Abstract
PURPOSE
Hereditary breast and ovarian cancer (HBOC) syndrome is primarily characterized by mutations in the BRCA1/2 genes. There are several barriers to the implementation of genetic testing and counseling in India that may affect clinical decisions. These consensus recommendations were therefore convened as a collaborative effort to improve testing and management of HBOC in India.
DESIGN
Recommendations were developed by a multidisciplinary group of experts from the Indian Society of Medical and Pediatric Oncology and some invited experts on the basis of graded evidence from the literature and using a formal Delphi process to help reach consensus. PubMed and Google Scholar databases were searched to source relevant articles.
RESULTS
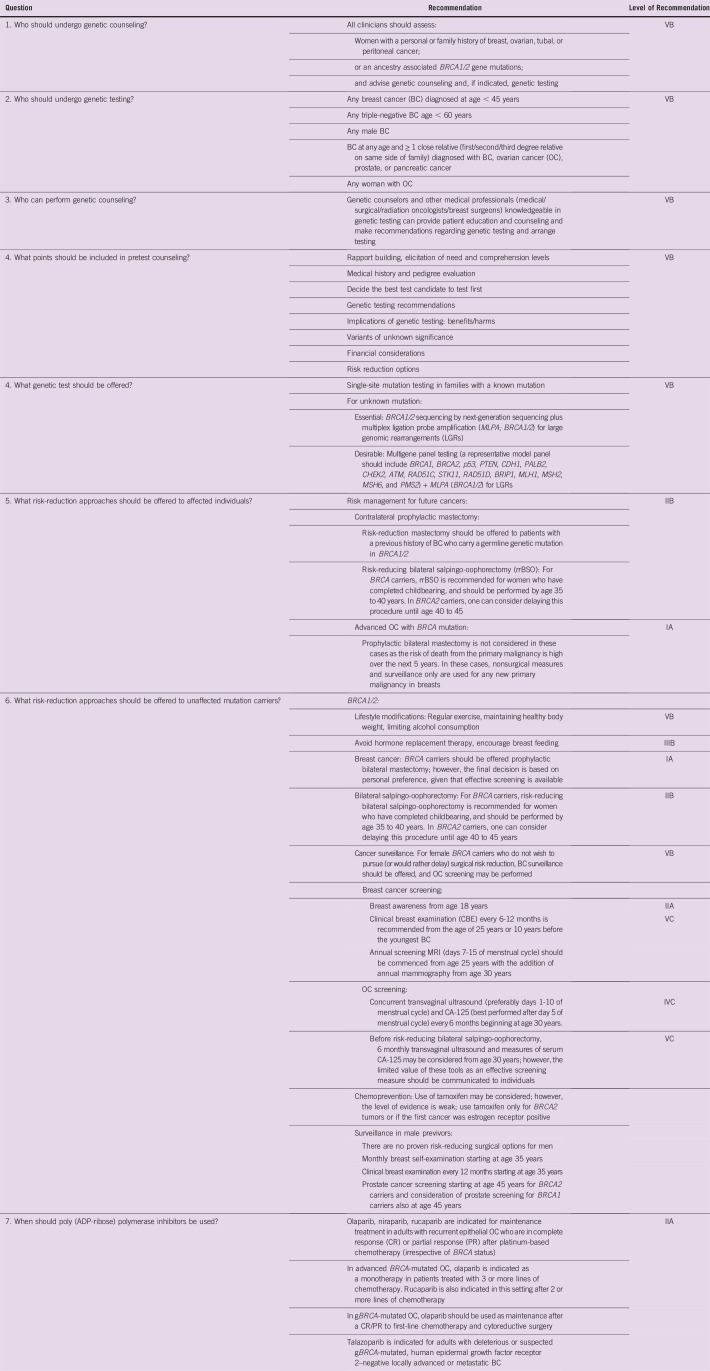
This consensus statement provides practical insight into identifying patients who should undergo genetic counseling and testing on the basis of assessments of family and ancestry and personal history of HBOC. It discusses the need and significance of genetic counselors and medical professionals who have the necessary expertise in genetic counseling and testing. Recommendations elucidate requirements of pretest counseling, including discussions on genetic variants of uncertain significance and risk reduction options. The group of experts recommended single-site mutation testing in families with a known mutation and next-generation sequencing coupled with multiplex ligation probe amplification for the detection of large genomic rearrangements for unknown mutations. Recommendations for surgical and lifestyle-related risk reduction approaches and management using poly (ADP-ribose) polymerase inhibitors are also detailed.
CONCLUSION
With rapid strides being made in the field of genetic testing/counseling in India, more oncologists are expected to include genetic testing/counseling as part of their clinical practice. These consensus recommendations are anticipated to help homogenize genetic testing and management of HBOC in India for improved patient care.
INTRODUCTION
Hereditary breast and ovarian cancer (HBOC) syndrome is characterized by an autosomal-dominant inheritance pattern with increased risk of early-onset breast cancer (BC) and ovarian cancer (OC) in multiple family members.1,2 HBOC syndrome is associated with 50% to 85% lifetime risk of BC and 15% to 30% risk of OC in women.3,4 Mutations in BRCA1 and BRCA2 are commonly implicated in HBOC.5 Founder mutations—specific mutations identified in a population with common ancestry—in BRCA1/2 have been identified in Ashkenazi Jews, French Canadians, and Icelanders, among other populations worldwide.6
Context
Key Objective
What are the current testing practices and effective approaches for advancing BRCA mutation testing and the management of hereditary breast and ovarian cancers in India?
Knowledge Generated
Women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations should undergo genetic counseling. The experts recommended single-site mutation testing in families with a known mutation and next-generation sequencing coupled with multiplex ligation probe amplification for detection of large genomic rearrangements for unknown mutations. Recommendations also include surgical and lifestyle-related risk-reduction approaches and management using poly (ADP-ribose) polymerase inhibitors.
Relevance
A growing number of oncologists in India are expected to implement genetic testing/counseling, and these consensus recommendations can be expected to standardize clinical practice for improved patient care.
In India, BC is the most common cancer in women as well as the most common cause of cancer-related death in women.7 The Indian scenario is characterized by younger median age of onset and a high incidence-to-mortality ratio compared with the West.8 However, the burden of BC attributable to inherited mutations is not well characterized.
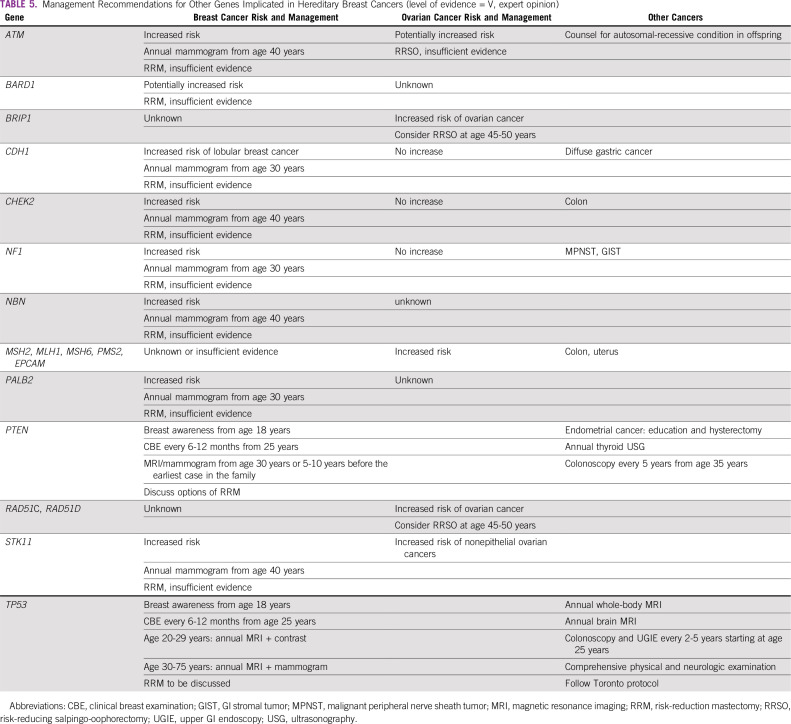
With the approval of poly (ADP-ribose) polymerase (PARP) inhibitors for both germline and somatic BRCA1/2 mutations and data indicating the efficacy of platinum-based chemotherapy in gBRCA mutant cases, genetic testing has the potential to affect treatment decisions. Genetic tests improve the understanding of the risk of future metachronous cancers in patients, which can be prevented by employing appropriate surgical or nonsurgical prophylactic measures.9 Clinician and genetic counselors can prevent an almost inevitable cancer in previvors. Appropriate preventive steps are available for several non-BRCA genes, like PALB2, CHEK2, and ATM1.10
The prevalence of germline mutations; their relative frequencies in high-, moderate-, and low-penetrance genes; and their founder status all vary with geography and ethnicity. Pathogenic genetic mutation is estimated to occur in 10% to 15% of all patients with BC, with BRCA1 and BRCA2 accounting for 40% to 50% of pathogenic/likely pathogenic mutations.11,12
Leveraging the recent developments in the management of HBOC, more than 32 international guidelines published between 2010 and 2018 provide recommendations for genetic counseling and screening, preventive or risk reduction approaches, and systemic management of BRCA-mutated BC and OC, but all these guidelines cater to issues of Western patients.13
These recommendations were convened to evaluate current testing practices and referral workflows, suggest effective testing methods, and provide practical insights to advance BRCA mutation testing in India with the ultimate goal of improving treatment outcome and patient care.
METHODOLOGY
Recommendations were developed by a multidisciplinary group of experts using evidence from phase III randomized controlled studies, relevant prospective and retrospective studies, and clinical experience as a guide.
The first meeting was organized on May 25, 2019, in Mumbai. The discussion centered on genetic counseling, methods of genetic testing, and challenges encountered during clinical practice and referrals with an Indian perspective in mind. The meeting involved extensive discussions of specific questions developed a priori by the committee chairpersons to aid the discussion, followed by voting to reach a consensus using the Delphi process.
The Expert Panel corresponded frequently through e-mail; progress on guideline development was driven primarily by committee chairpersons. All members participated in the preparation of the draft. PubMed and Google Scholar databases were searched using the following key words: “hereditary breast and ovarian cancer”; “HBOC”; “BRCA1/2 mutations”; “non-BRCA mutations”; “germline BRCA mutations”; “somatic BRCA mutations”; and “genetic testing”. Levels of evidence and grades of recommendation endorsed by the Infectious Diseases Society of America were applied.
GENETIC COUNSELING IN INDIA: IMPORTANCE AND AWARENESS
Despite recent progress, genetic testing in HBOC remains underutilized in India.14 The process of genetic counseling involves an attempt by one or more appropriately trained persons to help the individual or family to:
a) Comprehend the medical facts: diagnosis and probable course of the disorder and available management options
b) Understand how heredity contributes to the disorder and the risk of recurrence in first-degree mutation carrier relatives
c) Understand the alternatives for dealing with the risk of recurrence
d) Choose a course of action according to their risk and family goals
Genetic counseling before testing is endorsed by many international oncology working groups.1,19-21 Guidelines from several countries, including Europe and Australia, advocate pretest and post-test genetic counseling for BRCA1/2 by professionals who are adequately trained in genetics and clinical oncology.22,23 In India, oncologists are often the first point of contact for these patients.18,24
Components of HBOC Genetic Counseling
Pretest counseling.
The counselor would discuss the following issues to educate patients and suggests who should be tested first in the family. The following are key components:
Medical history and pedigree evaluation up to 3 generations
Application of mathematical risk assessment models/qualitative criteria (eg, National Comprehensive Cancer Network [NCCN])
Discussion of genetic testing recommendations
Implications of genetic testing: benefits/harms
Discussion of financial considerations
Discussion of legal protection against genetic discrimination.18
Assessment of family history.
Per the established standards, collection of complete family history should comprise a 3-generation pedigree analysis that includes information on age/year of birth for each individual, age at onset of cancer, age at death, cause of death (for deceased relatives), ethnic background of all grandparents (maternal and paternal), consanguinity, and any information on prior genetic testing, pregnancies, and half-siblings.25,26
Risk communication.
Information on genetic testing results; treatment implications of pathogenic, benign variants, and variants of uncertain significance (VUS) and associated risk for patients and predictive risk among relatives should be explained. A significant increase in medical knowledge and risk perception has been reported after pregenetic testing communication via face-to-face counseling, group discussion, and written communication—for example, information booklets—that eventually helped minimize anxiety in patients after receipt of test results.27-30
Post-test counseling session.
The post-test counseling session involves an assessment of understanding and recall of medical facts conveyed during counseling, change in anxiety level, severity of risk perception, reproductive plans, and satisfaction with the quality and extent of genetic counseling.18
GERMLINE BRCA TESTING
Assessment of Risk and Identifying Patients
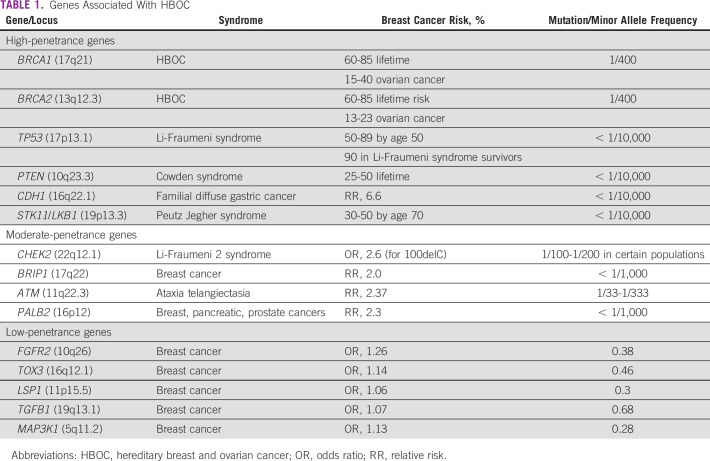
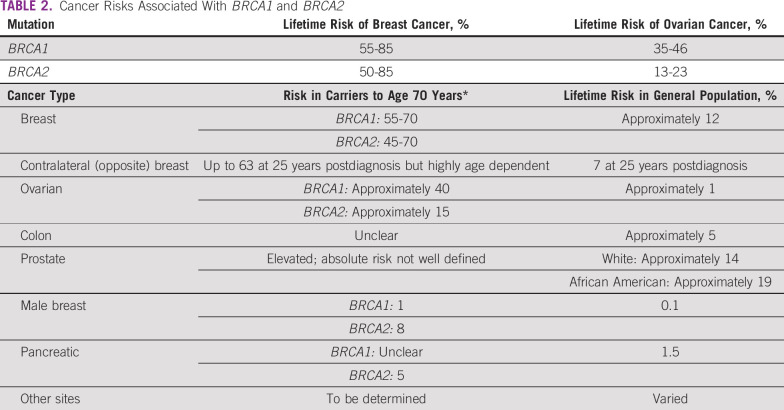
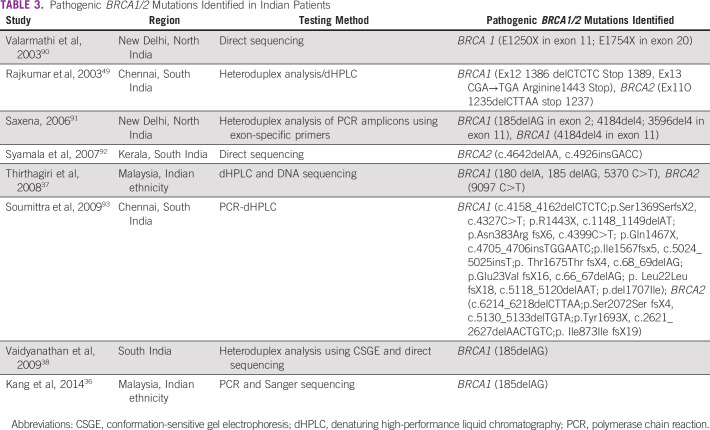
Germline mutations in BRCA1/2 genes are regarded as high penetrance—a cancer relative risk of greater than 5—and have been characterized in several populations globally. Mutations in other non-BRCA genes, such as PALB2, TP53, PTEN, CDH1, STK11, CHEK2, RAD51, and ATM, are also known to confer risk of BC and/or OC, albeit with lower frequency and penetrance31 (Tables 1 and 2). The lifetime risk of breast and ovarian malignancies is variable, with pathogenic mutations in BRCA1 (BC: 46% to 87%; OC: 39% to 63%) and BRCA2 (BC: 38% to 84%; OC: 17% to 27%). Other cancers associated with germline BRCA1/2 mutations include male BC (1% to 9%), prostate cancer (9% to 20%), pancreatic cancer (1% to 7%), and melanoma.32 The largest analysis of 1,010 high-risk families across India33 revealed BRCA mutations in 85% and non-BRCA mutations in 15% of families. Additional analysis based on age and family history showed a high prevalence of germline variants (75%) in younger patients age younger than 40 years with a first-degree family member affected with BC/OC.33 A recent study from North India reported a 30% prevalence of gBRCA mutation in patients with BC/OC qualifying for NCCN criteria for testing, including 5 novel mutations.34 A methodical review investigating the prevalence of germline variants in high-risk HBOC susceptibility genes in 1,028 patients of Indian descent with familial/early-onset/triple-negative BC or OC identified 18 BRCA1 and 16 BRCA2 variants that were not reported in the Breast Cancer Information Core or ClinVar databases.35 The putative Ashkenazi founder mutation BRCA1 185delAG was detected in a low proportion of patients (4.2%), the majority of whom were from South India or who were Malaysians of Indian origin.36-38 Table 3 provides a summary of deleterious germline mutations identified in Indian patients with HBOC.
TABLE 1.
Genes Associated With HBOC
TABLE 2.
Cancer Risks Associated With BRCA1 and BRCA2
TABLE 3.
Pathogenic BRCA1/2 Mutations Identified in Indian Patients
Until now, our clinical practice has been to test patients who fulfill NCCN criteria for testing (Box 1); however, recent publications have emphasized that using NCCN guidelines misses many patients with both BRCA and non-BRCA mutations who would otherwise benefit.39
BOX 1. NCCN Guidelines 2019 for gBRCA Risk Assessment
Individual from a family with a known BRCA1/2 pathogenic/likely pathogenic variant, including such variants found on research testing
-
Personal history of breast cancer (BC) plus one or more of the following:
○ Diagnosed age ≤ 45 years
○ Diagnosed age 46-50 years (an additional BC primary at any age or one or more close blood relative with BC at any age or one or more close blood relative with high-grade [Gleason score ≥ 7] prostate cancer)
○ An unknown or limited family history
○ Diagnosed age ≤ 60 years with triple-negative BC
○ Diagnosed at any age with: one or more close blood relative with BC diagnosed age ≤ 50 years; or OC, male BC, metastatic prostate cancer, or pancreatic cancer and two or more additional diagnoses of BC at any age in patient and/or close blood relatives)
○ Ashkenazi Jewish ancestry
Personal history of OC
Personal history of male BC
Personal history of pancreatic cancer
Personal history of metastatic prostate cancer
Personal history of high-grade prostate cancer (Gleason score ≥ 7) at any age with one or more close blood relative with ovarian carcinoma, pancreatic cancer, or metastatic prostate cancer at any age or BC age < 50 years; or two or more close blood relatives with BC, or prostate cancer (any grade) at any age; or Ashkenazi Jewish ancestry
An individual who does not meet the other criteria but with one or more 1 first- or second-degree blood relative meeting any of the above criteria
The US Preventive Services Task Force (August 2019) recommends that primary care clinicians assess women with a personal or family history of BC, OC, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing.40
WHOM TO TEST FIRST?
It is ideal to initiate genetic testing in a family member who is most likely to test positive for a pathogenic variant, which is usually a woman affected by early BC/OC (any age). Children should not be tested for BRCA before the age of 18 years.
Methods of Germline BRCA Detection
Germline genetic testing usually involves taking written informed consent for storage of biologic samples—blood sample, saliva, or cheek swab—and testing, followed by analysis of the sample for the detection of heritable germline mutations. Multigene panels using next-generation sequencing (NGS) coupled with the multiplex ligation probe amplification technique enables high-throughput genetic testing. The usual turnaround time to receive test results is 4 weeks. While selecting an NGS workflow, the following criteria should be considered to suit the genetic testing:
a) Enrichment method: polymerase chain reaction amplicon based or hybrid capture based;
b) Sequencing chemistry: sequencing by synthesis or pH mediation; and
c) Bioinformatic analysis
Studies examining NGS workflows for BRCA1/2 genes in HBOC samples have demonstrated excellent performance, with almost 100% sensitivity and specificity, and cost effectiveness compared with single-site mutation testing in these genes.41 Two studies from India have reported that the use of multigene panel testing by NGS for germline mutations in patients with HBOC.42,43 The majority of BRCA1/2 mutations may be single-base substitution missense or nonsense mutations. Other mutations are small insertions or deletions that result in a prematurely truncated nonfunctional protein. Some deleterious variants may also include splice junction alterations that lead to exon skipping or the inclusion of intronic region, resulting in a nonfunctional protein. It is important to remember that NGS can miss large genomic rearrangements, which are causal pathogenic mutations, in 5% of patients with HBOC. Some experts recommend that as multiplex ligation probe amplification technique allows for the identification of large genomic rearrangements, it should be performed in all patients who test negative by NGS who have a strong clinical suspicion of HBOC.44,45 The commonly used panels for HBOC syndrome include the following genes: ATM, BRCA1, BRCA2, BRIP1, CHEK2, RAD50, RAD51D, RAD51C, PALB2, BAARD1, P53, STK11, CDH1, MSH2, MSH6, MLH1, EPCAM, PMS2, ATM, PTEN, FGFR2, TOX3, LSP1, and MAP3K1.
Interpretation of Sequencing Results
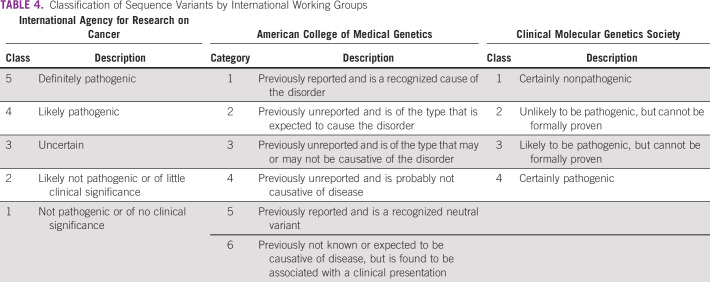
Genetic testing helps detect sequence changes that may be benign, pathogenic, or VUS. International working groups provide guidelines for the interpretation of germline sequence variants and categorize the DNA sequence alterations qualitatively on the basis of functional evidence, family history, allele frequency data, and computational and in silico predictions (Table 4).
TABLE 4.
Classification of Sequence Variants by International Working Groups
It is critical to note that when no deleterious germline mutation is detected in a proband, results should not be directly labeled as negative and the potential limitations of testing should be considered.44 Some possibilities include that the patient has a pathogenic variant in another gene not included in the multigene panel; that the tested gene has a sequence variant that cannot be easily detected by sequence analysis, such as large deletion; and that the patient has a sequence variant in a region, such as an intron or regulatory region of a gene, that may not be covered by the test.46
Interpretation of a variant for use in clinical decision making requires comprehensive knowledge of the patient’s phenotype, mode of inheritance for the disease gene, mutational mechanism (eg, haplo-insufficiency, dominant negative), protein structure/function, and the strength of the gene-disease relationship. Clinically relevant mutations are annotated using published variants in the literature and a set of disease databases—ClinVar, OMIM, GWAS, HGMD, and SwissVar. Nonsynonymous variant effects are calculated using multiple algorithms, such as PolyPhen-2, SIFT, Mutation Taster2, Mutation Assessor, and LRT. Only nonsynonymous and splice-site variants found in the hereditary cancer gene panel are used for clinical interpretation.
The test result obtained should be transcribed into a coherent genetic testing report that describes the test results and explains its significance for the proband and first-degree relatives. DNA change as a variant should be reported using the standard Human Genome Variation Society nomenclature that describes the nucleotide change in the cDNA as a c. and the consequent change in the amino acid and protein as a p., while mentioning the cDNA reference sequence used.
VALIDATION OF TEST RESULT
As results from genetic testing—for example, NGS—influence clinical treatment, validation of the test is critical.47,48 The joint consensus from the Association for Molecular Pathology and College of American Pathologists recommends the validation of every detected single-nucleotide variant or indel in the coding region that results in deleterious mutations and documenting it in terms of positive percentage agreement and positive predictive value.47 Samples in which a deleterious variant/mutation is detected should be reconfirmed using fresh DNA extraction from a different aliquot of cells from the same patient by Sanger sequencing, a recognized gold-standard method.49
HOW TO MANAGE VUS
VUS are genetic alterations that are usually single-base substitutions that result in a missense mutation and a different amino acid in the encoded protein. These alterations in the coding sites may be in the promoter regions, intronic regions close to exons, or may be small in-frame insertions and deletions and synonymous substitutions.50-52 It is estimated that more than 20,000 unique variants have been identified in the coding, splice site, and intervening sequences of BRCA genes.53 Almost 90% of BRCA1/2 mutations can be classified either as pathogenic or benign; however, approximately 10% of them cannot be classified as deleterious or neutral and are labeled as VUS. It is estimated that on complete analysis, approximately 30% to 50% of VUS might actually be pathogenic.54-56
A VUS is characterized by gathering evidence, such as its co-occurrence with a deleterious mutation, cosegregation with disease in families, functional characterization with available physiochemical, cellular and biologic assays, allelic frequency in databases that document well-characterized populations, and in silico assessment. Data-sharing initiatives, like the BRCA Challenge and the Evidence-Based Network for the Interpretation of Germline Mutant Alleles, aid in the assessment of VUS. The expanding database of HBOC genetic testing results and ongoing efforts targeted at determining the pathogenicity and categorizing VUS have resulted in a 13% decline in the rate of VUS detection between 2002 and 2013.57
As a result of the uncertainty of VUS, the International Agency for Research on Cancer does not recommend predictive genetic testing in at-risk relatives and emphasizes the need to treat VUS carriers as probands with no mutations. However, misinterpretation of VUS by clinicians has been reported, leading to unnecessary prophylactic surgery and patient anxiety.58
QUALITY OF GENETIC TESTING: THE BACKBONE OF CHARACTERIZING BRCA1/2 MUTATIONS
In an oncology setting, genetic testing addresses two purposes: identifying deleterious germline mutations in families with predisposition to cancers, followed by predictive genetic testing in these high-risk families; and identifying molecular markers or signatures in the tumors for treatment and prognosis. A robust methodology/algorithm in genetic testing is extremely important for maintaining test quality. The American Association of Pathologists’ Assistants and the College of American Pathologists have developed guidelines for NGS bioinformatics pipelines, and laboratories should follow them to reduce error rates.59 In addition, the guidelines emphasize the role of trained professionals to achieve optimal testing quality. Genetic testing that is based on national accreditation programs, various quality assessment programs, and participation in such schemes as the European Molecular Genetics Quality Network could help the testing laboratories maintain quality control.
SOMATIC OR TUMOR BRCA TESTING
Growing evidence suggests that tumors with somatically acquired BRCA1/2 pathogenic mutations respond to drugs that inhibit PARP. As mentioned in germline testing, informed consent of the participant should be obtained before testing. Testing for somatic mutations with NGS becomes a method of choice because of its sensitivity compared with Sanger sequencing. A limitation of somatic BRCA testing is DNA extraction from formalin-fixed, paraffin-embedded specimens. These samples have a variable mix of neoplastic and normal stroma cell tissue, and the quantity of DNA extracted is low and of poor quality.60 Furthermore, tissue preservation using formalin induces a chemical crosslinking reaction with nucleotides that results in artifactual sequence alterations and deamination of cytosine nucleotides. Use of shorter amplicons, de-crosslinking steps, and treatment with uracil-DNA glycosylase—DNA repair enzyme—to markedly reduce the number of sequence artifacts before polymerase chain reaction amplification are steps recommended to improve the quality of extracted DNA.60,61
The tumor content for somatic BRCA testing must be certified by a trained pathologist. DNA from the tissue sample should be extracted from a single representative block using a standardized and validated method. Known positive and negative controls should be included during testing. Somatic testing is generally recommended at 500× coverage to avoid a false-negative assessment. After testing, the bioinformatic pipeline should be able to filter out variants with 5% to 10% allele frequency on the basis of the initial tumor percentage.
The somatic testing report should include:
a) Suitability of tumor sample for tumor content and specific testing method
b) Number and names of genes tested (if using a multigene panel)
c) Depth of coverage for each gene
d) Details of mutation, if detected, with Human Genome Variation Society nomenclature
e) Reference sequence of the gene
f) Interpretation of results with reference to therapy
Interpretation of Somatic or Tumor BRCA Result and Its Role
Molecular signatures of homologous recombination deficiency from ovarian tumors and association with high loss of heterozygosity indicate genomic scaring and instability.62,63 Although regarded as uncommon, sporadic somatic BRCA1/2 mutations account for one third of BRCA mutations in OC and 4% to 15% of unselected triple-negative BC.64-66
In high-grade serous OC, BRCA1/2 germline and somatic mutations are frequent (17% to 25%), with somatic mutations representing 18% to 30% of all BRCA1/2 mutations. In a sequencing study, up to 9% of patients with OC had relevant somatic mutations in homologous recombinant genes (BRCA1/2, BRIP1, CHEK2, and RAD51C). Somatic mutations were highly predictive of primary platinum sensitivity and improved overall survival.67
Accumulating evidence suggests the role of somatically acquired BRCA1/2 pathogenic mutations, tumor pathology, and loss of heterozygosity as predictive biomarkers of clinical response to PARP inhibitor.68-70 In a phase II study, patients with platinum-sensitive relapsed serous OC with positive BRCA mutations had the highest likelihood of benefiting from olaparib (median progression-free survival, 11.2 months in BRCA mutation-positive v 7.4 months in wild-type BRCA patients; hazard ratio, 0.54 [95% CI, 0.34 to 0.85]; P = .0075).69
IMPLICATIONS OF TESTING BRCA (GERMLINE/TUMOR) MUTATIONS IN THE MANAGEMENT OF HBOC
The presence of pathogenic or likely pathogenic mutations in BRCA1 or BRCA2 has tremendous implications for the management of patients and unaffected relatives (previvors; Box 2).
BOX 2. SUMMARY OF THE INDIAN SOCIETY OF MEDICAL AND PEDIATRIC ONCOLOGY CONSENSUS DOCUMENT ON HEREDITARY BREAST AND OVARIAN CANCER
Levels of Evidence (adapted from the Infectious Diseases Society of America–US Public Health Service Grading System)
I: Evidence from at least one large randomized controlled trial of good methodologic quality (low potential for bias) or meta-analyses of well-conducted randomized trials without heterogeneity
II: Small randomized trials or large randomized trials with a suspicion of bias (lower methodologic quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity
III: Prospective cohort studies
IV: Retrospective cohort studies or case-control studies
V: Studies without control group, case reports, and/or expert opinions
Grades of Recommendation
A: Strong evidence for efficacy with a substantial clinical benefit, strongly recommended
B: Strong or moderate evidence for efficacy, but with a limited clinical benefit, generally recommended
C: Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs, etc), optional
D: Moderate evidence against efficacy or for adverse outcome, generally not recommended
E: Strong evidence against efficacy or for adverse outcome, never recommended
Risk Management for the Previvor (unaffected carrier of mutation)
Lifestyle modifications.
Regular exercise and maintaining a healthy body weight
Limiting alcohol consumption
Avoid hormone-replacement therapy
Encourage breast feeding
NCCN recommends that BRCA carriers be offered prophylactic bilateral mastectomy.19 In both retrospective and prospective observational studies, risk-reducing or prophylactic bilateral mastectomy decreases the incidence of BC by 90% or more in patients who are at risk for hereditary BC, with most studies focusing on BRCA mutation carriers.71-73
For BRCA1 carriers, risk-reducing bilateral salpingo-oophorectomy (rrBSO) is recommended for women who have completed childbearing and should be performed by age 35 to 40 years or individualized on the basis of age of onset of OC in the family.19 In BRCA2 carriers, this procedure can be delayed until age 40 to 45 years. rrBSO not only decreases the risk of OC in BRCA mutation carriers, but also decreases the risk of mortality.74-76 NCCN does not routinely recommend hysterectomy at the time of rrBSO and indicates that “salpingectomy alone is not the standard-of-care and is discouraged outside a clinical trial.”19
Cancer surveillance.
For female BRCA carriers who do not wish to pursue (or would rather delay) surgical risk reduction, BC surveillance should be offered, and OC screening may be performed.19
BC screening.
The following strategy is recommended by expert groups for women with BRCA pathogenic variants who have not undergone risk-reducing surgery and should be individualized as needed:
a) Breast awareness from 18 years of age
b) Clinical breast examination every 6 to 12 months is recommended from the age of 25 or 10 years before the youngest BC.
c) Annual screening magnetic resonance imaging (MRI; days 7 to 15 of the menstrual cycle) should be commenced from age 25 years with the addition of annual mammography with or without tomosynthesis from age 30 years.77
d) In women younger than age 30 years, breast ultrasonography can be considered if MRI is unavailable.
OC screening.
For carriers who have not undergone rrBSO, we recommend OC screening. This consists of concurrent transvaginal ultrasound, preferably day 1 to 10 of the menstrual cycle, and CA-125—best performed after day 5 of the menstrual cycle—every 6 months beginning at age 30 years or 5 to 10 years before the earliest age of first diagnosis in the family. Before rrBSO, 6 monthly transvaginal ultrasound and measure of serum CA-125 may be considered from age 30 years; however, the limited value of these tools as effective screening measures should be communicated to individuals.
Chemoprevention.
Use of tamoxifen may be considered; however, the level of evidence is weak.78
Prevention of other BRCA-related cancers.
No evidence-based data exist. BRCA2 carriers may consider annual skin and eye examination as screening for melanoma, and annual screening for pancreatic cancer with endoscopic ultrasound or MRI/magnetic resonance cholangiopancreatography. There is no consensus when screening should commence; however, age 50 years or 10 years before the earliest diagnosed case in the family would be reasonable (Table 5).
TABLE 5.
Management Recommendations for Other Genes Implicated in Hereditary Breast Cancers (level of evidence = V, expert opinion)
Reproductive counseling.
Pathogenic variants in many BC genes, including BRCA, are inherited in an autosomal-dominant pattern, meaning that there is a 50% chance that children of BRCA carriers will have inherited the cancer predisposition variant. Reproductive counseling of BRCA carriers includes education about prenatal diagnosis and assisted reproduction.19 One option is preimplantation genetic diagnosis, which is used to analyze embryos—obtained by in vitro fertilization—genetically before their transfer into the uterus.
Management for Patient
Contralateral prophylactic mastectomy.
Risk-reduction mastectomy is often offered to patients with or without a history of BC who carry a germline genetic mutation that confers a high risk for BC BRCA1/2, TP53, PTEN, CDH1, or STK11 mutation.79-82
rrBSO.
Recommendations are the same as those for previvors. There are conflicting data whether rrBSO reduces the risk of BC, with many recent studies not showing any association between rrBSO and BC risk.83-85 Larger studies are needed to validate these results.
Advanced OC with BRCA mutation.
Prophylactic bilateral mastectomy is not considered in these cases as the risk of death from the primary malignancy is high over the next 5 years.
Medical Implications of BRCA in BC
Olaparib is approved by the US Food and Drug Administration for patients with germline BRCA mutations and human epidermal growth factor receptor 2–negative BC previously treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic disease setting on the basis of the Olympiad trial.86
Talazoparib is US Food and Drug Administration approved for patients with germline BRCA mutations and human epidermal growth factor receptor 2–negative locally advanced or metastatic BC on the basis of the EMBRACA trial.87
Neoadjuvant platinum agents: Based on the GeparSixto and CALGB 40603 studies, platinum agents as neoadjuvant treatment improves pathological complete response in BRCA-positive patients. Improvement in disease-free survival was demonstrated in GeparSixto, but not in the CALGB trial.88,89
Medical Implications of BRCA in OC
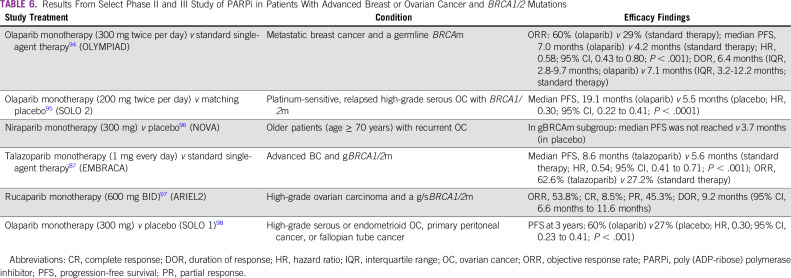
Olaparib, rucaparib, and niraparib have all been approved in OC for various indications (Table 6).
TABLE 6.
Results From Select Phase II and III Study of PARPi in Patients With Advanced Breast or Ovarian Cancer and BRCA1/2 Mutations
ACKNOWLEDGMENT
The authors thank Priya Ganpathy, MPharm, CMPP, (SIRO Clinpharm), for medical writing assistance and Sangita Patil, PhD, CMPP, (SIRO Clinpharm), for additional editorial support. The authors gratefully acknowledge AstraZeneca for help in organizing the meeting of experts at Mumbai.
Footnotes
This consensus statement represents the Indian Society of Medical and Pediatric Oncology expert subcommittee’s and other invited experts current thinking on the topic based on available evidence. This has been developed by national experts in the field and does not in any way bind a clinician to follow this verbatim. The treating physician is free to use an alternate mode of therapy/recommendation based on the discussions with the patient and with reference to institution, national, or international guidelines. The mention of recommendation for one particular type of testing does not constitute endorsement or recommendation for its use, but is a guidance for clinicians in complex decision making. The contributors to this document are acutely aware of the constant and continuous addition to the knowledge on the subject and in the field and the need for regular updates to this document and the fact that this needs to be living document requiring regular modification and revision.
AUTHOR CONTRIBUTIONS
Conception and design: Hemant Malhotra, Pradnya Kowtal, Raja Pramanik, Rajiv Sarin, Thangarajan Rajkumar, Sudeep Gupta, Ajay Bapna, Gouri Shankar Bhattacharyya, Sabhyata Gupta, Manish Singhal, B.K. Smruti, Somashekhar S.P., Moushumi Suryavanshi, Amit Verma
Administrative support: Ravindra Reddy Kundur, Moushumi Suryavanshi, Amit Verma
Provision of study materials or patients: Thangarajan Rajkumar, Rupinder Sekhon, B.K. Smruti, Amit Verma
Collection and assembly of data: Hemant Malhotra, Rajiv Sarin, Thangarajan Rajkumar, Sudeep Gupta, Gouri Shankar Bhattacharyya, Ashraf U. Mannan, Manish Singhal, B.K. Smruti, Amit Verma
Data analysis and interpretation: Hemant Malhotra, Pradnya Kowtal, Nikita Mehra, Rajiv Sarin, Thangarajan Rajkumar, Sudeep Gupta, Ajay Bapna, Gouri Shankar Bhattacharyya, Amita Maheshwari, Ashraf U. Mannan, Ravindra Reddy Kundur, Rupinder Sekhon, Manish Singhal, Moushumi Suryavanshi, Amit Verma
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thangarajan Rajkumar
Consulting or Advisory Role: Serum Institute of India
Patents, Royalties, Other Intellectual Property: The Cancer Institute has signed a memorandum of understanding with HLL Life Care for transfer of technology for a cervical cancer screening kit for which we have applied for a patent (Inst)
Sudeep Gupta
Research Funding: Roche (Inst), Sanofi (Inst), Johnson & Johnson (Inst), Amgen (Inst), Celltrion (Inst), OncoStem Diagnostics (Inst), Novartis (Inst)
Gouri Shankar Bhattacharyya
Honoraria: Vicus Therapeutics, Mylan, Biocon, Cipla, Intas
Consulting or Advisory Role: Vicus Therapeutics, Mylan, Biocon, Cipla, Zuventus, OncoStem Diagnostics
Speakers' Bureau: Biocon, Novartis, Cipla, Meda, Intas, Boehringer Ingelheim, AstraZeneca, OncoStem Diagnostics
Research Funding: Vicus Therapeutics
Ashraf U. Mannan
Employment: Strand Life Sciences
Manish Singhal
Honoraria: Pfizer, AstraZeneca
Speakers' Bureau: Novartis
Amit Verma
Employment: Max Healthcare
Consulting or Advisory Role: Foundation Medicine
No other potential conflicts of interest were reported.
REFERENCES
- 1.American College of Obstetricians and Gynecologists. ACOG Committee on Practice Bulletins—Gynecology. ACOG Committee on Genetics. et al. ACOG Practice Bulletin No. 103: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2009;113:957–966. doi: 10.1097/AOG.0b013e3181a106d4. [DOI] [PubMed] [Google Scholar]
- 2.Honrado E, Benítez J, Palacios J. The molecular pathology of hereditary breast cancer: Genetic testing and therapeutic implications. Mod Pathol. 2005;18:1305–1320. doi: 10.1038/modpathol.3800453. [DOI] [PubMed] [Google Scholar]
- 3.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 5.Gorodetska I, Kozeretska I, Dubrovska A. BRCA genes: The role in genome stability, cancer stemness and therapy resistance. J Cancer. 2019;10:2109–2127. doi: 10.7150/jca.30410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowalewski A, Szylberg Ł, Saganek M, et al. Emerging strategies in BRCA-positive pancreatic cancer. J Cancer Res Clin Oncol. 2018;144:1503–1507. doi: 10.1007/s00432-018-2666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghoncheh M, Momenimovahed Z, Salehiniya H. Epidemiology, incidence and mortality of breast cancer in Asia. Asian Pac J Cancer Prev. 2016;17(suppl 3):47–52. doi: 10.7314/apjcp.2016.17.s3.47. [DOI] [PubMed] [Google Scholar]
- 8.Hortobagyi GN, de la Garza Salazar J, Pritchard K, et al. The global breast cancer burden: Variations in epidemiology and survival. Clin Breast Cancer. 2005;6:391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 9.Rhiem K, Schmutzler R. Impact of prophylactic mastectomy in BRCA1/2 mutation carriers. Breast Care (Basel) 2014;9:385–389. doi: 10.1159/000369592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh D, Zori R. Genetic insights into familial cancers: Update and recent discoveries. Cancer Lett. 2002;181:125–164. doi: 10.1016/s0304-3835(02)00023-x. [DOI] [PubMed] [Google Scholar]
- 11.Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643–651. doi: 10.1002/1097-0142(19940201)73:3<643::aid-cncr2820730323>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Peto J, Collins N, Barfoot R, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst. 1999;91:943–949. doi: 10.1093/jnci/91.11.943. [DOI] [PubMed] [Google Scholar]
- 13.Forbes C, Fayter D, de Kock S, et al. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, genetic counseling, and treatment of BRCA-mutated breast cancer. Cancer Manag Res. 2019;11:2321–2337. doi: 10.2147/CMAR.S189627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen CG, Roberts M, Guan Y. Exploring predictors of genetic counseling and testing for hereditary breast and ovarian cancer: Findings from the 2015 U.S. National Health Interview Survey. J Pers Med. 2019;9:26. doi: 10.3390/jpm9020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vries AM, de Roten Y, Meystre C, et al. Clinician characteristics, communication, and patient outcome in oncology: A systematic review. Psychooncology. 2014;23:375–381. doi: 10.1002/pon.3445. [DOI] [PubMed] [Google Scholar]
- 16.Fallowfield L, Jenkins V. Effective communication skills are the key to good cancer care. Eur J Cancer. 1999;35:1592–1597. doi: 10.1016/s0959-8049(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs C, Patch C, Michie S. Communication about genetic testing with breast and ovarian cancer patients: A scoping review. Eur J Hum Genet. 2019;27:511–524. doi: 10.1038/s41431-018-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phadke SR, Pandey A, Puri RD, et al. Genetic counseling: The impact in Indian milieu. Indian J Pediatr. 2004;71:1079–1082. doi: 10.1007/BF02829818. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Genetic/familial high-risk assessment—Breast, Ovarian, and pancreatic (version 1.2020) doi: 10.6004/jnccn.2021.0001. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [DOI] [PubMed]
- 20.Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: Updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21:151–161. doi: 10.1007/s10897-011-9462-x. [DOI] [PubMed] [Google Scholar]
- 21.Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–3667. doi: 10.1200/JCO.2015.63.0996. [DOI] [PubMed] [Google Scholar]
- 22.National Pathology Accreditation Advisory Council . Classification of Human Genetic Testing. Canberra, ACT, Australia: Laboratory Accreditation Standards and Guidelines for Nucleic Acid Detection and Analysis; 2007. [Google Scholar]
- 23.Rantanen E, Hietala M, Kristoffersson U, et al. Regulations and practices of genetic counselling in 38 European countries: The perspective of national representatives. Eur J Hum Genet. 2008;16:1208–1216. doi: 10.1038/ejhg.2008.93. [DOI] [PubMed] [Google Scholar]
- 24.Darooei M, Poornima S, Salma BU, et al. Pedigree and BRCA gene analysis in breast cancer patients to identify hereditary breast and ovarian cancer syndrome to prevent morbidity and mortality of disease in Indian population. Tumour Biol. 2017;39:1010428317694303. doi: 10.1177/1010428317694303. [DOI] [PubMed] [Google Scholar]
- 25.Rich EC, Burke W, Heaton CJ, et al. Reconsidering the family history in primary care. J Gen Intern Med. 2004;19:273–280. doi: 10.1111/j.1525-1497.2004.30401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood ME, Kadlubek P, Pham TH, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: A pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol. 2014;32:824–829. doi: 10.1200/JCO.2013.51.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brédart A, Kop JL, De Pauw A, et al. Effect on perceived control and psychological distress of genetic knowledge in women with breast cancer receiving a BRCA1/2 test result. Breast. 2017;31:121–127. doi: 10.1016/j.breast.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Mancini J, Noguès C, Adenis C, et al. Impact of an information booklet on satisfaction and decision-making about BRCA genetic testing. Eur J Cancer. 2006;42:871–881. doi: 10.1016/j.ejca.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Quinn VF, Meiser B, Kirk J, et al. Streamlined genetic education is effective in preparing women newly diagnosed with breast cancer for decision making about treatment-focused genetic testing: A randomized controlled noninferiority trial. Genet Med. 2017;19:448–456. doi: 10.1038/gim.2016.130. [DOI] [PubMed] [Google Scholar]
- 30.Randall J, Butow P, Kirk J, et al. Psychological impact of genetic counselling and testing in women previously diagnosed with breast cancer. Intern Med J. 2001;31:397–405. doi: 10.1046/j.1445-5994.2001.00091.x. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute Susceptibility genes. https://www.cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq.
- 32.Petrucelli N, Daly MB, Pal T.BRCA1- and BRCA2-associated hereditary breast and ovarian cancerinAdam MP, Ardinger HH, Pagon RA, et al.(eds)GeneReviews Seattle, WA: University of Washington; 1993https://www.ncbi.nlm.nih.gov/books/NBK1247/ [PubMed] [Google Scholar]
- 33.Singh J, Thota N, Singh S, et al. Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi-gene panel: Prevalence of BRCA1/2 and non-BRCA mutations. Breast Cancer Res Treat. 2018;170:189–196. doi: 10.1007/s10549-018-4726-x. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal G, Pradeep PV, Aggarwal V, et al. Spectrum of breast cancer in Asian women. World J Surg. 2007;31:1031–1040. doi: 10.1007/s00268-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 35.Sharma-Oates A, Shaaban AM, Tomlinson I, et al. Heterogeneity of germline variants in high risk breast and ovarian cancer susceptibility genes in India. Precis Clin Med. 2018;1:75–87. doi: 10.1093/pcmedi/pby010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang PC, Phuah SY, Sivanandan K, et al. Recurrent mutation testing of BRCA1 and BRCA2 in Asian breast cancer patients identify carriers in those with presumed low risk by family history. Breast Cancer Res Treat. 2014;144:635–642. doi: 10.1007/s10549-014-2894-x. [Erratum: Breast Cancer Res Treat 150:699-700, 2015] [DOI] [PubMed] [Google Scholar]
- 37.Thirthagiri E, Lee SY, Kang P, et al. Evaluation of BRCA1 and BRCA2 mutations and risk-prediction models in a typical Asian country (Malaysia) with a relatively low incidence of breast cancer. Breast Cancer Res. 2008;10:R59. doi: 10.1186/bcr2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaidyanathan K, Lakhotia S, Ravishankar HM, et al. BRCA1 and BRCA2 germline mutation analysis among Indian women from south India: Identification of four novel mutations and high-frequency occurrence of 185delAG mutation. J Biosci. 2009;34:415–422. doi: 10.1007/s12038-009-0048-9. [DOI] [PubMed] [Google Scholar]
- 39.Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652–665. doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- 41.Feliubadaló L, Lopez-Doriga A, Castellsagué E, et al. Next-generation sequencing meets genetic diagnostics: Development of a comprehensive workflow for the analysis of BRCA1 and BRCA2 genes. Eur J Hum Genet. 2013;21:864–870. doi: 10.1038/ejhg.2012.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannan AU, Singh J, Lakshmikeshava R, et al. Detection of high frequency of mutations in a breast and/or ovarian cancer cohort: Implications of embracing a multi-gene panel in molecular diagnosis in India. J Hum Genet. 2016;61:515–522. doi: 10.1038/jhg.2016.4. [DOI] [PubMed] [Google Scholar]
- 43.Rajkumar T, Meenakumari B, Mani S, et al. Targeted resequencing of 30 genes improves the detection of deleterious mutations in South Indian women with breast and/or ovarian cancers. Asian Pac J Cancer Prev. 2015;16:5211–5217. doi: 10.7314/apjcp.2015.16.13.5211. [DOI] [PubMed] [Google Scholar]
- 44.Borg A, Haile RW, Malone KE, et al. Characterization of BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance in unilateral and bilateral breast cancer: The WECARE study. Hum Mutat. 2010;31:E1200–E1240. doi: 10.1002/humu.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sluiter MD, van Rensburg EJ. Large genomic rearrangements of the BRCA1 and BRCA2 genes: Review of the literature and report of a novel BRCA1 mutation. Breast Cancer Res Treat. 2011;125:325–349. doi: 10.1007/s10549-010-0817-z. [DOI] [PubMed] [Google Scholar]
- 46.Adam MP, Ardinger HH, Pagon RA, et al.(eds)Appendix: Interpretation of sequence analysis resultsinGeneReviews Seattle, WA: University of Washington; 2007 [Google Scholar]
- 47.Jennings LJ, Arcila ME, Corless C, et al. Guidelines for validation of next-generation sequencing-based oncology panels: A joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017;19:341–365. doi: 10.1016/j.jmoldx.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santani A, Murrell J, Funke B, et al. Development and validation of targeted next-generation sequencing panels for detection of germline variants in inherited diseases. Arch Pathol Lab Med. 2017;141:787–797. doi: 10.5858/arpa.2016-0517-RA. [DOI] [PubMed] [Google Scholar]
- 49.Rajkumar T, Soumittra N, Nancy NK, et al. BRCA1, BRCA2 and CHEK2 (1100 del C) germline mutations in hereditary breast and ovarian cancer families in South India. Asian Pac J Cancer Prev. 2003;4:203–208. [PubMed] [Google Scholar]
- 50.Nicolussi A, Belardinilli F, Mahdavian Y, et al. Next-generation sequencing of BRCA1 and BRCA2 genes for rapid detection of germline mutations in hereditary breast/ovarian cancer. PeerJ. 2019;7:e6661. doi: 10.7717/peerj.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park HS, Park SJ, Kim JY, et al. Next-generation sequencing of BRCA1/2 in breast cancer patients: Potential effects on clinical decision-making using rapid, high-accuracy genetic results. Ann Surg Treat Res. 2017;92:331–339. doi: 10.4174/astr.2017.92.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindor NM, Goldgar DE, Tavtigian SV, et al. BRCA1/2 sequence variants of uncertain significance: A primer for providers to assist in discussions and in medical management. Oncologist. 2013;18:518–524. doi: 10.1634/theoncologist.2012-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.BRCA Exchange Summary view. https://brcaexchange.org/
- 54.Lindor NM, Guidugli L, Wang X, et al. A review of a multifactorial probability-based model for classification of BRCA1 and BRCA2 variants of uncertain significance (VUS) Hum Mutat. 2012;33:8–21. doi: 10.1002/humu.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plon SE, Eccles DM, Easton D, et al. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofstra RM, Spurdle AB, Eccles D, et al. Tumor characteristics as an analytic tool for classifying genetic variants of uncertain clinical significance. Hum Mutat. 2008;29:1292–1303. doi: 10.1002/humu.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eggington JM, Bowles KR, Moyes K, et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin Genet. 2014;86:229–237. doi: 10.1111/cge.12315. [DOI] [PubMed] [Google Scholar]
- 58.Zuntini R, Ferrari S, Bonora E, et al. Dealing with BRCA1/2 unclassified variants in a cancer genetics clinic: Does cosegregation analysis help? Front Genet. 2018;9:378. doi: 10.3389/fgene.2018.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roy S, Coldren C, Karunamurthy A, et al. Standards and guidelines for validating next-generation sequencing bioinformatics pipelines: A joint recommendation of the Association for Molecular Pathology and the College of American Pathologists. J Mol Diagn. 2018;20:4–27. doi: 10.1016/j.jmoldx.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Wallace AJ. New challenges for BRCA testing: A view from the diagnostic laboratory. Eur J Hum Genet. 2016;24(suppl 1):S10–S18. doi: 10.1038/ejhg.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Do H, Wong SQ, Li J, et al. Reducing sequence artifacts in amplicon-based massively parallel sequencing of formalin-fixed paraffin-embedded DNA by enzymatic depletion of uracil-containing templates. Clin Chem. 2013;59:1376–1383. doi: 10.1373/clinchem.2012.202390. [DOI] [PubMed] [Google Scholar]
- 62.Hoppe MM, Sundar R, Tan DSP, et al. Biomarkers for homologous recombination deficiency in cancer. J Natl Cancer Inst. 2018;110:704–713. doi: 10.1093/jnci/djy085. [DOI] [PubMed] [Google Scholar]
- 63.Neff RT, Senter L, Salani R. BRCA mutation in ovarian cancer: Testing, implications and treatment considerations. Ther Adv Med Oncol. 2017;9:519–531. doi: 10.1177/1758834017714993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570–3576. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082–1089. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Press JZ, De Luca A, Boyd N, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ledermann J, Harter P, Gourley C. Correction to Lancet Oncol 2014; 15: 856. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2015;16:e158. doi: 10.1016/S1470-2045(15)70153-1. [DOI] [PubMed] [Google Scholar]
- 69.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 70.Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 71.Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 72.Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:159–164. doi: 10.1056/NEJM200107193450301. [DOI] [PubMed] [Google Scholar]
- 73.Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J Clin Oncol. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 74.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 76.Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 77.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 78.King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 79.Carbine NE, Lostumbo L, Wallace J, et al. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev. 2018;4:CD002748. doi: 10.1002/14651858.CD002748.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010;2010:CD002748. doi: 10.1002/14651858.CD002748.pub3. [DOI] [PubMed] [Google Scholar]
- 81.Pesce C, Liederbach E, Wang C, et al. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol. 2014;21:3231–3239. doi: 10.1245/s10434-014-3956-3. [DOI] [PubMed] [Google Scholar]
- 82.Wong SM, Freedman RA, Sagara Y, et al. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg. 2017;265:581–589. doi: 10.1097/SLA.0000000000001698. [DOI] [PubMed] [Google Scholar]
- 83.Heemskerk-Gerritsen BA, Seynaeve C, van Asperen CJ, et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: Revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107:djv033. doi: 10.1093/jnci/djv033. [DOI] [PubMed] [Google Scholar]
- 84.Kotsopoulos J, Huzarski T, Gronwald J, et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2016;109:djw177. doi: 10.1093/jnci/djw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Terry MB, Daly MB, Phillips KA, et al. Risk-reducing oophorectomy and breast cancer risk across the spectrum of familial risk. J Natl Cancer Inst. 2019;111:331–334. doi: 10.1093/jnci/djy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 87.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 90.Valarmathi MT, A A, Deo SS, et al. BRCA1 germline mutations in Indian familial breast cancer. Hum Mutat. 2003;21:98–99. doi: 10.1002/humu.9099. [DOI] [PubMed] [Google Scholar]
- 91.Saxena S, Chakraborty A, Kaushal M, et al. Contribution of germline BRCA1 and BRCA2 sequence alterations to breast cancer in Northern India. BMC Med Genet. 2006;7:75. doi: 10.1186/1471-2350-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Syamala V, Sreeja L, Syamala VS, et al. Novel germline mutations in BRCA2 gene among 96 hereditary breast and breast-ovarian cancer families from Kerala, South India. J Cancer Res Clin Oncol. 2007;133:867–874. doi: 10.1007/s00432-007-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soumittra N, Meenakumari B, Parija T, et al. Molecular genetics analysis of hereditary breast and ovarian cancer patients in India. Hered Cancer Clin Pract. 2009;7:13. doi: 10.1186/1897-4287-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 96.Fabbro M, Moore KN, Dørum A, et al. Efficacy and safety of niraparib as maintenance treatment in older patients (≥ 70 years) with recurrent ovarian cancer: Results from the ENGOT-OV16/NOVA trial. Gynecol Oncol. 2019;152:560–567. doi: 10.1016/j.ygyno.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 97.Oza AM, Tinker AV, Oaknin A, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol. 2017;147:267–275. doi: 10.1016/j.ygyno.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 98.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]