Supplemental Digital Content is available in the text.
Keywords: cangrelor, percutaneous coronary intervention, platelet aggregation, prasugrel hydrochloride, tirofiban
Abstract
Background:
Standard administration of newer oral P2Y12 inhibitors, including prasugrel or ticagrelor, provides suboptimal early inhibition of platelet aggregation (IPA) in patients with ST-segment–elevation myocardial infarction undergoing primary percutaneous coronary intervention. We aimed to investigate the effects of cangrelor, tirofiban, and prasugrel, administered as chewed or integral loading dose, on IPA in patients undergoing primary percutaneous coronary intervention.
Methods:
The FABOLUS-FASTER trial (Facilitation Through Aggrastat or Cangrelor Bolus and Infusion Over Prasugrel: A Multicenter Randomized Open-Label Trial in Patients with ST-Elevation Myocardial Infarction Referred for Primary Percutaneous Intervention) is an investigator-initiated, multicenter, open-label, randomized study. A total of 122 P2Y12-naive patients with ST-segment–elevation myocardial infarction were randomly allocated (1:1:1) to cangrelor (n=40), tirofiban (n=40) (both administered as bolus and 2-hour infusion followed by 60 mg of prasugrel), or 60-mg loading dose of prasugrel (n=42). The latter group underwent an immediate 1:1 subrandomization to chewed (n=21) or integral (n=21) tablets administration. The trial was powered to test 3 hypotheses (noninferiority of cangrelor compared with tirofiban using a noninferiority margin of 9%, superiority of both tirofiban and cangrelor compared with chewed prasugrel, and superiority of chewed prasugrel as compared with integral prasugrel, each with α=0.016 for the primary end point, which was 30-minute IPA at light transmittance aggregometry in response to 20 μmol/L adenosine diphosphate.
Results:
At 30 minutes, cangrelor did not satisfy noninferiority compared with tirofiban, which yielded superior IPA over cangrelor (95.0±8.9 versus 34.1±22.5; P<0.001). Cangrelor or tirofiban were both superior to chewed prasugrel (IPA, 10.5±11.0; P<0.001 for both comparisons), which did not provide higher IPA over integral prasugrel (6.3±11.4; P=0.47), despite yielding higher prasugrel active metabolite concentration (ng/mL; 62.3±82.6 versus 17.1±43.5; P=0.016).
Conclusions:
Cangrelor provided inferior IPA compared with tirofiban; both treatments yielded greater IPA compared with chewed prasugrel, which led to higher active metabolite concentration but not greater IPA compared with integral prasugrel.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02978040; URL: https://www.clinicaltrialsregister.eu; EudraCT 2017-001065-24.
Clinical Perspective.
What Is New?
The FABOLUS-FASTER trial (Facilitation Through Aggrastat or Cangrelor Bolus and Infusion Over Prasugrel: A Multicenter Randomized Open-Label Trial in Patients with ST-Elevation Myocardial Infarction Referred for Primary Percutaneous Intervention) is the first study comparing the pharmacodynamic effects of cangrelor, a direct parenteral P2Y12 inhibitor, with tirofiban, a glycoprotein IIb/IIIa inhibitor, as well as the pharmacodynamic and pharmacokinetic effects of a 60-mg chewed or integral pill intake of prasugrel, an oral indirect P2Y12 inhibitor among patients with ST-segment–elevation myocardial infarction undergoing primary percutaneous coronary intervention.
Cangrelor did not reach noninferiority with tirofiban at adenosine diphosphate–induced platelet aggregation; the latter showed superior platelet inhibition at 30 minutes as well as at all other time points.
Chewed prasugrel led to higher active metabolite concentration but not greater inhibition of platelet aggregation compared with integral prasugrel.
What Are the Clinical Implications?
This study supports the use of parenteral drugs to achieve immediate inhibition of platelet aggregation and to bridge the initial gap in platelet inhibition observed with oral P2Y12 inhibitors.
Cangrelor, unlike tirofiban, was associated with modest reductions of platelet reactivity during the drug infusion and during the transition toward oral P2Y12 inhibitors.
Tirofiban, by exerting more potent and consistent inhibition of platelet aggregation, may be more effective than cangrelor in reducing the risks of acute ischemic complications, which need to be ascertained in the context of studies powered for clinical end points.
Antithrombotic therapy, including oral or parenteral antiplatelet and anticoagulation agents, or both, mitigates the ischemic risks in patients with ST-segment–elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI).1–4 New-generation oral inhibitors of adenosine diphosphate (ADP)–activated platelet P2Y12 receptor (prasugrel or ticagrelor) are recommended, but their administration does not abrogate high residual platelet reactivity (HRPR) up to 4 or 6 hours after standard loading dose.5–7 Strategies to increase the bioavailability of oral P2Y12 inhibitors, such as crushing or chewing tablets, have been investigated, but pharmacokinetic and pharmacodynamic data remain limited.8–11 Parenteral antiplatelet agents, including tirofiban or cangrelor, have been shown to provide more rapid and sustained inhibition of platelet aggregation (IPA) as compared with prasugrel or ticagrelor. Pharmacodynamic data on cangrelor showed that it is able to achieve very high degrees (>80%) of platelet inhibition,12 suggesting that its effect could be equivalent to that of tirofiban; however, no comparative data on IPA are available between tirofiban or cangrelor in patients undergoing primary PCI, and it remains unclear how these treatment options compare with chewed prasugrel instead of standard administration of integral tablets.
We conducted the FABOLUS-FASTER trial (Facilitation Through Aggrastat or Cangrelor Bolus and Infusion Over Prasugrel: A Multicenter Randomized Open-Label Trial in Patients with ST-Elevation Myocardial Infarction Referred for Primary Percutaneous Intervention) to investigate the acute pharmacodynamic effects of cangrelor, tirofiban, or prasugrel, administered at standard 60 mg loading dose as integral or chewed tablets, in patients with STEMI undergoing primary PCI.
Methods
Study Design and Patient Population
The FABOLUS-FASTER trial (URL: https://www.clinicaltrials.gov; Unique identifier: NCT02978040; URL: https://www.clinicaltrialsregister.eu; EudraCT 2017-001065-24) is an investigator-initiated, multicenter, open-label, randomized trial.
The study rationale and design have been reported previously.13 Briefly, patients with suspected STEMI arriving to the catheterization laboratory with indication for coronary angiography were screened for eligibility. After informed consent was obtained, and coronary angiography confirmed the diagnosis, P2Y12-naive patients undergoing primary PCI (within 12 hours, or 24 hours if ongoing ischemia was demonstrated) were enrolled at 3 centers (Bern University Hospital, Switzerland; University Hospital Federico II, Naples, Italy; and University Hospital of Ferrara, Italy). The main exclusion criteria were inability to provide informed consent or to adhere with study protocols (prasugrel pills chewing or ingestion); contraindications or known hypersensitivity or allergy to aspirin, prasugrel, intravenous unfractionated heparin (UFH), cangrelor, or tirofiban; contraindications to the PCI procedure; recent (<7 days) treatment with study drugs (glycoprotein IIb/IIIa inhibitor [GPI] or P2Y12 inhibitors or cangrelor); dialysis; recent (<15 days) or ongoing major bleeding; recent (<15 days) major surgery; fibrinolytics administration (<30 days); oral anticoagulation therapy; prior stroke or transient ischemic attack; concomitant disease affecting life expectancy (<6 months); pregnant or breastfeeding; and recent (<30 days) recruitment in alternative trials on investigational drugs.
Patients were randomly allocated (1:1:1) to standard regimen of tirofiban (bolus + 2-hour infusion, followed by 60 mg loading dose prasugrel at the infusion discontinuation time), standard regimen of cangrelor (bolus + 2-hour infusion, followed by 60 mg loading dose prasugrel at the infusion discontinuation time), or prasugrel 60 mg (integral or chewed tablets) at PCI initiation. Patients in the prasugrel group immediately underwent a subrandomization (1:1) to chewed or integral tablets of prasugrel oral loading dose. Randomization was concealed and stratified according to center and to time from symptom onset to PCI (<3 hours, 3 to 6 hours, >6 hours) with randomly alternating blocks of 3 or 6 in the first randomization, and stratified according to center with randomly alternating blocks of 2 or 4 in the second randomization. The electronic data capture system delivered by AdvicePharma was used. The study complied with the Declaration of Helsinki and Good Clinical Practice and was approved by national agencies and local ethics committees. Further details are reported in the Data Supplement.
The data that support the findings of this study are available from the corresponding author on reasonable request.
Medications and Procedures
Aspirin was administered to all patients before primary PCI (150 to 300 mg orally or 80 to 150 mg intravenously, then 81 to 325 mg daily). Tirofiban was administered at a 25 µg/kg bolus + 0.15 µg/kg per minute infusion for 2 hours (or infusion at 0.075 µg/kg per minute if creatinine clearance was <30 mL/min). Cangrelor was administered at a 30 µg/kg bolus + 4 µg/kg per minute infusion for 2 hours. In both tirofiban and cangrelor arms, patients received integral pills of 60 mg loading dose prasugrel at the time of infusion discontinuation with a following maintenance dose of 10 mg daily (5 mg daily if body weight <60 kg or age >75 years). Patients in the prasugrel group had not to have received parenteral antiplatelet agents and received prasugrel (integral or chewed) at an identical loading dose of 60 mg, with a following maintenance dose of 10 mg daily (5 mg daily if body weight <60 kg or age >75 years). Patients in the chewed prasugrel group had to chew prasugrel tablets for at least 10 to 15 seconds and then swallow with water (≈150 mL) as described.10
UFH was used as an anticoagulant with an initial bolus of 50 to 70 UI/kg in tirofiban- and cangrelor-treated patients or 70 to 100 UI/kg bolus in prasugrel-treated patients, eventually followed by further boluses to reach an intraprocedural activated clotting time of at least 250 seconds. Protocol recommended to stop UFH at the end of PCI in the absence of strict clinical indications.
Blood samples were collected before study drug initiation (baseline), as well as at 15 minutes, 30 minutes, 1 hour, 2 hours, 3 hours, and between 4 and 6 hours after study drug administration (bolus completion or oral pills ingestion).
Pharmacodynamic and Pharmacokinetic Assessment
Pharmacodynamic assessments were performed using light transmittance aggregometry (LTA).14 Blood samples were collected in sodium citrate anticoagulated tubes. Platelet-rich plasma was stimulated with 5 and 20 μmol/L ADP and 5 and 15 μmol/L thrombin receptor agonist peptide (TRAP). Platelet aggregation (maximal percent of platelet aggregation after agonist stimulation) was collected and used to calculate IPA.7 The percent of late platelet aggregation was also collected.
Multiple electrode aggregometry (Multiplate, Roche Diagnostics, Switzerland) was also performed at 2 centers (Bern and Naples).15 Whole blood samples were collected in hirudin-anticoagulated tubes, then diluted with saline (1:2 with 0.9% NaCl) and stimulated with agonists (ADP or TRAP), and platelet aggregation was recorded continuously for 6 minutes. The area under the aggregation curve (AUC) value was used to express platelet inhibition.
A pharmacokinetic assessment was implemented within the prasugrel arm after 10 patients had already been included on request from the Italian Medicines Agency to compare integral versus chewed prasugrel. Prasugrel active metabolite (PAM) was measured at each time point by using precooled ethylenediaminetetraacetic acid tubes treated with 25 μL of 500 mM 3′-methoxyphenacyl bromide in acetonitrile within 30 seconds after collection to derivatize and stabilize the PAM as described previously.9,16 Plasma samples were centrifuged (2800 rpm for 15 minutes at 4°C) within 30 minutes and stored in polypropylene tubes at −20°C/−80°C. The analysis was performed at a central laboratory (University Hospital of Verona) through validated liquid chromatography methods and tandem mass spectrometric detection.
Study End Points
The primary end point was the 30-minute percentage of IPA (%IPA) assessed with LTA after stimulation of platelet-rich plasma with ADP 20 µmol/L. %IPA is defined as follows: 100% × (Baseline Platelet Aggregation − Platelet Aggregation at Time t)/Baseline Platelet Aggregation.
Secondary end points were LTA %IPA after TRAP (5 and 15 µmol/L), ADP (5 µmol/L) stimulation at all time frames, and ADP (20 µmol/L) at 15 minutes, 1 hour, 2 hours, 3 hours, and 4 to 6 hours.
Additional secondary end points were AUC values (residual platelet reactivity) measured by Multiplate after stimulation with ADP and TRAP at all time points.
HRPR was defined as percent of patients with platelet reactivity >59% after ADP 20 µmol/L or >46% after ADP 5 µmol/L or >46 U at Multiplate ADPtest as described previously.14,17,18
For pharmacokinetic assessment, the time to maximum plasma concentration, maximum observed plasma concentration, and the area under the plasma concentration versus time curve from time 0 to various times (AUC0 to30 minutes, AUC0 to1 hour, AUC0 to2 hours, AUC0 to4 hours) were analyzed.
Adverse clinical outcomes within 30 days were collected including all-cause mortality, cardiovascular mortality, myocardial infarction (MI), stroke, transient ischemic attack, definite or probable stent thrombosis, urgent target vessel revascularization, unplanned revascularization, bleeding occurrences, and net adverse clinical events. Events were blindly adjudicated by an independent clinical event committee. All deaths were categorized as cardiovascular or noncardiovascular. Cardiovascular death was defined as death resulting from an acute MI, sudden cardiac death, death from heart failure, death from stroke, death (immediate) from cardiovascular procedures, death from cardiovascular hemorrhage, and death from other cardiovascular causes. Noncardiovascular death was defined as any death not thought to be from a cardiovascular cause. MI was defined according to the fourth universal definition of MI.19 Stroke, categorized as ischemic or hemorrhagic or unknown, was defined as an acute episode of focal or global neurologic dysfunction caused by central nervous system (brain, spinal cord, and retina) vascular injury as a result of hemorrhage or infarction. Transient ischemic attack was defined as a new transient episode of neurologic dysfunction (usually 1 to 2 hours), always within 24 hours, caused by focal brain, spinal cord, or retinal ischemia, without acute infarction. Stent thrombosis was defined according to the Academic Research Consortium.20 Urgent target vessel revascularization was defined as an urgent coronary revascularization in a target coronary vessel (ie, a vessel treated during the index PCI), and unplanned revascularization as any revascularization that was not prespecified or staged after index PCI. Bleeding events were defined according to BARC (Bleeding Academic Research Consortium),21 TIMI (Thrombolysis in Myocardial Infarction),22 and GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries)23 classifications. Net adverse clinical events were defined as the composite of death, nonfatal MI, definite or probable stent thrombosis, nonfatal stroke, and BARC 2, 3, or 5 bleeding.
Statistical Analysis
All analyses were performed at the Clinical Trials Unit of Bern according to the prespecified statistical analysis plan. The sample size calculation was based on testing the noninferiority of cangrelor compared with tirofiban, superiority of both tirofiban and cangrelor compared with chewed prasugrel, and superiority of chewed compared with integral prasugrel.
The sample size estimation, which is partly based on previous evidence,7 followed these considerations (3 primary end points, each with α of 0.05/3=0.016): assuming at least 94% IPA with ADP 20 μmol/L 30 minutes after parenteral drug administration, 8% as SD, 9% as noninferiority margin (this margin was considered a clinically meaningful reduction in the %IPA to refute noninferiority), and 0.016 α, 40 patients per arm would provide a power of 99% to demonstrate noninferiority of cangrelor compared with tirofiban. If noninferiority of cangrelor was not met, then the superiority of tirofiban over cangrelor was tested (prespecified secondary end point); assuming 55% IPA with ADP 20 μmol/L 30 minutes after chewed prasugrel, a 24% SD, and 0.016 as α, the study power would be 100% to demonstrate superiority of tirofiban (n=40) compared with chewed prasugrel (n=20) and superiority of cangrelor (n=40) compared with chewed prasugrel (n=20); and assuming 55% IPA with ADP 20 μmol/L 30 minutes after chewed prasugrel, a 24% SD, and 0.016 as α, 20 patients per arm would provide a power of 82% to demonstrate superiority of chewed compared with integral prasugrel.
Clinical characteristics, procedural data, and medication use were summarized using means with SDs and counts with percentages, P values from t tests, Fisher exact tests, and χ2 tests. Pairwise mean differences in platelet inhibition (difference in %IPA) with 95% CIs were calculated (cangrelor versus tirofiban, cangrelor versus chewed prasugrel, tirofiban versus chewed prasugrel, chewed versus integral prasugrel; based on generalized mixed models, including data on all time points after drug administration, and random effects of patient as appropriate), and a z test was used to test noninferiority. Generalized mixed models (including data on all time points after drug administration) were used to test superiority (%IPA at LTA or AUC unit on Multiplate, as continuous variables or random effects of patient, as appropriate). P values are interpreted with a Bonferroni post hoc correction. Generalized mixed models used the appropriate link function according to the type of primary or secondary outcomes measured (continuous or binary) and adjusted for time point (minutes to hours after drug administration) and the interaction between randomized arm and time point, with random effects added of site and patient as appropriate. Wald χ2 test was used to compare event rates. Hazard ratios were estimated by fitting a Cox proportional hazards model (proportional hazards were tested and nonsignificant). The α used to claim statistical significance is 0.016 for the primary end point. Secondary end points were analyzed with a 2-sided α set at 0.05 to allow conventional interpretation of results. Statistical analyses were performed with Stata version 16.1.
Results
Between July 4, 2017, and August 26, 2019, we enrolled 122 patients with STEMI intended for primary PCI (cangrelor, n=40; tirofiban, n=40; prasugrel, n=42 [chewed, n=21; integral, n=21]) across 3 centers in Switzerland and Italy. Patient disposition is summarized in Figure 1.
Figure 1.
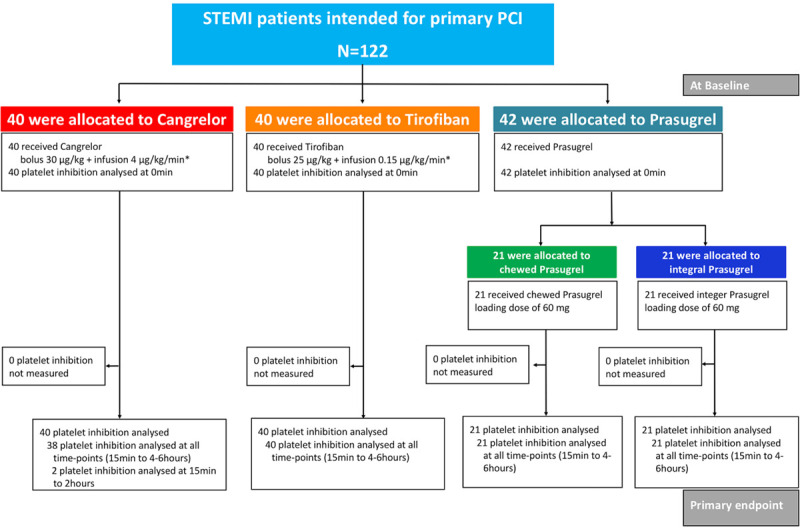
Flow chart of the FABOLUS-FASTER trial. *For 2 hours or to the end of the PCI. FABOLUS-FASTER indicates Facilitation Through Aggrastat or Cangrelor Bolus and Infusion Over Prasugrel: A Multicenter Randomized Open-Label Trial in Patients with ST-Elevation Myocardial Infarction Referred for Primary Percutaneous Intervention; PCI, percutaneous coronary intervention; and STEMI, ST-segment–elevation myocardial infarction.
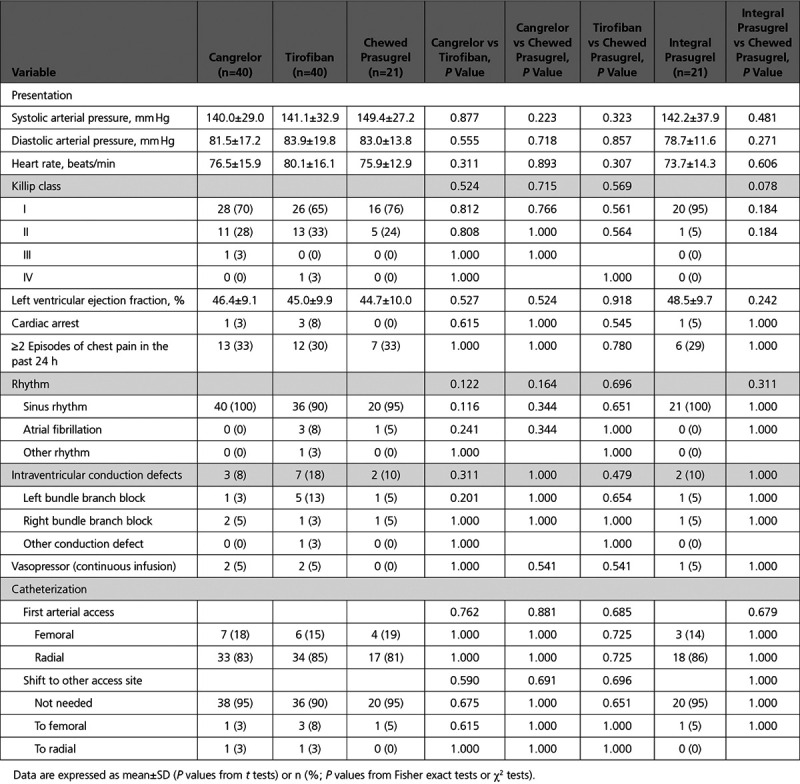
Baseline and procedural characteristics were well balanced across groups (Tables 1 and 2 and Tables I and II in the Data Supplement). Patients were predominantly male (76%), mean age was 64 years, 20% had diabetes mellitus, and 52% presented within 3 hours from symptom onset. Most patients presented in sinus rhythm, 27% had Killip class >1, 4% previously had cardiac arrest, and 4% required continuous infusion of vasopressors. Also, 21 (17%) patients were on chronic aspirin at the time of presentation and 115 (94%) received aspirin loading dose, administered mainly intravenously, whereas all patients received UFH. The radial was the primarily attempted access site in 84% of the patients, and 98% of the patients had TIMI flow 3 at the end of the procedure. Clinical outcomes at 30 days are shown in Table III in the Data Supplement.
Table 1.
Baseline Clinical Characteristics
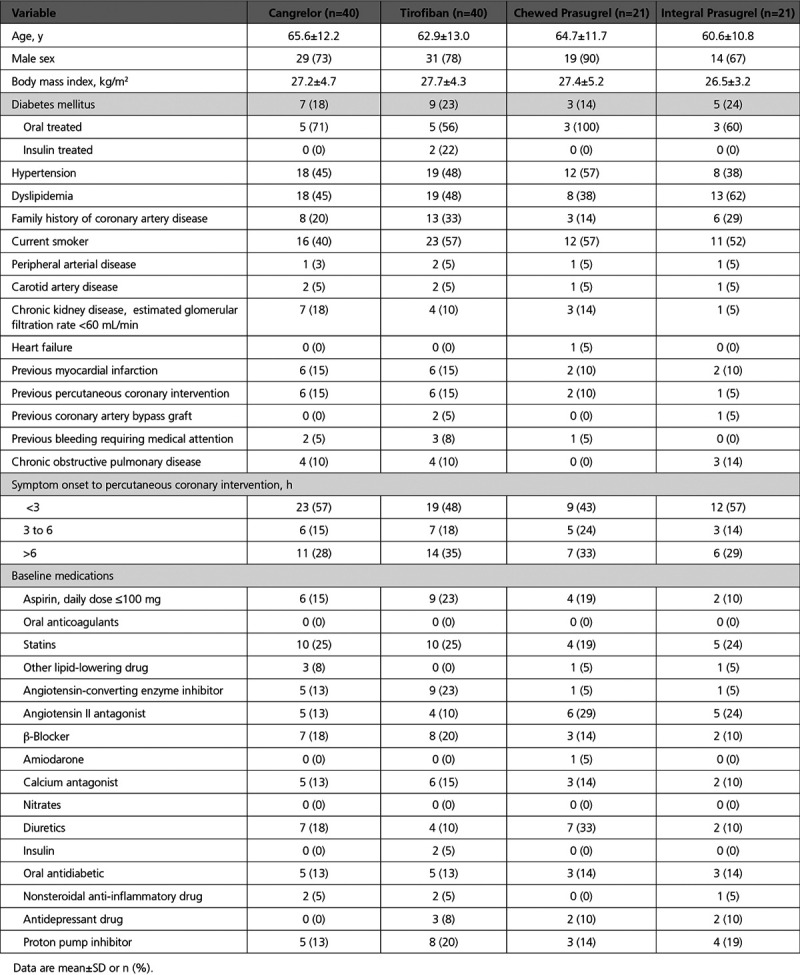
Table 2.
Clinical Presentation and Catheterization Laboratory Data
Pharmacodynamic Assessment
Light Transmittance Aggregometry
At baseline, there were no differences across groups in ADP- or TRAP-induced platelet aggregation. Figure 2 and Table IV in the Data Supplement show IPA with LTA ADP 20 µmol/L. At 30 minutes, cangrelor did not reach noninferiority with tirofiban, which, in turn, achieved superior IPA over cangrelor (IPA, 95.0±9.0 versus 34.1±22.5; P<0.001), cangrelor or tirofiban were both superior to chewed prasugrel (IPA, 10.5±11.0; P<0.001 for both comparisons), and chewed prasugrel did not reach superior IPA over integral prasugrel (6.3±11.4; P=0.47). At secondary analyses, tirofiban provided greater IPA at all time points compared with cangrelor or chewed prasugrel. Cangrelor provided greater IPA up to 1 hour compared with chewed prasugrel, but IPA did not differ at 2 hours and it became higher with chewed prasugrel compared with cangrelor at 3 and 4 to 6 hours. The proportions of patients with IPA >80% or 90% were consistently higher with tirofiban compared with cangrelor or chewed or integral prasugrel (Figure 2B and 2C). No patient with tirofiban had HRPR within the first 2 hours, whereas HRPR rates ranged from 50% to 58% during cangrelor infusion (Figure 3 and Table V in the Data Supplement). Cangrelor, in turn, provided significantly lower HRPR rates compared with chewed prasugrel at 15 and 30 minutes but not at 1 and 2 hours; at 3 hours and 4 to 6 hours, chewed prasugrel was associated with lower HRPR rates compared with cangrelor. HRPR rates trended lower with chewed versus integral prasugrel for up to 2 hours without reaching statistical significance. The primary end point for chewed versus integral prasugrel remained consistent when stratified by patients receiving opioids or not (Pinteraction=0.226; Table VI in the Data Supplement).
Figure 2.
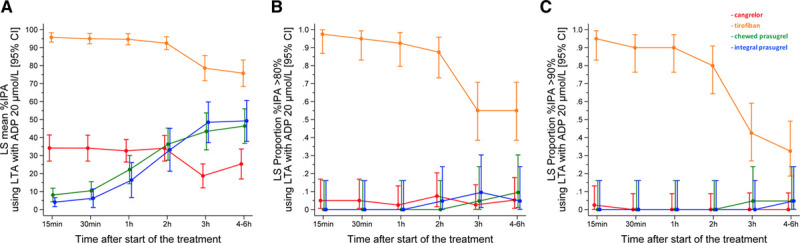
Pharmacodynamic effects of drugs measured by LTA after ADP 20 µmol/L stimulation. A, Percentage of inhibition of platelet aggregation (IPA). B, IPA >80%. C, IPA >90%. ADP indicates adenosine diphosphate; CI, confidence interval; LS, least square; and LTA, light transmittance aggregometry.
Figure 3.
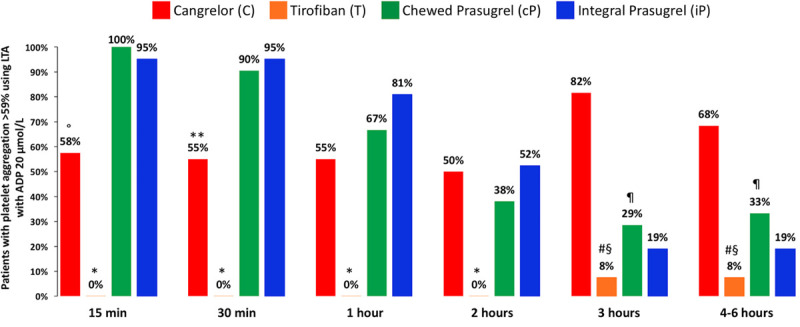
Rates of high residual platelet reactivity defined by platelet aggregation >59% at LTA after ADP 20 µmol/L stimulation. °P<0.001 versus cP; *P<0.001 versus C and cP; **P<0.05 versus cP; #P<0.001 versus C; §P<0.05 versus cP; ¶P<0.01 versus C. ADP indicates adenosine diphosphate; and LTA, light transmittance aggregometry.
The kinetics of platelet inhibition were consistent after 5 µmol/L ADP (Figure 4A and Tables V and VII in the Data Supplement).
Figure 4.
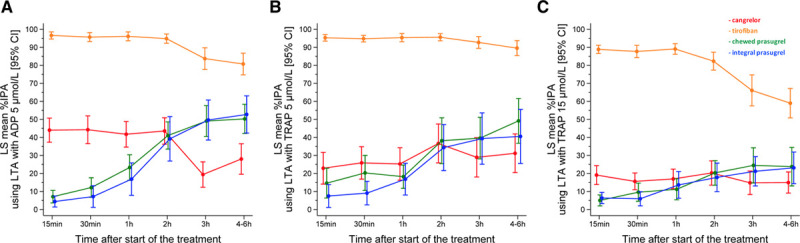
Pharmacodynamic effects of drugs measured by LTA. Pharmacodynamic effects of drugs measured by LTA after stimulation with ADP 5 µmol/L (A) and TRAP 15 (B) and 5 (C) µmol/L. ADP indicates adenosine diphosphate; CI, confidence interval; IPA, inhibition of platelet aggregation; LS, least square; LTA, light transmittance aggregometry; and TRAP, thrombin receptor agonist peptide.
Tirofiban was also associated with higher inhibition of 15 or 5 µmol/L TRAP-induced platelet aggregation at all time points compared with cangrelor or chewed prasugrel (Figure 4B and 4C and Table VII in the Data Supplement). IPA after 15 µmol/L TRAP was higher with cangrelor compared with chewed prasugrel at 15 minutes but not thereafter up to 2 hours, whereas at 3 and 4 to 6 hours chewed prasugrel was associated with greater IPA as compared with cangrelor. IPA after 5 µmol/L TRAP did not differ between cangrelor and chewed prasugrel up to 3 hours but was greater in the latter group at the 4 to 6 hours assessment.
Results remained consistent when the 2 prasugrel arms were analyzed as a single group against tirofiban or cangrelor or when IPA was calculated using late instead of maximal aggregation.
Multiplate
ADP-induced platelet aggregation was lower in tirofiban-treated patients compared with cangrelor or chewed prasugrel up to 2 hours, and in turn lower with cangrelor compared with chewed prasugrel from 15 minutes to 2 hours, but higher thereafter with cangrelor compared with chewed or integral prasugrel (Figure I and Table VII in the Data Supplement). Chewed prasugrel was associated with lower platelet aggregation than integral prasugrel at 30 minutes and 1 hour. Tirofiban was associated with higher TRAP-induced platelet aggregation compared with cangrelor or chewed prasugrel (P<0.001 at any time point for both comparisons) whereas there was no difference between cangrelor and chewed prasugrel or between the 2 prasugrel groups.
Pharmacokinetic Assessment
Chewed prasugrel, compared with integral prasugrel, was associated with higher PAM levels at 15, 30, and 60 minutes, but not thereafter. Maximum observed plasma concentration, time to maximum plasma concentration, AUC0 to2 hours and AUC0 to 4 hours did not differ between the 2 prasugrel groups. AUC0 to30 minutes and AUC0 to1 hour were both significantly higher with chewed compared with integral prasugrel (Figure 5 and Table 3).
Figure 5.
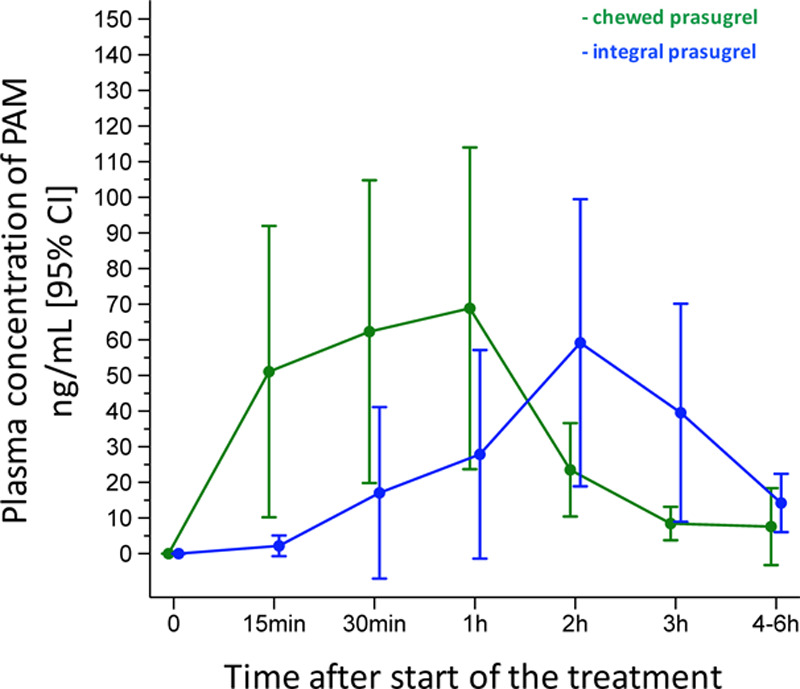
Pharmacokinetic analysis comparing PAM concentrations between chewed and integral prasugrel groups. CI indicates confidence interval; and PAM, prasugrel active metabolite.
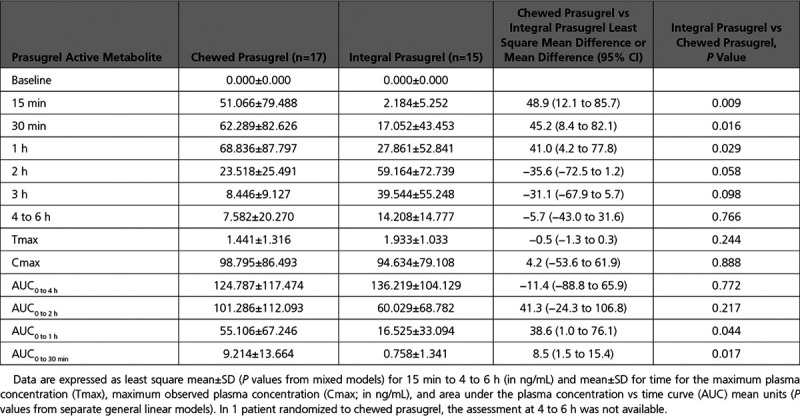
Table 3.
Pharmacokinetic Analysis Comparing Prasugrel Active Metabolite Concentrations Between Chewed and Integral Prasugrel Groups
Discussion
To our knowledge, our study is the first to compare the pharmacodynamic effects of cangrelor, a direct parenteral P2Y12 inhibitor, with tirofiban, a GPI, as well as the pharmacodynamic and pharmacokinetic effects of a 60-mg chewed or integral pill intake of prasugrel, an oral indirect P2Y12 inhibitor. The following are the main findings of the study (Figure 6):
Figure 6.
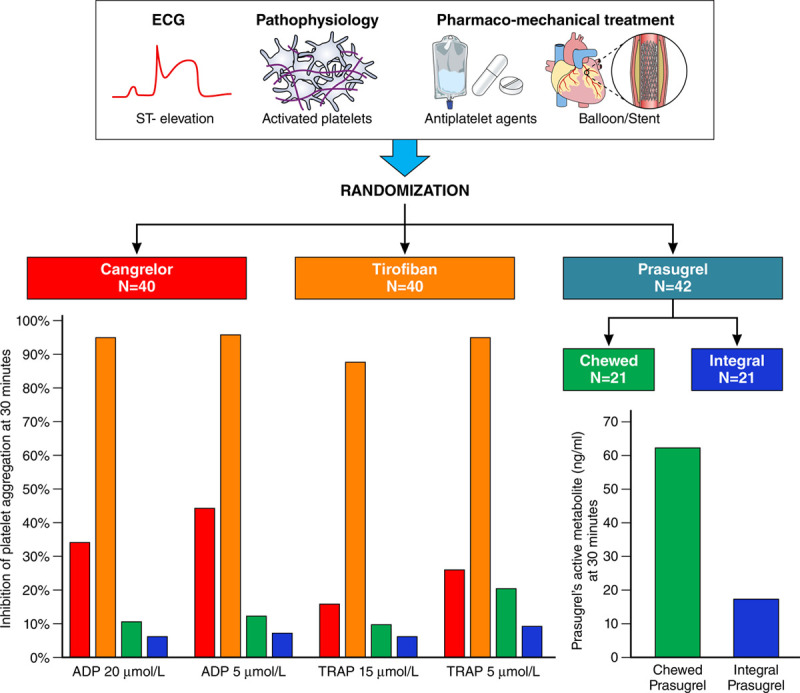
Pharmacodynamic and pharmacokinetic effects of cangrelor, tirofiban, and prasugrel (chewed or integral) in patients with ST-segment–elevation myocardial infarction undergoing primary percutaneous coronary intervention 30 minutes after drug administration. ADP indicates adenosine diphosphate; and TRAP, thrombin receptor agonist peptide.
Cangrelor did not reach noninferiority with tirofiban at ADP-induced platelet aggregation; the latter showed superior platelet inhibition at 30 minutes as well as at all other time points.
Cangrelor and tirofiban provided superior inhibition of platelet aggregation after ADP compared with chewed prasugrel at 30 minutes. Tirofiban remained associated with higher inhibition of ADP-induced platelet aggregation at all time points. Cangrelor, on the other hand, proved superior to chewed prasugrel at ADP-induced platelet aggregation test up to 1 hour from drug administration, but it provided similar treatment effect at 2 hours and lower IPA at later assessments.
Chewed prasugrel, compared with integral prasugrel, did not provide greater ADP-induced IPA despite providing greater active metabolite bioavailability up to 1 hour after drug administration.
TRAP-induced platelet aggregation was profoundly inhibited by tirofiban infusion whereas it was mildly affected by cangrelor or chewed or integral prasugrel at peak effects (ie, early after bolus administration for cangrelor and at 3 hours and 4 to 6 hours for both prasugrel arms).
On-treatment residual platelet reactivity measured at the time of intervention with LTA in response to ADP or at other platelet function assays correlates with myocardial damage, ST-segment–elevation resolution, procedural success, and clinical outcomes among patients with STEMI.24,25 Standard loading dose of newer P2Y12 inhibitors, even when administered at first medical contact, provides only limited IPA at the time of intervention,5,7 which led to the investigation of alternative treatment strategies aiming at enhancing early suppression of platelet reactivity, procedural success, and potentially improved outcomes among patients with STEMI.
Numerous studies have formed the basis for the currently approved cangrelor regimen.12,13 Yet, none of them included patients with STEMI and the majority of them evaluated multiple maintenance cangrelor regimens administered for several hours while omitting an initial bolus regimen. Among 14 patients with unstable angina or non–Q-wave MI, treated with aspirin, UFH, and cangrelor infusion at 4 μg/kg/min for up to 69 hours, mean IPA was 98.7±2.1% at 24 hours measured through whole blood impedance aggregometry and using ADP 3 μmol/L as agonist.26 In another randomized trial (composed of 2 parts) including overall 399 patients undergoing elective or urgent PCI, but excluding patients with STEMI, the mean %IPA measured with impedance aggregometry in heparinized blood in response to ADP 3 μmol/L was 99% for those receiving cangrelor infusion at 4 μg/kg/min.27 During part 2, the mean platelet inhibition was 100% before the end of infusion for both cangrelor 4 μg/kg/min and abciximab groups with 95% and 100% of patients achieving 100% platelet inhibition, respectively.27
Across these pivotal dose-finding studies, response to 3 μmol/L of ADP was chosen because this concentration was found to produce maximal or just submaximal activation when testing with impedance aggregometry in heparinized blood and a degree of activation similar to that seen with 5 to 20 μmol/L of ADP when using optical aggregometry in citrated platelet-rich plasma.27
We therefore anticipated a nearly maximal IPA in response to 20 μmol/L of ADP at optical aggregometry in citrated platelet-rich plasma and powered the study for noninferiority based on a stringent noninferiority margin. Our findings show that cangrelor provides a quicker onset of action among patients with STEMI as compared with prasugrel, administered with either chewed or integral pill loading dose. Yet, cangrelor yielded lower than expected and inferior IPA than tirofiban throughout the study duration as well as than prasugrel at 3 hours or 4 to 6 hours after drug administration. This latter finding likely reflects the fast recovery of platelet function at the end of cangrelor infusion and the time needed for prasugrel to provide sufficient IPA once cangrelor infusion is terminated. IPA in response to 5 or 20 µmol/L ADP was lower with cangrelor during drug infusion as compared with prasugrel peak effect at 3 hours or 4 to 6 hours. Patients with STEMI have higher platelet reactivity,28 have higher expression of P2Y12 receptor,29 and require greater concentration of antiplatelet agents to achieve similar IPA as compared with healthy subjects or patients without ongoing myocardial ischemia undergoing elective coronary artery disease.30 It was previously observed that, among patients with type 2 diabetes mellitus, in whom enhanced platelet P2Y12 receptor expression occurs, cangrelor yields lower IPA compared with nondiabetic patients.31 Whether an increased cangrelor regimen is required to yield greater IPA among STEMI, and in a similar order of magnitude as previously observed in patients undergoing elective procedures, remains speculative. The CANTIC study (Cangrelor and Crushed Ticagrelor in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention) assessed pharmacodynamic effects of standard cangrelor regimen versus placebo as P2Y12 reaction units (PRUs) by VerifyNow and platelet reactivity index by vasodilator-stimulated phosphoprotein in patients with STEMI undergoing PCI concomitantly treated with crushed ticagrelor.32 In the cangrelor plus ticagrelor arm, median PRU value was 63, with an interquartile range of 32 to 93. No patient with cangrelor had a PRU higher than 208 U during drug infusion. Yet, this cutoff value for defining nonresponsiveness to P2Y12 inhibitors was generated for the prediction of out-of-hospital events among patients who underwent elective PCI and it remains unclear whether it applies to patients with STEMI undergoing emergency treatment.33 It is interesting that 3 out of 22 patients remained nonresponders to cangrelor at vasodilator-stimulated phosphoprotein assay during drug infusion.32 Alexopoulos et al34 reported that HRPR was 6.7% at 15 minutes and 0% at 1 hour (using VerifyNow and a cutoff of PRU >208) in 15 patients with STEMI receiving cangrelor (pretreated with ticagrelor; none of them received morphine). Ubaid et al35 reported an HRPR rate of 10% (using VerifyNow and a cutoff of PRU >208) at the time of balloon inflation in 50 cangrelor-treated patients with STEMI. In Buchtele et al,36 16 cardiac arrest survivors with STEMI, who were treated with targeted temperature management, received cangrelor, and none had HRPR (using Multiplate Analyzer, platelet function had a median 18 U, interquartile range 10 to 25; <46 U was used as the cutoff). Our results show a much higher rate of HRPR during cangrelor infusion (50% to 58%) at LTA test after 20 µmol/L ADP stimulation, whereas HRPR ranged from 8.6% at 15 minutes to ≈14% at 30 minutes and 2 hours at Multiplate testing. Therefore, whereas the rates of HRPR varied across STEMI studies, most likely reflecting different platelet function tests and test-specific HRPR cutoffs, almost all STEMI studies have identified some degree of hyporesponsiveness to cangrelor.
Unlike cangrelor and prasugrel, tirofiban exerts a downstream IPA by blocking the glycoprotein IIb/IIIa receptor. This explains our observations on TRAP-induced platelet aggregation, which was profoundly inhibited by tirofiban but not with cangrelor or prasugrel. In keeping with previous observations,7 our results confirm that P2Y12 inhibition exerts a mild yet measurable effect on TRAP-induced platelet aggregation.
A strategy to crush a standard loading dose of ticagrelor or prasugrel has resulted in greater drug bioavailability and higher IPA in the first few hours after drug administration compared with whole tablets in patients with STEMI.11,13 However, crushing tablets requires available dedicated point-of-care devices. Recently, chewed ticagrelor resulted in a more rapid platelet inhibition compared with crushed or whole tablets among patients undergoing coronary angiography for stable angina.10 No study had so far examined whether chewed prasugrel increases PAM bioavailability and allows a more rapid IPA among patients with STEMI. In our study, chewed compared with integral prasugrel was associated with higher PAM levels up to 1 hour after drug administration. Despite numeric higher IPA with chewed prasugrel, at no time point was IPA significantly greater than integral prasugrel at LTA measurements. At Multiplate testing, IPA was greater with chewed as compared with integral prasugrel at 30 and 60 minutes. Yet, this assay was performed in only 2 out of 3 centers participating in the study and thus was available in a subset of patients (n=108).
Our results are consistent with previous observations suggesting that crumbling P2Y12 inhibitor tablets increases early drug availability. However, in the setting of our multicenter study, chewed tablets resulted in only a mild and lower than anticipated IPA increase in the first hour after drug administration.
Our study might have important clinical implications. First, the comparative efficacy data of intravenous agents with chewed prasugrel reinforces the superiority notion of parenteral over oral antiplatelet medications in the acute phase of STEMI treatment. Second, among parenteral drugs, tirofiban demonstrated superior efficacy than cangrelor, which in turn was associated with relatively high HRPR rates, suggesting that GPI might be preferable over cangrelor to minimize the risk of acute ischemic complications. A recent large retrospective study suggested that a short regimen of GPI was protective against stent thrombosis risk in morphine-treated patients with STEMI (who are potentially exposed to increased risk of acute stent thrombosis owing to delayed absorption of oral P2Y12 inhibitors).37 In recent years, the use of GPI has declined, mainly because of the perception that the ischemic benefits are counterbalanced by bleeding risks.3 Yet, clinical data on GPI are mainly based on prolonged postbolus drug infusion and femoral access site at the time of intervention. Large-scale trials reassessing the comparative risks and benefits of short infusion of parenteral platelet inhibitors such as cangrelor or GPI compared with newer oral P2Y12 receptor blockers alone in contemporary primary PCI practice remain desirable.
Limitations
Our study was mechanistic in nature and was not powered for clinical end points. Whereas multiple prior studies have shown an association between low residual platelet reactivity and better ischemic outcomes during and after PCI among patients undergoing elective procedures as well as patients with STEMI, the comparative efficacy and safety profile of cangrelor, tirofiban, or prasugrel in patients undergoing primary PCI remain to be established in an adequately powered clinical trial. A single study has suggested that prasugrel can be given at the start of cangrelor infusion, without evidence of drug–drug interaction.38 Whether this evidence applies to patients with STEMI is unknown and this treatment strategy remains off-label. Applicability to ticagrelor loading, which is mostly used in clinical practice at the beginning of cangrelor infusion or before primary PCI,39,40 cannot be claimed. However, on the basis of our observation that cangrelor followed by prasugrel associates with a rebound of platelet activation at 2 to 4 hours and on previous data showing some HRPR during and after cangrelor infusion,32,34 one could speculate that when cangrelor is used, ticagrelor might be the preferred oral P2Y12 inhibitor. It remains unclear whether the test-specific cutoffs used to define HRPR rates retain prognostic implications for the acute treatment of STEMI, considering that they were generated mainly for stable or stabilized patients receiving oral P2Y12 inhibitors.
Conclusions
Among patients with STEMI undergoing primary PCI, cangrelor provided inferior IPA compared with tirofiban; both parenteral treatment strategies yielded superior IPA during drug infusion compared with chewed prasugrel, which, despite achieving early higher active metabolite concentrations, did not lead to greater IPA compared with integral prasugrel.
Acknowledgments
The authors thank the study coordinators, doctors, nurses, and technicians at the 3 centers involved in the study for their support with blood samples and data collection.
Sources of Funding
This study is an investigator-initiated trial supported by a grant to the institution (Inselspital, University of Bern) from Medicure Inc, Winnipeg, Canada.
Disclosures
Dr Gargiulo reports consultant fees from Daiichi-Sankyo outside the submitted work. Dr Gragnano reports research grant support from the European Society of Cardiology. Dr Vranckx reports personal fees from Daiichi-Sankyo, AstraZeneca, Bayer Health Care, and Terumo outside the submitted work. Dr Leonardi reports personal fees from Bayer, Bristol-Myers Squibb SA, Chiesi, Daiichi-Sankyo, and AstraZeneca outside the submitted work. Dr Windecker reports research and educational grants to the institution from Abbott, Amgen, Bayer, BMS, Biotronik, Boston Scientific, CSL Behring, Edwards Lifesciences, Medtronic, Polares, and Sinomed outside the submitted work. Dr Valgimigli reports a grant to the institution from Medicure and grants and personal fees from Abbott, Alvimedica, Amgen, Bayer, Bristol-Myers Squibb SA, Coreflow, Daiichi-Sankyo, Vifor, Idorsia, Terumo, iVascular, and AstraZeneca outside the submitted work. The other authors report no disclosures.
Supplemental Materials
Data Supplement Tables I–VII
Data Supplement Figure I
Study Protocols (Swiss and Italian versions)
Statistical Analysis Plan
Supplementary Material
Footnotes
Sources of Funding, see page 452
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.046928.
References
- 1.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. ; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 201839119–177doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol 2016671235–1250doi: 10.1016/j.jacc.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 3.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. ; ESC Scientific Document Group 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 20194087–165doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 4.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 201839213–260doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, Koutsogiannis N, Damelou A, Tsigkas G, Davlouros P, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 20125797–804doi: 10.1161/CIRCINTERVENTIONS.112.972323 [DOI] [PubMed] [Google Scholar]
- 6.Parodi G, Valenti R, Bellandi B, Migliorini A, Marcucci R, Comito V, Carrabba N, Santini A, Gensini GF, Abbate R, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol 2013611601–1606doi: 10.1016/j.jacc.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 7.Valgimigli M, Tebaldi M, Campo G, Gambetti S, Bristot L, Monti M, Parrinello G, Ferrari R; FABOLUS PRO Investigators Prasugrel versus tirofiban bolus with or without short post-bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (Facilitation Through Aggrastat By Dropping or Shortening Infusion Line in Patients With ST-Segment Elevation Myocardial Infarction Compared to or on Top of Prasugrel Given at Loading Dose) trial. JACC Cardiovasc Interv 20125268–277doi: 10.1016/j.jcin.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 8.Parodi G, Xanthopoulou I, Bellandi B, Gkizas V, Valenti R, Karanikas S, Migliorini A, Angelidis C, Abbate R, Patsilinakos S, et al. Ticagrelor crushed tablets administration in STEMI patients: the MOJITO study. J Am Coll Cardiol 201565511–512doi: 10.1016/j.jacc.2014.08.056 [DOI] [PubMed] [Google Scholar]
- 9.Rollini F, Franchi F, Hu J, Kureti M, Aggarwal N, Durairaj A, Park Y, Seawell M, Cox-Alomar P, Zenni MM, et al. Crushed prasugrel tablets in patients with STEMI undergoing primary percutaneous coronary intervention: the CRUSH study. J Am Coll Cardiol 2016671994–2004doi: 10.1016/j.jacc.2016.02.045 [DOI] [PubMed] [Google Scholar]
- 10.Venetsanos D, Sederholm Lawesson S, Swahn E, Alfredsson J. Chewed ticagrelor tablets provide faster platelet inhibition compared to integral tablets: the inhibition of platelet aggregation after administration of three different ticagrelor formulations (IPAAD-Tica) study, a randomised controlled trial. Thromb Res 201714988–94doi: 10.1016/j.thromres.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 11.Serenelli M, Pavasini R, Vitali F, Tonet E, Bilotta F, Parodi G, Campo G. Efficacy and safety of alternative oral administrations of P2Y12-receptor inhibitors: systematic review and meta-analysis. J Thromb Haemost 201917944–950doi: 10.1111/jth.14434 [DOI] [PubMed] [Google Scholar]
- 12.Ferreiro JL, Ueno M, Angiolillo DJ. Cangrelor: a review on its mechanism of action and clinical development. Expert Rev Cardiovasc Ther 200971195–1201doi: 10.1586/erc.09.101 [DOI] [PubMed] [Google Scholar]
- 13.Gargiulo G, Esposito G, Cirillo P, Nagler M, Minuz P, Campo G, Gragnano F, Manavifar N, Piccolo R, Avvedimento M, et al. Facilitation through aggrastat or cangrelor bolus and infusion over prasugrel: a Multicenter Randomized Open-Label Trial in Patients With ST-Elevation Myocardial Infarction Referred for Primary Percutaneous Intervention (FABOLUS-FASTER) trial: design and rationale: the FABOLUS-FASTER trial [published online February 24, 2020]. J Cardiovasc Transl Res. doi: 10.1007/s12265-020-09969-4. doi: 10.1007/s12265-020-09969-4. https://link.springer.com/article/10.1007%2Fs12265-020-09969-4. [DOI] [PubMed] [Google Scholar]
- 14.Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019121521–1537doi: 10.1016/j.jcin.2019.03.034 [DOI] [PubMed] [Google Scholar]
- 15.Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schömig A, Kastrati A, von Beckerath N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol 200953849–856doi: 10.1016/j.jacc.2008.11.030 [DOI] [PubMed] [Google Scholar]
- 16.Farid NA, McIntosh M, Garofolo F, Wong E, Shwajch A, Kennedy M, Young M, Sarkar P, Kawabata K, Takahashi M, et al. Determination of the active and inactive metabolites of prasugrel in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 200721169–179doi: 10.1002/rcm.2813 [DOI] [PubMed] [Google Scholar]
- 17.Aradi D, Storey RF, Komócsi A, Trenk D, Gulba D, Kiss RG, Husted S, Bonello L, Sibbing D, Collet JP, et al. ; Working Group on Thrombosis of the European Society of Cardiology Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur Heart J 201435209–215doi: 10.1093/eurheartj/eht375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, et al. ; Working Group on High On-Treatment Platelet Reactivity Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 201056919–933doi: 10.1016/j.jacc.2010.04.047 [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018722231–2264doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, et al. ; Academic Research Consortium Standardized end point definitions for coronary intervention trials: the Academic Research Consortium–2 consensus document. Circulation 20181372635–2650doi: 10.1161/CIRCULATIONAHA.117.029289 [DOI] [PubMed] [Google Scholar]
- 21.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 20111232736–2747doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 22.Rao AK, Pratt C, Berke A, Jaffe A, Ockene I, Schreiber TL, Bell WR, Knatterud G, Robertson TL, Terrin ML. Thrombolysis in Myocardial Infarction (TIMI) Trial: phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol 1988111–11doi: 10.1016/0735-1097(88)90158-1 [DOI] [PubMed] [Google Scholar]
- 23.GUSTO investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993329673–682doi: 10.1056/NEJM199309023291001 [DOI] [PubMed] [Google Scholar]
- 24.Campo G, Valgimigli M, Gemmati D, Percoco G, Tognazzo S, Cicchitelli G, Catozzi L, Malagutti P, Anselmi M, Vassanelli C, et al. Value of platelet reactivity in predicting response to treatment and clinical outcome in patients undergoing primary coronary intervention: insights into the STRATEGY study. J Am Coll Cardiol 2006482178–2185doi: 10.1016/j.jacc.2005.12.085 [DOI] [PubMed] [Google Scholar]
- 25.Frossard M, Fuchs I, Leitner JM, Hsieh K, Vlcek M, Losert H, Domanovits H, Schreiber W, Laggner AN, Jilma B. Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation 20041101392–1397doi: 10.1161/01.CIR.0000141575.92958.9C [DOI] [PubMed] [Google Scholar]
- 26.Storey RF, Oldroyd KG, Wilcox RG. Open multicentre study of the P2T receptor antagonist AR-C69931MX assessing safety, tolerability and activity in patients with acute coronary syndromes. Thromb Haemost 200185401–407 [PubMed] [Google Scholar]
- 27.Greenbaum AB, Grines CL, Bittl JA, Becker RC, Kereiakes DJ, Gilchrist IC, Clegg J, Stankowski JE, Grogan DR, Harrington RA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J 2006151689.e1–689.e10doi: 10.1016/j.ahj.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 28.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 20073572482–2494doi: 10.1056/NEJMra071014 [DOI] [PubMed] [Google Scholar]
- 29.Liverani E, Kilpatrick LE, Tsygankov AY, Kunapuli SP. The role of P2Y2 receptor and activated platelets during inflammation. Curr Drug Targets 201415720–728doi: 10.2174/1389450115666140519162133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabbani SS, Aggarwal A, Terrien EF, DiBattiste PM, Sobel BE, Schneider DJ. Suboptimal early inhibition of platelets by treatment with tirofiban and implications for coronary interventions. Am J Cardiol 200289647–650doi: 10.1016/s0002-9149(01)02319-0 [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Chang L, Zhang Y, Zhai L, Zhang S, Qi Z, Yan H, Yan Y, Luo X, Zhang S, et al. Platelets express activated P2Y12 receptor in patients with diabetes mellitus. Circulation 2017136817–833doi: 10.1161/CIRCULATIONAHA.116.026995 [DOI] [PubMed] [Google Scholar]
- 32.Franchi F, Rollini F, Rivas A, Wali M, Briceno M, Agarwal M, Shaikh Z, Nawaz A, Silva G, Been L, et al. Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation 20191391661–1670doi: 10.1161/CIRCULATIONAHA.118.038317 [DOI] [PubMed] [Google Scholar]
- 33.Campo G, Fileti L, de Cesare N, Meliga E, Furgieri A, Russo F, Colangelo S, Brugaletta S, Ferrari R, Valgimigli M; 3T/2R Investigators Long-term clinical outcome based on aspirin and clopidogrel responsiveness status after elective percutaneous coronary intervention: a 3T/2R (tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel) trial substudy. J Am Coll Cardiol 2010561447–1455doi: 10.1016/j.jacc.2010.03.103 [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulos D, Pappas C, Sfantou D, Xanthopoulou I, Didagelos M, Kikas P, Ziakas A, Tziakas D, Karvounis H, Iliodromitis E. Cangrelor in ticagrelor-loaded STEMI patients undergoing primary percutaneous coronary intervention. J Am Coll Cardiol 2018721750–1751doi: 10.1016/j.jacc.2018.07.041 [DOI] [PubMed] [Google Scholar]
- 35.Ubaid S, Ford TJ, Berry C, Murray HM, Wrigley B, Khan N, Thomas MR, Armesilla AL, Townend JN, Khogali SS, et al. Cangrelor versus ticagrelor in patients treated with primary percutaneous coronary intervention: impact on platelet activity, myocardial microvascular function and infarct size: a randomized controlled trial. Thromb Haemost 20191191171–1181doi: 10.1055/s-0039-1688789 [DOI] [PubMed] [Google Scholar]
- 36.Buchtele N, Herkner H, Schorgenhofer C, Merrelaar A, Laggner R, Gelbenegger G, Spiel AO, Domanovits H, Lang I, Jilma B, et al. High platelet reactivity after transition from cangrelor to ticagrelor in hypothermic cardiac arrest survivors with ST-segment elevation myocardial infarction. J Clin Med. 2020;9:E583. doi: 10.3390/jcm9020583. doi: 10.3390/jcm9020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zwart B, Yazdani M, Ow KW, Richardson JD, Iqbal J, Gunn JP, Storey RF. Use of glycoprotein IIb/IIIa antagonists to prevent stent thrombosis in morphine-treated patients with ST-elevation myocardial infarction. Platelets 202031174–178doi: 10.1080/09537104.2019.1665642 [DOI] [PubMed] [Google Scholar]
- 38.Hochholzer W, Kleiner P, Younas I, Valina CM, Löffelhardt N, Amann M, Bömicke T, Ferenc M, Hauschke D, Trenk D, et al. Randomized comparison of oral P2Y12-receptor inhibitor loading strategies for transitioning from cangrelor: the ExcelsiorLOAD2 trial. JACC Cardiovasc Interv 201710121–129doi: 10.1016/j.jcin.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 39.Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, Sibbing D, So DYF, Trenk D, Alexopoulos D, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation 20171361955–1975doi: 10.1161/CIRCULATIONAHA.117.031164 [DOI] [PubMed] [Google Scholar]
- 40.Grimfjärd P, Lagerqvist B, Erlinge D, Varenhorst C, James S. Clinical use of cangrelor: nationwide experience from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J Cardiovasc Pharmacother 20195151–157doi: 10.1093/ehjcvp/pvz002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


