Abstract
This review will compare and contrast the costs and access to novel drugs for treating chronic lymphocytic leukemia (CLL) and lymphoma in the United States and India during the last 5 years. Clinical outcomes for patients with hematologic malignancies have improved significantly since the approval of immunotherapeutic and targeted therapies. These new treatments have had an impact on overall outcomes and have helped determine the design for translational research and future trials. Although most of these novel drugs called “innovators” are initially approved and marketed in the United States, several have also become available in countries such as India. With the expiration of patents, generic versions of innovator drugs have increased and accessibility has improved for patients. The advent of biosimilars is another route for expanding access to biologic compounds. As a result, the development costs for developing these drugs are lower, and consequently, the costs for the patient are often lower. Although the delivery of cancer care is not the same in India as it is in the United States, the introduction of biosimilars and generics has helped bridge the gap. This has made treatment of CLL and lymphoma similar in both countries and has had the same impact on patient outcomes and quality of life. Compulsory licensing for essential medications, as stipulated by the Doha Declaration, and capping of drug prices could improve global access to treatments for CLL and lymphoma.
INTRODUCTION
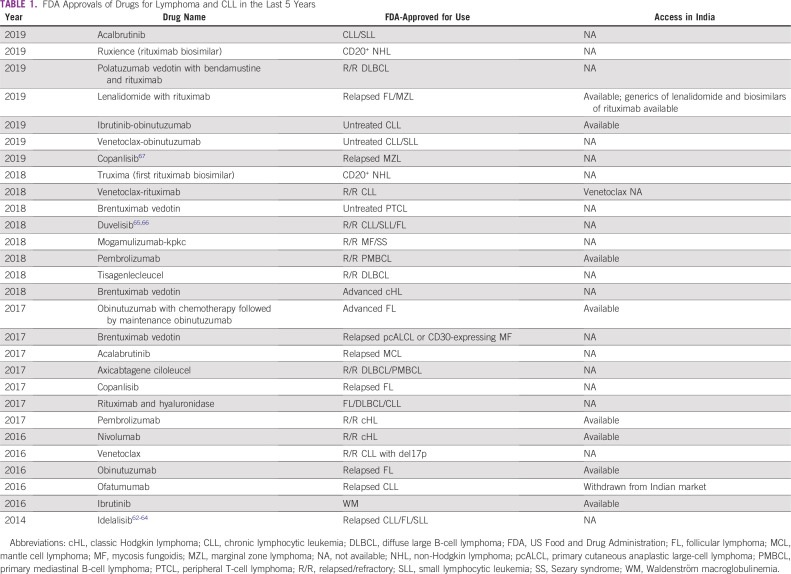
Blood cancers are a significant public health problem worldwide and a leading cause of death in the United States and India. According to GLOBOCAN 2018, the annual incidence of lymphoma is 82,548 in the United States and 37,225 in India.1 The annual incidence of chronic lymphocytic leukemia (CLL) was 50,149 in the United States and 42,055 in India.2 Over the past 5 years, the US Food and Drug Administration (FDA) has approved 24 new indications in lymphoma and 11 in CLL (Table 1).3 Most clinical trials for these novel drugs are conducted in the United States, and the drugs are available for use soon after, but only in the United States. The approval process for new drugs in India is managed by the Central Drugs Standard Control Organisation and generally lags behind approval in the United States by at least 2 years.4 In addition, a decade can elapse before generics and biosimilars reach clinical practice. Our study aims to increase understanding of the similarities and differences regarding cancer care delivery, accessibility, cost, and the potential impact on survival for patients in the United States and India with the advent of these novel drugs for lymphoma and CLL.
TABLE 1.
FDA Approvals of Drugs for Lymphoma and CLL in the Last 5 Years
CONTEXT
Key Objective
Blood cancers are a leading health problem in the United States and India. We compare the cost of and access to novel drugs for treating chronic lymphocytic leukemia (CLL) and lymphoma between the United States and India during the last 5 years.
Knowledge Generated
Delivery of cancer care in the United States is different from that in India. In the United States, around 90% of the population has health insurance. In India, a majority of the population pays for medical expenses out of pocket. The cost of drug development is high, and most novel drugs are initially developed and marketed in the United States, but it takes several years before the drugs become available in India. The development of biosimilars has increased access to and affordability of biologics for the treatment of CLL and lymphoma in India.
Relevance
The development of biosimilars has increased access to and affordability of biologics for the treatment of CLL and lymphoma in India. The overall outcome and quality of life is rather similar in the two countries with the advent of biosimilars and generics. Future strategies to ensure universal access include expanding the availability of biosimilars, capping drug prices, expanding insurance coverage, and constructing a hub-and-spoke rural outreach model to make novel drugs accessible to all patients.
DELIVERY OF CANCER CARE
The cancer care delivery systems in the United States and India illustrate the differences between health care in a developed and in a developing nation. The United States spends 17.8% of its gross domestic product on health care and often is the first country to adopt new therapies.5 Most novel drugs recently approved for treating cancer are priced at more than $100,000 per year and are thus affordable only with health insurance.6-9 Approximately 90% of the US population is covered by health insurance.10 In addition, the Patient Protection and Affordable Care Act (2010) has expanded insurance coverage to include cancer care.11 Individuals with lymphoma who live in rural areas and those who are uninsured have inferior outcomes probably because they have less access to cancer care.12-16
Cancer care delivery in India is the opposite. India spends around 3.89% of its gross domestic product on health care.17 The low patient:oncologist ratio in India (1:2,000) adds to the increasing demands of cancer care.18 Most people pay out of pocket for medical expenses, and only a minority have health insurance.19,20 A substantial portion of the population lives in rural areas with little or no awareness of what health care options are available, and they have limited resources to pay for specialized health care. We have not captured comparisons between countries in terms of rural accessibility. The regional cancer centers in India provide health care at a subsidized rate only to a limited population.21 The National Cancer Grid is a recent initiative by the Government of India under the Ayushman Bharat-Pradhan Mantri Jan Arogya Yojana, which extends annual financial support up to $7,000 for patients with cancer.22
DRUG PRICING IN THE UNITED STATES
The average cost of new drug development is approximately $100 million, and the projected cost for a successful approval is approximately $1 billion, so the prices for marketed drugs are higher. Generic medicines and the biosimilars that enter the system later can help to reduce the cost of the drugs for the consumer.23,24 The Drug Price Competition and Patent Term Restoration Act (Hatch-Waxman Act, 1984) has led to accelerated methods for approving generic drugs.25 However, in some cases, prices for cancer drugs are kept high by pharmaceutical company lobbying, which can also potentially delay the entry of generics into the market.26 The benchmark for drug reimbursement is set by Centers for Medicare & Medicaid Services. Effective January 1, 2017, payment for infusion drugs is based on Section 1847A of the Social Security Act and implies that most of the payments will be based on the average sales price of these drugs.27-29 Accordingly, Medicare pays 106% of the average sales price for most drugs covered under Part B.
BIOSIMILARS AND GENERICS
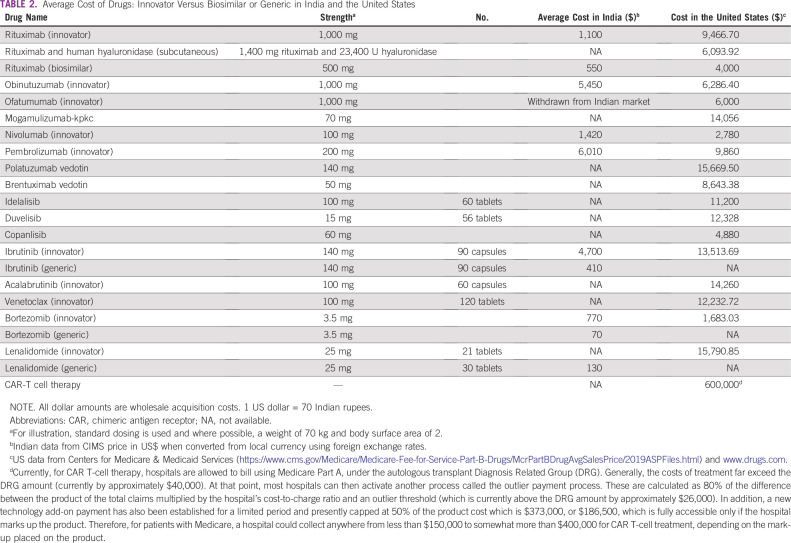
Once the patents for the innovative drug expire after a stipulated period, a window of opportunity opens for producing a similar product called a biosimilar at a much lower cost without compromising efficacy.30 To date, the number of these agents that receive approval in the United States has been limited. Relevant agents include the pegfilgrastim biosimilars and the two biosimilars of rituximab.31,32 Because of a thriving pharmaceutical industry, the generics and biosimilars are more affordable in India. The first biosimilar in India was approved in 2007, and it reached a market value of $2.2 billion in 2017.33 Table 2 lists cost information for drugs in the United States and India for standard therapies for lymphomas and CLL. The costs appear to be different because of the method used to convert local currencies into US dollars. The World Bank classifies India as a low- and middle-income country. Although the current prices for drugs have been converted by using foreign exchange rates, the prices can be different if the conversion uses purchasing power parity.34 To illustrate, there are 70.84 Indian rupees to 1 US dollar using foreign exchange rates, but only 18.128 rupees to 1 US dollar using purchasing power parity.
TABLE 2.
Average Cost of Drugs: Innovator Versus Biosimilar or Generic in India and the United States
NOVEL DRUGS FOR LYMPHOMA AND CLL
Several novel drugs have shown promising results and have improved patient outcome in CLL and lymphoma. This section describes the FDA approvals and access to novel drugs in the United States and India during the last 5 years. Table 1 summarizes the new drug approvals and Table 2 provides an average cost of these agents in the United States and India.
Antibodies
Rituximab.
Rituximab is an anti-CD20 monoclonal antibody approved in the United States for treating B-cell non-Hodgkin lymphoma. It is also approved for use in untreated and relapsed CLL, as an induction and maintenance therapy in follicular lymphoma (FL), and in combination with ibrutinib for Waldenström macroglobulinemia.35,36 Recent approvals include rituximab combined with lenalidomide for treating relapsed FL and marginal zone lymphoma (MZL) and rituximab combined with venetoclax for treating relapsed CLL.3 Truxima (rituximab-abbs) and Ruxience (rituximab-pvvr) are rituximab biosimilars recently approved by the FDA in the United States that cost less than rituximab.31,32
The use of anti-CD20 antibody in India is quite similar to the way it is used in the United States. The innovative drug rituximab has been available since the early 2000s at a higher price; when the biosimilar Reditux became available in 2007, the price dropped and the drug was made available across the country.37,38 In a retrospective study of 173 patients with diffuse large B-cell lymphoma (DLBCL), overall response rate (ORR), toxicity, progression-free survival (PFS), and overall survival (OS) were similar between Reditux and rituximab.39 The pharmacokinetic and toxicity profiles in 21 patients who received Reditux (biosimilar) were found to be comparable to those of patients receiving rituximab.40 With an effective patient assistance program, the access to rituximab biosimilars has improved in India. The subcutaneous formulation of rituximab, which was approved for treating FL, is not available in India.41,42
Obinutuzumab.
The fully humanized anti-CD20 antibody obinutuzumab was initially approved for treating relapsed CLL in 2013 and was subsequently approved as part of induction followed by maintenance in untreated advanced FL on the basis of results from the Gallium trial (ClinicalTrials.gov identifier: NCT013322968). Patients were randomly assigned to obinutuzumab plus chlorambucil chemotherapy or rituximab plus chlorambucil followed by obinutuzumab or rituximab maintenance in responders. The estimated 3-year PFS rate was 80.0% versus 73.3% (hazard ratio [HR], 0.66; P = .001) favoring the obinutuzumab arm. Adverse events (AEs) of grade 3 or higher were seen more often in the obinutuzumab group (74.6% v 67.8%).43 Other additional indications included ibrutinib-obinutuzumab and venetoclax-obinutuzmab for untreated CLL.44,45 Obinutuzumab is currently manufactured and marketed by Genentech in the United States. In India, it is used sparingly used because of its high cost.
Ofatumumab.
This second-generation CD20 antibody46 was initially available in India but was withdrawn from commercial use in 2018.47
Mogamulizumab-kpkc.
Mogamulizumab is a humanized monoclonal antibody against the chemokine receptor CCR-4 and was approved for adult patients with relapsed or refractory (R/R) mycosis fungoides or Sezary syndrome. In the phase III MAVORIC trial (ClinicalTrials.gov identifier: NCT01728805), patients on the mogamulizumab arm had a median PFS of 7.7 months versus 3.1 months for those on the vorinostat arm (HR, 0.53; P < .001); 41% had grade 3 or 4 AEs.48 This drug is currently not available in India.
Nivolumab and pembrolizumab.
Nivolumab is currently FDA approved for adult patients with R/R classic Hodgkin lymphoma (cHL) with previous autologous stem cell transplantation (ASCT). In 95 patients with relapsed cHL after ASCT and brentuximab vedotin, nivolumab produced a 65% ORR, and the median duration of response was 8.7 months. Serious AEs were reported in 21% of patients.49 Pembrolizumab is approved for treating refractory cHL and was recently granted accelerated approval for treating refractory primary mediastinal large B-cell lymphoma. The KEYNOTE-170 trial (ClinicalTrials.gov identifier: NCT02576990) was a multicentre, single-arm trial in 53 patients with R/R primary mediastinal large B-cell lymphoma and had an ORR of 45%. Although 61% had AEs, they were mostly grade 1 to 2 and were manageable.50,51 Nivolumab and pembrolizumab are available in India and are accessible to patients at an average cost of approximately $1,420 (100 mg) and $6,010 (200 mg), respectively.52-54
Antibody-Drug Conjugates
Polatuzumab vedotin.
The FDA recently approved the novel CD79b-directed antibody-drug conjugate polatuzumab vedotin in combination with bendamustine and rituximab for adult patients with refractory DLBCL who had disease progression after 2 lines of chemotherapy. In a multicenter clinical trial (ClinicalTrials.gov identifier: NCT02257567) of 80 patients with relapsed DLBCL, the complete response (CR) rates and the ORR was higher with the polatuzumab vedotin plus benadmustine and rituximab as compared to the control arm of bendmustine plus rituximab (40% vs 18%; 63% vs 25% respectively). The combination had higher grade 3 to 4 cytopenias.55 Currently, polatuzumab is unavailable in India.56
Brentuximab vedotin.
Brentuximab vedotin was initially approved in 2011 for patients with relapsed Hodgkin lymphoma whose disease had progressed after ASCT or after 2 previous chemotherapy treatments and who were ineligible for a transplantation; an additional indication was added for patients with relapsed anaplastic large-cell lymphoma.57 In March 2018, brentuximab vedotin was approved by the FDA for treatment-naive advanced cHL on the basis of the ECHELON-1 trial (ClinicalTrials.gov identifier: NCT01712490). In all, 1,334 patients were randomly assigned to receive either brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (AVD) or bleomycin plus AVD. There was a 23% reduction in the risk of a PFS event in the brentuximab vedotin arm. Occurrence of neutropenia and peripheral neuropathy were higher in the brentuximab vedotin arm (58% v 45% and 67% v 43%, respectively) as compared with AVD plus bleomycin.58
In 2018, the FDA also approved brentuximab vedotin for the treatment of untreated systemic anaplastic large-cell lymphoma (ALCL) or other CD30+ T-cell lymphomas (TCLs) with cyclophosphamide, doxorubicin, and prednisone (CHP). The phase III ECHELON-2 trial (ClinicalTrials.gov identifier: NCT01777152) was a randomized study of brentuximab vedotin plus CHP versus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in 452 patients with newly diagnosed CD30+ TCL. The median PFS was 48.2 months with brentuximab vedotin plus CHP and 20.8 months with CHOP, with an HR of 0.71; there was improvement in OS (HR, 0.66). AEs were similar in both arms.59 Brentuximab vedotin is currently not available in India; TCL is treated with CHOP with or without etoposide followed by ASCT.60 R/R cHL is treated with immune checkpoint inhibitors or salvage chemotherapy in India, and occasionally brentuximab vedotin is imported and incorporated into some treatment regimens.61
BTK Inhibitors and PI3 Kinase Inhibitors
Ibrutinib.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is FDA approved for relapsed and untreated CLL, relapsed mantle cell lymphoma (MCL), relapsed MZL, and Waldenström macroglobulinemia.62-67 In the phase III trial (ClinicalTrials.gov identifier: NCT01886872) of ibrutinib versus ibrutinib with rituximab or bendamustine and rituximab in 547 elderly patients with untreated CLL, the median PFS was higher with patients receiving ibrutinib alone or with rituximab. The grade 3 to 4 hematologic AEs were greater with bendamustine and rituximab (61%) than with ibrutinib (41%) or with ibrutinib plus rituximab (39%).68 In the extended follow up of the RESONATE 2 trial (Clinical Trials.gov identifier: NCT01722487), the overall response rate was 92% with ibrutinib. At a median follow-up of 60 months, the PFS and OS for ibrutinib versus chlorambucil was-PFS estimates at 5 years: 70% vs 12%; HR: 0.146; 95% CI, 0.098 to 0.218; OS estimates at 5 years: 83% vs 68%; HR: 0.450; 95% CI, 0.266 to 0.761). There was a 88% reduction in risk of progression or death in the ibrutinib arm (P <0.001). Grade 3/4 AEs decreased over time except for atrial fibrillation.63,69 In 197 patients treated with ibrutinib, similar ORR, PFS, and OS were noted in 37 patients (19%) receiving a reduced median dose of 4.3 mg/kg per day compared with standard doses.70 A smaller Indian study also reported a similar efficacy with low-dose ibrutinib.71
Ibrutinib has also been approved for treating R/R MZL on the basis of a phase II trial in 63 patients who had an ORR of 46%. The median time to initial response was 4.5 months and PFS was 14 months.65 Ibrutinib is available for patients in India, but the cost remains high at this time ($4,700 for 90 capsules [140 mg]). Recently, a generic formulation has become available in India at $410 for 90 capsules (140 mg).72
Acalabrutinib.
Acalabrutinib is a second-generation BTK inhibitor approved by the FDA for treating patients with refractory MCL on the basis of the ACE LY-004 trial (ClinicalTrials.gov identifier: NCT02213926). The ORR was 81%, and the median duration of response was not reached, with a median follow-up of 15.2 months. Grade 3 or worse AEs were neutropenia in 10%, anemia in 9%, and pneumonia in 5%; there was 1 instance of hemorrhage.73 The impressive PFS results in patients with relapsed CLL, including those with high-risk cytogenetics in the phase III ASCEND trial (ClinicalTrials.gov identifier: NCT02970318), led to approval for this indication.3,74 In combination with obinutuzumab (ClinicalTrials.gov identifier: NCT02475681), there was an improvement in PFS compared with obinutuzumab-chlorambucil in more than 500 patients with previously untreated CLL.3,75 Acalabrutinib is not available in India. Patients with R/R MCL are being treated with ibrutinib or lenalidomide with rituximab; patients eligible for ASCT undergo induction with salvage chemotherapies followed by ASCT. Although the outcomes may be similar, a single-agent therapy would be more convenient and less toxic for patients compared with chemotherapy with ASCT or lenalidomide with rituximab. In addition, the recent availability of the generic ibrutinib in India at an affordable price may delay entry of acalabrutinib to India.
The approved PI3 kinase inhibitors, such as idelalisib,76-78 duvelisib,79,80 and copanlisib,81 are not available in India. Because of the increased number of AEs compared with those with BTK inhibitors in CLL and lenalidomide in indolent lymphomas, this lack of access has no real impact.
BCL-2 Inhibitor
Venetoclax is an oral BCL-2 inhibitor that received FDA approval for treating patients with relapsed CLL who had a 17p deletion.82 Subsequently, on the basis of the MURANO study (ClinicalTrials.gov identifier: NCT02005471), venetoclax with rituximab was approved for treating patients with CLL or small lymphocytic lymphoma who had received at least 1 previous therapy. The comparison arm was bendamustine plus rituximab. Tumor lysis syndrome and neutropenia were higher with venetoclax.83 The combination of venetoclax-obinutuzumab in a trial of 432 elderly patients with untreated CLL also has been approved, and it showed a benefit in the high-risk group (ClinicalTrials.gov identifier: NCT02242942). The 2-year PFS was higher in the venetoclax-obinutuzumab arm (88.2% v 64.1%, with 28 months of follow-up) as compared with chlorambucil plus obinutuzumab. The combination was well tolerated and it had similar rates of neutropenia and infections as the control arm of chlorambucil plus obinutuzumab.45 Patients in India currently do not have access to venetoclax.
Proteosome Inhibitor
Bortezomib is a protease inhibitor approved for patients with relapsed MCL and subsequently for treating patients with untreated MCL who are ineligible for ASCT.84,85 The generic version is affordable and is mainly used for managing multiple myeloma. It has not had an impact on care for patients with lymphoma in India because lenalidomide, rituximab, and ibrutinib are used to treat patients with relapsed MCL.
Immunomodulators
Lenalidomide.
Lenalidomide is an immunomodulatory agent approved for patients with relapsed MCL.86 It has been approved in the United States for R/R FL or MZL, based on the AUGMENT randomized clinical trial (ClinicalTrials.gov identifier: NCT01472562). The lenalidomide-rituximab arm had a median PFS of 39 months versus 14 months for rituximab alone. A higher percentage of infections (63% v 49%), neutropenia (58% v 23%), and rash (32% v 12%) was seen in the combination arm, but overall the AEs were tolerable in both arms.87 The data from several studies show an improvement in PFS in patients with relapsed lymphomas and CLL and also in elderly patients with DLBCL who received lenalidomide as maintenance therapy after rituximab plus CHOP (R-CHOP).88-91 Because this drug is available as an affordable generic, access to this novel drug has expanded significantly in India.
CAR T-cell therapy.
In October 2017, the FDA approved axicabtagene ciloleucel for patients with R/R DLBCL on the basis of a single-arm multicenter trial of 111 adult patients (ZUMA-1; ClinicalTrials.gov identifier: NCT02348216). The ORR was 72% and the CR rate was 51%. The responses were sustained, and the median OS was not reached at 2 years, but an estimated 50.5% of patients were still alive at 24 months.92,93 In May 2018, the FDA approved tisagenlecleucel for adults with relapsed DLBCL after 2 lines of chemotherapy on the basis of a phase II single-arm study of 92 patients. The ORR was 50%, and the CR was 32%.94 Neutropenia, anemia, thrombocytopenia, and chimeric antigen receptor (CAR) T-cell–specific AEs such as cytokine release syndrome and neurologic toxicities were seen in both trials. Therapy using CAR T cells involves substantial cost, expertise, and infrastructure. CAR T-cell therapy may be the best treatment currently available for patients with relapsed lymphomas who otherwise have a dismal prognosis, but it is not available in India.29,95,96
Possible Strategies for Improving the Accessibility of Drugs in the United States and India
Effective management of lymphoid cancers can often provide patients with long-term survival if they are treated appropriately with novel drugs. Thus, the role of compulsory licensing is worth considering. The Doha Declaration on mandatory licensing allowed member countries to circumvent patent rights so that patients would have access to essential medications. There are several hurdles in this path that need to be addressed. A step in the right direction would be to expand the market for biosimilars and generics in the United States and India. Furthermore, global trials for these drugs might enhance accessibility.97,98 In India, the Prime Minister’s health insurance scheme caps the drug costs to reduce the financial burden.22 Another step toward improving accessibility would be to provide universal insurance coverage. A systematic effort from the government agencies and use of a hub-and-spoke model for rural outreach may be required to improve access in remote areas of India. In the United States, by allowing Medicare and the FDA to have a say in drug prices, laws that enable importing drugs and negotiating drug prices can help reduce drug costs.99 Here, we have outlined the differences in availability and affordability of cancer drugs between India and the United States.
In conclusion, the advent of biosimilars has reduced the cost of and made treatment of CLL and lymphoma similar in the United States and India in terms of survival and patient quality of life. Innovative strategies would help expand access to other novel agents to patients in India as well. In addition, implementing the Doha Declaration, having compulsory licensing, capping drug prices, expanding insurance coverage, and making cancer care available to all people irrespective of their economic, social, racial, and geographical backgrounds would make treatment for cancer globally accessible.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Christopher R. Flowers
Administrative support: Christopher R. Flowers
Collection and assembly of data: Christopher R. Flowers, Swaminathan P. Iyer
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Vishwanath Sathyanarayanan
Honoraria: Dr. Reddy’s Laboratories, Alkem Laboratories, Lupin
Christopher R. Flowers
Consulting or Advisory Role: Abbvie, Bayer, Celgene (unpaid), Denovo Biopharma, Genentech/Roche, Gilead, OptumRx, Karyopharm, Pharmacyclics/ Janssen, Spectrum
Research Funding: Abbvie, Acerta, Celgene, Gilead, Genentech/Roche, Janssen Pharmaceutical, Millennium/Takeda, Pharmacyclics, TG Therapeutics, Burroughs Wellcome Fund, V Foundation
Swaminathan P. Iyer
Honoraria: Seattle Genetics
Consulting or Advisory Role: Daiichi Sankyo, Seattle Genetics, Legend Biotech
Research Funding: Bristol-Myers Squibb (Inst), Takeda (Inst), Novartis (Inst), Genentech (Inst), Rhizen Pharmaceuticals (Inst), Seattle Genetics (Inst), CRISPR Therapeutics (Inst), Spectrum Pharmaceuticals (Inst),
Trillium Therapeutics (Inst), Affimed Therapeutics (Inst)
Travel, Accommodations, Expenses: Takeda, Gilead Sciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Saxena R, Kumar R, Sazawal S, et al. CLL in India may have a different biology from that in the West. Blood. 2016;128 abstract 5574. [Google Scholar]
- 3.US Food and Drug Administration Hematology/Oncology (Cancer) Approvals & Safety Notifications. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications
- 4.Central Drugs Standard Control Organisation Homepagehttps://www.cdscoonline.gov.in/CDSCO/homepage
- 5.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024–1039. doi: 10.1001/jama.2018.1150. [DOI] [PubMed] [Google Scholar]
- 6.Collins SR, Gunja MZ, Doty MM. How Well Does Insurance Coverage Protect Consumers From Health Care Costs? Findings from the Commonwealth Fund Biennial Health Insurance Survey, 2016. commonwealthfund.org/publications/issue-briefs/2017/oct/how-well-does-insurance-coverage-protect-consumers-health-care
- 7.Kantarjian H, Steensma D, Rius Sanjuan J, et al. High cancer drug prices in the United States: Reasons and proposed solutions. J Oncol Pract. 2014;10:e208–e211. doi: 10.1200/JOP.2013.001351. [DOI] [PubMed] [Google Scholar]
- 8.Light DW, Kantarjian H. Market spiral pricing of cancer drugs. Cancer. 2013;119:3900–3902. doi: 10.1002/cncr.28321. [DOI] [PubMed] [Google Scholar]
- 9.Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32:306–311. doi: 10.1200/JCO.2013.52.9123. [DOI] [PubMed] [Google Scholar]
- 10.Barnett JC, Berchick ER. Health Insurance Coverage in the United States: 2016. https://www.census.gov/content/dam/Census/library/publications/2017/demo/p60-260.pdf Current Population Reports.
- 11.Obama B. United States health care reform: Progress to date and next steps. JAMA. 2016;316:525–532. doi: 10.1001/jama.2016.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halpern MT, Brawley OW. Insurance status, health equity, and the cancer care continuum. Cancer. 2016;122:3106–3109. doi: 10.1002/cncr.30158. [DOI] [PubMed] [Google Scholar]
- 13.Shah NN, Xi Y, Liu Y, et al. Racial and socioeconomic disparities in mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19:e312–e320. doi: 10.1016/j.clml.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JS, Nastoupil LJ, Han X, et al. Disparities in survival by insurance status in follicular lymphoma. Blood. 2018;132:1159–1166. doi: 10.1182/blood-2018-03-839035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keegan TH, DeRouen MC, Parsons HM, et al. Impact of treatment and insurance on socioeconomic disparities in survival after adolescent and young adult Hodgkin lymphoma: A population-based study. Cancer Epidemiol Biomarkers Prev. 2016;25:264–273. doi: 10.1158/1055-9965.EPI-15-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han X, Jemal A, Flowers CR, et al. Insurance status is related to diffuse large B-cell lymphoma survival. Cancer. 2014;120:1220–1227. doi: 10.1002/cncr.28549. [DOI] [PubMed] [Google Scholar]
- 17.National Health Mission National Health Accounts Estimates for India. http://nhsrcindia.org/sites/default/files/NHA%20Estimates%20Report%20-%20Final%20Web%20Optimized%20PDF%20Version%20-%2022.11.17_0.pdf
- 18.Noronha V, Tsomo U, Jamshed A, et al. A fresh look at oncology facts on south central Asia and SAARC countries. South Asian J Cancer. 2012;1:1–4. doi: 10.4103/2278-330X.96489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joe W. Distressed financing of household out-of-pocket health care payments in India: Incidence and correlates. Health Policy Plan. 2015;30:728–741. doi: 10.1093/heapol/czu050. [DOI] [PubMed] [Google Scholar]
- 20.Rajpal S, Kumar A, Joe W. Economic burden of cancer in India: Evidence from cross-sectional nationally representative household survey, 2014. PLoS One. 2018;13:e0193320. doi: 10.1371/journal.pone.0193320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ministry of Health and Family Welfare. Addresses of regional cancer centers. https://main.mohfw.gov.in/sites/default/files/Addresses%20Of%20Regional%20Cancer%20Centres.pdf.
- 22.Angell BJ, Prinja S, Gupt A, et al. The Ayushman Bharat Pradhan Mantri Jan Arogya Yojana and the path to universal health coverage in India: Overcoming the challenges of stewardship and governance. PLoS Med. 2019;16:e1002759. doi: 10.1371/journal.pmed.1002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Light D, Warburton R. Demythologizing the high costs of pharmaceutical research. BioSocieties. 2011;6:34–50. [Google Scholar]
- 24.Vincent Rajkumar S. The high cost of prescription drugs: causes and solutions. Blood Cancer J. 10:71, 2020. doi: 10.1038/s41408-020-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mossinghoff GJ. Overview of the Hatch-Waxman Act and its impact on the drug development process. Food Drug Law J. 1999;54:187–194. [PubMed] [Google Scholar]
- 26.Scherer FM. The F.T.C., Oligopoly, and Shared Monopoly. https://research.hks.harvard.edu/publications/getFile.aspx?Id978 Faculty Research Working Paper Series. Harvard Kennedy School. September 2013.
- 27.CMS.gov, Centers for Medicare & Medicaid Services 2019 ASP Drug Pricing Files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles.html Homepage.
- 28.Drugs.com Find Drugs & Conditions. www.drugs.com Homepage.
- 29.Jacobson C, Emmert A, Rosenthal MB. CAR T-cell therapy: A microcosm for the challenges ahead in Medicare. JAMA. doi: 10.1001/jama.2019.10194. [epub ahead of print on July 29, 2019] [DOI] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration Biosimilar and interchangeable products. https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products
- 31.US Food and Drug Administration FDA approves Truxima as biosimilar to Rituxan for non-Hodgkin’s lymphoma. https://www.fda.gov/drugs/fda-approves-truxima-biosimilar-rituxan-non-hodgkins-lymphoma
- 32.Business Wire FDA approves Pfizer’s biosimilar, RUXIENCE™ (rituximab-pvvr), for certain cancers and autoimmune conditions. https://www.businesswire.com/news/home/20190723005823/en/FDA-Approves-Pfizer%E2%80%99s-Biosimilar-RUXIENCE%E2%84%A2-rituximab-pvvr-Cancersrituximab-pvvr
- 33.Pharmaceutical Technology Expanding from generics to biosimilars in India. https://www.pharmaceutical-technology.com/features/expanding-generics-biosimilars-in-india/
- 34.Organisation for Economic Co-operation and Development Purchasing power parities (PPP) https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm
- 35.Drugs.com Rituxan approval history. https://www.drugs.com/history/rituxan.html
- 36.US Food and Drug Administration Rituximab (marketed as Rituxan) Information. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/rituximab-marketed-rituxan-information
- 37.Biswas G, Parikh PM, Nair R, et al. Rituximab (anti-CD20 monoclonal antibody) in lymphoproliferative malignancies: Tata Memorial experience. J Assoc Physicians India. 2006;54:29–33. [PubMed] [Google Scholar]
- 38.Kamoda S, Ishikawa R, Kakehi K. Capillary electrophoresis with laser-induced fluorescence detection for detailed studies on N-linked oligosaccharide profile of therapeutic recombinant monoclonal antibodies. J Chromatogr A. 2006;1133:332–339. doi: 10.1016/j.chroma.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Roy PS, John S, Karankal S, et al. Comparison of the efficacy and safety of Rituximab (Mabthera™) and its biosimilar (Reditux™) in diffuse large B-cell lymphoma patients treated with chemo-immunotherapy: A retrospective analysis. Indian J Med Paediatr Oncol. 2013;34:292–298. doi: 10.4103/0971-5851.125248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gota V, Karanam A, Rath S, et al. Population pharmacokinetics of Reditux™, a biosimilar Rituximab, in diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2016;78:353–359. doi: 10.1007/s00280-016-3083-x. [DOI] [PubMed] [Google Scholar]
- 41.Davies A, Merli F, Mihaljevic B, et al. Pharmacokinetics and safety of subcutaneous rituximab in follicular lymphoma (SABRINA): Stage 1 analysis of a randomised phase 3 study. Lancet Oncol. 2014;15:343–352. doi: 10.1016/S1470-2045(14)70005-1. [DOI] [PubMed] [Google Scholar]
- 42.Davies A, Berge C, Boehnke A, et al. Subcutaneous rituximab for the treatment of B-cell hematologic malignancies: A review of the scientific rationale and clinical development. Adv Ther. 2017;34:2210–2231. doi: 10.1007/s12325-017-0610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377:1331–1344. doi: 10.1056/NEJMoa1614598. [DOI] [PubMed] [Google Scholar]
- 44.Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. doi: 10.1016/S1470-2045(18)30788-5. [DOI] [PubMed] [Google Scholar]
- 45.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–2236. doi: 10.1056/NEJMoa1815281. [DOI] [PubMed] [Google Scholar]
- 46.Robak T, Warzocha K, Govind Babu K, et al. Ofatumumab plus fludarabine and cyclophosphamide in relapsed chronic lymphocytic leukemia: Results from the COMPLEMENT 2 trial. Leuk Lymphoma. 2017;58:1084–1093. doi: 10.1080/10428194.2016.1233536. [DOI] [PubMed] [Google Scholar]
- 47.WCG FDA News Novartis withdraws chronic leukemia drug Arzerra from non-U.S. markets. https://www.fdanews.com/articles/185419-novartis-withdraws-chronic-leukemia-drug-arzerra-from-non-us-markets
- 48.Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192–1204. doi: 10.1016/S1470-2045(18)30379-6. [DOI] [PubMed] [Google Scholar]
- 49.Kasamon YL, de Claro RA, Wang Y, et al. FDA approval summary: Nivolumab for the treatment of relapsed or progressive classical Hodgkin lymphoma. Oncologist. 2017;22:585–591. doi: 10.1634/theoncologist.2017-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130:267–270. doi: 10.1182/blood-2016-12-758383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The Economic Times MSD launches blockbuster cancer drug Keytruda in India. https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/msd-launches-blockbuster-cancer-drug-keytruda-in-india/articleshow/60487138.cms
- 53.1mg Opdyta 100mg Injection. https://www.1mg.com/drugs/opdyta-100mg-injection-369756
- 54.Bristol-Myers Squibb Company Transparency. https://www.bms.com/in/transparency.html
- 55.Sehn LH, Herrera AF, Matasar MJ, et al. Polatuzumab vedotin (Pola) plus bendamustine (B) with rituximab (R) or obinutuzumab (G) in relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL): Updated results of a phase (Ph) Ib/II study. Blood. 2018;132 abstr 1683. [Google Scholar]
- 56.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drugs.com Adcetris approval history. https://www.drugs.com/history/adcetris.html
- 58.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horwitz S, O’Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet. 2019;393:229–240. doi: 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellin F, Landström J, Jerkeman M, et al. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood. 2014;124:1570–1577. doi: 10.1182/blood-2014-04-573089. [DOI] [PubMed] [Google Scholar]
- 61.Moskowitz CH, Walewski J, Nademanee A, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132:2639–2642. doi: 10.1182/blood-2018-07-861641. [DOI] [PubMed] [Google Scholar]
- 62.Barr PM, Robak T, Owen C, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: Extended phase 3 results from RESONATE-2. Haematologica. 2018;103:1502–1510. doi: 10.3324/haematol.2018.192328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129:2224–2232. doi: 10.1182/blood-2016-10-747345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dimopoulos MA, Salman Z, Buske C. Ibrutinib and rituximab in Waldenström’s macroglobulinemia. N Engl J Med. 2018;379:1975–1976. doi: 10.1056/NEJMc1809505. [DOI] [PubMed] [Google Scholar]
- 67.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–443. doi: 10.1056/NEJMoa1817073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–2528. doi: 10.1056/NEJMoa1812836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34:787–798. doi: 10.1038/s41375-019-0602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mato AR, Timlin C, Ujjani C, et al. Comparable outcomes in chronic lymphocytic leukaemia (CLL) patients treated with reduced-dose ibrutinib: Results from a multi-centre study. Br J Haematol. 2018;181:259–261. doi: 10.1111/bjh.14540. [DOI] [PubMed] [Google Scholar]
- 71.Lad DP, Malhotra P, Khadwal A, et al. Reduced dose ibrutinib due to financial toxicity in CLL. Indian J Hematol Blood Transfus. 2019;35:260–264. doi: 10.1007/s12288-018-1011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.The Economic Times Natco launches cut price versions of cancer drug ibrutinib in India. https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/natco-launches-cut-price-versions-of-cancer-drug-ibrutinib-in-india/articleshow/72894811.cms?from=mdr
- 73.Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): A single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–667. doi: 10.1016/S0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghia P, Pluta A, Wach M, et al. ASCEND: Phase III randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. doi: 10.1200/JCO.19.03355. [epub ahead of print on May 27, 2020] [DOI] [PubMed] [Google Scholar]
- 75.AstraZeneca Calquence Phase III ELEVATE-TN trial met primary endpoint at interim analysis in previously-untreated chronic lymphocytic leukaemia. https://www.astrazeneca.com/media-centre/press-releases/2019/calquence-phase-iii-elevate-tn-trial-met-primary-endpoint-at-interim-analysis-in-previously-untreated-chronic-lymphocytic-leukaemia06062019.html June 6, 2019.
- 76.Drugs.com Zydelig approval history. https://www.drugs.com/history/zydelig.html
- 77.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: Duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132:2446–2455. doi: 10.1182/blood-2018-05-850461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: A phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37:912–922. doi: 10.1200/JCO.18.00915. [DOI] [PubMed] [Google Scholar]
- 81.Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35:3898–3905. doi: 10.1200/JCO.2017.75.4648. [DOI] [PubMed] [Google Scholar]
- 82.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–778. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 83.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–1120. doi: 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 84.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: Updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372:944–953. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 86.Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: A phase 1/2 clinical trial. Lancet Oncol. 2012;13:716–723. doi: 10.1016/S1470-2045(12)70200-0. [DOI] [PubMed] [Google Scholar]
- 87.Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: A phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37:1188–1199. doi: 10.1200/JCO.19.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance) J Clin Oncol. 2015;33:3635–3640. doi: 10.1200/JCO.2014.59.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zinzani PL, Pellegrini C, Argnani L, et al. Prolonged disease-free survival in elderly relapsed diffuse large B-cell lymphoma patients treated with lenalidomide plus rituximab. Haematologica. 2016;101:e385–e386. doi: 10.3324/haematol.2016.147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2017;35:2473–2481. doi: 10.1200/JCO.2017.72.6984. [DOI] [PubMed] [Google Scholar]
- 92.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neelapu SS, Ghobadi A, Jacobson CA, et al. 2-Year follow-up and high-risk subset analysis of Zuma-1, the pivotal study of axicabtagene ciloleucel (Axi-Cel) in patients with refractory large B cell lymphoma. Blood. 2018;132 abstr 2967. [Google Scholar]
- 94.Schuster SJ, Bishop MR, Tam C, et al. Sustained disease control for adult patients with relapsed or refractory diffuse large B-cell lymphoma: An updated analysis of Juliet, a global pivotal phase 2 trial of tisagenlecleucel. abstr 1684 Blood. 2018;132 [Google Scholar]
- 95.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: The MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166:891–901. doi: 10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 97.Bognar CLFB, Bychkovsky BL, Lopes GL., Jr Compulsory licenses for cancer drugs: Does circumventing patent rights improve access to oncology medications? J Glob Oncol. 2016;2:292–301. doi: 10.1200/JGO.2016.005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nicol D, Owoeye O. Using TRIPS flexibilities to facilitate access to medicines. Bull World Health Organ. 2013;91:533–539. doi: 10.2471/BLT.12.115865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones GH, Carrier MA, Silver RT, et al. Strategies that delay or prevent the timely availability of affordable generic drugs in the United States. Blood. 2016;127:1398–1402. doi: 10.1182/blood-2015-11-680058. [DOI] [PMC free article] [PubMed] [Google Scholar]