR (400 MHz, CDCl3): δ 9.16 (s, 1H), 7.49 (dd, J = 7.5, 2.3 Hz, 2H), 7.41 (d, J = 2.7 Hz, 1H), 7.24 (t, J = 7.8 Hz, 1H), 7.19–7.10 (m, 1H), 7.09–6.98 (m, 3H), 3.60 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 169.70, 140.26, 137.65, 133.98, 128.69, 125.67, 125.46, 125.32, 124.72, 122.27, 122.18, 115.29, 102.05, 52.05.
5-Methyl-3-(phenylthio)-1H-indole (4f)90
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (64.6 mg, 90%), mp 160.9–161.3 °C; 1H NMR (400 MHz, CDCl3): δ 8.19 (s, 1H), 7.32 (s, 1H), 7.27 (d, J = 2.8 Hz, 1H), 7.18 (d, J = 8.4 Hz, 1H), 7.08–7.03 (m, 2H), 7.02–6.92 (m, 4H), 2.31 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 139.61, 134.87, 131.08, 130.47, 129.48, 129.20, 128.82, 125.72, 124.77, 119.20, 111.41, 101.84, 21.55.
5-Methoxy-3-(phenylthio)-1H-indole (4g)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a pale yellow oil (71.6 mg, 94%); 1H NMR (400 MHz, CDCl3): δ 8.38 (s, 1H), 7.40 (d, J = 2.8 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 7.18–7.13 (m, 2H), 7.11–7.02 (m, 4H), 6.90 (dd, J = 8.8, 2.8 Hz, 1H), 3.76 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 155.24, 139.46, 131.47, 130.08, 128.83, 125.77, 124.83, 113.71, 112.57, 102.24, 100.90, 55.91.
5-Chloro-3-(phenylthio)-1H-indole (4h)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (72.1 mg, 93%), mp 167.2–168.1 °C; 1H NMR (400 MHz, CDCl3): δ 8.45 (s, 1H), 7.59 (d, J = 1.6 Hz, 1H), 7.51 (d, J = 2.8 Hz, 1H), 7.35 (d, J = 8.4 Hz, 1H), 7.21 (dd, J = 8.4, 2.0 Hz, 1H), 7.20–7.15 (m, 2H), 7.11–7.04 (m, 3H); 13C NMR (101 MHz, CDCl3): δ 138.79, 132.15, 128.94, 126.00, 125.16, 123.70, 123.64, 120.68, 119.20, 112.82, 112.50, 102.85.
6-Fluoro-3-(phenylthio)-1H-indole (4i)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (84.4 mg, 93%), mp 161.7–162.5 °C; 1H NMR (400 MHz, CDCl3): δ 8.36 (s, 1H), 7.50 (dd, J = 8.8, 5.2 Hz, 1H), 7.46 (d, J = 2.8 Hz, 1H), 7.19–7.14 (m, 2H), 7.13–7.04 (m, 4H), 6.91 (td, J = 9.6, [2.0 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 161.80, 138.97, 136.59, 136.46, 131.02, 130.98, 128.89, 126.06, 125.08, 120.80, 120.70, 110.03, 109.79, 103.42, 98.28, 98.01; 19F NMR (376 MHz, CDCl3): δ −119.72 (s).
6-Bromo-3-(phenylthio)-1H-indole (4j)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (86.2 mg, 95%), mp 160.9–161.3 °C; 1H NMR (400 MHz, CDCl3): δ 8.43 (s, 1H), 7.56 (d, J = 1.2 Hz, 1H), 7.47–7.40 (m, 2H), 7.27–7.22 (m, 1H), 7.19–7.12 (m, 2H), 7.07 (d, J = 8.0 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 138.81, 137.35, 131.26, 128.90, 128.12, 126.06, 125.14, 124.40, 121.11, 116.79, 114.69, 103.57.
7-Methyl-3-(phenylthio)-1H-indole (4k)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a blue oil (70.2 mg, 98%); 1H NMR (400 MHz, CDCl3): δ 8.28 (s, 1H), 7.45 (d, J = 7.2 Hz, 1H), 7.38 (s, 1H), 7.14–7.01 (m, 7H), 2.47 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 139.36, 136.16, 130.57, 128.80, 128.77, 125.93, 124.87, 123.67, 121.16, 120.92, 117.43, 103.11, 16.54,
7-Methoxy-3-(phenylthio)-1H-indole (4l)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a blue solid (69.1 mg, 91%), mp 115.2–116.3 °C; 1H NMR (400 MHz, CDCl3): δ 8.62 (s, 1H), 7.40 (d, J = 2.8 Hz, 1H), 7.20 (d, J = 8.0 Hz, 1H), 7.17–6.99 (m, 6H), 6.68 (d, J = 7.6 Hz, 1H), 3.95 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 146.41, 139.48, 130.66, 130.31, 128.78, 127.16, 125.92, 124.82, 121.43, 112.31, 103.14, 102.88, 55.54.
2-Methyl-3-(phenylthio)-1H-indole (4m)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (70.3 mg, 92%), mp 118.3–120.0 °C; 1H NMR (400 MHz, CDCl3): δ 8.16 (s, 1H), 7.54 (d, J = 7.6 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.21–7.14 (m, 2H), 7.14–7.08 (m, 3H), 7.05–6.99 (m, 3H), 2.45 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 141.29, 139.45, 135.54, 130.38, 128.80, 125.58, 124.62, 122.27, 120.79, 119.06, 110.79, 99.35, 12.24.
2-Phenyl-3-(phenylthio)-1H-indole (4n)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (86.1 mg, 96%); 1H NMR (400 MHz, CDCl3): δ 8.39 (s, 1H), 7.66–7.60 (dt, J = 6.8, 1.6 Hz, 2H), 7.54 (d, J = 7.9 Hz, 1H), 7.34–7.26 (m, 4H), 7.20–7.14 (td, J = 7.0, 4.7 Hz, 1H), 7.09–6.99 (m, 5H), 6.97–6.91 (tt, J = 7.6, 1.7 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 142.09, 139.28, 135.87, 131.42, 131.21, 128.86, 128.81, 128.75, 128.17, 125.60, 124.67, 123.41, 121.22, 120.02, 111.23, 99.39.
5-Chloro-2-methyl-3-(phenylthio)-1H-indole (4o)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (80.5 mg, 98%), mp 135.9–136.7 °C; 1H NMR (400 MHz, CDCl3): δ 8.28 (s, 1H), 7.51 (d, J = 2.0 Hz, 1H), 7.23 (m, 1H), 7.19–7.09 (m, 3H), 7.08–6.98 (m, 3H), 2.48 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 142.15, 139.34, 135.93, 131.48, 131.26, 128.92, 128.87, 128.80, 128.23, 125.66, 124.73, 123.47, 121.27, 120.08, 111.29, 99.45.
1-Methyl-3-(phenylthio)-1H-indole (4p)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (69.1 mg, 91%), mp 116.7–118.5 °C; 1H NMR (400 MHz, CDCl3): δ 7.66 (d, J = 8.0 Hz, 1H), 7.41–7.31 (m, 2H), 7.21–7.14 (m, 3H), 7.13–7.10 (m, 4H), 3.80 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 138.63, 135.96, 129.32, 128.79, 127.53, 126.72, 126.32, 124.12, 121.20, 120.52, 110.32, 31.25.
3-(Phenylthio)-1H-pyrrolo(2,3-b) Pyridine (4q)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a pale yellow solid (55.1 mg, 82%), mp 147.6–147.9 °C; 1H NMR (400 MHz, CDCl3): δ 11.67 (s, 1H), 8.41 (dd, J = 4.8, 1.2 Hz, 1H), 7.95 (dd, J = 7.6, 1.2 Hz, 1H), 7.70 (s, 1H), 7.22–7.12 (m, 3H), 7.14–7.03 (m, 3H); 13C NMR (101 MHz, CDCl3): δ 149.27, 143.45, 138.89, 132.02, 128.93, 128.67, 126.15, 125.21, 122.31, 116.97, 101.58.
3-(p-Tolylthio)-1H-indole (4r)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (64.9 mg, 91%), mp 137.6–138.8 °C; 1H NMR (400 MHz, CDCl3): δ 8.35 (s, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.47 (d, J = 2.0 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.28–7.23 (m, 2H), 7.18–7.13 (m, 1H), 7.03 (d, J = 8.4 Hz, 2H), 6.97 (d, J = 8.0 Hz, 2H), 2.24 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 136.59, 135.60, 134.77, 130.53, 129.61, 129.25, 126.39, 120.96, 119.84, 111.65, 103.68, 21.00.
3-((4-Methoxyphenyl)thio)-1H-indole (4s)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a white solid (68.8 mg, 90%), mp 138.0–138.6 °C; 1H NMR (400 MHz, CDCl3): δ 8.34 (s, 1H), 7.62 (d, J = 8.0 Hz, 1H), 7.45–7.36 (m, 2H), 7.27–7.20 (m, 1H), 7.16–7.09 (m, 3H), 6.75–6.71 (m, 2H), 3.71 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 157.88, 136.56, 133.79, 130.14, 128.67, 123.05, 120.89, 119.76, 115.28, 114.60, 111.64, 104.71, 55.46.
3-((4-Nitrophenyl)thio)-1H-indole (4t)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a yellow solid (75.3 mg, 93%), mp 121.2–121.7 °C; 1H NMR (400 MHz, CDCl3): δ 8.71 (s, 1H), 8.02–7.96 (m, 2H), 7.58–7.45 (m, 3H), 7.36–7.26 (m, 1H), 7.23–7.15 (m, 1H), 7.15–7.08 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 150.00, 144.98, 136.72, 131.38, 128.54, 125.20, 123.98, 123.64, 121.51, 119.30, 112.10, 100.19.
Phenyl(2,4,6-trimethoxyphenyl)sulfane (5a)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a white solid (65.2 mg, 79%), mp 119.6–121.5 °C; 1H NMR (400 MHz, CDCl3): δ 7.18–7.12 (m, 2H), 7.07–6.99 (m, 3H), 6.22 (s, 2H), 3.87 (s, 3H), 3.81 (s, 6H); 13C NMR (101 MHz, CDCl3): δ 163.05, 162.68, 138.81, 128.60, 125.76, 124.48, 98.85, 91.34, 56.44, 55.57.
(4-Methoxyphenyl)(2,4,6-trimethoxyphenyl)sulfane (5b)91
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to give the product as a colorless oil (59.7 mg, 65%); 1H NMR (400 MHz, CDCl3): δ 7.08 (d, J = 2.2 Hz, 1H), 7.06 (d, J = 2.2 Hz, 1H), 6.74 (d, J = 2.2 Hz, 1H), 6.72 (d, J = 2.1 Hz, 1H), 6.19 (s, 2H), 3.85 (s, 3H), 3.81 (s, 6H), 3.74 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 162.70, 162.44, 157.67, 129.36, 128.69, 114.37, 100.73, 91.33, 56.42, 55.54, 55.42.
(4-Nitrophenyl)(2,4,6-trimethoxyphenyl)sulfane (5c)40
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the product as a bright yellow solid (92.2 mg, 96%), mp 131–132 °C; 1H NMR (400 MHz, CDCl3): δ 8.00 (d, J = 9.0 Hz, 1H), 7.05 (d, J = 9.0 Hz, 1H), 6.24 (s, 1H), 3.89 (s, 2H), 3.80 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 163.90, 162.53, 149.56, 144.71, 124.92, 123.82, 96.02, 91.40, 77.16, 56.42, 55.65.
1-(Phenylthio)naphthalen-2-ol (5d)94
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:100) as the eluent to give the product as a yellow liquid (34.1 mg, 45%); 1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 8.3 Hz, 1H), 7.92 (d, J = 8.8 Hz, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.51 (t, J = 7.5 Hz, 1H), 7.44–7.32 (m, 2H), 7.17 (d, J = 8.5 Hz, 3H), 7.15–7.09 (m, 1H), 7.04 (d, J = 6.6 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 157.14, 135.58, 135.49, 132.97, 129.63, 129.31, 128.71, 128.09, 126.51, 126.03, 124.83, 124.00, 117.00, 108.18.
2,5-Bis(phenylthio)-1H-pyrrole (6a)61
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:100) as the eluent to give the product as a light gray liquid (42.7 mg, 51%); 1H NMR (400 MHz, DMSO-d6): δ 12.21 (s, 1H), 7.31 (s, 1H), 7.30 (s, 2H), 7.28 (s, 1H), 7.15 (t, J = 7.4 Hz, 2H), 7.05 (d, J = 1.2 Hz, 2H), 7.03 (d, J = 1.0 Hz, 2H), 6.58 (d, J = 2.4 Hz, 2H); 13C NMR (101 MHz, DMSO-d6): δ 135.11, 134.74, 129.72, 126.29, 119.67, 118.99, 20.45.
2,5-Bis(p-tolylthio)-1H-pyrrole (6b)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:100) as the eluent to give the product as a light gray liquid (37.8 mg, 41%); 1H NMR (400 MHz, DMSO-d6): δ 12.13 (s, 1H), 7.11 (d, J = 8.0 Hz, 4H), 6.99–6.95 (m, 4H), 6.50 (s, 2H), 2.23 (s, 6H); 13C NMR (101 MHz, DMSO-d6): δ 135.11, 134.74, 129.72, 126.29, 119.67, 118.99, 20.45. HRMS (ESI): m/z calcd for C18H17NS2+ (M + Na)+, 334.0695; found, 334.0691.
2,5-Bis((4-nitrophenyl)thio)-1H-pyrrole (6c)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:50) as the eluent to give the product as a bright yellow solid (91.8 mg, 82%), mp 86.7–89.2 °C; 1H NMR (400 MHz, DMSO-d6): δ 12.55 (s, 1H), 8.46–7.87 (m, 4H), 7.42–7.03 (m, 4H), 6.76 (d, J = 2.4 Hz, 2H); 13C NMR (101 MHz, DMSO-d6): δ 148.44, 145.02, 125.33, 124.38, 120.69, 117.74. HRMS (ESI): m/z calcd for C16H11N3O4S2+ (M + Na)+, 396.0083; found, 396.0089.
1-Methyl-2,5-bis(phenylthio)-1H-pyrrole (6d)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:100) as the eluent to give the product as a colorless liquid (62.5 mg, 71%); 1H NMR (400 MHz, CDCl3): δ 7.24 (dd, J = 12.5, 4.8 Hz, 1H), 7.12 (t, J = 7.4 Hz, 1H), 6.99 (t, J = 7.5 Hz, 1H), 6.69 (s, 1H), 3.50 (s, 1H); 13C NMR (101 MHz, CDCl3): δ 138.07, 129.20, 125.92, 125.67, 122.25, 119.54, 31.50. HRMS (ESI): m/z calcd for C17H15NS2+ (M + H)+, 298.0719; found, 298.0724.
1-Methyl-2,5-bis(p-tolylthio)-1H-pyrrole (6e)83
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:100) as the eluent to give the product as a colorless liquid (57.8 mg, 60%); 1H NMR (400 MHz, CDCl3): δ 7.08 (d, J = 8.0 Hz, 1H), 6.95 (d, J = 8.3 Hz, 1H), 6.70 (s, 1H), 3.54 (s, 1H), 2.33 (s, 1H); 13C NMR (101 MHz, CDCl3): δ 135.51, 134.38, 129.94, 126.24, 122.58, 119.16, 31.46, 21.01.
2,5-Dimethyl-3,4-bis(phenylthio)-1H-pyrrole (6f)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:100) as the eluent to give the product as a white solid (75.6 mg, 81%), mp 89.7–91.5 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.59 (s, 1H), 7.14 (t, J = 7.7 Hz, 4H), 7.04–6.97 (tt, J = 7.4, 1.3 Hz, 2H), 6.89 (dd, J = 8.4, 1.1 Hz, 4H), 2.22 (s, 6H); 13C NMR (101 MHz, DMSO-d6): δ 139.69, 133.40, 128.60, 124.77, 124.25, 107.52, 11.63. HRMS (ESI): m/z calcd for C18H17NS2+ (M + Na)+, 334.0695; found, 334.0700.
3-Phenyl-4-(phenylthio)-1-tosyl-1H-pyrazol-5-amine (6g)
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:100) as the eluent to give the product as a white solid (60.8 mg, 65%); 1H NMR (400 MHz, CDCl3): δ 7.93 (d, J = 8.4 Hz, 1H), 7.83 (dd, J = 6.5, 3.3 Hz, 1H), 7.32 (dd, J = 11.8, 4.9 Hz, 2H), 6.98 (d, J = 8.0 Hz, 1H), 6.85 (s, 1H), 5.48 (s, 1H), 2.43 (s, 2H), 2.26 (s, 2H). 13C NMR (101 MHz, CDCl3): δ 157.44, 153.17, 146.00, 135.38, 134.18, 133.04, 131.29, 130.09, 129.98, 129.33, 128.24, 128.08, 128.04, 125.36, 86.84, 77.48, 77.16, 76.84, 21.87, 20.97. HRMS (ESI): m/z calcd for C18H17NS2+ (M + Na)+, 436.1150; found, 436.1143.
General Procedure for Gram-Scale Experiment
A mixture of 6-bromonaphthalen-2-amine or 6-bromo-1H-indole (10 mmol), ethyl benzenesulfinate (20 mmol), TBAI (2 equiv), and H2O (25 mL) were added into a Schlenk tube. The solution was stirred and heated to 100 °C for 48 h in air. After completion of the reaction, the mixture was extracted with dichloromethane (10 mL × 5). The aqueous solution (I) was collected, and the organic phase was concentrated in vacuum. The concentrate was dissolved in ethyl acetate (20 mL), and the insoluble material TBAI (I) was filtered. Subsequently, the ethyl acetate solution was suction-filtered on a 4 cm thick sand core funnel with a thickness of 4 cm of basic alumina, and the basic alumina (15 mL × 3) was washed with ethyl acetate, and the filtrate was concentrated to about 10 mL. The filtrate was washed with distilled water (5 mL × 3). The aqueous solution (II) was retained, and the organic phase was concentrated to give the product 3p or 4j.
Typical Procedure for the Synthesis of 7a
To a 25 mL tube were added 6-bromo-1-(phenylthio)naphthalen-2-amine (3p) (33.0 mg, 0.1 mmol), p-tolylboronic acid (20.4 mg, 0.15 mmol), K2CO3 (27.6 mg, 0.2 mmol), and Pd(PPh3)4 (1.2 mg, 0.01 mmol) in 2 mL of EtOH sequentially. The reaction vessel was allowed to stir at 80 °C for 3 h under an argon atmosphere. After completion of the reaction, the mixture was quenched with the saturated solution of NaCl (5 mL) and extracted with dichloromethane (3 × 10 mL). The organic phase was dried over Na2SO4 and concentrated in vacuum, and the crude mixture was purified via column chromatography using ethyl acetate/PE (1:30) as the eluent to afford the final product 7a in 84% yield (28.9 mg).
1-(Phenylthio)-6-(p-tolyl)naphthalen-2-amine (7a)
White solid, mp 125.9–127.2 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.09 (d, J = 8.8 Hz, 1H), 8.02 (d, J = 1.9 Hz, 1H), 7.87 (d, J = 8.8 Hz, 1H), 7.70 (dd, J = 8.8, 2.0 Hz, 1H), 7.62 (d, J = 8.2 Hz, 2H), 7.26 (d, J = 7.9 Hz, 2H), 7.24–7.21 (td, J = 2.3 Hz, 1H), 7.21–7.17 (m, 2H), 7.11–7.05 (tt, d, J = 7.3, 1.3 Hz, 1H), 7.00 (dd, J = 8.4, 1.2 Hz, 2H), 6.06 (s, 2H), 2.34 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δ 150.44, 137.07, 136.91, 136.14, 135.50, 133.04, 131.92, 129.51, 129.03, 127.54, 126.32, 126.24, 125.58, 125.56, 124.97, 123.60, 118.58, 100.50, 20.66. HRMS (ESI): m/z calcd for C18H17NS2+ (M + Na)+, 334.0695; found, 334.0700.
Typical Procedure for the Synthesis of 7b
To a 25 mL tube were added 6-bromo-1-(phenylthio)naphthalen-2-amine (3p) (66.0 mg, 0.2 mmol), phenylacetylene (51.1 mg, 0.5 mmol), PdCl2 (3.5 mg, 0.02 mmol), CuI (7.6 mg, 0.04 mmol), and PPh3 (5.3 mg, 0.02 mmol) in 2 mL of Et3N sequentially. The reaction vessel was allowed to stir at 60 °C for 13 h under an argon atmosphere. After completion of the reaction, the mixture was quenched with the saturated solution of NaCl (5 mL) and extracted with dichloromethane (3 × 10 mL). The organic phase was dried over Na2SO4 and concentrated in vacuum. The crude mixture was purified via column chromatography using ethyl acetate/PE (1:10) as the eluent to give the final product 7b in 75% yield (52.7 mg).
6-(Phenylethynyl)-1-(phenylthio)naphthalen-2-amine (7b)
White solid, mp 79.9–82.6 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.04 (d, J = 8.7 Hz, 1H), 8.00 (d, J = 1.6 Hz, 1H), 7.84 (d, J = 8.9 Hz, 1H), 7.59–7.53 (m, 2H), 7.50 (dd, J = 8.7, 1.8 Hz, 1H), 7.45–7.39 (m, 3H), 7.22 (dd, J = 14.6, 8.3 Hz, 3H), 7.09 (tt, J = 6.7, 1.1 Hz, 1H), 7.01–6.95 (dt, J = 8.2, 1.4 Hz, 2H), 6.26 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δ 151.42, 136.58, 136.23, 131.86, 131.56, 131.20, 129.77, 129.07, 128.73, 128.46, 126.76, 125.57, 125.08, 123.34, 122.66, 118.96, 114.68, 100.65, 90.07, 88.64. HRMS (ESI): m/z calcd for C24H17NS+ (M + H)+, 352.1152; found, 352.1153.
S-Phenyl Benzenesulfonothioate (8a)52
The crude mixture was purified via column chromatography using ethyl acetate/PE (1:100) as the eluent to give the product as a white solid, mp 48–49 °C; 1H NMR (400 MHz, CDCl3): δ 7.61–7.54 (m, 3H), 7.50–7.39 (m, 3H), 7.37–7.28 (m, 4H); 13C NMR (101 MHz, CDCl3): δ 143.07, 136.72, 133.76, 131.54, 129.57, 128.93, 127.96, 127.68.
Acknowledgments
We gratefully acknowledge the financial support of this work by the National Natural Science Foundation of China (nos. 21563025, 2171101076 and 21872060) and the Shihezi University (no. CXRC201602).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02590.
Copies of 1H, 13C NMR spectra for all products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mansy S. S.; Cowan J. A. Iron-sulfur cluster biosynthesis: Toward an understanding of cellular machinery and molecular mechanism. Acc. Chem. Res. 2004, 37, 719–725. 10.1021/ar0301781. [DOI] [PubMed] [Google Scholar]
- Wang X.; Cui L.; Zhou N.; Zhu W.; Wang R.; Qian X.; Xu Y. A highly selective and sensitive near-infrared fluorescence probe for arylamine N-acetyltransferase 2 in vitro and in vivo. Chem. Sci. 2013, 4, 2936–2940. 10.1039/c3sc51079d. [DOI] [Google Scholar]
- Block E. Fifty years of smelling sulfur. J. Sulfur Chem. 2013, 34, 158–207. 10.1080/17415993.2012.717294. [DOI] [Google Scholar]
- Liu L.; Stelmach J. E.; Natarajan S. R.; Chen M.-H.; Singh S. B.; Schwartz C. D.; Fitzgerald C. E.; O’Keefe S. J.; Zaller D. M.; Schmatz D. M.; Doherty J. B. SAR of 3, 4-Dihydropyrido [3, 2-d] pyrimidone p38 Inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 3979–3982. 10.1016/j.bmcl.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Kaldor S. W.; Kalish V. J.; Davies J. F.; Shetty B. V.; Fritz J. E.; Appelt K.; Burgess J. A.; Campanale K. M.; Chirgadze N. Y.; Clawson D. K.; Dressman B. A.; Hatch S. D.; Khalil D. A.; Kosa M. B.; Lubbehusen P. P.; Muesing M. A.; Patick A. K.; Reich S. H.; Su K. S.; Tatlock J. H. Viracept (Nelfinavir Mesylate, AG1343): A Potent, Orally Bioavailable Inhibitor of HIV-1 Protease. J. Med. Chem. 1997, 40, 3979–3985. 10.1021/jm9704098. [DOI] [PubMed] [Google Scholar]
- Somei M.; Yamada F. Simple indole alkaloids and those with a non-rearranged monoterpenoid unit. Nat. Prod. Rep. 2005, 22, 73–103. 10.1039/b316241a. [DOI] [PubMed] [Google Scholar]
- Gupta L.; Talwar A.; Chauhan P. M. Bis and tris indole alkaloids from marine organisms: new leads for drug discovery. Curr. Med. Chem. 2007, 14, 1789–1803. 10.2174/092986707781058904. [DOI] [PubMed] [Google Scholar]
- Patil S. A.; Patil S. A.; Patil R. Medicinal applications of (benz) imidazole-and indole-based macrocycles. Chem. Biol. Drug Des. 2017, 89, 639–649. 10.1111/cbdd.12802. [DOI] [PubMed] [Google Scholar]
- Kochanowska-Karamyan A. J.; Hamann M. T. Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S.; Cammerer S.; Filali S.; Frohner W.; Knoll J.; Krahl M.; Reddy K.; Knolker H.-J. Novel routes to pyrroles, indoles and carbazoles-applications in natural product synthesis. Curr. Org. Chem. 2005, 9, 1601–1614. 10.2174/138527205774370496. [DOI] [Google Scholar]
- Halim M.; Yee D. J.; Sames D. Imaging induction of cytoprotective enzymes in intact human cells: coumberone, a metabolic reporter for human AKR1C enzymes reveals activation by panaxytriol, an active component of red ginseng. J. Am. Chem. Soc. 2008, 130, 14123–14128. 10.1021/ja801245y. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prieto M. A.; Miatello R. M. Organosulfur compounds and cardiovascular disease. Mol. Aspects Med. 2010, 31, 540–545. 10.1016/j.mam.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Eichman C. C.; Stambuli J. P. Transition metal catalyzed synthesis of aryl sulfides. Molecules 2011, 16, 590–608. 10.3390/molecules16010590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T.; Mitsudo T.-a. Metal-catalyzed carbon-sulfur bond formation. Chem. Rev. 2000, 100, 3205–3220. 10.1021/cr9902749. [DOI] [PubMed] [Google Scholar]
- Chauhan P.; Mahajan S.; Enders D. Organocatalytic carbon-sulfur bond-forming reactions. Chem. Rev. 2014, 114, 8807–8864. 10.1021/cr500235v. [DOI] [PubMed] [Google Scholar]
- Fernández-Rodríguez M. A.; Shen Q.; Hartwig J. F. A general and long-lived catalyst for the palladium-catalyzed coupling of aryl halides with thiols. J. Am. Chem. Soc. 2006, 128, 2180–2181. 10.1021/ja0580340. [DOI] [PubMed] [Google Scholar]
- Wong Y.-C.; Jayanth T. T.; Cheng C.-H. Cobalt-catalyzed aryl-sulfur bond formation. Org. Lett. 2006, 8, 5613–5616. 10.1021/ol062344l. [DOI] [PubMed] [Google Scholar]
- Shen C.; Zhang P.; Sun Q.; Bai S.; Hor T. S. A.; Liu X. Recent advances in C-S bond formation via C-H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. 10.1039/c4cs00239c. [DOI] [PubMed] [Google Scholar]
- Choudhury P.; Roy B.; Basu B. Sustainable and site-selective C-H sulfenylation of aromatic compounds with thiol using catalytic graphene oxide and NaI. Asian J. Org. Chem. 2017, 6, 1569–1574. 10.1002/ajoc.201700275. [DOI] [Google Scholar]
- Dong D.-Q.; Hao S.-H.; Yang D.-S.; Li L.-X.; Wang Z.-L. Sulfenylation of C-H bonds for C-S bond formation under metal-free conditions. Eur. J. Org. Chem. 2017, 2017, 6576–6592. 10.1002/ejoc.201700853. [DOI] [Google Scholar]
- Liu Y.; Xiong J.; Wei L. Recent advances in the C(sp2)-S bond formation reactions by transition metal-free C(sp2)-H functionalization. Chin. J. Org. Chem. 2017, 37, 1667–1680. 10.6023/cjoc201702009. [DOI] [Google Scholar]
- Hu F.; Gao W.; Chang H.; Li X.; Wei W. Progress in iodine-mediated sulfonylation reactions. Chin. J. Org. Chem. 2015, 35, 1848–1860. 10.6023/cjoc201504039. [DOI] [Google Scholar]
- Xu X.-M.; Yang H.-L.; Li W.-Z. Transition metal-free direct C-H bond sulfenylation of alkenes and arenes. Chin. J. Org. Chem. 2020, 10.6023/cjoc201912044. [DOI] [Google Scholar]
- Harry N. A.; Radhika S.; Neetha M.; Anilkumar G. Recent advances and prospects of organic reactions “on water”. ChemistrySelect 2019, 4, 12337–12355. 10.1002/slct.201903360. [DOI] [Google Scholar]
- Simon M.-O.; Li C.-J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. 10.1039/c1cs15222j. [DOI] [PubMed] [Google Scholar]
- Yang J.; Mei F.; Fu S.; Gu Y. Facile synthesis of 1, 4-diketones via three-component reactions of α-ketoaldehyde, 1, 3-dicarbonyl compound, and a nucleophile in water. Green Chem. 2018, 20, 1367–1374. 10.1039/c7gc03644b. [DOI] [Google Scholar]
- Lai B.; Mei F.; Gu Y. Bifunctional solid catalyst for organic reactions in water: simultaneous anchoring of acetylacetone ligands and amphiphilic ionic liquid “tags” by using a dihydropyran linker. Chem.–Asian J. 2018, 13, 2529–2542. 10.1002/asia.201800567. [DOI] [PubMed] [Google Scholar]
- Xu J.; Huang W.; Bai R.; Queneau Y.; Jérôme F.; Gu Y. Utilization of bio-based glycolaldehyde aqueous solution in organic synthesis: application to the synthesis of 2, 3-dihydrofurans. Green Chem. 2019, 21, 2061–2069. 10.1039/c8gc04000a. [DOI] [Google Scholar]
- Kitanosono T.; Masuda K.; Xu P.; Kobayashi S. Catalytic organic reactions in water toward sustainable society. Chem. Rev. 2018, 118, 679–746. 10.1021/acs.chemrev.7b00417. [DOI] [PubMed] [Google Scholar]
- Clarke C. J.; Tu W.-C.; Levers O.; Bröhl A.; Hallett J. P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. 10.1021/acs.chemrev.7b00571. [DOI] [PubMed] [Google Scholar]
- Liu P.; Zhou L.; Li X.; He R. Bis(imino)pyridine palladium(II) complexes: Synthesis, structure and catalytic activity. J. Organomet. Chem. 2009, 694, 2290–2294. 10.1016/j.jorganchem.2009.03.021. [DOI] [Google Scholar]
- Liu P.; Zhang W.; He R. Preparation and catalytic properties of bis(imino)pyridine palladium(II) complexes as efficient catalysts for Suzuki cross-coupling reaction in water. Appl. Organomet. Chem. 2009, 23, 135–139. 10.1002/aoc.1481. [DOI] [Google Scholar]
- Liu P.; Yan M.; He R. Bis(imino)pyridine palladium(II) complexes as efficient catalysts for the Suzuki-Miyaura reaction in water. Appl. Organomet. Chem. 2010, 24, 131–134. 10.1002/aoc.1591. [DOI] [Google Scholar]
- Liu Y.; Ma X.; Xie J.; Liu P.; Dai B.; He R. Metallomicelles of palladium(II) complexes as efficient catalysts for the Suzuki-Miyaura reaction in neat water. Appl. Organomet. Chem. 2013, 27, 494–498. 10.1002/aoc.3021. [DOI] [Google Scholar]
- Gu N.; Liu Y.; Liu P.; Ma X.; Yan L.; Dai B. Synthesis of terphenyl derivatives by Pd-catalyzed Suzuki-Miyaura reaction of dibromobenzene Using 2N2O-salen as a ligand in aqueous solution. Chin. J. Chem. 2015, 33, 1189–1193. 10.1002/cjoc.201500446. [DOI] [Google Scholar]
- Liu Y.; Gu N.; Liu P.; Xie J.; Dai B.; Liu Y. A simple and efficient 2N2O-Cu(II) complex as a catalyst for N-arylation of imidazoles in water. Appl. Organomet. Chem. 2015, 29, 468–470. 10.1002/aoc.3317. [DOI] [Google Scholar]
- Liu Y.-s.; Gu N.-n.; Liu P.; Ma X.-w.; Liu Y.; Xie J.-w.; Dai B. Water-soluble salen-Pd complex as an efficient catalyst for Suzuki-Miyaura reaction of sterically hindered substrates in pure water. Tetrahedron 2015, 71, 7985–7989. 10.1016/j.tet.2015.08.070. [DOI] [Google Scholar]
- Xiao F.; Chen S.; Li C.; Huang H.; Deng G.-J. Copper-catalyzed three-component one-pot synthesis of aryl sulfides with sulfur powder under aqueous conditions. Adv. Synth. Catal. 2016, 358, 3881–3886. 10.1002/adsc.201600642. [DOI] [Google Scholar]
- Lin Y.-m.; Lu G.-p.; Wang G.-x.; Yi W.-b. Odorless, regioselective synthesis of diaryl sulfides and α-thioaryl carbonyls from sodium arylsulfinates via a metal-free radical strategy in water. Adv. Synth. Catal. 2016, 358, 4100–4105. 10.1002/adsc.201600846. [DOI] [Google Scholar]
- Feng Q.; Chen D.; Hong M.; Wang F.; Huang S. Phenyliodine (III) bis (trifluoroacetate)(PIFA)-mediated synthesis of aryl sulfides in water. J. Org. Chem. 2018, 83, 7553–7558. 10.1021/acs.joc.8b00435. [DOI] [PubMed] [Google Scholar]
- Saima S.; Equbal D.; Lavekar A. G.; Sinha A. K. Cooperative catalysis by bovine serum albumin-iodine towards cascade oxidative coupling-C(sp2)-H sulfenylation of indoles/hydroxyaryls with thiophenols on water. Org. Biomol. Chem. 2016, 14, 6111–6118. 10.1039/c6ob00930a. [DOI] [PubMed] [Google Scholar]
- Huang X.; Chen Y.; Zhen S.; Song L.; Gao M.; Zhang P.; Li H.; Yuan B.; Yang G. Cobalt-catalyzed aerobic cross-dehydrogenative coupling of C-H and thiols in water for C-S formation. J. Org. Chem. 2018, 83, 7331–7340. 10.1021/acs.joc.7b02718. [DOI] [PubMed] [Google Scholar]
- Brownbridge P.; Jowett I. C. One-pot synthesis of sulphinic esters from disulphides. Synthesis 1988, 1988, 252–254. 10.1055/s-1988-27535. [DOI] [Google Scholar]
- Xia M.; Chen Z.-C. Hypervalent iodine in synthesis XXIV: a facile method for the preparation of arylsulfinic esters: oxidation of disulfides or thiophenols by phenyliodine (III) bis (trifluoroacetate) in the presence of alcohols. Synth. Commun. 1997, 27, 1321–1326. 10.1080/00397919708006060. [DOI] [Google Scholar]
- Fernández I.; Khiar N.; Roca A.; Benabra A.; Alcudia A.; Espartero J.; Alcudia F. A generalization of the base effect on the diastereoselective synthesis of sulfinic and phosphinic esters. Tetrahedron Lett. 1999, 40, 2029–2032. 10.1016/s0040-4039(99)00171-9. [DOI] [Google Scholar]
- Hajipour A. R.; Falahati A. R.; Ruoho A. E. An efficient and novel method for the synthesis of sulfinate esters under solvent-free conditions. Tetrahedron Lett. 2006, 47, 2717–2719. 10.1016/j.tetlet.2006.02.080. [DOI] [Google Scholar]
- Tranquilino A.; Andrade S. R. C. P.; da Silva A. P. M.; Menezes P. H.; Oliveira R. A. Non-expensive, open-flask and selective catalytic systems for the synthesis of sulfinate esters and thiosulfonates. Tetrahedron Lett. 2017, 58, 1265–1268. 10.1016/j.tetlet.2017.02.025. [DOI] [Google Scholar]
- Wei J.; Sun Z. tert-Butyl sulfoxide as a starting point for the synthesis of sulfinyl containing compounds. Org. Lett. 2015, 17, 5396–5399. 10.1021/acs.orglett.5b02743. [DOI] [PubMed] [Google Scholar]
- Shyam P. K.; Kim Y. K.; Lee C.; Jang H.-Y. Copper-catalyzed aerobic formation of unstable sulfinyl radicals for the synthesis of sulfinates and thiosulfonates. Adv. Synth. Catal. 2016, 358, 56–61. 10.1002/adsc.201500785. [DOI] [Google Scholar]
- Zhou C.; Tan Z.; Jiang H.; Zhang M. A sustainable oxidative esterification of thiols with alcohols by a cobalt nanocatalyst supported on doped carbon. Green Chem. 2018, 20, 1992–1997. 10.1039/c8gc00441b. [DOI] [Google Scholar]
- Du B.; Li Z.; Qian P.; Han J.; Pan Y. Copper-catalyzed aerobic oxidative reaction of sulfonyl hydrazides with alcohols: an easy access to sulfinates. Chem.–Asian J. 2016, 11, 478–481. 10.1002/asia.201501262. [DOI] [PubMed] [Google Scholar]
- Chen L.; Pu J.; Liu P.; Dai B. Facial synthesis of sulfinic esters via copper-catalyzed reaction of sulfonyl hydrazides with alcohols in air. J. Saudi Chem. Soc. 2019, 23, 1102–1108. 10.1016/j.jscs.2018.10.007. [DOI] [Google Scholar]
- Lujan-Montelongo J. A.; Estevez A. O.; Fleming F. F. Alkyl sulfinates: formal nucleophiles for synthesizing TosMIC analogs. Eur. J. Org. Chem. 2015, 2015, 1602–1605. 10.1002/ejoc.201403615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resek J. E.; Meyers A. I. Unsaturation of ketones, nitriles and lactams with methyl phenylsulfinate. Tetrahedron Lett. 1995, 36, 7051–7054. 10.1016/0040-4039(95)01461-p. [DOI] [Google Scholar]
- Ruano J. G.; Parra A.; Yuste F.; Mastranzo V. M. Mild and general method for the synthesis of sulfonamides. Synthesis 2008, 2008, 311–319. 10.1055/s-2007-1000850. [DOI] [Google Scholar]
- Maldonado M. F.; Sehgelmeble F.; Bjarnemark F.; Svensson M.; Åhman J.; Arvidsson P. I. Synthesis and arylation of unprotected sulfonimidamides. Tetrahedron 2012, 68, 7456–7462. 10.1016/j.tet.2012.06.072. [DOI] [Google Scholar]
- Yuste F.; Hernández Linares A.; Mastranzo V. M.; Ortiz B.; Sánchez-Obregón R.; Fraile A.; García Ruano J. L. Methyl sulfinates as electrophiles in Friedel-Crafts reactions. synthesis of aryl sulfoxides. J. Org. Chem. 2011, 76, 4635–4644. 10.1021/jo2006335. [DOI] [PubMed] [Google Scholar]
- Nguyen N.-L.; Vo H.-T.; Duus F.; Luu T. Dramatic influence of ionic liquid and ultrasound irradiation on the electrophilic sulfinylation of aromatic compounds by sulfinic esters. Molecules 2017, 22, 1458–1469. 10.3390/molecules22091458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. E.; Lazarova T. I. Preparation of (phenylsulfinyl) phenols from aryl phenylsulfinates: “Thia-Fries rearrangement”. Tetrahedron Lett. 1996, 37, 7–8. 10.1016/0040-4039(95)02068-3. [DOI] [Google Scholar]
- Tata R. R.; Hampton C. S.; Harmata M. Preparation of propargylic sulfinates and their [2, 3]-sigmatropic rearrangement to allenic sulfones. Adv. Synth. Catal. 2017, 359, 1232–1241. 10.1002/adsc.201600986. [DOI] [Google Scholar]
- Chen L.; Zhang J.; Wei Y.; Yang Z.; Liu P.; Zhang J.; Dai B. NH4I/1, 10-phenanthroline catalyzed direct sulfenylation of N-heteroarenes with ethyl arylsulfinates. Tetrahedron 2019, 75, 130664–130671. 10.1016/j.tet.2019.130664. [DOI] [Google Scholar]
- El-Harairy A.; Yiliqi; Lai B.; Vaccaro L.; Li M.; Gu Y. A Sulfone-containing imidazolium-based Brønsted acid ionic liquid catalyst enables replacing dipolar aprotic solvents with butyl acetate. Adv. Synth. Catal. 2019, 361, 3342–3350. 10.1002/adsc.201900246. [DOI] [Google Scholar]
- Wu J.; Liu Y.; Ma X.; Liu P.; Gu C.; Dai B. Highly selective copper-catalyzed oxidation of benzyl alcohols to aromatic aldehydes in water at room temperature. Appl. Organomet. Chem. 2016, 30, 577–580. 10.1002/aoc.3473. [DOI] [Google Scholar]
- Wu J.; Liu Y.; Ma X.; Liu P.; Gu C.; Dai B. Metal-free oxidation of secondary benzylic alcohols using aqueous TBHP. Synth. Commun. 2016, 46, 1747–1758. 10.1080/00397911.2016.1223307. [DOI] [Google Scholar]
- Liu Y.; Liu P.; Liu Y.; Wei Y. Synthesis of novel 1,4-substituted 1,2,3-triazoles by water-soluble (salicyladimine)2Cu complex catalyzed azide-alkyne cycloaddition in water. Lett. Org. Chem. 2017, 14, 557–565. 10.2174/1570178614666170609072912. [DOI] [Google Scholar]
- Wu J.; Liu Y.; Liu P.; Gu C. A simple, mild and efficient oxidation of benzylic alcohols in the presence of NBS/KOAc in aqueous solution. Lett. Org. Chem. 2017, 14, 254–260. 10.2174/1570178614666170221142818. [DOI] [Google Scholar]
- Wu J.; Liu Y.; Ma X.; Liu P.; Gu C.; Dai B. Cu(II)-Catalyzed ligand-free oxidation of diarylmethanes and second alcohols in water. Chin. J. Chem. 2017, 35, 1391–1395. 10.1002/cjoc.201700115. [DOI] [Google Scholar]
- El-Harairy A.; Yue M.; Fan W.; Popowycz F.; Queneau Y.; Li M.; Gu Y. Novel non-toxic and non-hazardous solvent systems for the chemistry of indoles: use of a sulfone-containing Brønsted acid ionic liquid catalyst in butyl acetate. ChemCatChem 2019, 11, 4403–4410. 10.1002/cctc.201900784. [DOI] [Google Scholar]
- Li M.; Wu F.; Gu Y. Brönsted acidic ionic liquid catalyzed synthesis of benzo[a]carbazole from renewable acetol and 2-phenylindoles in a biphasic system. Chin. J. Catal. 2019, 40, 1135–1140. 10.1016/s1872-2067(19)63370-x. [DOI] [Google Scholar]
- Ravichandiran P.; Lai B.; Gu Y. Aldo-X bifunctional building blocks for the synthesis of heterocycles. Chem. Rec. 2017, 17, 142–183. 10.1002/tcr.201600042. [DOI] [PubMed] [Google Scholar]
- Taheri A.; Pan X.; Liu C.; Gu Y. Brønsted acid ionic liquid as a solvent-conserving catalyst for organic reactions. ChemSusChem 2014, 7, 2094–2098. 10.1002/cssc.201402220. [DOI] [PubMed] [Google Scholar]
- Huang W.; Xu J.; Liu C.; Chen Z.; Gu Y. Lewis acid-catalyzed synthesis of benzofurans and 4, 5, 6, 7-tetrahydrobenzofurans from acrolein dimer and 1, 3-dicarbonyl compounds. J. Org. Chem. 2019, 84, 2941–2950. 10.1021/acs.joc.9b00270. [DOI] [PubMed] [Google Scholar]
- Gandeepan P.; Kaplaneris N.; Santoro S.; Vaccaro L.; Ackermann L. Biomass-derived solvents for sustainable transition metal-catalyzed C-H activation. ACS Sustainable Chem. Eng. 2019, 7, 8023–8040. 10.1021/acssuschemeng.9b00226. [DOI] [Google Scholar]
- Santoro S.; Marrocchi A.; Lanari D.; Ackermann L.; Vaccaro L. Towards sustainable C-H functionalization reactions: The emerging role of bio-based reaction media. Chem.—Eur. J. 2018, 24, 13383–13390. 10.1002/chem.201801114. [DOI] [PubMed] [Google Scholar]
- Mahmoudi H.; Valentini F.; Ferlin F.; Bivona L. A.; Anastasiou I.; Fusaro L.; Aprile C.; Marrocchi A.; Vaccaro L. A tailored polymeric cationic tag-anionic Pd(II) complex as a catalyst for the low-leaching Heck-Mizoroki coupling in flow and in biomass-derived GVL. Green Chem. 2019, 21, 355–360. 10.1039/c8gc03228a. [DOI] [Google Scholar]
- Nalbandian C. J.; Miller E. M.; Toenjes S. T.; Gustafson J. L. A conjugate Lewis base-Brønsted acid catalyst for the sulfenylation of nitrogen containing heterocycles under mild conditions. Chem. Commun. 2017, 53, 1494–1497. 10.1039/c6cc09998j. [DOI] [PubMed] [Google Scholar]
- Xiao F.; Xie H.; Liu S.; Deng G.-J. Iodine-catalyzed regioselective sulfenylation of indoles with sodium sulfinates. Adv. Synth. Catal. 2014, 356, 364–368. 10.1002/adsc.201300773. [DOI] [Google Scholar]
- Rafique J.; Saba S.; Franco M. S.; Bettanin L.; Schneider A. R.; Silva L. T.; Braga A. L. Direct, metal-free C(sp2)-H chalcogenation of indoles and imidazopyridines with dichalcogenides catalysed by KIO3. Chem.—Eur. J. 2018, 24, 4173–4180. 10.1002/chem.201705404. [DOI] [PubMed] [Google Scholar]
- Ohkado R.; Ishikawa T.; Iida H. Flavin-iodine coupled organocatalysis for the aerobic oxidative direct sulfenylation of indoles with thiols under mild conditions. Green Chem. 2018, 20, 984–988. 10.1039/c8gc00117k. [DOI] [Google Scholar]
- Bering L.; D’Ottavio L.; Sirvinskaite G.; Antonchick A. P. Nitrosonium ion catalysis: aerobic, metal-free cross-dehydrogenative carbon-heteroatom bond formation. Chem. Commun. 2018, 54, 13022–13025. 10.1039/c8cc08328b. [DOI] [PubMed] [Google Scholar]
- Chen M.; Luo Y.; Zhang C.; Guo L.; Wang Q.; Wu Y. Graphene oxide mediated thiolation of indoles in water: a green and sustainable approach to synthesize 3-sulfenylindoles. Org. Chem. Front. 2019, 6, 116–120. 10.1039/c8qo00726h. [DOI] [Google Scholar]
- Yang Y.; Zhang S.; Tang L.; Hu Y.; Zha Z.; Wang Z. Catalyst-free thiolation of indoles with sulfonyl hydrazides for the synthesis of 3-sulfenylindoles in water. Green Chem. 2016, 18, 2609–2613. 10.1039/c6gc00313c. [DOI] [Google Scholar]
- Wang F.-X.; Zhou S.-D.; Wang C.; Tian S.-K. N-Hydroxy sulfonamides as new sulfenylating agents for the functionalization of aromatic compounds. Org. Biomol. Chem. 2017, 15, 5284–5288. 10.1039/c7ob01390f. [DOI] [PubMed] [Google Scholar]
- Deng C.-L.; Xu W.; Hei Y.-Y.; Song J.-L.; Zhan X.-C.; Zhang X.-G. Copper (I)-catalyzed thiolation of C-H bonds for the synthesis of sulfenyl pyrroles and indoles. Synthesis 2019, 51, 545–551. 10.1055/s-0037-1610295. [DOI] [Google Scholar]
- Xu Z.-b.; Lu G.-p.; Cai C. Acid-induced chemoselective arylthiolations of electron-rich arenes in ionic liquids from sodium arylsulfinates: the reducibility of halide anions in [Hmim] Br. Org. Biomol. Chem. 2017, 15, 2804–2808. 10.1039/c6ob02823c. [DOI] [PubMed] [Google Scholar]
- Cao L.; Luo S.-H.; Jiang K.; Hao Z.-F.; Wang B.-W.; Pang C.-M.; Wang Z.-Y. Disproportionate coupling reaction of sodium sulfinates mediated by BF3·OEt2: an approach to symmetrical/unsymmetrical thiosulfonates. Org. Lett. 2018, 20, 4754–4758. 10.1021/acs.orglett.8b01808. [DOI] [PubMed] [Google Scholar]
- Yang X.; Bao Y.; Dai Z.; Zhou Q.; Yang F. Catalyst-free sulfenylation of indoles with sulfinic esters in ethanol. Green Chem. 2018, 20, 3727–3731. 10.1039/c8gc01764f. [DOI] [Google Scholar]
- Katrun P.; Hongthong S.; Hlekhlai S.; Pohmakotr M.; Reutrakul V.; Soorukram D.; Jaipetch T.; Kuhakarn C. Iodine-PPh3-mediated C3-sulfenylation of indoles with sodium sulfinates. RSC Adv. 2014, 4, 18933–18938. 10.1039/c4ra02607a. [DOI] [Google Scholar]
- Tranquilino A.; Andrade S. R. C. P.; da Silva A. P. M.; Menezes P. H.; Oliveira R. A. Non-expensive, open-flask and selective catalytic systems for the synthesis of sulfinate esters and thiosulfonates. Tetrahedron Lett. 2017, 58, 1265–1268. 10.1016/j.tetlet.2017.02.025. [DOI] [Google Scholar]
- Huang S.; Li H.; Xie T.; Wei F.; Tung C.-H.; Xu Z. Scandium-catalyzed electrophilic alkene difunctionalization: regioselective synthesis of thiosulfone derivatives. Org. Chem. Front. 2019, 6, 1663–1666. 10.1039/c9qo00138g. [DOI] [Google Scholar]
- Chen L.; Liu P.; Wu J.; Dai B. Cu-catalyzed direct C-H thiolation of electron-rich arenes with arylsulfonyl hydrazides. Tetrahedron 2018, 74, 1513–1519. 10.1016/j.tet.2018.02.014. [DOI] [Google Scholar]
- Kang X.; Yan R.; Yu G.; Pang X.; Liu X.; Li X.; Xiang L.; Huang G. Iodine-Mediated Thiolation of substituted naphthols/naphthylamines and arylsulfonyl hydrazides via C(sp2)-H bond functionalization. J. Org. Chem. 2014, 79, 10605–10610. 10.1021/jo501778h. [DOI] [PubMed] [Google Scholar]
- Mohammadinezhad A.; Akhlaghinia B. CoII immobilized on an aminated magnetic metal-organic framework catalyzed C-N and C-S bond forming reactions: a journey for the mild and efficient synthesis of arylamines and arylsulfides. New J. Chem. 2019, 43, 15525–15538. 10.1039/c9nj03400e. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.