Abstract
PURPOSE
The terms undertreatment and overtreatment are often used to describe inappropriate management of older adults with cancer. We conducted a comprehensive scoping review of the literature to clarify the meanings behind the use of the terms.
METHODS
We searched PubMed (National Center for Biotechnology Information), Embase (Elsevier), and CINAHL (EBSCO) for titles and abstracts that included the terms undertreatment or overtreatment with regard to older adults with cancer. We included all types of articles, cancer types, and treatments. Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived through qualitative analysis. Within a random subset of articles, C.D. and K.P.L. independently performed this analysis to determine final categories and then independently assigned these categories to assess inter-rater reliability.
RESULTS
Articles using the terms undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion in our review (n = 256). Only 14 articles (5.5%) explicitly provided formal definitions; for the remaining, we inferred the implicit definitions from the terms’ surrounding context. There was substantial agreement (κ = 0.81) between C.D. and K.P.L. in independently assigning categories of definitions within a random subset of 50 articles. Undertreatment most commonly implied less than recommended therapy (148; 62.7%) or less than recommended therapy associated with worse outcomes (88; 37.3%). Overtreatment most commonly implied intensive treatment of an older adult in whom the harms of treatment outweigh the benefits (38; 53.5%) or intensive treatment of a cancer not expected to affect an older adult in his/her remaining lifetime (33; 46.5%).
CONCLUSION
Undertreatment and overtreatment of older adults with cancer are imprecisely defined concepts. We propose new, more rigorous definitions that account for both oncologic factors and geriatric domains.
INTRODUCTION
Older adults make up the growing majority of patients with cancer yet continue to be under-represented in clinical trials.1,2 This under-representation has led to a lack of knowledge with regard to how risk factors unique to older adults, such as multimorbidity, functional dependency, and frailty, interact with novel treatments studied in trials.3 Under-representation has also led to a lack of knowledge about how cancer and its treatment affect function and quality of life (QOL), outcomes older adults value as much as, if not more than, survival.4,5 These knowledge gaps create clinical uncertainty.6 The terms undertreatment and overtreatment are often used when uncertainty is believed to lead to inappropriate treatment decisions. On one side of the spectrum, physiologically robust older adults who may benefit from intensive medical, surgical, and/or radiation therapies are often precluded from receiving them on the basis of chronologic age alone. On the other side, frail older adults may not tolerate guideline-based treatments that are grounded in evidence from research enrolling predominantly younger patients with minimal comorbidities and good performance status.7,8
CONTEXT
Key Objective
The terms undertreatment and overtreatment are often used to describe the mismanagement of older adults with cancer. However, no consensus definition of under- or overtreatment currently exists. We conducted a comprehensive scoping review of the literature to clarify the meanings behind the use of these terms.
Knowledge Generated
The majority of articles using the terms undertreatment or overtreatment with regard to older adults with cancer do so without explicit definitions, and we found significant variability in the implicit meanings. Survival and surrogate treatment outcomes are overemphasized, while important outcomes like functional status and patient preferences are underemphasized.
Relevance
The limitations and imprecision in the current concepts of undertreatment and overtreatment carry potentially harmful implications for older adults with cancer. We propose new, more rigorous definitions that synthesize the findings of our review and the current evidence from geriatric oncology. These definitions aim to better match treatment intensity with age-associated vulnerability and align relevant outcomes with patient preferences.
The prevention of undertreatment and overtreatment of older adults with cancer is more possible now than ever. Significant progress has been made in risk stratifying older patients beyond age and traditional performance status scales (eg, Eastern Cooperative Oncology Group performance status); ASCO and other cancer-focused organizations now recommend a geriatric assessment for all older adults with cancer considering systemic therapy to detect biophysical, functional, and psychosocial impairments that can increase the likelihood of toxicity from cancer therapies.9 However, no universal consensus definition of under- or overtreatment exists, and there may be implicit meanings of these concepts that vary across disciplines, providers, and patients. It is critical to identify and clarify these underlying meanings because the terms undertreatment and overtreatment assert a claim: that a given treatment prescribed to an older adult was too little or too much relative to some optimal treatment. Prevention of the undertreatment and overtreatment of older adults with cancer must begin with rigorously defining what constitutes undertreatment and overtreatment.
The objective of this review is to clarify the meanings behind the use of the terms undertreatment and overtreatment as applied to older patients with cancer. To date, no such review exists. We conducted a comprehensive scoping review of the literature to identify articles of older adults with cancer that include these terms. We extracted explicit or implicit definitions to assess for commonalities, differences, and limitations. Finally, we propose more rigorous definitions of undertreatment and overtreatment that are grounded in evidence-based medicine and account for both oncologic factors and geriatric domains.
METHODS
Methodology of Scoping Review
To explore the meanings behind the current uses of the terms undertreatment and overtreatment, we conducted a scoping review to map key concepts associated with a topic and clarify definitions.10 Neither a systematic review nor a meta-analysis were possible given the imprecision in the uses of the terms undertreatment and overtreatment that preclude the combination of outcomes across studies. Rather than analyze the effectiveness of one or more interventions in articles using these terms with a specified set of outcomes, we investigated authors’ consideration of the interventions and outcomes believed to comprise undertreatment and overtreatment in older adults with cancer. To guide our review, we developed an a priori protocol that adheres to the latest standards recommended by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews11,12 (Data Supplement, online only).
Article Search, Selection, and Data Extraction
We searched PubMed (National Center for Biotechnology Information), Embase (Elsevier), and CINAHL (EBSCO) for records representing cancer studies in older adults that included the terms undertreatment or overtreatment in the title and/or abstract. Although Embase contains some gray literature, we sought only published articles. The searches, carried out on April 28, 2018, were restricted to articles written in English. The search strategies are included in the Data Supplement. Next, C.D. screened the titles and abstracts of all search results for inclusion and exclusion criteria. All types of articles (primary and secondary research articles, including interventional studies, observational studies, reviews, letters to the editor, and news articles), treatments (medical, surgical, and/or radiation therapies), and cancers (solid or hematologic) were included. We excluded studies of patients exclusively < 60 years of age as well as studies that included younger patients but did not specifically delineate a subgroup of older patients. Relevant data from all articles were extracted, including study design, location, type of cancer and treatment studied, outcomes, and article sections where the terms undertreatment and/or overtreatment were used. We surveyed whether each article recommended the geriatric assessment and whether any analyses adequately accounted for geriatric domains.
Analysis
Articles were identified by C.D. (a geriatrician) that reported explicit definitions of undertreatment and overtreatment. For the remainder, implicit definitions were inferred by examining the terms’ surrounding context while incorporating any associated analyses conducted by the authors. A qualitative analysis that was based on grounded theory was then conducted in the following manner.13 First, definitions were reviewed, and one or more common categories underlying these definitions were derived through an iterative process. Second, the derived subcategories were reviewed for common, underlying categories. Third, a second author (K.P.L., an oncologist) independently repeated these two steps on a randomly selected (by computer algorithm) subset of 50 articles. C.D. and K.P.L. discussed and agreed on the final subcategories and categories that encompassed extracted definitions. Then, C.D. and K.P.L. independently assigned these categories in this subset of 50 articles to assess for inter-rater reliability. Any differences in assignment within this subset were resolved by C.D. and K.P.L. followed by C.D. re-reviewing all remaining articles for accurate assignment of the final categories.
RESULTS
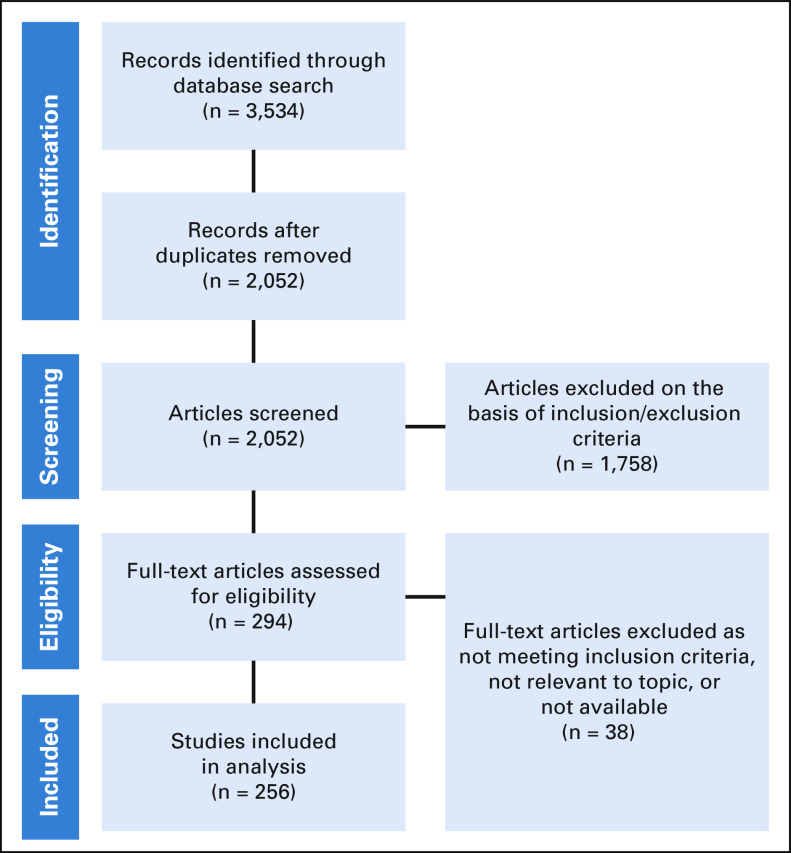
Figure 1 shows a flow diagram of article inclusion in our review. Our searches returned 3,534 records (PubMed, 1,675; Embase, 1,474; CINAHL, 395). After removal of duplicates, 2,052 records remained. Of these, 1,758 failed to meet the inclusion criteria or met one or more exclusion criteria. The full text of the remaining 294 articles was examined, after which an additional 38 articles were excluded on the basis of not meeting the inclusion criteria, lacking relevance to our topic, or unavailability, which left 256 articles for our analysis.
FIG 1.
Flow diagram of article inclusion in review.
Table 1 lists the characteristics of the included articles that used the terms undertreatment and/or overtreatment with regard to older adults with cancer (n = 256). Overall, 236 articles used the term undertreatment at least once, 71 used the term overtreatment, and 51 used both terms. Of the 63 primary research articles, the largest proportion was cohort studies (48; 18.8%); of the 193 secondary research articles, the largest proportion was reviews (95; 37.1%). Fifty-five articles (21.5%) focused on multiple cancers or did not specify cancer type, whereas the remainder focused on specific cancer types, including breast (75; 29.3%), lung (30; 11.7%), and colorectal (28; 10.9%), among others. The majority of articles (165; 64.5%) focused on multiple treatments or combinations of treatments (medical, surgical, and/or radiation therapies). Similarly, the majority of articles focused on multiple outcomes (158; 61.7%), whereas 63 (24.6%) focused mainly on survival or surrogates of survival, 32 (12.5%) on decision making, and only 3 (1.2%) on QOL. Eighty articles (31.3%) did not discuss QOL, patient values, and/or patient preferences at any point.
TABLE 1.
Characteristics of Included Articles
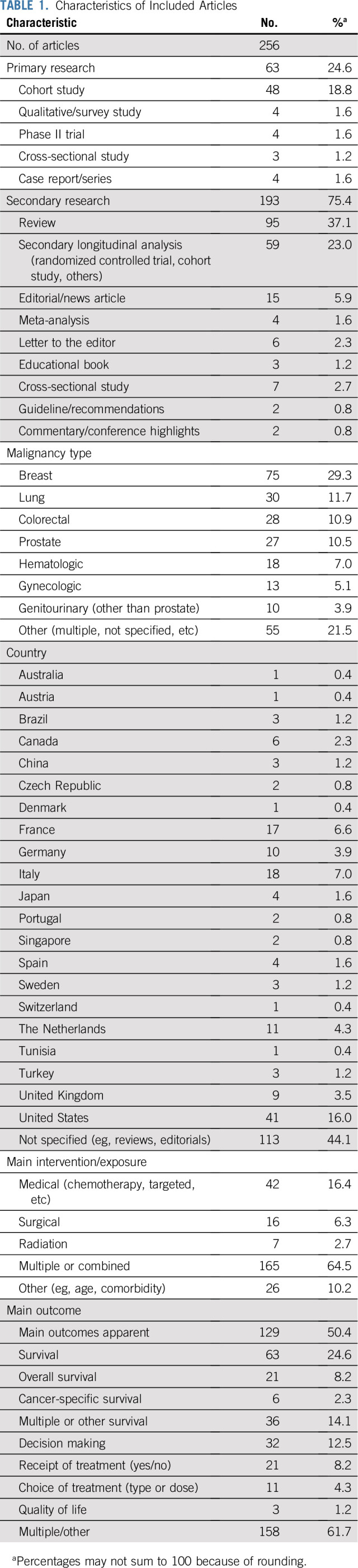
Fourteen articles (5.5%) included an explicit definition that accompanied their use of the terms undertreatment and/or overtreatment. For the remaining articles, the implicit definitions of undertreatment and overtreatment were inferred from the terms’ surrounding context. These explicit and implicit definitions for all articles, as well as the location in each article where the terms were used, are listed in the Data Supplement.
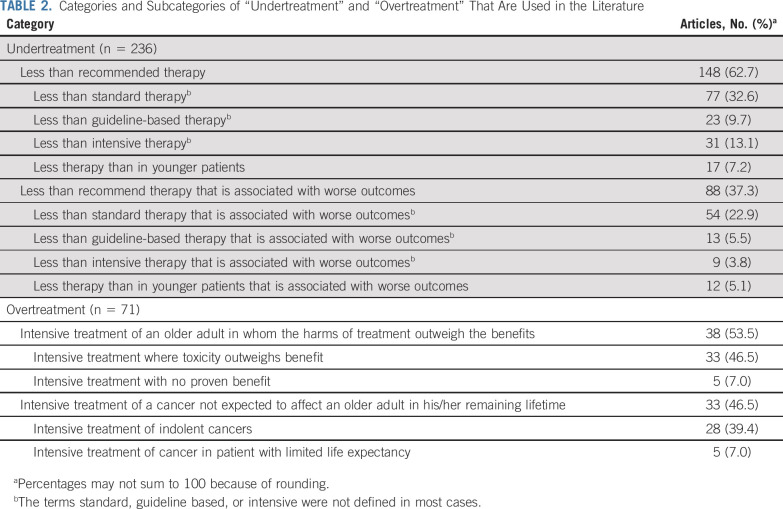
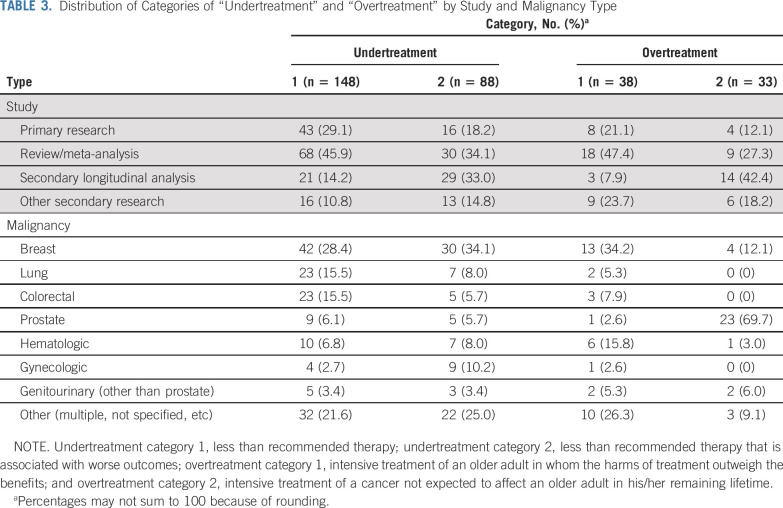
Through qualitative analysis, 12 distinct subcategories emerged from all of the extracted explicit and implicit definitions: eight subcategories that pertained to the definitions of undertreatment and four that pertained to the definitions of overtreatment (Table 2). These subcategories were then synthesized into broader, encompassing categories on the basis of common and distinguishing features. Table 3 lists the distribution of these categories by study and malignancy type. Inter-rater agreement between C.D. and K.P.L. on their independent assignment of these categories within a random subset of 50 articles was 87.1%, with a κ-coefficient of 0.81.
TABLE 2.
Categories and Subcategories of “Undertreatment” and “Overtreatment” That Are Used in the Literature
TABLE 3.
Distribution of Categories of “Undertreatment” and “Overtreatment” by Study and Malignancy Type
Categories Underlying the Reviewed Definitions of Undertreatment
Table 2 lists the two broad categories and eight subcategories that emerged from the explicit and implicit definitions of the term undertreatment. In the first broad category, 148 (62.7%) of the 236 articles that included the term undertreatment defined it as prescribing less than a recommended therapy (medical, surgical, radiation, or a combination of these). Authors either used the words less than standard (77; 32.6%), less than guideline or guideline based (23; 9.7%), less than intensive or aggressive (31; 13.1%), or less than in younger patients (17; 7.2%). These four subcategories all shared the common feature of qualifying undertreatment as less therapy in older adults either in dose, proportion of patients prescribed, or modifications of a surgery or procedure compared with some reference or recommended treatment. The terms used to represent what this recommended treatment was (standard, guideline, intensive) were most often not defined themselves. Nearly half of the articles in this category were review articles (68; 45.9%; Table 3).
In the second broad category, 88 (37.3%) of the 236 articles using the term undertreatment defined it as less than recommended therapy that is associated with worse outcomes. The four subcategories that were included in this category were similar to the first category in the types of recommended therapy used as a reference (ie, less than standard, less than guideline based, less than intensive, less than younger patients). However, the distinguishing feature for the second category was that undertreatment was not merely less than recommended therapy but that this lesser therapy contributed to worse outcomes compared with a recommended therapy. Seventy-eight of these articles (88.6%) defined worse outcomes as worse survival or surrogate measures of survival (Data Supplement).
Many observational cohort studies supported this definition of undertreatment with a primary or secondary analysis (39 [44%] of 88 articles listed in the Data Supplement). These analyses showed that older adults who received a predefined recommended therapy had better survival (overall, disease-specific, or surrogate of survival) than older adults who received less than recommended therapy, or alternatively, that compared with younger patients, older patients received lower rates of recommended therapy and had worse survival (in separate analysis). There were variable approaches to account for factors that may have confounded the relationship between the receipt of recommended therapy and survival. Eleven of these 39 studies performed only univariable analyses without any adjustment,14-24 16 performed multivariable analyses that adjusted mainly for disease-based factors (eg, tumor stage) but not for any geriatric assessment domains,25-40 and 12 performed limited adjustment beyond disease-based factors (eg, adjusted for performance status and/or comorbidity but not for other important geriatric assessment domains, such as functional status, mobility, and cognition).41-52 Toxicity associated with treatment was reported in 3 of these 39 studies. Comments on other analyses that were included in articles but not used as evidence to support undertreatment or overtreatment are listed in the Data Supplement.
Categories Underlying the Reviewed Definitions of Overtreatment
Table 2 lists the two broad categories and four subcategories that emerged from the explicit and implicit definitions of the term overtreatment. In the first broad category, 38 (53.5%) of the 71 articles that included the term overtreatment defined it as intensive treatment of an older adult in whom the harms of treatment outweigh the benefits. The first subcategory included definitions that referred to treatment with possible benefit but with excessive toxicity that outweighs this benefit (33; 46.5%). This first subcategory often referred to excessive toxicity in a frail or vulnerable older adult whose physiologic reserves could not tolerate intensive therapy. The second subcategory included definitions that referred to treatment that has no proven benefit regardless of the patient’s health status (eg, axillary lymph node dissection when sentinel lymph node biopsy is sufficient in women with breast cancer [5; 7.0%]).53
In the second broad category, 33 (46.5%) of the 71 articles that included the term overtreatment defined it as intensive treatment of a cancer not expected to affect an older adult in his/her remaining lifetime. The majority of these articles focused on prostate cancer (23; 69.7%; Table 3). Authors focused on either the first component of this definition in the first subcategory—treating an indolent, slow-growing cancer (eg, low-risk prostate cancer [28 articles; 39.4%])—or the latter component of this definition in the second subcategory—treating a patient with limited life expectancy independently of the aggressiveness of their cancer (eg, a patient more likely to die as a result of a comorbidity other than prostate cancer54 [5 articles; 7.0%]). These subcategories shared the common feature of focusing on the cancer and/or the patient with the cancer, wherein the time to the development of symptoms from the cancer exceeded the expected time to death of the patient. Intensive treatment of the cancer in this circumstance was deemed overtreatment. Although there is some overlap in concepts between the first and second broad categories of overtreatment, the first category focuses more on the harm/benefit ratio of the treatment itself, whereas the second category focuses on the time to benefit of the treatment—a function of the aggressiveness of the cancer and the life expectancy of the patient.
Geriatric Assessment
Just over half of all included articles advocated for the use of the geriatric assessment to risk stratify older adults for treatment selection (135 [52.7%] of 256; Data Supplement). Fewer advocated for its use to detect age-related vulnerabilities (eg, cognitive impairment, functional dependency) for further management alongside cancer treatment (67; 26.2%).
DISCUSSION
To our knowledge, this is the first comprehensive scoping review to date that has sought to clarify the meaning of the terms undertreatment or overtreatment used to describe the management of older patients with cancer. Through our qualitative analysis of reviewed definitions, we derived 12 distinct subcategories of definitions for undertreatment and overtreatment, which coalesced into two categories for undertreatment and two categories for overtreatment. Our review demonstrates that there are limitations and imprecision in the current concepts of undertreatment and overtreatment, and this imprecision carries potentially harmful implications, which we describe next. We conclude by proposing new, more rigorous definitions of undertreatment and overtreatment that synthesize the findings of our review and the current evidence in geriatric oncology.
Articles that defined undertreatment as offering older adults less than recommended therapy correctly argue against age bias in withholding potentially beneficial treatments but understate the limitation that older adults are often not included in the trials used to support these recommended therapies.2,7,8,55,56 The second category of undertreatment improves upon the first by considering whether less than recommended therapy actually leads to worse outcomes. However, the majority of articles in this second category defined worse outcomes as worse survival, with many focusing on disease-specific survival and surrogate survival measures (eg, progression-free survival).25,32,41,51 This overemphasis on survival and surrogates raises several issues.
First, vulnerable older patients treated with intensive therapy may actually have higher all-cause mortality as a result of treatment toxicity, even if their cancer-specific mortality is lower.57 Second, surrogate survival outcomes, such as progression-free survival, may correlate poorly with patient-centered outcomes, such as QOL.58-60 Third, any improvement in survival may be outweighed by treatment-associated declines in function and QOL. Older adults often value function and QOL just as much as, if not more than, survival, and many may not accept a cancer treatment if it is associated with declines in these outcomes, even if it prolongs life.4,5,61 This harm/benefit imbalance is magnified when the gains in survival are minimal and the costs to QOL are large.62-64 There is limited high-quality evidence to inform this balance given the under-representation of vulnerable/frail older adults in trials leading to recommended cancer therapies.2,65
Finally, the articles that conducted observational studies showing worse outcomes in older patients treated with less intensive therapy (compared with recommended therapy) did not sufficiently measure and account for the impact of age-related vulnerabilities21,23,26,29,31 (Data Supplement). Not accounting for geriatric domains such as multimorbidity, cognitive impairment, or functional dependency opens the possibility for significant unmeasured confounding in the association between treatment intensity and outcomes (eg, confounding by indication/contraindication).66 Vulnerable patients with geriatric domain deficits in addition to their cancer are less likely to be treated with intensive therapies, but their higher mortality may be mediated through these age-related deficits rather than through the receipt of lower intensity cancer treatment.9,67-71 Indeed, several studies outside this review report decreased benefits and increased harms when intensive guideline-recommended therapies are prescribed to vulnerable older adults in real-world practice.3,72-74
The first category of overtreatment denotes a mismatch between the intensity of cancer therapy and the vulnerability of an older patient, whereas the second category defines overtreatment as cancer therapy without benefit in an older patient’s remaining lifetime. Both categories highlight distinct, important features of overtreatment but when considered separately, are incomplete definitions. Many articles that used the first category focused on applying recommended intensive treatments (eg, open surgery, stem-cell transplantation, combined therapies) in vulnerable or frail older patients who could not tolerate the burden or toxicity of the treatment.75-77 However, even lower intensity treatments (eg, localized surgery such as a lumpectomy78) can exceed the reduced physiologic reserves of vulnerable or frail older patients and contribute to functional decline and/or death.
Moreover, many articles in the first category failed to advocate for using geriatric assessment to better define vulnerability in older adults for risk stratification, and even more failed to recommend using geriatric assessment–guided interventions that target reversible causes of vulnerability/frailty.9,79 Articles using the second category of overtreatment related overtreatment to the better-known concept of overdiagnosis (detecting with a screening test a cancer that will not cause symptoms in a patient’s lifetime), and the National Cancer Institute similarly defines overtreatment through linking it with overdiagnosis.80 However, overtreatment pertains not only to screen-detected malignancies but also to cancers diagnosed from symptoms. In either scenario, clinicians must consider both the aggressiveness of the cancer and the life expectancy of the patient to estimate whether a cancer treatment can confer any benefit in an older adult’s remaining lifetime.9 In addition, providers must weigh the harms of the treatment against the benefits, as reflected in the first category of overtreatment discussed previously.
Examination of the categories that emerged from this review raises important issues with regard to the current understanding of what it means to undertreat or overtreat an older adult with cancer. First, although the majority of articles used these terms without an explicit definition, we identified variability in the implied meanings. Second, an overemphasis on disease-specific and survival measures neglects other risk factors and outcomes important in older adults.81 Third, nearly a third of articles made no reference to patient values, preferences, and/or QOL, and those that did often did so peripherally to their use of the terms undertreatment or overtreatment.82-85 A discussion of what outcomes matter most to an individual older patient should come first in defining and avoiding under- or overtreatment.86,87 Establishment of priorities for an older patient, who may have many comorbidities and functional limitations aside from cancer, can be nuanced and vary from patient to patient.88 An older patient who values and believes that his/her life can be prolonged by cancer treatment may be willing to tolerate the burden and toxicity of an intensive treatment to achieve this benefit.5 When facing the same treatment options, a different older patient who prioritizes QOL over quantity of life may view intensive treatment as a net harm, not as a net benefit.
Consequently, the variability in meanings behind undertreatment and overtreatment, the overemphasis on disease-specific and survival measures, and the underemphasis on patient preferences may dangerously lead to overtreatment when trying to avoid undertreatment and vice versa. For example, some clinicians may view that withholding guideline-concordant definitive surgery in a 75-year-old with early-stage breast cancer is undertreating this patient and may elevate her risk of cancer-specific mortality.89 However, if this 75-year-old is vulnerable with multimorbidity, cognitive, and/or functional deficits, then she may be at higher risk of other-cause mortality that is unaffected by or even exacerbated by surgery.78 If the goals of the patient and her family are to preserve function and QOL given her vulnerability and limited prognosis at baseline, then surgery would be overtreatment. Conversely, if this patient is community dwelling, fit, and values survival even with potential tradeoffs, then not offering definitive surgery would be undertreatment.90 Some clinicians may view that full definitive treatment in this 75-year-old is overtreatment on the basis of a perceived risk of toxicity and/or limited benefit associated with (chronologic) age alone; this view would be uninformed without rigorous assessment of age-related vulnerabilities and a discussion of patient preferences. If such treatment decisions are made, recorded in a database with mainly disease-based measures, and retrospectively analyzed years later by a separate investigator, then any interpretation of under- and overtreatment runs the risk of the same limitations identified in this review.91 Without a singular theoretical framework and more rigorous, consistent definitions, undertreatment and overtreatment will continue to be applied in an imprecise manner across researchers, clinicians, and patients.92
We thus propose new definitions of undertreatment and overtreatment that synthesize the findings of our review and the current evidence in geriatric oncology (Table 4). Although some may challenge the very attempt at forming universally accepted definitions, we believe that this attempt must be made given the variability and harmful implications of the status quo. Our proposed definitions account for both oncologic factors and geriatric domains important in the care of older adults with cancer. We further ground our definitions in the framework of evidence-based medicine, which advocates for the incorporation of the latest best evidence with patient values to optimize decision making.93 Figures 2A-D illustrate the essential concepts with examples of our definitions.
TABLE 4.
Newly Proposed Definitions of Undertreatment and Overtreatment in Older Adults With Cancer
FIG 2.
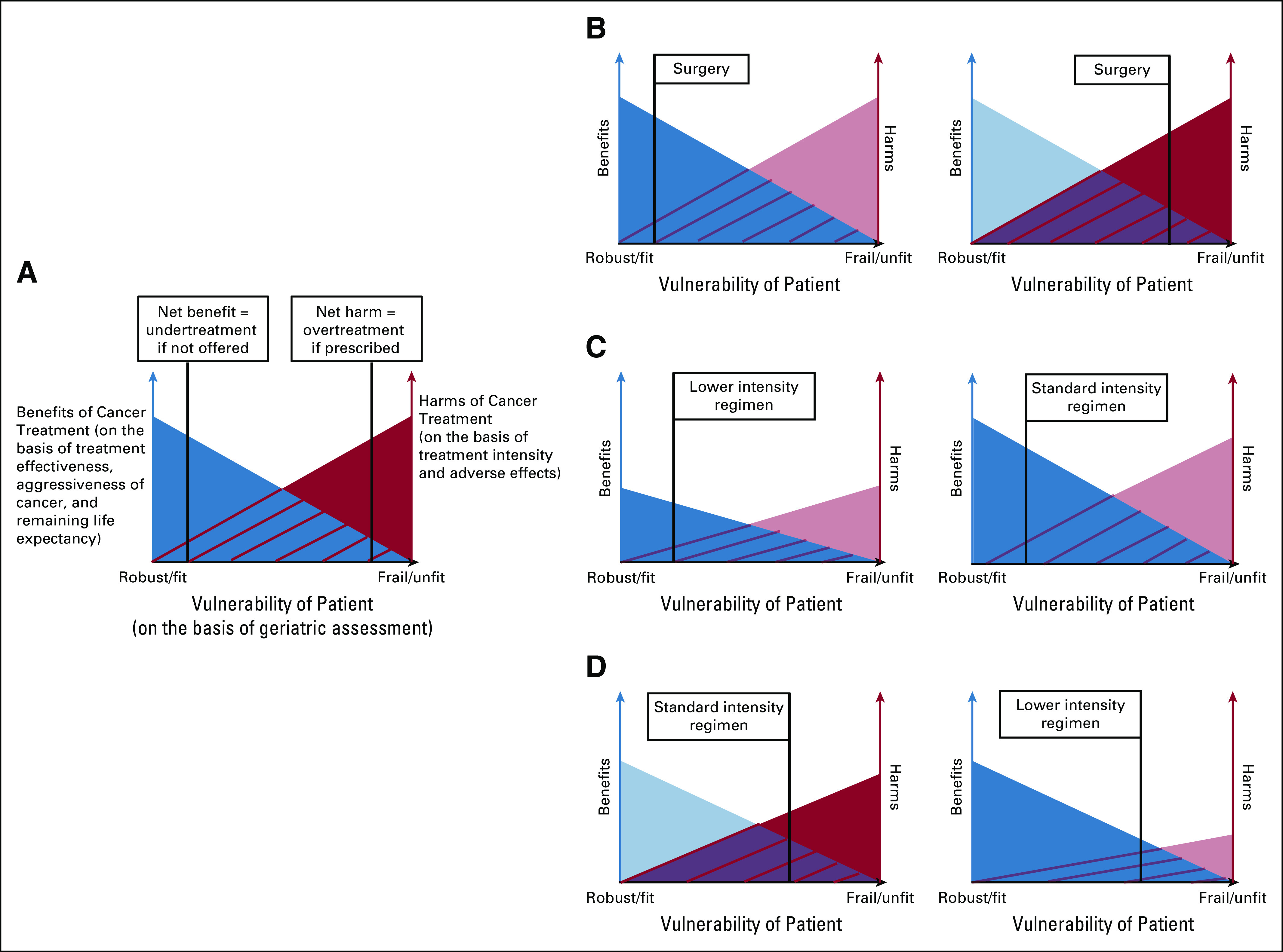
Graphical illustration of essential concepts of proposed definitions of undertreatment and overtreatment. (A) General graph of relationships among benefits of cancer treatment, harms of treatment, and vulnerability of older patient. Benefits of cancer treatment are a function of the effectiveness of the treatment, the aggressiveness of the cancer, and the remaining life expectancy of the patient. Harms of a particular treatment are a function of treatment intensity and adverse effects. Vulnerability is a function of geriatric assessment deficits (eg, cognitive impairment, functional dependency). As patient vulnerability increases, treatment benefits decrease and harms increase. For a given vulnerability on the x-axis, the blue shading represents a treatment where the benefits outweigh the harms (net benefit = undertreatment if not offered). For more severe vulnerability on the x-axis, the red shading represents a treatment where the harms outweigh the benefits (net harm = overtreatment if prescribed). The more intensive color shading in the next figure panels helps to illustrate net harm v net benefit for a given patient and treatment. Individual patient preferences should inform the balance between benefits and harms of a given treatment. (B) The left panel shows an example of undertreatment when considering definitive surgery in a fit older patient: A 75 year-old community-dwelling female with few comorbidities has early-stage breast cancer and values survival even with tradeoffs but is not offered definitive cancer surgery on the basis of her age alone. The right panel shows an example of overtreatment when considering definitive cancer surgery in a vulnerable older patient: A 75-year-old female nursing home resident with limited life expectancy has early-stage breast cancer and values quality of life over survival but is recommended to undergo definitive surgery that leads to an irreversible decline in function.78 (C) Example of undertreatment when considering variable intensities of chemotherapy in a fit patient: A 75-year-old male with intact cognitive and physical function has multiple myeloma and values survival even with tradeoffs but is treated with a lower intensity chemotherapy regimen on the basis of age alone (left panel) when a standard intensity regimen (right panel) offers greater prolongation of life.96 (D) Example of overtreatment when considering variable intensities of chemotherapy in a vulnerable patient: A 75-year-old male with advanced osteoarthritis and sarcopenia has gastroesophageal cancer but is prescribed a higher intensity chemotherapy regimen (left panel) when a lower-intensity regimen (right panel) offers similar survival benefit with less toxicity.97
As an example in applying our definition of overtreatment, the ESOGIA trial compared the use of geriatric assessment in determining chemotherapy allocation to usual care for older adults with lung cancer.94 Although older adults in the geriatric assessment arm did not experience an improvement in the primary surrogate outcome of treatment failure–free survival, they received less intense chemotherapy, had less toxicity, and had better QOL, all while maintaining similar overall survival.95 The trial was deemed negative on the basis of the surrogate outcome. However, it should be interpreted as positive in preventing overtreatment in the older patients who achieved similar survival benefit with less toxicity: a greater net benefit. This more accurate interpretation of the trials’ results stems from our more accurate definition of overtreatment and illustrates how our definition can be used not only in practice but also as an outcome to assess in efficacy research studying older adults with cancer.
A limitation of our review was that the requirement of the term undertreatment or overtreatment in our search strategy potentially excluded other relevant articles that discuss related concepts. This specific search strategy was used given that the intent of this scoping review was to clarify the meaning behind the use of the terms undertreatment and overtreatment themselves because these terms are most commonly used to refer to inappropriate management of an older adult with cancer. Our rationale was to select for articles that made these terms an essential focus of their content by placing them in their titles and/or abstracts.
In conclusion, the undertreatment and overtreatment of older adults with cancer are imprecisely defined concepts. We propose new, more rigorous definitions that shift disease-centric criteria to patient-centered criteria, with a broader focus on not only survival but also on function and QOL. Additional research must investigate how cancer and its treatment interact with geriatric domains to affect these three outcomes through both enrolling more older adults in clinical trials and incorporating geriatric measures in well-designed observational studies.55 This evidence will better delineate the harms and benefits of cancer treatments in older adults. However, application of this evidence to treatment decisions—and in approaching decisions where evidence is limited—requires new standard criteria for undertreatment and overtreatment to maximize net benefit and avoid net harm. Our proposed definitions seek to meet this need by better matching treatment intensity to age-related vulnerability and aligning anticipated outcomes with patient values.
ACKNOWLEDGMENT
We thank Beatriz Korc-Grodzicki, MD, PhD, for her review of our manuscript. We also thank the Cancer and Aging Research Group for its feedback with regard to our study.
PRIOR PRESENTATION
Presented at the American Society of Clinical Oncology 2019 Annual Meeting, Chicago, IL, May 31-June 4, 2019, and 19th Annual Conference of the International Society of Geriatric Oncology, Geneva, Switzerland, November 14-16, 2019.
SUPPORT
Supported by the Harvard Translational Research in Aging Training Program (National Institute on Aging grant T32AG023480; C.D.); Wilmot Research Fellowship and National Cancer Institute (K99CA237744; K.P.L.); Murphy Family Fund from the Dana-Farber Cancer Institute (G.A.); and National Institutes of Health grants U13AG038151 (W.D. with S. Mohile), R21AG059206 (W.D. with S. Mohile); and 5K24AG055693-02 and 5R25CA183723 (W.D. with M. Loscalzo and S. Mohile).
AUTHOR CONTRIBUTIONS
Conception and design: Clark DuMontier, Kah Poh Loh, Paul A. Bain, Rebecca A. Silliman, Gregory A. Abel, Benjamin Djulbegovic Jane Driver, William Dale
Collection and assembly of data: Clark DuMontier, Kah Poh Loh, Paul A. Bain
Data analysis and interpretation: Clark DuMontier, Kah Poh Loh, Rebecca A. Silliman, Tammy Hshieh, Gregory A. Abel, Benjamin Djulbegovic, Jane A. Driver, William Dale
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Defining Undertreatment and Overtreatment in Older Adults With Cancer: A Scoping Literature Review
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kah Poh Loh
Consulting or Advisory Role: Pfizer, Seattle Genetics
Paul A. Bain
Employment: PhAST Diagnostics (I)
Stock and Other Ownership Interests: Paratek Pharmaceuticals
Benjamin Djulbegovic
Research Funding: GlaxoSmithKline (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Shapiro CL. Cancer survivorship. N Engl J Med. 2018;379:2438–2450. doi: 10.1056/NEJMra1712502. [DOI] [PubMed] [Google Scholar]
- 2.Ludmir EB, Mainwaring W, Lin TA, et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. 2019;5:1769. doi: 10.1001/jamaoncol.2019.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ethun CG, Bilen MA, Jani AB, et al. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67:362–377. doi: 10.3322/caac.21406. [DOI] [PubMed] [Google Scholar]
- 4.Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 5.Loh KP, Mohile SG, Epstein RM, et al. Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults. Cancer. 2019;125:2506–2513. doi: 10.1002/cncr.32074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han PK. Conceptual, methodological, and ethical problems in communicating uncertainty in clinical evidence. Med Care Res Rev. 2013;70:14S–36S. doi: 10.1177/1077558712459361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passaro A, Spitaleri G, Gyawali B, et al. Immunotherapy in non–small-cell lung cancer patients with performance status 2: Clinical decision making with scant evidence. J Clin Oncol. 2019;37:1863–1867. doi: 10.1200/JCO.18.02118. [DOI] [PubMed] [Google Scholar]
- 8.Sanoff HK, Chang Y, Lund JL, et al. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist. 2016;21:1113–1120. doi: 10.1634/theoncologist.2015-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36:2326–2347. doi: 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 12. Peters MDJ, Godfrey C, McInerney P, et al: Chapter 11: Scoping reviews, in Aromataris E, Munn Z (eds): Joanna Briggs Institute Reviewer’s Manual. Adelaide, South Australia, Australia, Joanna Briggs Institute, 2017. [Google Scholar]
- 13. Strauss A, Corbin J: Grounded theory methodology, in Denzin N, Lincoln Y (eds):Handbook of Qualitative Research. Thousand Oaks, CA, Sage, 1994, pp 273-285. [Google Scholar]
- 14.Bastiaannet E, Liefers GJ, de Craen AJ, et al. Breast cancer in elderly compared to younger patients in the Netherlands: Stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat. 2010;124:801–807. doi: 10.1007/s10549-010-0898-8. [DOI] [PubMed] [Google Scholar]
- 15.Bastiaannet E, Portielje JE, van de Velde CJ, et al. Lack of survival gain for elderly women with breast cancer. Oncologist. 2011;16:415–423. doi: 10.1634/theoncologist.2010-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrero A, Fuso L, Tripodi E, et al. Ovarian cancer in elderly patients: Patterns of care and treatment outcomes according to age and modified frailty index. Int J Gynecol Cancer. 2017;27:1863–1871. doi: 10.1097/IGC.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 17.Mangiola A, Maira G, De Bonis P, et al. Glioblastoma multiforme in the elderly: A therapeutic challenge. J Neurooncol. 2006;76:159–163. doi: 10.1007/s11060-005-4711-1. [DOI] [PubMed] [Google Scholar]
- 18.Noon AP, Albertsen PC, Thomas F, et al. Competing mortality in patients diagnosed with bladder cancer: Evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013;108:1534–1540. doi: 10.1038/bjc.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petera J, Dušek L, Sirák I, et al. Cancer in the elderly in the Czech Republic. Eur J Cancer Care (Engl) 2015;24:163–178. doi: 10.1111/ecc.12287. [DOI] [PubMed] [Google Scholar]
- 20.Poupon C, Bendifallah S, Ouldamer L, et al. Management and survival of elderly and very elderly patients with endometrial cancer: An age-stratified study of 1228 women from the FRANCOGYN Group. Ann Surg Oncol. 2017;24:1667–1676. doi: 10.1245/s10434-016-5735-9. [DOI] [PubMed] [Google Scholar]
- 21.Van Leeuwen BL, Rosenkranz KM, Feng LL, et al. The effect of under-treatment of breast cancer in women 80 years of age and older. Crit Rev Oncol Hematol. 2011;79:315–320. doi: 10.1016/j.critrevonc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Wallwiener CW, Hartkopf AD, Grabe E, et al. Adjuvant chemotherapy in elderly patients with primary breast cancer: Are women ≥65 undertreated? J Cancer Res Clin Oncol. 2016;142:1847–1853. doi: 10.1007/s00432-016-2194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. doi: 10.1007/s10143-014-0528-8. Zouaoui S, Darlix A, Fabbro-Peray P, et al: Oncological patterns of care and outcomes for 265 elderly patients with newly diagnosed glioblastoma in France. Neurosurg Rev 37:415-423, 2014; discussion 423-424. [DOI] [PubMed] [Google Scholar]
- 24.Dialla PO, Quipourt V, Gentil J, et al. In breast cancer, are treatments and survival the same whatever a patient’s age? A population-based study over the period 1998-2009. Geriatr Gerontol Int. 2015;15:617–626. doi: 10.1111/ggi.12327. [DOI] [PubMed] [Google Scholar]
- 25.Al-Refaie WB, Habermann EB, Dudeja V, et al. Extremity soft tissue sarcoma care in the elderly: Insights into the generalizability of NCI cancer trials. Ann Surg Oncol. 2010;17:1732–1738. doi: 10.1245/s10434-010-1034-z. [DOI] [PubMed] [Google Scholar]
- 26.Camilon PR, Stokes WA, Nguyen SA, et al. Are the elderly with oropharyngeal carcinoma undertreated? Laryngoscope. 2014;124:2057–2063. doi: 10.1002/lary.24660. [DOI] [PubMed] [Google Scholar]
- 27.Chang GJ, Skibber JM, Feig BW, et al. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg. 2007;246:215–221. doi: 10.1097/SLA.0b013e318070838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa GJ, de Mello MJG, Ferreira CG, et al. Undertreatment trend in elderly lung cancer patients in Brazil. J Cancer Res Clin Oncol. 2017;143:1469–1475. doi: 10.1007/s00432-017-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fourcadier E, Trétarre B, Gras-Aygon C, et al. Under-treatment of elderly patients with ovarian cancer: A population based study. BMC Cancer. 2015;15:937. doi: 10.1186/s12885-015-1947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grumpelt AM, Ignatov A, Tchaikovski SN, et al. Tumor characteristics and therapy of elderly patients with breast cancer. J Cancer Res Clin Oncol. 2016;142:1109–1116. doi: 10.1007/s00432-015-2111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inwald EC, Ortmann O, Koller M, et al. Screening-relevant age threshold of 70 years and older is a stronger determinant for the choice of adjuvant treatment in breast cancer patients than tumor biology. Breast Cancer Res Treat. 2017;163:119–130. doi: 10.1007/s10549-017-4151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaloshi G, Psimaras D, Mokhtari K, et al. Supratentorial low-grade gliomas in older patients. Neurology. 2009;73:2093–2098. doi: 10.1212/WNL.0b013e3181c6781e. [DOI] [PubMed] [Google Scholar]
- 33.Pignata S, Gallo C, Daniele B, et al. Characteristics at presentation and outcome of hepatocellular carcinoma (HCC) in the elderly. A study of the Cancer of the Liver Italian Program (CLIP) Crit Rev Oncol Hematol. 2006;59:243–249. doi: 10.1016/j.critrevonc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Shervani S, Lu G, Sager K, et al. Prognostic factors and hazard ratios in colorectal cancer patients over 80 years of age: A retrospective, 20-year, single institution review. J Gastrointest Oncol. 2018;9:254–262. doi: 10.21037/jgo.2018.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. doi: 10.1245/s10434-013-3115-2. Weiss A, Noorbakhsh A, Tokin C, et al: Hormone receptor-negative breast cancer: Undertreatment of patients over 80. Ann Surg Oncol 20:3274-3278, 2013 [Erratum: Ann Surg Oncol 21:S785, 2014] [DOI] [PubMed] [Google Scholar]
- 36.Deng F, Xu X, Lv M, et al. Age is associated with prognosis in serous ovarian carcinoma. J Ovarian Res. 2017;10:36. doi: 10.1186/s13048-017-0331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herlemann A, Buchner A, Kretschmer A, et al. Postoperative upgrading of prostate cancer in men ≥75 years: A propensity score-matched analysis. World J Urol. 2017;35:1517–1524. doi: 10.1007/s00345-017-2045-1. [DOI] [PubMed] [Google Scholar]
- 38.Horton JK, Gleason JF, Jr, Klepin HD, et al. Age-related disparities in the use of radiotherapy for treatment of localized soft tissue sarcoma. Cancer. 2011;117:4033–4040. doi: 10.1002/cncr.25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monroe MM, Myers JN, Kupferman ME. Undertreatment of thick head and neck melanomas: An age-based analysis. Ann Surg Oncol. 2013;20:4362–4369. doi: 10.1245/s10434-013-3160-x. [DOI] [PubMed] [Google Scholar]
- 40.Sacher AG, Le LW, Lau A, et al. Real-world chemotherapy treatment patterns in metastatic non-small cell lung cancer: Are patients undertreated? Cancer. 2015;121:2562–2569. doi: 10.1002/cncr.29386. [DOI] [PubMed] [Google Scholar]
- 41.Akre O, Garmo H, Adolfsson J, et al. Mortality among men with locally advanced prostate cancer managed with noncurative intent: A nationwide study in PCBaSe Sweden. Eur Urol. 2011;60:554–563. doi: 10.1016/j.eururo.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez R, Esteves S, Chacim S, et al. What determines therapeutic choices for elderly patients with DLBCL? Clinical findings of a multicenter study in Portugal. Clin Lymphoma Myeloma Leuk. 2014;14:370–379. doi: 10.1016/j.clml.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21:3580–3587. doi: 10.1200/JCO.2003.02.046. [DOI] [PubMed] [Google Scholar]
- 44.Eggemann H, Ignatov T, Burger E, et al. Management of elderly women with endometrial cancer. Gynecol Oncol. 2017;146:519–524. doi: 10.1016/j.ygyno.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Eggemann H, Ignatov T, Geyken CH, et al. Management of elderly women with cervical cancer. J Cancer Res Clin Oncol. 2018;144:961–967. doi: 10.1007/s00432-018-2617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu N, Molena D, Stem M, et al. Underutilization of treatment for regional gastric cancer among the elderly in the USA. J Gastrointest Surg. 2018;22:955–963. doi: 10.1007/s11605-018-3691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. doi: 10.1016/j.clgc.2017.07.011. Parker WP, Smelser W, Lee EK, et al: Utilization and outcomes of radical cystectomy for high-grade non-muscle-invasive bladder cancer in elderly patients. Clin Genitourin Cancer 16:PE79-PE97, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Peake MD, Thompson S, Lowe D, et al. Ageism in the management of lung cancer. Age Ageing. 2003;32:171–177. doi: 10.1093/ageing/32.2.171. [DOI] [PubMed] [Google Scholar]
- 49.Pignata S, Ferrandina G, Scarfone G, et al. Poor outcome of elderly patients with platinum-sensitive recurrent ovarian cancer: Results from the SOCRATES retrospective study. Crit Rev Oncol Hematol. 2009;71:233–241. doi: 10.1016/j.critrevonc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Serra-Rexach JA, Jimenez AB, García-Alhambra MA, et al. Differences in the therapeutic approach to colorectal cancer in young and elderly patients. Oncologist. 2012;17:1277–1285. doi: 10.1634/theoncologist.2012-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takuwa H, Tsuji W, Yotsumoto F. Overall survival of elderly patients with breast cancer is not related to breast-cancer specific survival: A single institution experience in Japan. Breast Dis. 2018;37:177–183. doi: 10.3233/BD-170280. [DOI] [PubMed] [Google Scholar]
- 52.Yamano T, Yamauchi S, Kimura K, et al. Influence of age and comorbidity on prognosis and application of adjuvant chemotherapy in elderly Japanese patients with colorectal cancer: A retrospective multicentre study. Eur J Cancer. 2017;81:90–101. doi: 10.1016/j.ejca.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 53.Tran P, Fentiman IS. Better treatment for breast cancer in older patients. Expert Rev Anticancer Ther. 2009;9:1081–1090. doi: 10.1586/era.09.67. [DOI] [PubMed] [Google Scholar]
- 54.Lunardi P, Ploussard G, Grosclaude P, et al. Current impact of age and comorbidity assessment on prostate cancer treatment choice and over/undertreatment risk. World J Urol. 2017;35:587–593. doi: 10.1007/s00345-016-1900-9. [DOI] [PubMed] [Google Scholar]
- 55.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32:2587–2594. doi: 10.1200/JCO.2013.55.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burstein HJ, Krilov L, Aragon-Ching JB, et al. Clinical cancer advances 2017: Annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1341–1367. doi: 10.1200/JCO.2016.71.5292. [DOI] [PubMed] [Google Scholar]
- 57.Mailankody S, Prasad V. Overall survival vs disease-specific survival-reply. JAMA Oncol. 2018;4:586–587. doi: 10.1001/jamaoncol.2017.3865. [DOI] [PubMed] [Google Scholar]
- 58.Hwang TJ, Gyawali B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int J Cancer. 2019;144:1746–1751. doi: 10.1002/ijc.31957. [DOI] [PubMed] [Google Scholar]
- 59.Chen EY, Raghunathan V, Prasad V. An overview of cancer drugs approved by the US Food and Drug Administration based on the surrogate end point of response rate. JAMA Intern Med. 2019;179:915–921. doi: 10.1001/jamainternmed.2019.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med. 2019;179:906–913. doi: 10.1001/jamainternmed.2019.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Celis ESPD, Li D, Sun C-L, et al: Patient-defined goals and preferences among older adults with cancer starting chemotherapy (CT). J Clin Oncol 36, 2018 (suppl; abstr 10009) [Google Scholar]
- 62.Gyawali B, Niraula S. Cancer treatment in the last 6 months of life: When inaction can outperform action. Ecancermedicalscience. 2018;12:826. doi: 10.3332/ecancer.2018.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity Patient-centered care for older adults with multiple chronic conditions: A stepwise approach from the American Geriatrics Society: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60:1957–1968. doi: 10.1111/j.1532-5415.2012.04187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee H, Shi SM, Kim DH. Home time as a patient-centered outcome in administrative claims data. J Am Geriatr Soc. 2019;67:347–351. doi: 10.1111/jgs.15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rocque GB, Williams GR. Bridging the data-free zone: Decision making for older adults with cancer. J Clin Oncol. 2019;37:3469–3471. doi: 10.1200/JCO.19.02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cnossen MC, van Essen TA, Ceyisakar IE, et al. Adjusting for confounding by indication in observational studies: A case study in traumatic brain injury. Clin Epidemiol. 2018;10:841–852. doi: 10.2147/CLEP.S154500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 68.Clough-Gorr KM, Stuck AE, Thwin SS, et al. Older breast cancer survivors: Geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28:380–386. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohile SG, Magnuson A, Pandya C, et al. Community oncologists’ decision-making for treatment of older patients with cancer. J Natl Compr Canc Netw. 2018;16:301–309. doi: 10.6004/jnccn.2017.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw. 2017;15:894–902. doi: 10.6004/jnccn.2017.0122. [DOI] [PubMed] [Google Scholar]
- 71.Chaïbi P, Magné N, Breton S, et al. Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients. Crit Rev Oncol Hematol. 2011;79:302–307. doi: 10.1016/j.critrevonc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Clough-Gorr KM, Thwin SS, Stuck AE, et al. Examining five- and ten-year survival in older women with breast cancer using cancer-specific geriatric assessment. Eur J Cancer. 2012;48:805–812. doi: 10.1016/j.ejca.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caires-Lima R, Cayres K, Protásio B, et al. Palliative chemotherapy outcomes in patients with ECOG-PS higher than 1. Ecancermedicalscience. 2018;12:831. doi: 10.3332/ecancer.2018.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Audisio RA. Preoperative evaluation of the older patient with cancer. J Geriatr Oncol. 2016;7:409–412. doi: 10.1016/j.jgo.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Björkholm M, Svedmyr E, Sjöberg J. How we treat elderly patients with Hodgkin lymphoma. Curr Opin Oncol. 2011;23:421–428. doi: 10.1097/CCO.0b013e328348c6c1. [DOI] [PubMed] [Google Scholar]
- 77.Rosko A, Giralt S, Mateos MV, et al. Myeloma in elderly patients: When less is more and more is more. Am Soc Clin Oncol Educ Book. 2017;37:575–585. doi: 10.14694/EDBK_175171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang V, Zhao S, Boscardin J, et al. Functional status and survival after breast cancer surgery in nursing home residents. JAMA Surg. 2018;153:1090–1096. doi: 10.1001/jamasurg.2018.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wynter-Blyth V, Moorthy K. Prehabilitation: Preparing patients for surgery. BMJ. 2017;358:j3702. doi: 10.1136/bmj.j3702. [DOI] [PubMed] [Google Scholar]
- 80. National Cancer Institute: NCI Dictionary of Cancer Terms: Overtreatment. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/overtreatment.
- 81.Le Saux O, Falandry C, Gan HK, et al. Changes in the use of end points in clinical trials for elderly cancer patients over time. Ann Oncol. 2017;28:2606–2611. doi: 10.1093/annonc/mdx354. [DOI] [PubMed] [Google Scholar]
- 82.Byrne A, Carney DN. Cancer in the elderly. Curr Probl Cancer. 1993;17:149–218. doi: 10.1016/0147-0272(93)90007-o. [DOI] [PubMed] [Google Scholar]
- 83.Cheong KA, Chrystal K, Harper PG. Management of the elderly patient with advanced non-small cell lung cancer. Int J Clin Pract. 2006;60:340–343. doi: 10.1111/j.1368-5031.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- 84.Daskivich TJ, Tan HJ, Litwin MS, et al. Life expectancy and variation in treatment for early stage kidney cancer. J Urol. 2016;196:672–677. doi: 10.1016/j.juro.2016.03.133. [DOI] [PubMed] [Google Scholar]
- 85.Kong TK. Managing the elderly person with cancer: A geriatrician’s perspective. Hong Kong J Radiol. 2013;16:209–218. [Google Scholar]
- 86.Tinetti M, Dindo L, Smith CD, et al. Challenges and strategies in patients’ health priorities-aligned decision-making for older adults with multiple chronic conditions. PLoS One. 2019;14:e0218249. doi: 10.1371/journal.pone.0218249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179:1688. doi: 10.1001/jamainternmed.2019.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fried TR, Tinetti ME, Iannone L, et al. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171:1858–1858. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dimitrakopoulos FI, Kottorou A, Antonacopoulou AG, et al. Early-stage breast cancer in the elderly: Confronting an old clinical problem. J Breast Cancer. 2015;18:207–217. doi: 10.4048/jbc.2015.18.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) Lancet Oncol. 2012;13:e148–e160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 91.Levit LA, Singh H, Klepin HD, et al. Expanding the evidence base in geriatric oncology: Action items from an FDA-ASCO workshop. J Natl Cancer Inst. 2018;110:1163–1170. doi: 10.1093/jnci/djy169. [DOI] [PubMed] [Google Scholar]
- 92.Djulbegovic B, Elqayam S, Dale W. Rational decision making in medicine: Implications for overuse and underuse. J Eval Clin Pract. 2018;24:655–665. doi: 10.1111/jep.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: A quarter century on. Lancet. 2017;390:415–423. doi: 10.1016/S0140-6736(16)31592-6. [DOI] [PubMed] [Google Scholar]
- 94.Corre R, Greillier L, Le Caër H, et al. Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non-small-cell lung cancer: The phase III randomized ESOGIA-GFPC-GECP 08-02 study. J Clin Oncol. 2016;34:1476–1483. doi: 10.1200/JCO.2015.63.5839. [DOI] [PubMed] [Google Scholar]
- 95.Gajra A, Loh KP, Hurria A, et al. Comprehensive geriatric assessment-guided therapy does improve outcomes of older patients with advanced lung cancer. J Clin Oncol. 2016;34:4047–4048. doi: 10.1200/JCO.2016.67.5926. [DOI] [PubMed] [Google Scholar]
- 96.Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet. 2017;389:519–527. doi: 10.1016/S0140-6736(16)31594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hall PS, Swinson D, Waters JS, et al: Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): The GO2 phase III trial. J Clin Oncol 37, 2019 (suppl; abstr 4006) [Google Scholar]