Abstract
PURPOSE
Breast cancer is the most common cancer in women in India, with higher incidence rates of aggressive subtypes, such as triple-negative breast cancer (TNBC).
METHODS
A systematic review was performed to compute pooled prevalence rates of TNBC among patients with breast cancer, and clinical features at presentation were systematically compared with non-TNBC in an Indian cohort of 20,000 patients.
RESULTS
Combined prevalence of TNBC among patients with breast cancer was found to be on the higher side (27%; 95% CI, 24% to 31%). We found that the estrogen receptor (ER) expression cutoff used to determine ER positivity had an influence on the pooled prevalence and ranged from 30% (ER/progesterone receptor [PR] cut ff at 1%) to 24% (ER/PR cutoff at 10%). Odds for TNBC to present in the younger age-group were significantly higher (pooled odds ratio [OR], 1.35; 95% CI, 1.08 to 1.69), with a significantly younger mean age of incidence (weighted mean difference, −2.75; 95% CI, −3.59 to −1.92). TNBC showed a significantly higher odds of presenting with high grade (pooled OR, 2.57; 95% CI, 2.12 to 3.12) and lymph node positivity (pooled OR, 1.39; 95% CI, 1.21 to 1.60) than non-TNBC.
CONCLUSION
Systematic review and meta-analysis of 34 studies revealed a high degree of heterogeneity in prevalence of TNBC within Indian patients with breast cancer, yet pooled prevalence of TNBC is high in India. High proportions of patients with TNBC present with aggressive features, such as high grade and lymph node positivity, compared with patients without TNBC. We emphasize the need for standardized methods for accurate diagnosis in countries like India.
INTRODUCTION
Breast cancer is the most common cancer in India, with the highest numbers of new cancer incidence per year (14%) and with a high incidence-to-mortality ratio (approximately 50%) according to GLOBOCAN 2018.1 At present, breast cancer is classified into 4 molecular subtypes on the basis of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Positive expression of ER/PR and/or HER2 determines the ER-positive and/or HER2-positive subtype, while absence of ER, PR, and HER2 expression defines triple-negative breast cancer (TNBC).2 Both, ER-positive and HER2-positive subtypes are effectively and routinely treated with respective targeted therapy.3 In contrast, TNBCs lack targeted therapy and are still treated with systemic chemotherapy drugs. In addition, TNBCs tend to present with more aggressive clinical features4 and tend to recur earlier and with higher frequency, which make them a most aggressive subtype of breast cancer.5,6
CONTEXT
Key Objective
The meta-analysis systematically compared prevalence of triple-negative breast cancer (TNBC) in a large cohort of 20,000 Indian/Indian-origin patients from 34 studies.
Knowledge Generated
Indian patients with TNBC present with high rates (27%) of prevalence, although with a high degree of variability. To our knowledge, this is the first time a possible source of variability in TNBC prevalence among the studies has been objectively analyzed. Our study reveals and emphasizes the need for standardized methods for a standardized diagnostic protocol across the country.
Relevance
Even with the variable prevalence, patients with TNBC in India present at a significantly younger age compared with patients without TNBC and with a higher odds ratio of high-grade disease and lymph node involvement. Understanding the high rates of prevalence and clinical features of the most aggressive, triple-negative subtype may help to clarify and better interpret breast cancer outcomes in India.
TNBC incidence in the West is at 12.2%-13% of all breast cancers,4,6 with the highest prevalence in Blacks (22.5%-23.7%).4,6 In India, several reports have suggested that TNBC incidence is higher and up to 31%.7,8
Having a higher incidence of TNBC may translate into a higher proportion of the aggressive disease that is clinically difficult to target, which contributes to higher mortality rates in India. Moreover, there is a high degree of variability in TNBC prevalence among individual studies.7,8 We conducted a systematic review and meta-analysis to assess the effect of detection method for ER/PR positivity that determines triple-negative status of the disease because such methods are reportedly varied across centers in India.9,10 Clinical features of TNBC and non-TNBC at incidence, such as age, grade, and lymph node involvement, were systematically compared with the understanding of whether TNBC in Indian cohorts present with a higher degree of aggressive features, as has been observed in the West.6
METHODS
Search Criteria
The key terms used to search for the breast cancer reports in Indian cohorts were as follows: breast cancer, breast carcinoma, triple negative, ER, PR, HER2, TNBC, and Indian or India. The studies that were peer reviewed and listed in PubMed until October 2019 were included. To be certain that breast cancer studies with patients from India or of Indian origin were included in the analysis; individual studies/reports were manually curated for the following: studies conducted at and published from an Indian center (assuming that all the patients were of Indian origin) or studies conducted in countries other than India, with data clearly annotated for Indian-origin patients. With these inclusion criteria, 49 studies were identified7-9,11-55 (Data Supplement).
Exclusion Criteria
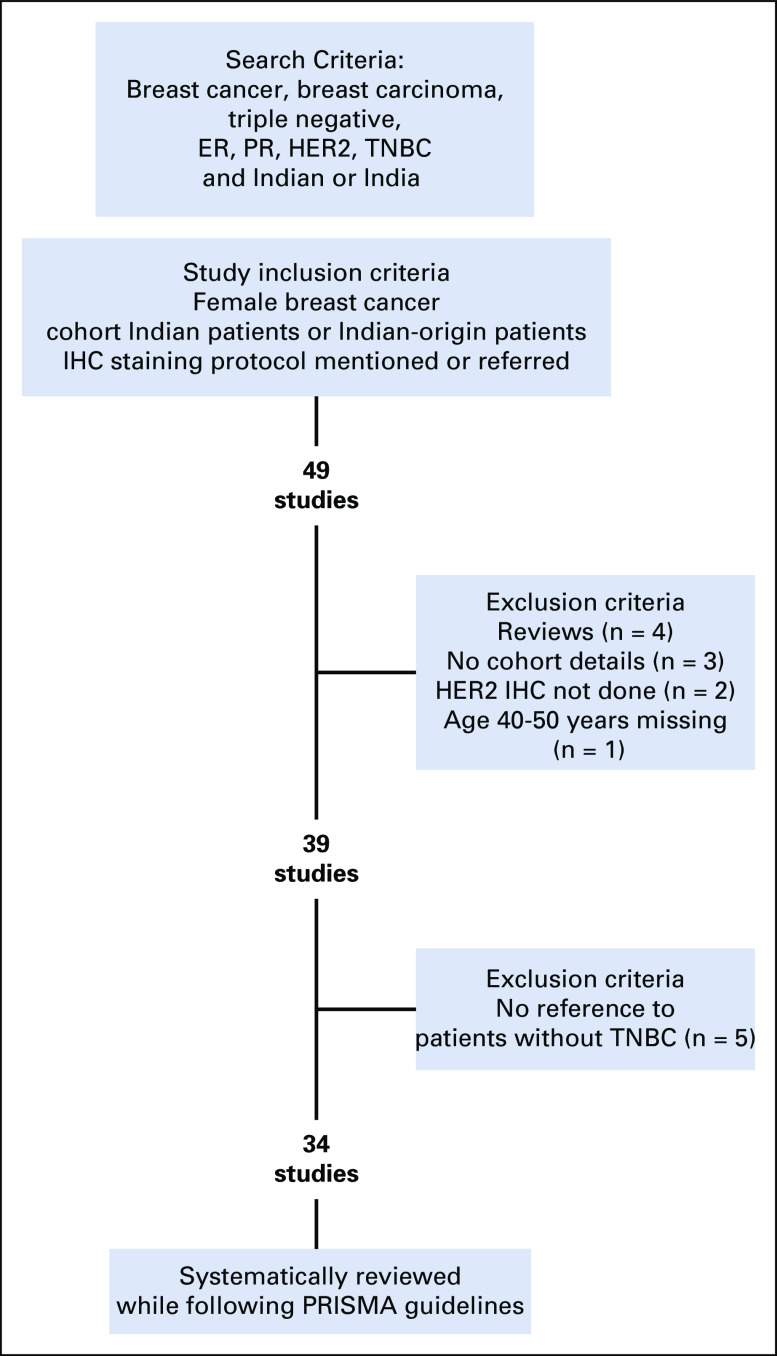
Of the 49 studies identified, those that did not mention criteria for defining HER2 positivity or negativity, subtype prevalence, or cohort details; had information missing for part of the cohort; or were review articles were excluded. In total, 15 articles were excluded for reasons shown in Figure 1. The remaining 34 studies were considered for the systematic comparison between TNBC and non-TNBC for prevalence and clinical features at incidence, such as age, grade, and lymph node involvement.
FIG 1.
Search and inclusion/exclusion criteria of the studies. The flowchart depicts search criteria used to select the studies with breast cancer cohorts of Indian and/or Indian-origin patients. Exclusion and inclusion criteria are explained and led to the inclusion of 34 studies in the systematic review. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2, IHC, immunohistochemistry; PR, progesterone receptor; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; TNBC, triple-negative breast cancer.
Quality Assessment
Quality of the studies was assessed independently by 2 authors while following Newcastle-Ottawa Scale criteria for selection, comparability, and outcome. Consensus scores for each study are listed in the Data Supplement. Overall, 33 studies scored good for quality, with 3 stars in the selection domain, 2 stars in the comparability domain, and 2 stars in the outcome/exposure domain. One study scored fair for quality, with 2 stars in the selection domain.36
Data Extraction
For the articles included in the study, data were independently extracted by 2 investigators (A.K. and N.P.) for cohort number, ER/PR and HER2 expression reported by immunohistochemistry (IHC) while following standard guidelines given by ASCO-College of American Pathologists (CAP); TNBC and non-TNBC; number of patients; age at incidence; grade; and lymph node involvement. Extracted data were confirmed for each study (M.K).
In total, 34 studies reported the cohort numbers for patients with breast cancer with the molecular subtypes (Data Supplement). These studies were screened for their assessment of ER/PR status. According to 2010 ASCO-CAP guidelines, > 1% ER/PR expression is considered positive.56 Before these guidelines, > 5%-10% ER/PR expression was set as a cutoff for ER/PR positivity.57 On the basis of the reports, we observed that it has taken time to implement the guidelines across India, and a few articles published later than 2010 still followed the older guidelines. To assess the impact of this variability in defining TNBC status, we evaluated each study with respect to the ER expression cutoff used. First, all the studies that followed ASCO-CAP guidelines were evaluated here. The studies were segregated into 3 subgroups as follows: the ones that used 1% expression as cutoff to define ER/PR positivity (subgroup 1; n = 15), the ones that used 5%-10% expression as cutoff (subgroup 2; n = 5), and the ones that did not mention the percent expression cutoff or did not refer to the year of ASCO-CAP guidelines but did refer to ASCO-CAP guidelines (subgroup 3; n = 14).
Method for HER2 positivity assessment was also screened. All the studies evaluated here referred to the ASCO-CAP guidelines to determine HER2 positivity. The studies in subgroups 1 and 2 either referred to ASCO-CAP 2007 guidelines (n = 5) or determined HER2 positivity with HER2 IHC scores of 3+ or 2+ with positive fluorescence in situ hybridization (FISH; n = 14). The studies from subgroup 3 referred to ASCO-CAP guidelines without referring to the year, except for 3 studies that defined HER2 positivity with HER2 IHC scores of 3+ or 2+ with positive FISH.
Twenty-three of 39 studies reported age at incidence either as mean or as grouped by younger and older age. Mean age at incidence was reported by 12 studies, and 8 studies reported age-groups for patients with or without TNBC. Fourteen of 34 studies reported grade and lymph node status for patients with and without TNBC. In these 14 studies, grade was grouped into low (grade 1 and 2) and high (grade 3) categories. Lymph node involvement was referred to as positive when ≥ 1 lymph nodes were reported to be involved on the basis of a pathologic report, as mentioned in the studies.
Statistical Analysis
The reported number of patients with TNBC within the breast cancer total cohort of a given study was used to calculate TNBC proportions. These proportions were logit transformed to calculate effect size and study weight. Individual effect sizes and study weights were then pooled in fixed- and random-effects models and back transformed to proportions. The analysis was done on the basis of the inverse variance method, using the DerSimonian-Laird estimator for τ2 and Clopper-Pearson CI as described in Wang et al.58 Data were analyzed using the metafor (2.1.0) package59 in R version 3.6.160 for Windows (Microsoft Corporation, Redmond, WA).
Moderator analysis allowed us to test for sources of heterogeneity in the pooled analysis. We used the reported IHC cutoff for ER positivity as the moderator for subgroup analysis. The 3 subgroups created for ER positivity were those described in the Data Extraction section. These subgroups were analyzed individually using a random-effects model, which assumed a difference between study variance (τ2) across subgroups. Finally, the subgroups were pooled using a mixed-effects model, and the moderator effect was assessed by Wald test.
The statistical significance for the difference in mean age at incidence for 12 studies was analyzed using t test. Of those 12 studies, 9 reported a mean age with either standard deviation, significance values, or CIs. Standard deviation was computed for all significance values or CIs. These data were pooled and a meta-analysis performed for the continuous data. Mean differences in the mean age at incidences between TNBC and non-TNBC for the 12 studies were plotted in a forest plot with both the random-effects and the fixed-effects models because the heterogeneity (I2) was close to 50%.
For meta-analysis of categorical data, namely binned age at incidence, high grade (grade 3), and lymph node positivity within TNBC and non-TNBC cohorts, pooled odds ratios (ORs) of the binary outcomes were estimated. When the heterogeneity index (I2) was > 50% and/or significant (P < .05), the DerSimonian-Laird method was used for the pooled estimate of the ORs using the random-effects model. Otherwise, the Mantel-Haenszel method was used to obtain the fixed-effects model of the pooled ORs. Data were analyzed using the meta package in R.61 Study outliers are assessed by visual inspection of the funnel plot for OR against SE. Data were re-analyzed and forest plots replotted after removal of the outliers to examine outlier effect.
RESULTS
To assess the variability and the source of variability in the prevalence rates of TNBC in Indian patients with breast cancer, we selected and curated studies in breast cancer cohorts of Indian and/or Indian-origin patients (Fig 1). A systematic review of 34 such research articles published during 2009-2019 that reported incidence numbers and clinical parameters of patients with breast cancer according to molecular subtype is compiled in the Data Supplement. The average cohort size was 608 patients (range, 72-5,436 patients), with a total number of 20,678 patients. The meta-analysis was performed for prevalence of TNBC. Clinical parameters associated with the aggressive characteristic of the disease, such as age at incidence, high grade, and lymph node positivity, were compared between TNBC and non-TNBC using summary OR with 95% CIs.
Prevalence of TNBC Within Indian Cohorts
Pooled prevalence of TNBC for all 34 studies was 27% (95% CI, 24% to 31%; Fig 2). The lowest prevalence observed was 7%12 and 11.8%,62 while the highest prevalence was 47%14 and 50%40 (Fig 2). Thus, significantly high heterogeneity was observed among the 34 studies (I2 = 95.1%), with > 50% of the studies falling outside the CIs (Data Supplement).
FIG 2.
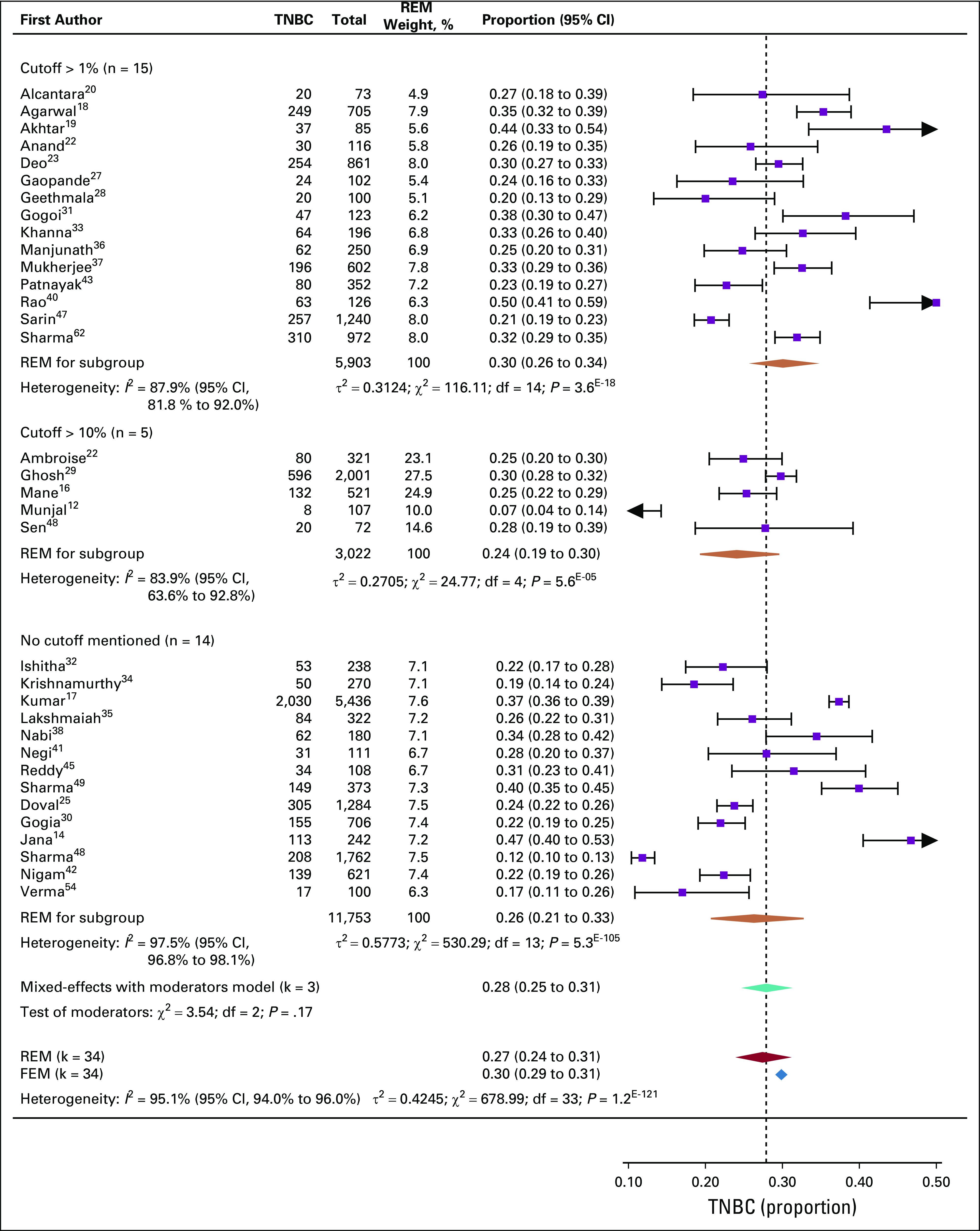
Prevalence of triple-negative breast cancer (TNBC) in Indian cohorts. Forest plot of prevalence (%) of TNBC within 34 studies that reported data on Indian patients with breast cancer. Subgroups were made by guidelines followed for estrogen receptor (ER)/progesterone receptor (PR) positivity: studies that used 1% expression of ER/PR as a cutoff, 10% expression of ER/PR as a cutoff, or studies that did not mention the ER/PR expression criteria. Heterogeneity (I2) is noted to be significantly high for each subgroup as well as for all 34 studies together. Combined pooled prevalence for all 34 studies estimated by a random-effects model (REM) is shown in purple. The fixed-effects estimate is also shown. Pooled prevalence for each subgroup computed with random effects is shown as orange diamonds. The subgroup variances are pooled together in a mixed-effects model (FEM; teal diamond). Intergroup variance in pooled prevalence is tested by Wald test, and test of moderators are not significant. The spread of each diamond represents the 95% CIs for the pooled estimate. The dotted line represents the proportion calculated from the mixed-effects model with moderators.
In an attempt to understand the source of heterogeneity, we tested whether different ER expression cutoffs used to define ER positivity had any influence on the TNBC prevalence. Accordingly, the 34 studies were grouped into 3 subgroups. Heterogeneity analysis was computed for TNBC prevalence for each subgroup independently (Data Supplement). All 3 subgroups had high heterogeneity. The highest heterogeneity was observed for subgroup 3, where the studies did not mention a cutoff for ER/PR positivity. Combined prevalence was computed for each subgroup using a random-effects model. Subgroup 1, which used 1% ER expression as cutoff, showed a higher prevalence of TNBC (30%; 95% CI, 26% to 34%) compared with subgroup 2, which used 5%-10% ER expression as the cutoff (24%; 95% CI, 19% to 30%; Fig 2). Subgroup 3 (no mention of cutoff) showed a prevalence of 26% (95% CI, 21% to 33%). Among subgroups, prevalence variation was not significant, as revealed by the test of moderators, indicating that the heterogeneity within subgroups as well as in all 34 studies may be due to factors other than ER expression cutoff used.
Age at Incidence
Twenty of the 34 studies reported age at incidence for patients with and without TNBC in the cohort. Mean age at incidence for TNBC was 47.52 ± 3 years, which is significantly younger than that for non-TNBC (51.02 ± 2.4; P = 0.005; n = 12; Data Supplement). In 9 of 12 studies, mean difference in the mean age at incidence between TNBC and non-TNBC was plotted as a continuous forest plot using a fixed-effects model. A weighted mean difference of −2.75 (95% CI, −3.59 to −1.92) significantly favored younger age at incidence for TNBC (Fig 3A). The studies were highly homogeneous, as observed in the funnel plot shown in Data Supplement.
FIG 3.
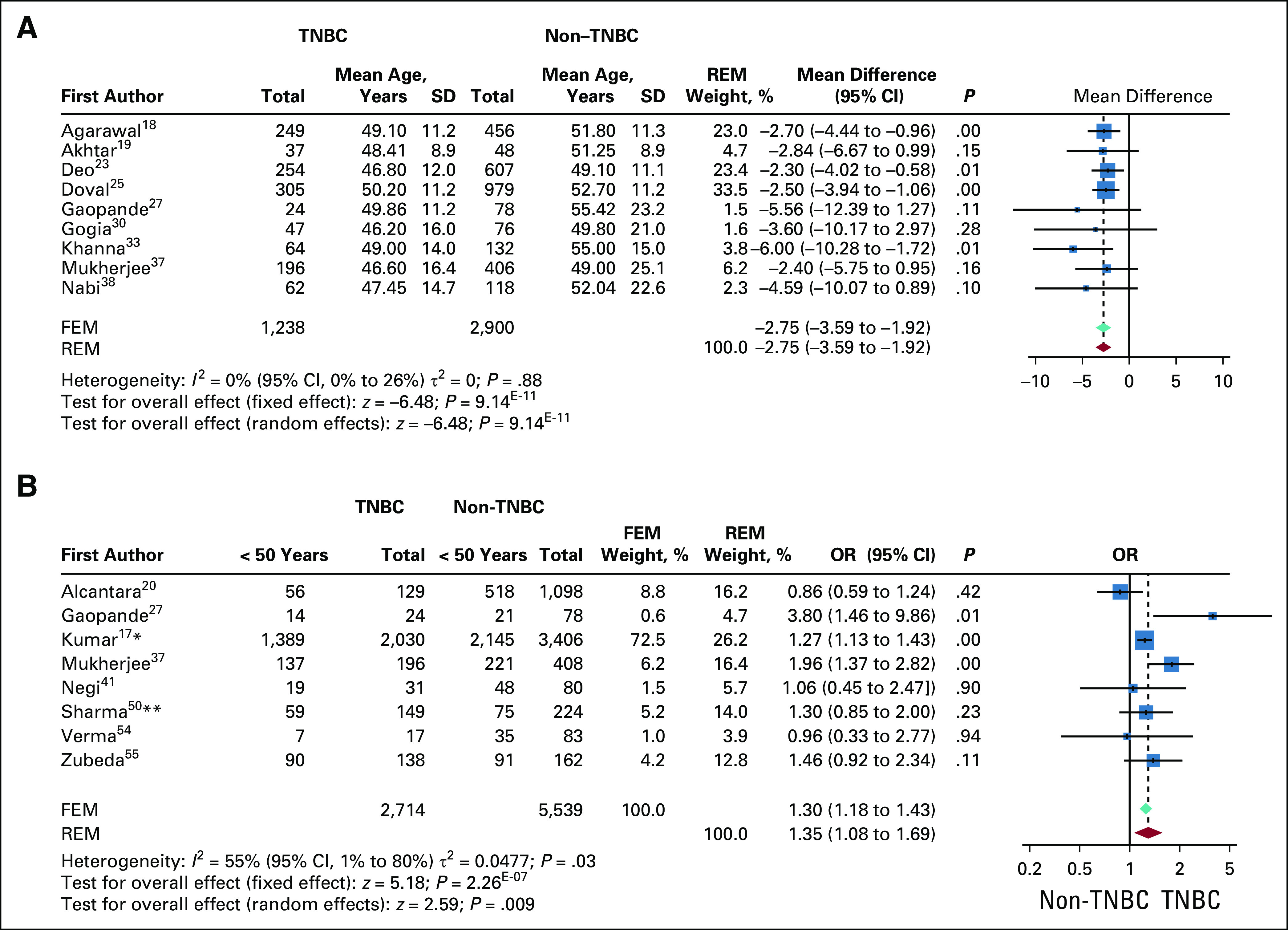
Age at incidence compared for triple-negative breast cancer (TNBC) and non-TNBC. (A) Forest plot for weighted mean difference in mean age at incidence between TNBC and non-TNBC for 9 studies. Studies were highly homogenous, with significant odds for younger age at incidence in patients with TNBC. The P value for comparison within each study is also shown. The red diamond and dotted line represent the random-effects pooled estimate of the weighted mean difference in age at incidence between patients with and without TNBC, while the teal diamond represents the fixed-effects model (FEM). (B) Binned age at incidence. The pooled odds ratio (OR) is computed by both FEM and random-effects model (REM) because the heterogeneity among 8 studies was found to be close to 50%. The pooled OR of the FEM is represented by a teal diamond, and the REM is represented by a red diamond. The dotted line represents the OR of the REM. OR of individual studies are represented by blue boxes. The boxes are weighted by the study weights in the REM. The P value for each study is mentioned. (*)The bins represent a cutoff of 55 years instead of 50 years. (**)Cutoff of 40 years. SD, standard deviation.
For the studies that reported age at incidence in bins of < 50 years and > 50 years, the ORs for incidence at < 50 years were computed and plotted as a dichotomous forest plot (n = 8). Study heterogeneity was observed to be 55%; hence, both random- and fixed-effects models were used. Both models showed significantly higher odds for age < 50 years for incidence of TNBC compared with non-TNBC (pooled OR, 1.35; 95% CI, 1.08 to 1.69) in the random-effects model (Fig 3B). The studies are more or less homogeneous as observed in the funnel plot (Data Supplement). Either as mean age or grouped age, TNBC in Indian cohorts seem to consistently present at a significantly younger age than non-TNBC.
Tumor Grade
Higher histologic grade of tumor at incidence is a significant predictor of poor prognosis. Patients with TNBC were more likely to have higher histologic tumor grade than those without TNBC.63,64 We compared grade records for TNBC versus non-TNBC within the Indian cohorts. Fourteen of the 34 studies had grade records for patients with and without TNBC. We compared the ORs for the proportion of grade 3 within TNBC with respect to grade 3 within non-TNBC (Fig 4A). The heterogeneity analysis using funnel plot was significantly high with 3 outliers (Data Supplement). The outliers were removed and ORs pooled and re-analyzed for the 11 studies. Heterogeneity within the 11 studies was < 50%; hence, the forest plot for ORs was plotted using the fixed-effects model. For these 11 studies, patients with TNBC had a 2.57 times higher odds of presenting with high-grade (grade 3) disease compared with those without TNBC, with a highly significant overall effect (Fig 4B).
FIG 4.
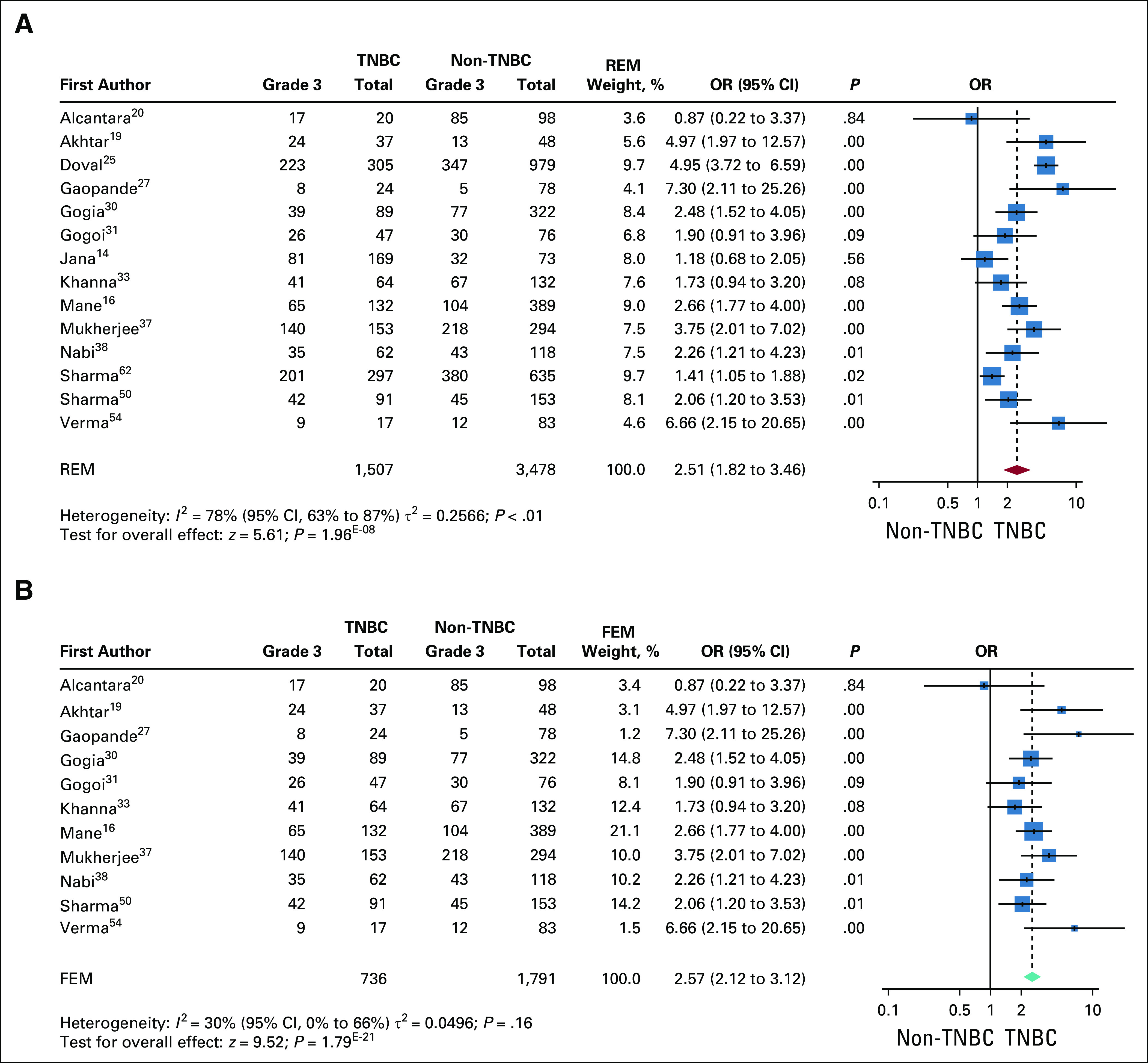
Comparison of high grade at incidence between triple-negative breast cancer (TNBC) and non-TNBC. (A) Forest plot of odds ratio (OR) for high grade in TNBC v non-TNBC for 14 studies. Studies were significantly heterogenous and showed higher ORs for high grade at incidence in TNBC. The P value for comparison within each study is also shown. The red diamond and the dotted line represent the random effects pooled estimate of the OR, while the teal diamond represents the fixed-effects model (FEM). (B) Forest plot of ORs for high grade after removal of outliers. The analysis shows 3 studies (Doval et al,25 Sharma et al,49 and Jana et al14) to be outliers in the pooled OR (Data Supplement). These studies were excluded and the pooled OR recomputed. Because the heterogeneity among the remaining the 10 studies is reduced (I2 = 30%), the fixed-effects estimate of the pooled OR is shown (green diamond). The pooled OR shows a significantly increased OR for high grade in TNBC v non-TNBC. FEM, fixed-effects model; REM, random-effects model.
Lymph Node Positivity
Tumor metastasis to axillary lymph nodes is one of the prognostic factors for breast cancer recurrence.65 We analyzed Indian cohorts for any difference in proportions of lymph node positivity between TNBC and non-TNBC at presentation. Fourteen of 34 studies reported data on lymph node status for patients with and without TNBC. The pooled data for lymph node positivity in TNBC and non-TNBC showed high heterogeneity and were therefore analyzed by random-effects model. The pooled OR favored lymph node positivity in TNBC (OR, 1.35; 95% CI, 0.94 to 1.92), but the overall effect was not significant (Fig 5A). Visual inspection of the funnel plot for these data showed 3 outliers.18,31,37 Re-analysis of the pooled data from the remaining 11 studies after removal of the outliers showed a marked decrease in heterogeneity. The pooled OR showed that lymph node positivity was favored in TNBC over non-TNBC (OR, 1.39; 95% CI, 1.21 to 1.60), with a significant overall effect (Fig 5B).
FIG 5.
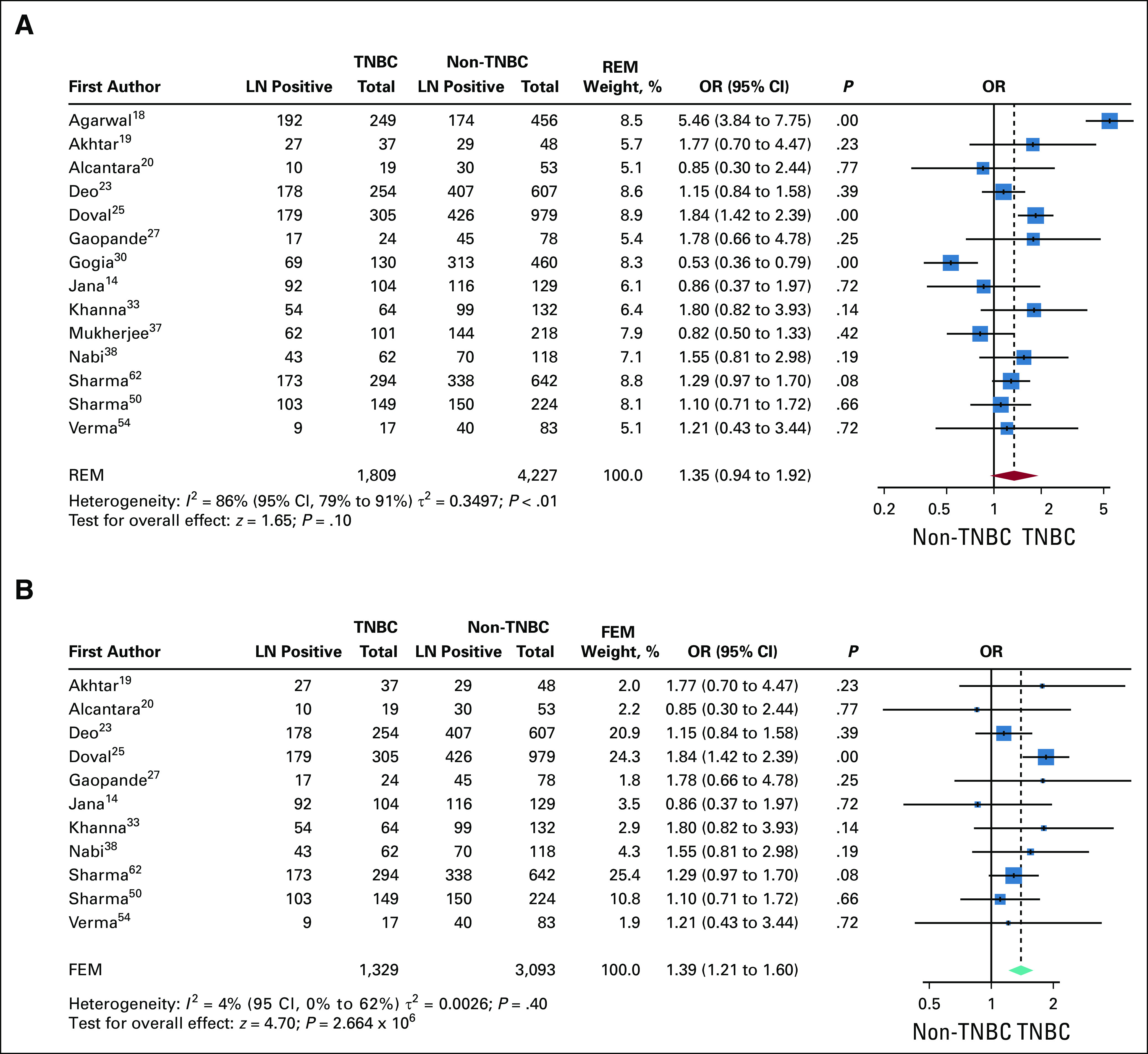
Comparison of lymph node (LN) positivity at incidence between triple-negative breast cancer (TNBC) and non-TNBC. (A) Forest plot of odds ratios (ORs) of LN positivity between TNBC and non-TNBC in 14 studies. Studies were heterogenous, and increased ORs for LN positivity in TNBC were not significant except for Doval et al.25 The pooled OR is also not significant. The P value for comparison within each study is also shown. The red diamond and dotted line represent the random effects pooled estimate of the OR. (B) Comparison of LN-positive incidence after removal of outliers. Funnel plot analysis shows that 3 studies (Gogoi et al,31 Mukherjee et al,37 and Agarwal et al18) were outliers of the pooled OR (Data Supplement). These studies were excluded and the pooled OR recomputed. Because the heterogeneity among the remaining 11 studies is low (I2 = 4%), the fixed-effects estimate of the pooled OR was computed (teal diamond). The overall effect without the outliers shows a significantly higher OR for LN positivity in TNBC v non-TNBC. FEM, fixed-effects model; REM, random-effects model.
DISCUSSION
To our knowledge, this systematic review of 34 studies is one of a kind, covering the highest number of peer-reviewed articles published so far for Indian patients with breast cancer, and for the first time, a possible source of heterogeneity observed in the prevalence of TNBC and OR of clinical features at presentation within Indian cohorts has been systematically explored. Pooled prevalence from all 34 studies was found to be high at 27% (95% CI, 24% to 31%), with high variation (range, 7%-50%). The reviews published in an earlier report indicated similar variation, although with a fewer number of articles.7,8 We reason that the differences in defining ER positivity may have contributed to the variation in prevalence. Revised ASCO-CAP guidelines for ER/PR positivity were published in 2010, yet we did encounter studies published as late as 201516 that followed the 10% ER expression cutoff as a guideline to call out ER-positive breast cancer. Half of the studies did not mention the cutoff they used to define ER positivity. Irrespective of the guidelines followed, all the subgroups presented with high heterogeneity as well as variation in TNBC prevalence.
Thirty percent prevalence in the subgroup with 1% cutoff for ER/PR positivity is alarmingly high, as pointed out earlier.8 Significantly high heterogeneity within this subgroup may indicate inconsistent IHC diagnostic methods. It is highly likely that factors like uneven tissue fixation, inadequate retrieval methods, and use of different and/or unconventional antibodies contributed to lower expression of ER and hence, high TNBC prevalence (reviewed in Shet9). Similar to ER-positive selection criteria, some of the studies used HER2 score with no reference to FISH. Definitions for both ER/PR and HER2 positivity are determining factors in defining TNBC. Inconsistent methods as well as variation in detection methods across centers may have contributed to false-negative reporting of ER/PR and HER2 expression, especially for tumors that inherently express these receptors at low levels. Of the 34 studies reviewed here, 1 reported that with the improvement in the detection methods and protocol over a period of 6 years, the percentage of patients with TNBC reduced from 40% to 26% in its cohort.17
Our meta-analysis reveals that the variations in tissue processing and detection protocols and/or standards as well the lack of reporting thereof (eg, subgroup 3 in our analysis) might be important and major contributing factors toward the observed variation in TNBC prevalence in Indian cohorts. An analysis of SEER data in the United States after 1988 showed that women of Asian Indian/Pakistani origin had significantly higher rates of ER/PR-negative cancer than White women (30.6% v 21.8%; P = .0095).66 This cohort may be considered as representative of a population of Indian immigrants and their descendants in the United States.67 Because this data set did not include HER2 status, ER/PR-negative cancers represent both HER2 and TNBC. This cohort was found to be significantly younger (< 50 years of age) and presented with higher-grade cancer. More recently, Plasilova et al6 analyzed updated data from SEER, which included HER2 by IHC and FISH. In detailed race analysis of cancer subtypes, the authors reported subtype data for Asian Indians. The proportion of TNBC in the Asian Indian group (15.4%) was found to be significantly higher than in the White group (11.6%; P = .0003), and the second highest among all the races reported. Although significantly higher, the TNBC incidence rate for the Asian Indian population (15.6%) is substantially lower than the pooled TNBC prevalence of 27% shown in Figure 2. Even with greater standardization of diagnostic procedures and reporting, the TNBC rate in a biased Indian-origin subpopulation is high compared with White rates. This reinforces the conclusion that TNBC rates in an Indian/Indian-origin population are intrinsically high; however, some of the observed increase in our meta-analysis may stem from diagnostic limitations across the country.
With this backdrop, it will be interesting to systematically review the IHC protocols used in Indian centers as a source of variation. High TNBC in Indian cohorts may be partly due to a lack of standardized protocols and stringent guidelines in diagnostic centers leading to inadequate assessment of hormone receptor expression. Moving forward, standardized protocols with unified and stringent guidelines are essential in the Indian setting for accurate assessment of hormone receptor expression in breast cancers. In the meantime, performance of PAM50 on select and representative cohorts may give a true assessment of TNBC proportions in Indian cohorts.68 The difference between the biologically high TNBC prevalence and the observed TNBC prevalence may become clearer as health care standards improve across the country.
Regardless of high prevalence and variation in the prevalence, the clinical features that TNBC presents within Indian cohorts tend to be aggressive compared with non-TNBC, similar to the observations in western cohorts.6 All the studies reviewed here reported that patients with TNBC are significantly younger and that significantly higher proportions have high-grade tumors compared with non-TNBC (except for Alcantara et al20), with studies being highly homogeneous. For lymph node positivity, heterogeneity was low (after removal of outliers), and all the studies except 2 (Alcantara et al and Jana et al14) showed higher odds for TNBC to present with lymph node positivity. The pooled OR is significantly in favor of TNBC after the removal of outliers.
In summary, the systematic review of Indian cohorts once again reflects a higher prevalence of TNBC within Indian cohorts. Indian patients with TNBC presented at a younger age compared with those without TNBC and with a significantly higher proportion of aggressive clinical features, such as high grade and lymph node positivity. In India, TNBC with its higher prevalence and clinically aggressive features needs focus and consistent efforts to identify an appropriate and effective treatment regimen to tackle this clinically challenging disease. Before that, however, India needs to standardize the detection methods that identify this aggressive subtype of breast cancer with accuracy.
SUPPORT
Supported by Science and Engineering Research Board JC Bose Fellowship (L.S.S.); research grant from Bajaj Auto Limited (C.B.K.); and Department of Biotechnology, Ministry of Science and Technology, Ramalingaswami Re-entry Fellowship (M.K.).
AUTHOR CONTRIBUTIONS
Conception and design: Lingadahalli S. Shashidhara, Chaitanyanand B. Koppiker, Madhura Kulkarni
Financial support: Lingadahalli S. Shashidhara, Chaitanyanand B. Koppiker, Madhura Kulkarni
Administrative support: Lingadahalli S. Shashidhara, Chaitanyanand B. Koppiker, Madhura Kulkarni
Collection and assembly of data: Apurv Kulkarni, Nidhi Parikh, Madhura Kulkarni
Data analysis and interpretation: Apurv Kulkarni, Devaki A. Kelkar, Madhura Kulkarni
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.3322/caac.21492. Bray F, Ferlay J, Soerjomataram I, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018. [DOI] [PubMed]
- 2.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Cronin KA, Kurian AW, et al. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27:619–626. doi: 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plasilova ML, Hayse B, Killelea BK, et al: Features of triple-negative breast cancer: Analysis of 38,813 cases from the National Cancer Database. Medicine (Baltimore) 95:e4614, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandhu GS, Erqou S, Patterson H, et al. Prevalence of triple-negative breast cancer in India: Systematic review and meta-analysis. J Glob Oncol. 2016;2:412–421. doi: 10.1200/JGO.2016.005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Singh R, Gupta S, Pawar SB, Pawar RS, Gandham SV, Prabhudesai S. Evaluation of ER, PR and HER-2 receptor expression in breast cancer patients presenting to a semi urban cancer centre in Western India. J Cancer Res Ther. 2014;10:26–28. doi: 10.4103/0973-1482.131348. [DOI] [PubMed] [Google Scholar]
- 8.Thakur KK, Bordoloi D, Kunnumakkara AB. Alarming burden of triple-negative breast cancer in India. Clin Breast Cancer. 2018;18:e393–e399. doi: 10.1016/j.clbc.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Shet T. Improving accuracy of breast cancer biomarker testing in India. Indian J Med Res. 2017;146:449–458. doi: 10.4103/ijmr.IJMR_896_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee S, Arun I, Agrawal S, et al. Immunohistochemistry heterogeneity in reported breast cancer demographics from India: Triple-negative breast cancer rates could be lower than suggested in pooled meta-analysis. J Glob Oncol. 2016;3:180–181. doi: 10.1200/JGO.2016.006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandhu GS, Erqou S, Patterson H, et al. Hormone receptor status (estrogen receptor, progesterone receptor), human epidermal growth factor-2 and p53 in South Indian breast cancer patients: A tertiary care center experience. Asian Pac J Cancer Prev. 2017;7:95. doi: 10.4103/0971-5851.158844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munjal K, Ambaye A, Evans MF, et al: Immunohistochemical analysis of ER, PR, HER2 and CK5/6 in infiltrative breast carcinomas in Indian patients. Asian Pac J Cancer Prev 10:773-778, 2009. [PubMed] [Google Scholar]
- 13.Sharma P, Klemp JR, Kimler BF, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: Implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat. 2014;145:707–714. doi: 10.1007/s10549-014-2980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jana D, Sarkar DK, Ganguly S, et al. Can molecular subtyping replace axillary nodal status as prognostic marker in breast cancer? Indian J Surg Oncol. 2014;5:282–289. doi: 10.1007/s13193-014-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao C, Shetty J, Prasad KH. Immunohistochemical profile and morphology in triple - negative breast cancers. J Clin Diagn Res. 2013;7:1361–1365. doi: 10.7860/JCDR/2013/5823.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mane A, Khatib KI, Deshmukh SP, et al. A comparison of clinical features, pathology and outcomes in various subtypes of breast cancer in Indian women. J Clin Diagn Res. 2015;9:PC01–PC04. doi: 10.7860/JCDR/2015/15253.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar RV, Panwar D, Amirtham U, et al. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 status in breast cancer: A retrospective study of 5436 women from a regional cancer center in South India. South Asian J Cancer. 2018;7:7–10. doi: 10.4103/sajc.sajc_211_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal G, Nanda G, Lal P, et al. Outcomes of triple-negative breast cancers (TNBC) compared with non-TNBC: Does the survival vary for all stages? World J Surg. 2016;40:1362–1372. doi: 10.1007/s00268-016-3422-4. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar M, Dasgupta S, Rangwala M. Triple negative breast cancer: An Indian perspective. Breast Cancer (Dove Med Press) 2015;7:239–243. doi: 10.2147/BCTT.S85442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcantara VS, Lim GH, Lim SH, et al. Incidence and prognosis of non-metastatic triple negative breast cancer (TNBC) among different races in Southeast Asia. J Surg Oncol. 2017;115:523–537. doi: 10.1002/jso.24559. [DOI] [PubMed] [Google Scholar]
- 21. Ambroise M, Ghosh M, Mallikarjuna VS, et al: Immunohistochemical profile of breast cancer patients at a tertiary care hospital in South India. Asian Pac J Cancer Prev 12:625-629, 2011. [PubMed]
- 22.Anand A, Singh KR, Kumar S, et al. Androgen receptor expression in an Indian breast cancer cohort with relation to molecular subtypes and response to neoadjuvant chemotherapy - A prospective clinical study. Breast Care (Basel) 2017;12:160–164. doi: 10.1159/000458433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deo SVS, Shukla NK, Gogia A, et al. A comparative study of clinical profile and relapse patterns in triple-negative and non-triple-negative breast cancer patients treated with curative intent. Indian J Surg Oncol. 2017;8:291–297. doi: 10.1007/s13193-017-0634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dogra A, Doval DC, Sardana M, et al. Clinicopathological characteristics of triple negative breast cancer at a tertiary care hospital in India. Asian Pac J Cancer Prev. 2014;15:10577–10583. doi: 10.7314/apjcp.2014.15.24.10577. [DOI] [PubMed] [Google Scholar]
- 25.Doval DC, Sharma A, Sinha R, et al. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in New Delhi, India. Asian Pac J Cancer Prev. 2015;16:4959–4964. doi: 10.7314/apjcp.2015.16.12.4959. [DOI] [PubMed] [Google Scholar]
- 26.Chandra D, Suresh P, Sinha R, et al. Eight year survival analysis of patients with triple negative breast cancer in India. Asian Pac J Cancer Prev. 2016;17:2995–2999. [PubMed] [Google Scholar]
- 27.Gaopande V, Joshi S, Kulkarni M, et al. A clinicopathologic study of triple negative breast cancer. J Sci Soc. 2015;42:12. [Google Scholar]
- 28. Geethamala K, Murthy VS, Vani BR, et al: Hormone receptor expression in breast carcinoma at our hospital: An experience. Clin Cancer Investig J 4:511-515, 2015.
- 29.Ghosh J, Gupta S, Desai S, et al. Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer. 2011;48:391–396. doi: 10.4103/0019-509X.92245. [DOI] [PubMed] [Google Scholar]
- 30. Gogia A, Raina V, Deo SVS, et al: Triple-negative breast cancer: An institutional analysis. Indian J Cancer 51:163-166, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Gogoi G, Borgohain M, Saikia P, et al. Profile of molecular subtypes of breast cancer with special reference to triple negative: A study from Northeast India. Clin Cancer Investig J. 2016;5:374. [Google Scholar]
- 32.Ishitha G, Manipadam MT, Backianathan S, et al. Clinicopathological study of triple negative breast cancers. J Clin Diagn Res. 2016;10:EC05–EC09. doi: 10.7860/JCDR/2016/20475.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanna R, Meena RN, Bansal A, et al. Triple negative breast cancer: Experience from a North Indian tertiary care center. Indian J Surg. 2018;80:474–478. [Google Scholar]
- 34.Krishnamurthy S, Poornima R, Challa VR, et al. Triple negative breast cancer - Our experience and review. Indian J Surg Oncol. 2012;3:12–16. doi: 10.1007/s13193-012-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakshmaiah KC, Das U, Suresh TM, et al. A study of triple negative breast cancer at a tertiary cancer care center in southern India. Ann Med Health Sci Res. 2014;4:933–937. doi: 10.4103/2141-9248.144917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manjunath S, Prabhu JS, Kaluve R, et al. Estrogen receptor negative breast cancer in India: Do we really have higher burden of this subtype? Indian J Surg Oncol. 2011;2:122–125. doi: 10.1007/s13193-011-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mukherjee G, Lakshmaiah KC, Vijayakumar M, et al: Analysis of clinico-pathological characteristics of Indian breast cancers shows conservation of specific features in the hormone receptor sub-types. J Integr Oncol 5:1000159, 2016.
- 38.Nabi MG, Ahangar A, Wahid MA, et al. Clinicopathological comparison of triple negative breast cancers with non-triple negative breast cancers in a hospital in North India. Niger J Clin Pract. 2015;18:381–386. doi: 10.4103/1119-3077.153248. [DOI] [PubMed] [Google Scholar]
- 39.Nandi M, Mahata A, Mallick I, et al. Hypofractionated radiotherapy for breast cancers--Preliminary results from a tertiary care center in eastern India. Asian Pac J Cancer Prev. 2014;15:2505–2510. doi: 10.7314/apjcp.2014.15.6.2505. [DOI] [PubMed] [Google Scholar]
- 40.Rao C, Shetty J, Kishan Prasad HL. Morphological profile and receptor status in breast carcinoma: An institutional study. J Cancer Res Ther. 2013;9:44–49. doi: 10.4103/0973-1482.110358. [DOI] [PubMed] [Google Scholar]
- 41.Negi M, Kumar S, Devi S, et al. Profiling estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2/neu in breast carcinoma: Study of 111 consecutive cases. J Sci Soc. 2018;45:13. [Google Scholar]
- 42.Nigam JS, Yadav P, Sood N. A retrospective study of clinico-pathological spectrum of carcinoma breast in a West Delhi, India. South Asian J Cancer. 2014;3:179–181. doi: 10.4103/2278-330X.136804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patnayak R, Jena A, Rukmangadha N, et al. Hormone receptor status (estrogen receptor, progesterone receptor), human epidermal growth factor-2 and p53 in South Indian breast cancer patients: A tertiary care center experience. Indian J Med Paediatr Oncol. 2015;36:117–122. doi: 10.4103/0971-5851.158844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rangarajan B, Shet T, Wadasadawala T, et al: Breast cancer: An overview of published Indian data. South Asian J Cancer 5:86-92, 2016. [DOI] [PMC free article] [PubMed]
- 45.Reddy GM, Suresh PK, Pai RR. Clinicopathological features of triple negative breast carcinoma. J Clin Diagn Res. 2017;11:EC05–EC08. doi: 10.7860/JCDR/2017/21452.9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sable M, Pai TD, Shet T, et al. Triple-negative breast cancer: A comprehensive study of clinical, histomorphological, and immunohistochemical features in Indian patients. Int J Surg Pathol. 2017;25:230–237. doi: 10.1177/1066896916667815. [DOI] [PubMed] [Google Scholar]
- 47.Sarin R, Khandrika L, Hanitha R, et al. Epidemiological and survival analysis of triple-negative breast cancer cases in a retrospective multicenter study. Indian J Cancer. 2016;53:353–359. doi: 10.4103/0019-509X.200682. [DOI] [PubMed] [Google Scholar]
- 48. Sen S, Gayen R, Das S, et al: A clinical and pathological study of triple negative breast carcinoma: Experience of a tertiary care centre in eastern India. J Indian Med Assoc 110:686-689, 705, 2012. [PubMed]
- 49. doi: 10.4103/0019-509X.123616. Sharma B, Kalwar SA, Sharma N, et al: Five year retrospective survival analysis of triple negative breast cancer in North-West India. Indian J Cancer 50:330-332, 2013. [DOI] [PubMed]
- 50.Sharma D, Singh G. An institutional analysis of clinicopathological features of triple negative breast cancer. Indian J Cancer. 2016;53:566–568. doi: 10.4103/ijc.IJC_534_16. [DOI] [PubMed] [Google Scholar]
- 51.Shet T, Agrawal A, Nadkarni M, et al. Hormone receptors over the last 8 years in a cancer referral center in India: What was and what is? Indian J Pathol Microbiol. 2009;52:171–174. doi: 10.4103/0377-4929.48909. [DOI] [PubMed] [Google Scholar]
- 52.Sultana R, Kataki AC, Borthakur BB, et al. Imbalance in leptin-adiponectin levels and leptin receptor expression as chief contributors to triple negative breast cancer progression in Northeast India. Gene. 2017;621:51–58. doi: 10.1016/j.gene.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 53.Suresh P, Batra U, Doval DC. Epidemiological and clinical profile of triple negative breast cancer at a cancer hospital in North India. Indian J Med Paediatr Oncol. 2013;34:89–95. doi: 10.4103/0971-5851.116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verma S, Bal A, Joshi K, et al. Immunohistochemical characterization of molecular subtypes of invasive breast cancer: A study from North India. APMIS. 2012;120:1008–1019. doi: 10.1111/j.1600-0463.2012.02933.x. [DOI] [PubMed] [Google Scholar]
- 55.Zubeda S, Kaipa PR, Shaik NA, et al. Her-2/neu status: A neglected marker of prognostication and management of breast cancer patients in India. Asian Pac J Cancer Prev. 2013;14:2231–2235. doi: 10.7314/apjcp.2013.14.4.2231. [DOI] [PubMed] [Google Scholar]
- 56. Hammond H, Hayes DF, Dowsett M, et al: American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134:e48-e72, 2010. [DOI] [PubMed]
- 57. doi: 10.6004/jnccn.2009.0079. Allred DC, Carlson RW, Berry DA, et al: NCCN task force report: Estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Compr Canc Netw 7:S1-S21, 2009; quiz S22-S23. [DOI] [PubMed] [Google Scholar]
- 58. Wang, N.: How to Conduct a Meta-Analysis of Proportions in R: A Comprehensive Tutorial. New York, NY, John Jay College of Criminal Justice, 0–62, 2018.
- 59.Viechtbauer W. Conducting meta-analysis in R with metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 60. R Core Team: R: A language and environment for statistical computing, 2019. https://www.r-project.org.
- 61.Swarzer G. meta: An R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 62.Sharma M, Sharma JD, Sarma A, et al. Triple negative breast cancer in people of North East India: Critical insights gained at a regional cancer centre. Asian Pac J Cancer Prev. 2014;15:4507–4511. doi: 10.7314/apjcp.2014.15.11.4507. [DOI] [PubMed] [Google Scholar]
- 63.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 64.Urru SAM, Gallus S, Bosetti C, et al. Clinical and pathological factors influencing survival in a large cohort of triple-negative breast cancer patients. BMC Cancer. 2018;18:56. doi: 10.1186/s12885-017-3969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9:606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 66. doi: 10.1186/1471-2407-10-191. Kakarala M, Rozek L, Cote M, et al: Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.—A SEER analysis. BMC Cancer 10:191, 2010. [DOI] [PMC free article] [PubMed]
- 67. Zong J, Batalova J: Indian Immigrants in the United States, 2017. migrationpolicy.org/article/indian-immigrants-united-states. [Google Scholar]
- 68.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]