Abstract
PURPOSE
Burkitt lymphoma is an aggressive B-cell lymphoma curable with dose-intensive chemotherapy derived from pediatric leukemia regimens. Treatment is acutely toxic with late sequelae. We hypothesized that dose-adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab (DA-EPOCH-R) may obviate the need for highly dose-intensive chemotherapy in adults with Burkitt lymphoma.
METHODS
We conducted a multicenter risk-adapted study of DA-EPOCH-R in untreated adult Burkitt lymphoma. Low-risk patients received three cycles without CNS prophylaxis, and high-risk patients received six cycles with intrathecal CNS prophylaxis or extended intrathecal treatment if leptomeninges were involved. The primary endpoint was event-free survival (EFS), and secondary endpoints were toxicity and predictors of EFS and overall survival (OS).
RESULTS
Between 2010 and 2017, 113 patients were enrolled across 22 centers, and 98 (87%) were high risk. The median age was 49 (range, 18-86) years, and 62% were ≥ 40 years. Bone marrow and/or CSF was involved in 29 (26%) of patients, and 28 (25%) were HIV positive. At a median follow-up of 58.7 months, EFS and OS were 84.5% and 87.0%, respectively, and EFS was 100% and 82.1% in low- and high-risk patients. Therapy was equally effective across age groups, HIV status, and International Prognostic Index risk groups. Involvement of the CSF identified the group at greatest risk for early toxicity-related death or treatment failure. Five treatment-related deaths (4%) occurred during therapy. Febrile neutropenia occurred in 16% of cycles, and tumor lysis syndrome was rare.
CONCLUSION
Risk-adapted DA-EPOCH-R therapy is effective in adult Burkitt lymphoma regardless of age or HIV status and was well tolerated. Improved therapeutic strategies for adults with CSF involvement are needed (funded by the National Cancer Institute; ClinicalTrials.gov identifier: NCT01092182).
INTRODUCTION
Burkitt lymphoma is a highly aggressive B-cell lymphoma characterized by rapidly dividing malignant cells that may involve the bone marrow and/or CNS.1,2 It is the most common B-cell lymphoma in children, but accounts for only 1%-2% of adult lymphoma.3,4
CONTEXT
Key Objective
Burkitt lymphoma is curable with high-dose chemotherapy derived from pediatric regimens but poorly tolerated in adult patients. Acute toxicity often requires inpatient care and limits treatment of older patients and those with comorbidities. We hypothesized that infusional dose-adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab (DA-EPOCH-R) chemotherapy obviates the need for high-dose treatment and is a curative and less toxic strategy in adult Burkitt lymphoma irrespective of age or comorbidities.
Knowledge Generated
This multicenter study of risk-adapted DA-EPOCH-R was curative in most adults patients with low- or high-risk Burkitt lymphoma. Low-risk patients received abbreviated therapy without CNS prophylaxis, and high-risk patients received full therapy with CNS prophylaxis or active therapy if the CNS was involved. Patients with involvement of the CNS were at the greatest risk of death related to toxicity or treatment failure.
Relevance
Risk-adapted DA-EPOCH-R was highly effective and well tolerated, and can be administered in the outpatient setting in adults with Burkitt lymphoma.
Burkitt lymphoma is curable with highly dose-intensive chemotherapy regimens developed for children and young adults.5,6 These regimens rely on rapid cycling of chemotherapy and hyperfractionation of cyclophosphamide, and include agents that specifically penetrate the CNS. Although most children are cured, rates decline with advancing age.7-10 Acute treatment-related toxicities are problematic and include profound myelosuppression, particularly in older patients or those with comorbid conditions, including HIV.9,11-15 Patients also risk late sequelae, including cognitive effects, second malignancies, infertility, and disabling neuropathy.16,17
We explored several strategies to reduce toxicity while maintaining efficacy in this study. Key among these is infusional chemotherapy, which is based on the hypothesis that drug exposure time and not peak concentration is the relevant pharmacodynamic principle to optimize the cell death of rapidly proliferating tumor cells.18 Although pediatric regimens partially address exposure time through high doses, hyperfractionation, and rapid cycling times, the high peak drug concentrations significantly increase toxicity. We tested infusional chemotherapy for prolonged exposure time without high peak drug concentrations in a pilot study of dose-adjusted infusional etoposide, doxorubicin, and vincristine with prednisone, cyclophosphamide, and rituximab (EPOCH-R) and showed high efficacy in adult Burkitt lymphoma.19 We incorporated rituximab, which has subsequently improved outcomes in sporadic and HIV-associated Burkitt lymphoma.9,13,20 A third strategy employs risk adaptation, where low-risk patients receive fewer cycles of DA-EPOCH-R without CNS prophylaxis.19 The final strategy incorporates pretreatment CSF flow cytometry to determine the intensity of the intrathecal methotrexate schedule. We present results of a phase II multicenter study of risk-adapted DA-EPOCH-R in adult Burkitt lymphoma, including findings for patients with HIV.
METHODS
Study Design and Participants
We conducted a multicenter study of risk-adapted DA-EPOCH-R in adults with untreated Burkitt lymphoma. The study started on February 24, 2010, patients enrolled between June 2010 and May 2017, and data were locked in May 2019 (Data Supplement). Eligibility included a confirmed histologic diagnosis, age ≥ 18 years, adequate organ function unless disease related, negative pregnancy test in women of childbearing potential, and other criteria (Data Supplement).21 Patients had received no prior treatment except limited-field radiotherapy or short courses of steroids and/or cyclophosphamide.
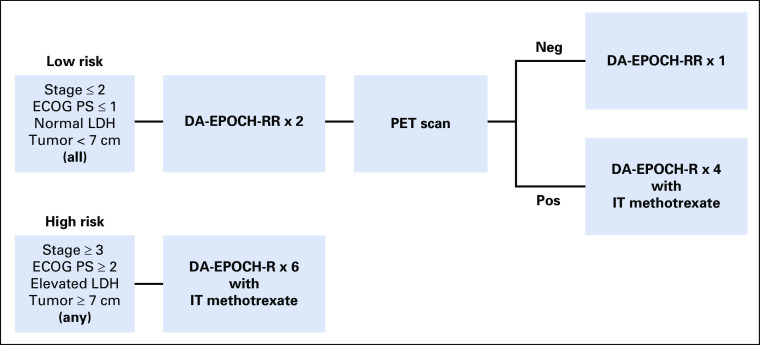
Pretreatment evaluation included laboratory investigations, computed tomography (CT) scans, bone marrow aspirate and biopsy, peripheral blood flow cytometry, CSF analysis by flow cytometry and cytology, and brain magnetic resonance imaging (MRI)/CT, if indicated (Data Supplement).22 Pretreatment CSF flow cytometry determined the intensity and schedule of intrathecal methotrexate (Data Supplement). A single dose of intrathecal therapy was allowed at diagnostic lumbar puncture. Patients were divided into low-risk and high-risk categories for risk-adapted treatment (Fig 1). Low-risk disease was defined as stage I or II disease, normal lactate dehydrogenase levels, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1, and no tumor mass ≥ 7 cm.
FIG 1.
Treatment was risk stratified based on pretreatment characteristics. Patients were considered low risk if they had all of the following: stage I or II disease, Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, normal serum lactate dehydrogenase (LDH) levels, and no tumor mass with a diameter ≥ 7 cm. Low-risk patients were treated with two cycles of dose-adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab on days 1 and 5 (DA-EPOCH-RR), followed by an interim positron emission tomography (PET) scan. If the PET scan was considered negative (neg), these patients received only one additional cycle of DA-EPOCH-RR and no CNS prophylaxis. If the PET scan was considered positive (pos), patients were treated for a full six cycles of therapy and CNS prophylaxis with intrathecal (IT) methotrexate was given. Patients were considered high risk if they had any of the following: stage III or IV disease, ECOG PS of 2-4, elevated serum LDH levels, or any tumor mass ≥ 7 cm. High-risk patients were treated with six cycles of DA-EPOCH-R (rituximab on day 1 only) along with either CNS prophylaxis or active CNS therapy with IT methotrexate, as indicated.
Tumor response was assessed per the revised response criteria for malignant lymphoma.23,24 Patients underwent CT scan after two and six cycles and every 4 months for 2 years. Positron emission tomography (PET) scans were performed after cycles two and six (if positive after cycle 2). Radiologic scans were reviewed at each participating site and were not centrally reviewed (Data Supplement). In low-risk patients, interim PET scans determined treatment duration, but not in high-risk patients.
Trial Oversight
The study was coordinated by the Lymphoid Malignancies Branch of the National Cancer Institute, and the study sponsor was the Cancer Therapy Evaluation Program, with support from the Cancer Trials Support Unit (Data Supplement). Rituximab was provided by Genentech, which had no role in trial design or data interpretation. The study was registered at ClinicalTrials.gov (NCT01092182) and was conducted in accordance with the principles of the Declaration of Helsinki. The study was approved by local institutional review boards of participating institutions, and all patients signed informed consent forms. Investigators submitted data using centralized electronic databases. Data were analyzed and interpreted by the lead authors and made available to all authors. All authors approved the manuscript and vouch for the completeness and accuracy of the data and the fidelity of the trial to the protocol.
Treatment
DA-EPOCH-R was administered and pharmacodynamically dose adjusted (Data Supplement).25 Patients started at dose-level 1 and received subsequent cycles of dose-adjusted etoposide, doxorubicin, and cyclophosphamide based on neutrophil nadir (Data Supplement). Complete blood counts were monitored twice weekly at least 3 days apart, and if the neutrophil nadir was above 500/μL, the next cycle was increased by 1 dose level. Low-risk patients received two cycles with rituximab on days 1 and 5 (DA-EPOCH-RR), followed by a PET scan (Fig 1). If the interim PET scan was negative, patients received one more cycle of DA-EPOCH-RR without CNS prophylaxis. If the interim PET scan was positive, patients were treated with 4 additional cycles of DA-EPOCH-R and CNS prophylaxis.
High-risk patients received two cycles of DA-EPOCH-R therapy followed by a PET scan. Unless there was disease progression, an additional 4 cycles were given, and therapy was not altered based on the interim PET scan (Fig 1). High-risk patients without CNS involvement received prophylactic intrathecal methotrexate on days 1 and 5 of cycles three to six, for a total of eight doses (Data Supplement). Patients with active CSF involvement as determined by flow cytometry and/or cytology received treatment with methotrexate intrathecally twice weekly for at least 4 weeks, then weekly for 6 weeks, and then monthly for 6 months. Intravenous methotrexate was not permitted.
Statistical Analysis
The primary objective was to estimate event-free survival (EFS) from the date of enrollment until the date of progression, last documentation of active disease at or after the last treatment cycle, death, or last follow-up on an intention-to-treat-basis. Median follow-up was calculated as median intervals from study enrollment until data cut-off. Overall survival (OS) was calculated from the enrollment date until the date of death or last follow-up; we used the Kaplan-Meier method with exact log-rank tests to identify the degree of difference. Secondary objectives included toxicity and the predictive value of interim PET scans. Exploratory analyses for differences in EFS and OS were assessed according to the International Prognostic Index (IPI), age groups, HIV status, and bone marrow and/or CSF involvement.
RESULTS
Patient Characteristics
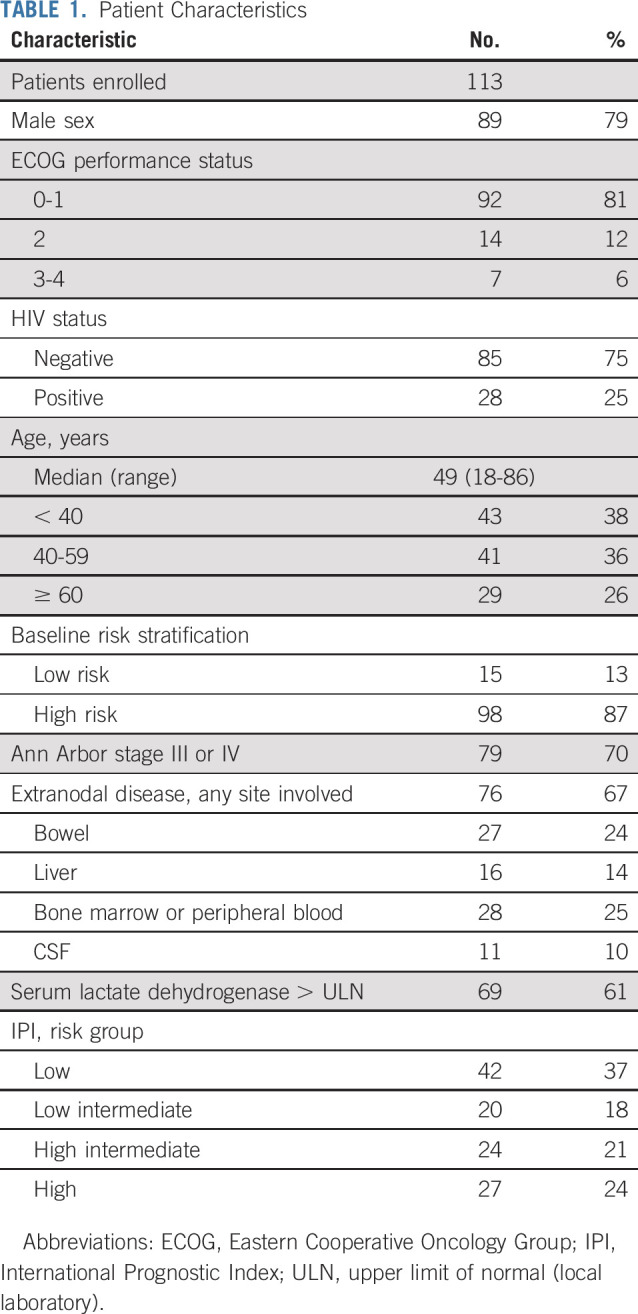
One hundred thirteen patients were enrolled. Clinical characteristics included male sex in 89 patients (79%) and ECOG performance status ≥ 2 in 21 (18%; Table 1). The median age was 49 (range, 18-86) years, and 70 patients (62%) were ≥ 40 years of age, including 29 patients (26%) ages ≥ 60 years. Fifteen patients (13%) were low risk, and 98 (87%) were high risk. Ann Arbor stage was III or IV in 79 patients (70%), and 76 (67%) had extranodal involvement. Twenty-eight patients (25%) had involvement of the peripheral blood and/or bone marrow, and 11 (10%) had involvement of the CSF. No patients had brain parenchymal involvement. Serum lactate dehydrogenase was elevated in 69 patients (61%), and 51 patients (45%) had high-intermediate–risk or high-risk disease by the IPI. HIV was positive in 28 patients (25%), with a median CD4+ T-cell count of 268 (range, 22 to 886) cells/mm3.
TABLE 1.
Patient Characteristics
Clinical Outcome
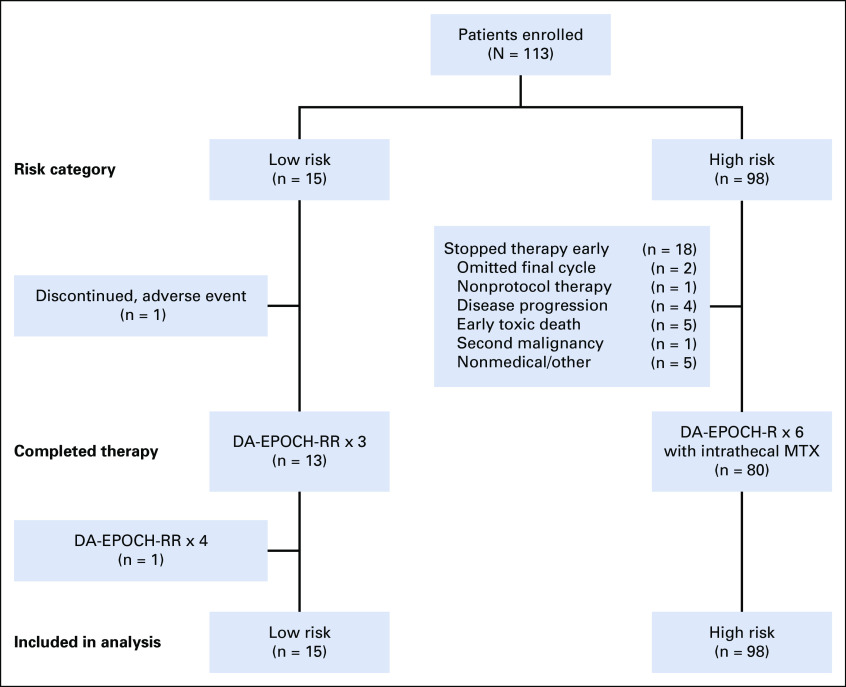
Of 15 low-risk patients, 13 (87%) received three treatment cycles (Fig 2). One patient developed severe hyponatremia during the first cycle and received modified EPOCH-R. Another low-risk patient received four cycles despite a negative interim PET scan. In five low-risk patients, surgical tumor resection was performed before systemic treatment and 4 high-risk patients had surgical debulking before systemic treatment. Among 98 high-risk patients, 80 (82%) received six treatment cycles (Fig 2). Among 18 patients who did not complete six treatment cycles, two patients received five treatment cycles, and one patient received four treatment cycles and radiotherapy at physician discretion. Four patients experienced disease progression during treatment, and one successfully received salvage treatment (Data Supplement). Five patients died of treatment-related early death related to toxicity. One early toxicity-related death occurred in a patient 74 years of age who completed four cycles and died of respiratory failure. Four early toxicity-related deaths occurred during the first cycle of therapy, including three patients with CSF involvement. Two patients 50 and 72 years of age with CSF involvement died of sepsis during the first cycle. Two patients, both 59 years of age with stage IV disease and evidence of spontaneous tumor lysis syndrome, died of multisystem organ failure during the first cycle. One patient, age 25 years, was diagnosed with cholangiocarcinoma and died after two cycles of therapy. Five high-risk patients did not complete protocol therapy for nonmedical reasons, including three patients because of noncompliance, one because of financial reasons, and one because of loss of insurance.
FIG 2.
Patients were enrolled and treated according to baseline risk category. Fifteen low-risk patients were enrolled, and 13 (87%) received only 3 treatment cycles per protocol. One low-risk patient discontinued after one cycle, and another received four cycles. Among 98 high-risk patients, 80 (82%) received the planned six treatment cycles. The reasons for not completing protocol therapy included physician discretion (n = 3), disease progression (n = 4), early toxicity-related death (n = 5), second malignancy (n = 1), and nonmedical reasons (n = 5). Study analysis was based on intention to treat and included all patients. DA-EPOCH-R, dose-adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab on day 1 only; DA-EPOCH-RR, dose-adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab on days 1 and 5; MTX, methotrexate.
The median follow-up was 58.7 months, the 4-year EFS for all 113 patients was 84.5% (95% CI, 76% to 90%), and the 4-year OS was 87.0% (95% CI, 79% to 92%; Figs 3A and 3B). Two patients without evidence of active disease received consolidation with autologous stem cell transplantation (n = 1) and radiotherapy (n = 1). All low-risk patients are in remission (Fig 3C). Among 98 high-risk patients, the 4-year EFS and OS were 82.1% (95% CI, 73% to 89%; Fig 3D) and 84.9% (95% CI, 76% to 91%; data not shown), respectively. Two patients whose disease progressed are alive without disease after successful salvage therapy (Data Supplement).
FIG 3.
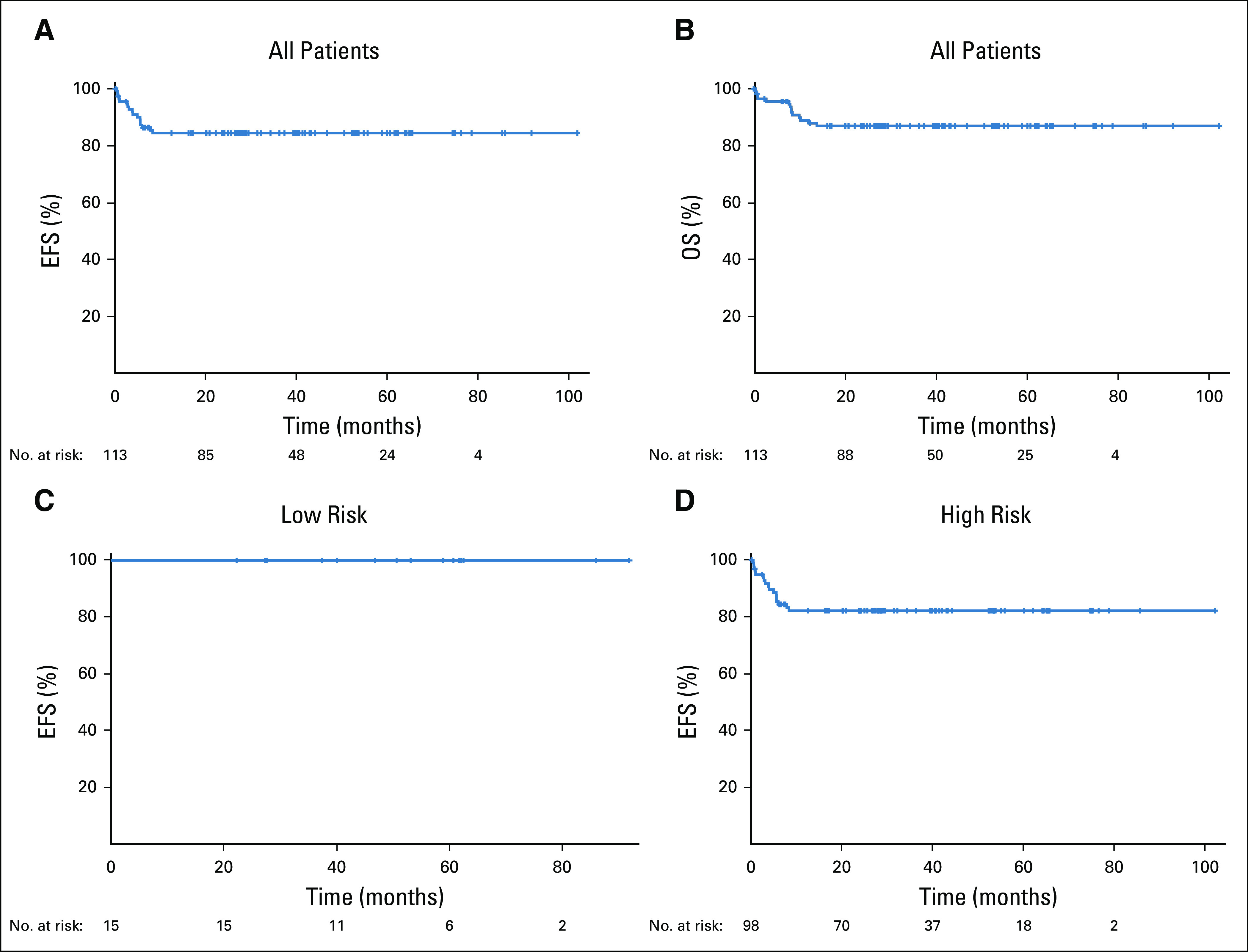
Kaplan-Meier estimates of the event-free survival (EFS) and overall survival (OS) of patients enrolled with Burkitt lymphoma. Median follow-up was 58.7 months. (A) The 4-year EFS of all patients (N = 113) was 84.5% (95% CI, 76.3% to 90.1%). (B) The 4-year OS of all patients (N = 113) was 87.0 (95% CI, 79.0% to 92.1%). (C) The 4-year EFS of low-risk patients (n = 15) was 100%. (D) The 4-year EFS of high-risk patients (n = 98) was 82.1% (95% CI, 72.7% to 88.5%).
Relapses in the CNS after therapy were uncommon. Among 81 patients with high-risk disease and no pretreatment evidence of CSF involvement, there were two relapses (2%) in the brain parenchyma despite CNS prophylaxis (Data Supplement). Among 11 patients with CSF involvement at presentation, six patients experienced disease progression or died (Data Supplement): three died during cycle one from sepsis and/or multiorgan failure, and three experienced progression on therapy, two at peripheral sites only and one in both a peripheral site and brain parenchyma.
Prognostic Analysis
We explored variables associated with survival, including interim PET scans. In the low-risk arm, 14 patients had an interim PET scan, which were all interpreted as negative. In the high-risk arm, 85 patients underwent interim PET scans, with 51 (60%) interpreted as negative and 34 (40%) interpreted as positive. The 4-year EFS was not significantly different among high-risk patients with a negative versus a positive interim PET scan: 90.0% (95% CI, 78% to 96%) versus 78.7% (95% CI, 61% to 89%; P = .12), respectively (Fig 4A).
FIG 4.
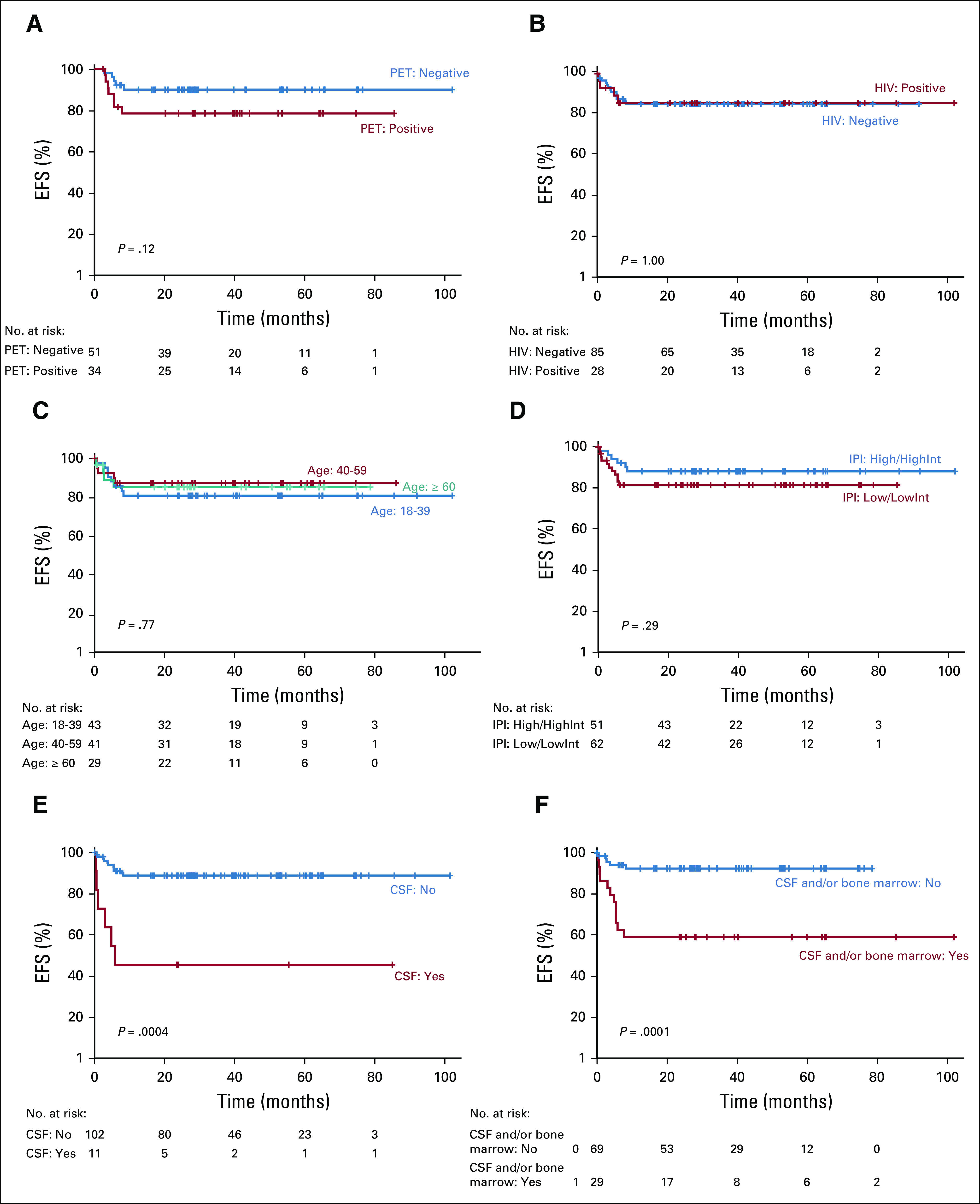
Kaplan-Meier estimates of the 4-year event-free survival (EFS) according to prognostic variables. (A) EFS of negative interim positron emission tomography (PET) scans (n = 51) versus positive interim PET scans (n = 34) in high-risk patients was 90.0% (95% CI, 77.7% to 95.7%) and 78.7% (95% CI, 60.5% to 89.2%; P = .12), respectively. (B) EFS of HIV-negative (n = 85) versus HIV-positive (n = 28) patients was 84.5% (95% CI, 74.8% to 90.7%) and 84.9% (95% CI, 64.5% to 94.0%; P = 1.00), respectively. (C) EFS across age group categories of 18-39 (n = 43) versus 40-59 (n = 41) versus ≥ 60 years (n = 29) was 81.1% (95% CI, 65.8% to 90.1%), 87.5% (95% CI, 72.5% to 94.6%), and 85.4% (95% CI, 65.6% to 94.3%; P = .77), respectively. (D) EFS according to high-/high-intermediate–risk (HighInt) score on the International Prognostic Index (IPI) risk score (n = 51) versus low-/low-intermediate–risk (LowInt) IPI risk score (n = 62) was 88.2% (95% CI, 75.5% to 94.5%) and 81.5% (95% CI, 69.1% to 89.3%; P = .29), respectively. (E) EFS for patients with no CSF involvement (n = 102) versus CSF involvement (n = 11) was 89.9% (95% CI, 82.0% to 94.4%) and 45.5% (95% CI, 16.7% to 70.7%; P = .0004), respectively. (F) EFS for patients with no involvement of the CSF or bone marrow (n = 69) versus involvement of either (n = 29) was 92.4% (95% CI, 83 to 97) and 58.6% (95% CI, 39 to 74; P = .0001), respectively.
HIV coinfection, age, and IPI had no effect on survival. The 4-year EFS in HIV-positive compared with HIV-negative patients was 84.9% (95% CI, 65% to 94%) and 84.5% (95% CI, 75% to 91%; P = 1.00), respectively (Fig 4B). Outcome was assessed in 3 age groups: 18 to 39 years, 40 to 59 years, and ≥ 60 years. The 4-year EFS of these groups was 81.1% (95% CI, 66% to 90%), 87.5% (95% CI, 73% to 95%), and 85.4% (95% CI, 66% to 94%; overall P = .77), respectively (Fig 4C). Patients with low-/low-intermediate–risk (0-2) and high-intermediate–/high-risk (3-5) IPI had a 4-year EFS of 81.5% (95% CI, 69% to 89%) and 88.2% (95% CI, 76% to 95%; P = .29), respectively (Fig 4D).
The most important variable associated with survival was involvement of the CSF. In high-risk disease, patients with and without CSF involvement at presentation had a 4-year EFS of 45.5% (95% CI, 17 to 71) and 89.9% (95% CI, 82 to 94; P = .0004), respectively (Fig 4E). We also assessed the outcome of high-risk patients with and without involvement of the peripheral blood, bone marrow, and/or CSF, and found a 4-year EFS of 58.6% (95% CI, 39% to 74%) and 92.4% (95% CI, 83% to 97%; P = .0001), respectively (Fig 4F). In high-risk patients without CSF involvement, those with and without bone marrow or peripheral involvement had a 4-year EFS of 66.7% (95% CI, 40% to 83%) compared with 92.4% (95% CI, 83% to 97%; P = .0086), respectively (Data Supplement).
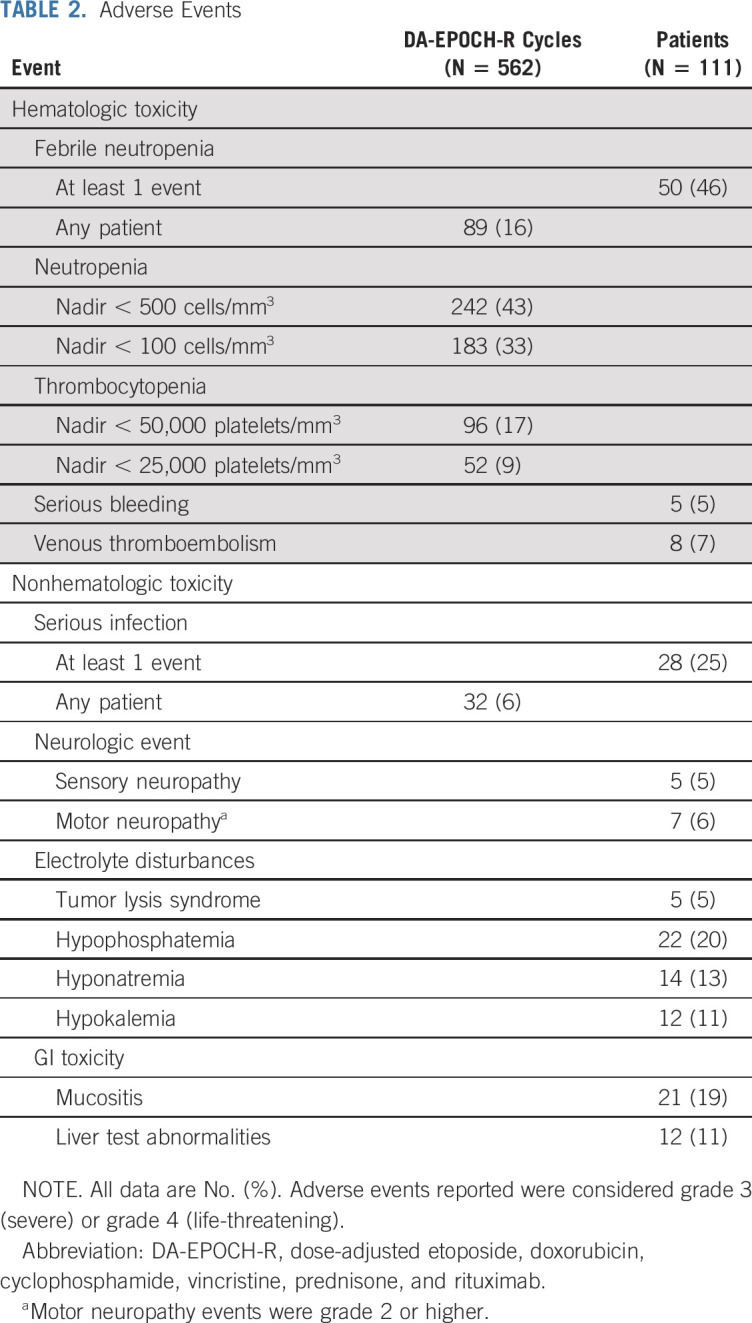
Dose Intensity and Toxicity
Patient disposition was assessed across 481 cycles, and most were delivered as an outpatient. Toxicity data were assessed across 562 cycles in 111 patients (Table 2). Younger patients achieved higher maximum dose levels. Dose-level 1 was the maximum level in 27 patients (24%) with a median age of 59 (range, 23-76) years, level 2 was the maximum in 29 patients (26%) with a median age of 49 (range, 20-86) years, level 3 was the maximum in 29 (26%) with a median age of 38 (range, 18-69) years, level 4 was the maximum in 20 patients (18%) with a median age of 32 (range, 19-69) years, and level 5 was the maximum in 8 patients (7%) with a median age of 28 (range, 18-66) years. Grade 3 or 4 thrombocytopenia occurred in 96 cycles (17%), and fever with neutropenia occurred in 89 cycles (16%). Tumor lysis syndrome occurred in 5 patients (5%), and 21 patients (19%) had grade 3 or 4 mucositis. Grade 3 or 4 sensory neuropathy occurred in 5 patients (5%), and grade 2 or higher motor neuropathy occurred in 7 patients (6%), respectively. Of 14 deaths in the study, 7 (50%) were due to nonlymphoma causes. Four patients died as a result of multisystem organ failure and/or sepsis during the first cycle, and one died of respiratory failure after four cycles. One patient died of cholangiocarcinoma diagnosed after two cycles, and one patient died of a heart attack 4 months after therapy. Other serious toxicities were uncommon (Data Supplement).
TABLE 2.
Adverse Events
DISCUSSION
We demonstrated that risk-adapted DA-EPOCH-R is effective in adults with Burkitt lymphoma, irrespective of HIV status, with tolerable toxicity across all age groups. With a median follow-up of 58.7 months, all patients with low-risk disease achieved durable remissions, and patients with high-risk disease achieved an EFS of 82%. Interim PET scans in high-risk patients did not reliably identify patients at risk for treatment failure. These results support our treatment strategies to ameliorate toxicity while maintaining efficacy. Indeed, they suggest highly dose-intensive chemotherapy is unnecessary for cure, and carefully defined low-risk patients may be treated with limited chemotherapy. Furthermore, they suggest that risk-adapted intrathecal therapy prevents most CNS relapses without high-dose intravenous methotrexate.
Intensive multiagent chemotherapy regimens have cured Burkitt lymphoma for decades.5,6 Various regimens have been tested with fractionated schedules of cyclophosphamide or ifosfamide along with doxorubicin, vincristine, steroids, and the CNS-penetrating agents intravenous cytarabine and high-dose methotrexate.11,26-29 The high dose intensity of these regimens requires prolonged hospitalization. The acute toxicity limits broad applicability, and late sequelae are indefinite risks. Indeed, population trends in Burkitt lymphoma demonstrate that the greatest benefit of highly dose-intensive chemotherapy is for younger patients.7 Use of these regimens in older adults and HIV frequently require dose modifications.12,13,30 The results of DA-EPOCH-R in this study significantly improve on the complexity, cost, and toxicity profile of other regimens, and the regimen is a treatment administered on an outpatient basis, often including the first cycle of therapy because of the low risk of tumor lysis syndrome.
Recent studies have shown a benefit of rituximab in Burkitt lymphoma.9,13-15,20,31,32 A randomized study in adults with Burkitt lymphoma demonstrated rituximab with highly dose-intensive chemotherapy improved the 3-year EFS compared with chemotherapy alone (75%; 95% CI, 66% to 82% v 62%; 95% CI, 53% to 70%; P = .024).20 Other multicenter studies of rituximab with highly dose-intensive chemotherapy have reported an EFS in the 69%-74% range.9,13,14 Despite less dose-intensive therapy, our results in high-risk disease compare favorably with a 4-year EFS of 82.1%.
Our results show risk-adapted DA-EPOCH-R is tolerated by all age groups, and pharmacologic dose-level increases occurred in 76% of patients. Infectious complications and hematologic toxicity are major limiting factors of highly dose-intensive regimens. Serious infections with these regimens occur in 15%-20% of cycles, and only younger adults reliably complete the planned treatment regimen.8 In contrast, serious infections were observed in 6% of cycles in our study, despite the inclusion of patients with HIV. Over all cycles, the incidence of grade 4 neutropenia was 43%, but was associated with fever in only 16% of cycles. Most serious complications occurred early in therapy and were associated with impaired performance status. Of 5 treatment-related deaths, 4 resulted from sepsis or multiorgan failure during the first cycle in patients > 50 years of age with an ECOG performance status of ≥ 2. Given the known toxicity profile of DA-EPOCH-R, it is highly unlikely that alternative Burkitt lymphoma regimens would have been more tolerable.7,33-35 Impaired performance status does not preclude successful treatment with DA-EPOCH-R, however. Seven patients with ECOG 3 or 4 were included in our study, and 5 of them are alive without disease.
A remaining unmet medical need is management of patients with active CSF involvement, which occurs in 10%-15% of patients. In our study, patients with CSF involvement were at the greatest risk of treatment failure, with a 4-year EFS of 45%, although a small number of patients with active CSF involvement were enrolled. Notably, events were evenly distributed between disease progression (n = 3) and early toxicity-related death (n = 3) during the first treatment cycle. The use of highly dose-intensive chemotherapy would likely increase the risk of early toxicity-related death and may not overcome treatment resistance. Indeed, in a recent study of dose-intensive chemotherapy with high-dose methotrexate and cranial irradiation, 25 patients (39%) with active CNS disease did not achieve remission.20 A preliminary report of a retrospective study suggested that patients treated with DA-EPOCH-R have a higher incidence of CNS progression than regimens that included high-dose methotrexate. This underscores the critical role of CSF flow cytometry to identify patients who should be treated with CNS-directed therapy more intensive than prophylactic schedules of intrathecal methotrexate alone.36 Alternative strategies including a prephase course of steroids may have a role to improve performance status before therapy.
Another important issue is the occurrence of parenchymal brain relapse, which occurred in 2 patients (2%) despite intrathecal CNS prophylaxis. These results are not dissimilar to studies of chemotherapy regimens in adults that include high-dose methotrexate and rituximab and also report CNS relapses, often as the most common site of relapse.9,14 An alternative strategy to overcome treatment resistance in high-risk patients is the addition of targeted agents, such as inhibitors of the phosphoinositide 3-kinase pathway, which have demonstrated clinically relevant CNS penetration.37,38
In conclusion, risk-adapted DA-EPOCH-R is highly effective in adults with Burkitt lymphoma irrespective of HIV status, and its relative tolerance allows broad applicability across patients of all ages. In patients with high-risk features such as CSF involvement, the addition of targeted agents with CNS penetration should be studied.37
ACKNOWLEDGMENT
The authors thank all the patients and their caregivers who participated in this clinical trial. Genentech provided rituximab for the following institutions: Dana-Farber Cancer Institute, Massachusetts General Hospital, and MD Anderson Cancer Center.
PRIOR PRESENTATION
Presented in part at the 14th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 14-17, 2017, and the 59th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 9-12, 2017.
SUPPORT
The Cancer Therapy Evaluation Program and Lymphoid Malignancies Branch, National Cancer Institute, National Institutes of Health provided funding for this study. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Nos. U10CA180888 and UG1CA233230, as well as the AIDS Malignancy Consortium Grant No. UMI CA121947. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CLINICAL TRIAL INFORMATION
DATA AVAILABILITY STATEMENT
De-identified clinical data will be provided to the Protocol Registration and Results System of ClinicalTrials.gov within 1 year of publication. Information on data sharing may be obtained from ClinicalTrials.gov Web site.
AUTHOR CONTRIBUTIONS
Conception and design: Kieron Dunleavy, Seth M. Steinberg, Richard F. Little, Ariela Noy, Wyndham H. Wilson
Financial support: Wyndham H. Wilson
Administrative support: Andrea N. Lucas, Richard F. Little, Wyndham H. Wilson
Provision of study materials or patients: Kieron Dunleavy, Jeremy S. Abramson, Brian K. Link, Deepa Jagadeesh, Ronald T. Mitsuyasu, David Peace, Christopher Melani, Stefania Pittaluga, Elaine S. Jaffe, Jonathan W. Friedberg, Brad S. Kahl, Michelle A. Fanale, Ariela Noy, Wyndham H. Wilson
Collection and assembly of data: Mark Roschewski, Kieron Dunleavy, Jeremy S. Abramson, Brian K. Link, Prapti Patel, Philip J. Bierman, Deepa Jagadeesh, David Peace, Michelle A. Fanale, Wahid T. Hanna, Christopher Melani, Andrea N. Lucas, Stefania Pittaluga, Elaine S. Jaffe, Brad S. Kahl, Nancy L. Bartlett, Ariela Noy, Wyndham H. Wilson
Data analysis and interpretation: Mark Roschewski, Seth M. Steinberg, Ariela Noy, Wyndham H. Wilson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multicenter Study of Risk-Adapted Therapy With Dose-Adjusted EPOCH-R in Adults With Untreated Burkitt Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kieron Dunleavy
Honoraria: AbbVie, Celgene, Karyopharm Therapeutics, Seattle Genetics, Astra Zeneca, Amgen, Kymera, Atara
Consulting or Advisory Role: Seattle Genetics, Karyopharm Therapeutics, Celgene, AbbVie, Astra Zeneca, Kymera, Atara, Amgen
Jeremy S. Abramson
Consulting or Advisory Role: Gilead Sciences, Celgene, Novartis, Juno Therapeutics, Verastem, Bayer, AbbVie, Janssen, Merck, Kite Pharma, Genentech, EMD Serono, MorphoSys, Allogene, Karyopharm Therapeutics, Bristol-Myers Squibb,
Research Funding: Seattle Genetics (Inst), Celgene (Inst), AI Therapeutics (Inst)
Bayard L. Powell
Honoraria: Jazz Pharmaceuticals, Novartis, Pfizer
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Cornerstone Pharmaceuticals
Speakers' Bureau: Jazz Pharmaceuticals
Research Funding: Sunesis Pharmaceuticals, Pfizer, Novartis, Celator, Incyte
Travel, Accommodations, Expenses: Celgene, Novartis, Jazz Pharmaceuticals, Pfizer
Brian K. Link
Consulting or Advisory Role: Genentech, AbbVie, Karyopharm
Research Funding: Pharmacyclics/Janssen (Inst)
Travel, Accommodations, Expenses: Genentech
Prapti Patel
Consulting or Advisory Role: Celgene, Agios
Travel, Accommodations, Expenses: Rafael Pharmaceuticals, Forty Seven
Deepa Jagadeesh
Consulting or Advisory Role: Seattle Genetics, Atara Biotherapeutics, Verastem, Kyowa Hakko Kirin
Research Funding: Seattle Genetics (Inst), Regeneron (Inst), MEI Pharma (Inst), Debiopharm Group (Inst), Seattle Genetics (Inst)
Ronald T. Mitsuyasu
Stock and Other Ownership Interests: Amgen
Research Funding: Calimmune (Inst), Sangamo Bioscience (Inst)
David Peace
Stock and Other Ownership Interests: Bristol-Myers Squibb, Amgen, Merck
Research Funding: Seattle Genetics, F Hoffman La Roche, Celgene
Patents, Royalties, Other Intellectual Property: U.S. Patent # 8,557,777 B2: Methods of Treating Prostate Cancer Using Prostate Specific Antigen and Tumor Endothelial Marker Peptides. October 15, 2013 (Inst), U.S. Patent # 8,557,777 B2: Methods of Treating Prostate Cancer Using Prostate Specific Antigen and Tumor Endothelial Marker Peptides. October 15, 2013 (Inst)
Open Payments Link: https://https://openpaymentsdata.cms.gov/physician/345598
Peter R. Watson
Stock and Other Ownership Interests: Managed fund
Elaine S. Jaffe
Stock and Other Ownership Interests: Gilead Sciences, Merck, Teva
Jonathan W. Friedberg
Consulting or Advisory Role: Bayer, Astellas Pharma, Portola, Seattle Genetics (Inst), Kite Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Patient on bone marrow microenvironment signals (I)
Travel, Accommodations, Expenses: Roche
Brad S. Kahl
Consulting or Advisory Role: Celgene, Juno Therapeutics, AbbVie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, TG Therapeutics, Kite/Gilead, MorphoSys, Janssen
Research Funding: Genentech (Inst), Acerta Pharma (Inst), Therapeutics (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: Celgene, Juno Therapeutics, Genentech, AbbVie, Millennium, Seattle Genetics
Nancy L. Bartlett
Consulting or Advisory Role: Seattle Genetics
Research Funding: Seattle Genetics (Inst), Genentech (Inst), Kite Pharma (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), Immune Design (Inst), Forty Seven (Inst), Janssen (Inst), Pharmacyclics (Inst), Millennium (Inst), ADC Therapeutics (Inst), Autolus (Inst)
Ariela Noy
Consulting or Advisory Role: MorphoSys
Speakers' Bureau: Prime Oncology
Research Funding: Pharmacyclics, Rafael Pharmaceuticals
Travel, Accommodations, Expenses: Pharmacyclics, Janssen Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dalla-Favera R, Bregni M, Erikson J, et al. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci USA. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler JL, Bluming AZ, Morrow RH, et al. Central nervous system involvement in Burkitt’s lymphoma. Blood. 1970;36:718–728. [PubMed] [Google Scholar]
- 3.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: State of the science. Br J Haematol. 2009;144:24–40. doi: 10.1111/j.1365-2141.2008.07393.x. [DOI] [PubMed] [Google Scholar]
- 5.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SB, Bowman WP, Abromowitch M, et al. Results of treatment of advanced-stage Burkitt’s lymphoma and B cell (SIg+) acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. J Clin Oncol. 1986;4:1732–1739. doi: 10.1200/JCO.1986.4.12.1732. [DOI] [PubMed] [Google Scholar]
- 7.Costa LJ, Xavier AC, Wahlquist AE, et al. Trends in survival of patients with Burkitt lymphoma/leukemia in the USA: An analysis of 3691 cases. Blood. 2013;121:4861–4866. doi: 10.1182/blood-2012-12-475558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly JL, Toothaker SR, Ciminello L, et al. Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin Lymphoma Myeloma. 2009;9:307–310. doi: 10.3816/CLM.2009.n.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoelzer D, Walewski J, Döhner H, et al. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: Report of a large prospective multicenter trial. Blood. 2014;124:3870–3879. doi: 10.1182/blood-2014-03-563627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhardt B, Oschlies I, Klapper W, et al. Non-Hodgkin’s lymphoma in adolescents: Experiences in 378 adolescent NHL patients treated according to pediatric NHL-BFM protocols. Leukemia. 2011;25:153–160. doi: 10.1038/leu.2010.245. [DOI] [PubMed] [Google Scholar]
- 11. doi: 10.1093/annonc/mdf253. Mead GM, Sydes MR, Walewski J, et al: An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: Results of United Kingdom Lymphoma Group LY06 study. Ann Oncol 13:1264-1274, 2002 [Erratum: Ann Oncol 13:1961, 2020] [DOI] [PubMed] [Google Scholar]
- 12.Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: Preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45:761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 13.Noy A, Lee JY, Cesarman E, et al. AMC 048: Modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma. Blood. 2015;126:160–166. doi: 10.1182/blood-2015-01-623900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzieri DA, Johnson JL, Byrd JC, et al. Improved efficacy using rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or aggressive lymphomas: Cancer and Leukemia Group B study 10 002. Br J Haematol. 2014;165:102–111. doi: 10.1111/bjh.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evens AM, Carson KR, Kolesar J, et al. A multicenter phase II study incorporating high-dose rituximab and liposomal doxorubicin into the CODOX-M/IVAC regimen for untreated Burkitt’s lymphoma. Ann Oncol. 2013;24:3076–3081. doi: 10.1093/annonc/mdt414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 17.Rizzieri DA, Johnson JL, Niedzwiecki D, et al. Intensive chemotherapy with and without cranial radiation for Burkitt leukemia and lymphoma: Final results of Cancer and Leukemia Group B Study 9251. Cancer. 2004;100:1438–1448. doi: 10.1002/cncr.20143. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor PM, Wassermann K, Sarang M, et al. Relationship between DNA cross-links, cell cycle, and apoptosis in Burkitt’s lymphoma cell lines differing in sensitivity to nitrogen mustard. Cancer Res. 1991;51:6550–6557. [PubMed] [Google Scholar]
- 19.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369:1915–1925. doi: 10.1056/NEJMoa1308392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribrag V, Koscielny S, Bosq J, et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: A randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:2402–2411. doi: 10.1016/S0140-6736(15)01317-3. [DOI] [PubMed] [Google Scholar]
- 21.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: The role of flow cytometry versus cytology. Blood. 2005;105:496–502. doi: 10.1182/blood-2004-05-1982. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 24.Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652–4661. doi: 10.1200/JCO.2005.01.891. [DOI] [PubMed] [Google Scholar]
- 25.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717–2724. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patte C, Philip T, Rodary C, et al. Improved survival rate in children with stage III and IV B cell non-Hodgkin’s lymphoma and leukemia using multi-agent chemotherapy: Results of a study of 114 children from the French Pediatric Oncology Society. J Clin Oncol. 1986;4:1219–1226. doi: 10.1200/JCO.1986.4.8.1219. [DOI] [PubMed] [Google Scholar]
- 27.Patte C, Auperin A, Michon J, et al. The Société Française d’Oncologie Pédiatrique LMB89 protocol: Highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 28.Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: It is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diviné M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: A prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16:1928–1935. doi: 10.1093/annonc/mdi403. [DOI] [PubMed] [Google Scholar]
- 31.Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 32.Goldman S, Smith L, Galardy P, et al. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: A Children’s Oncology Group Report. Br J Haematol. 2014;167:394–401. doi: 10.1111/bjh.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: Clinical outcomes of the phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol. 2019;37:1790–1799. doi: 10.1200/JCO.18.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. doi: 10.1016/j.leukres.2019.106287. Silva WF, Garibaldi PM, Da Rosa LI, et al: Outcomes of HIV-associated Burkitt lymphoma in Brazil: High treatment toxicity and refractoriness rates - A multicenter cohort study. Blood 89:106287, 2019. [DOI] [PubMed] [Google Scholar]
- 35.Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. 2012;156:234–244. doi: 10.1111/j.1365-2141.2011.08947.x. [DOI] [PubMed] [Google Scholar]
- 36.Zayac A, Evens AM, Stadnik A, et al. Outcomes of patients with newly-diagnosed Burkitt lymphoma (BL) and central nervous system (CNS) involvement treated in the modern era: A multi-institutional real-world analysis. Blood. 2019;134(suppl 1):402. [Google Scholar]
- 37.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grommes C, Gavrilovic I, Miller AM, et al. Phase Ib of copanlisib in combination with ibrutinib in recurrent/refractory primary CNS lymphoma (PCNSL) Blood 1341598.2019suppl 131558468 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified clinical data will be provided to the Protocol Registration and Results System of ClinicalTrials.gov within 1 year of publication. Information on data sharing may be obtained from ClinicalTrials.gov Web site.