Abstract
Background
Vitamin A plays a pivotal role in respiratory infection, accurate estimation of vitamin A status was recommended in planning and implementing interventions. As infections affect serum vitamin A productions, the real status need to be adjusted by acute phase protein (APP). Mycoplasma pneumoniae is an important cause of respiratory infection in children, the association between vitamin A concentrations and refractory Mycoplasma pneumoniae pneumonia (RMPP) remains unclear.
Methods
181 MPP patients were enrolled in this retrospective study, adjusted vitamin A concentrations and other parameters were compared between RMPP and general-MPP (GMPP) patients. Multivariate logistic regression test was performed to evaluate the association between vitamin A levels and RMPP incidence, linear correlation tests were applied to evaluate correlation between vitamin A concentrations and fever duration, length of stay (LOS).
Results
Vitamin A concentrations in RMPP group were significantly lower than those in GMPP patients (P < 0.05), vitamin A (OR = 0.795, 95% C. I 0.669–0.946) and CRP (OR = 1.050, 95% C. I 1.014–1.087) were independently associated with RMPP incidence. Linear correlation tests found vitamin A concentrations were negatively correlated with fever duration and LOS (P < 0.001).
Conclusions
Serum vitamin A concentrations were independently associated with RMPP incidence, which may correlate with reduced incidence of RMPP.
Keywords: Vitamin a, Retinol-binding protein (RBP), Mycoplasma Pneumoniae pneumonia (MPP)
Background
Mycoplasma pneumoniae (M. pneumoniae) is the predominant pathogen of community-acquired pneumonia (CAP), which contributes to approximately 10 to 40% of CAP cases in children [1–3]. Most pediatric cases of M. pneumoniae pneumonia (MPP) are benign and self-limited, however, there still are some cases showing clinical and radiological deterioration despite of macrolides antibiotic therapy for 7 days or longer, which are defined as refractory Mycoplasma pneumoniae pneumonia (RMPP) [4, 5]. The exact mechanisms of RMPP are not fully clarified, reducing the incidence of RMPP and improving its prognosis remain challenges. Our previous study and other literatures demonstrated glucocorticoid therapy attenuated the clinical manifestations, radiological findings and length of stay (LOS) of RMPP children [6, 7], indicating excessive inflammation involved in RMPP pathogenesis [8].
Micronutrients share inter-dependent relationships with host’s infection immunity [9, 10]. As acute infection affects concentrations of some micronutrients (including vitamin A, ferritin) [11–13], those circulating concentrations should be adjusted by acute phase protein (APP) to eliminate the impact of infection and reflect real micronutrient status [13–15]. The most two commonly used APPs are C-reactive protein (CRP) and α-1-acid glycoprotein (AGP) [14, 15]. Vitamin A is an essential micronutrient governs broad range of biological processes [16]. Recent studies highlight the interactions between vitamin A status and immune response [9, 17], demonstrating vitamin A deficiency (VAD) may cause imbalance between pro- and anti-inflammatory factors and excessive immune response [18], which emerged in RMPP. Serum retinol or retinol-binding protein (RBP) concentrations represent vitamin A status. High-performance liquid chromatography (HPLC) is recommended for serum retinol assessment, while it’s expensive and technically challenging. The method of RBP assessment takes the advantages of being more robust for sample collection and handling processes. Therefore, RBP is often substituted as an indicator of vitamin A status. Literatures have showed higher incidence of VAD in MPP children than healthy children, and VAD was associated with MPP severity, which indicated vitamin A levels could be associated with RMPP incidence [19]. Based on all above, we hypothesis that vitamin A levels could be associated with RMPP incidence. Therefore, we constructed this retrospective study to investigate adjusted vitamin A concentrations in MPP children and clarify the association between adjusted vitamin A levels and RMPP incidence, trying to provide more evidence for RMPP intervention.
Methods
Study population
This study was retrospectively conducted in Children’s Hospital of Chongqing Medical University, a 1500-bed tertiary level III teaching hospital in Chongqing, China. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University. Data from patients were analyzed anonymously for hospitalized mycoplasma pneumoniae pneumonia (MPP) children from 1 September 2018 to 31 December 2019 retrospectively. The inclusions had the following characteristics: (i) inpatients MPP children; (ii) age between 6 months and 18 years old. The exclusion criteria included any of the following: (i) patients who had an underlying organ dysfunction;(ii) patients coinfected with other pathogens. According to the diagnostic criteria of RMPP, patients were divided in to RMPP group and general M. pneumoniae pneumonia (GMPP) group. Besides, 65 children with micronutrients measurements in Physical Examination Center matched with age, gender and testing time were selected as the healthy control group.
Definitions
MPP was diagnosed based on the followings: (i) clinical presentation (fever, cough, etc); (ii) chest imaging with infiltrates; (iii) having the positive results for MP polymerase chain reaction (PCR) tests of nasopharyngeal secretions with serum anti-MP IgM titer ≥1:160. The diagnosis of refractory M. pneumoniae pneumonia (RMPP) was based on the presence of persistent fever and clinical manifestations as well as radiological deterioration after regular macrolides treatment for 7 days or longer [4, 5], the other cases were defined as general M. pneumoniae pneumonia (GMPP). The body mass index (BMI) was calculated by weight in kilograms divided by height in meters squared (kg/m2). Extrapulmonary presentations include liver function abnormalities, myocarditis, encephalitis, rash, proteinuria, hemolytic anemia and arthritis. VAD was defined as RBP concentration lower than 0.7 μmol/L (15 mg/L) [20].
M. pneumoniae detection
The specific antibodies against M. pneumoniae (IgG and IgM) were detected with passive particle agglutination (SERODIA-MYCO II, Japan) in nearly 2 ml serum samples of children on admission, MP antibody > 1:160 is a positive finding. Nasopharyngeal aspirate (NPA) was used for M. Pneumoniae DNA detection. In accordance with the manufacturer instructions, NPA was centrifugated 12,000 g for 5 min at 4 °C, the sediment was collected for DNA extraction with a real-time PCR commercial kit (Daan Gene Co. Ltd., Guangzhou, China). The DNA was then amplified using PCR primers and probes. Quantification curves were plotted using several concentrations of standard control samples (Daan Gene Co. Ltd., Guangzhou, China).
Micronutrients detection
Blood samples were collected from all inpatients during the first 24 h of admission. Serum micronutrients included ferritin, vitamin A, vitamin D, folate and vitamin B12 productions. Vitamin A concentrations were measured by retinol-binding protein (RBP). Vitamin D productions were measured by 25-hydroxy vitamin D (25(OH)D). Ferritin, folate, vitamin B12 and 25(OH)D concentrations were evaluated by Chemi Luminescence (Siemens, Germany), RBP levels were evaluated by Turbidimetric inhibition immunoassay (Homa Biological, Beijing, China). For accurate estimation, vitamin A and ferritin concentrations were adjusted by CRP, using regression correction (RC) approach [14, 21].
Adjustment approach
Adjusted vitamin A concentrations were obtained through RC approach [14, 21]. The RC approach was applied according to BRINDA methods articles, which uses linear regression to adjust RBP by the concentration of CRP. Briefly, the adjusted RBP equation was calculated by subtracting the influence of CRP, and RMPP as follows:
RBP adjusted = RBP unadjusted -β1 (CRP observe - CRP reference) -β2 (RMPP),
According to available data, β1 is the CRP regression coefficient, β2 is the RMPP regression coefficient, the reference of non-logged CRP is 8 mg/L, the minimum threshold of CRP measurement. CRP and RBP are all ln transformed, CRP and RBP are continuous variables, and RMPP is a dichotomous variable. The correction was only applied to individuals with CRP > 8 mg/L to avoid over adjustment. The same approach was applied to adjust ferritin.
BALF cytokines measurement
Mycoplasma pneumoniae pneumonia patients who received bronchoalveolar lavage, the bronchoalveolar lavage fluid (BALF) was extracted and collected, then delivered to the Center Laboratory Medicine immediately (no more than half an hour), kinds of cytokines including IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, IL-17a were measured by Cytometric Bead Array (CBA) (Cell-Gene, Hangzhou, China).
Data collection
Demographic characteristics (age, gender, weight, BMI), extrapulmonary manifestation, serum inflammatory factors (CRP, PCT, LDH, prealbumin), BALF inflammatory cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, IL-17a), serum micronutrients (ferritin, vitamin A, vitamin D, folate and vitamin B12), oxygenation, fever duration and length of stay (LOS) were retrospectively collected from all children who were included in the study.
Statistical analysis
The continuous variables were presented as medians with 25 and 75% quartiles (interquartile range, IQR); the non-parametric test and Mann-Whitney U test were used for analysis. The categorical variables were presented as counts (percentages), and assessed by Kruskal-Wallis or the Fisher exact test. Correlations between variables were analyzed by Pearson Correlation. Univariate and multivariate logistic regression tests were used to evaluate the association between RMPP incidence and other variables. All the statistical analyses were conducted using SPSS 25.0 and Graphpad Prism 7.0 for Windows.
Results
Study population
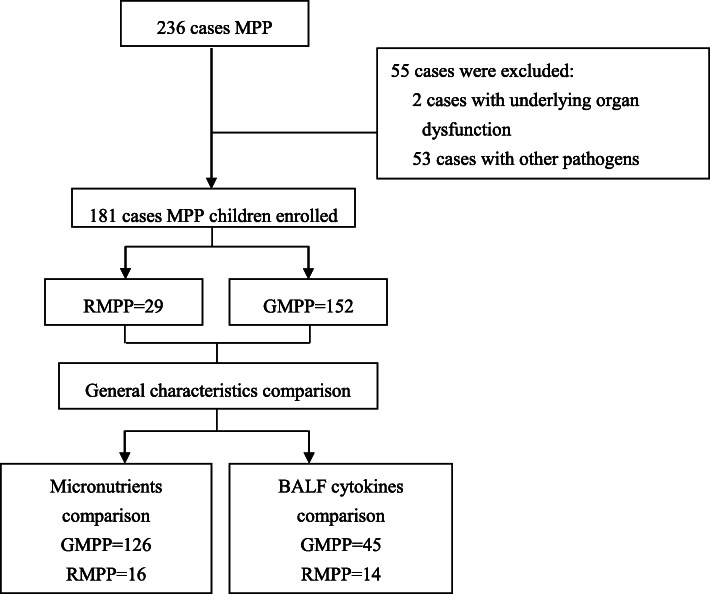
A total of 236 children diagnosed with MPP enrolled in this study, after exclusion, 181 MPP children were involved in general characteristics comparison. Among them, 142 children with micronutrients measurements were compared with micronutrient measurements of the controls. The grouped comparisons of cytokines were applied to the patients who received bronchoalveolar lavage only. The detailed breakdown of the participants is showing in Fig. 1.
Fig. 1.
Flow diagram of the participants. As shown, a total of 291 cases met the inclusion criteria, after exclusion, 181 MPP patients were enrolled according to the inclusion and exclusion criteria, and then divided into RMPP group (n = 29) and GMPP group (n = 152). The vitamin A concentrations and other characteristics of each group were then determined
General characteristics of RMPP and GMPP patients
One hundred and eighty-one children diagnosed with MPP were divided into RMPP (n = 29) and GMPP (n = 152) according to the diagnostic criteria. No significant difference was found in age, gender and BMI between the two groups. The levels of CRP [20.00 (0.00–32.50) vs. 0 (0.00–11.00), mg/L, P < 0.05], PCT [0.188 (0.105–0.716) vs. 0.074 (0.043–0.132), mg/L, P < 0.05], LDH [388.00 (298.50–480.80) vs. 331.50 (288.00–381.25), U/L, P < 0.05] were significantly higher in RMPP than those in GMPP patients, while prealbumin productions were significantly lower in RMPP than that in GMPP patients [82.00 (68.00–100.00) vs. 115.00 (97.00–151.00), g/L, P < 0.05]. As expected, RMPP group had longer fever duration (10.00 (9.00–13.00) vs. 5.00 (2.00–8.00), days, P < 0.05) and length of stay (LOS) (9.00 (7.50–10.00) vs. 6.00 (5.00–7.75), days, P < 0.05), higher incidence of VAD (68.75% vs. 31.75%), extrapulmonary manifestations (58.62% vs. 14.47%) and oxygenation (100.00% vs. 45.39%) when compared to GMPP patients. (Table 1).
Table 1.
General characteristics of MPP children
| General characteristics | GMPP (n = 152) | RMPP (n = 29) | P |
|---|---|---|---|
| Age (month), Median (IQR) | 46.00 (26.25–72.25) | 55.00 (37.00–83.00) | 0.062 |
| Male/Female | 82/70 | 18/11 | 0.422 |
| BMI (kg/m2), Median (IQR) | 16.44 (15.08–17.78) | 15.92 (14.58–16.40) | 0.168 |
| Extrapulmonary manifestations (n, %) | 22 (14.47%) | 17 (58.62%) | < 0.001* |
| Oxygenation (n, %)a | 69 (45.39%) | 29 (100.00%) | < 0.001* |
| Fever duration (day), Median (IQR) | 5.00 (2.00–8.00) | 10.00 (9.00–13.00) | < 0.001* |
| CRP (mg/L), Median (IQR)b | 0 (0.00–11.00) | 20.00 (0.00–32.50) | < 0.001* |
| PCT (mg/L), Median (IQR) | 0.074 (0.043–0.132) | 0.188 (0.105–0.716) | < 0.001* |
| Prealbumin (g/L), Median (IQR) | 115.00 (97.00–151.00) | 82.00 (68.00–100.00) | < 0.001* |
| LDH (U/L), Median (IQR) | 331.50 (288.00–381.25) | 388.00 (298.50–480.80) | 0.017* |
| LOS (day), Median (IQR) | 6.00 (5.00–7.75) | 9.00 (7.50–10.00) | < 0.001* |
| VAD (n, %) | 40 (31.75%) | 11 (68.75%) | 0.004* |
* showed difference between RMPP and GMPP groups (P < 0.05)
a including nasal oxygen breath and Continuous Positive Airway Pressure (CPAP)
b CRP values under minimum threshold of measurement (8 mg/L) were taken as 0 mg/L
Serum micronutrients status in RMPP and GMPP patients
Serum micronutrients in RMPP (n = 16), GMPP(n = 126) patients and control children (n = 65) were presented in Table 2. Both serum unadjusted-vitamin A concentrations [10.90 (9.38–14.98) vs. 16.95 (13.38–22.63) vs. 25.10(21.90–29.05), mg/L, < 0.001] and adjusted- vitamin A [12.23(9.83–15.43) vs. 17.00(13.53–22.93) vs. 25.10(21.90–29.05), mg/L, < 0.001] in RMPP patients were significantly lower than those in healthy children and GMPP patients. Conversely, RMPP patients had remarkably higher serum unadjusted-ferritin [179.50 (109.23–290.85) vs. 95.85 (60.68–143.25) vs. 47.00 (34.55–67.10), mg/L, < 0.001] and adjusted-ferritin [171.45(104.90–238.55) vs. 96.40(61.00–143.20) vs. 47.00 (34.55–67.10), mg/L, < 0.001] productions when compared to GMPP patients and control children. No significant difference of vitamin B 12, vitamin D and folate levels was found among the three groups.
Table 2.
Micronutrients measurements comparison of participant children
| Micronutrients | Healthy control (n = 65) | GMPP (n = 126) | RMPP (n = 16) | P |
|---|---|---|---|---|
| Unadjusted-ferritin (mg/L), Median (IQR) | 47.00 (34.55–67.10) | 95.85 (60.68–143.25) | 179.50 (109.23–290.85) | < 0.001* |
| Adjusted-ferritin (mg/L), Median (IQR) a | 47.00 (34.55–67.10) | 96.40 (61.00–143.20) | 171.45 (104.90–238.55) | < 0.001* |
| Vitamin B12 (pg/mL), Median (IQR) | 902.00 (737.00–1031.00) | 1090.00 (901.00–1604.00) | 942.00 (685.00–1333.00) | 0.165 |
| Folate (ng/mL), Median (IQR) | 16.45 (12.81–19.85) | 16.64 (13.00–20.53) | 15.87 (14.66–19.75) | 0.803 |
| Vitamin D (ng/mL), Median (IQR) | 19.93 (16.28–24.06) | 22.10 (15.53–31.54) | 24.92 (16.54–33.94) | 0.227 |
| Unadjusted-vitamin A (mg/L), Median (IQR) | 25.10 (21.90–29.05) | 16.95 (13.38–22.63) | 10.90 (9.38–14.98) | < 0.001* |
|
Adjusted-vitamin A (mg/L), Median (IQR) a |
25.10 (21.90–29.05) | 17.00 (13.53–22.93) | 12.23 (9.83–15.43) | < 0.001* |
* showed difference among all groups (P < 0.05)
a Adjusted by CRP using the Regression Correction (RC) approach
BALF inflammatory cytokines in RMPP and GMPP patients
The inflammatory cytokines in BALF were compared between RMPP and GMPP patients (Table 3). The levels of IL-6 [302.27 (141.45–726.08) vs. 122.00 (43.92–294.49), pg/mL, P < 0.05] and TNF-α [29.41 (5.01–79.13) vs. 5.56 (0.00–11.04), pg/mL, P < 0.05] were significantly higher in RMPP than those in GMPP patients. No significant differences of IL-2, IL-4, IL-10, IFN-γ, IL-17a productions was found.
Table 3.
BALF inflammatory cytokines between GMPP and RMPP children
| BALF inflammatory cytokines | GMPP (n = 45) | RMPP (n = 14) | P |
|---|---|---|---|
| IL-6 (pg/mL) | 122.00 (43.92–294.49) | 302.27 (141.45–726.08) | 0.017* |
| TNF-α (pg/mL) | 5.56 (0.00–11.04) | 29.41 (5.01–79.13) | 0.014* |
| IL-2 (pg/mL) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.314 |
| IL-4 (pg/mL) | 0.00 (0.00–2.06) | 0.00 (0.00–0.74) | 0.841 |
| IL-10 (pg/mL) | 3.09 (0.00–9.27) | 9.74 (0.00–37.48) | 0.227 |
| IFN-γ (pg/mL) | 3.87 (0.00–12.26) | 6.74 (2.40–46.56) | 0.143 |
| IL-17a (pg/mL) | 3.70 (0.00–7.76) | 6.82 (0.38–10.74) | 0.214 |
* showed difference between RMPP and GMPP groups (P < 0.05)
Independent associated factors of RMPP
Regression analysis was used to find the associated factors of RMPP and the results were presented in Table 4. Univariate logistic regression analysis showed serum levels of adjusted-vitamin A, CRP, PCT, LDH, prealbumin and adjusted-ferritin, TNF-α productions in BALF were significantly associated with RMPP. Multivariate logistic regression analysis stated serum CRP and adjusted-vitamin A concentrations were independently associated with RMPP. Vitamin A is a protective factor, every unit decrease of adjusted-vitamin A (mg/L) resulted in 0.205 odds increase in RMPP (95% C. I 0.669–0.946); CRP is a risk factor, every unit increase of CRP (mg/L) resulted in 0.050 odds increase in RMPP (95% CI 1.014–1.087).
Table 4.
Multivariate logistic regression analysis of RMPP
| variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% C.I) | P | OR (95% C.I) | P | |
| CRP | 1.043 (1.021–1.065) | < 0.001 | 1.050 (1.014–1.087) | 0.007 |
| Adjusted-vitamin A a | 0.747 (0.634–0.882) | 0.001 | 0.795 (0.669–0.946) | 0.010 |
| PCT | 12.534 (3.587–43.794) | < 0.001 | – | – |
| LDH | 1.005 (1.002–1.009) | 0.005 | – | – |
| Prealbumin | 0.980 (0.965–0.995) | 0.011 | – | – |
| Ferritin1 | 1.009 (1.003–1.016) | 0.002 | – | – |
| TNF-α | 1.046 (1.016–1.077) | 0.002 | – | – |
a Adjusted by CRP using the Regression Correction (RC) approach
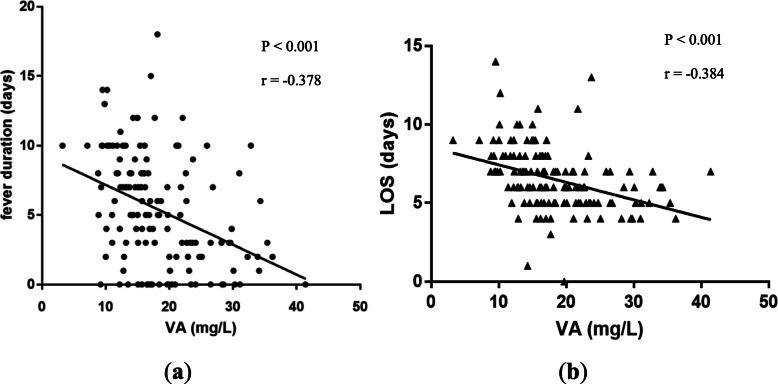
Correlation between vitamin a and fever duration, LOS in MPP children
To further evaluate the correlation between serum adjusted-vitamin A levels and fever duration as well as LOS, linear correlation tests were constructed in MPP children with adjusted-vitamin A measurements (n = 143). Results showed serum adjusted-vitamin A levels were negatively correlated with fever duration (Fig. 2a, r = − 0.378, P < 0.001) and LOS (Fig. 2b, r = − 0.384, P < 0.001).
Fig. 2.
Correlation between serum vitamin A levels and clinical finding in MPP children. a Correlation between serum adjusted-vitamin A concentrations and fever duration in MPP children (r = − 0.378, P < 0.001). b Correlation between serum adjusted-vitamin A concentrations and LOS in MPP children (r = − 0.384, P < 0.001)
Correlation between vitamin a and BALF cytokine levels in MPP children
To assess the correlation between adjusted-vitamin A and lung immunity, the linear correlation tests were applied for children who received bronchoalveolar lavage, results were shown in Fig. 3. We found a significantly negative correlation between adjusted-RBP and IL-6 levels (r = − 0.321, P = 0.032), while no significances were found with IL-2, IL-4, IL-10, TNF-α and IFN-γ.
Fig. 3.
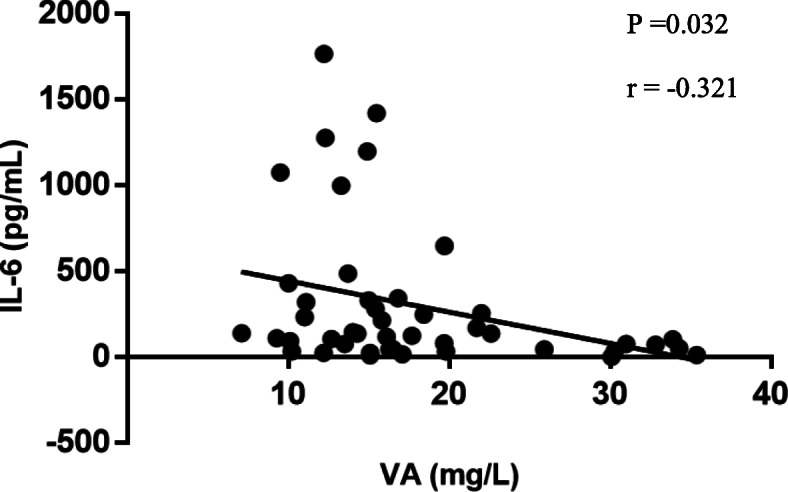
Correlation between serum adjusted-vitamin A levels and IL-6 concentrations in BALF in MPP children (r = − 0.321, P = 0.032)
Discussion
M. pneumoniae is a leading cause of CAP, some MPP children could progress to RMPP. Researches have already noticed the important role of vitamin A in respiratory infection [22, 23], and emphasized accurate estimation of vitamin A deficiency [14]. Serum retinol or RBP concentrations provide vital information of vitamin A status and vitamin A deficiency (VAD) severity. Serum vitamin A concentrations could be affected by infection, for accurate estimation, adjusting it by CRP and/or AGP was recommended [14, 15]. As compared with other adjustment approaches, RC approach adjusting vitamin A in a continuous manner that better reflects the association between vitamin A and APPs. Thus, vitamin A concentrations were adjusted by CRP with RC approach in this study. To our knowledge, this is the first study to investigate the association between the real vitamin A status and RMPP incidence. We demonstrated serum vitamin A concentrations were significantly lower in RMPP children than those in GMPP patients. Insufficient serum vitamin A concentration was independently associated with RMPP incidence.
The overall incidence of RMPP in MPP patients was 16.02% in this study, which was similar with previous studies [24, 25]. However, we found the prevalence of VAD was 35.92% in this study, which was relatively lower than others’ reports [23, 26]. As serum vitamin A levels could be affected by infection [13], using vitamin A without adjusted by APPs could overestimate the prevalence of VAD in MPP children. The adjustments estimated VAD by mathematically removing or reducing the effect of elevated CRP in this study, which is important for decisions regarding nutrition interventions, programs, and policies.
Another important finding of our study is that sufficient serum vitamin A served as an independently protective factor for RMPP, every one unit decrease of adjusted-vitamin A (mg/L) resulted in 0.205 odds increase in RMPP incidence. Vitamin A is essential for the airway epithelium integrity [27], lung immune function and inflammation regulation [28], VAD may result in impaired mucosal barrier [29], disordered immune response [29, 30] and excessive cytokines release [31]. As we known, M. pneumoniae adhere to the host airway epithelium during MPP, followed by local airway epithelium damage and inflammatory cytokines release. Therefore, the decreased vitamin A during M. pneumoniae infection could deteriorate pulmonary injuries and clinical manifestations [32], which contributes to RMPP development together with longer fever duration and LOS. In malnourished children, vitamin A supplementation showed beneficial effects in acute lower respiratory infection (ALRI) children [23, 33, 34], those are evidences indicated the protective role of vitamin A in RMPP development. However, some studies indicated vitamin A supplementation in ALRI children had no benefits or modestly adverse effect in well-nourished children, which demonstrated the importance of accurate estimation of vitamin A status in planning and implementing interventions.
We also documented that one unit increase of CRP (mg/L) resulted in 0.05 odds increase in RMPP incidence, the median CRP concentrations in RMPP children were significantly higher than those in GMPP patients, which was in line with other studies [35, 36]. CRP is wildly known as a kind of acute phase protein, which rises rapidly and acutely in response to an inflammatory stimulus and reflect the individual immune response, translating into unfavorable conditions such as RMPP development. Meanwhile, prealbumin was found to be significantly lower in RMPP than GMPP patients, which correlated with RMPP incidence. Prealbumin is a carrier protein synthesized in the liver, it serves as an nonspecific host defense substance by eliminating toxic metabolites during infection [37]. Hrnciarikova [38] et al. found it negatively correlated with CRP and could serve as a negative acute phase protein, suggesting the reduction of prealbumin has similar significance with the increase of CRP in RMPP development.
In addition, we found LDH was significantly higher in RMPP children, univariate regression test also found its correlation with RMPP incidence [35, 39, 40]. It was confirmed that LDH elevated in many kinds of pulmonary diseases and reported to be associated with RMPP. LDH is released from cells after cell damage and can be used to monitor cell and tissue damage. Lung parenchymal cells, local inflammatory cells, including alveolar macrophages and neutrophils might be potential sources of LDH in pulmonary disorders [41, 42]. Thus, the higher level of LDH could translated into excessive inflammatory cell infiltration and severe lung injury, indicating increased RMPP incidence.
Besides, there were trends for correlations with ferritin and TNF-α, which agreed with other’ studies [43, 44]. In the linear correlation test, we found a significantly negative correlation between IL-6 and vitamin A. M. pneumoniae infections are closely related to stimulation of macrophages via toll-like receptors that release immunomodulatory and inflammatory cytokines and chemokines [33]. Ferritin is a kind of non-specific marker of inflammation induced by activated macrophages, which could also produce TNF-α [45] and interplay with IL-6 [46]. Thus, the increased level of ferritin and cytokines can reflect excessive inflammation and RMPP development. However, no significance was found between TNF-α and vitamin A in linear correlation analysis, this may relate to the small sample of bronchoalveolar lavage, which could underestimate the correlation between vitamin A and BALF cytokines.
There are potential limitations of this study. First, this is a single-center study, the data were collected from one academic teaching hospital in China, the results may not easily extrapolate to patients admitted to other regions. Second, the relatively small sample size of our study may reduce the ability to determine the statistical significance of the variables. Third, as the data were collected from the records retrospectively, some information was unfortunately missed, which may lead to imbalanced group sample size. A larger prospective study could help to evaluate the role of vitamin A for RMPP in different age groups, geographical locations.
Conclusions
Serum vitamin A concentrations are independently associated with RMPP incidence, vitamin A levels may correlate with reduced incidence of RMPP.
Acknowledgments
We would like to thank staff the Department of Respiratory Medicine, Children’s Hospital of Chongqing Medical University.
Abbreviations
- M. pneumoniae
Mycoplasma pneumoniae
- CAP
Community-acquired pneumonia
- MPP
M. pneumoniae pneumonia
- RMPP
Refractory Mycoplasma pneumoniae pneumonia
- LOS
Length of stay
- APP
Acute phase protein
- CRP
C-reactive protein
- AGP
α-1-acid glycoprotein
- VAD
Vitamin A deficiency
- RBP
Retinol-binding protein
- HPLC
High-performance liquid chromatography
- GMPP
General M. pneumoniae pneumonia
- PCR
Polymerase chain reaction
- BMI
Body mass index
- NPA
Nasopharyngeal aspirates
- RC
Regression correction
- BALF
Bronchoalveolar lavage fluid
- CBA
Cytometric Bead Array
- IQR
Interquartile range
- OR
Odds ratio
- CI
Confidence interval
- LDH
Lactate dehydrogenase
Authors’ contributions
ZXL designed the experiments; YYL performed the experiments and wrote the manuscript; ZYG contributed to drawing the figures; GLZ helped in the statistical analyses; XYT drew the tables; QYL, DPC helped to collect the figures. All authors have read and approved the manuscript.
Funding
This work was supported in part by the fund of National Key Clinical Specialty Discipline Construction Program of China (2011–873). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The data-sets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University (File No.: 201813). All methods were performed in accordance with the relevant guidelines and regulations. All study participants provided written consents for future research, guardians provided the consents on behalf of patients under 16 years.
Consent for publication
Not applicable.
Competing interests
The authors declare no financial and non-financial competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Principi N, Esposito S. Emerging role of mycoplasma pneumoniae and chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis. 2001;1:334–344. doi: 10.1016/S1473-3099(01)00147-5. [DOI] [PubMed] [Google Scholar]
- 3.Principi N, Esposito S, Blasi F, et al. Role of mycoplasma pneumoniae and chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001;32:1281–1289. doi: 10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 4.Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association The Editorial Board, Chinese Journal of Pediatrics. [Guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (II)]. Zhonghua Er Ke Za Zhi. 2013. 51(11): 856–62. [PubMed]
- 5.Tamura A, Matsubara K, Tanaka T, Nigami H, Yura K, Fukaya T. Methylprednisolone pulse therapy for refractory mycoplasma pneumoniae pneumonia in children. J Inf Secur. 2008;57(3):223–228. doi: 10.1016/j.jinf.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Z, Luo J, Liu E, et al. Effects of prednisolone on refractory mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2014;49(4):377–380. doi: 10.1002/ppul.22752. [DOI] [PubMed] [Google Scholar]
- 7.Okumura T, Kawada JI, Tanaka M, et al. Comparison of high-dose and low-dose corticosteroid therapy for refractory mycoplasma pneumoniae pneumonia in children. J Infect Chemother. 2019;25(5):346–350. doi: 10.1016/j.jiac.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17(4):697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel N, Penkert RR, Jones BG, et al. Baseline Serum Vitamin A and D Levels Determine Benefit of Oral Vitamin A&D Supplements to Humoral Immune Responses Following Pediatric Influenza Vaccination. Viruses. 2019;11(10):907. [DOI] [PMC free article] [PubMed]
- 10.Finkelstein JL, Colt S, Layden AJ, et al. Micronutrients, immunological parameters, and dengue virus infection in coastal Ecuador: a nested case-control study in an infectious disease surveillance program. J Infect Dis. 2020;221(1):91–101. doi: 10.1093/infdis/jiz427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raiten DJ, Sakr Ashour FA, Ross AC, et al. Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE) J Nutr. 2015;145(5):1039S–1108S. doi: 10.3945/jn.114.194571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galloway P, McMillan DC, Sattar N. Effect of the inflammatory response on trace element and vitamin status. Ann Clin Biochem. 2000;37(Pt 3):289–297. doi: 10.1258/0004563001899429. [DOI] [PubMed] [Google Scholar]
- 13.Bresnahan KA, Tanumihardjo SA. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr. 2014;5(6):702–711. doi: 10.3945/an.114.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson LM, Namaste SM, Williams AM, et al. Adjusting retinol-binding protein concentrations for inflammation: biomarkers reflecting inflammation and nutritional determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):390S–401S. doi: 10.3945/ajcn.116.142166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92(3):546–555. doi: 10.3945/ajcn.2010.29284. [DOI] [PubMed] [Google Scholar]
- 16.Bang BR, Li M, Tsai KN, et al. Regulation of Hepatitis C Virus Infection by Cellular Retinoic Acid Binding Proteins through the Modulation of Lipid Droplet Abundance. J Virol. 2019;93(8):e02302-18. [DOI] [PMC free article] [PubMed]
- 17.McGill JL, Kelly SM, Guerra-Maupome M, et al. Vitamin a deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves. Sci Rep. 2019;9(1):15157. doi: 10.1038/s41598-019-51684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 19.郭艳霞, 冯艳芳, 沈丹华等. 儿童普通及难治性支原体肺炎维生素A水平及免疫功能的临床分析. 广西医科大学学报. 2019;36:23–6.
- 20.de Pee S, Dary O. Biochemical indicators of vitamin a deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132:2895S–2901S. doi: 10.1093/jn/132.9.2895S. [DOI] [PubMed] [Google Scholar]
- 21.Namaste SM, Aaron GJ, Varadhan R, Peerson JM, Suchdev PS. BRINDA working group. Methodologic approach for the biomarkers reflecting inflammation and nutritional determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):333S–347S. doi: 10.3945/ajcn.116.142273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurwitz JL, Jones BG, Penkert RR, et al. Low retinol-binding protein and vitamin D levels are associated with severe outcomes in children hospitalized with lower respiratory tract infection and respiratory syncytial virus or human Metapneumovirus detection. J Pediatr. 2017;187:323–327. doi: 10.1016/j.jpeds.2017.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing Y, Sheng K, Xiao X, et al. Vitamin a deficiency is associated with severe mycoplasma pneumoniae pneumonia in children. Ann Transl Med. 2020;8(4):120. doi: 10.21037/atm.2020.02.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y, Chu C, Li Y, et al. High expression of HMGB1 in children with refractory mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2018;18:439. doi: 10.1186/s12879-018-3346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao X, Qian-qian LI, Xiang Z, et al. Clinical features and treatment of refractory Mycoplasma pneumoniae pneumonia in children. J Clin Pediatr. 2015;33(11):958–61.
- 26.许颖, 苏艳琦, 郎会利. 肺炎支原体肺炎患儿血清维生素A与维生素E水平调查%An investigation of vitamin A and E levels in serum of children with mycoplasma pneumoniae pneumonia. 中国中西医结合儿科学. 2019 .
- 27.Sirisinha S. The pleiotropic role of vitamin a in regulating mucosal immunity. Asian Pac J Allergy Immunol. 2015;33(2):71–89. [PubMed] [Google Scholar]
- 28.Timoneda J, Rodríguez-Fernández L, Zaragozá R, et al. Vitamin A Deficiency and the Lung. Nutrients. 2018;10(9):1132. [DOI] [PMC free article] [PubMed]
- 29.Cassani B, Villablanca EJ, De Calisto J, Wang S, Mora JR. Vitamin a and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol Asp Med. 2012;33(1):63–76. doi: 10.1016/j.mam.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Feng L, Jiang WD, et al. Vitamin a deficiency suppresses fish immune function with differences in different intestinal segments: the role of transcriptional factor NF-κB and p38 mitogen-activated protein kinase signalling pathways. Br J Nutr. 2017;117(1):67–82. doi: 10.1017/S0007114516003342. [DOI] [PubMed] [Google Scholar]
- 31.Cui W, Zhang P, Gu J, et al. Vitamin a deficiency promotes inflammation by induction of type 2 cytokines in experimental ovalbumin-induced asthma murine model. Inflammation. 2016;39(5):1798–1804. doi: 10.1007/s10753-016-0415-2. [DOI] [PubMed] [Google Scholar]
- 32.Waites KB, Balish MF, Atkinson TP. New insights into the pathogenesis and detection of mycoplasma pneumoniae infections. Future Microbiol. 2008;3(6):635–648. doi: 10.2217/17460913.3.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nacul LC, Kirkwood BR, Arthur P, Morris SS, Magalhães M, Fink MC. Randomised, double blind, placebo controlled clinical trial of efficacy of vitamin a treatment in non-measles childhood pneumonia. BMJ. 1997;315(7107):505–510. doi: 10.1136/bmj.315.7107.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fawzi WW, Mbise RL, Fataki MR, et al. Vitamin a supplementation and severity of pneumonia in children admitted to the hospital in Dar Es Salaam, Tanzania. Am J Clin Nutr. 1998;68(1):187–192. doi: 10.1093/ajcn/68.1.187. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhou Y, Li S, Yang D, Wu X, Chen Z. The clinical characteristics and predictors of refractory mycoplasma pneumoniae pneumonia in children. PLoS One. 2016;11(5):e0156465. doi: 10.1371/journal.pone.0156465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu JR, Peng Y, Yang HM, Li HM, Zhao SY, Jiang ZF. Clinical characteristics and predictive factors of refractory mycoplasma pneumoniae pneumonia. Zhonghua Er Ke Za Zhi. 2012;50(12):915–918. [PubMed] [Google Scholar]
- 37.Ning J, Shao X, Ma Y, Lv D. Valuable hematological indicators for the diagnosis and severity assessment of Chinese children with community-acquired pneumonia: Prealbumin. Medicine (Baltimore) 2016;95(47):e5452. doi: 10.1097/MD.0000000000005452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hrnciarikova D, Juraskova B, Hyspler R, et al. A changed view of serum prealbumin in the elderly: prealbumin values influenced by concomitant inflammation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(2):273–276. doi: 10.5507/bp.2007.046. [DOI] [PubMed] [Google Scholar]
- 39.Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate dehydrogenase as a biomarker for prediction of refractory mycoplasma pneumoniae pneumonia in children. Respir Care. 2015;60(10):1469–1475. doi: 10.4187/respcare.03920. [DOI] [PubMed] [Google Scholar]
- 40.Izumikawa K. Clinical features of severe or fatal mycoplasma pneumoniae pneumonia. Front Microbiol. 2016;7:800. doi: 10.3389/fmicb.2016.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cobben NA, Drent M, Jacobs JA, et al. Relationship between enzymatic markers of pulmonary cell damage and cellular profile: a study in bronchoalveolar lavage fluid. Exp Lung Res. 1999;25(2):99–111. doi: 10.1080/019021499270321. [DOI] [PubMed] [Google Scholar]
- 42.Liu TY, Lee WJ, Tsai CM, et al. Serum lactate dehydrogenase isoenzymes 4 plus 5 is a better biomarker than total lactate dehydrogenase for refractory mycoplasma pneumoniae pneumonia in children. Pediatr Neonatol. 2018;59(5):501–506. doi: 10.1016/j.pedneo.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Li G, Fan L, Wang Y, et al. High co-expression of TNF-α and CARDS toxin is a good predictor for refractory mycoplasma pneumoniae pneumonia. Mol Med. 2019;25(1):38. doi: 10.1186/s10020-019-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao J, Ji X, Wang Y, Wang X. Clinical role of serum interleukin-17A in the prediction of refractory mycoplasma pneumoniae pneumonia in children. Infect Drug Resist. 2020;13:835–843. doi: 10.2147/IDR.S240034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruscitti P, Cipriani P, Di Benedetto P, et al. H-ferritin and proinflammatory cytokines are increased in the bone marrow of patients affected by macrophage activation syndrome. Clin Exp Immunol. 2018;191(2):220–228. doi: 10.1111/cei.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali ET, Jabbar AS, Mohammed AN. A comparative study of interleukin 6, inflammatory markers, ferritin, and hematological profile in rheumatoid arthritis patients with Anemia of chronic disease and Iron deficiency Anemia. Anemia. 2019;2019:3457347. doi: 10.1155/2019/3457347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data-sets analyzed during the current study are available from the corresponding author on reasonable request.