Abstract
PURPOSE
The WHO now recommends thermal ablation as an alternative to cryotherapy within “screen-and-treat” cervical cancer programs in low- and middle-income countries (LMICs). We conducted a safety and acceptability clinical trial of thermal ablation in a Kenyan Ministry of Health hospital among women living with HIV (WLWH; ClinicalTrials.gov identifier: NCT04191967).
METHODS
Between August 2019 and February 2020, WLWH age 25-65 years underwent human papillomavirus (HPV) self-collection in western Kenya. HPV-positive women underwent visual inspection with acetic acid, biopsy, and treatment with thermal ablation performed by a nonphysician clinician, if eligible by standard guidelines. A questionnaire was administered after treatment to assess for pain and treatment acceptability. Adverse events (AEs) were evaluated 4-6 weeks after treatment with a standardized grading tool.
RESULTS
A total of 293 HPV-positive WLWH underwent thermal ablation in the study period. The mean age was 40.4 years (standard deviation, 8.7 years). After treatment, 15 (5.1%), 231 (78.8%), 42 (14.3%), and 5 (1.8%) reported none, mild, moderate, and severe pain with treatment, respectively. At follow-up, spotting, vaginal discharge, and pelvic pain were reported by 99 (37.8%), 258 (98.5%), and 46 (17.6%), respectively, for a median of 3.3 (interquartile range [IQR], 2-3), 14 (IQR, 7-21), and 7 (IQR, 3-7) days, respectively. Most participants graded their AEs as mild (grade 1): 94 (95.0%) for bleeding, 125 (48.5%) for vaginal discharge, and 37 (80.4%) for pelvic pain. No grade 3 or 4 AEs were reported. The vast majority (99.2%) were satisfied with the treatment and would recommend it to a friend.
CONCLUSION
Thermal ablation performed by nonphysicians in the public health sector in Kenya proved safe and highly acceptable in treating HPV-positive WLWH.
INTRODUCTION
Although cervical cancer is preventable, in 2018 an estimated 570,000 new cases occurred, with 90% in low- and middle-income countries (LMICs).1 Cervical cancer is an AIDS-defining malignancy, and women living with HIV (WLWH) are at increased risk because of high incidence and persistence of high-risk human papillomavirus (HPV) infection, the causative agent.2 Compared with women without HIV, WLWH develop precancerous lesions at a younger age and have faster progression to cervical cancer, making prevention efforts among this group particularly urgent.3 Low-income countries have been unable to implement cytology-based screening programs because of significant infrastructure and human resource requirements that are not feasible in these settings.4 In 2013, the WHO recommended cervical cancer screening using visual inspection with acetic acid (VIA) or HPV testing in LMICs, followed by immediate treatment with cryotherapy, in a “screen-and-treat” strategy.5
CONTEXT
Key Objective
Is thermal ablation (TA) for treatment of precancerous cervical lesions among women living with HIV (WLWH) in low- and middle-income countries (LMICs) safe and acceptable?
Knowledge Generated
In this study among WLWH in Kenya, TA for treatment of precancerous lesions by a nonphysician provider was found to be safe and highly acceptable. Most participants report grade 1 (mild) adverse events (AEs) after treatment, with no grade 3 or 4 AEs. Although acceptability was high (99.2%), 16.1% reported moderate or severe pain with treatment, higher than reported in prior studies. Compared with women < 40 years of age, women ≥ 40 years were more likely to report moderate or severe pain with treatment (odds ratio, 2.6; P = .060).
Relevance
Our findings support ongoing efforts to increase access to treatment of precancerous lesions with TA among WLWH in LMIC settings. Additional studies on predictors of moderate to severe pain may support widespread acceptability.
Although multiple studies demonstrated the safety and efficacy of cryotherapy for use within screen-and-treat programs in LMICs,6-8 widespread implementation has been limited.9 Challenges associated with cryotherapy include bulky equipment, limiting the feasibility of mobile treatment, and the need for refrigerant gas, which is expensive and of variable quality in rural areas.9 An evaluation of 25 health facilities with cryotherapy services in Uganda showed that almost half of them were not operational owing to lack of gas.9 In Malawi, over a 5-year period, only 43.3% of women who screened VIA-positive accessed treatment owing to challenges with delivering cryotherapy.10 To achieve the WHO’s cervical cancer elimination strategy, which includes targets of 70% of women screened for cervical cancer using an HPV test, and 90% of those with a positive result adequately treated by 2030,11 accessible treatment options are an urgent priority.
Thermal ablation (TA) is an alternative treatment method that uses heat instead of refrigerant gas to ablate abnormal cervical tissue and has recently been investigated for use in LMICs.12 Compared with cryotherapy, TA has several advantages that may enable successful scale-up in screen-and-treat programs in low-resource settings. Newer-generation battery-powered devices are light and highly portable, weighing 2-5 kg compared with 15-20 kg for each cryotherapy gas cylinder.9 Thermal ablation also allows for faster treatment—a 20- to 40-second application (single or multiple) of a reusable probe heated to 100°C, compared with 12-15 minutes for cryotherapy.9,13 Widely accepted WHO criteria for eligibility for treatment with TA are similar to that for cryotherapy.9 Similar to cryotherapy, it is widely accepted that TA is provided without local analgesia.14
Following evidence primarily from high-income countries showing similar efficacy of TA for treatment of precancerous lesions,13,15 the WHO has issued guidelines for the use of TA in LMICs.14 While calling for more context-specific evidence, the WHO issued a conditional recommendation for providing TA as an alternative to cryotherapy for women with histologically confirmed cervical intraepithelial neoplasia grade ≥ 2 (CIN2+) or who are high-risk HPV positive and are eligible for ablation treatment.14 This recommendation includes treatment of WLWH.14 To ensure treatment access, the guidelines suggest that trained nurses and midwives may perform TA in addition to physicians.
Although several studies from high-income countries have described the safety and acceptability of TA,16-20 few data exist from LMICs.21-23 Available studies among HIV-negative women, primarily from high-income countries, report mild to moderate adverse events (AEs) associated with TA. These include mild cramping in 25%-79%,2,19,22 moderate pain in 10.5%,20 and severe pain in 3.5%.20 The majority of studies did not provide analgesia during TA,13 including all studies in low-income settings. After treatment, reported AEs were mild, including vaginal discharge,2,21 pain,2,22 and, rarely, local cervical infection (in 1.1%).17 Only 1 published study describes safety and acceptability of TA among WLWH in sub-Saharan Africa—a recently published randomized pilot trial comparing TA to cryotherapy and loop electrosurgical excision procedure for treating VIA-positive women in Zambia.23 In this study in which 52% (392) of women were HIV positive, TA was found to be safe, with few associated AEs and no reported complications.23 The majority of women undergoing TA reported no (46%), or little (52%) pain, with 1% reporting moderate and < 1% severe pain with treatment. Although TA was found to be highly acceptable, no data on adverse effects associated with treatment were reported. Given the limited data among WLWH in low-income countries, we sought to evaluate the safety and acceptability of TA for the treatment of HPV-positive, HIV-positive women in a low-resource setting in sub-Saharan Africa.
METHODS
We conducted a prospective cohort study at the Family AIDS Care & Education (FACES)–supported Ministry of Health clinics in Kisumu County, in Western Kenya. At FACES, WLWH age 25-65 years are offered cervical cancer screening using HPV testing of self-collected vaginal specimens. Nonpregnant women age 25-65 years with no history of cervical cancer or precancer treatment were eligible to participate in this study. Study recruitment occurred from August 2019 to February 2020. Counseling on HPV, cervical cancer, and the screening process was offered during routine HIV clinics in group and individual settings. Participants were then provided self-sampling instructions, a collection kit, and a private area to perform self-collection. The self-collected HPV samples were labeled, stored, and processed in batches of 90 on the careHPV system, which tests for DNA of 14 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68).24 HPV-positive women were invited for a return visit, where they underwent a pelvic examination and VIA to determine eligibility for ablative therapy. Those eligible for ablation were offered treatment per the WHO recommendation, regardless of whether a lesion was seen on VIA.5 Women were considered candidates for ablation if the squamocolumnar junction was fully visualized; cervical lesions, if present, took up < 75% of the cervix; and there was no endocervical component of the lesion or suspicion for cancer.5 Ineligible women were referred for excision or other evaluation by a gynecologist. Before ablation, all women underwent colposcopically directed biopsies of abnormal lesions or, in the absence of lesions on VIA, a random biopsy at 6 or 12 o’clock for disease status ascertainment. Thermal ablation was performed using the Liger thermocoagulator device (Cure Medical Global, Lehi, UT).25 Thermal ablation was performed by a nonphysician clinician who had undergone a 5-day training on VIA and ablation treatment with both the thermocoagulator and cryotherapy. Treatment was performed per the WHO recommendations,14 with a treatment length of 20 seconds, as previously described.22 No local anesthesia was used. The probe was decontaminated with alcohol and heated to 100°C or soaked in Cidex solution for 20 minutes for sterilization before reuse.22
After ablation, a questionnaire was administered to participants to evaluate their experience with treatment, including a 4-point visual analog scale to evaluate pain, as well as treatment acceptability. Participants then received counseling on expected symptoms after treatment, including mild cramping and vaginal discharge.26 Participants were advised to abstain from sexual intercourse for 4 weeks after treatment and to present to the clinic in case of any concerning adverse effects, including severe pain, heavy bleeding, or fever.26 All women were given a 4- to 6-week phone or in-person follow-up appointment, per their preference. At this appointment, AEs, as a measure of safety, were evaluated using the Division of AIDS table for grading the severity of female genital symptoms (grade 0 is normal, 1 is mild, 2 is moderate, 3 is severe, and 4 is life threatening).27 Complications, including severe bleeding during or after treatment or infection, were noted and any concerning symptoms evaluated in person.28 Participants were also asked whether they would recommend the treatment to others who needed it as a measure of acceptability.
Data were collected via tablets, using a REDCap database, and analyzed by Stata version 13.1 (StataCorp, College Station, TX). Clinical and demographic characteristics were obtained from participant interviews or abstracted from clinical data. Baseline and demographic characteristics for all participants were compared with those with a diagnosis of cervical intraepithelial neoplasia grade 2, 3, or invasive cancer (CIN2+). The prevalence of AEs was reported as proportions with 95% CIs, and pain during treatment was reported on a visual scale ranging from 1-4 (none, mild, moderate, severe, potentially life threatening). To test for association between a diagnosis of CIN2+ and clinical and demographic variables, we performed χ2, Fisher’s exact, or Student t tests as appropriate. We used logistic regression to explore the association of demographic and clinical characteristics with participant reports of moderate or severe pain during treatment. The institutional review boards of Maseno University and the University of California San Francisco approved this study. Participants provided written informed consent.
RESULTS
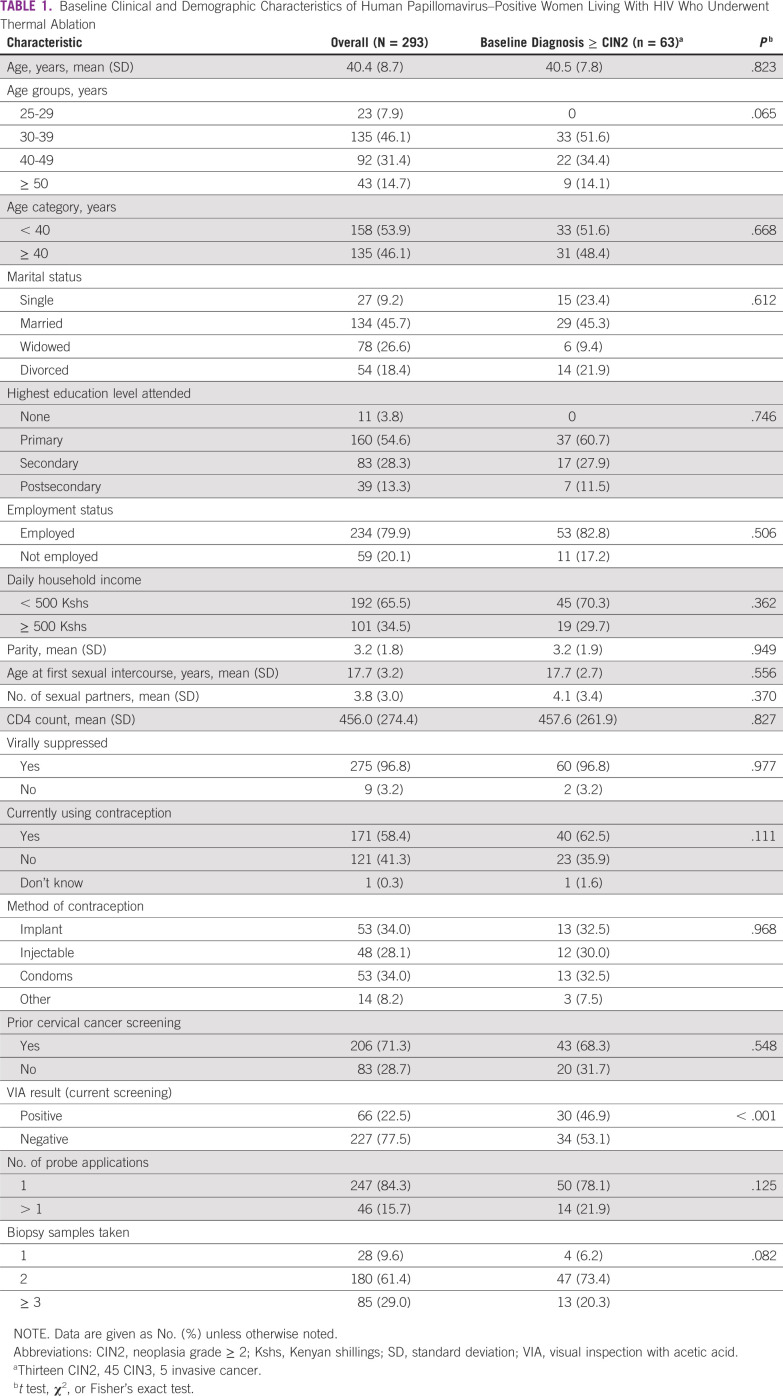
A total of 293 WLWH at the FACES clinics tested positive for HPV and underwent treatment with TA between August 2019 and February 2020. The mean age was 40.4 years (standard deviation [SD], 8.7 years). Approximately half of women were married (45.7%) and had at least a primary school education (54.6%; Table 1). The majority (79.9%) had no formal employment, and 65.5% had a daily household income of < US $5 (500 Kenyan shillings). All women were on antiretroviral therapy, with 96.8% having achieved HIV viral load suppression. Only 58.4% reported current contraception use, with 34.0% using the implant, 28.1% using injectable contraception, and 34.0% using condoms. Of the 293 HPV-positive WLWH included, 63 (21.5%) had CIN2+ on colposcopically directed biopsy. Of these 63 women, 13 (20.6) had CIN2, 45 (71.4.2%) had CIN3, 4 (6.3%) had squamous cell carcinoma, and 1 (1.6%) had endocervical carcinoma. On bivariate analyses, women with CIN2+ or worse on pathology were statistically more likely to be VIA positive (P < .001; Table 1).
TABLE 1.
Baseline Clinical and Demographic Characteristics of Human Papillomavirus–Positive Women Living With HIV Who Underwent Thermal Ablation
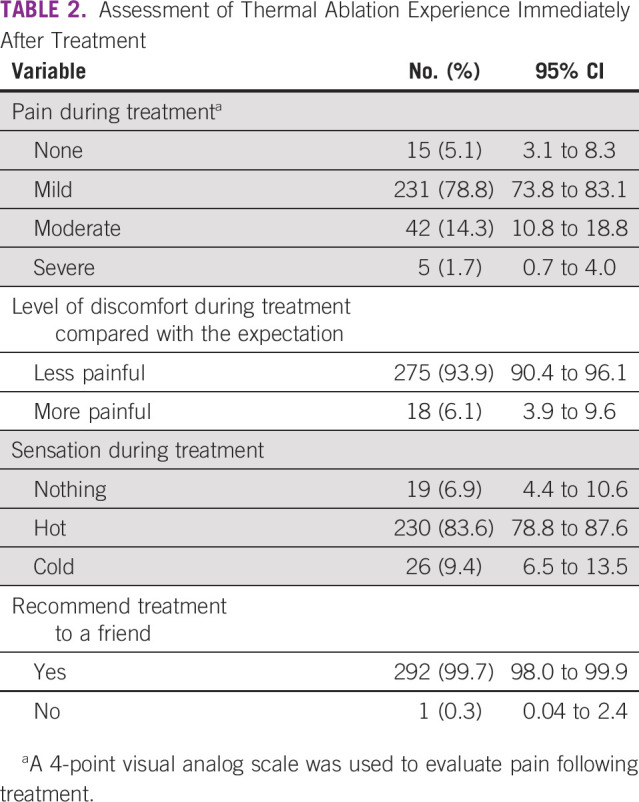
After TA, 15 (5.1%) participants reported no pain, 231 (78.8%) reported mild pain, 42 (14.3%) reported moderate pain, and 5 (1.8%) reported severe pain during the procedure (Table 2). The vast majority of participants (275; 93.9%) reported the experience to be less painful than anticipated. Most women (230; 88.6%) reported experiencing a sensation of heat during the procedure. After the procedure, the vast majority of women (292; 99.7%) reported that they would recommend TA to a friend if they needed it.
TABLE 2.
Assessment of Thermal Ablation Experience Immediately After Treatment
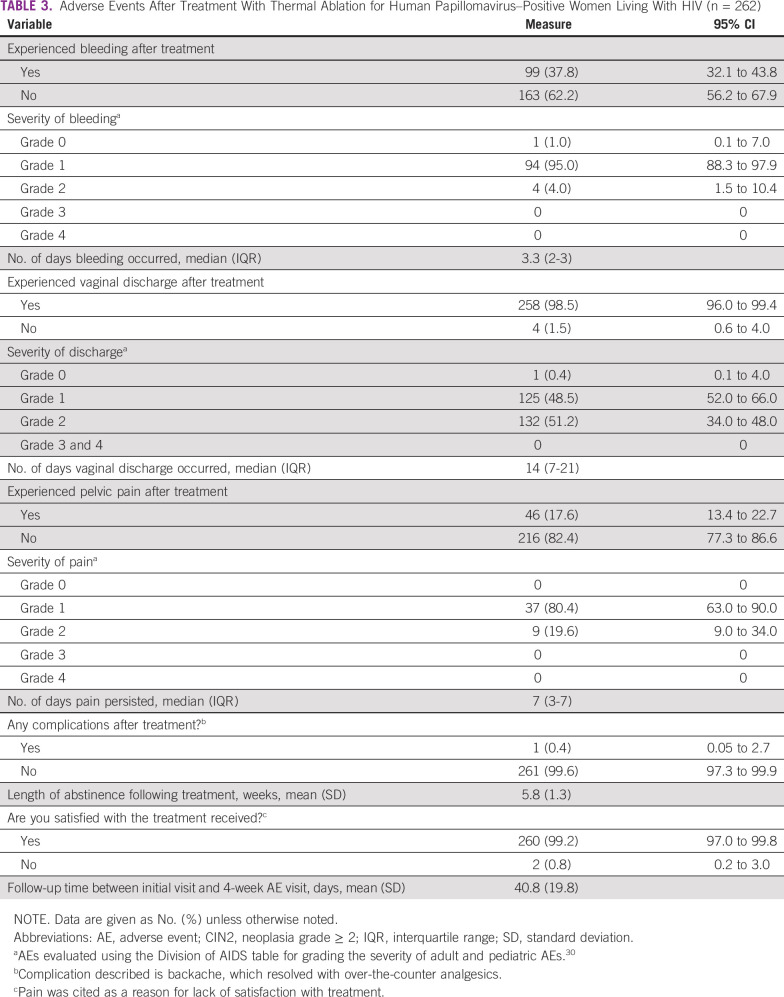
Data from the 4- to 6-week follow-up assessment are available for 292 participants (99.7%). The median follow-up period was 40.8 days after treatment (SD, 19.8 days). The mean reported abstinence period after treatment was 5.8 weeks (SD, 1.3 weeks). Spotting or bleeding, vaginal discharge, and pelvic pain were reported by 99 (37.8%), 258 (98.5%), and 46 (17.6%), respectively (Table 3). The majority of participants graded their symptoms as mild (grade 1) or moderate (grade 2): 94 (95%) for bleeding, 132 (58.8%) for vaginal discharge, and 37 (80.4%) for pelvic pain (Table 3). No grade 3 or 4 AEs were reported. Two women had presented for evaluation after treatment with vaginal discharge and were prescribed antibiotics, with resolution of their symptoms. The median number of days of bleeding, vaginal discharge, and pelvic pain symptoms was 3.3 (interquartile range [IQR], 2-3), 14 (IQR, 7-21), and 7 (IQR, 3-7), respectively. When asked about other complications associated with treatment, 1 participant (0.5%) reported associated back pain, which resolved with over-the-counter analgesics and did not warrant further evaluation. At the follow-up evaluation, 260 (99.2%) of women reported overall satisfaction with TA. The 2 participants who were not satisfied with treatment reported pain as the primary concern.
TABLE 3.
Adverse Events After Treatment With Thermal Ablation for Human Papillomavirus–Positive Women Living With HIV (n = 262)
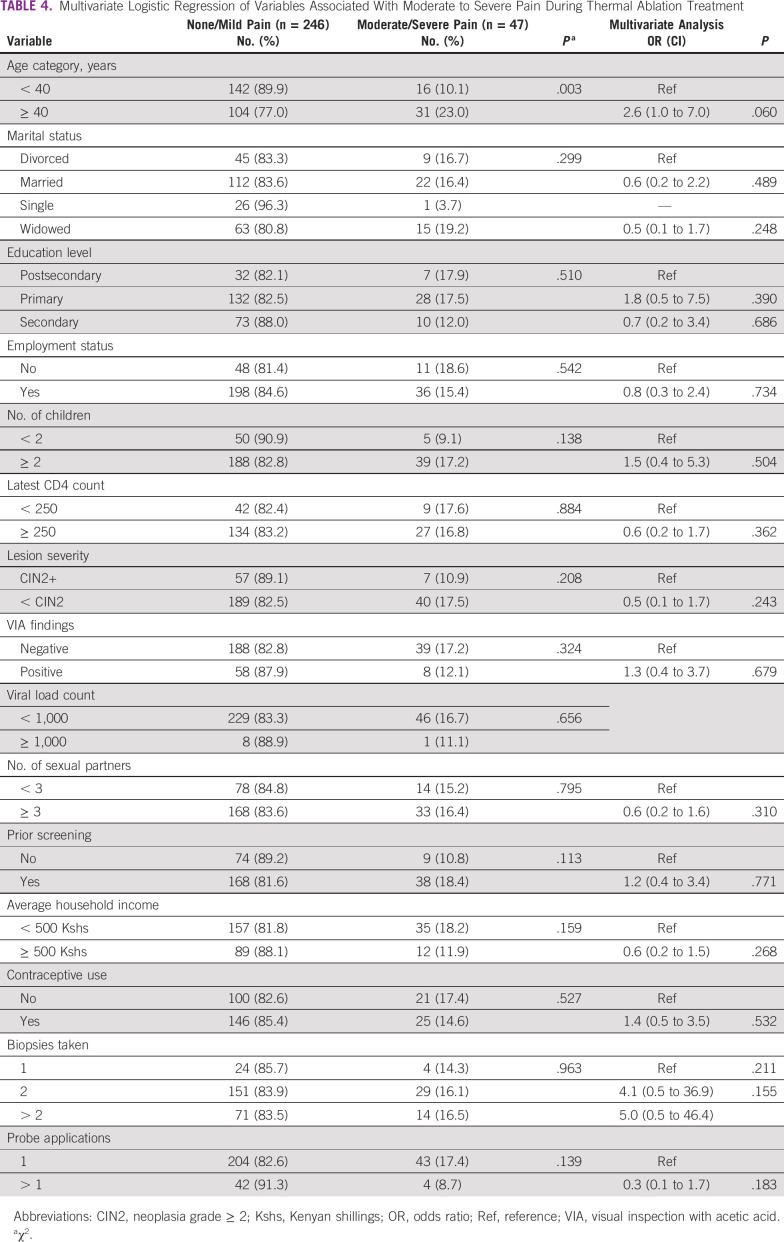
We performed a multivariate logistic regression to evaluate factors associated with women reporting moderate or severe pain during treatment (Table 4). Compared with women < 40 years of age, women ≥ 40 years were more likely to experience moderate or severe pain during TA, although this was not statistically significant (odds ratio [OR], 2.6; 95% CI, 1.0 to 7.0; P = .060). Lesion severity on biopsy, VIA findings, number of biopsy samples taken, and the number of probe applications were not associated with moderate or severe pain with TA.
TABLE 4.
Multivariate Logistic Regression of Variables Associated With Moderate to Severe Pain During Thermal Ablation Treatment
DISCUSSION
In this study evaluating the safety and acceptability of TA among HPV-positive WLWH in sub-Saharan Africa, TA was found to be a safe and highly acceptable ablative treatment method, with no significant complications or AEs reported in this population. The majority of treated women (83.9%) reported no or mild pain during the procedure, and all participants who started the treatment completed it. The study also found a low rate of AEs after treatment, with the majority of symptoms rated as mild on follow-up evaluation. Among reported AEs, vaginal discharge was the most common, with 98.5% reporting discharge for a median of 14 days. Participants were able to adhere to the abstinence recommendation, with a mean reported abstinence period of 5.8 weeks after treatment. Nearly all participants were satisfied with the treatment and would recommend it to a friend.
Our findings are largely consistent with other studies in HIV-negative women and the 1 published study among WLWH reporting high levels of safety and acceptability of TA, but they also raise specific issues that may warrant further evaluation. The low rates of AEs after TA are consistent with the literature on AEs after cryotherapy. In a study in Western Kenya evaluating safety of cryotherapy among VIA-positive women, the most commonly reported adverse effects were vaginal discharge in 95.7% (compared with 99.0% in our study) and mild or moderate vaginal bleeding in 26.1% (compared with 40.8% in our study).29 Our findings of low rates of significant bleeding after TA (3.8% grade 2, and no grade 3 or 4 cases) are similar to the 0.7% rate among HIV-positive women in India after cryotherapy, despite the use of different scales.6
Among studies from LMICs, although using different scales, hence limiting comparability, pain with TA has largely been reported as little to mild (35%-98% of surveyed women),2,21-23 with only 1 study reporting moderate or severe pain in 2% undergoing TA.23 This is compared with our findings, where 16.0% in our study reported moderate or severe pain (14.3% moderate, 1.7% severe). On multivariate analysis, age was associated with reporting moderate or severe pain with TA, with women ≤ 40 years being more likely to report higher pain scores compared with younger women (OR, 2.6; P = .060), although this did not reach statistical significance. This is in comparison with a study among HIV-uninfected women in Cameroon, where women with < 2 children were more likely to report a higher mean pain score (4.2 [SD, 2.0] v 2.9 [SD, 1.5]; P = .016).21 Despite these findings of a higher proportion reporting moderate or severe pain with TA, no women stopped treatment because of pain, and we report no AEs like syncope associated with treatment. Before treatment, all women were evaluated visually for cervicitis but could have had a subclinical infection that may affect treatment tolerability. This finding may warrant additional investigation, because pain as a measure of acceptability is an important consideration if TA is to be scaled within see-and-treat programs in low-resource settings.
Our results on the safety of TA treatment are consistent with published findings both from high-income countries and LMICs, which support a high safety profile of TA. A systematic review and meta-analysis evaluating safety and efficacy of TA, including 6 studies from LMICs, found mild to moderate adverse effects to be common, with rare severe AEs, consistent with our results.15 Similar to our findings, vaginal discharge was common after TA in published studies,15,21 reported in 99.1% of women in Cameroon, with a mean duration of 16.2 days (SD, 8.4 days).21 Only a very small percentage of women in that study (2.8%), as in our study (0.96%), received antibiotics for discharge. Of note, the 2 women prescribed antibiotics in our study for foul-smelling vaginal discharge had no fever or other evidence of systemic infection. The majority of women in our study, 181/208 (89.6%), reported experiencing a sensation of heat during treatment, compared with only 13 (25%) in the Brazil study.22 Our findings of a high rate of acceptability (98% satisfaction, and 100% would recommend TA to a friend) are consistent with Pinder et al,23 the only study evaluating TA acceptability in an LMIC, who also found that 100% of treated women would recommend TA to others.
There are several limitations to our study. As part of the study protocol, a biopsy was performed for all participants before TA. This may have confounded the pain rating, as women may have had a difficult time differentiating pain or discomfort from the biopsy to that related to treatment, despite an active effort by the study team to orient participants to the different parts of study procedures. Some2,22 but not all21,23 cited studies reporting pain after TA included biopsy in their protocol, making direct comparisons difficult. In addition, as the accepted duration of treatment with TA is not uniform, ranging from 20-60 seconds, this may affect a patient’s perceived pain with treatment. Our study used a treatment time of 20 seconds, similar to Naud et al,22 compared with 40 seconds,23 45 seconds,2 and 60 seconds.21 Similar to most published studies, we rely on patient recall in reporting AEs after treatment, which can be subject to recall bias. In addition, our average length of follow-up after treatment is 44.9 days. Hence, it is possible that other treatment-related AEs may occur after our assessment, although this is unlikely, because the median length of reported adverse effects after ablation have ranged in the 2- to 3-week window.28
In conclusion, our findings add to the growing evidence of safety and acceptability of TA when performed by a nonphysician provider in an LMIC. In particular, we report an excellent safety profile and high acceptability of TA treatment among HPV-positive WLWH in sub-Saharan Africa. Although data on the efficacy of TA for treatment of precancerous lesions among HIV-positive women are limited and are the subject of several ongoing studies (including by our group, which will report data on the efficacy of TA for treating biopsy-proven CIN2/3 among WLWH at 12 months), these findings support ongoing efforts to increase access to treatment of precancerous lesions with TA in LMICs.
ACKNOWLEDGMENT
We thank the FACES Lumumba staff and leadership for support throughout this study.
SUPPORT
Supported by the Fogarty International Center of the National Institutes of Health (NIH) Award No. D43TW009343, the University of California Global Health Institute, and the National Institutes of Mental Health of the US Public Health Service Grant No. T32 MH19105.
AUTHOR CONTRIBUTIONS
Conception and design: Chemtai Mungo, Cirilus Ogollah Osongo, Jackton Omoto, Craig R. Cohen, Megan Huchko
Administrative support: Cirilus Ogollah Osongo, Magdalene A. Randa, Craig R. Cohen
Financial support: Megan Huchko
Provision of study material or patients: Magdalene A. Randa, Jackton Omoto, Megan Huchko
Collection and assembly of data: Cirilus Ogollah Osongo, Jeniffer Ambaka, Magdalene A. Randa
Data analysis and interpretation: Chemtai Mungo, Cirilus Ogollah Osungo, Magdalene A. Randa, Megan Huchko
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Craig R. Cohen
Honoraria: Lupin Pharmaceuticals, Miyarisan Pharmaceutical
No other potential conflicts of interest were reported.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018. [DOI] [PubMed]
- 2.Joshi S, Sankaranarayanan R, Muwonge R, et al. Screening of cervical neoplasia in HIV-infected women in India. AIDS. 2013;27:607–615. doi: 10.1097/QAD.0b013e32835b1041. [DOI] [PubMed] [Google Scholar]
- 3.Gichangi PB, Bwayo J, Estambale B, et al. Impact of HIV infection on invasive cervical cancer in Kenyan women. AIDS. 2003;17:1963–1968. doi: 10.1097/00002030-200309050-00015. [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.1016/j.vaccine.2006.05.121. Denny L, Quinn M, Sankaranarayanan R: Chapter 8: Screening for cervical cancer in developing countries. Vaccine 24:71-77, 2006 (suppl 3) [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. 2013 www.who.int/about/licensing/copyright_form/en/index.html [PubMed]
- 6.Sankaranarayanan R, Rajkumar R, Esmy PO, et al. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96:738–743. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nene BM, Hiremath PS, Kane S, et al. Effectiveness, safety, and acceptability of cryotherapy by midwives for cervical intraepithelial neoplasia in Maharashtra, India. Int J Gynaecol Obstet. 2008;103:232–236. doi: 10.1016/j.ijgo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Santesso N, Schünemann H, Blumenthal P, et al. World Health Organization Guidelines: Use of cryotherapy for cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2012;118:97–102. doi: 10.1016/j.ijgo.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Castle PE, Murokora D, Perez C, et al. Treatment of cervical intraepithelial lesions. Int J Gynaecol Obstet. 2017;138(suppl 1):20–25. doi: 10.1002/ijgo.12191. [DOI] [PubMed] [Google Scholar]
- 10.Msyamboza KP, Phiri T, Sichali W, et al. Cervical cancer screening uptake and challenges in Malawi from 2011 to 2015: Retrospective cohort study. BMC Public Health. 2016;16:806. doi: 10.1186/s12889-016-3530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binagwaho A, Garcia PJ, Gueye B, et al. Eliminating deaths from cervical cancer-report of a panel at the 7th Annual Symposium on Global Cancer Research, a satellite meeting at the Consortium of Universities for Global Health 10th Annual Meeting. J Glob Oncol. doi: 10.1200/JGO.19.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.1200/JGO.2016.003731. Maza M, Schocken CM, Bergman KL, et al: Cervical Precancer treatment in low- and middle-income countries: A technology overview. J Glob Oncol 10.1200/JGO.2016.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolman L, Sauvaget C, Muwonge R, et al. Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: A systematic review. BJOG. 2014;121:929–942. doi: 10.1111/1471-0528.12655. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization: WHO guidelines for the use of thermal ablation for cervical pre-cancer lesions. 2019. [PubMed]
- 15.Randall TC, Sauvaget C, Muwonge R, et al. Worthy of further consideration: An updated meta-analysis to address the feasibility, acceptability, safety and efficacy of thermal ablation in the treatment of cervical cancer precursor lesions. Prev Med. 2019;118:81–91. doi: 10.1016/j.ypmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Loobuyck HA, Duncan ID. Destruction of CIN 1 and 2 with the Semm cold coagulator: 13 years’ experience with a see-and-treat policy. Br J Obstet Gynaecol. 1993;100:465–468. doi: 10.1111/j.1471-0528.1993.tb15273.x. [DOI] [PubMed] [Google Scholar]
- 17.Singh P, Loke K-L, Hii J, et al. Cold coagulation versus cryotherapy for treatment of cervical intraepithelial neoplasia: Results of a prospective randomized trial. J Gynecol Surg. 1988;4:211–221. [Google Scholar]
- 18.Goodman JD, Sumner D. Patient acceptability of laser and cold coagulation therapy for pre-malignant disease of the uterine cervix. Br J Obstet Gynaecol. 1991;98:1168–1171. doi: 10.1111/j.1471-0528.1991.tb15372.x. [DOI] [PubMed] [Google Scholar]
- 19.Hussein IY, Galloway RK. Use of the cold coagulator in the treatment of cervical intra-epithelial neoplasia. J Obstet Gynaecol. 1985;6:62–64. [Google Scholar]
- 20. de Cristofaro D, Trioni G, Pezzoli C: Treatment of CIN with cold coagulation. J Exp Clin Cancer Res 9:133, 1990. [Google Scholar]
- 21.Viviano M, Kenfack B, Catarino R, et al. Feasibility of thermocoagulation in a screen-and-treat approach for the treatment of cervical precancerous lesions in sub-Saharan Africa. BMC Womens Health. 2017;17:2. doi: 10.1186/s12905-016-0355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naud PSV, Muwonge R, Passos EP, et al. Efficacy, safety, and acceptability of thermocoagulation for treatment of cervical intraepithelial neoplasia in a hospital setting in Brazil. Int J Gynaecol Obstet. 2016;133:351–354. doi: 10.1016/j.ijgo.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 23.Pinder LF, Parham GP, Basu P, et al. Thermal ablation versus cryotherapy or loop excision to treat women positive for cervical precancer on visual inspection with acetic acid test: Pilot phase of a randomised controlled trial. Lancet Oncol. 2020;21:175–184. doi: 10.1016/S1470-2045(19)30635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiruneh FN, Chuang KY, Ntenda PAM, et al. Individual-level and community-level determinants of cervical cancer screening among Kenyan women: A multilevel analysis of a Nationwide survey. BMC Womens Health. 2017;17:109. doi: 10.1186/s12905-017-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cure Medical: Thermocoagulator kitshttp://www.curemedicalglobal.com/thermocoagulator-kits/
- 26. Sellors JW, Sankaranarayanan R (eds): Colposcopy and Treatment of Cervical Intraepithelial Neoplasia: A Beginner’s Manual. Lyon, France, World Health Organization, 2003. [Google Scholar]
- 27. Division of AIDS, National Institute of Allergy and Infectious Diseases: Division of AIDS Table Grading the Severity of Adult and Pediatric Adverse Events. 2004. https://rsc.niaid.nih.gov/sites/default/files/addendum-1-female-genital-grading-table-v1-nov-2007.pdf.
- 28. Castro W, Gage J, Gaffikin L, et al: Effectiveness, safety and acceptability of cryotherapy: A systematic literature review. Cervical Cancer Prevention Issues in Depth, Seattle, WA, Alliance for Cervical Cancer Prevention, 2003. [Google Scholar]
- 29.Lewis KDC, Sellors JW, Dawa A, et al. Report on a cryotherapy service for women with cervical intraepithelial neoplasia in a district hospital in western Kenya. Afr Health Sci. 2011;11:370–376. [PMC free article] [PubMed] [Google Scholar]
- 30. Division of AIDS, National Institute of Allergy and Infectious Diseases: Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events: Corrected version 2.1. https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf.