Abstract
Background
Delirium may be one of the presenting symptoms of COVID-19, complicating diagnosis and care of elderly patients with dementia. We aim to identify the prevalence and prognostic significance of delirium as the sole onset manifestation of COVID-19.
Methods
This is a retrospective single-centre study based on review of medical charts, conducted during the outbreak peak (March 27-April 18, 2020) in a Lombard dementia facility, including 59 elderly subjects with dementia and laboratory-confirmed COVID-19.
Findings
Of the 59 residents, 57 (96⋅6%) tested positive (mean age: 82⋅8; women: 66⋅7%). Comorbidities were present in all participants, with 18/57 (31⋅6%) having three or more concomitant diseases. Delirium-Onset COVID-19 (DOC) was observed in 21/57 (36⋅8%) subjects who were chiefly older (mean age: 85⋅4 y/o) and with multiple comorbidities. Eleven/21 DOC patients (52⋅4%) had hypoactive delirium, while hyperactive delirium occurred in ten/21 (47⋅6%). Lymphopenia was present in almost all subjects (median: 1⋅3 × 109/L). Overall mortality rate was 24⋅6% (14/57) and dementia severity per se had no impact on short-term mortality due to COVID-19. DOC was strongly associated with higher mortality (p<0⋅001). Also, DOC and male gender were independently associated with increased risk of mortality (OR: 17⋅0, 95% CI: 2⋅8–102⋅7, p = 0⋅002 and 13⋅6, 95% CI: 2⋅3–79⋅2, p = 0⋅001 respectively).
Interpretation
Delirium occurrence in the elderly with dementia may represent a prodromal phase of COVID-19, and thus deserves special attention, especially in the presence of lymphopenia. Hypoxia and a severe inflammatory state may develop subsequently. DOC cases have higher short-term mortality rate.
Funding
None.
1. Introduction
Italy was the first European country severely affected by the COVID-19 pandemic. According to a statistical report from the Italian National Institute of Health (Istituto Superiore di Sanità) (1), 27,955 COVID-19 patients nationwide have succumbed to the disease by the first week of May 2020, with more than 50% of deceased patients coming from the northern region of Lombardy. The median age of patients who died from COVID-19 was 20 years higher than that of the total patients who contracted the infection (median age 81y vs 62y), with nearly three-quarters of deaths occurring in the 70–79 (28⋅1%) and 80–89 (40⋅8%) age groups. (1)
COVID-19 induces a massive cytokine storm causing a variety of symptoms, including neurological manifestations. Neurological symptoms associated with COVID-19 may include vegetative or peripheral manifestations, such as nausea, vomiting, hypotension, severe asthenia, myalgia, neuralgia, headache, olfactory, and gustatory dysfunction, or even Guillain-Barré syndrome; and central nervous system (CNS) symptoms like psychomotor retardation, dizziness, confusion, delirium and, in rare cases, ataxia, encephalitis, stroke, and seizures. (2, 3, 4, 5, 6) Neurological symptoms appear more commonly in severe COVID-19 patients showing less pronounced typical symptoms (fever, cough and dyspnoea). (2) Delirium may develop during the clinical course of COVID-19 but it may also notably be one of the presenting symptoms, thus further complicating diagnosis and management particularly in people with cognitive impairment. (7, 8, 9, 10, 11)
Older adults living in skilled nursing facilities with multiple comorbidities are the most susceptible to severe COVID-19. (4,12) Unfortunately, residential care facilities where “elderly people or people with disabilities and severe neurological diseases live in close contact with their fellow residents” represent the weakest link in preventing the spread of COVID-19. In fact, the infection has spread very rapidly in many of these facilities. (13,14) Since individuals ≥65 years old (y/o) represent about 23% of the Italian population, the high case-fatality rates observed in Italy may be attributable to the higher number of older patients. (15) Despite being a prevalent and troublesome disease in this specific population, there are currently no studies that deal with the manifestations of COVID-19 in this particular setting.
On the basis of available evidence and our clinical observations, we hypothesized that SARS-CoV-2 might have early and particularly harmful effects on the brain functions of elderly patients with dementia, manifesting as delirium which might be frequent in the prodromal phase of the infection when other typical symptoms have not yet emerged. The primary objective of this paper is to determine the prevalence of delirium as the sole onset manifestation of COVID-19 in elderly patients with dementia living in a Dementia Special Care Unit (DSCU), and its prognostic value for mortality. Our secondary aim is to verify the possible occurrence of this “atypical” onset in other settings.
2. Methods
Study design and setting. This is an observational study based on a retrospective review of medical records, conducted in the area of Milan (Lombardy) at the DSCU of the “C. Golgi” Geriatric Institute, which specializes in dealing with patients with behavioural problems associated with dementia. The residents of this facility are receiving long-term care and are constantly being monitored. The DSCU remained isolated throughout the duration of this study — admissions and discharges were discontinued, and visitors were banned — to reduce the risk of further contagion. For our secondary aim, additional two settings were considered for comparison: the Golgi-Cenci Foundation Geriatric Consultation Service (GFGCS), which provides outpatient clinic and house call services to elderly people, and the general medical Emergency Room (ER) of San Carlo Borromeo Hospital. Data from all patients were collected from March 27 to April 18, 2020, which corresponded to the peak of the SARS-CoV-2 exposure.
Ethics: This study was performed in accordance with the principles outlined in the Declaration of Helsinki of 1964, including all amendments and revisions (the last in October 2013). The local Institutional Review Board approved this study and waived the need for written informed consent due to its retrospective nature and the urgency to collect data given the swift spreading of the disease. Nevertheless, before receiving medical assistance, all patients from each setting had signed a consent form for collection and processing of sensitive personal data intended for medical purposes, including research on condition that data be anonymised.
Participants. All subjects aged 65 and above with dementia and concomitant COVID-19 were included. The definite diagnosis of COVID-19 was determined through reverse-transcription polymerase chain reaction (RT-PCR) testing of pharyngeal swabs. Exclusion criteria were as follows: 1) other active infections or 2) other conditions causing delirium (e.g. new-onset acute medical condition other than pre-existing comorbidities, medications or substance intoxication). All eligible participants who developed delirium at onset of COVID-19 without manifesting any of the typical symptoms (e.g. fever, cough, dyspnoea) were grouped under Delirium-Onset COVID-19 (DOC), while the remaining represented the control group and were classified as Non-Delirium-Onset COVID-19 (N-DOC).
Variables and data sources/measurements: General and laboratory clinical data were collected, including comorbidities, initial peripheral oxygen saturation (SpO2), temperature, lymphocyte count, C-reactive protein (CRP), and creatine phosphokinase (CPK). The DSM-5 criteria (16) were used to define the mental state and the pre-existing cognitive dysfunction. In DSM-5, delirium is defined as a disturbance in attention and awareness, which develops over a short period of time (hours-days), fluctuates, and represents a change from baseline behavioural state as a consequence of an underlying medical condition. Dementia, referred to as major neurocognitive disorder (major-NCD) in DSM-5, is a significant cognitive decline from a previous level of performance in one or more cognitive domains which interfere with independence in everyday activities. The Clinical Dementia Rating (CDR) (17) is a 5-point scale used to assess six domains of cognitive and functional performance such as memory, orientation, judgement & problem solving, community affairs, home & hobbies performance, and personal care. An overall CDR score is generally used to track the level of impairment of patients with dementia, including Alzheimer's disease and other dementias (see the Appendix for dementias classification): CDR 0 - no dementia, CDR 0.5 - mild neurocognitive impairment, CDR 1 - mild dementia, CDR 2 - moderate dementia, CDR 3 – severe dementia, CDR 4 - very severe dementia and CDR 5 - terminal dementia. (18) (19) In this study, delirium was initially suspected by nurses upon observation of acute behavioural changes, defined as the emergence of sudden-onset psychomotor retardation or agitation, oppositional behaviour, emotional changes, or even psychotic symptoms (hallucinations or delusions). Based on this suspicion, the medical staff would use the 4-item Confusion Assessment Method (CAM) (20) to make the diagnosis of delirium. According to CAM, delirium is diagnosed when an acute and fluctuating confusion and inattention are accompanied by either disorganized thinking or altered level of consciousness. Additionally, we defined hypoactive delirium as the presence of any of the following: lethargy, psychomotor retardation, inertness or anorexia. On the other hand, hyperalert mental status, psychomotor agitation or resistance to care characterized hyperactive delirium. Although our main focus was on the initial delirium diagnosis, CAM was repeated weekly in the DSCU to detect any changes.
All clinical data (including CAM and CDR scores) were documented in medical records by trained physicians (geriatricians, neurologists or ER specialists) and subsequently reviewed by a team of neurologists with expertise in neurocognitive disorders.
Our outcome was short-term mortality. A comparison of mortality between DOC and N-DOC was performed to determine the prognostic significance of delirium-onset. Only participants who died within our study period were considered.
Bias and study size: We examined a limited number of subjects belonging to a dementia facility. In order to counteract a selection bias and to verify the occurrence of DOC in other settings, we estimated its prevalence in other two facilities, as described above.
Statistical analysis: Quantitative variables were reported as means ± standard deviations when distributed normally or as median and interquartile ranges (IQR) when skewed, whereas qualitative variables were reported as counts and percentages. For statistical analysis, participants were divided into groups based on presenting clinical manifestation (DOC or N-DOC) or outcome (deceased or alive). Finally, we compared mortality between DOC and N-DOC. Differences between continuous variables were analysed using Mann–Whitney U or Student t-test as indicated by the normal or non-normal distribution of data. Categorical data were compared using Chi-square or Fisher's exact test where appropriate. To reduce type I error from multiple comparisons, Bonferroni corrections were applied. The relationship between behavioural onset and mortality was tested using logistic regression, adjusted for gender while age, CRP, comorbidity and CDR were excluded by forward conditional model. Survival curves were obtained using Kaplan-Meier analysis. All statistical analyses were performed using statistical package for social science (IBM®SPSS®-PC Statistics, release 20.0, 2011).
Role of funding source: There was no funding source for this study.
3. Results
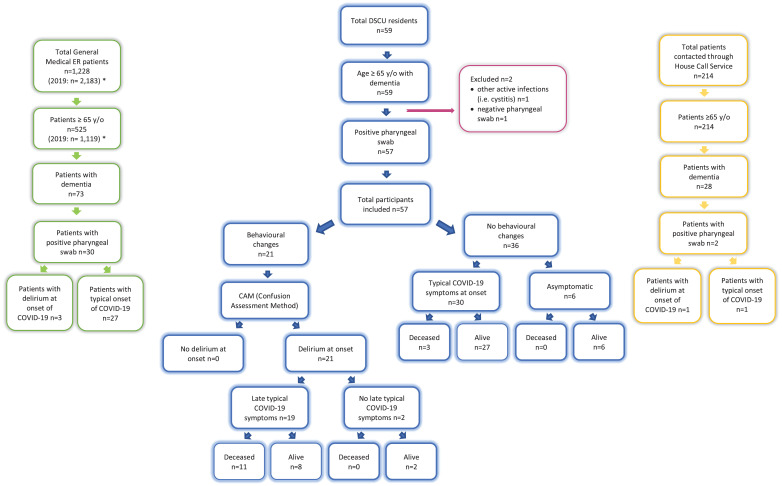
Participants and overall descriptive data: The total number of DSCU residents was 59; all of whom were older than 65 y/o and had a clinical diagnosis of dementia (major-NCD). Of all the 59 residents who underwent repeated pharyngeal swabs testing, 57 (96⋅6%,) resulted positive and were included in the study, while the remaining two (3⋅4%) were excluded because one subject had a negative swab (repeated twice) and the other had urosepsis coupled with negative RT-PCR results (see Fig. 1). The mean age of participants was 82.8 years (± 6⋅8), and the majority consisted of women, 38/57 (66⋅7%). The included subjects were affected by major-NCD due to: Alzheimer's Disease (AD, 38), multiple aetiologies (vascular and degenerative: MixD, ten), Vascular Dementia (VaD, seven), Parkinson's disease/Lewy body dementia (two). For aetiological classification of dementias see the Appendix. All participants had other comorbidities, with 18/57 (31⋅6%) having three or more concomitant diseases (see table 1). Overall, median (IQR) laboratory values were as follows: SpO2 (%) - 96⋅0 (93⋅5–97⋅0); lymphocyte count (x109/L) - 1⋅3 (1⋅1–1⋅6); CPK (μkat/L) - 1⋅1 (0⋅5–2⋅2); and CRP (nmol/L) - 152⋅4 (29⋅5–361⋅9). Some lymphocyte count, CPK and CRP values were missing from six, 26 and seven subjects, respectively, as these tests were never performed. (see table 2).
Fig. 1.
Flow chart of the participants seen during the study period (May 27-April 18,2020). Blue = DSCU residents; Green = ER patients; Yellow = House Call Service patients *Data referring to the same period of the previous year: March 27 – April 18, 2019.
Table 1.
Demographic and clinical characteristics of Dementia Special Care Unit (DSCU) residents grouped by clinical onset of SARS-CoV-2 infection (DOCs or N-DOCs) during the observation period.
| OVERALL (N = 57) | DOCs (N = 21) | N-DOCs (N = 36) | P VALUE | |
|---|---|---|---|---|
| Demographics and clinical characteristics | ||||
| Age y/o (mean ± SD) | 82⋅8 ± 6⋅8 | 85⋅4 ± 5⋅0 | 81⋅2 ± 7⋅6 | 0⋅024# |
| Gender n. (%) | ||||
| Male | 19 (33⋅3) | 9 (42⋅9) | 10 (27⋅8) | 0⋅261* |
| Female | 38 (66⋅7) | 12 (57⋅1) | 26 (72⋅2) | |
| Comorbidity (number of active diseases) | ||||
| ≥ 3 | 18 (31⋅6) | 11 (52⋅4) | 7 (19⋅4) | 0⋅017* |
| < 3 | 39 (68⋅4) | 10 (47⋅6) | 29 (80⋅6) | |
| Existing Comorbidities n. (%) | ||||
| Hypertension | 31 (54⋅4) | 14 (66⋅7) | 17 (47⋅2) | 0⋅155 |
| Cardiovascular Disease | 18 (31⋅6) | 6 (28⋅6) | 12 (33⋅3) | 0⋅709 |
| Diabetes Mellitus | 11 (19⋅3) | 6(28⋅6) | 5 (13⋅9) | 0⋅296* |
| Chronic Pulmonary Disease | 8 (14⋅0) | 3 (14⋅3) | 5 (13⋅9) | 1⋅000* |
| Malignancy | 6 (10⋅5) | 4(19⋅0) | 2(5⋅6) | 0⋅179* |
| Clinical Dementia Rating (CDR) n. (%) | ||||
| CDR1 | 2 (3⋅5) | ⋅⋅ | 2 (5⋅6) | 0⋅028* |
| CDR2 | 9 (15⋅8) | 5 (23⋅8) | 4 (11⋅1) | |
| CDR3 | 33 (57⋅9) | 15 (71⋅4) | 18 (50⋅0) | |
| CDR4 | 13 (22⋅8) | 1 (4⋅8) | 12 (33⋅3) | |
Data are shown as means ± SD or n (%). Bonferroni-adjusted alpha level = 0⋅05/9 = 0⋅0055.
# Student's t-test * Fisher's Exact Test.
CDR = Clinical Dementia Rating; DOC = delirium-onset COVID-19; N-DOC = no delirium-onset COVID-19.
Table 2.
Laboratory tests of Dementia Special Care Unit (DSCU) residents grouped by clinical onset of SARS-CoV-2 infection (DOCs or N-DOCs) in the observation period.
| N | SpO2 (%) | N | Lymphocyte count (n.r.: 1⋅5 – 4 × 109/L) | N | CPK(n.r.: 0⋅6 – 3⋅2 μkat/L) | N | CRP(n.r.: 2⋅9 – 76⋅2 nmol/L) | |
|---|---|---|---|---|---|---|---|---|
| Laboratory values | ||||||||
| Overall | 57 | 96⋅0 (93⋅5–97⋅0) | 51 | 1⋅3 (1⋅1–1⋅6) | 31 | 1⋅1 (0⋅5–2⋅2) | 50 | 152⋅4 (29⋅5–361⋅9) |
| DOC | 21 | 96⋅0 (95⋅0–97⋅0) | 20 | 1⋅5 (1⋅1–1⋅7) | 19 | 1⋅6 (1⋅0–2⋅7) | 19 | 219⋅1 (81⋅9–390⋅5) |
| N-DOC | 36 | 95⋅5 (93⋅0–97⋅0) | 31 | 1⋅3 (1⋅1–1⋅6) | 12 | 0⋅5 (0⋅4–1⋅0) | 31 | 133⋅3 (29⋅5–352⋅4) |
| p –value° | 0⋅360 | 0⋅434 | 0⋅003 | 0⋅230 | ||||
Data are shown as medians (Q1-Q3). N is the number of available data per given laboratory test. Data in bold represent statistically significant p-values Independent samples Mann Whitney U test.
CPK = creatine phosphokinase; CRP = C-reactive protein; DOC = Delirium-onset COVID-19; N-DOC = non-Delirium-onset COVID-19; n.r.: normal range; SpO2 = Peripheral oxygen saturation.
3.1. Main results and outcome
-
A)
Presenting manifestation of COVID-19: Sudden onset behavioural changes were observed in 21/57 (36.8%) DSCU residents — all of whom were later diagnosed with delirium through CAM. Delirium onset was not related to gender but was more frequent with older age (mean age 85⋅4 y., SD: ± 5⋅0 in DOC versus 81⋅2 y., SD: ± 7⋅6 in N-DOC; p = 0⋅024), and in the presence of multiple comorbidities (p = 0⋅017), regardless of the type (see table 1). Delirium-onset COVID-19 was seldom observed in patients with very severe dementia (CDR 4) but it was clearly predominant in moderate and severe dementia (CDR 2–3). On initial evaluation, none of the 21 DOCs presented typical symptoms of fever, cough or dyspnoea and their median temperature and SpO2 were 36⋅6 °C (36⋅2 – 36⋅6) and 96⋅0% (95⋅0–97⋅0), respectively. Fever or other typical symptoms appeared 24–96 h after delirium onset in 19/21 DOCs (90⋅4%), 11 of whom died due to respiratory failure (overall mortality rate in DOC: 52⋅4%). Two/21 DOCs (9⋅5%) never had any typical symptom during the entire course of COVID-19 (see Fig. 1). The remaining 36/57 (63⋅2%) did not manifest delirium at onset thereby constituting the N-DOC group. The majority of this group (30/36) developed typical symptoms but it is noteworthy that six/36 (16⋅6%, all female) remained completely asymptomatic during the entire clinical course. In the N-DOC group, only three/36 (8⋅3%) developed stupor due to respiratory failure and subsequently died. Regarding laboratory results, median SpO2 (%) values were similar in both DOCs and N-DOCs (96⋅0 [95⋅0–97⋅0] and 95⋅5 [93⋅0–97⋅0], respectively). Median lymphocyte counts (x109/L) were low in both groups (DOC: 1⋅5 [1⋅1- 1⋅7]; N-DOC: 1⋅3 [1⋅1- 1⋅6]) and CRP (nmol/L) was moderately high (DOC: 219⋅1 [81⋅9–390⋅5]; N-DOC: 133⋅3 [29⋅5–352⋅4]) but without significant differences between the two groups. For CPK (μkat/L), delirium-onset was significantly associated with higher values (1⋅6 [1⋅0–2⋅7] vs 0⋅5 [0⋅4–1⋅0]; p = 0⋅003), although within the normal range (see table 2).
-
B)
Mortality: The overall mortality rate was 24⋅6% (14/57 patients), see table 3. The mean age of the participants who died was higher than those who survived (85⋅5 ± 7⋅1 vs 81⋅9 ± 6⋅6). The strongest association was found between the type of COVID-19 onset (delirium vs typical symptoms) and mortality (p<0.001); specifically, the majority (11/14 [78⋅6%]) of those who died had delirium as initial manifestation and only a few ones (3/14 [21.4%]) had a typical onset (see also Kaplan-Meier curves in Supplementary figure 1). The presence of DOC was found to independently increase the risk for COVID-19 mortality (OR: 17⋅0, 95% CI: 2⋅8 – 102⋅7; p = 0⋅002). Consistent with other studies, male gender was found to be significantly associated with short-term mortality (p = 0⋅001) and was revealed to be an independent risk factor for poor outcome (OR: 13⋅6, 95% CI: 2⋅3 - 79⋅2). Moreover, multiple (≥3) comorbidities were also a frequent finding in those who did not survive (p = 0⋅006), regardless of the type. Also, VaD and MixD appeared to be associated with higher mortality than AD (p = 0⋅062, data not shown). The CDR (dementia severity) had no impact on mortality due to COVID-19 (p = 0⋅788). Laboratory tests (see table 4) showed that CRP (nmol/L) was higher in deceased compared to alive group (367⋅6 [196⋅2–740⋅0] vs 88⋅6 [29⋅5–269⋅5]; p = 0⋅002). Median lymphocyte counts (x109/L) were low in both deceased and alive groups without significant differences (1⋅5 [1⋅1–1⋅9] and 1⋅3 [1⋅1 – 1⋅6], respectively). SpO2 (%) and CPK (μkat/L) values were similar in both groups (deceased: 95⋅5 [91⋅5–97⋅0] and 1⋅3 [0⋅9–2⋅8]; alive: 96⋅0 [94⋅0–97⋅0] and 0⋅9 [0⋅4–2⋅2], respectively).
Table 3.
Demographic and clinical characteristics of Dementia Special Care Unit (DSCU) residents grouped by outcome (alive or deceased) during the observation period.
| Alive(N = 43) | Deceased(N = 14) | P VALUE | |
|---|---|---|---|
| Demographics and clinical characteristics | |||
| AGE y/o (mean ± SD) | 81⋅9 ± 6⋅6 | 85⋅5 ± 7⋅1 | 0⋅080# |
| Gender n. (%) | |||
| Male | 9 (20⋅9) | 10 (71⋅4) | 0⋅001* |
| Female | 34 (79⋅1) | 4 (28⋅6) | |
| Comorbidity (number of active diseases) | |||
| ≥ 3 | 9 (20⋅9) | 9 (64⋅3) | 0⋅006* |
| < 3 | 34 (79⋅1) | 5 (35⋅7) | |
| Existing Comorbidities n. (%) | |||
| Hypertension | 21 (48⋅8) | 10 (71⋅4) | 0⋅121* |
| Cardiovascular Disease | 13 (30⋅2) | 5 (35⋅7) | 0⋅736* |
| Diabetes Mellitus | 6 (14⋅0) | 5 (35⋅7) | 0⋅115* |
| Chronic Pulmonary Disease | 6 (14⋅0) | 2 (14⋅3) | 1⋅000* |
| Malignancy | 3 (7⋅0) | 3 (21⋅1) | 0⋅151* |
| Clinical Dementia Rating (CDR) n. (%) | |||
| CDR1 | 2 (4⋅7) | ⋅⋅ | 0⋅788* |
| CDR2 | 6 (13⋅9) | 3 (21⋅4) | |
| CDR3 | 26 (60⋅5) | 7 (50⋅0) | |
| CDR4 | 9 (20⋅9) | 4 (28⋅6) | |
| COVID-19 onset symptoms | |||
| Typical | 33 (76⋅7) | 3 (21.4) | <0.001 |
| Atypical – Delirium-onset | 10 (23⋅3) | 11 (78⋅6) | |
Data are shown as means ± SD or n (%). Data in bold represent statistically significant p-values before Bonferroni adjustment (Bonferroni-adjusted alpha level = 0⋅05/10 = 0⋅005).
# Student's t-test * Fisher's Exact Test.
CDR = Clinical Dementia Rating; DOC = delirium-onset COVID-19; N-DOC = no delirium-onset COVID-19.
Table 4.
Laboratory tests of Dementia Special Care Unit (DSCU) residents grouped by outcome (alive or deceased) in the observation period.
| N | SpO2 (%) | N | Lymphocyte count (n.r.: 1⋅5 – 4 × 109/L) | N | CPK(n.r.: 0⋅6 – 3⋅2 μkat/L) | N | CRP(n.r.: 2⋅9 – 76⋅2 nmol/L) | |
|---|---|---|---|---|---|---|---|---|
| Laboratory values | ||||||||
| Overall | 57 | 96⋅0 (93⋅5–97⋅0) | 51 | 1⋅3 (1⋅1–1⋅6) | 31 | 1⋅1 (0⋅5–2⋅2) | 50 | 152⋅4 (29⋅9–361⋅9) |
| Alive | 43 | 96⋅0 (94⋅0–97⋅0) | 38 | 1⋅3 (1⋅1–1⋅6) | 20 | 0⋅9 (0⋅4–2⋅2) | 38 | 88⋅6 (29⋅5–269⋅5) |
| Deceased | 14 | 95⋅5 (91⋅5–97⋅0) | 13 | 1⋅5 (1⋅1–1⋅9) | 11 | 1⋅3 (0⋅9–2⋅8) | 12 | 367⋅6 (196⋅2–740⋅0) |
| P –VALUE° | 0⋅305 | 0⋅531 | 0⋅113 | 0⋅002 | ||||
Data are shown as medians (Q1-Q3). N is the number of available data per given laboratory test. Data in bold represent statistically significant p-values Independent samples Mann Whitney U test.
CPK = creatine phosphokinase; CRP = C-reactive protein; DOC = Delirium-onset COVID-19; N-DOC = non-Delirium-onset COVID-19; n.r.: normal range; SpO2 = Peripheral oxygen saturation.
Other analysis: All acute behavioural symptoms observed in DOC patients were described in Table 5. The majority of DOC had hypoactive delirium with slowing down of both mental and motor functions (in 11/21, 52⋅4%), while hyperactive delirium with agitation and aggressive behaviour occurred slightly less frequently (in 10/21, 47⋅6%). Psychotic symptoms were observed in 4/21 (19⋅0%). A switch from Hyperactive to Hypoactive delirium was observed in some cases. Other atypical symptoms such as myalgia, asthenia, anorexia, and/or diarrhoea were rarely reported. amongst the 214 older adults (≥65 y/o) contacted through our house call service (GFGCS), 28 had dementia, of which only two had a positive pharyngeal swab. One of these two COVID-19 patients, an 85 y/o man, displayed hypoactive delirium at onset with psychomotor retardation and oppositional behaviour (e.g. inertness and anorexia). His-initial blood tests revealed lymphopenia (0⋅8 × 109/L), high CPK (15⋅2 μkat/L) and normal CRP (34⋅3 nmol/L). He subsequently developed typical symptoms and died. Conversely, the other home bound patient exhibited typical symptoms early in the course of the disease and survived (see Fig. 1). In the ER setting, a total of 1228 visits were made during our considered study period (almost half of the 2183 ER accesses recorded during the same timeframe last year). amongst these patients, 525 were ≥65 y/o (compared to the 1119 from the previous year), and 73 of them had dementia. Of the 30 laboratory-diagnosed COVID-19 elderly patients with dementia, only three (10⋅0%) were detected with delirium (see Fig. 1): two arrived at the ER already with apathy and lethargy, while the other one was hyperalert, agitated and restless, constantly complaining, resisting care and showing aggression. The latter also suffered from LBD and hallucinations. His-laboratory tests demonstrated lymphocytic count to the lower limit (2⋅0 × 109/L), very high CPK (46⋅7 μkat/L), and high CRP (268⋅6 nmol/L).
Table 5.
Delirium symptoms seen at onset of COVID-19 in Dementia Special Care Unit (DSCU) patients.
| Delirium-onset COVID-19 (DOC) DSCU residents(n = 21) | |
|---|---|
| Delirium – CAM | |
| Acute and fluctuating course | 21 (100⋅0) |
| Inattention | 21 (100⋅0) |
| Disorganized thinking | 12 (57⋅1) |
| Altered level of consciousness | 19 (90⋅5) |
| Lethargic | 11/19 (57⋅9) |
| Hyperalert | 8/19 (42⋅1) |
| Psychomotor retardation | 11 (52⋅4) |
| Psychomotor agitation / anxiety | 10 (47⋅6) |
| Incessant talking / Constant complaining | 3 (14⋅3) |
| Restlessness / Irritability | 10 (47⋅6) |
| Wandering / Intrusiveness | 4 (19⋅0) |
| Physical and verbal aggression | 8 (38⋅1) |
| Oppositional behaviour | 18 (85⋅7) |
| Apathy / Inertness | 10 (47⋅6) |
| Anorexia | 12 (57⋅1) |
| Resistance to care | 10 (47⋅6) |
| Psychotic symptoms | 4 (19⋅0) |
| Hallucinations | 1 (4⋅8) |
| Delusions | 4 (19⋅0) |
Data are shown as n (%).
DOC = Delirium-onset COVID-19; DSCU = Dementia Special Care Unit.
4. Discussion
This study was conducted in Lombardy, the epicentre of SARS-CoV-2 infection carrying 46% of the Italian cases, during the peak of the COVID-19 outbreak. Our results show that: a) delirium represented the initial manifestation of COVID-19 in a relevant percentage of elderly patients (36⋅8%) in the DSCU; b) delirium was strongly associated with mortality (p<0⋅001; see table 3 and Supplementary figure 1) and it was found to be independently associated with increased mortality (OR: 17⋅0, p = 0⋅002); c) the atypical onset of COVID-19 with delirium was also observed in one/two homebound subjects and in three/30 (10%) ER patients older than 65 y/o with dementia.
This is a retrospective study based on review of medical charts from a limited number of patients with dementia and COVID-19 (n = 57) belonging to a single centre. To verify the possible occurrence of this “atypical” early manifestation in other settings, we also considered a medical ER and a homebound group. Due to the escalating widespread turmoil during our study period, a limited amount of data was collected from these two additional settings, where a number of COVID-19 patients may not have been properly identified and were lost to follow-up. The study was conducted in a short period of time corresponding to the peak of COVID-19 outbreak in Italy; consequently, it was not possible to obtain information regarding long-term morbidity and sequelae of the infection.
Our results are in line with a recent report from the British Geriatrics Society describing the results of a Twitter survey, which demonstrated a high frequency of atypical presentations in elderly subjects including delirium (hypo and hyperactive), diarrhoea, lethargy, anorexia and myalgias. At the same time, the survey showed a relatively low frequency of typical symptoms. (8), (21) In our DSCU setting, patient identification and isolation based solely on the presence of typical symptoms (fever, cough, dyspnoea) did not and could not have prevented the spread of the virus within the facility – 96⋅6% of the residents were infected with tragic consequences (24⋅6% mortality). Our overall data are in accordance with previous reports, (2,8,22), (23), (24), (25) in which male sex, multiple (>three) comorbidities, elevated CRP and low lymphocyte count were observed in the majority of COVID-19 deaths. It is noteworthy that in our case series: the severity of dementia per se did not appear to affect short-term mortality due to COVID-19 (table 3); lymphocyte counts were low in almost all subjects and could be potentially considered a predictor of COVID-19 although it did not have a clear impact on mortality; median CPK values were significantly higher in the DOC group probably due to the increased muscle activity induced by hyperactive delirium; delirium may manifest when hypoxia, fever or a severe inflammatory state had not yet occurred; DOC was more frequent in older patients with multiple comorbidities and moderate to severe dementia (CDR 2–3) and was rare in cases with very severe dementia (CDR 4), in which behavioural changes were less detectable (see table 1); both male gender and DOC were independently associated with mortality with a significantly higher number of deaths in the male and DOC groups compared to the female and N-DOC groups (71⋅4% and 78⋅6% vs 28⋅6% and 21⋅4%, p = 0⋅001 and p<0.001, respectively). Thus, DOC patients tended to have a worse prognosis. They also frequently developed more severe late symptoms, including abundant bronchial secretions, severe hypotension (systolic blood pressure <80 mmHg), poorly controlled hyperpyrexia, pulmonary oedema, persistently low SpO2 (< 90% despite maximum oxygen supply), stupor or seizures. Varying combinations of these serious clinical manifestations, especially refractory respiratory failure, characterized terminally ill patients requiring palliative care. In these occasions, the use of morphine to reduce pain and dyspnoea and benzodiazepines for anxiety management and prevention of seizures is recommended by current Lombard guidelines for palliative care. (26)
Six out of 57 DSCU patients, all females, remained asymptomatic during the entire course of COVID-19 whereas two/57 patients experienced only hyperkinetic delirium. Together, they accounted for eight/57 (14%) cases. As they initially went unrecognized, these cases may have contributed to the spread of the infection in the high-risk DSCU residents. (27) Moreover, ten patients presented with psychomotor agitation, wandering, intrusiveness, or aggression. Most of them refused to wear a masque, consequently favouring the spread of the virus. The early recognition of these cases is crucial for the timely use of anti-viral drugs and anti-contagion measures which are needed to thwart the spread of the virus. In particular, special rooms with adequate ventilation should be used to isolate infected patients unable/unwilling to wear a masque with a tendency to carelessly wander around. Sufficient personal protective equipment should also be provided to all personnel.
To the best of our knowledge, this is the first study to consider delirium as the only initial presentation of COVID-19. The DSCU represents the ideal setting to study in detail the onset of COVID-19 in this specific group of patients. Indeed, all the residents in our study had repeated pharyngeal swab testing to confirm the diagnosis. They remained in the facility and were constantly monitored throughout the observation period; therefore, any typical or atypical symptoms were detected and recorded through the entire course of the disease. These conditions are not met in the ER and homebound settings. Another consideration to be made when investigating these two settings is the fact that, as per quarantine rules, everyone especially the elderly was forced to stay at home. On the one hand, this produced a lower frequency of contagion amongst those who were homebound (only 2/28 dementia subjects: 7⋅1%), on the other, it led to a drastic reduction in medical ER access. We indeed observed a drastic reduction in ER visits by elderly people at San Carlo Borromeo Hospital during the pandemic peak compared to the same period of the previous year (525 vs 1119; see flow-chart in Fig. 1). This is consistent with previous reports. (28) (29) Additionally, both caregivers and physicians did not perceive delirium as a possible initial symptom of COVID-19. Thus, homebound patients presenting with delirium were not advised to seek specific medical assistance for COVID-19 and those who visited the ER were not always subjected to a pharyngeal swab. Nonetheless, as reported by others, (10), (11) the occurrence of delirium-onset was observed also in these patient groups, although its prevalence was probably underestimated.
As demonstrated by this work, the brains of older people with dementia may be particularly prone to injury induced by SARS-CoV-2, either through direct infection or by means of different indirect inflammatory pathways. Indeed, neurons and glial cells possess the viral-binding and entry receptor ACE-2 (Angiotensin-Converting Enzyme 2) and CNS invasion by SARS-CoV-1 had been previously demonstrated, although without a topographical description of the infection. (30), (31) Due to high homology between SARS-CoV-1 and SARS-CoV-2, they probably share common properties such as neurotropism and CNS localization. (32) However, compared to SARS-CoV-1, SARS-CoV-2 may have a greater affinity for ACE2 and, therefore, could have a greater neuro-invasiveness. (33) SARS-CoV-2 may infect the CNS through different routes: 1) the intranasal trans-synaptic route to the brainstem and, possibly, the limbic structures, or 2) the endothelial-astrocytic route by crossing the blood brain barrier or through transport by infected leukocytes (Trojan horse mechanism). (4,34,35) Current autopsy findings on the brains of COVID-19 patients remain unspecific and equivocal (brain congestion and oedema, neuronal damage, and inflammatory infiltrates with pan-encephalitis and meningitis), and do not clearly establish the presence of SARS-CoV-2 in the brain. (36, 37, 38) Data on ACE-2 topography in CNS (39) and observed clinical findings (alterations of behaviour, consciousness, sense of smell, breath, and other vegetative functions) should specifically guide the search for the virus in the limbic structures, thalamus, temporal lobe, olfactory bulbs, brainstem, and ventricular ependymal cells. The pathological confirmation of virus invasion and a detailed characterization and topography of inflammatory infiltrate are needed to correctly interpret the mechanisms underlying delirium in COVID-19.
As with other diseases, COVID-19 shows atypical or peculiar manifestations in elderly patients. In the event of a new COVID-19 outbreak, the occurrence of sudden behavioural changes and subsequent delirium deserve special attention, especially in the presence of lymphopenia, and even before the unequivocal onset of widespread inflammation or hypoxia. Indeed, such a clinical presentation in elderly patients with dementia might represent a prodromal phase of SARS-CoV-2 infection. As also suggested by other authors, (10,27) we believe that a symptom-based strategy centred on the identification of typical symptoms is not an effective approach for preventing the spread of the virus in this specific population and setting, and should therefore be revised to include delirium and other atypical symptoms. A test-based strategy involving prompt and more extensive pharyngeal swab testing should also be encouraged. Also, DOC patients tend to have a worse prognosis. In these circumstances, establishing an empathetic relationship with next-of-kin is particularly important for an effective communication of possible poor prognosis and palliative care interventions. Further studies should be directed towards the investigation of the epidemiological and clinical relevance of delirium. Longitudinal follow-up of DOC patients may provide important information on their long-term prognosis. Finally, the neuropathology underlying the neurological manifestations of SARS-CoV-2 infection deserves further analysis to clarify the disease pathogenesis in the brain.
5. Research in context
5.1. Evidence before this study
PubMed was consulted in the period between March 30 and June 28, 2020, searching for articles that documented the clinical manifestation of COVID-19 in elderly people, particularly in subject with cognitive impairment, using the search terms: (“COVID-19″ OR “SARS-COV-2″ OR “novel coronavirus”) AND (“dementia” OR “neurological symptoms” OR “elderly”) with no language or time restrictions, and national registries were also periodically checked for updates on the spreading of the pandemic. In the hardest-hit Italian region of Lombardy, the elderly, in particular those living in residential care facilities, represented the most vulnerable population with the highest mortality rate and in whom atypical symptoms, including behavioural changes and delirium could occur. Nonetheless, there are currently no studies that deal with these atypical COVID-19 manifestations in patients with dementia living in residential care facilities.
5.2. Added value of this study
This research shows that delirium may represent the initial manifestation of SARS-CoV-2 infection in a relevant percentage (36⋅8%) of elderly patients with dementia. This atypical presentation was found to be independently associated with increased risk of short-term mortality (OR: 17⋅0, 95% CI: 2⋅8–102⋅7, p = 0⋅002). Additionally, about 14% of cases did not manifest any of the typical COVID-19 symptoms and, as they initially went unrecognized, these cases might have contributed to the spread of the infection in the high-risk facilities.
5.3. Implications of all the available evidence
To thwart a new spread of the virus in the event of a second wave of COVID-19, special attention should be given to elderly patients who manifest acute behavioural changes, namely delirium, especially in the presence of lymphopenia. In these circumstances, establishing an empathetic relationship with next-of-kin is particularly important for an effective communication of possible poor prognosis and palliative care interventions. The brain pathology underlying delirium as a symptom of COVID-19 deserves further investigation to clarify disease mechanisms.
Contributors
T.E.P. and A.F.C. contributed equally to the original research idea conception, study design, literature search, data collection, data analysis and interpretation, figure and table formatting, and writing of the manuscript. M.C., C.C., V.M., E.M., and D.F. contributed equally to data collection and analysis and assisted in the writing of the text. A.G., P.B., A.D., A.C. contributed equally to data collection and analysis. Ar.C., Cr.C., Ma.C., L.T., S.V. contributed equally to data analysis. A.G. supervised the study and contributed to data analysis. All authors provided intellectual content and critical review of the manuscript.
Data sharing statement
Our dataset is available upon request.
Declaration of Competing Interest
None
Acknowledgments
Acknowledgments
We would like to thank the following DSCU nurses: Massimo Molgora, Michele Magliano, Patrizia Pastori, Alma and Oliver Baca, Adriana Fadda, Nadia Volpati, Luigi Tabbi, Marco Cannata. We also wish to dedicate this work to all the victims of the COVID-19 pandemic and to the healthcare personnel who courageously cared for the sick and continue to do so every day.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100490.
Appendix. Supplementary materials
References
- 1.Istituto Superiore di Sanità. Characteristics of COVID-19 patients dying in Italy report based on available data on May 7th, 2020[Internet]. 2020 [cited 2020 May 11]. p. 1–6.Available from:https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_7_May_2020.pdf
- 2.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol [Internet] 2020:1–8. doi: 10.1001/jamaneurol.2020.1127. http://www.ncbi.nlm.nih.gov/pubmed/32275288 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol [Internet] 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020:1–10. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci [Internet] 2020;77:8–12.. doi: 10.1016/j.jocn.2020.05.017. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baig A.M., Sanders E.C. Potential neuroinvasive pathways of SARS‐CoV‐2: deciphering the spectrum of neurological deficit seen in coronavirus disease 2019 (COVID‐19) J Med Virol. 2020;2019(May) doi: 10.1002/jmv.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinese Society of Geriatric Psychiatry Alzheimer's disease Chinese (ADC), Chinese society of psychiatry. Chin Psychiatr Assoc CA MH. Mental Health Psyochosoc Support Persons Dementia During Outbreak COVID-19 [Internet]. 2020 https://www.alz.co.uk/sites/default/files/pdfs/MHPSS-Keymessages-EN-min.pdf [cited 2020 Apr 1]Available from: [Google Scholar]
- 8.British Geriatrics Society Atypical Covid-19 presentations in older people - the need for continued vigilance [Internet] Br Geriatr Soc Blog. 2020 Apr 17 https://www.bgs.org.uk/blog/atypical-covid-19-presentations-in-older-people-the-need-for-continued-vigilance [citedAvailable from. [Google Scholar]
- 9.Wang H. Dementia care during COVID-19. Lancet. 2020:19–20. doi: 10.1016/S0140-6736(20)30755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchetti A., Rozzini R., Guerini F., Boffelli S., Ranieri P., Minelli G. Clinical presentation of COVID19 in dementia patients. J Nutr Heal Aging. 2020;24(6):560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beach S.R., Praschan N.C., Hogan C., Dotson S., Merideth F., Kontos N. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020 doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. People Who are at increased risk for severe illness [Internet]. 2020[cited 2020 Jun 28]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-increased-risk.html
- 13.Istituto Superiore di Sanità. A survey on COVID-19 infection in long-stay residential care homes. Third Report (Survey nazionale sul contagio COVID-19 nelle strutture residenziali e sociosanitarie Terzo report) [Internet]. [cited2020 May 1]. p. 1–24. Available from: https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-survey-rsa-rapporto-3.pdf
- 14.Barnett M.L., Grabowski D.C. Nursing homes are ground zero for COVID-19 pandemic [Internet] JAMA Health Forum. 2020 May 11 doi: 10.1001/jamahealthforum.2020.0369. https://jamanetwork.com/channels/health-forum/fullarticle/2763666 citedAvailable from. [DOI] [PubMed] [Google Scholar]
- 15.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association . American Psychiatric Publishing; 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [Internet]https://books.google.it/books?id=-JivBAAAQBAJ Available from. [Google Scholar]
- 17.Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982 doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 18.Heyman A., Wilkinson W.E., Hurwitz B.J., Helms M.J., Haynes C.S., Utley C.M. Early-onset alzheimer's disease: clinical predictors of institutionalization and death. Neurology. 1987 doi: 10.1212/wnl.37.6.980. [DOI] [PubMed] [Google Scholar]
- 19.Dooneief G., Marder K., Tang M.X., Stern Y. The clinical dementia rating scale: community-based validation of “profound” and “terminal” stages. Neurology. 1996 doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- 20.Inouye S.K., Van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990 doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 21.Holroyd-Leduc J., Gandell D., Miller A., Petrov D. COVID-19 in older adults [Internet] Reg Geriatr Program Toronto. 2020 May 2 https://www.rgptoronto.ca/wp-content/uploads/2020/04/COVID-19-Presentations-in-Frail-Older-Adults-U-of-C-and-U-fo-T.pdf citedAvailable from. [Google Scholar]
- 22.Boccia S., Ricciardi W., Ioannidis J.P.A. What other countries can learn from Italy during the COVID-19 pandemic. JAMA Intern Med [Internet] 2020 doi: 10.1001/jamainternmed.2020.1447. Available from. [DOI] [PubMed] [Google Scholar]
- 23.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next. Lancet [Internet] 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet [Internet] 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet [Internet] 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rete Terapia del Dolore - Milano. La complessa scelta del medico nella gestione terapeutica del SARS-CoV-2. Pratici suggerimenti e principali interazioni farmacologiche per il trattamento del dolore, dell'analgo-sedazione, del delirium, dello stato d'ansia e dell'insonnia in pazienti COVI [Internet]. [cited2020 Jun 28]. Available from: https://www.ospedaleniguarda.it/uploads/default/attachments/in_evidenza/in_evidenza_m/28/files/allegati/39/trattamento_dolore_pazienti_covid-19.pdf
- 27.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020:1–10. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocher K.E., Macy M.L. Emergency department patients in the early months of the coronavirus disease 2019 (COVID-19) pandemic—What have we learned? [Internet]. JAMA Health Forum. 2020 Jun 26 doi: 10.1001/jamahealthforum.2020.0705. https://jamanetwork.com/channels/health-forum/fullarticle/2767238 citedAvailable from. [DOI] [PubMed] [Google Scholar]
- 29.Cofano F., Tartara F., Zenga F., Penner F., Lanotte M., Garbossa D. Letter: back pain and accesses to emergency departments during COVID-19 lockdown in Italy. Neurosurgery. 2020 doi: 10.1093/neuros/nyaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Zhong S., Liu J., Li L., Li Y., Wu X. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis [Internet] 2005 Oct 15;41(8):1089–1096. doi: 10.1086/444461. https://pubmed.ncbi.nlm.nih.gov/16163626 2005/09/12Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis virus transmission pathways. J Pathol. 2004 doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. 2020:24–27. doi: 10.1002/jmv.25728. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020 doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steardo L., Steardo L., Zorec R., Verkhratsky A. Neuroinfection may potentially contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020 doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baig A.M., Sanders E.C.Potential neuroinvasive pathways of SARS-CoV-2: deciphering the spectrum of neurological deficit seen in coronavirus disease 2019 (COVID-19). 2020; [DOI] [PMC free article] [PubMed]
- 36.National Health Commission. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment (7th edition). 2020. p. 9.
- 37.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020 doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S. Neuropathological features of Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen R., Wang K., Yu J., Chen Z., Wen C., Xu Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. BioRxiv. 2020 doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.