Abstract
PURPOSE
Atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP) demonstrated survival benefit versus bevacizumab, carboplatin, and paclitaxel (BCP) in chemotherapy-naïve nonsquamous non–small-cell lung cancer (NSCLC). We present safety and patient-reported outcomes (PROs) to provide additional information on the relative impact of adding atezolizumab to chemotherapy with and without bevacizumab in nonsquamous NSCLC.
METHODS
Patients were randomly assigned to receive atezolizumab, carboplatin, and paclitaxel (ACP), ABCP, or BCP. Coprimary end points were overall survival and investigator-assessed progression-free survival. The incidence, nature, and severity of adverse events (AEs) were assessed. PROs, a secondary end point, were evaluated using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-Core 30 and EORTC QLQ-Lung Cancer 13.
RESULTS
Overall, 400 (ACP), 393 (ABCP), and 394 (BCP) patients were safety evaluable (ie, intention-to-treat population that received one or more doses of any study treatment). More patients had grade 3/4 treatment-related AEs during the induction versus maintenance phase (ACP, 40.5% v 8.2%; ABCP, 48.6% v 21.2%; BCP, 44.7% v 11.1%). During induction, the incidence of serious AEs (SAEs) was 28.3%, 28.5%, and 26.4% in the ACP, ABCP, and BCP arms, respectively. During maintenance, SAE incidences were 20.0%, 26.3%, and 13.0%, respectively. Completion rates of the PRO questionnaires were > 88% at baseline and remained ≥ 70% throughout most study visits. Across arms, patients on average reported no clinically meaningful worsening of global health status or physical functioning scores through cycle 13. Patients across arms rated common symptoms with chemotherapy and immunotherapy similarly.
CONCLUSION
ABCP seems tolerable and manageable versus ACP and BCP in first-line nonsquamous NSCLC. Treatment tolerability differed between induction and maintenance phases across treatment arms. PROs reflect a minimal treatment burden (eg, health-related quality of life, symptoms) with each regimen.
INTRODUCTION
Treatment options for patients with metastatic non–small-cell lung cancer (NSCLC) have expanded with the availability of immune checkpoint inhibitor therapies,1,2 particularly programmed death-ligand 1 (PD-L1) and programmed death-1 (PD-1) inhibitors.3-6 In the first-line setting, these options include anti-PD-1 monotherapy in patients with PD-L1 expression on ≥ 1% of tumor cells or combination approaches of anti-PD-L1 or anti-PD-1 with platinum doublet chemotherapy with and without pemetrexed or bevacizumab.1,7-12 Because these combinations become more prevalent in the clinical setting, a better understanding of the safety profiles of these agents within the combination backgrounds is needed. Symptoms at presentation as well as symptomatic adverse events (AEs) that arise from treatment, as part of the treatment burden, adversely affect health-related quality of life (HRQOL).13-15 It is therefore important to characterize a patient’s overall experience to determine the net benefit of treatment combination strategies and ensure that delayed tumor progression or increased survival does not come at the expense of their HRQOL.16-22
CONTEXT
Key Objective
Atezolizumab in combination bevacizumab and chemotherapy is a first-line treatment option in nonsquamous non-small-cell lung cancer (NSCLC). The safety and patient-reported outcomes (PROs) data provide complementary evidence of the tolerability of this multi-regimen therapy.
Knowledge Generated
Standardized safety reporting combined with patients’ ratings of the severity of commonly experienced treatment-related symptoms and health-related quality of life, confirm that adding immunotherapy (atezolizumab) to bevacizumab and chemotherapy in nonsquamous NSCLC is not adding significant treatment burden while improving survival.
Relevance
Provide additional patient-centric evidence to inform the tolerability of atezolizumab administered in combination with bevacizumab and chemotherapy as first-line treatment in nonsquamous NSCLC.
Atezolizumab (anti-PD-L1) has demonstrated overall survival (OS) benefit versus docetaxel in previously treated NSCLC, regardless of PD-L1 expression.3 Patient-reported outcomes (PROs) also showed improved HRQOL with atezolizumab versus docetaxel in second-line NSCLC, supporting the benefit and tolerability observed with atezolizumab versus docetaxel.23 Bevacizumab (anti–vascular endothelial growth factor [VEGF]) is an anti-angiogenic agent that also inhibits VEGF-mediated immunosuppression.24 Therefore, bevacizumab may enhance the antitumor activity of atezolizumab. Atezolizumab combined with bevacizumab, carboplatin, and paclitaxel chemotherapy (ABCP) showed progression-free survival (PFS) and OS benefit versus bevacizumab, carboplatin, and paclitaxel (BCP) chemotherapy7,25 (PFS hazard ratio, 0.59 [95% CI, 0.50 to 0.69]; OS hazard ratio, 0.76 [95% CI, 0.63 to 0.93]; data cutoff, January 22, 2018).7 The combination is approved in the United States, European Union, and other regions for the first-line treatment of metastatic nonsquamous NSCLC.26,27 The overall safety profile of ABCP was consistent with that of the individual medicines,3,28 and no new safety signals were observed.7
We further report on the safety of atezolizumab plus carboplatin and paclitaxel (ACP), ABCP, and BCP in the IMpower150 study. PROs of HRQOL, physical functioning, and treatment-related symptoms show the relative impact of these treatment regimens.
METHODS
Study Design, Patients, and Treatment
IMpower150 is a global, open-label, randomized, phase III study.7 Patients were enrolled if they had chemotherapy-naïve, metastatic, nonsquamous NSCLC. Details of the study design, patient population, and treatments were previously described7; a brief description is included in the Data Supplement.
This study was conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and all patients gave written informed consent. The protocol, informed consent forms, any information provided to patients, and relevant supporting information were reviewed and approved by the institutional review board/ethics committee.
Objective
The objective of this analysis was to evaluate the safety and tolerability of atezolizumab, as reported per standardized safety procedures and using data collected from patients on PRO rating scales for each of the two treatment comparisons (ABCP v BCP and ACP v BCP). The PRO end points aimed to capture patients’ ratings of the severity of commonly experienced symptoms with these treatments and characterize their impact on HRQOL and physical functioning.
Safety and PRO Assessments
All-cause AEs, treatment-related AEs (TRAEs; related to any study treatment or individual study treatments), and AEs of special interest (AESIs) were assessed. The incidence, nature, and severity of these events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Atezolizumab AESIs were defined as important identified risks, potential risks, and class effects that have been associated with atezolizumab and other immune checkpoint inhibitors, including immune-related AEs (irAEs) and infusion-related reactions. The irAEs were defined using Medical Dictionary for Regulatory Activities Preferred Terms that included both diagnosed immune conditions and signs and symptoms potentially representative of immune-related events, regardless of investigator-assessed causality. Post hoc safety analyses included the incidence, nature, and severity of AEs reported by treatment phase (induction v maintenance); time to onset and duration of irAEs; and TRAE rates that led to treatment discontinuation. For analysis of AEs by treatment phase, AEs that occurred during induction had an onset on or after the first study drug treatment and up to 1 day before the date of the first dose of the maintenance therapy, and AEs that occurred during maintenance had an onset on or after the first dose of maintenance therapy.
PROs were assessed using validated and reliable self-report measures: the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-Core 30 (C30)29,30 and the EORTC QLQ-Lung Cancer 13 (LC13).31 The EORTC QLQ-C30 consists of 30 questions that assess five aspects of patient functioning (physical, emotional, role, cognitive, and social), three symptom scales (fatigue, nausea and vomiting, and pain), global health/QOL, and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). The EORTC QLQ-LC13 module, which assesses the severity of lung cancer–specific symptoms, incorporates one multiple-item scale to assess dyspnea and a series of single items that assess pain, cough, sore mouth, dysphagia, peripheral neuropathy, alopecia, and hemoptysis. Questionnaires were completed at each scheduled study visit during treatment and 3 and 6 months after disease progression or until loss of clinical benefit in atezolizumab-treated patients who had continued treatment with atezolizumab after radiographic disease progression (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1). Patients recorded their answers to the PRO questionnaires before any meaningful medical discussion or test to minimize assessment bias. Completion took approximately 15 minutes per EORTC QLQ module. Sites did not have access to patients’ answers and were not expected to use PRO information in discussions with patients about their treatment or health status. Answers were recorded on an electronic device (ePRO tablet), and data were transmitted automatically to a centralized database at the ePRO vendor through a prespecified transmission method (eg, web or wireless) and could be accessed by appropriate study personnel securely through the Internet. PROs were analyzed as a change from baseline in mean scores. A ≥ 10-point score change from baseline within a patient was considered the threshold of clinically meaningful change.32 The PRO and safety data were elicited per different standardized processes; therefore, no attempt was made to reconcile the two data sets.33
Statistical Analysis
All PRO scores were derived according to developers’ guidelines. Missing scores were not imputed. Descriptive analyses were conducted on the safety population per treatment received to complement traditional safety reporting and to quantify treatment and symptom burden from the patients’ perspective. The most frequent and clinically relevant disease-related symptoms according to patients with NSCLC (eg, cough, chest pain, dyspnea) were examined in the safety population because these symptoms might not be solely attributed to tumor growth and, therefore, are part of the symptom burden. Other symptom ratings provided insight into patients’ experiences with fatigue, nausea/vomiting, constipation, diarrhea, sore mouth, and neuropathic pain. Mean changes from baseline in patient-reported HRQOL, physical functioning, treatment-related symptoms, and lung cancer‒related symptoms were analyzed descriptively at each cycle on treatment. For selected scales, changes from baseline are presented for the induction and maintenance phases. Good practices for reporting and analyzing PROs were followed.34
RESULTS
Patients and Treatment
Overall, 1,202 patients were enrolled, and 402, 400, and 400 patients were randomly assigned to receive ACP, ABCP, and BCP, respectively (Data Supplement). The data cutoff date for this analysis was January 22, 2018; the minimum duration of follow-up was 13.5 months (median, 19.6, 19.6, and 19.7 months for ACP, ABCP, and BCP, respectively).
Safety
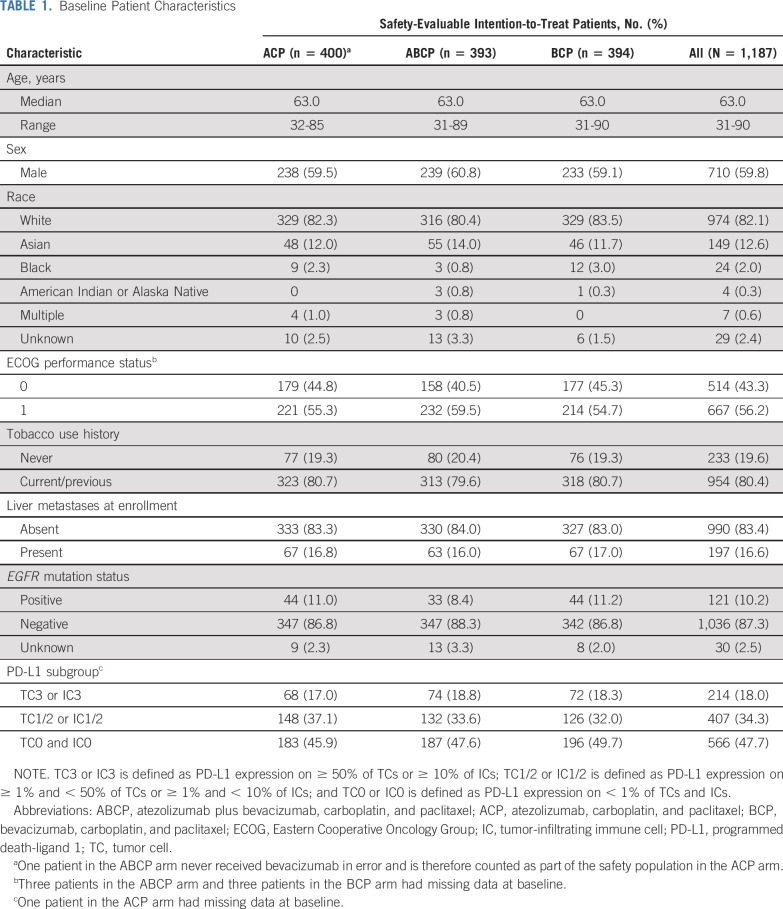
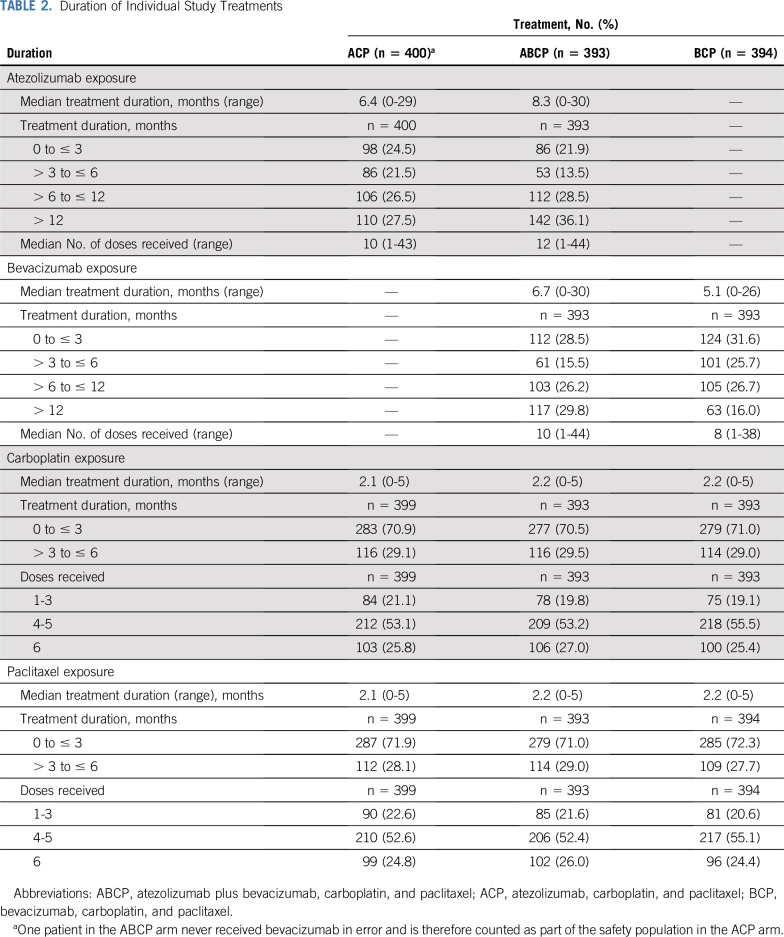
Baseline characteristics were well-balanced across treatment arms in the safety-evaluable intention-to-treat population, which included randomly assigned patients who received any amount of any component of study treatment (Table 1). For the safety analyses, patients were grouped according to whether any amount of atezolizumab was received, including when it was received in error. This population comprised 400 patients treated with ACP, 393 treated with ABCP, and 394 treated with BCP. The median duration of treatment with each individual study treatment is listed in Table 2.
TABLE 1.
Baseline Patient Characteristics
TABLE 2.
Duration of Individual Study Treatments
The incidences of all-cause AEs, TRAEs, and serious AEs (SAEs) by treatment phase are listed in Table 3. A higher incidence of grade 3/4 all-cause and TRAEs was observed during the induction versus maintenance phase across all treatment arms. While the incidence of grade 5 all-cause AEs was similar across treatment phases in all arms, the incidence of grade 5 TRAEs was higher during the induction versus maintenance phase in the ABCP arm. Incidence of grade 3/4 and grade 5 atezolizumab-related AEs was comparable across the ACP and ABCP arms (Data Supplement); a similar observation was noted for bevacizumab-related AEs in the ABCP and BCP arms.
TABLE 3.
Safety Summary by Treatment Phase
The majority of the most common AEs (≥ 20% overall incidence in any arm) reported across treatment phases were grade 1/2 (Fig 1A). Alopecia, nausea, and fatigue were the most common AEs reported during the induction phase in the ABCP arm, while alopecia, anemia, nausea, and peripheral neuropathy were the most common AEs reported during this phase in the ACP arm (Fig 1B). Hypertension was the most commonly reported AE during the maintenance phase in both the ABCP and the BCP arms, while dyspnea was the most common AE reported during this phase in the ACP arm (Fig 1C). The most common bleeding/hemorrhage AESIs with bevacizumab were epistaxis (ACP, 3.8%; ABCP, 16.8%; BCP, 22.1%), hemoptysis (ACP, 3.5%; ABCP, 6.9%; BCP, 5.1%), and hematuria (ACP, 2.8%; ABCP, 3.3%; BCP, 1.8%). The majority of these events were grade 1/2 and were consistent with the known safety profile of bevacizumab.
FIG 1.
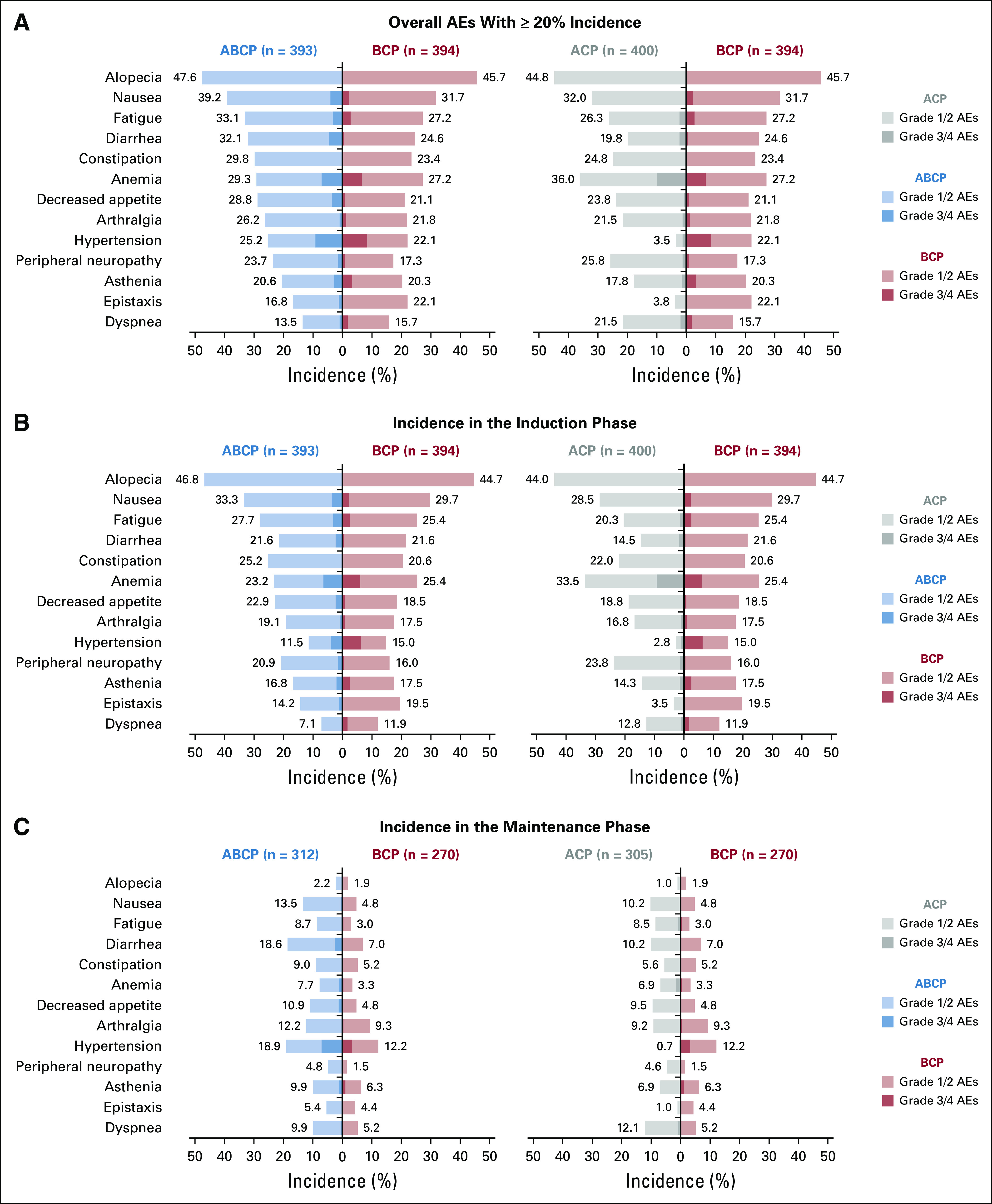
Most common adverse events (AEs) overall and by phase of treatment. (A) Incidences of the most common (≥ 20% overall incidence) AEs reported in any treatment arm. Incidences of the most common AEs in the (B) induction and (C) maintenance phases. AEs that occurred during the induction phase had an onset on or after the first study drug treatment and up to 1 day before the date of the first dose of the maintenance therapy. AEs that occurred during the maintenance phase had an onset on or after the first dose of maintenance therapy. Overall AEs represent the sum of AEs with onset during the induction phase, the maintenance phase, and/or the AE reporting period. The AE reporting period includes those patients who discontinued induction therapy, never received maintenance therapy, and had an AE with onset > 30 days after the last dose of study treatment and during the AE reporting period as defined in the protocol. ABCP, atezolizumab plus bevacizumab, carboplatin, and paclitaxel; ACP, atezolizumab, carboplatin, and paclitaxel; BCP, bevacizumab, carboplatin, and paclitaxel.
The incidence of SAEs was higher during the induction versus maintenance phase across all arms (Table 3). During induction, the incidence of SAEs was 28.3%, 28.5%, and 26.4% in the ACP, ABCP, and BCP arms, respectively. During maintenance, SAE incidences were 20.0%, 26.3% and 13.0%, respectively. The most common SAEs (≥ 2% overall incidence in any arm) are shown in the Data Supplement. The rate of these SAEs decreased from the induction to the maintenance phase of treatment; however, the incidences of diarrhea, pneumonia, and pneumonitis in the ABCP arm were similar across treatment phases.
The overall incidence of AEs leading to discontinuation of any study treatment was 13.3% with ACP, 33.8% with ABCP, and 24.9% with BCP. Discontinuation of only carboplatin and paclitaxel because of AEs occurred in 3.3%, 6.1%, and 6.1% of patients in the ACP, ABCP, and BCP arms, respectively. During induction, the incidence of AEs that led to discontinuation of any study treatment was higher with ABCP (22.4%) and BCP (17.8%) than with ACP (9.5%). The most commonly reported AEs that led to any treatment discontinuation during induction (≥ 1% of patients) were peripheral sensory neuropathy (ACP, 1.0%; ABCP, 2.0%; BCP, 1.0%), peripheral neuropathy (ACP, 1.0%; ABCP, 1.8%; BCP, 0.8%), pulmonary embolism (ACP, 0%; ABCP, 1.3%; BCP, 1.3%), thrombocytopenia (ACP, 0%; ABCP, 1.0%; BCP, 1.0%), and febrile neutropenia (ACP, 0%; ABCP, 0.5%; ACP, 1.0%). During maintenance, the incidence of AEs that led to discontinuation of any treatment was higher with ABCP than with BCP or ACP. The most commonly reported events that led to any treatment discontinuation during maintenance (≥ 1% of patients) were proteinuria (ACP, 0%; ABCP, 2.9%; BCP, 2.6%), hypertension (ACP, 0%; ABCP, 1.9%; BCP, 0%), increased ALT (ACP, 0%; ABCP, 1.0%; BCP, 0%), and diarrhea (ACP, 0%; ABCP, 1.0%; BCP, 0%). Atezolizumab discontinuation because of AEs during the induction and maintenance phases occurred in 7.6% and 8.3% of patients in the ABCP arm and 4.5% and 4.6% in the ACP arm, respectively. The most common event that led to atezolizumab discontinuation (≥ 1% of patients) was pneumonitis in both the induction (ACP, 1.0%; ABCP, 1.0%) and the maintenance (ACP, 1.0%; ABCP, 0.6%) phases. Bevacizumab discontinuation because of AEs in either phase occurred in 13.5% and 13.1% of patients in the ABCP arm and 11.4% and 9.3% in the BCP arm, respectively. The most common events that led to bevacizumab discontinuation during induction (≥ 1% of patients) were pulmonary embolism (ABCP, 1.3%; BCP, 1.3%) and hemoptysis (ABCP, 1.0%; BCP, 0%); during maintenance, the most common events were proteinuria (ABCP, 2.9%; BCP, 2.6%) and hypertension (ABCP, 0%; BCP, 1.9%).
The incidence of atezolizumab AESIs was similar between treatment phases in the ACP and ABCP arms, and most were grade 1/2 (Table 3). The AESI of hepatitis was divided into two categories: AEs of liver-related abnormal investigations (hepatitis laboratory abnormalities) and AEs of noninfectious hepatitis, hepatic failure, cirrhosis, and liver damage–related conditions (hepatitis diagnosis). The most common irAEs (≥ 1% overall incidence in any arm) were rash, hypothyroidism, and hepatitis laboratory abnormalities (Data Supplement). The incidence of irAEs was similar across treatment phases in the ACP and ABCP arms, except for a lower incidence of hypothyroidism during the induction versus maintenance phase in both arms and a lower incidence of pneumonitis during the induction versus maintenance phase in the ACP arm. The time to onset and duration of the most common irAEs in the ACP and ABCP arms are shown in the Data Supplement. Most irAEs appeared within the first 3-4 months of treatment and persisted for approximately 2 months. Because systemic corticosteroids are often given to treat irAEs, corticosteroid use is also reported (Data Supplement).
PROs
Of the 400 patients in the ACP arm who received their intended treatment, 371 (92.8%) and 370 (92.5%), respectively, completed the EORTC QLQ-C30 and EORTC QLQ-LC13 at baseline. The respective rates of completion were 356 (90.6%) of 393 and 349 (88.8%) of 393 in the ABCP arm and 360 (91.4%) of 394 and 354 (89.8%) of 394 in the BCP arm. The completion rates documented in the intention-to-treat population were for patients who were active participants in the study at each time point, and they remained at ≥ 70% completion through cycle 18 of treatment in all arms. PRO data were only interpreted up to cycle 13, at which point ≤ 25% of patients in the BCP arm were still receiving treatment; in the intention-to-treat population, this limited the generalizability of the findings.
Patient-reported disease burden was comparable among treatment arms at baseline (cycle 1, day 1; Figs 2 and 3). Patients generally reported moderate to high functioning (higher scores indicate better HRQOL) and minimal symptom burden (lower scores indicate lower symptom severity) across arms (Data Supplement).
FIG 2.
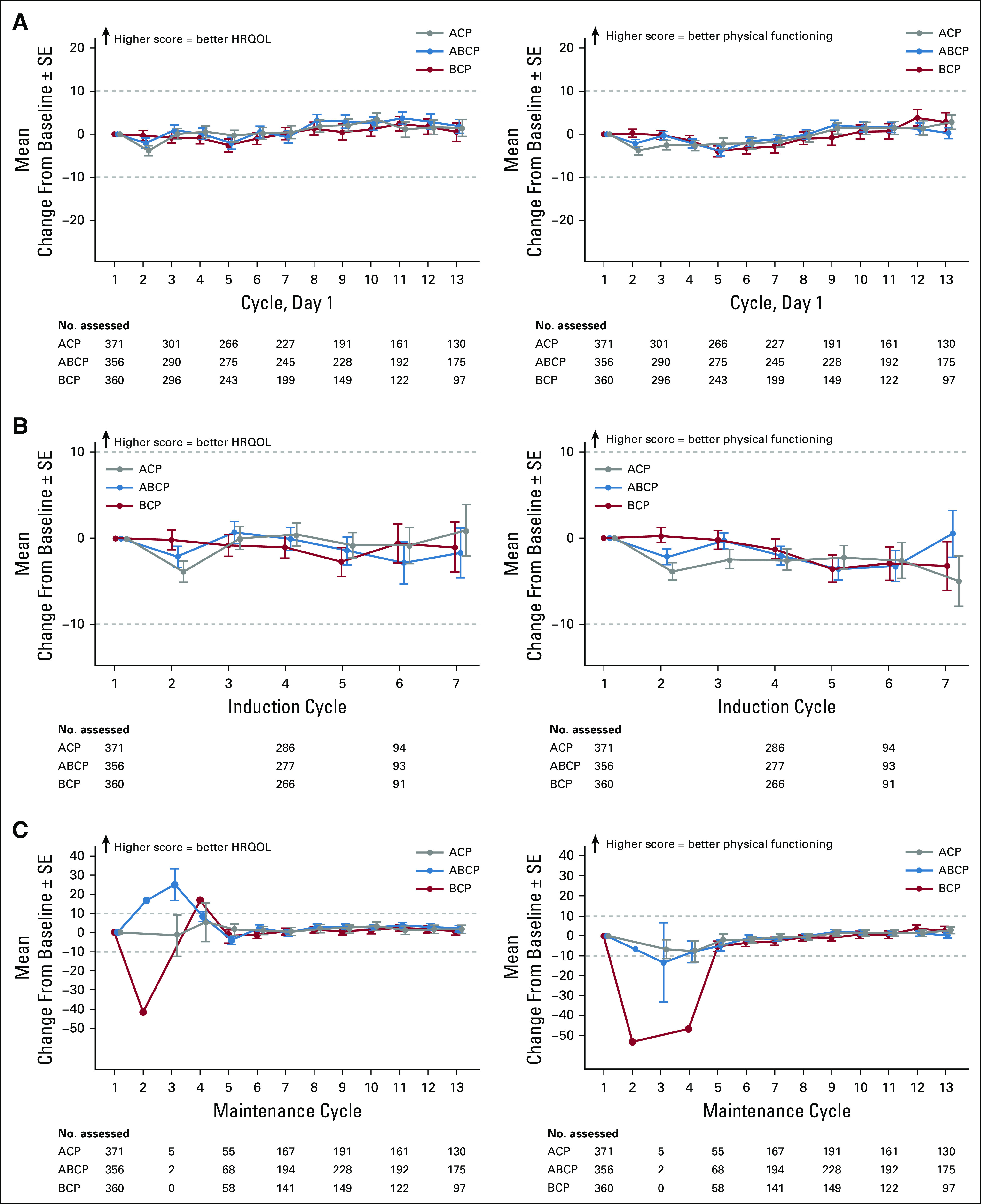
Mean change in baseline score of patient-reported health-related quality of life (HRQOL) and physical functioning overall and by phase of treatment. (A) Mean change in baseline scores for global health status and physical functioning overall and during the (B) induction and (C) maintenance phases. Induction was defined as four or six 21-day cycles of atezolizumab plus bevacizumab, carboplatin, and paclitaxel (ABCP); atezolizumab, carboplatin, and paclitaxel (ACP); or bevacizumab, carboplatin, and paclitaxel (BCP). Maintenance started at cycle 4 or 6, with 21-day cycles of atezolizumab plus bevacizumab (in the ABCP arm), atezolizumab (in the ACP arm), or bevacizumab (in the BCP arm).
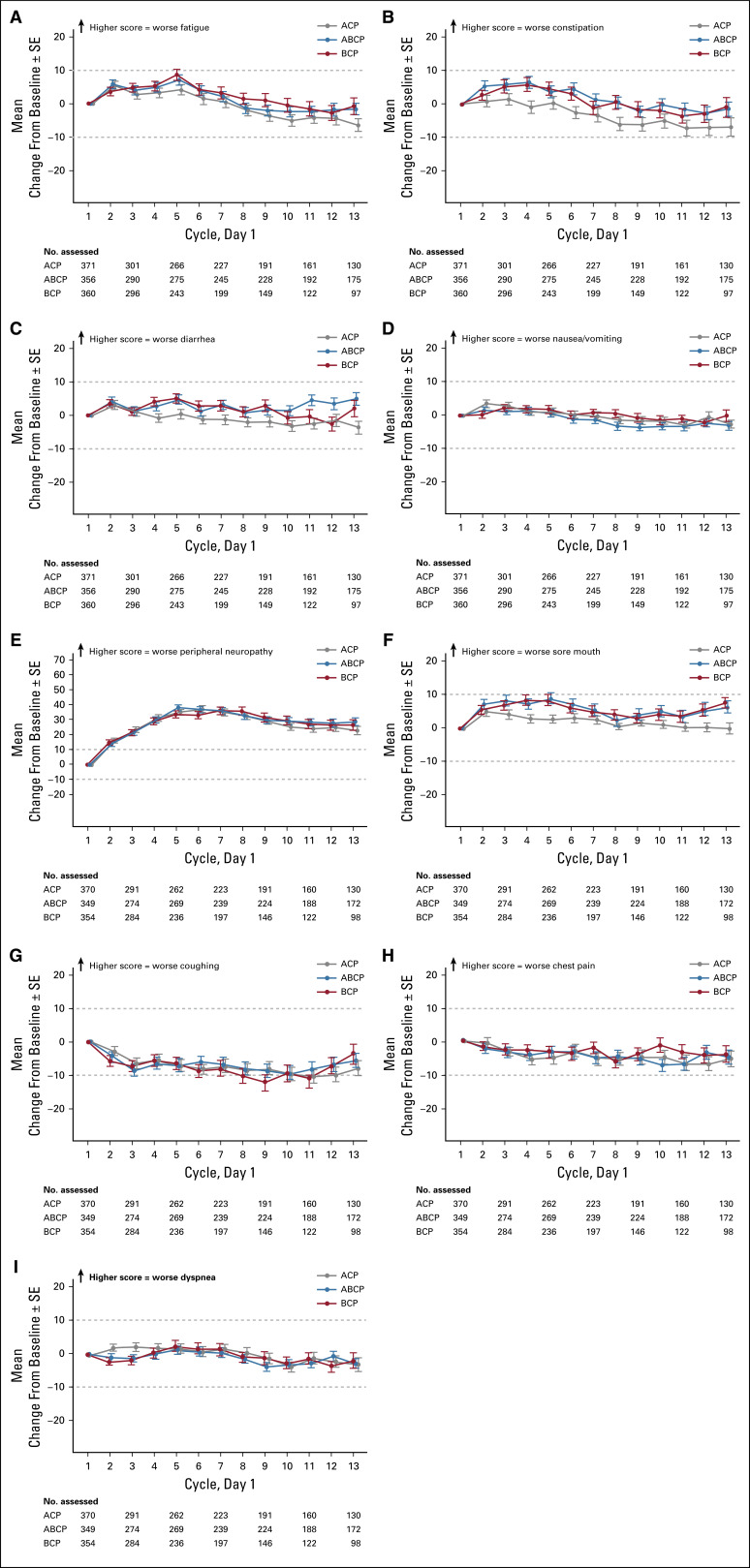
FIG 3.
Mean change in baseline scores of patient-reported symptom severity. Mean change in baseline score through cycle 13 for (A) fatigue, (B) constipation, (C) diarrhea, (D) nausea/vomiting, (E) peripheral neuropathy, (F) sore mouth, (G) coughing, (H) chest pain, and (I) dyspnea. ABCP, atezolizumab plus bevacizumab, carboplatin, and paclitaxel; ACP, atezolizumab, carboplatin, and paclitaxel; BCP, bevacizumab, carboplatin, and paclitaxel.
Patients on average did not report clinically meaningful worsening of global health status or physical functioning scores at any point through cycle 13 in any treatment arm (Fig 2A). When analyzed by treatment phase, average global health status and physical functioning scores remained mostly similar during induction (Fig 2B), and physical functioning showed a trend toward improvement during maintenance (Fig 2C). Mean global health status and physical functioning scores were mostly similar in the ABCP and BCP arms across all time points analyzed.
Mean treatment-related symptom scores are shown in Figures 3A-3F; clinically meaningful worsening in peripheral neuropathy symptom severity was reported. Mean lung cancer–related symptom scores numerically improved in all treatment arms from baseline through cycle 13 (Figs 3G-3I), with a clinically meaningful improvement in coughing scores observed in the BCP arm at one or more treatment cycles.
DISCUSSION
No new safety signals were identified with ABCP, which thus confirms that the four-drug ABCP regimen had no additive toxicities compared with the three-drug ACP and BCP regimens in the phase III IMpower150 study.7 In addition, the improved PFS and OS observed with ABCP versus BCP7 was achieved while maintaining good HRQOL and physical functioning. Despite the higher grade 3/4 TRAE rates in the ABCP arm than in the ACP and BCP arms, these findings support the safety and tolerability of ABCP and provide insight on the ABCP regimen to the medical community beyond its regulatory approval.
Patients had longer exposure to study treatment with ABCP versus ACP or BCP. The AEs observed in the ABCP arm were mostly low grade and manageable, with numerically higher rates of treatment discontinuation compared with the BCP arm. The addition of atezolizumab to BCP did not lead to premature withdrawal from chemotherapy compared with BCP alone. Despite the addition of atezolizumab to the BCP regimen, the overall safety profile for atezolizumab in the combination remains similar to that of atezolizumab monotherapy.3
Across arms, patients reported no clinically meaningful worsening in mean HRQOL, physical functioning, or symptom scores at any point through cycle 13, except for patient-rated severity of peripheral neuropathy. PROs of treatment-related symptoms and safety particularly improved in the maintenance phase, a phenomenon that is well-known and not surprising considering the discontinuation of chemotherapy. The PRO data suggest a minimal difference in treatment burden among arms and highlight an overall sense of the patient’s well-being, which may not necessarily be reflected in the reporting of clinical safety, potentially because of the episodic nature of patient-reported symptoms. With the new options now available, including immunotherapy, future studies should explore approaches to reduce the burden of chemotherapy (eg, fewer cycles in subgroups that are more likely to benefit from immunotherapy) that might obscure the potential HRQOL advantages of immunotherapy.
Strengths of the PRO analyses conducted in this study include the large number of patients evaluated and the high rates of questionnaire completion across study arms through cycle 13. The EORTC QLQ-C30 and lung cancer–specific EORTC QLQ-LC13 are the most commonly used instruments for measuring HRQOL in patients with lung cancer and can be considered the standard option for HRQOL assessment in this population.35 As a limitation, IMpower150 is an open-label trial, which could influence how patients perceived HRQOL. However, to date there is no evidence to indicate that receiving active treatments across the study arms would bias patients’ assessments of symptom severity and impact on life.36,37 Furthermore, as a result of the reduction in the number of patients considered PRO evaluable over time, specifically in the BCP arm, PRO data were interpretable only up to cycle 13. The small patient numbers beyond this time point also limited longer-term PRO analyses. In addition, the EORTC QLQ-C30 and -LC13 were developed before the availability of cancer immunotherapy and, therefore, may miss certain symptoms (eg, rash) that could be experienced with such treatments. It should also be noted that IMpower150 has a select clinical trial population, with minimal symptoms that probably differ from those of real-life patients. Furthermore, this publication does not include a cost-effectiveness analysis, and as such, the value of this therapy given its reported clinical benefits, tolerability, and cost will need to be determined by stakeholders, including payers, health systems, clinicians, and patients. Overall, safety and PRO data from IMpower150 support the positive benefit-risk profile demonstrated by the clinical data with atezolizumab plus bevacizumab and chemotherapy in first-line nonsquamous NSCLC.
ACKNOWLEDGMENT
We thank the participating patients and their families, investigators, and clinical sites. We acknowledge Thomas Karagiannis for contributions to the PRO study design and analysis.
PRIOR PRESENTATION
Presented at the 2018 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2018, and European Society for Medical Oncology 2018 Congress, Munich, Germany, October 19-23, 2018.
SUPPORT
Supported by F. Hoffmann-La Roche and Genentech. Support for third-party writing assistance for this article, furnished by Kia C. E. Walcott, PhD, of Health Interactions, was provided by Genentech.
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to individual patient-level data through the clinical study data request platform: www.clinicalstudydatarequest.com. Additional details on Roche’s criteria for eligible studies are available at https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, go to https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Martin Reck, Shelley Coleman, Geetha Shankar, Hina Patel, Claudia Kelsch, Anthony Lee, Elisabeth Piault, Mark A. Socinski
Provision of study material or patients: Martin Reck, Thomas Wehler, Francisco Orlandi, Carlo Barone, Denis Moro-Sibilot, Mikhail Shtivelband, Wei Yu, Mark A. Socinski
Collection and assembly of data: Martin Reck, Thomas Wehler, Francisco Orlandi, Naoyuki Nogami, Carlo Barone, Denis Moro-Sibilot, Mikhail Shtivelband, Jose Luis González Larriba, Martin Früh, Shelley Coleman, Geetha Shankar, Anthony Lee, Elisabeth Piault, Mark A. Socinski
Data analysis and interpretation: Martin Reck, Thomas Wehler, Naoyuki Nogami, Carlo Barone, Denis Moro-Sibilot, Jose Luis González Larriba, Jeffrey Rothenstein, Wei Yu, Yu Deng, Shelley Coleman, Geetha Shankar, Hina Patel, Claudia Kelsch, Anthony Lee, Elisabeth Piault, Mark A. Socinski
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety and Patient-Reported Outcomes of Atezolizumab Plus Chemotherapy With or Without Bevacizumab Versus Bevacizumab Plus Chemotherapy in Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Martin Reck
Consulting or Advisory Role: Eli Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Roche, Genentech, AbbVie, Amgen
Speakers’ Bureau: Roche, Genentech, Eli Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen
Thomas Wehler
Honoraria: Roche, Genentech, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol Myers Squibb
Consulting or Advisory Role: AstraZeneca, Roche, Genentech, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, AbbVie, Merck Serono, Boehringer Ingelheim
Research Funding: AstraZeneca, Roche, Genentech, Boehringer Ingelheim
Travel, Accommodations, Expenses: Pfizer, Celgene, Roche, Genentech, Boehringer Ingelheim, AstraZeneca
Francisco Orlandi
Honoraria: Roche, Genentech
Consulting or Advisory Role: AstraZeneca, Roche, Genentech, Bristol Myers Squibb, MSD Oncology, Eli Lilly, Pfizer, Bristol Myers Squibb, Novartis, Sanofi
Speakers’ Bureau: AstraZeneca, MedImmune, Roche
Research Funding: AstraZeneca, MedImmune, Amgen, Genentech, Roche, Boehringer Ingelheim, Astellas, Medivation, MSD Oncology, Bristol Myers Squibb, Celltrion, Pfizer, mAbxience, Nektar, Sanofi
Travel, Accommodations, Expenses: Pfizer, MSD Oncology, AstraZeneca, MedImmune, Roche, Bristol Myers Squibb, Genentech
Naoyuki Nogami
Honoraria: Pfizer, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Kyowa Hakko Kirin, Ono Pharmaceutical, Bristol Myers Squibb Japan, MSD
Carlo Barone
Consulting or Advisory Role: Servier
Speakers’ Bureau: MSD Oncology, Celgene, Eli Lilly, Servier Italia, Bristol Myers Squibb
Research Funding: Novartis (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: Merck Serono, Servier
Uncompensated Relationships: Merck Serono
Denis Moro-Sibilot
Consulting or Advisory Role: Roche, Genentech, Boehringer Ingelheim, Eli Lilly, ImClone, Sanofi, Novartis, Amgen, Pfizer, AstraZeneca, Clovis Oncology, MSD Oncology, ARIAD Pharmaceuticals, Bristol Myers Squibb, Takeda Pharmaceuticals, AbbVie
Research Funding: AbbVie (Inst), Boehr (Inst)
Expert Testimony: MSD Oncology
Travel, Accommodations, Expenses: Roche, Genentech, Eli Lilly, ImClone, Pfizer, MSD Oncology, Bristol Myers Squibb
Jose Luis González Larriba
Honoraria: MSD Oncology, AstraZeneca, Roche, Pfizer, Janssen-Cilag, Novartis, Astellas Pharma, Bristol Myers Squibb
Consulting or Advisory Role: MSD Oncology, Janssen-Cilag, Bristol Myers Squibb, Boehringer Ingelheim
Speakers’ Bureau: MSD Oncology
Research Funding: Roche, Novartis, Boehringer Ingelheim, AbbVie, Celgene, Pfizer, Mirati Therapeutics, Ignyta, PharmaMar, AstraZeneca, OncoMed, Bristol Myers Squibb, Bayer AG, Astellas Pharma, Janssen-Cilag
Travel, Accommodations, Expenses: MSD Oncology, Takeda Pharmaceuticals, Bristol Myers Squibb, Roche, Pfizer, Janssen-Cilag
Jeffrey Rothenstein
Honoraria: Bristol Myers Squibb, Roche Canada, AstraZeneca, Merck, Pfizer
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Roche Canada, AstraZeneca, Takeda Pharmaceuticals
Martin Früh
Consulting or Advisory Role: Bristol Myers Squibb (Inst), AstraZeneca (Inst), MSD (Inst), Takeda Pharmaceuticals (Inst), Roche (Inst)
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Wei Yu
Employment: Genentech, Roche
Stock and Other Ownership Interests: Roche, Genentech
Yu Deng
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Travel, Accommodations, Expenses: Genentech
Shelley Coleman
Employment: Genentech
Stock and Other Ownership Interests: Roche, Genentech, Gilead Sciences, Johnson & Johnson, TEVA Pharmaceuticals Industries, Bristol Myers Squibb
Travel, Accommodations, Expenses: Genentech
Geetha Shankar
Employment: Genentech, Roche
Stock and Other Ownership Interests: Roche, Genentech, Exelixis
Hina Patel
Stock and Other Ownership Interests: Roche, Genentech
Claudia Kelsch
Employment: Roche, Genentech
Stock and Other Ownership Interests: Exelixis, Roche, Genentech
Travel, Accommodations, Expenses: Roche, Genentech
Anthony Lee
Employment: Roche, Genentech
Stock and Other Ownership Interests: Roche, Genentech
Elisabeth Piault
Employment: Genentech, Roche
Stock and Other Ownership Interests: Genentech, Roche
Research Funding: Genentech, Roche
Travel, Accommodations, Expenses: Genentech, Roche
Mark A. Socinski
Honoraria: Genentech, Bristol Myers Squibb, Celgene, AstraZeneca, Guardant Health, Bayer AG, Merck, Roche, Genentech, Eli Lilly
Consulting or Advisory Role: Genentech, AstraZeneca, MedImmune, Eli Lilly
Speakers’ Bureau: Genentech, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Bayer AG
Research Funding: Genentech (Inst), Spectrum (Inst), AstraZeneca (Inst), MedImmune (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. V4.2019. Plymouth Meeting, PA, 2019.
- 2.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v1–v27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 3.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 11.Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 12. Keytruda (pembrolizumab) [package insert]. Whitehouse Station, NJ, Merck Sharp & Dohme, 2020.
- 13.Hopwood P, Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: Implications for the evaluation of palliative treatment. Br J Cancer. 1995;71:633–636. doi: 10.1038/bjc.1995.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarna L, Evangelista L, Tashkin D, et al. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest. 2004;125:439–445. doi: 10.1378/chest.125.2.439. [DOI] [PubMed] [Google Scholar]
- 15.Gralla RJ. Quality-of-life considerations in patients with advanced lung cancer: Effect of topotecan on symptom palliation and quality of life. Oncologist. 2004;9:14–24. doi: 10.1634/theoncologist.9-90006-14. [DOI] [PubMed] [Google Scholar]
- 16.Cherny NI, Dafni U, Bogaerts J, et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28:2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 17.Ho MY, Mackey JR. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag Res. 2014;6:253–259. doi: 10.2147/CMAR.S40601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giesinger JM, Kuijpers W, Young T, et al: Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: Physical functioning, emotional functioning, fatigue and pain. Health Qual Life Outcomes 14:87, 2016. [DOI] [PMC free article] [PubMed]
- 19.Koller M, Warncke S, Hjermstad MJ, et al. Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: A systematic review of the literature 20 years after its development. Cancer. 2015;121:4300–4323. doi: 10.1002/cncr.29682. [DOI] [PubMed] [Google Scholar]
- 20.Basch E, Geoghegan C, Coons SJ, et al. Patient-reported outcomes in cancer drug development and US regulatory review: Perspectives from industry, the Food and Drug Administration, and the patient. JAMA Oncol. 2015;1:375–379. doi: 10.1001/jamaoncol.2015.0530. [DOI] [PubMed] [Google Scholar]
- 21. Kluetz PG, Chingos DT, Basch EM, et al: Patient-reported outcomes in cancer clinical trials: Measuring symptomatic adverse events with the National Cancer Institute’s patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book 35:67-73, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Basch E, Campbell A, Hudgens S, et al: A Friends of Cancer Research White Paper: Broadening the Definition of Tolerability in Cancer Clinical Trials to Better Measure the Patient Experience. https://www.focr.org/sites/default/files/Comparative%20Tolerability%20Whitepaper_FINAL.pdf.
- 23.Bordoni R, Ciardiello F, von Pawel J, et al. Patient-reported outcomes in OAK: A phase III study of atezolizumab versus docetaxel in advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19:441–449.e4. doi: 10.1016/j.cllc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–124. doi: 10.1016/j.semcancer.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 26. TECENTRIQ (atezolizumab) [package insert]. South San Francisco, CA, Genentech, 2019.
- 27. TECENTRIQ: Atezolizumab [summary of product characteristics]. Grenzach-Wyhlen, Germany, Roche Registration GmbH, 2019. [Google Scholar]
- 28.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 29.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 30. Fitzsimmons D, Johnson CD, George S, et al: Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer 35:939-941, 1999. [DOI] [PubMed]
- 31.Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: A modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur J Cancer. 1994;30:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 32.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Singh H, Ayalew K, et al. Use of PRO measures to inform tolerability in oncology trials: Implications for clinical review, IND safety reporting, and clinical site Inspections. Clin Cancer Res. 2018;24:1780–1784. doi: 10.1158/1078-0432.CCR-17-2555. [DOI] [PubMed] [Google Scholar]
- 34.Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA. 2013;309:814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 35.Bouazza YB, Chiairi I, El Kharbouchi O, et al. Patient-reported outcome measures (PROMs) in the management of lung cancer: A systematic review. Lung Cancer. 2017;113:140–151. doi: 10.1016/j.lungcan.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Atkinson TM, Wagner JS, Basch E. Trustworthiness of patient-reported outcomes in unblinded cancer clinical trials. JAMA Oncol. 2017;3:738–739. doi: 10.1001/jamaoncol.2016.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roydhouse JK, King-Kallimanis BL, Howie LJ, et al. Blinding and patient-reported outcome completion rates in US Food and Drug Administration cancer trial submissions, 2007-2017. J Natl Cancer Inst. 2019;111:459–464. doi: 10.1093/jnci/djy181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform: www.clinicalstudydatarequest.com. Additional details on Roche’s criteria for eligible studies are available at https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, go to https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.