Abstract
A novel coronavirus, SARS-CoV-2, was first reported as a respiratory illness in December 2019 in Wuhan, China. Since then, the World Health Organization (WHO) Emergency Committee declared a global health. COVID-19 has now spread worldwide and is responsible of more than 472,216 persons, out of 9,100,090 officially diagnosed worldwide since 23 of June. In the context of cancer patients, COVID-19 has a severe impact, regarding pulmonary infection but also cancer treatments in this fragile and immunocompromised population, and ICU admission for cancer patients in the context of COVID-19 requires ethical and clinical consideration. In our cancer center, intensivists, oncologists, pharmacists, and hospital administrators had to prepare for a substantial increase in critical care bed capacity (from 10 ICU beds, 6 medical intensive care beds, and 12 surgical intensive care beds, bed capacity was increased to 28 medical intensive care beds with ventilating capacity) and to adapt infrastructure (i.e., ICU beds), supplies (i.e., drugs, ventilators, protective materials), and staff (i.e., nurses and medical staff). Overall, thirty-three COVID-19 patients were admitted in our ICU, 17 cancer-free and 16 with cancer, and 23 required mechanical ventilation, resulting in 4 deaths (of them two patients with cancer). We report here management of a dedicated intensive care unit of a cancer center during the COVID-19 infection pandemic, considering resource allocation and redistribution of healthcare workers.
Keywords: COVID-19, Coronavirus, Cancer, Pandemic, Management, Intensive care unit
Introduction
A worldwide public health emergency of international concern has emerged since December 2019, named coronavirus disease 2019 (COVID-19) and caused by a novel coronavirus SARS-CoV-2. First detected in China as a respiratory illness, this disease spread all around the world and achieved pandemic spread [1]. It is now responsible for the death of 472,216 persons, out of 9,100,090 officially diagnosed worldwide since 23 of June [2]. This new respiratory illness is characterized by a rapid human-to-human transmission, with a broad range of symptoms and severity, from asymptomatic cases to acute respiratory distress syndrome [1]. Importantly, the most severe cases require intensive care for high-flow oxygen therapy up to mechanical ventilation and management of potential other organ failure [3]. In a Chinese review, 6.1% of patients were classified as critical (i.e., with respiratory failure, shock, or multiple organ dysfunction or failure) and 13.8% were classified as severe (i.e., with a respiratory rate ≥ 30 breaths per min, dyspnea, oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio < 300 mmHg, or increase in lung infiltrates > 50% within 24–48 h) [4]. In Italy, until March 29, 2020, around 12% of all positive patients required ICU admission [5, 6].
In France, the first patient was diagnosed on January 24, 2020. COVID-19 then rapidly spread, and an urgent need for more intensive care unit (ICU) beds was noted. Indeed, with large numbers of infected patients and rapidly increasing numbers of diagnosed and severe patients, COVID-19 is a challenge for healthcare systems [7]. In France, an inter- and intra-hospital reorganization led to a creation of more than 8000 ICU beds, more specifically in the east of France and in Paris area, where the pandemic was the most virulent. The limited number of ICU beds raised ethical consideration, with a reality of rationing care in a context of limited resources.
In this context, cancer patients are a particular population with their own specificity, and one must realize that cancer mortality remains substantial [8]. In a situation of predictable shortage of beds and resources due to patients with COVID-19 requiring intensive care, the usual perception of cancer with a poor life expectancy population may lead to a limitation of aggressive management of this cohort. Oncologists may face an unacceptable reality of rationing care for their patients. Moreover, oncologists should also reason in terms of potential cancer progression due to treatment interruption for COVID-19 infection. In this regard, some cancer centers have their own ICU (dedicated to cancer patients only) that would receive COVID-19 patients in the context of pandemic. Moreover, these specific ICU might be reassigned to host non-cancer patients to cope with the global influx of COVID-19 patients. Nevertheless, these cancer-dedicated centers must keep on cancer treatments and oncologic emergency. Consequently, these specific ICUs have adapted to meet national requirements while continuing taking care of cancer patients.
Here, we report management of such a specific ICU during the COVID-19 infection pandemic, considering resource allocation and redistribution of healthcare workers, and anticipation of the influx of patients.
Chronology of pandemic in our cancer center
Anticipation of the influx of patients
Even before the first COVID-19 patient admitted at our hospital, a general reorganization of the hospital was planned, regarding oncology wards, surgery wards as well as ICU wards. Case definition for COVID-19 infection in our center was a positive reverse transcriptase-polymerase chain reaction test (RT-PCR) for COVID-19 or evocative symptoms with typical radiological images ion CT scan. In ICU, all patients had a positive RT-PCR for COVID-19. All patients with evocative symptoms were tested (such as fever, upper or lower tract respiratory symptoms) or at physician discretion. All patients with planned surgery or interventional gesture were also tested. Patients requiring hospital admission who met case definition for COVID-19 were admitted in a specific ward awaiting test results since the microbiology lab is located in the hospital. Results were available within 24 h in March and then within 6 to 8 h since April. Test was repeated if the patient met clinical case definition, and the first test was negative.
A close flexibility was given on the needed number of beds in ICU, to reassigned human (medical, paramedical, administrative, and technic staff) and material resources (beds, respirator, drugs such as narcotics and curare), depending on the anticipated needs. To increase ICU capacity, and to reduce the number of patients admitted in the post-surgical care ward, a large number of non-urgent surgery were postponed, as well as cancer treatments that were adapted to preserve available beds in ICU. Therefore, some respirator form surgical rooms were reassigned to ICU in order to increase ventilating capacity. We increased our capacity for extra renal purification in 2 new ICU beds. Work has been done to open windows (needs to reach negative pressure inside the room to limit public area exposure to the virus) and separate COVID-positive and COVID-negative zone [3].
The ICU ward usually consists of three wards with 10 ICU beds, 6 medical intensive care beds, and 12 surgical intensive care beds. The intensive care beds are not supplied with ventilators. Almost all beds were converted to ICU beds raising the capacity to 28 potential ventilated patients. At first, specific parts of the ward were dedicated to COVID-19 patients with dedicated medical and paramedical staff in order to avoid nosocomial contamination of non-COVID-19 patients. The 16 of March, 6 beds were dedicated to COVID-19 patients, then 16 one week later and the 1st of April, 28 beds were available (i.e., then an increase by > 400% in just 12 days). Due to their ICU hospitalization at the start of the crisis, only four beds were devoted to non-COVID-19 cancer-patients.
ICU facing the COVID-19 wave
The influx of patients in our cancer-dedicated center was 2 weeks behind other Paris area hospitals, giving time to anticipation. Our ICU is usually dedicated to cancer patients which specific concerns such as critical adverse events, disease complication, and any other medical condition requiring ICU. Notably, these patients are not prioritized in most non-cancer ICU. In this context, our ICU had to reorganize to face COVID-19 patients influx in our frail population of cancer patients.
From March 14 to April 15, on a total of 1302 patients tested by PCR in the cancer center, 12% was COVID-19 positive. The median age was 61 (range 21–90), and 58% was female. A total of 33 patients (11%) were admitted in ICU; among them, 17 were cancer-free, and 13 patients were treated in Gustave Roussy Cancer Center for a cancer.
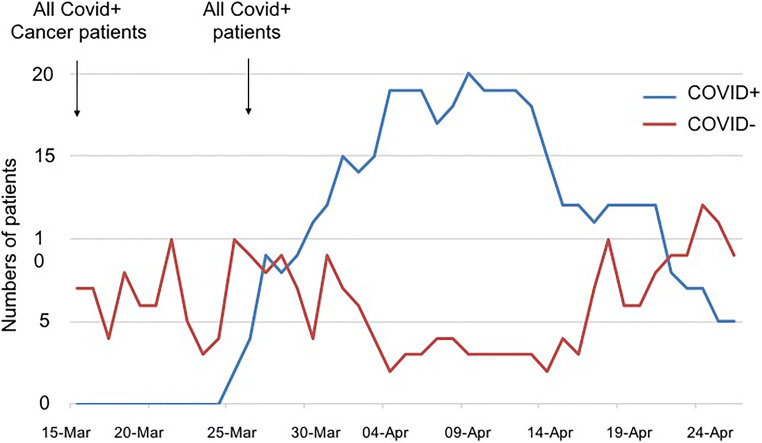
Figure 1 shows the number of COVID-19 patients in ICU from March 15, 2020 (beginning of lockdown in France), to April 26, 2020. Regional health agency ordered on March 16 to welcome cancer patients with COVID-19 even if not managed in our hospital. Four cancer patients with COVID-19 were then admitted from other hospital. On March 27, considering the large influx of patients in Paris area, order was given to admit COVID-19 patients even without cancer. Then, 13 ventilated cancer-free patients and 2 cancer patients (included 1 ventilated) were admitted in 4 days (Fig. 1).
Fig. 1.
Numbers of patients admitted in ICU, according to their COVID status (COVID positive (blue) or negative (red)), from March 15, 2020, to April 26, 2020
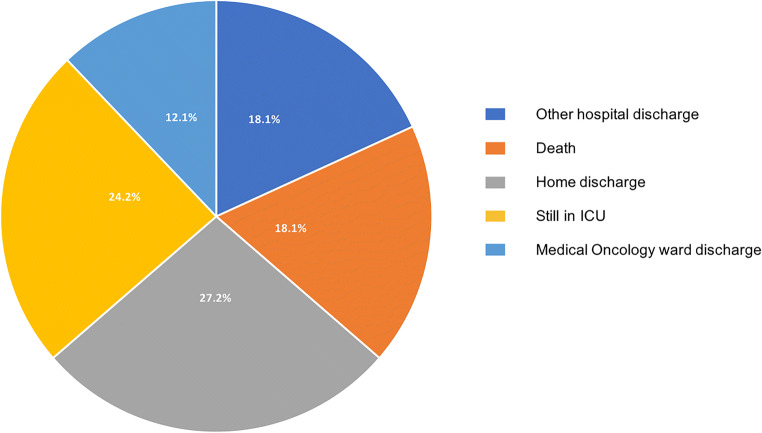
To date (update April 26), 33 COVID-19 patients were admitted in our ICU, 17 cancer-free and 16 with cancer. Twenty-three patients required mechanical ventilation, resulting in 4 deaths (of them two patients with cancer). Two patients required extracorporeal membrane oxygenation (ECMO) and were referred to specialized ICU. Ten patients did not require mechanical ventilation (all with cancer), resulting in two deaths (in a context of limited additional invasive intervention). Eight patients were still in ICU under mechanical ventilation, 6 died (18%), and 9 are home discharged (27%) (Fig. 2). Those results are confident with the ICU outcomes in Milan, Italy (death 23.3%, 31.5% discharged) [9].
Fig. 2.
Outcomes of COVID-19+ ICU patients
A new organization for ICU
To adapt to COVID-19 pandemic, new organization was decided, regarding human and material resources.
Human resources
New nurses and caregivers were assigned in ICU. First, 16 specialized nurses from surgical rooms were trained for ICU by colleagues, and 8 new nurses and 8 new caregivers were then available for ICU. Four nurses that previously worked in our ICU came back from nurse school (n = 2) or oncology/hematology wards (n = 2). They received a time-limited training resulting in a 2-day observation course with their ICU colleagues before caring ICU patients. Each nurse was in responsibility of 2 patients, each caregiver for 4 patients, with one extra nurse from surgical room occasionally. Considering spatial organization of ICU, each nurse from ICU and extra nurses alternated for a better integration of the new ICU actors.
A special team for prone positioning was developed with the help of surgeons whose surgical activity had been restricted. Four of them were assigned each day to help to turn on or turn back ventilated patients every morning and every afternoon. They were trained during two sessions and then were fully efficient and appreciated, while 6–7 people per patient were required.
Considering medical resources, a team of 12 anesthetists and 2 residents were requested to manage 14 ICU beds with their own night shift list. Other beds were managed by 6 senior intensivists and 4 residents. Moreover, 4 residents from medical oncology wards that previously worked for one trainee ship in ICU during their residency were reassigned for at least 1 month in ICU for day and night shifts.
Importantly, the oldest workers or workers with chronic illness were relocated in non-COVID-19 wards of the hospital. All workers of COVID-19 units were volunteers. Notably, some people were allocated to work with COVID-1 patients only and others with non-COVID-19 patients only, as far as possible, to prevent nosocomial COVID-19 infection.
Once the COVID ICU ward is fully opened, management of the patient influx was a challenging and determinant part of the organization. The incoming patients could therefore came from (i) inside medical patients (non-COVID), (ii) inside surgical patients, (iii) inside COVID patients (cancer patients and staff), and (iv) outside COVID patients requiring ICU care. In order to optimize organization, each day one doctor was in charge of admission decisions. More than managing the resource allocations, the purpose of this function was to distribute mental load due to complex ethical decisions. This global approach and close collaboration between oncologists, surgeons, and ICU staff demonstrated the solidarity and multidisciplinarity of cancer centers.
It is worth to note the huge adaptability and flexibility of all healthcare actors, to work with other colleagues, in different wards, with different kinds of patients, different software, and different rhythms. In order to preserve health status of these professionals, a training for protective measures and proper dressing was organized as well as meals with social distancing. To date, no healthcare worker was diagnosed positive for COVID-19 while working in the ICU ward (4 nurses from surgical room were screened positive before being reassigned in ICU). Of note, a PCR testing was performed for all symptomatic workers and was not systematic at first.
As our cancer-dedicated hospital includes psychologists and psychiatrists, a dedicated consultation was set up to prevent burnout. Estheticians and sophrologists that used to work with cancer patient dedicated part of their time every day to ICU workers. All these allowed the best possible conditions for all workers.
Material resources management
Material resource allocation can be challenging in a context of medical supply shortage and infectious disease.
ICU procedures were adjusted to enable caregivers and patients safety, with dressing protocol for nursing COVID-positive patients, the use of close-loop system for endotracheal access in intubated patients, or bedside diagnostic procedures that were promoted (fibroscopy, ultrasound, radiography). The efficiency of those protocols was dependent of supply chain, and several innovative solutions have been found to secure it.
A specific intubation protocol was followed:
-
(i)
Appropriate respiratory protective equipment for all providers present during airway management.
-
(ii)
Performed by the most experienced provider.
-
(iii)
High-quality heat and moisture exchange filter was placed between the face mask and reservoir bag during preoxygenation and between the endotracheal tube and ventilator circuit.
-
(iv)
The use of videolaryngoscopy was used to optimize success of intubation and distance the laryngoscopist from the patient’s oral and nasal mucosa.
-
(v)
Systematic rapid sequence induction to avoid manual bag-mask ventilation (short action curares).
-
(vi)
Systematic control of the tracheal cuff of the endotracheal tube.
-
(vii)
Initiation of the positive pressure ventilation after control of the cuff and correct connection to the machine.
ICU was always provided with surgical and FFP2 masks, since early order was performed by the hospital. New ways of supply were found for dressing material such as blouses or gloves. Particulars helped to provide apron and protective visors. The pharmacy department has produced its own hydro-alcoholic solution. There were no exceptions to security rules. Therefore, no shortage in personal protective equipment (PPE) supplies was observed in our center. When taking care of patients, PPE standard was pajamas and blouses, as well as visors and FFP2 masks, shoe protections, and hair protections. When not taking care of patients, PPE standard was pajamas and FFP2 masks.
A more worrying problem was medication shortage, especially narcotics and curares. The pharmacy department faced a real challenge to provide enough essential ICU drugs since a national (and international) penury was observed. The first step consisted in needs analysis and anticipation. Pharmacists and medical staff revised all sedative protocols and proposed therapeutic alternatives to restrict product use and to avoid a shortage. We establish the daily need per bed to guarantee the supply during this period for the 28 beds (Table 1). We set up a dedicated logistic loop twice a day monitoring any deviation with the calculated needs in order to correct it the fastest way possible. The second step consisted in elaborate a next strategy of supply. Instead of ordering massive amount to usual supplier, we asked reasonable quantities to all our supplier panel. During the most epidemic period, the drug consumption drastically increases to 1000% for curare and 400% for sedatives (midazolam and propofol) versus the same period last year. In the most critical situation, the entire capacity of treatment is about 4 days (Table 1). The third step was to optimize drug administration. A close monitoring of curare effects through the train of four and sedative drugs with the bispectral index allowed more effective administration. The adjunction of other pharmacologic class to the sedative arsenal, such as ketamine, dexmedetomidine, and sevoflurane gas, allowed us to reduce the supply chain tension.
Table 1.
Estimated and real use of sedative drugs
| March 14 to 23 (9 days) |
March 23 to 31 (8 days) |
March 31 to April 21 (22 days) | April 21 to 26 (5 days) |
|
|---|---|---|---|---|
| COVID-19+ beds (n) | 6 | 16 | 28 | 16 |
|
Treatments days (number of bed × days in period study) |
54 | 128 | 616 | 80 |
| Midazolam | ||||
| Estimation of daily need (number of vials) for all patients treated with midazolam | 42 | 112 | 196 | 112 |
| Number of vials in pharmacy (estimated lowest stock capacity if all beds need drug) |
457 (10.8 days) |
1402 (12.5 days) |
975 (4.9 days) |
1200 (10.7 days) |
| Patients really treated with midazolam (n) | 0 | 2 | 7 | 5 |
| Cumulated days with really used treatment (n) | NA | 5 | 92 | 28 |
| Vials used for really treated patients (n) | NA | 21 | 510 | 80 |
|
Average number of vials used daily (maximal use for 1 day) |
NA | 2.6 (5) | 22.2 (40) | 13.3 (20) |
| Adjusted stock capacity with real drug use at maximal daily use | > 100 days | > 100 days | 24 days | 60 days |
| Propofol | ||||
| Estimation of daily need (number of vials) for all patients treated with propofol | 108 | 288 | 504 | 288 |
| Number of vials in pharmacy (estimated lowest stock capacity if all beds need drug) |
590 (5.4 days) |
570 (2 days) |
1400 (2.7 days) |
1420 (4.9 days) |
| Patients really treated with propofol (n) | 2 | 10 | 18 | 5 |
| Cumulated days with really used treatment (n) | 2 | 27 | 112 | 18 |
| Vials used for really treated patients (n) | 8 | 66 | 369 | 45 |
| Average number of vials used daily (maximal use for 1 day) | 4 | 10 (25) | 16 (30) | 5 (21) |
| Adjusted stock capacity with real drug use at maximal daily use | > 100 days | 21 days | 46 days | 67 days |
| Cisatracurium | ||||
| Estimation of daily need (number of vials) for all patients treated with cisatracurium | 18 | 48 | 84 | 48 |
| Number of vials in pharmacy (estimated lowest stock capacity if all beds need drug) |
132 (7.3 days) |
232 (4.8 days) |
102 (1.2 days) |
74 (1.5 days) |
| Patients really treated with cisatracurium (n) | 1 | 12 | 18 | 5 |
| Cumulated days with really used treatment (n) | 1 | 35 | 147 | 14 |
| Vials used for really treated patients (n) | 4 | 124 | 639 | 18 |
| Average number of vials used daily (maximal use for 1 day) | 4 | 17 (53) | 30 (67) | 3 (9) |
| Adjusted stock capacity with real drug use at maximal daily use | 33 days | 4.3 days | 1.5 days | 8.2 days |
Midazolam, 50 mg/10 ml® MYLAN; propofol® 500 mg/50 ml (BBRAUN); cisatracurium 150 mg/30 ml (Nilbex®, ASPEN)
For each drug, estimation of daily needs (number of vials) per bed was calculated for an average weight of 90 kg
Midazolam = 0.15 mg/kg/h; the total dose calculated for 1 day = 324 mg; estimation of 7 vials per patient for 1 day
Propofol = 4 mg/kg/h; the total dose calculated for 1 day = 8640 mg; estimation of 18 vials per patient for 1 day
Cisatracurium = 0.18 mg/kg/h; the total dose calculated for 1 day = 390 mgs estimation of 3 vials per patient for 1 day
Estimation of daily need (number of vials) for all beds treated with one drug is calculated as the number of vials need for one bed during 1 day multiplied by the number of beds opened for COVID19+ inpatient multiplied by the number of days
Stock capacity in pharmacy is expressed in number of treatment day = number of vials in stock/daily need estimated for with all COVID 19+ beds opened
The number of patients really treated, the number of cumulated treatment days, and the number of vials used were extracted from the electronic prescribing system (grimoire®)
In ICU, specific COVID-19 treatment consisted in hydroxychloroquine depending on ONCOVID protocol (NCT04341207) for cancer patients and tocilizumab (CORIMUNO-TOCI, NCT04331808). Most patients received only symptomatic treatment.
Working management
Since the ICU staff was strengthened with adjunction of several various physicians, the daily activity was reconsidered. The main objective was to maintain a constant quality of care without spreading the virus among caregivers. The ICU was divided into independent functional units, each one with different medical transmissions schedules and night shifts. Meetings requiring outside stakeholders (ethical or infectious disease staff) were partially phone conducted. A consistency of patient care was ensured through the presence of one intensivist in every medical staff but also through the creation of dedicated medical protocols for COVID patients that were implemented in the prescription software. Finally, every medical information was available for any ICU physician through secured local database.
Families management
In accordance with hospital recommendation, there was a restrictive access to ICU, which was a new paradigm. This was decided to decrease population movement and to protect non-COVID-19 patients as well as families. No visit was allowed, except in case of life ending. All families could phone every day to be informed of every clinical change of their relative. Additionally, a digital tablet has been acquired to allow visual contact between patients and their relatives.
An absolute compliance to these new and difficult rules was observed, with a great agreement of every family. Of note, the strict limitation of family access was applied the same way in all the hospital, to reduce the number of people within the hospital.
COVID-19 in the context of cancer
As the pandemic spread, it was inevitable that patient with COVID-19 would present at our hospital, for specific symptoms or as an accidental diagnosis.
To maximized protection of non-COVID-19 patients, a specific ward was early dedicated for COVID-19 cancer patients. A daily ethical cross-disciplinary meeting allowed to discuss eventual clinical limitations for ICU admission for every patient in respiratory degradation (with oncologist, intensivist, infectious disease specialist, and supportive care specialist). An ethical committee and psycho-oncological team was also set up that could meet at every moment, days and nights, for difficult decisions that could require a multidisciplinary discussion. In particular, a member from ICU team attended each ethical meeting.
In the context of cancer, and regardless COVID-19 pandemic, ICU admission is discussed depending (1) patient wish; (2) general state and comorbidities; (3) reversibility of the supposed acute failure (number and type of organ failure, short-term prognosis); and (4) cancer prognosis.
COVID-19 respiratory illness is considered as reversible, but (1) COVID-19 ARDS is worse than usual ARDS with an estimated mortality of 60–70% when mechanical ventilation is required [10]; (2) ARDS lasts often several weeks; (3) rehabilitation time post-ARDS (respiratory, neuromuscular) is very long and proportional to duration of ICU stay [11–14]; (4) treatment limitation can be necessary during ICU stay; and (5) the prognosis is worse in cancer patients, especially when treatment is ongoing [15]. Most patients that survived this severe ARDS are young, without comorbidity and in good general state, without use of mechanical ventilation [16].
Therefore, a formalized list of criteria for ICU admission was edited by the ethical committee and discussed for every patient. Importantly, it was decided to discuss this potential ICU admission before any clinical criteria for intensive care and irrespective of ICU available beds. Importantly, no patient was recused from ICU for a lack of ICU bed. Moreover, a COVID-19 protocol was applied at the entry of the hospital. All patients had body temperature measured before any hospitalization and tested if febrile. A questionnaire was also filled with the help of nurses at the entry. All patients with planned surgery or any interventional gesture were also tested 24 to 48 h before hospitalization. Lastly, all patients with self-reported specific symptoms were also tested. If positive, patients were addressed at the specific COVID-19 ICU ward.
To define the level of therapeutic commitment, the patient general state is preponderant. Characteristics related to cancer were assessed in perspective of the initial seriousness of COVID-19, as well as the predictable duration of intensive care and rehabilitation. In this regard, an oncologic prognosis of less than 12 months, considering an interruption of cancer treatments (for a three-month to six-month period), seems incompatible with mechanical ventilation. Therefore, the only indication of intubation for COVID-19 ARDS is a good general state, without heavy comorbidities, and an oncologic prognosis of more than 12 months. Age itself is not a limiting factor per se.
Cancer in the context of COVID-19
As a referral center for cancer, our hospital was due to continue providing care for all cancer patients. Nevertheless, it should address the security of patient and workers.
In a series of 79 patients from Taiwan enrolled in clinical trials published in 2004 during SARS epidemic, a questionnaire showed that almost two-third of patients were afraid coming to hospital for fear of being acquiring SRAS and three patients ceased further chemotherapy for this reason [17]. In our hospital, a surgical mask and hydro-alcoholic friction was mandatory to enter the hospital, to prevent nosocomial and healthcare workers infections. A dedicated circuit was set up for suspicion of COVID-19 to avoid too many COVID-19 patients in the emergency room.
Some patients undergoing major surgery for cancer or systemic treatment (chemotherapy, immunotherapy) might require intensive care support. Having ICU with COVID-19 patients reduced the capacity and available beds for non-COVID-19 patients, and therefore diminished ability to perform major planned surgery. Surgical room reopened as soon as the influx of COVID-19 patients decreased, with more available ICU beds, in order not to induce a lot of opportunity in case of postponed surgery for a resectable cancer.
In this regard, some guidelines from various Cancer Core Europe have been edited and were unanimous that priority should be given to neoadjuvant therapies and curative surgery if limited access to ICU [18]. All urgent medical and surgical treatments were maintained and realized. Moreover, radiotherapy treatments were continued the same way for most patients.
Despite COVID-19 crisis, our institute was able to keep on his core mission, which relies on care and research. More than 150 patients were included into 4 different interventional trials (one sponsored by our institute), 3 translational studies, and 10 observational studies (four sponsored by our institute), with an active participation to national databases. A biobanking was also realized to further explore COVID-19 in the context of cancer.
Conclusions
COVID-19 pandemic highlighted the crucial role of intensive care, and our cancer-dedicated hospital ICU was involved for national requirement and general solidarity (and non-cancer patients admitted for the first time of the hospital institute) but also for continuity of care for cancer patients. In this regard, ethical discussion provided a learning and insightful experience. This pandemic impacts directly our organization, with very serious patients requiring heavy care, with the reassignment of new health workers in ICU that had to integrate and to be fully competent in a short delay, and with a change of practice especially regarding therapeutic commitment. Uncertainty remains regarding successive waves, and anticipation of the recovery of standard activity is difficult, but facing this pandemic requires adaptability and solidarity.
Authors’ contribution
Alice Boilève, Florence Netzer, Annabelle Stoclin, and Florian Scotté contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Alice Boilève, Florence Netzer, Fabrice Barlesi, Florent Varin, Florian Scotté, and Annabelle Stoclin. The first draft of the manuscript was written by Alice Boilève, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
F.S. has conflict of interests with Mylan, BMS, Tesaro, Sanofi, Roche, MSD, TEVA, Norgine, Prostrakan, Leo pharma, Janssen, Hospira, Boehringer, AMGEN, Pierre Fabre Oncologie, Vifor Pharma, Arrow, Mundi Pharma. F.B. reports personal fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly Oncology, F. Hoffmann-La Roche Ltd., Novartis, Merck, MSD, Pierre Fabre, Pfizer and Takeda, outside the submitted work. Other authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(2020) Worldometer COVID-19 coronavirus pandemic
- 3.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, Shrestha BR, Arabi YM, Ng J, Gomersall CD, Nishimura M, Koh Y, du B, Asian Critical Care Clinical Trials Group Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;2020:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(2020) WHO-China Joint Mission Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)
- 5.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 7.Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, Zhang C, Boyle C, Smith M, Phillips JP. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zangrillo A, Beretta L, Scandroglio AM, et al (2020) Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc 2020; Online ahead of print [DOI] [PMC free article] [PubMed]
- 10.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;2020:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angus DC, Musthafa AA, Clermont G, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1389–1394. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 12.Davidson TA, Rubenfeld GD, Caldwell ES, et al. The effect of acute respiratory distress syndrome on long-term survival. Am J Respir Crit Care Med. 1999;160:1838–1842. doi: 10.1164/ajrccm.160.6.9903058. [DOI] [PubMed] [Google Scholar]
- 13.Schelling G, Stoll C, Vogelmeier C, Hummel T, Behr J, Kapfhammer HP, Rothenhäusler HB, Haller M, Durst K, Krauseneck T, Briegel J. Pulmonary function and health-related quality of life in a sample of long-term survivors of the acute respiratory distress syndrome. Intensive Care Med. 2000;26:1304–1311. doi: 10.1007/s001340051342. [DOI] [PubMed] [Google Scholar]
- 14.Weinert CR, Gross CR, Kangas JR, et al. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med. 1997;156:1120–1128. doi: 10.1164/ajrccm.156.4.9611047. [DOI] [PubMed] [Google Scholar]
- 15.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balzer F, Menk M, Ziegler J, et al. Predictors of survival in critically ill patients with acute respiratory distress syndrome (ARDS): an observational study. BMC Anesthesiol. 2016;16:108. doi: 10.1186/s12871-016-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y-M, Perng R-P, Chu H, Tsai CM, Whang-Peng J. Impact of severe acute respiratory syndrome on the status of lung cancer chemotherapy patients and a correlation of the signs and symptoms. Lung Cancer. 2004;45:39–43. doi: 10.1016/j.lungcan.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26:665–671. doi: 10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]