Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, coronavirus disease 2019, intensive care unit, mortality, severe acute respiratory syndrome coronavirus 2, surge capacity
Abstract
Objectives:
In many countries, large numbers of critically ill patients with coronavirus disease 2019 are admitted to the ICUs within a short period of time, overwhelming usual care capacities. Preparedness and reorganization ahead of the wave to increase ICU surge capacity may be associated with favorable outcome. The purpose of this study was to report our experience in terms of ICU organization and anticipation, as well as reporting patient characteristics, treatment, and outcomes.
Design:
A prospective observational study.
Setting:
The division of intensive care at the Geneva University Hospitals (Geneva, Switzerland).
Patients:
All consecutive adult patients with acute respiratory failure due to coronavirus disease 2019 admitted in the ICU between March 9, 2020, and May 19, 2020, were enrolled. Patients’ demographic data, comorbidities, laboratory values, treatments, and clinical outcomes were collected.
Interventions:
None.
Measurements and Main Results:
The ICU was reorganized into cells of six to eight patients under the care of three physicians and five nurses. Its capacity increased from 30 to 110 beds, fully equipped and staffed, transforming the surgical intermediate care unit, the postoperative care facility, and operating theaters into ICUs. Surge capacity has always exceeded the number of patients hospitalized. Among 129 critically ill patients with severe acute hypoxemic respiratory failure, 96% required invasive mechanical ventilation. A total of 105 patients (81%) were discharged alive and 24 died, corresponding to a mortality of 19%. Patients who died were significantly older, with higher severity scores at admission, had higher levels of d-dimers, plasma creatinine, high-sensitive troponin T, C-reactive protein, and procalcitonin, and required more frequent prone sessions.
Conclusions:
A rapid increase in ICU bed capacity, including adequate equipment and staffing, allowed for a large number of critically ill coronavirus disease 2019 patients to be taken care of within a short period of time. Anticipation and preparedness ahead of the wave may account for the low mortality observed in our center. These results highlight the importance of resources management strategy in the context of the ongoing coronavirus disease 2019 pandemic.
On February 24, 2020, the first infected person with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was diagnosed in Switzerland. On March 9, 2020, the first patient requiring mechanical ventilation was admitted to the intensive care division at Geneva University Hospitals, the largest healthcare complex in Switzerland. The complex serves a population of 506,000 inhabitants, with a 30-bed medical and surgical ICU and a full technical plateau (1). Switzerland has been one of the most affected countries in the world, with a prevalence increasing from 7.2 to 357 cases per 100,000 inhabitants between March 9, 2020, and May 19, 2020 (2, 3).
At the end of February 2020, Geneva University Hospitals received a mandate from the health authorities to prepare to receive an important wave of patients with coronavirus disease 2019 (COVID-19). It was decided that the university hospital would become the “COVID-19 hospital,” and the surrounding private hospitals would care for non-COVID-19 patients. Consecutively all patients diagnosed with COVID-19 in surrounding hospitals as well as ambulatory patients with suspected COVID-19 requiring hospitalization were transferred to Geneva University Hospitals. A hospital preparedness committee was initiated on February 28, 2020, and transformed into a crisis cell on March 18, 2020. The Swiss government decided on March 13, 2020, to partially confine the population (4). Predictions based on early local epidemiological data estimated that Geneva may need more than 180 ICU beds at the peak. At that time, it was reported that 5% to 32% of COVID-19 patients required ICU admission (5, 6), with very high mortality rates both in China and in the United States (7–11). An Italian study of 1,591 ICU patients reported a mortality of 26% for the whole cohort, but of 61% when considering only patients discharged from the ICU (12).
The purpose of this study was to report the experience of our center in terms of ICU organization and anticipation, as well as reporting patient characteristics, treatment, and outcomes.
MATERIALS AND METHODS
Study Design and Participants
This single-center prospective observational study was conducted at the ICU of University Hospitals of Geneva (Geneva, Switzerland), between March 9, 2020, and May 19, 2020. All adult patients admitted to the ICU with acute respiratory failure due to SARS-CoV-2 infection were included. SARS-CoV-2 infection was defined by a positive reverse transcriptase-polymerase chain reaction testing on a nasopharyngeal swab and/or bronchoalveolar lavage (BAL) fluid. Criteria for ICU admission followed Swiss triage and ethics guidelines (13). Only patients requiring mechanical ventilation or Fio2 greater than 80% were admitted to the ICU. The institutional ethics committee approved the study (BASEC Number: 2020-00917). An informed consent was obtained either from the patient or the next-of-kin.
ICU Preparedness and Reorganization
The ICU was reorganized to accommodate a larger number of patients by transforming the surgical intermediate care unit, the postoperative care facility, and operating theaters into ICUs (Fig. S1, Supplemental Digital Content 1, http://links.lww.com/CCX/A249). ICU cells of 8–10 patients were created, and each cell was staffed with two physicians, five nurses, and two assistant nurses. One attending physician and one physical therapist were available for two cells. The trigger for opening a new ICU cell was dependent on the inflow of new COVID-19 patients. New cells were prepared and staffed in order to always have 10 to 15 beds available for new incomings. The opening of new units was decided by an ICU direction cell composed of the chief of the ICU and two attending physicians. Medical and nursing staff was mainly recruited from anesthesia since all elective surgery was stopped. Only a few physicians, nurses, and assistant nurses were recruited from internal medicine and PICU. Former intensivists, both physicians and nurses, working elsewhere in the hospital were also recruited. Intermediate care units were densified and expanded to other locations in order to provide pre- and post-ICU care with trained and dedicated staff. In these units, patients could receive noninvasive ventilation, continuous positive airway pressure, and high-flow nasal oxygenation (HFNO).
Two shock rooms for handling incoming COVID-19 patients were set up at the entrance of the ICU with a team of experienced physicians and nurses, wearing Tyvek gowns, filter facepiece class 2 (FFP2) masks, and eye protections (Fig. 1). The incoming patient was intubated and fully equipped before being taken care of in the COVID-19 cells (14). The ventilator management was under the primary responsibility of the physicians taking care of the patients in the ICU cell. Nurses with specific ICU formation could manage the ventilators mainly during the weaning period but always under control of the physician in charge. Physical therapy resources have indeed been very helpful in the management of ventilated patients. Physical therapists helped managing the ventilators for patients needing noninvasive ventilation or during the weaning and extubation phases. The team of physical therapists of the ICU has been reinforced by physical therapists from other wards of the hospital and worked 24/7 during that period of time.
Figure 1.
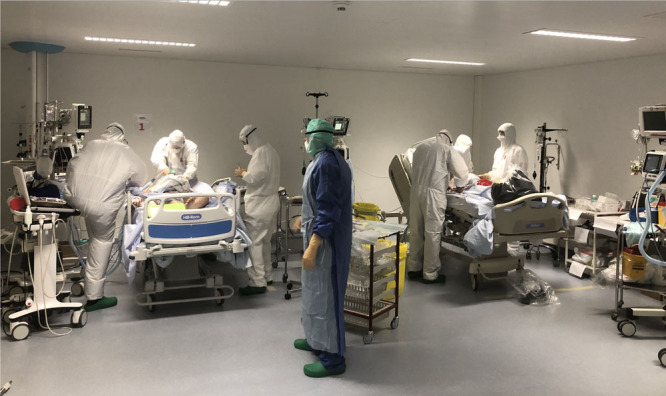
Shock room with two patients requiring intubation by a dedicated team of intensivists and anesthetists, wearing Tyvek gowns, filter facepiece class 2 masks, gloves, and goggles.
ICU-specific procedures and therapies were protocolized and taught using regular teaching and e-learning modules. Medical care followed international standards, such as lung-protective ventilation (15), neuromuscular blockade (16), prone positioning (17), inhaled nitric oxide (iNO), and venovenous extracorporeal membrane oxygenation (ECMO). Systemic glucocorticoids were considered in patients remaining with poor oxygenation from day 10 onwards after intubation (18). Usual strategies for the prevention and control of infection, surveillance, and diagnosis of healthcare-associated infections were maintained, including the use of a ventilator-associated pneumonia (VAP) bundle for all patients (19). A detailed description of ICU organization and protocols for admission criteria, prevention and infection control, mechanical ventilation, including weaning, use of neuromuscular blocking agents and sedative drugs, prone positioning, use of noninvasive mechanical ventilation (NIV) and HFNO in nonintubated patients, renal replacement therapy (RRT), venovenous ECMO, tracheostomy, thromboprophylaxis, nutrition, family communication, and end-of-life care can be found in the ICU Preparedness and the COVID-19 specific ICU protocols sections of the Supplemental Digital Content (http://links.lww.com/CCX/A249), respectively.
Wellness of Caregivers
The institution organized free meals delivered to the ICU, free parking, free accommodation in neighboring hotels, grocery pick-up, and hairdressers at the hospital. Two psychologic therapists were present in the ICU, interacting, and debriefing with healthcare workers (HCWs). Hypnosis sessions, massages, and spiritual support were also a daily resource available during breaks or after work.
Contacts With Families
Next-of-kin were not allowed to visit patients in the ICU. Families were called every day using a dedicated smartphone using video calls and were able to see and talk to the patients directly. If distress was detected, family members could call a hotline number and be directed to psychologic therapists. An electronic diary where caregivers and families could write or put pictures in was also set up and given to patients or families upon discharge or death. Families, protected according to COVID-19 hospital hygiene standards, were allowed to come to the ICU in the context of end-of-life to see the patient before and after death.
Data Collection
Patients were classified in two groups: nonsurvivors and patients discharged alive from the ICU on May 19, 2020. Demographics, comorbidities, severity scores, and a panel of biological variables were collected at the time of admission. Treatments such as mechanical ventilation, antibiotics, vasoactive drugs, corticosteroids, iNO and venovenous ECMO, and potential antivirals were recorded. Complications during the ICU stay were recorded prospectively, including venous thromboembolic events, VAP, septic shock (20), and acute renal failure requiring RRT and pressure sores. Patients were followed-up until hospital discharge or death.
Statistical Analysis
Continuous variables are presented as median and interquartile range (IQR). Categorical variables are expressed as the number of patients (percentage). Proportions are presented with 95% CI. Patients were grouped by vital status at ICU discharge (discharged alive and nonsurvivors). The Mann-Whitney U test was used to compare nonparametric continuous variables among two groups. chi-square or Fisher exact test was used for categorical variables, as appropriate. We estimated the cumulative incidence of death in the ICU and discharge by time from admission to the ICU (d) using a competing risk survival model based on Fine and Gray proportional sub hazards model. We calculated the incidence of VAP per 1,000 days of ventilation and the 95% CI within each group. All reported p values are two-sided and statistical significance was defined as p value of less than 0.05. Analyses were performed using Stata IC 16.0 (StataCorp, College Station, TX) and R Version 3.6.2 (R project, St. Louis, MI).
RESULTS
A total of 129 patients with acute respiratory failure due to COVID-19 were admitted to the ICU during the study period. SARS-CoV-2 infection was confirmed by nasopharyngeal swab and BAL in 119 and 10 patients, respectively. We reported data by vital status at ICU discharge according to those discharged alive (n = 105) and those who died in the ICU (n = 24). ICU mortality was 19% (95% CI, 13–27%).
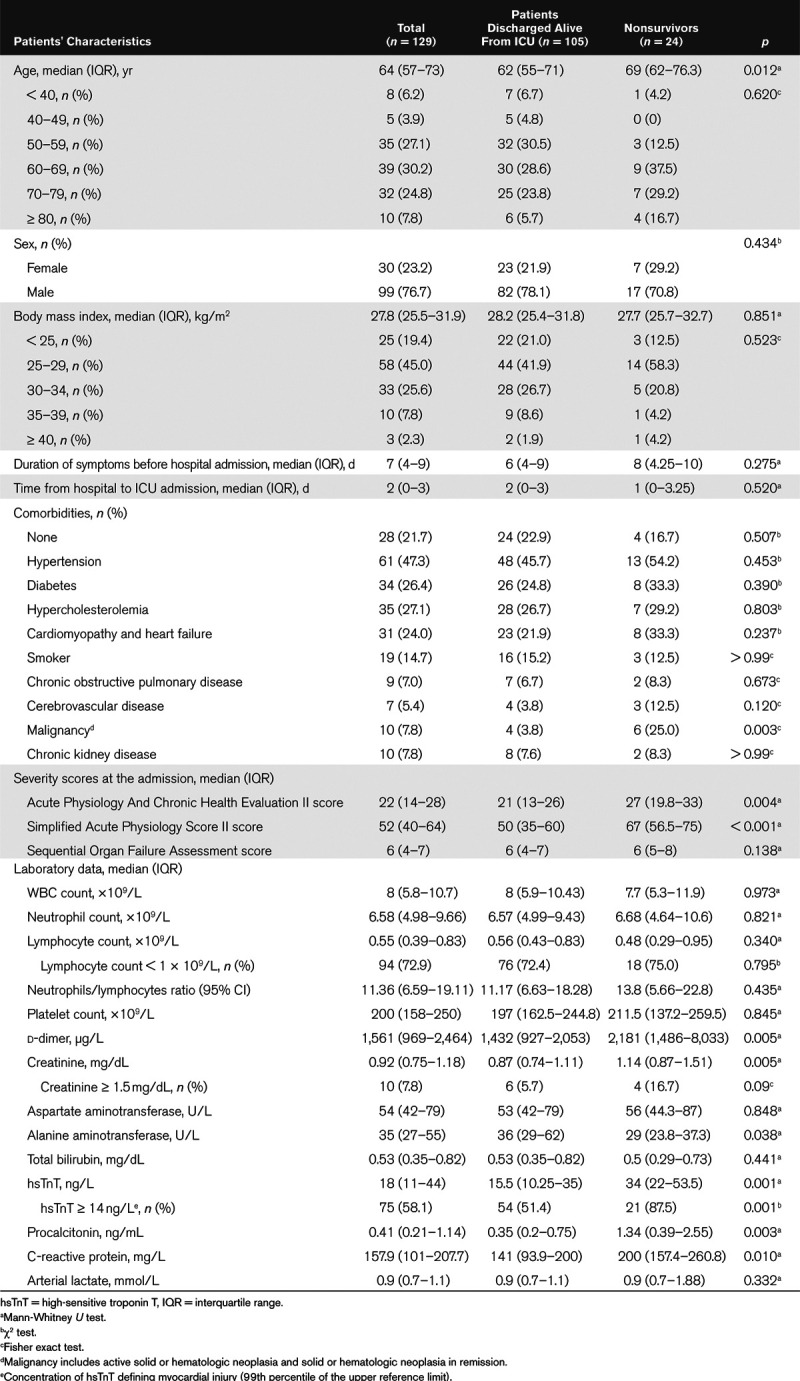
Table 1 shows the demographic and clinical characteristics of patients. Overall, 99 patients (76.7%) were male and similarly distributed among the two groups. Median age was 64 years (range, 25–86 yr). Patients who died in the ICU were significantly older than those discharged alive. Patients were similar among the two groups with regards to their body mass index. More than 80% (104 patients) were overweight, including 35.7% (46 patients) obese patients.
Table 1.
Demographic and Clinical Characteristics of Patients Admitted to the ICU
The COVID-19 intermediate care units cared for a total of 295 patients during that period of time. Of those, 61 were transferred to the ICU and 87 were transferred from the ICU. Thirty-three patients were admitted to the ICU from a medical ward.
One-hundred one patients (78.3%) had at least one comorbidity. Hypertension was the most common comorbidity, affecting 61 patients (47.3%). Other frequent comorbidities were diabetes (26.4%), hypercholesterolemia (27.1%), and cardiomyopathy or heart failure (24%). No difference was observed in the proportion of patients affected by each comorbidity among the two groups, except for malignancy that was significantly more represented in the group of patients who died in the ICU. Only 19 patients (14.7%) were active smokers. Patients who died in the ICU had higher severity scores at admission compared with those discharged alive.
Regarding laboratory data, patients were admitted with severe lymphopenia; 94 (72.9%) had less than 109 lymphocytes/L, irrespective of the groups. d-dimers, creatinine, high-sensitive troponin T (hsTnT), procalcitonin, and C-reactive protein were all significantly higher in the group of nonsurvivors.
Table 2 shows treatments, complications, and outcomes of COVID-19 patients admitted to the ICU. Almost all received invasive mechanical ventilation (96.1%). All patients had moderate to severe acute respiratory distress syndrome (ARDS) (21). At the time of admission, the oxygenation and ventilator variables were similar among the two groups. In nonsurvivors, the proportion of patients receiving iNO and the number of prone sessions were significantly higher. Eleven patients (8.5%) required venovenous ECMO. Twenty-three patients (17.8%) were tracheostomized. Regarding other medical treatment, the proportions of patients treated with antibiotics and their duration were similar across the two groups. The proportion of patients treated with glucocorticoids for ARDS was higher among nonsurvivors. The proportion of patients treated with hydroxychloroquine was higher among the group of patients discharged alive from the ICU. The distribution of other anti-SARS-CoV-2 treatments were similar across groups.
Table 2.
Treatments, Complications, and Outcome of Patients Admitted to the ICU
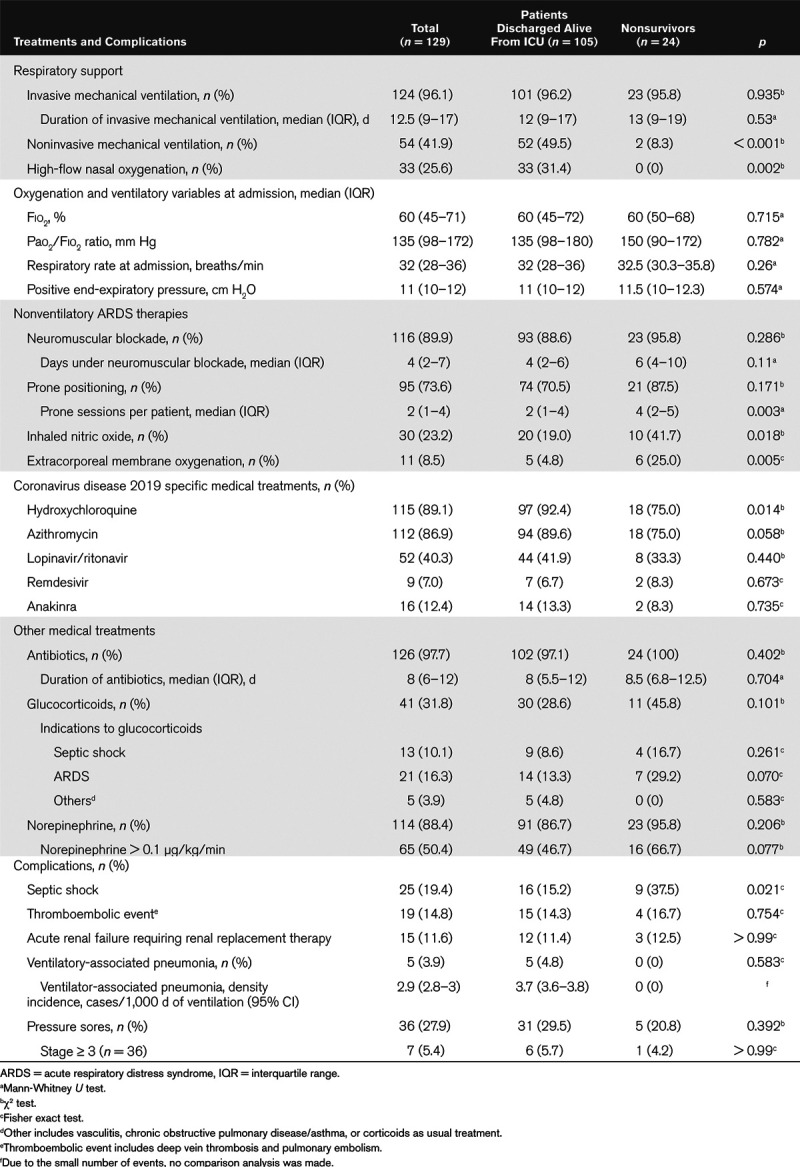
Regarding complications, only septic shock significantly differed among the two groups, and it was significantly higher in patients who died in the ICU. The incidence density of VAP was low (2.9 VAP episodes/1,000 ventilation days). Fifteen of 120 patients (11.6%) required RRT.
Among patients discharged alive from the ICU, 87 (82.9%) were transferred to an intermediate care ward and 18 (17.1%) to a medical care ward; two patients died after limitation of care on the ward after ICU discharge. Median duration of ICU stay was 15 days (IQR, 10–21 d), but it was not significantly longer in patients discharged alive from the ICU (median, 16 d [IQR, 11–21 d] vs 12.5 d [IQR, 9–18 d] for nonsurvivors (p = 0.346). Eighty-five patients (81%) were discharged from the acute care facility on May 19, 2020, after a median stay of 28 days (IQR, 21–36 d). Fifty-two patients (61.2%) required rehabilitation and 33 (38.8%) went home.
The cumulative mortality rate in the ICU by time from admission and the cumulative incidence of those discharged alive from the ICU are shown in Figure 2. The cumulative mortality rate plateaued after 15 days from admission. The cumulative incidence of discharge alive from the ICU increased gradually after 7 days from admission to the ICU and plateaued after more than 30 days. Patients died following therapeutic withdrawal in the context of refractory hypoxemia and/or multiple organ failure (21 patients), sudden hypoxemic cardiac arrest (two patients), and cerebral hemorrhage under venovenous ECMO (one patient).
Figure 2.
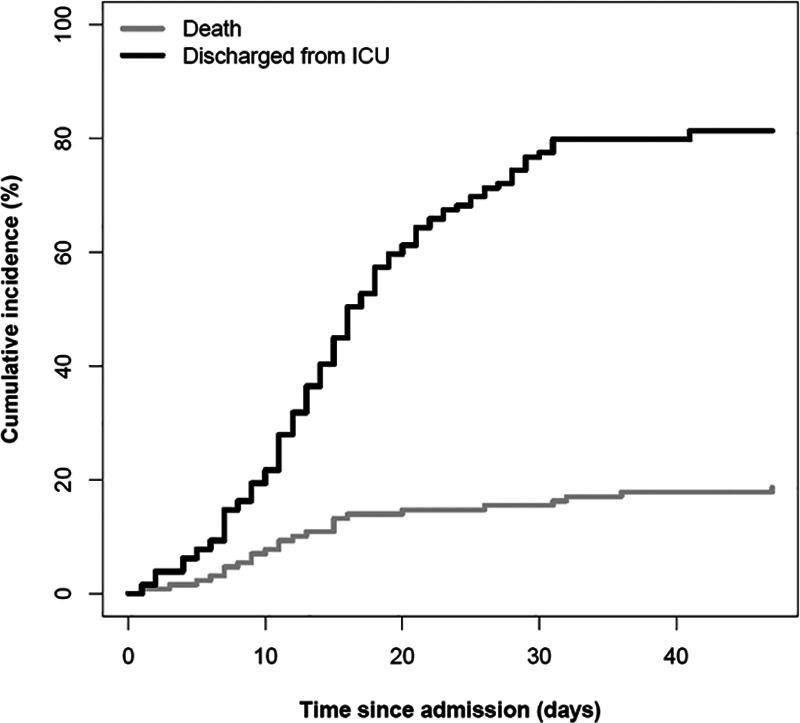
Cumulative mortality rate within the ICU by time from admission and the cumulative incidence of patients discharged alive from the ICU. The gray line represents the cumulative mortality rate in the ICU by time of admission, and the black line shows the cumulative incidence of patients discharged alive from the ICU.
Total bed capacity was constantly above the number of beds occupied by COVID-19 patients (Fig. 3A). The evolution and balance over time between patients admitted and discharged are shown in Figure 3B. The total number of HCWs increased by a factor of 2.3; medical staff increased from 45 to 146; nursing staff (nurses and assistant nurses); and physical therapists increased from 150 to 296 and from 8 to 18, respectively. Temporal increase of staff compared to bed occupancy is shown in Figure S2a and S2b (Supplemental Digital Content 1, http://links.lww.com/CCX/A249). During the study period, 21 HCWs (21/460; 4.6%), five physicians, 14 nurses, and two physical therapists were found to be positive with SARS-CoV-2. They all had minor symptoms.
Figure 3.
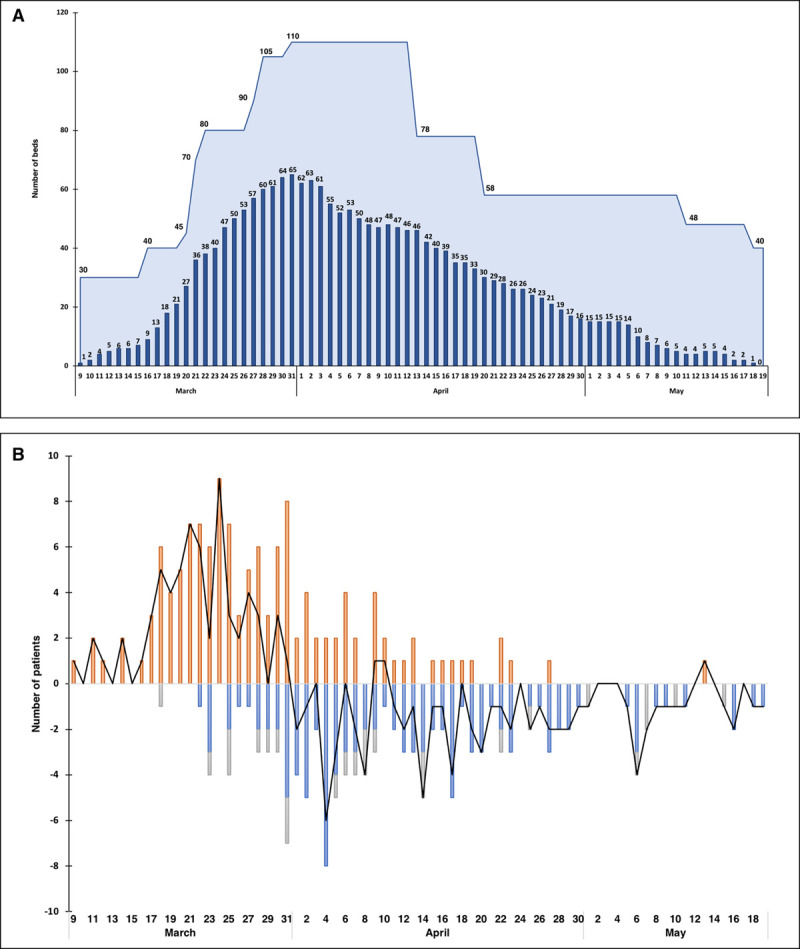
Capacity and occupancy of ICU beds by coronavirus disease 2019 patients, and balance of patients with time. A, Capacity and occupancy of ICU beds by coronavirus disease 2019 patients. Navy blue bars represent the actual bed occupancy across time. The light blue area represents the total bed capacity. Total bed capacity constantly exceeded the number of occupied beds. B, Balance (black line) of patients across time between admissions (red bars), patients discharged from the ICU (blue bars), and deaths (gray bars).
DISCUSSION
On May 19, 2020, 30,672 COVID-19 cases were laboratory-confirmed in Switzerland for a population of 8.6 million, corresponding to an incidence of 357 cases/100,000 inhabitants, with 1,636 COVID-19-related deaths. In the canton of Geneva, the incidence was 1,043 cases/100,000 inhabitants, with 267 deaths (2). At that time, the local epidemic curve reached zero, with no new admission.
In this study, we describe the rapid adaptive ICU reorganization to the incoming wave of severe COVID-19 patients, as well as the care and the outcome of the 129 critically ill COVID-19 patients admitted to our unit. This represents the first report of a complete wave of COVID-19 critically ill patients admitted to an ICU. We report a mortality rate lower to that previously reported in COVID-19 ICU patients in other countries with similar severity patients (6, 8, 12, 22, 23), with a mortality rate of 19%. We associate this favorable outcome with an effective upstream preparation and organization to care for the sickest COVID-19 patients and a rapid expansion of ICU beds to novel nearby locations, together with the necessary human resources, material, training, protocolized care, and a strong program to prevent secondary infections (24). At no time during this period, we experienced a shortage of staff, beds, ventilators, sedatives, neuromuscular blocking agents, antibiotics, disposable items, gowns, and FFP2 or surgical masks. To staff additional beds, a rapid recruitment of physicians, essentially anesthetists, and nurses was made possible by the interruption of all elective surgery.
Almost all patients were intubated. Patients with marked hypoxemia requiring HFNO or NIV were generally kept out of the ICU in well-equipped intermediary care units. Caring only for the sickest patients may explain that initial oxygenation variables were more severe in our patients compared with other patient cohorts (11, 12). We used neuromuscular blockade in practically all intubated patients (16) and prone positioning frequently (73.6% of patients) at the beginning of mechanical ventilation, which may also have had a favorable impact on patient outcome (17). In the most severely ill patients who did not respond to this treatment, nonventilatory additional treatments were used, such as iNO and venovenous ECMO. Mortality rate of patients requiring venovenous ECMO was in line with mortality rates recently reported in severe COVID-19 patients (25).
Prevention of secondary bacterial infections was a priority from the beginning, including a VAP prevention bundle (19), initial empirical antibiotic therapy, and antibiotic stewardship. The low measured VAP incidence density of 2.9 VAP/1,000 ventilation days during this period was in the same range as those measured previously in 2018 (5.4) and in 2019 (2.5) in our unit, which may have contributed to the favorable outcome. Among other complications, the incidence of septic shock was low compared with other reports (26). Venous thromboembolic events were diagnosed in 14.8% of patients. Incidence of thromboembolic events in our cohort was lower than recently reported (27). The use of a thromboprophylaxis regimen with an increased dose of heparin, rather than standard dose, may account for these results.
Obesity has been associated with a higher risk for the need of invasive mechanical ventilation in COVID-19 patients (28). Most of our patients were overweighed (80.6%), and frequently obese (35.7%), suggesting a link between obesity and severe SARS-CoV-2 infection. Similar to other studies (10–12, 29), we found that most COVID-19 patients had comorbidities (73.3%), mainly hypertension, diabetes, hypercholesterolemia, and cardiac disease. Surprisingly, active smokers were underrepresented in our patients compared to the proportion of smokers in the Swiss population (14.7% vs 27.1%, respectively) (30). Similarly, a low proportion of smokers was also found in COVID-19 patients from China (5, 8). However, smoking seems to be associated with a worse outcome (31). Patients who died were older and had a higher Simplified Acute Physiology Score II score. Patients requiring frequent prone sessions, as well as those who developed secondary sepsis, were also more likely to die. Higher levels of d-dimers, plasma creatinine, hsTnT, C-reactive protein, and procalcitonin at the time of admission were found in patients who died, possibly related to a more important inflammatory response (6). Patients were admitted with more profound lymphopenia than in previously described cohorts (9–11). In contrast with the study of Liu et al (32), the neutrophil/lymphocyte ratio was not different between survivors and nonsurvivors.
Some patients arriving from the ward were treated with lopinavir/ritonavir; this was generally stopped at ICU admission. Most patients received an initial treatment with hydroxychloroquine and azithromycin. Patients who died in the ICU were significantly less frequently treated with hydroxychloroquine. This result should be taken with caution due to the small number of subjects. Most of the recent studies report a lack of efficacity of hydroxychloroquine (33–36). However, at this time, no randomized double-blind controlled trial has been published yet.
As families were not allowed to visit patients, we set up daily teleconferences using dedicated smartphones and offered the possibility of support in the case of psychologic distress through a hotline. In contrast with some centers, we encouraged families to come during the end-of-life period, in a dedicated room, using appropriate personal protective measures. An electronic diary was implemented during ICU stay where both caregivers and families could write and post pictures. By these measures, it is our hope that these measures will help families to prevent stress and anxiety (37).
Our study has some limitations. First, it is monocentric. Second, the small number of nonsurvivors did not allow to identify independent prognostic factors in a multivariate analysis. Although probable, a direct causality link between ICU preparedness, surge capacity, and the outcome of patients cannot be ascertained in our setting. In a trauma setting, overwhelmed hospitals lead to supplemental deaths (38); such analysis has not been described in the context of the current COVID-19 pandemic.
In conclusion, the anticipation and preparation ahead of the wave of incoming severe COVID-19 patients by upgrading massively the number of ICU beds and staffing with external aid allowed to maintain a good quality of care, which translated into a low mortality rate.
ACKNOWLEDGMENTS
We wish to thank our physician colleagues who have been involved in coronavirus disease 2019 patient care, we also wish to deeply thank the work of nurses and assistant nurses from the ICU who came from the adult ICU, anesthesiology, the PICU, and other divisions as well as the physical therapists, with all maintaining high-quality care, despite a substantially increased workload. These also include the head nurses and physical therapist team. We are also indebted to our secretaries, medical assistants and receptionists, equipment and material logistics, our quality officers, and our information technology specialists. We also wish to thank the 6th year medical students who volunteered to come to the ICU, take care of patients, collect data, and manage administrative aspects. We also acknowledge the unconditional support of the direction of Geneva University Hospitals and key actors of the crisis management: the support of our Swiss minister of health; the minister of health of the canton of Geneva; the chair of the hospital board; the direction of the cantonal health services; and the departmental crisis cell. We are also thankful for the tremendous help of private hospitals, in particular La Tour Hospital with their ICU division. We could not have accomplished our tasks without the great help and dedication of the cleaning staff, stretcher-bearers, pharmacists, chaplains, and the Swiss army for providing us with ventilators and soldiers. Finally, we wish to express our deep gratitude to our Italian and French intensivists colleagues who provided us with key and valuable information about their early experience with the surge of patients in their centers.
Supplementary Material
Footnotes
Drs. Primmaz, Le Terrier, and Suh contributed equally to this work.
Drs. Primmaz, Le Terrier, Suh, Ventura, and J. Pugin had the idea and designed the study. They had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Primmaz, Le Terrier, Suh, and Ventura collected the data. Drs. Primmaz, Le Terrier, Suh, Ventura, Dolet, Gayet-Ageron, and J. Pugin analyzed the data. Drs. Primmaz, Le Terrier, Suh, Ventura, and J. Pugin drafted the article, and all authors interpreted the data and critically revised the article for important intellectual content and gave final approval for the version to be published. Drs. Primmaz, Le Terrier, and Suh contributed equally to this work. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
This study was performed at the Division of Intensive Care, Geneva University Hospitals, Geneva, Switzerland.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Pugin J. Le service de soins intensifs (réanimation) adultes à Genève, Suisse. Anesthésie & Réanimation. 2020; 6:182–187 [Google Scholar]
- 2.Swiss Federal Office of Public Health: COVID-19. Situation Suisse: Répartition par canton, âge et sexe. 2020 Available at: https://covid-19-schweiz.bagapps.ch/fr-1.html. Accessed April 22, 2020.
- 3.Coronavirus Resource Center: COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). COVID-19 Map 2020, Baltimore, MD: Johns Hopkins University of Medicine; Available at: https://coronavirus.jhu.edu/map.html. Accessed April 22, 2020 [Google Scholar]
- 4.Swiss Federal Council: Le Conseil fédéral renforce les mesures contre le coronavirus pour protéger la santé de la population et soutient les secteurs touchés. 2020. Available at: https://www.admin.ch/gov/fr/accueil/documentation/communiques.msg-id-78437.html. Accessed April 22, 2020
- 5.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020; 395:1014–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 2600:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020; 323:1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020; 368:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020; 323:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swiss Academy Of Medical Sciences. COVID-19 pandemic: Triage for intensive-care treatment under resource scarcity. Swiss Med Wkly. 2020; 150:w20229. [DOI] [PubMed] [Google Scholar]
- 14.Canelli R, Connor CW, Gonzalez M, et al. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020; 382:1957–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998; 338:347–354 [DOI] [PubMed] [Google Scholar]
- 16.Papazian L, Forel JM, Gacouin A, et al. ; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363:1107–1116 [DOI] [PubMed] [Google Scholar]
- 17.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 18.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: A randomized controlled trial. JAMA. 1998; 280:159–165 [DOI] [PubMed] [Google Scholar]
- 19.Landelle C, Nocquet Boyer V, Abbas M, et al. Impact of a multifaceted prevention program on ventilator-associated pneumonia including selective oropharyngeal decontamination. Intensive Care Med. 2018; 44:1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 22.Intensive Care and National Audit and Research Centre (ICNARC): ICNARC Report on COVID-19 in Critical Care. Apr 24, 2020. Available at: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. Accessed April 26, 2020.
- 23.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020; 10022:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin KM, Karas MG, Ivascu NS, et al. Hospital preparedness for COVID-19: A practical guide from a critical care perspective. Am J Respir Crit Care Med. 2020; 201:1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs JP, Stammers AH, St. Louis J, et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: Experience with 32 patients. ASAIO J. 2020; 66:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020; 191:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonnet A, Chetboun M, Poissy J, et al. ; LICORN and the Lille COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020; 28:1195–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao J, Hu X, Cheng W, et al. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020; 46:851–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swiss Federal Office of Public Health: Faits et chiffres: Tabac. 2020. Available at: https://www.bag.admin.ch/bag/fr/home/zahlen-und-statistiken/zahlen-fakten-zu-sucht/zahlen-fakten-zu-tabak.html. Accessed April 20, 2020.
- 31.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020; 133:1032–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020; 81:e6–e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020; 12203:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020; 382:2411–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahévas M, Tran V, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with Covid-19 pneumonia who require oxygen: Observational comparative study using routine care data. BMJ. 2020; 2019:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehra MR, Desai SS, Ruschitzka F, et al. Articles hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020; 6736:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Wakam GK, Montgomery JR, Biesterveld BE, et al. Not dying alone — modern compassionate care in the Covid-19 pandemic. N Engl J Med. 2020; 96:NEJMp2007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins PC, Richardson CR, Norton EC, et al. Trauma surge index: Advancing the measurement of trauma surges and their influence on mortality. J Am Coll Surg. 2015; 221:729–738.e1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


