Abstract
PURPOSE
Until human papillomavirus (HPV)–based cervical screening is more affordable and widely available, visual inspection with acetic acid (VIA) is recommended by the WHO for screening in lower-resource settings. Visual inspection will still be required to assess the cervix for women whose screening is positive for high-risk HPV. However, the quality of VIA can vary widely, and it is difficult to maintain a well-trained cadre of providers. We developed a smartphone-enhanced VIA platform (SEVIA) for real-time secure sharing of cervical images for remote supportive supervision, data monitoring, and evaluation.
METHODS
We assessed programmatic outcomes so that findings could be translated into routine care in the Tanzania National Cervical Cancer Prevention Program. We compared VIA positivity rates (for HIV-positive and HIV-negative women) before and after implementation. We collected demographic, diagnostic, treatment, and loss-to-follow-up data.
RESULTS
From July 2016 to June 2017, 10,545 women were screened using SEVIA at 24 health facilities across 5 regions of Tanzania. In the first 6 months of implementation, screening quality increased significantly from the baseline rate in the prior year, with a well-trained cadre of more than 50 health providers who “graduated” from the supportive-supervision training model. However, losses to follow-up for women referred for further evaluation or to a higher level of care were considerable.
CONCLUSION
The SEVIA platform is a feasible, quality improvement, mobile health intervention that can be integrated into a national cervical screening program. Our model demonstrates potential for scalability. As HPV screening becomes more affordable, the platform can be used for visual assessment of the cervix to determine amenability for same-day ablative therapy and/or as a secondary triage step, if needed.
INTRODUCTION
In February 2020, the 146th WHO Executive Board recommended a draft resolution on the elimination of cervical cancer as a public health problem be adopted by the 73rd World Health Assembly.1 The Draft Strategy2 includes the targets “90/70/90”: 90% of girls should be vaccinated against human papillomavirus (HPV) by 15 years of age, 70% of women should be screened by 35 and 45 years of age, and 90% of women with cervical disease should receive treatment (90% of women with precancer treated; 90% of women with invasive cancer managed). However, despite WHO’s inclusion in its “Best Buy” category,3 the availability and affordability of HPV testing remains profoundly limited in many settings.4 Although the Papanicolaou test (cytology-based) screening has reduced cervical cancer deaths in many high-income countries, it has not been successfully implemented in many low- and middle-income (LMIC) settings for reasons including costs and logistical challenges,5 with multiple steps from specimen collection, transport to laboratories for processing and interpretation, and communication to providers and participants.5-9
CONTEXT
Key Objective
To evaluate the 1-year programmatic outcomes of incorporating our smartphone visual inspection with acetic acid platform (SEVIA) into the national cervical cancer screening program (CECAP) of Tanzania.
Knowledge Generated
The SEVIA mobile health platform improved programmatic effectiveness after > 10,000 women were screened by mostly nurse providers, demonstrating (1) effectiveness of SEVIA's supportive-supervision model to enhance the quality of visual inspection of the cervix; (2) capacity of our cascade (training of trainers) model; and (3) effectiveness of real-time data acquisition, monitoring, and evaluation via our online dashboard to support the quality control efforts of the country's CECAP program.
Relevance
Smartphone-based platforms like SEVIA can be used and adapted to improve the quality of provider assessment and can facilitate quality control of visual assessment for treatment as human papillomavirus DNA testing becomes more widely available. Additional functionalities, including image capture for machine-learning algorithms and semiautomated processes for patient navigation, can be added to platforms like SEVIA.
As such, visual inspection with acetic acid (VIA) has been the mainstay of cervical screening, and despite its shortcomings, is still among the recommended strategies in lower-resource settings where HPV testing is not yet widely available.5 Moreover, visual inspection will still be required to assess the cervix for same-visit ablative therapy (ie, with thermal coagulation or cryotherapy) for women who screen positive for high-risk HPV.
VIA can be incorporated into a task-sharing model and can be performed by a variety of trained health workers, including midwives and nurses.5,10 Treatment of precancers can be performed by nonphysician health workers, enabling a single visit screen-and-treat approach in many settings.5,10 However, this can be challenging in settings where monitoring and evaluation, programmatic oversight, and sustainable financing are lacking.7,8 In addition, quality of VIA can vary widely, and it is difficult to maintain a well-trained cadre of providers.7,8 Interpreting VIA with the naked eye is subjective and can be highly variable between providers, an issue that supportive supervision and quality assurance (QA) can help to overcome.5,7,9 The Pan American Health Organization (PAHO) and WHO describe a process of QA and quality control (QC) of VIA-based cervical screening programs11 that acknowledges the challenges of maintaining quality of service provision and programmatic outcomes in lower-resource settings. QC processes include “co-assessment” and supportive supervision by established providers that a country considers as adequately skilled (ie, “experts”). In a previously unscreened population, the expected VIA-positivity (VIA+) rate is between 5% and 10%,12 and up to 20% for women living with HIV, which is cited in the Tanzanian National Cervical Cancer Prevention and Control program guidelines. To address some of the quality issues in successful implementation of VIA, Parham et al13,14 developed a single-visit point-of-care enhanced digital imaging of the cervix (digital cervicography) program in Zambia. The method uses a digital single-lens reflex camera and a monitor that allows for “peer review, QA, continuing medical education, an objective record of screening test results, and expert opinion through immediate distance consultation, if needed”14. However, scalability and portability have been hampered by the technical requirements.
In Tanzania, VIA+ rates have historically been lower than expected (based on global estimates) within the national cervical cancer screening (CECAP) program and have been fraught with wide ranges, as reviewed by Runge et al.15 This can be attributed, in part, to a lack of QA mechanisms to ensure VIA skills are maintained among health providers. To improve VIA quality and to evaluate the impact of a QA program within the CECAP program, we developed a smartphone-enhanced VIA program (SEVIA). Our proof-of-concept study in Tanzania16 evaluated the sharing of images and clinical information within a closed user group on an Android smartphone to improve the VIA skills of cervical cancer screening providers. SEVIA allows secure, real-time sharing of cervical images and clinical information acquired by health providers, which are assessed for quality of VIA interpretation by expert reviewers as part of a supervision and mentorship program for nonphysician health providers in the CECAP program. This report presents the results of the first 12 months of the SEVIA program after its integration in 5 regions in Tanzania.
METHODS
SEVIA allows expert reviewers to be assigned to VIA screening providers. Reviewers receive a notification within the smartphone application that provides immediate feedback to the provider to enhance the quality of screening in an efficient and supportive manner from a remote location. Our online monitoring and evaluation dashboard allows programmatic oversight, with access to real-time data, showing screening activities of health providers, quality of screening (VIA interpretation), and epidemiologic data to inform government programming. Once training was completed, screening providers were able to securely share, with informed consent, a woman’s de-identified data (including cervical images) with their assigned expert reviewers for feedback and supervision. All data were also collected and stored (once submitted) within the dashboard.
Study Setting
The transition-to-scale program was launched in July 2016 in collaboration with the Ministry of Health, Community Development, Gender, Elderly and Children (MoHCDGEC). At the time of implementation, 96% of health facilities offering SEVIA provided same-day treatment with cryotherapy. Loop electrosurgical excision procedure (LEEP) services were provided at 17% of health facilities offering SEVIA (3 regional referral hospitals and 1 zonal hospital). Unless a woman received screening at a hospital with LEEP services, she was referred to the nearest government facility where LEEP was available.
Study Design
We assessed the effectiveness and the programmatic outcomes so that research findings could be translated into routine care. Because the SEVIA platform was designed as both a training and QA tool, we evaluated the change in mean VIA+ rate at each program site from the same 6 calendar months in the year before implementation of SEVIA (among both HIV-positive [HIV+] and HIV negative [HIV−] women) compared with the VIA+ rate 6 months after implementation. We collected demographic, diagnostic, treatment, and loss-to-follow-up data.
Study Participants
Although the national screening program encourages women to undergo screening between ages 30 and 49 years, women living with HIV are invited to screen earlier. Study participants included women ≥ 25 years of age attending their local reproductive health facility for routine care, including cervical cancer screening as part of a screening campaign, or who were receiving care in an HIV clinic. Participants were asked to provide informed consent to have their data and images collected for program evaluation. Informed consent was obtained verbally through individual and/or group consent through an information session delivered by a trained health provider before the participant underwent screening with SEVIA. If the participant declined to have cervical images taken, she received screening with VIA only. During the consent process, participants also provided consent to be contacted through the mobile phone number they provided for follow-up purposes, which is part of the CECAP program care pathway.
Ethical Oversight
participants gave informed consent to the provider before initiating the screening process. Because SEVIA was incorporated into routine care under the direction and authority of the Tanzanian government (MoHCDGEC), the Ministry provided oversight of all program operations. It should be noted that the pilot work16 was conducted with institutional review board (IRB) approval by the National Institute of Medical Research and Queen’s University in Canada, and amendments to include this SEVIA scale-up program were approved by both IRBs before implementing this study.
SEVIA Providers
Five regions were approved by the MoHCDGEC for integration of the SEVIA program into the existing CECAP program. Health providers were selected to be trained in SEVIA, with input from regional, district, and facility-based health management teams from each of the participating regions. Providers included mainly nurses, clinical officers, assistant medical officers, and obstetricians/gynecologists who had completed the 6-day MoHCDGEC cervical cancer screening course, screened more than 50 women, and treated more than 5 participants with cryotherapy.17 LEEP providers who were selected to be trained in SEVIA were obstetricians/gynecologists, medical officers, and assistant medical officers. Health facilities were selected if they had providers already trained in VIA and were actively providing cervical screening services. Fifty-one providers already performing SEVIA screening at 24 facilities were provided with refresher VIA and cryotherapy training, in addition to technical training with SEVIA, before the program launch. SEVIA providers thus had variable levels of hands-on VIA experience and limited exposure to VIA refresher training in the years before the SEVIA program launch.
SEVIA Reviewers
The 16 reviewers for the SEVIA program included obstetrician/gynecologists, assistant medical officers, clinical officers, and registered nurses, as well as 1 medical officer, 1 nursing officer, and 1 assistant nursing officer.
SEVIA Functions
Image acquisition, reviewing, and feedback process.
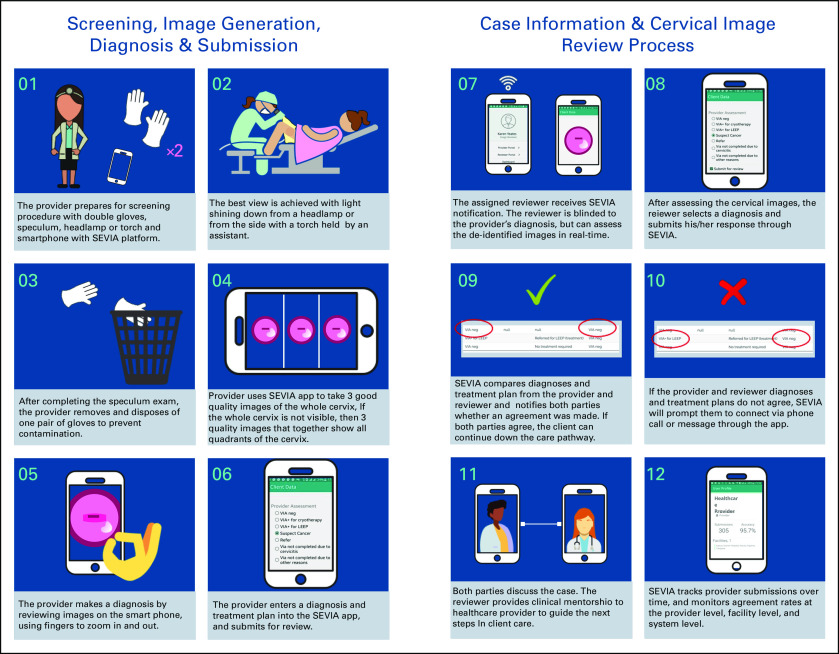
To ensure feasibility within the screen-and-treat approach, the SEVIA program was founded on the principle of providing real-time feedback on the health provider’s VIA diagnosis, treatment, and follow-up plan. Figure 1 includes a basic infographic showing how SEVIA is used by providers to enhance the screening process, including image capture and data sharing with the offsite reviewers. Providers are considered to have graduated from the SEVIA training program based on the number of women screened, the concordance/agreement with reviewers, and the threshold for the number of VIA+ patients in their participant history. The highest performing providers were invited to become reviewers.
FIG 1.
Smartphone-enhanced visual inspection with acetic acid (SEVIA) image capture and review process. Client, participant.
Data collection and monitoring.
SEVIA captures indicators required for the CECAP program, including demographics, HIV status, previous screening history, cervical screening results, and treatment plans. In the event of network issues, data uploads and communication with reviewers occurs once the network becomes functional again. Aggregated data across facilities were collected in real time and populated on the monitoring and evaluation dashboard, with key indicators, disaggregated by HIV status and visit type (initial visit, routine screening after negative result at first screening, and follow-up visit 1 year after treatment), downloaded as CSV (comma-separated values) files to IBM SPSS (version 24 for Windows; Armonk, NY). Figure 2 shows examples from the SEVIA provider portal, as well as the dashboard. All data were de-identified. All personal health information was de-identified, and all cervical images submitted by SEVIA providers were uploaded, encrypted, and stored on the platform. Images were never visible on the dashboard to program administrative staff and managers. All data were uploaded to a secure server in the Tanzanian Commission for Science and Technology.
FIG 2.
Screenshots of the smartphone-enhanced visual inspection with acetic acid (SEVIA) platform.
Statistical Analysis
Baseline VIA+ rates were collected from facilities for the same 6-month period in the year preceding SEVIA implementation (July 1, 2015, to December 31, 2015). Data were audited for quality and completeness, and corrections were sought in 2 regions in Tanzania where data appeared to have possible errors. A health facility was considered an outlier if it had a VIA+ rate of (1) < 0.5%; or (2) > 50%. Two facilities from 1 region were excluded because the VIA+ rate of 1 facility was < 0.5% and the other > 50%. A third facility was brought online during the second half of the demonstration program (January 2017) and therefore was excluded from the VIA+ rate comparison. Pearson χ2 tests were was used to test the overall difference between VIA+ rates at baseline and after SEVIA program implementation at 21 facilities participating in the demonstration program, as well as for subsets of HIV+, HIV−, and first-time participants.
RESULTS
From July 1, 2016, to June 30, 2017, 10,545 women underwent cervical screening by providers using the SEVIA platform at 24 government and faith-based reproductive health facilities across 5 regions of Tanzania. The demographic characteristics of the women screened are presented in Table 1. The majority of participants were between the ages of 35 and 50 years and were multiparous. A small proportion of women < 25 years of age were offered screening, including some women who were HIV negative. A higher proportion of HIV+ participants had previously attended cervical cancer screening relative to HIV− participants (27.3% v 4.6%).
TABLE 1.
Demographic Characteristics of Women Screened in the First Year of SEVIA Implementation by HIV Status
Nearly all SEVIA images of participants screened were reviewed by an offsite expert reviewer (99.8%), with only 22 participants submitted to the platform not receiving formal review. Overall, 45.6% of participants were reviewed in < 10 minutes, 17.4% of were reviewed within 10 minutes to 2 hours of submission, and 15.4% were reviewed within 2 to 24 hours of submission. Because of reviewers’ schedules, mobile network outages, and down time on the SEVIA platform for maintenance, 21.7% of participants submitted were reviewed within 24 to 72 hours (data not shown).
Table 2 displays the comparison of baseline VIA+ rates from a 6-month period in the year before SEVIA implementation with VIA+ rates in the same 6-month period in the year after SEVIA implementation. Results are shown overall for participants ages 30-49 years, by HIV serostatus, and for participants who were screened for the first time. We also assessed provider and reviewer concordance over 1 year, which fluctuated with the integration of new, untrained reviewers over time. Provider and reviewer concordance rates were approximately 90% over the 1-year period, although there was some fluctuation as new, untrained providers were integrated into the program over time (data not shown).
TABLE 2.
Comparison of VIA Positivity Rates by HIV Status at Project Baseline (July 1 to December 31, 2015) and During SEVIA Program Implementation (July 1 to December 3, 2016)
Table 3 shows the mean VIA+ rate for HIV+ and HIV− participants during the first year of the program. Table 4 shows the uptake of treatment of precancers as well as among women who required LEEP and/or referral to a higher level of care. Of those women who screened positive with VIA and were amenable to cryotherapy, 48.9% received cryotherapy on the day of screening. The losses to follow-up were high for those requiring treatment that was not available at the same visit. A total of 64 VIA+ participants were asked to return for cryotherapy at a later date, some because of cervicitis (requiring antibiotic treatment), others because of lack of available resources, such a CO2 laser therapy.
TABLE 3.
No. of Women Screened and VIA-Positivity Rate by HIV Status in the First Year of Implementation of the SEVIA Program (ages 25-49 years only)

TABLE 4.
Treatment Uptake of VIA-Positive Women by HIV Status in the First Year of Implementation of the SEVIA Program in Tanzania (ages 25-49 only)
DISCUSSION
Despite the existence of highly effective and cost-effective prevention strategies, cervical cancer remains the fourth most common cancer in women globally, and the most common cause of cancer-related deaths among women in more than 42 countries, mostly in sub-Saharan Africa.18 Challenges are well recognized in scaling programs to achieve the population coverage needed to reduce cervical cancer mortality.7,9,10,17 We evaluated the VIA rates for the first 9,142 women ages 25-49 years screened by SEVIA providers within Tanzania’s national CECAP. VIA+ rates over the first 6 months increased compared with the same 6-month period in the preceding year (before the introduction of SEVIA), for HIV+ and HIV− participants and for first-time participants. Although we did not compare our VIA results against biopsy-proven cervical intraepithelial neoplasia 2+, we believe this reflects the clinical utility of SEVIA to improve quality of health care delivery in this real-world setting. This QC indicator, that is, providers’ average VIA+ results compared with the country’s reference standard, is in keeping with WHO/PAHO11 and the Tanzanian CECAP guidelines.12
Of the 9,142 women screened (ages 25-49 years), 136 were deemed to require referral to a higher level of care for LEEP or evaluation of suspected invasive cancer. It is possible these women did receive evaluation and treatment as needed, but we were unable to track them within the existing system at that time.
Among our program strengths is that SEVIA was developed in partnership with key stakeholders within the Ministry of Health in Tanzania. Oversight and training of health providers and reviewers was conducted in close collaboration with the Ministry; as such, SEVIA was integrated into the existing CECAP program. Our train-the-trainer model, whereby qualified graduates of the SEVIA curriculum can go on to become trainers and/or reviewers, demonstrates the potential for scalability and sustainability via a cascade training model. Improvements can now be readily implemented, such as random auditing of (graduated) providers’ SEVIA results, which would provide an entry point for refresher training.
Some quality improvement might have been due in part to the VIA refresher training before SEVIA training. Given the high incidence of cervical cancer in Tanzania, the true VIA+ rate is likely somewhat higher that what we report; ideally, the SEVIA model of training and QA should be tested against histopathology from cervical biopsies of VIA+ patients. However, PAHO/WHO guidance for QC in real-world settings11 states that the gold standard is as the country defines it (ie, according local experience and/or comparisons among similar populations). Although we estimated the costs of using SEVIA at less than US $1.00 per screen, including hardware, phones, data bundles, information technology support, and hosting and maintenance of the platform, we did not conduct a formal cost analysis.
The high loss to follow-up for women who required referral to higher levels of care has been previously reported in Tanzania19 and speaks to the difficulties in retaining participants in large-scale screening programs in settings with fragile health systems. Here, patient navigation services can be an acceptable strategy to improve retention.20 Automated reminders can facilitate the provider’s role as navigation tools in the mobile health (mHealth) platform; however, all such interventions should be studied to learn whether and how such efforts can serve to improve health service delivery and, ultimately, population health.21 Smartphones can support other aspects of the cervical screening pathway, including recruitment of patients to screening, as we report in a related study in Tanzania.22 The WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening23 highlights challenges and opportunities to strengthen health systems. In partnership with the Information Telecommunications Union, WHO published Be Healthy, Be Mobile: A Handbook on How to Implement mCervicalCancer,24 outlining pathways to integrate an mHealth framework into new or existing programs. In designing SEVIA, cited in the WHO handbook, we incorporated core features of the framework.
Although HPV DNA testing and cytology-based screening are considered more effective methods, costs and logistical barriers in their implementation present significant challenges for most low-income and many middle-income countries.25 In the meantime, as HPV-based screening makes its way into LMICs, smartphone and related platforms such as SEVIA might continue to play a role as a secondary “triage” for women testing positive for the oncogenic HPV subtypes and can facilitate QC of visual assessment for treatment suitability. Platforms like SEVIA can also aid in the development and testing of machine-learning algorithms26,27 to improve quality of assessment and clinical decision support.
ACKNOWLEDGMENT
The authors acknowledge Grand Challenges Canada, Global Affairs Canada, Groesbeck Parham, Pamoja Tunaweza Women’s Centre, and Ministry of Health-Tanzania/CECAP program.
SUPPORT
The smartphone-enhanced visual inspection with acetic acid (SEVIA) mobile platform and implementation research program was developed in partnership with the Ministry of Health in Tanzania and was funded by a Transition to Scale grant from Grand Challenges Canada. The funders of the study had no role in its design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the article for publication. The lead, second, third, and senior author had full access to all the data in the study and all authors had final responsibility for the decision to submit for publication.
AUTHOR CONTRIBUTIONS
Conception and design: Karen Yeates, Zac Mtema, Frank Magoti, Olola Oneko, Godwin Macheku, Carter Smith, Ophira Ginsburg
Administrative support: Karen Yeates, Zac Mtema, Safina Yuma, Agnes Feksi Mtei, Nicola West, Ashley Newcomb
Provision of study materials or patients: Simoni Nkumbugwa, Olola Oneko, Godwin Macheku
Collection and assembly of data: Karen Yeates, Zac Mtema, Frank Magoti, Simoni Nkumbugwa, Safina Yuma, Olola Oneko, Godwin Macheku, Agnes Feksi Mtei, Carter Smith, Linda Andrews, Nicola West, Ophira Ginsburg
Data analysis and interpretation: Karen Yeates, Erica Erwin, Zac Mtema, Frank Magoti, Simoni Nkumbugwa, Wilma M. Hopman, Alyssa Ferguson, Carter Smith, Milena Dalton, Ashley Newcomb, Ophira Ginsburg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Simon Nkumbugwa
Consulting or Advisory Role: Tanzania
No other potential conflicts of interest were reported.
REFERENCES
- 1.World Health Organization WHO EB recommends the adoption of the strategy for elimination of cervical cancer. https://www.who.int/news-room/detail/05-02-2020-who-eb-recommends-the-adoption-of-the-strategy-for-elimination-of-cervical-cancer
- 2.World Health Organization : Draft: Global strategy towards eliminating cervical cancer as a public health problem. https://www.who.int/docs/default-source/cervical-cancer/cerv-cancer-elimn-strategy-16dec-12pm.pdf?sfvrsn=3cd24074_8.
- 3.World Health Organization . “Best Buys” and other recommended interventions for the prevention and control of noncommunicable diseases. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 4.Denny L, de Sanjose S, Mutebi M, et al. Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries. Lancet. 2017;389:861–870. doi: 10.1016/S0140-6736(16)31795-0. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Comprehensive cervical cancer control: A guide to essential practice. Geneva, Switzerland: World Health Organization; 2014. [PubMed] [Google Scholar]
- 6.Mezei AK, Armstrong HL, Pedersen HN, et al. Cost-effectiveness of cervical cancer screening methods in low- and middle-income countries: A systematic review. Int J Cancer. 2017;141:437–446. doi: 10.1002/ijc.30695. [DOI] [PubMed] [Google Scholar]
- 7. Holme F, Kapambwe S, Nessa A, et al: Scaling up proven innovative cervical cancer screening strategies: Challenges and opportunities in implementation at the population level in low- and lower-middle-income countries. Int J Gynecol Obstet 138:63-68, 2017 (suppl) [DOI] [PubMed]
- 8.Tsu VD, Ginsburg O. The investment case for cervical cancer elimination. Int J Gynaecol Obstet. 2017;138(suppl 1):69–73. doi: 10.1002/ijgo.12193. [DOI] [PubMed] [Google Scholar]
- 9.Basu P, Meheus F, Chami Y, et al. Management algorithms for cervical cancer screening and precancer treatment for resource-limited settings. Int J Gynaecol Obstet. 2017;138(suppl 1):26–32. doi: 10.1002/ijgo.12183. [DOI] [PubMed] [Google Scholar]
- 10.Shastri SS, Mittra I, Mishra GA, et al. Effect of VIA screening by primary health workers: Randomized controlled study in Mumbai, India. J Natl Cancer Inst. 2014;106:dju009. doi: 10.1093/jnci/dju009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization and Pan-American Health Organization: Monitoring national cervical cancer prevention and control programmes: Quality control and quality assurance for visual inspection with acetic acid (VIA)-based programmes. (2013)
- 12.The United Republic of Tanzania National Cervical Cancer Prevention and Control Program : Guideline of Quality Improvement for VIA Based Screening Approach (2016)
- 13.Parham GP, Mwanahamuntu MH, Pfaendler KS, et al. eC3--a modern telecommunications matrix for cervical cancer prevention in Zambia. J Low Genit Tract Dis. 2010;14:167–173. doi: 10.1097/LGT.0b013e3181cd6d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parham GP, Mwanahamuntu MH, Kapambwe S, et al. Population-level scale-up of cervical cancer prevention services in a low-resource setting: Development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLoS One. 2015;10:e0122169. doi: 10.1371/journal.pone.0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Runge AS, Bernstein ME, Lucas AN, et al: Cervical cancer in Tanzania: A systematic review of current challenges in six domains. Gynecol Oncol Rep 29:40-47, 2019. [DOI] [PMC free article] [PubMed]
- 16.Yeates KE, Sleeth J, Hopman W, et al. Evaluation of a smartphone-based training strategy among health care workers screening for cervical cancer in northern Tanzania: The Kilimanjaro Method. J Glob Oncol. 2016;2:356–364. doi: 10.1200/JGO.2015.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starrs AM, Ezeh AC, Barker G, et al. Accelerate progress-sexual and reproductive health and rights for all: Report of the Guttmacher-Lancet Commission. Lancet. 2018;391:2642–2692. doi: 10.1016/S0140-6736(18)30293-9. [DOI] [PubMed] [Google Scholar]
- 18.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 19. Bateman LB, Blakemore S, Koneru A: Barriers and facilitators to cervical cancer screening, diagnosis, follow-up care and treatment: Perspectives of human immunodeficiency virus-positive women and health care practitioners in Tanzania. Oncologist 24:69-75, 2018. [DOI] [PMC free article] [PubMed]
- 20.Koneru A, Jolly P.E., Blakemore S, et al. Acceptance of peer navigators to reduce barriers to cervical cancer screening and treatment among women with HIV infection in Tanzania. Int J Gynaecol Obstet. 2017;138:53–61. doi: 10.1002/ijgo.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal S, LeFevre AE, Lee J, et al. Guidelines for reporting of health interventions using mobile phones: Mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. 2016;352:i1174. doi: 10.1136/bmj.i1174. [DOI] [PubMed] [Google Scholar]
- 22.Erwin E, Aronson KJ, Day A, et al. SMS behaviour change communication and eVoucher interventions to increase uptake of cervical cancer screening in the Kilimanjaro and Arusha regions of Tanzania: A randomised, double-blind, controlled trial of effectiveness. BMJ Innov. 2019;5:28–34. doi: 10.1136/bmjinnov-2018-000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization: WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening. Geneva, Switzerland, World Health Organization, 2019. [PubMed] [Google Scholar]
- 24.World Health Organization and the Information Telecommunications Union . Be Healthy, Be Mobile: A Handbook on How to Implement mCervicalCancer. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 25.Ogilvie G, Nakisige C, Huh WK, et al. Optimizing secondary prevention of cervical cancer: Recent advances and future challenges. Int J Gynaecol Obstet. 2017;138(suppl 1):15–19. doi: 10.1002/ijgo.12187. [DOI] [PubMed] [Google Scholar]
- 26.Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. 2019. [DOI] [PMC free article] [PubMed]
- 27. Hu L, Horning M, Dipayan B, et al: Deep learning-based image evaluation for cervical precancer screening with a smartphone targeting low-resource settings: Engineering approach. Presented at the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society in conjunction with the 43rd Annual Conference of the Canadian Medical and Biological Engineering Society, Montreal, Quebec, Canada, July 20-24, 2020. [DOI] [PubMed] [Google Scholar]