Abstract
PURPOSE
Three new therapies have been approved recently for the adjuvant treatment of stage III melanoma, substantially reducing the risk of tumor recurrences. This study evaluates 3 independent data sets to clarify the survival probabilities of patients with stage III melanoma.
PATIENTS AND METHODS
The Central Malignant Melanoma Registry (CMMR) evaluated 1,553 patients with a primary diagnosis of stage III melanoma from 2000 to 2012. Studies from the European Organisation for Research and Treatment of Cancer (EORTC), of 573 patients in the observation arm of the 18991 study and 445 patients in the placebo arm of the 18071 study, were evaluated as reference cohorts. The survival outcomes were compared with the published American Joint Committee on Cancer version 8 (AJCCv8) stage III survival data.
RESULTS
For the CMMR stage III cohort versus the AJCCv8 cohort, the melanoma-specific survival (MSS) rates at 5 years were 67% versus 77%, and at 10 years were 56% versus 69%, respectively. For stage IIIA, the MSS rates at 5 years were 80% versus 93%, and at 10 years were 71% versus 88%; for stage IIIB, the MSS rates at 5 years were 75% versus 83%, and at 10 years were 61% versus 77%. The MSS rates of the EORTC studies either overlapped with or were lower than, the CMMR data.
CONCLUSION
The MSS rates in the CMMR and EORTC cohorts over the entire stage III are less favorable than those published in AJCCv8. This is particularly true for substages IIIA and IIIB.
INTRODUCTION
The incidence of cutaneous melanoma has been increasing continuously for decades, and so far, no change in trend has been observed in Europe or the United States.1 The proportion of metastatic melanomas is largely stable and shows an increasing trend in absolute numbers,2 including patients with regional lymph node or skin metastasis classified as stage III in the 8th version of the American Joint Committee on Cancer (AJCC) classification (AJCCv8).
CONTEXT
Key Objectives
Is the prognosis of patients with stage III melanoma, especially IIIA and IIIB, really as favorable as that published in the American Joint Committee on Cancer version 8 (AJCCv8) classification?
Knowledge Generated
In 3 independent cohorts of patients with stage III disease in the Central Malignant Melanoma Registry and the European Organisation for Research and Treatment of Cancer studies 18991 and 18071, we found significantly less favorable survival probabilities than those published in the AJCCv8 classification. The 5-year melanoma-specific survival rates were 73% to 80% in stage IIIA, instead of 93% according to AJCCv8, and 56% to 75% in stage IIIB, instead of 83% according to AJCCv8.
Relevance
The difference shown here should be taken into account in clinical decision making (eg, on initiation of adjuvant therapy) and in the planning of clinical trials.
A new era of adjuvant therapy has begun for stage III melanoma.3 Two immunotherapies and 1 targeted therapy were approved for stage III melanoma in 2018 in Europe and the United States on the basis of improved relapse-free, distant metastases-free, and in part overall survival (OS) benefits.4 The exact prognostic outcomes should be taken into consideration in adjuvant treatment recommendations.5
The TNM classification of solid tumors, as established by the AJCC and the Union International Control Cancer, provides the backbone for prognostic classification and treatment decisions for solid tumors.6 The first AJCC classification of melanoma was published in 1977 and was valid until 1983.7 Starting with the AJCCv6 classification in 2002, Breslow's tumor thickness and ulceration were used for the T classification, and the distinction between micro- and macrometastasis and the number of nodes was used for the N classification.8 The AJCCv7 classification of melanoma contained only small differences compared with the 6th classification.9 The AJCCv8 of melanoma contains minor changes for the T classification, the N classification has been changed in several aspects, and substages of stage III disease have been newly defined.10
In the AJCCv8 classification, stage III melanoma is defined both by the N classification with 9 subgroups and by the T classification, also with 9 subgroups, resulting in 81 different combination possibilities, which in 78 cases become relevant for the stage classification and makes staging complicated.11 The combinations are presented in a field diagram and then assigned to the prognostically relevant substages of stage III: the 5- and 10-year melanoma-specific survival (MSS) rates for stage IIIA (combining 6 fields) were 93% and 88%, respectively; for stage IIIB (combining 19 fields), 83% and 77%, respectively; for stage IIIC (combining 48 fields), 69% and 60%, respectively; and for stage IIID (combining 3 fields), 32% and 24%, respectively. For the entire stage III population, the 5- and 10-year MMS rate were 77% and 69%, respectively.10
The favorable outcome in both stage IIIA and stage IIIB, as well as throughout stage III, in the AJCCv8 was considered surprising and can be explained partially by upstaging to stage III some patients who were classified previously as stage II. This has stirred vigorous discussions in the community regarding acceptable risk-benefit ratios and whether adjuvant therapy should be recommended at all to patients in stage IIIA or IIIB.12,13
Taking that into consideration, the current study examines the prognosis of patients with melanoma in stage III and its substages according to the definitions of the AJCCv8 classification. A database of the German Central Malignant Melanoma Registry (CMMR) containing patients with primary stage III melanoma diagnosed from 2000 to 2012 is used for this purpose. Furthermore, 2 databases from studies of the European Organisation for Research and Treatment of Cancer (EORTC) are analyzed, so that the prognostic course can be verified independently on the basis of 3 different data sets. The results of these analyses are of particular relevance because a thorough risk-benefit evaluation is of the utmost importance, especially in stages IIIA and IIIB, but also in stage III as a whole, for patient information and treatment recommendations, and also for future clinical study designs.
PATIENTS AND METHODS
Three Patient Cohorts
CMMR cohort.
Between January 2000 and December 2012, 60,289 patients with melanoma were documented in the nationwide CMMR.14 Only patients with cutaneous melanoma presenting with stage III at primary diagnosis were included in the current analysis. In the period from 2000 to 2012, patients with primary melanoma in stage IB and higher were staged with sentinel lymph node biopsy. Patients with ocular or mucosal melanoma and follow-up of less than 3 months were excluded. Follow-up was performed according to the valid version of the guidelines of the German Society of Dermatology. For stage III patients, physical examination, lymph node ultrasound, and blood testing including the tumor marker protein S100B, were performed every 3 months; whole-body computed tomography scans and brain magnetic resonance imaging were carried out every 6 months.
Reference cohorts: EORTC 18991 and 18071 trials.
To estimate the validity and reliability of the CMMR survival data, we used 2 reference cohorts from EORTC studies conducted in patients with stage III melanoma over the past 15 years. The first reference cohort consisted of patients from the observation arm of the EORTC 18991 study (recruitment period, June 2000 through August 2003), in which adjuvant treatment with pegylated interferon-alpha was tested.15,16 The second reference cohort consisted of patients from the placebo arm of the EORTC 18071 study (recruitment period from July 2008 to August 2011), which tested adjuvant treatment with ipilimumab.17-19 In both studies, eligible patients underwent complete regional lymphadenectomy and had not received previous systemic therapy for melanoma. Patients with in-transit metastasis (EORTC 18991 and 18071) and those with 1-3 lymph node micrometastases ≤ 1 mm in diameter (EORTC 18071) were excluded. Both patient cohorts are described in detail in the original publications.16,18
Outcomes
The outcomes of interest were MSS and OS. MSS was defined as the time between the date of primary diagnosis of stage III cutaneous melanoma and the date of death from melanoma; the follow-up of patients still alive was censored at the last date to be alive, and of those who died as a result of other causes, at the date of death. OS was calculated from the date of primary diagnosis of stage III cutaneous melanoma to the date of last follow-up (censored observation) or the date of death as a result of any cause. For the EORTC trials, the starting point of these end points was the date of random assignment.
Statistical Analysis
Quantitative clinical and histopathologic data were expressed as medians with interquartile ranges (IQRs), and categoric data were presented as absolute numbers and proportions. The Kaplan-Meier method was used to estimate MSS and OS, and differences between the substages IIIA, IIIB, IIIC, and IIID were assessed by means of the log-rank test. Estimated survival rates were expressed as percentages with standard errors. Cumulative incidences of death as a result of melanoma and not as a result of melanoma were estimated using competing risk methods.
All statistical tests were 2 sided, with a P value < .05 considered statistically significant. Statistical calculations were performed with IBM SPSS Statistics Version 25.0 (IBM SPSS, Chicago, IL) or SAS Version 6.4 (Cary, NC) statistical software.
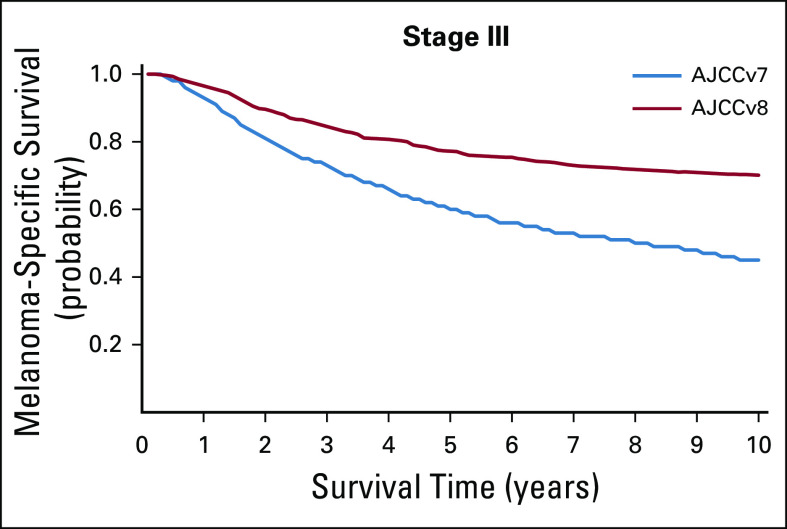
In sensitivity analyses, we compared the MSS curves for stage III patients classified according to AJCCv8 with those classified according to AJCCv7. For this purpose, Kaplan-Meier survival curves were identified from 2 publications describing the AJCCv79,20 and the AJCCv8.10 The curves were scanned, extracted, and digitized manually using an interactive digitizing software21 as described previously.22,23 This software creates sampling points and allows curve construction by linear interpolation between these points.
RESULTS
Patient Characteristics
Clinical and histopathologic characteristics are summarized in Table 1. The CMMR cohort comprised 1,553 stage III patients according to the AJCCv8, of whom 41.3% were classified as stage IIIC. Only patients with data for AJCCv8 classification were selected. Because of incomplete information, 664 patients from a total of 2,217 patients were excluded, which corresponds to 30% of the whole stage III CMMR collective. There were no differences in terms of age and sex between the patients included and excluded. There was a statistically significant difference in tumor characteristics: patients excluded from the analysis had a median tumor thickness of 4.95 mm compared with 2.80 mm in patients included (P < .001). A higher proportion of patients excluded had stage IIIC (AJCCv7) disease compared with patients included in the analysis (65.2% v 30.7%; P < .001).
TABLE 1.
Clinical and Histopathologic Characteristics of the CMMR Cohort and the EORTC 18991 and 18071 Cohorts of Patients With Stage III Melanoma, and Their Follow-Up
The stage III melanoma cohort of the EORTC 18991 trial comprised 573 patients from the observation arm, and the stage III cohort of the EORTC 18071 trial consisted of 445 patients from the placebo arm. In both studies, only patients with complete data were included in this analysis. A total of 56 patients (8.9%) in the EORTC 18991 study and 31 patients (6.5%) in the EORTC 18701 study were excluded. There was no significant difference in terms of age and sex between the 2 groups of excluded and included patients. Tumor thickness was slightly higher in the excluded patients compared with the included patients: in the EORTC 18991 study, 2.8 mm versus 2.2 mm, and in the EORTC 18701 study, 2.5 mm versus 2.2 mm, respectively.
In the CMMR cohort, melanoma-specific death occurred in 441 patients (28.4%) and death from other cause in 66 patients (4.2%). The median follow-up time was 58.0 months (IQR, 34.0-93.0 months). In the EORTC studies 18991 and 18071, melanoma-specific deaths occurred in 297 patients (51.8%) and in 200 patients (44.9%), respectively. In the 18991 study, 14 patients (2.4%), and in the 18071 study, 7 patients (1.6%), died as a result of other causes. The median follow-up time was 90.5 months (IQR, 54.5-99.4 months) in the EORTC 18991 cohort and 86.4 months (IQR, 64.4-97.2 months) in the EORTC 18071 cohort.
Survival Analysis
We compared the MSS rates for all 3 cohorts with data from the International Melanoma Database and Discovery Platform (IMDDP) analysis presented in the publication of the AJCCv8 classification (Table 2).10 In the IMDDP analysis, the 5- and 10-year MSS rates for all stage III patients were 77% and 69%, higher than those calculated for all our collectives. In the CMMR cohort, the 5- and 10-year MSS rates were 67% and 56%. In the EORTC 18991 and 18071 studies, the 5-year MSS rates were even lower (52.7% and 55.5%, respectively), whereas the 10-year MSS rates were not available.
TABLE 2.
Estimated MSS Rates at 5, 7, and 10 Years Corresponding to IMDDP and CMMR Databases and EORTC 18991 and EORTC 18071 Trials, in Patients With Stage III Melanoma According to the AJCCv8 Classification
The 5-year stage-specific MSS in the IMDDP cohort was 93% in stage IIIA, 83% in stage IIIB, 69% in stage IIIC, and 32% in stage IIID. The 5-year MSS rates in the CMMR cohort and in EORTC cohorts 18991 and 18071 were systematically lower: in stage IIIA, 80% (18991: 80%; 18071: 73%); in stage IIIB, 75% (18991: 56%; 18071: 66%); in stage IIIC, 56% (18991: 42%; 18071: 48%); and in stage IIID, 30% (18991: 20%; 18071: 28%; Table 2, Figs 1-3).
FIG 1.

Kaplan-Meier curves for melanoma-specific survival (MSS) in the Central Malignant Melanoma Registry cohort. (A) In all stage III patients. (B) According to American Joint Committee on Cancer version 8 (AJCCv8) classification: stage IIIA, IIIB, IIIC, and IIID. HR, hazard ratio.
FIG 3.

Kaplan-Meier curves for melanoma-specific survival (MSS) in European Organisation for Research and Treatment of Cancer cohort 18071, placebo group. (A) In all stage III patients. (B) According to American Joint Committee on Cancer version 8 (AJCCv8) classification: stage IIIA, IIIB, IIIC, and IIID. HR, hazard ratio.
FIG 2.

Kaplan-Meier curves for melanoma-specific survival (MSS) in European Organisation for Research and Treatment of Cancer cohort 18991, observation group. (A) In all stage III patients. (B) According to American Joint Committee on Cancer version 8 (AJCCv8) classification: stage IIIA, IIIB, IIIC, and IIID. HR, hazard ratio.
For the study cohorts CMMR and EORTC 18991 and 18071, the estimated OS rates and OS curves are presented in the Appendix Table A1 and Appendix Figs A1-A3 (online only). Because the rates of nonmelanoma causes of death were < 5% (Table 1), the OS and MSS rates were similar; the difference was between 2% and 5%. The 5-, 7-, and 10-year cumulative incidences of death as a result of melanoma and not as a result of melanoma are indicated in the Appendix (Table A2, online only). For the CMMR cohort, the 10-year cumulative incidence of death as a result of melanoma was 28.8% for stage IIIA patients and 37.7% for stage IIIB patients.
DISCUSSION
In this investigation, we show that MSS in AJJCv8 stage III is less favorable in 3 independent databases than as reported by the IMDDP database leading to the AJCCv8 classification of melanoma. This is true for the prognosis of the entire stage III cohort and is particularly true for the substages IIIA and IIIB. According to the IMDDP data, on which the survival calculations of the AJCCv8 classification of melanoma are based, the 5- and 10-year MSS rates in stage IIIA were 93% and 88%, and in stage IIIB, 83% and 77%. Corresponding MSS rates from the CMMR at 5 and 10 years in stage IIIA were 80% and 71%, respectively, and in stage IIIB, 75% and 61%, respectively. Therefore, the Kaplan-Meier estimation of the 10-year rate of dying as a result of melanoma in stage IIIA was 12% according to AJCCv8 data and 29% according to CMMR data. In stage IIIB, it was 23% according to AJCCv8 data and 38% according to CMMR data. The CMMR outcomes in stage III melanoma were similar to the data from the EORTC 18991 and 18071 studies. The data of the CMMR, as well as both EORTC cohorts, are also well in line with 2 German cohorts published recently that reported 5-year MSS rates of 89% for stage IIIA, 73% for stage IIIB, 56% for stage IIIC, and 52% for stage IIID.24 Moreover, another study, by the Swedish cancer registry in 2,067 patients, reported 5- and 10-year MSS rates of 87% and 80% for stage IIIA, 69% and 55% for stage IIIB, and 50% and 43% for stage IIIC.25
Three adjuvant stage III therapies for melanoma have been approved since 2018, all of which are effective in extending relapse-free survival, distant-metastases–free survival and (in part) OS.26 First, it was shown that adjuvant treatment with ipilimumab versus placebo in stage III melanoma resulted in a hazard ratio for relapse-free survival of 0.76, and another study comparing nivolumab and ipilimumab for relapse-free survival showed a hazard ratio of 0.65 (product value of both hazard ratios = 0.49).4,18,27 Nivolumab was subsequently approved in both the United States and Europe for the adjuvant treatment of stage III melanoma.5, The estimated hazard ratio for relapse-free survival comparing the programmed cell death protein 1 antibody pembrolizumab with placebo was 0.57, and for the targeted therapy (with the combination of dabrafenib and trametinib) with placebo was 0.47. Both therapies were approved for adjuvant treatment in the United States, Europe, and many other countries.28,29 The estimated hazard ratios for distant-metastasis–free survival were generally in the same range as for relapse-free survival. At a median follow-up of 2.8 years, the combined treatment with dabrafenib and trametinib showed promising results regarding OS (estimated hazard ratio, 0.57), but more mature follow-up results are required to draw conclusions.29 Overall, there is no other cancer for which such effective adjuvant therapies are available.
The survival curves generated from the IMDDP database leading to the AJCC classification have a great impact on the clinical management of patients with melanoma.20,30 They are used for patient information, risk calculation, and decision making, including risk-benefit evaluations of therapeutic measures (eg, adjuvant therapies). Furthermore, the AJCC survival estimates are of great value because they are used for sample size calculations in the planning of clinical studies. Survival curves are critically dependent on the completeness and length of the follow-up of all patients. Therefore, loss of follow-up is a critical quality parameter that is not reported in the IMDDP publication.
Interesting in this context is the comparison of the MSS rates of the entire stage III patients from the AJCCv7 and AJCCv8 publications. Despite the fact that the definitions of the entire stage III remained unchanged between AJCCv7 and AJCCv8, a large difference was observed regarding MSS between these 2 versions. For stage III, the 5-year MSS rates were 60% for AJCCv7 and 77% for AJCCv8, and the 10-year MSS rates were 45% for AJCCv7 and 69% for AJCCv8 (Appendix Fig A4, online only). These differences in MSS cannot be explained by any therapeutic progress.
How could these apparently favorable survival data in the IMPDD cohort come into existence? The most likely explanation is an underreporting of melanoma-related deaths. The IMDDP database consists of a total of 10 clinical registry data sets going back to 1998, collected in different centers, under different national and cultural conditions, which also implicates different follow-up schedules. In such data sets, the recording of deaths is a critical matter, because many patients do not die in hospitals. For future evaluations of relevant survival data, not only MSS should be calculated, but also OS, which includes all different causes of death. If there are significant differences between the 2 curves, it is likely that melanoma-specific deaths have been under-recorded.
The databases of the CMMR and of the EORTC studies were created using a prospective data collection. The clinical trials are particularly carefully controlled for deaths. The CMMR also systematically identifies deaths by asking residents' registration offices. In this respect, a high quality of the evaluated data can be assumed.
One disadvantage of these databases is that they do not contain population-related data, but this is also the case for the IMDDP data set used for the AJCCv8 classification of melanoma. Regarding the EORTC data, 1 limitation is that there was a trial-specific selection; however, this probably excluded only a small proportion of patients. In the EORTC 18071 study, stage IIIA patients with a more favorable prognosis (1-3 micrometastases, ≤ 1 mm in the greatest diameter) were excluded, which may explain in part the less favorable prognosis of this collective in comparison with the CMMR data.
In conclusion, the data presented here may indicate that MSS in stage III according to the AJCCv8 classification of melanoma is less favorable than that published for the IMDDP cohort. This is particularly true for stages IIIA and IIIB.
ACKNOWLEDGMENT
We thank the German Central Malignant Melanoma Registry and the European Organisation for Research and Treatment of Cancer.
Appendix
FIG A1.

Kaplan-Meier curves for overall survival in the Central Malignant Melanoma Registry cohort. (A) In all stage III patients. (B) According to American Joint Committee on Cancer version 8 (AJCCv8) classification: stage IIIA, IIIB, IIIC, and IIID. HR, hazard ratio.
FIG A2.

Kaplan-Meier curves for overall survival in the European Organisation for Research and Treatment of Cancer cohort 18991, observation group. (A) In all stage III patients. (B) According to American Joint Committee on Cancer version 8 (AJCCv8) classification: stage IIIA, IIIB, IIIC, and IIID. HR, hazard ratio.
FIG A3.

Kaplan-Meier curves for overall survival in the European Organisation for Research and Treatment of Cancer cohort 18071, placebo group. (A) In all stage III patients. (B) according to American Joint Committee on Cancer version 8 (AJCCv8) classification: stage IIIA, IIIB, IIIC, and IIID. HR, hazard ratio.
FIG A4.
Reconstructed melanoma-specific survival curves for patients staged according to American Joint Committee on Cancer version 7 (AJCCv7) and American Joint Committee on Cancer version 8 (AJCCv8) for all stage III patients.
TABLE A1.
Estimated OS Rates at 5, 7, and 10 Years of CMMR Database and EORTC 18991 and EORTC 18071 Trials in Patients With Stage III Melanoma According to AJCCv8 Classification
TABLE A2.
Estimated Cumulative Incidence of Death as a Result of Melanoma and as a Result of Another Cause at 5, 7, and 10 Years for the CMMR Database and EORTC 18991 and EORTC 18071 Trials in Patients With Stage III Melanoma
SUPPORT
Supported by the German Central Malignant Melanoma Registry and by the European Organisation for Research and Treatment of Cancer.
AUTHOR CONTRIBUTIONS
Conception and design: Claus Garbe, Stefan Suciu, Teresa Amaral, Axel Hauschild, Dirk Schadendorf, Rudolf Stadler, Ulrich Keilholz, Alessandro Testori, Ulrike Leiter, Alexander M. M. Eggermont
Financial support: Rudolf Stadler
Administrative support: Thomas K. Eigentler, Rudolf Stadler
Provision of study material or patients: Axel Hauschild, Lucie Heinzerling, Dirk Schadendorf, Rudolf Stadler, Cord Sunderkötter, Thomas Tüting, Jochen Utikal, Uwe Wollina, Christos C. Zouboulis, Ulrich Keilholz, Alessandro Testori, Alexander M. M. Eggermont
Collection and assembly of data: Claus Garbe, Ulrike Keim, Stefan Suciu, Teresa Amaral, Thomas K. Eigentler, Anja Gesierich, Axel Hauschild, Lucie Heinzerling, Felix Kiecker, Dirk Schadendorf, Rudolf Stadler, Cord Sunderkötter, Thomas Tüting, Jochen Utikal, Uwe Wollina, Christos C. Zouboulis, Alessandro Testori, Ulrike Leiter
Data analysis and interpretation: Claus Garbe, Ulrike Keim, Stefan Suciu, Teresa Amaral, Thomas K. Eigentler, Axel Hauschild, Lucie Heinzerling, Dirk Schadendorf, Rudolf Stadler, Cord Sunderkötter, Uwe Wollina, Ulrich Keilholz, Alessandro Testori, Peter Martus, Ulrike Leiter
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prognosis of Patients With Stage III Melanoma According to American Joint Committee on Cancer Version 8: A Reassessment on the Basis of 3 Independent Stage III Melanoma Cohorts
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Claus Garbe
Honoraria: Amgen, Bristol Myers Squibb, MSD Oncology, NeraCare GmbH, Novartis, Philogen, Roche/Genentech, Sanofi
Consulting or Advisory Role: Amgen, Bristol Myers Squibb, MSD Oncology, NeraCare GmbH, Novartis, Philogen, Roche/Genentech, Sanofi
Research Funding: Bristol Myers Squibb, Novartis, NeraCare GmbH, Roche/Genentech
Teresa Amaral
Honoraria: Bristol Myers Squibb, Pierre Fabre
Research Funding: Novartis (Inst), NeraCare GmbH (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Novartis, Bristol Myers Squibb
Thomas K. Eigentler
Consulting or Advisory Role: Bristol Myers Squibb, Roche Pharma AG, MSD, Pierre Fabre, LEO Pharma, Sanofi/Regeneron, Novartis
Speakers' Bureau: Roche Pharma AG, MSD, Bristol Myers Squibb, Novartis
Anja Gesierich
Honoraria: Novartis, Bristol Myers Squibb, Roche Pharma AG
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, MSD Oncology, Roche Pharma AG, Pierre Fabre, Sanofi/Aventis, Pfizer
Travel, Accommodations, Expenses: Novartis, Bristol Myers Squibb, Roche Pharma AG, MSD Oncology, Pierre Fabre
Axel Hauschild
Honoraria: Amgen, Bristol Myers Squibb, Merck Serono, Merck Sharp & Dohme, Novartis, OncoSec, Roche, Philogen, Provectus, Regeneron, Sanofi/Aventis
Consulting or Advisory Role: Amgen, Bristol Myers Squibb, Merck Serono, Merck Sharp & Dohme, Novartis, OncoSec, Roche, Philogen, Provectus, Regeneron, Sanofi/Aventis
Research Funding: Amgen, Bristol Myers Squibb, Merck Serono, Merck Sharp & Dohme, Novartis, Roche, Regeneron
Travel, Accommodations, Expenses: Amgen, Bristol Myers Squibb, Merck Serono, Merck Sharp & Dohme, Novartis, Roche, Sanofi/Aventis
Lucie Heinzerling
Consulting or Advisory Role: Bristol Myers Squibb, MAS, Sanofi, Pierre Fabre, Amgen, Curevac, Roche, Novartis
Research Funding: Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Predictive and soluble prognostic biomarker
Dirk Schadendorf
Honoraria: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Immunocore, Merck Serono, Array BioPharma, Incyte, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Mologen, Sanofi/Regeneron, Neracare, Sun Pharma, InflarxGmbH, Ultimovacs, Sandoz
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Incyte, 4SC, Pierre Fabre, Mologen, Sanofi/Regeneron
Speakers' Bureau: Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Incyte, Pierre Fabre, Sanofi/Regeneron, Merck KGaA
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Bristol Myers Squibb, Merck Serono, Novartis, Merck Sharp & Dohme, Pierre Fabre, Sanofi/Regeneron
Rudolf Stadler
Honoraria: 4SC, Takeda, Merck
Consulting or Advisory Role: Takeda
Cord Sunderkötter
Honoraria: InfectoPharm, Novartis, Pfizer, LEO Pharma, Celgene
Consulting or Advisory Role: InfectoPharm, Novartis, Pfizer, LEO Pharma, Roche, Celgene
Research Funding: Celgene
Travel, Accommodations, Expenses: Celgene, Novartis
Thomas Tüting
Honoraria: Novartis, Bristol Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Dynavax
Research Funding: Novartis (Inst)
Jochen Utikal
Honoraria: Bristol Myers Squibb, Novartis, MSD Oncology, Roche, Pierre Fabre, Sanofi
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, MSD Oncology, Novartis, Pierre Fabre, Amgen, Sanofi
Research Funding: Apogenix (Inst), Noxxon Pharma (Inst), Elsalys Biotech (Inst), TILT Biotherapeutics (Inst)
Travel, Accommodations, Expenses: MSD Oncology, Roche, Novartis, Pierre Fabre, Bristol Myers Squibb, Amgen, Sanofi
Christos C. Zouboulis
Honoraria: PPM, Sobi, Galderma
Consulting or Advisory Role: Incyte, Inflarx, Novartis, UCB, Almirall Hermal GmbH, PPM, AccureAcne, Regeneron
Travel, Accommodations, Expenses: InflarxGmbH, Galderma, PPM, SOBI
Uncompensated Relationships: AbbVie
Ulrich Keilholz
Honoraria: Bristol Myers Squibb, Merck KGaA, MSD Oncology, AstraZeneca, Novartis, Pfizer, Glycotope GmbH, Roche/Genentech, Bayer Schering Pharma
Consulting or Advisory Role: Bristol Myers Squibb, Merck Serono, AstraZeneca, MSD Oncology, Pfizer
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb, Novartis, Merck Serono, AstraZeneca, Bayer Schering Pharma
Research Funding: Pfizer (Inst), AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Merck Serono, MSD Oncology, Ipsen, Pierre Fabre, Bayer Schering Pharma
Alessandro Testori
Consulting or Advisory Role: Agenus
Travel, Accommodations, Expenses: Agenus
Ulrike Leiter
Honoraria: Roche, Novartis, Sanofi, MSD Oncology, MSD Oncology
Consulting or Advisory Role: Roche, MSD Oncology, Sanofi, Novartis
Research Funding: MSD Oncology
Travel, Accommodations, Expenses: Novartis
Alexander M. M. Eggermont
Stock and Other Ownership Interests: RiverD, Skyline Diagnostics, Theranovir
Honoraria: Bristol Myers Squibb, Ellipses Pharma, GlaxoSmithKline, ISA Pharmaceuticals, MSD, Novartis, Pfizer, Sanofi, Sellas Life Sciences, Skyline Diagnostics, BIOCAD, CatalYm, BIOINVENT, IO Biotech, NEKTAR
Consulting or Advisory Role: Bristol Myers Squibb, Ellipses Pharma, GlaxoSmithKline, Incyte, ISA Pharmaceuticals, MSD, Novartis, Pfizer, Sanofi, Sellas Life Sciences, Skyline Diagnostics, BIOINVENT, IO Biotech, CatalYm, NEKTAR
Speakers' Bureau: MSD
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Bristol Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Garbe C, Keim U, Eigentler TK, et al. Time trends in incidence and mortality of cutaneous melanoma in Germany. J Eur Acad Dermatol Venereol. 2019;33:1272–1280. doi: 10.1111/jdv.15322. [DOI] [PubMed] [Google Scholar]
- 2.Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol. 2014;810:120–140. doi: 10.1007/978-1-4939-0437-2_7. [DOI] [PubMed] [Google Scholar]
- 3.Eggermont AMM, Robert C, Ribas A. The new era of adjuvant therapies for melanoma. Nat Rev Clin Oncol. 2018;15:535–536. doi: 10.1038/s41571-018-0048-5. [DOI] [PubMed] [Google Scholar]
- 4.Kudchadkar RR, Michielin O, van Akkooi ACJ. Practice-changing developments in stage III melanoma: Surgery, adjuvant targeted therapy, and immunotherapy. Am Soc Clin Oncol Educ Book. 2018;38:759–762. doi: 10.1200/EDBK_200241. [DOI] [PubMed] [Google Scholar]
- 5.Grob JJ, Garbe C, Ascierto P, et al. Adjuvant melanoma therapy with new drugs: Should physicians continue to focus on metastatic disease or use it earlier in primary melanoma? Lancet Oncol. 2018;19:e720–e725. doi: 10.1016/S1470-2045(18)30596-5. [DOI] [PubMed] [Google Scholar]
- 6.Boland GM, Gershenwald JE. Principles of melanoma staging. Cancer Treat Res. 2016;167:131–148. doi: 10.1007/978-3-319-22539-5_5. [DOI] [PubMed] [Google Scholar]
- 7. Crompton JG, Gilbert E, Brady MS: Clinical implications of the eighth edition of the American Joint Committee on Cancer melanoma staging. J Surg Oncol 119:168-174, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gershenwald JE, Scolyer RA, Hess KR, et al: Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:472-492, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grob JJ, Schadendorf D, Lorigan P, et al. Eighth American Joint Committee on Cancer (AJCC) melanoma classification: Let us reconsider stage III. Eur J Cancer. 2018;91:168–170. doi: 10.1016/j.ejca.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Crocetti E, Stanganelli I, Mancini S, et al. Evaluation of the agreement between TNM 7th and 8th in a population-based series of cutaneous melanoma. J Eur Acad Dermatol Venereol. 2019;33:521–524. doi: 10.1111/jdv.15285. [DOI] [PubMed] [Google Scholar]
- 13.Napolitano S, Brancaccio G, Argenziano G, et al. It is finally time for adjuvant therapy in melanoma. Cancer Treat Rev. 2018;69:101–111. doi: 10.1016/j.ctrv.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Garbe C, McLeod GR, Buettner PG. Time trends of cutaneous melanoma in Queensland, Australia and Central Europe. Cancer. 2000;89:1269–1278. doi: 10.1002/1097-0142(20000915)89:6<1269::aid-cncr11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: Final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 16.Eggermont AM, Suciu S, Testori A, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–3818. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 17.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 18.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: Long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer. 2019;119:1–10. doi: 10.1016/j.ejca.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Balch CM, Gershenwald JE, Soong SJ, et al. Update on the melanoma staging system: The importance of sentinel node staging and primary tumor mitotic rate. J Surg Oncol. 2011;104:379–385. doi: 10.1002/jso.21876. [DOI] [PubMed] [Google Scholar]
- 21. DigitizeIt: http://www.digitizeit.de.
- 22.Ugurel S, Röhmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies. Eur J Cancer. 2016;53:125–134. doi: 10.1016/j.ejca.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Ugurel S, Röhmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies-update 2017. Eur J Cancer. 2017;83:247–257. doi: 10.1016/j.ejca.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 24. Kanaki T, Stang A, Gutzmer R, et al: Impact of American Joint Committee on Cancer 8th edition classification on staging and survival of patients with melanoma. Eur J Cancer 119:18-29, 2019. [DOI] [PubMed] [Google Scholar]
- 25. Isaksson K, Katsarelias D, Mikiver R, et al: A population-based comparison of the AJCC 7th and AJCC 8th editions for patients diagnosed with stage III cutaneous malignant melanoma in Sweden. Ann Surg Oncol 26:2839-2845, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber J, Qureshi A, Ascierto PA. Adjuvant therapy in resected melanoma. N Engl J Med. 2018;378:680. doi: 10.1056/NEJMc1716071. [DOI] [PubMed] [Google Scholar]
- 27.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 28.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 29.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 30. Gershenwald JE, Scolyer RA: Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann Surg Oncol 25:2105-2110, 2018 [Erratum: Ann Surg Oncol 2018] [DOI] [PubMed] [Google Scholar]