Abstract
It is now reported that coronavirus disease 2019 ICU patients are at increased risk of thrombosis. Expert opinion and scientific societies recommend a higher dose of low-molecular-weight heparin, but definitive data is lacking. We report our adapted thromboprophylaxis practice of low-molecular-weight heparin administration in coronavirus disease 2019 ICU patients. One-hundred six measurements in 19 ICU patients were collected. Despite enoxaparin 60 mg once daily, only two measurements of the trough anti-Xa were in the upper end of prophylactic range. Anti-Xa activity peaks increased significantly after administration, but all measurements were under the optimal prophylactic ranges. Despite an adapted protocol, three of the 19 patients (16%) developed venous thromboembolism. We show in coronavirus disease 2019 ICU patients, despite higher prophylactic low-molecular-weight heparin administration due to body mass index, anti-Xa activity was well below peak serum levels in our cohort of critically ill coronavirus disease 2019 patients. This evaluation suggests the need for rapid studies on adequate thromboprophylaxis in these patients.
Keywords: coronavirus disease 2019, low-molecular-weight heparin, thromboembolism, thromboprophylaxis
To the Editor:
Abnormalities in coagulation with increased venous thromboembolism (VTE) are now reported in critically ill coronavirus disease 2019 (COVID-19) patients (1, 2). Potential explanations of the activation of coagulation in COVID-19 ICU patients are the vessel wall inflammation and the cytokine storm that activate the coagulation pathways associated perhaps with decreased concentrations of physiologic anticoagulant proteins (2–5). This imbalance favors thrombus formation which is associated with poor outcome. COVID-19 ICU patients also have more thrombotic events than patients suffering from non-COVID-19 pneumonia (2). It seemed a crucial issue to ensure optimized thromboprophylaxis in all COVID-19 patients and, in particular, in ICU patients.
Although several expert opinion and scientific societies reported VTE guidelines for critically ill COVID-19 patients with significantly raised d-dimer concentrations, the optimal dose of low-molecular-weight heparin (LMWH) remains unclear (4–8). Furthermore, LMWH has also anti-inflammatory properties that might be beneficial in COVID-19 patients (4, 5, 7). Here, we report our adapted thromboprophylaxis practice of LMWH administration in COVID-19 ICU patients with a body mass index (BMI) adjusted dose of enoxaparin (40 mg for BMI < 30, 60 mg for BMI > 30 and < 40, and 80 mg for BMI > 40 kg/m2) in patients with a creatinine clearance greater than or equal to 30 mL/min and with platelets count greater than or equal to 100,000/mm3. This made it possible to obtain homogeneous doses of at least 1 mg/kg of body weight of enoxaparin in agreement with some guidelines (2, 5, 9). Due to the higher frequency of acute renal failure in ICU patients (2), we measured especially the trough anti-Xa activity. In some patients, we also reported the anti-Xa activity peaks.
We performed 106 measurements in 19 COVID-19 ICU patients during the month of April 2020 in the ICU from the CHU-Charleroi Marie Curie after approval by the Ethical Committee. The median age was 58 years, all except five were mechanically ventilated for acute respiratory distress syndrome due to severe acute respiratory syndrome coronavirus 2 infection. ICU mortality was 26%. Demographic and biological data are reported in Table 1. Trough anti-Xa for all patients is reported in Figure 1.
Table 1.
Demographic and Biological Data at ICU Admission
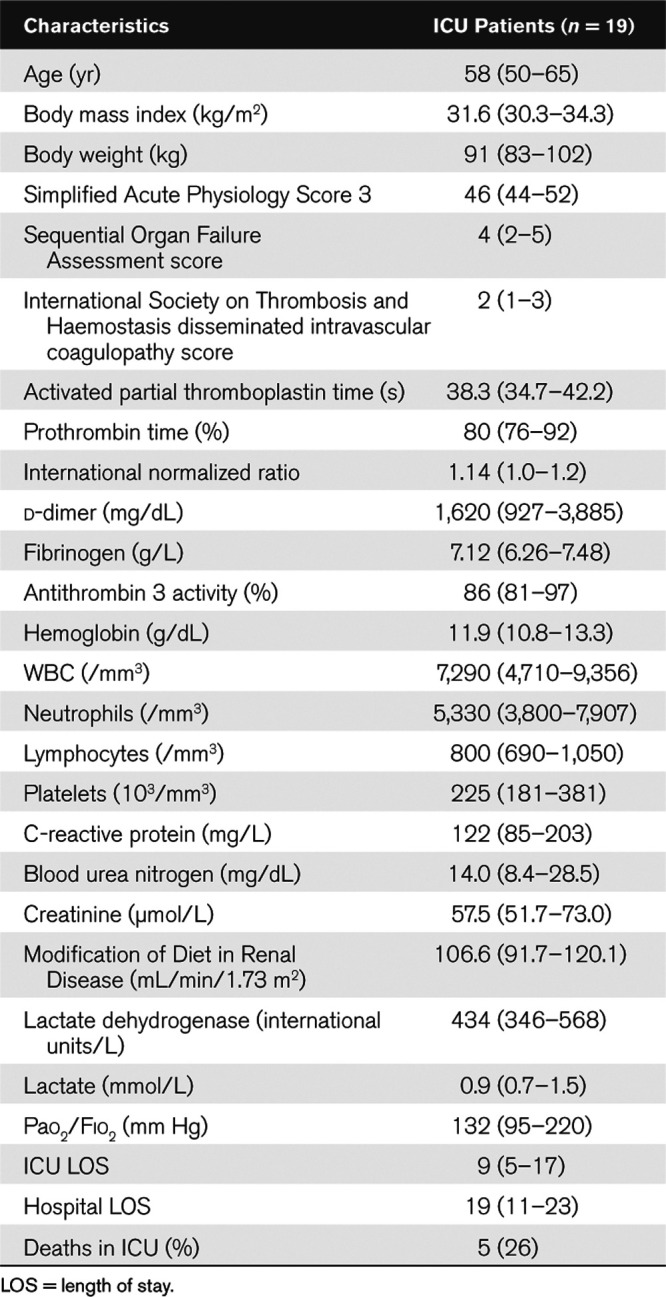
Figure 1.
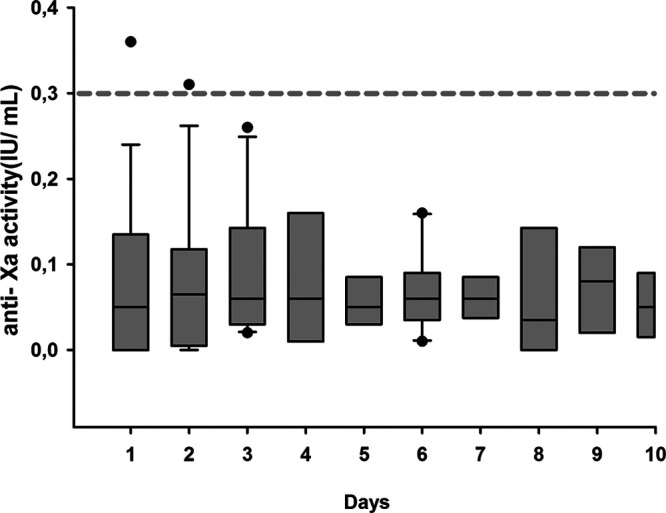
Trough anti-Xa activity in ICU patients with coronavirus disease 2019. Box plot for the through anti-Xa activity. The hatched line represents the limit of the trough anti-Xa activity. IU = international units.
Despite enoxaparin 60 mg once daily, only two measurements were at the upper end of the prophylactic range (< 0.3 anti-Xa international units [IU]/mL). In eight patients, anti-Xa activity peaks were performed and increased significantly after administration (0.059 ± 0.085 vs 0.277 ± 0.151 anti-Xa IU/mL; p = 0.009; paired t test) but all measurements were under the optimal prophylactic ranges of 0.65 ± 0.17 anti-Xa IU/mL. Three of the 19 patients (16%) developed VTE between 6 and 8 days after the adaptation of the protocol of thromboprophylaxis.
Our results showed that despite higher prophylactic LMWH administration due to BMI in COVID-19 ICU patients as recommended, trough anti-Xa activity remains in the majority low and without risk of bleeding. Anti-Xa activity was well below peak serum levels in our cohort of critically ill COVID-19 patients. Despite treatment, three patients developed VTE during this adapted protocol.
Our evaluation suggests that current thromboprophylaxis guidelines are ineffective in COVID-19 ICU patients. Thrombotic complications were frequently reported and associated with higher mortality in critically ill COVID-19 patients. The medical community needs to rapidly determine optimal thromboprophylaxis for COVID-19 patients, especially in high-risk critically ill patients.
The authors have disclosed that they do not have any potential conflicts of interest.
Dr. Piagnerelli developed the protocol, collected, analyzed the data, and wrote the article. Drs. Cauchie and Wautrecht developed the protocol, collected, analyzed the data, and approved the article. Drs. Vancutsem, Thooft, Zouaoui Boudjeltia, and Biston collected, analyzed data, and approved article. Dr. Piagnerelli had full access to all the data in the study and final responsibility for the decision to submit for publication.
This study was approved by appropriate regulatory committee (EC ISPPC OM008, Charleroi, Belgium) in accordance with national regulation. The requirement for written informed consent was waived.
REFERENCES
- 1.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020; 191:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020; 46:1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395:1417–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020; 7:e438–e440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iba T, Levy JH, Levi M, et al. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020. May 27. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai Z, Li C, Chen Y, et al. ; Prevention treatment of VTE associated with COVID-19 infection consensus statement group. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: A consensus statement before guidelines. Thromb Haemost. 2020; 120:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B, Madhavan MS, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020; 75:2950–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020; 18:1020–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spyropoulos AC, Levy JH, Ageno W, et al. ; Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis, Haemostasis. Scientific and standardization committee communication: Clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020. May 27 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


