Abstract
The canalicular system (CS) has been defined as: 1) an inward, invaginated membrane connector that supports entry into and exit from the platelet; 2) a static structure stable during platelet isolation; and 3) the major source of plasma membrane (PM) for surface area expansion during activation. Recent analysis from STEM tomography and serial block face electron microscopy has challenged the relative importance of CS as the route for granule secretion. Here, We used 3D ultrastructural imaging to reexamine the CS in mouse platelets by generating high-resolution 3D reconstructions to test assumptions 2 and 3. Qualitative and quantitative analysis of whole platelet reconstructions, obtained from immediately fixed or washed platelets fixed post-washing, indicated that CS, even in the presence of activation inhibitors, reorganized during platelet isolation to generate a more interconnected network. Further, CS redistribution into the PM at different times, post-activation, appeared to account for only about half the PM expansion seen in thrombin-activated platelets, in vitro, suggesting that CS reorganization is not sufficient to serve as a dominant membrane reservoir for activated platelets. In sum, our analysis highlights the need to revisit past assumptions about the platelet CS to better understand how this membrane system contributes to platelet function.
Keywords: Canalicular System, Platelet Granules, Platelet Organelles, Platelet Ultrastructure, Hemostasis, Electron Microscopy
INTRODUCTION
The discovery of the platelet canalicular system (CS), an open network of randomly distributed, often interconnected, membrane-limited, tubular channels, dates to the late 1960s [1,2]. This discovery was attributed then to “improved” methods for electron microscopy sample preparation [3]. Based on its morphology, CS has drawn attention as a possible pathway for the uptake of external substances to platelet organelles [4], as a putative conduit for the extrusion of α-granule stored proteins [3–6], and as a membrane reservoir for expansion of platelet surface area during platelet activation/spreading [7]. A fourth, possible general role as a structural intermediate in membrane trafficking processes, e.g., endocytosis, phagocytosis, and exocytosis has been questioned [8–12]. The proposed roles of the CS as a structural conduit to and from the platelet interior and as a membrane reservoir could well be logically considered to be time-shared between resting and activated platelet states. In brief, the CS’s properties, and by extension its proposed function, appear varied and the subject of some controversy [for a recent review, see 13]
The emergence of improved three-dimensional (3D) electron microscopy techniques such as serial block face (SBF)- and focused ion beam (FIB)- scanning electron microscopy (SEM) offers opportunities to reassess and revalidate CS structure and perhaps to illuminate function. With the advent of these techniques, the electron lucent elements of a putative CS system can be analyzed in 3D at high 5–7 nm in XY plane of the block face qnd ~5–35 nm Z-steps perpendicular to the block-face, to give full platelet volumes without the need for image confounding electron-dense tracers to track membrane continuities. When applied to human or mouse platelets, these techniques [10,11,14] as well as scanning transmission electron (STEM) tomography [9], all indicate that a portion of apparent CS elements fail to connect to the plasma membrane. Hence, the designation of two morphologically defined CS classes: closed CS (CCS) with no apparent continuities to the plasma membrane and open CS (OCS) that does display such continuities. Although many of the CCS elements could be scattered elements of Golgi apparatus [15] or endosomes. However, based on morphology, only the smallest structures dimensions less than 100 nm can be readily excluded, i.e., vesicles of various origins. Significantly, the portion of closed and open CS elements vary between platelet preparations and activation states suggesting an underappreciated set of CS reorganizations and an uncontrolled experimental variable.
The aim of the present study was to investigate the comparative organization of the open and closed CS following 1) platelet isolation, immediately fixed vs. isolated washed platelets, the typical starting point for many in vitro experiments or 2) agonist-induced, thrombin activation. We have shown from STEM tomography of thick sections that such an isolation procedure affects a platelet’s morphologically defined activation state [9] and we thus predict that the isolation procedure could be an important experimental variable in quantitative ultrastructural measurements of 3D CS organization. Additionally, we posited that OCS would be rapidly depleted with similar kinetics to cell surface expansion if it were the dominant membrane reservoir for platelet activation. To test these predictions, we analyzed both SBF- and FIB-SEM image stacks of mouse platelets. We found the CS in washed platelets to be swollen and more interconnected than that of immediately fixed platelets suggesting that preparation methods do have an effect. Physiologically, this indicates that CS architecture reorganizes in response to even small changes in platelet activation state. With thrombin stimulation, the OCS was found to be rapidly depleted. However, quantitatively, it accounted for <50% of the expanded platelet surface area. Consistent with evidence that Golgi enzymes are secreted during platelet activation [16], we found that CCS was also depleted, albeit less rapidly and less extensively, consistent with a separate biogenic origin.
METHODS
Preparation of washed mouse platelets-
Mouse platelets were prepared essentially as described previously [10]. In brief, animals were euthanized by CO2 asphyxiation and blood was collected immediately by cardiac puncture in a 1 mL Tuberculin slip trip syringe with a 26G 3 1/8 needle. The syringe was prefilled with 3.8% sodium citrate, 100 ng/mL PGI2 and 2 U/mL apyrase. The needle was inserted into the ventricle and blood was slowly drawn as to prevent bubbles. The blood was collected in one draw. The needle was removed and the blood from 6 mice each was transferred to a 15 mL conical tube and was diluted with HEPES Tyrode buffer (pH 7.4, 20 mM HEPES/KOH, 128 mM NaCl, 2.8 mM KCl, 1 mM MgCl2, 5 mM D-glucose, 12 mM NaHCO3, 0.4 mM NaH2PO4) in 1:1 ratio in presence of 0.2 U/mL apyrase and 10 ng/mL PGI2. After incubation at room temperature for 5 min, samples were centrifuged at 215 × g for 8 min in a Sorvall Legend RT centrifuge (Thermo Fisher Scientific) at room temperature (RT) to yield a platelet rich plasma (PRP). PRP was then supplemented with 0.2 U/mL apyrase and 10 ng/mL PGI2 for 5 min at RT and centrifuged at 657 × g for 10 min to collect platelets. The platelet pellet was gently resuspended in HEPES Tyrode buffer (pH 7.4) with 1 mM EGTA, 0.2 U/mL apyrase and 10 ng/mL PGI2 and centrifuged a final time at 657 × g for 10 min to collect washed platelets. The washed platelet pellet was gently resuspended in a small volume of HEPES Tyrode’s buffer (pH 7.4).
Platelet concentrations were measured using a Z2 Counter (Beckman Coulter, Inc., Miami, FL) and adjusted with HEPES Tyrode buffer (pH 7.4) to the 2.5 × 108/mL. The platelet suspensions were then supplemented with 0.7 mM CaCl2 and incubated at RT for 5 min before thrombin stimulation.
Initial fixation for electron microscopy studies-
For block face SEM, blood from 6 mice (genders were mixed) was pooled for each sample time point. For an immediate (direct) fixed sample, blood was drawn by cardiac puncture into a syringe prefilled with 3.8% sodium citrate and an equal volume of 2X fixative (6% PFA and 0.2% glutaraldehyde in 1x PBS) was added directly to the 3.8% sodium citrate blood. The blood was fixed at RT for 30 min before collecting Platelet Rich Plasma (PRP) by centrifugation at 237 × g for 8 min in a Sorvall Legend RT centrifuge. Platelets were then isolated from the PRP as previously described [10]
For a stimulation time series, washed platelets were supplemented with 0.7 mM CaCl2. Washed platelets were fixed at 0 sec/0 time with 2X fixative (6% PFA and 0.2% glutaraldehyde). The activation samples were stimulated with a final concentration of 0.1U/mL of thrombin. The reaction was stopped at either 90 or 300 sec by adding 2X fixative for 30 min at RT.
Sequential block face-SEM sample staining [14,15]-
Isolated platelet suspensions were further fixed using 0.1 M cacodylate buffer containing 2.5% glutaraldehyde and 2 mM CaCl2 for 1 h in ice. Cells were washed three times with cold 0.1 M sodium cacodylate buffer containing 2 mM CaCl2 and spun at 600 × g for 5 min. Samples were fixed in 3% potassium ferrocyanide in 0.3 M cacodylate buffer with 4 mM CaCl2 combined with an equal volume of 4% osmium tetroxide for 1 h in ice. After washing five times with H2O samples were then placed in a 0.22 μm-Millipore-filtered 1% thiocarbohydrazide (TCH) solution in ddH2O for 20 min following five washes with double-distilled water (ddH2O) at RT each for 3 min. Samples were fixed in 2% osmium tetroxide in ddH2O for 30 min at RT following five washes with ddH2O at RT each for 3 min and then placed in 1% uranyl acetate (aqueous) for overnight at 4°C. The next day, samples were washed five times with ddH2O at RT each for 3 min and processed for en bloc Walton’s lead aspartate staining at 60°C for 30 min following five washes with ddH2O at RT each for 3 min. Samples were dehydrated and proceed for resin embedding.
Serial block face scanning electron microscopy (SBF-SEM) and focused ion beam SEM (FIB-SEM)-
SBF-SEM was performed with a Gatan 3View system mounted in a Zeiss Sigma SEM as previously described [14,15]. Maximum accelerating voltage was 1.8 kV and XY pixel size was 6.7 nm. FIB- SEM was performed with a Zeiss Crossbeam 550 SEM at an accelerating voltage of 2 kV and XY pixel size of 5 nm. An energy selective backscatter detector was used with a bias of 530 V to filter out low energy backscattered electrons that could originate from deep in the specimen. SBF-SEM samples were sliced at 35 (immediately fixed/resting and washed/0 sec time mouse platelets) or 40 nm (90 and 300 sec stimulated) nominal step size. FIB-SEM samples were milled at 30 kV Ga beam amd am ion current of 700 pA to give a 5 nm nominal step interval.
Surface rendering and quantitative analysis of platelet features –
Platelet features in the 3D image volumes were segmented manually in the Amira Software 6.5.0 environment (FEI SAS, part of Thermo Fisher Scientific) as previously described [10,14]. SBF-SEM images were spatially filtered to eliminate the inclusion of membrane trafficking and/or other small vesicles by requiring continuity across at least 3-Z planes. Polygons were then generated to create surface models of the corresponding features. Fractional weights, automatically generated during segmentation, were used to produce smooth boundary interfaces. From known voxel dimensions, distances, areas, and volumes were quantified within the software. Results were then exported to Excel spreadsheets for further analysis, e.g., the generation of means and standard errors. In past experiments with human platelets, CS and only CS data have been corrected on the basis of stereology for missing Z-information [10,17]. To test whether such a correction factor was required for mouse CS data, we compared the outcome for total CS surface area in resting (immediately fixed platelets) and washed platelets. As shown in Table 1, no significant differences in surface area were found for CS quantified either by software driven segmentation analysis or by stereology. Hence, since all the rendering had already been done in software, we report Amira program values as a rapid quantitative output of platelet and organelle parameters. A full user guide for image processing and analysis using Amira software is available as a free download (no registration required) at https://www.fei.com/software/amira-user-guide/.
Table 1.
Comparison of Alternate Methods for CS Surface Area Quantification in Mouse Platelets: Software Segmentation vs Stereology
|
Software Segmentation (Amira 6.5) |
Stereology | |
|---|---|---|
| Resting platelets | 3.97 μm2 ± 2.06 μm2 | 4.83 μm2 ± 1.41 μm2 |
| Washed platelets | 7.89 μm2 ± 2.03 μm2 | 7.17 μm2 ± 1.71 μm2 |
Values are total CS surface area per platelet ± standard error of the mean. N =5 platelets each. The blood from 6 mice, mixed gender, was pooled for each data point.
Statistical analysis --
Student t-tests were used to calculate P values using Kaleidagraph software (Synergy Software, Reading, PA, USA)
Data sharing –
Raw image stacks are available electronically via a Google drive download to qualified investigators.
RESULTS
Preparation Matters: Comparison of CS in immediately fixed and washed mouse platelets
Platelet isolation in the absence of inhibitors results in so called “handling activated” platelets [9,18] containing a reorganized CS in which the OCS is more interconnected and CCS is less prominent, based on qualitative STEM microscopy of nearly, whole platelet volumes [9]. How pronounced these changes may be in platelets isolated in the presence of prostaglandin and apyrase treatments, to control activation, is unknown. In preliminary thin section experiments, some extension of pseudopods was noted [10]. To probe 3D CS morphology under these conditions, we compared CS organization both qualitatively and quantitatively in immediately fixed and inhibitor washed mouse platelets through serial block face SEM.. Immediately-fixed platelets have been suggested to be more representative of circulating platelets [9] while platelets washed in the presence of inhibitors typically serve as the 0 time reference for biochemical or morphological analysis of platelet activation in vitro [or review, see 19]. Preparation differences in CS organization in immediately fixed vs. washed platelets could explain the comparatively low apparent levels of OCS vs. CCS reported previously for resting human platelets analyzed by STEM and SBF-SEM [9,14]. FIB-SEM analysis at ~20 nm resolution along the Z axis shows both discontinuous and continuous CS element in washed platelets [11]. In our analysis, blood from 6 mice (genders were mixed) was pooled for each sample preparation (see Methods for further details). For the purposes of this communication, we defined OCS and CCS on the basis of tracking membrane continuities in 3D space, individual image slice to image slice, with OCS physically connected to the platelet plasma membrane, sometimes by a tortuous route, and CCS displaying no detectable physical continuity with the plasma membrane. Small vesicles, presumably endosomes, were excluded from consideration by filtering against structures that were not found in at least 3 consecutive block face images (i.e., image “slices/sections”).
As shown through morphology or surface and 3D CS rendering (Figure 1A, E, I), immediately fixed mouse platelets were generally discoid in shape with little to no evidence of pseudopodal extensions. The platelets contained membrane limited, electron lucent elements, deemed CS (Figure 1A, arrows); some, upon 3D rendering (Figure 1I), were surface connected (OCS, yellow) and some were not (CCS, blue). As shown qualitatively in Figure 2, abundance and relative amounts of OCS vs. CCS varied from resting platelet to resting platelet but, on average, CCS was more abundant than OCS (Figure 3). The biological significance of this variation is presently unknown, The extent of interconnection between CS elements appeared low in immediately-fixed platelets. Washed platelets, prepared in the presence of activation inhibitors (prostaglandin I2, apyrase, and in Ca++-free media), were more rounded and frequently displayed short pseudopods (Figure 1B). Similar results are displayed in washed platelet image stacks published by others (e.g., Supplemental Movie 1, Eckley et al., 2016 [11]). Strikingly, washed platelets contained enlarged CS elements that appeared, in both single image planes and 3D renderings, to be interconnected (Figure 1B, F, J, Figure 4). Thin section quantification of the major and minor axes of the planar CS elements showed the typical CS element to be about 1.6-fold larger than that found in immediately-fixed platelets. As shown by visual comparisons between immediately-fixed, resting platelets (Figure 1A,E, I; Figure 2) and isolated, washed platelets (Figure 1B, F, J), CS appeared more abundant, centralized and interconnected in washed than in resting platelets. Quantitatively, both segmentation and stereological analysis showed an almost 2-fold difference in CS surface area between the two states (Table 1, Figure 3). As both platelet and α-granule surface area also increased (Figure 3), we attribute these differences to a generalized platelet swelling during the preparation of washed platelets. OCS swelling was a surprising finding for a non-closed membrane system, a system for which swelling is not expected, and suggests that the CS is much more dynamic than previously expected. The quantitative trend in the data sets (p = 0.12 to 0.23) when comparing resting and washed platelets is reinforced by the fact that the magnitude of these differences was similar in all 4 comparisons (Figure 3).
Figure 1. CS morphology in immediately fixed (resting) platelets versus washed platelets and agonist-stimulated (0.1 U/mL thrombin) mouse platelets.
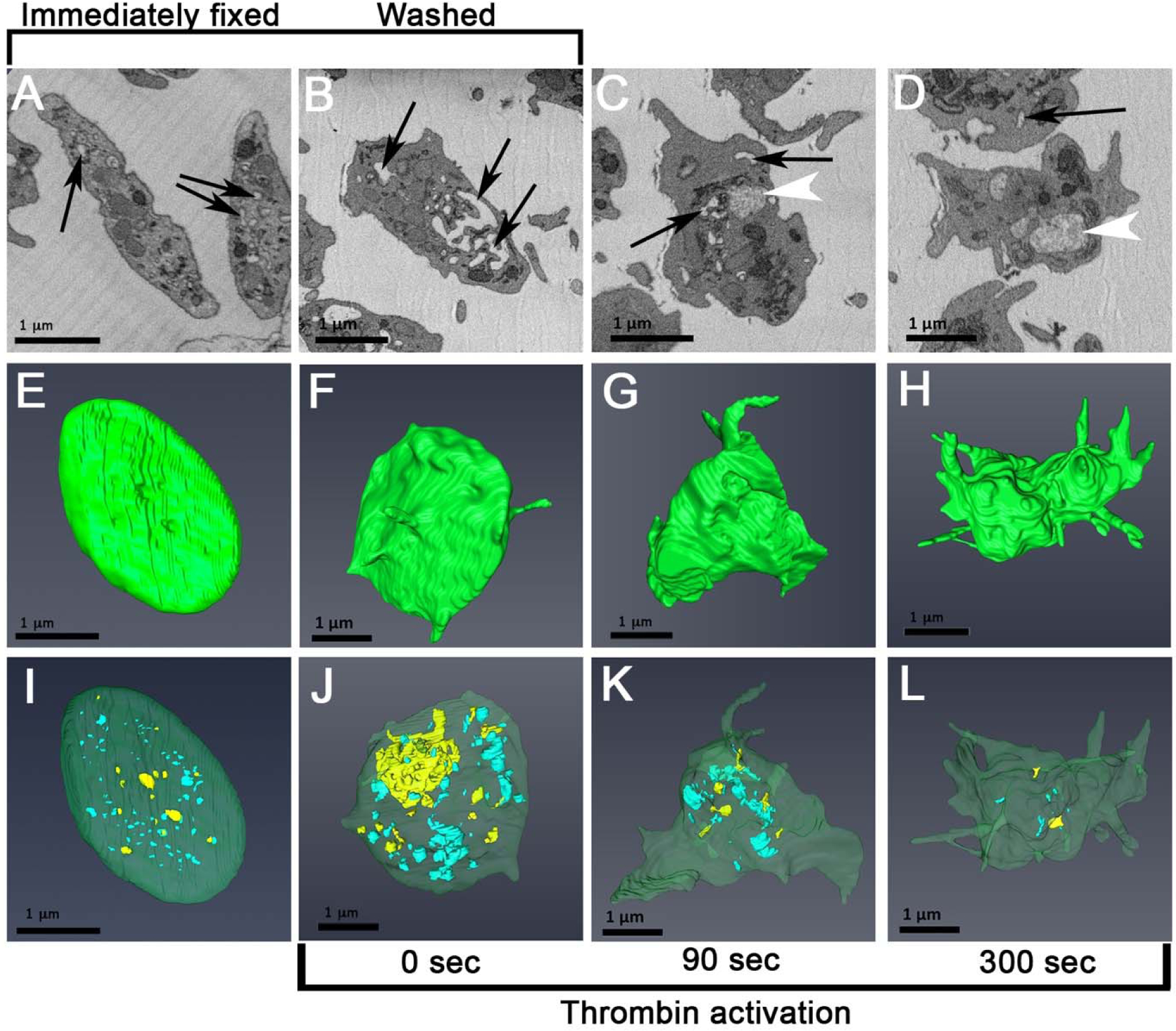
A-D, platelet morphology in individual serial block face images (SBF-SEM) at various stages in platelet preparation or activation. Arrows point to various examples of putative CS elements. White arrowheads point to decondensed α-granules in activated platelets. E-H, Highlighted surface renderings of platelet at various stages in platelet preparation or activation. I-L, Morphology of rendered CCS (blue) and OCS (yellow) at various stages in platelet preparation or activation. Green, plasma membrane; scale bar = 1 μm.
Figure 2. Rendered SBF-SEM (A-J) image sets of CCS (blue) and OCS (yellow) in 10 different immediately fixed (resting) mouse platelets.

Plasma membrane, green; scale bar = 1 μm.
Figure 3. Dot plot of surface area per platelet of closed and open CS, platelet plasma membrane, and α-granules in resting (red symbols) and washed (blue symbols) mouse platelets.
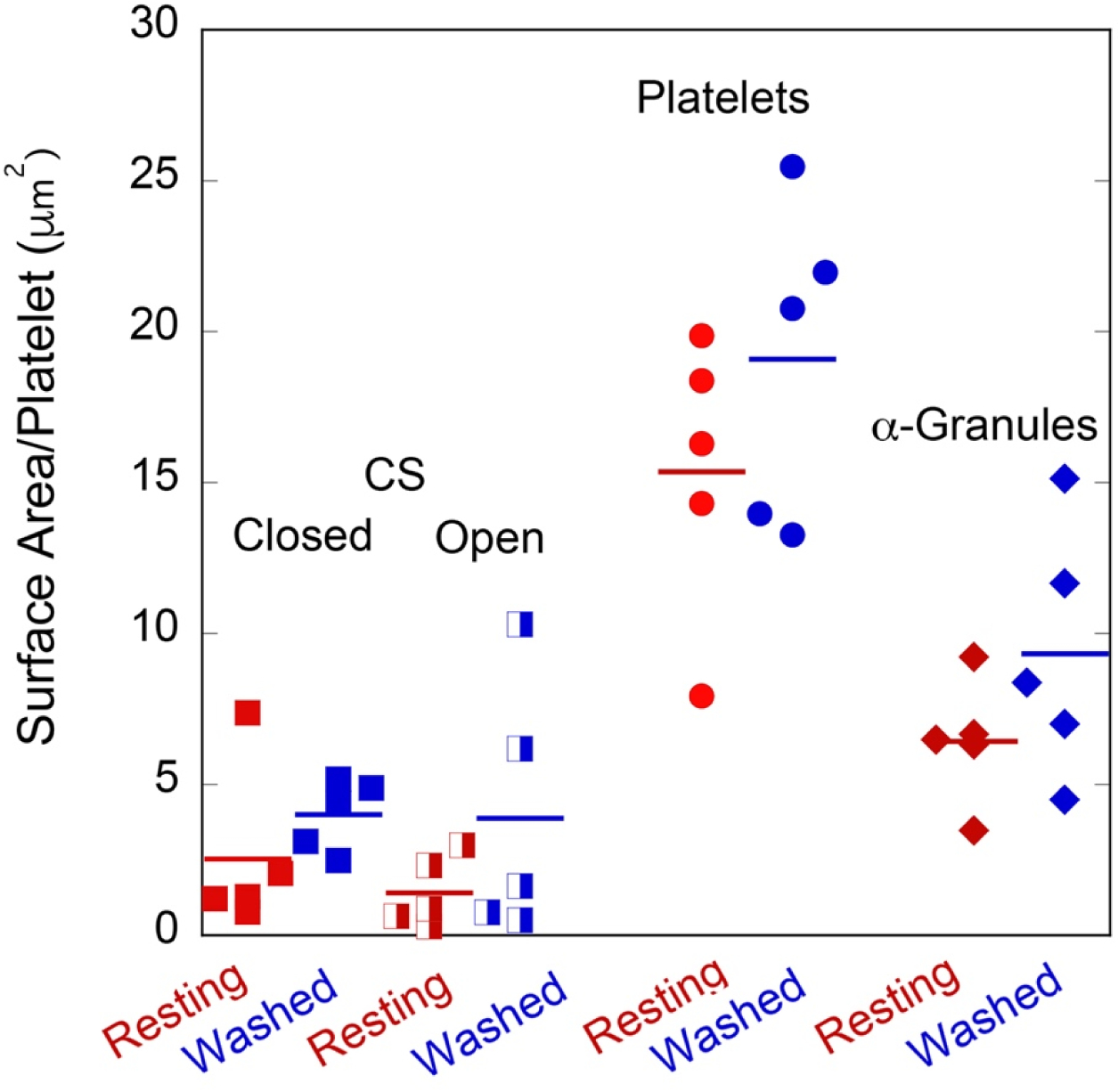
Resting platelets were fixed immediately upon blood draw while Washed platelets were fixed after platelet isolation and repeated rounds of centrifugation. Paired P values: Resting versus Washed preparations, OCS = 0.23, CS = 0.21; Resting versus Washed Platelets = 0.12, Resting versus Washed preparations, α-granules = 0.20. The quantitative and qualitative trend is the same in all cases, i.e., expansion, Washed versus Resting platelet preparations. For each condition, the pooled blood from 6 mice, gender mixed, was used. N = 5 platelets quantified for each comparison.
Figure 4. Comparative Rendered CCS (blue) and OCS (yellow) in 5 different washed platelets imaged either by SBF-SEM (A-E) or by FIB-SEM (F-J).

Arrowheads in H and J point to examples of thin continuities detected at the higher resolution of FIB-SEM versus SBF-SEM. Note that the thin continuities are rare, low in incidence and the examples pointed to are each of CCD, not OCS. The two imaging methodologies appear to give similar outcomes. Scale bar = 1 μm.
SBF-SEM resolution in Z is limited to 30–40 nm by knife and plastic properties, therefore some interconnections between CS elements could have been missed or exaggerated. To test whether, Z resolution was a factor in our analysis, we compared qualitatively 3D renderings of washed mouse platelets imaged by SBF-SEM with those imaged by CrossBeam FIB-SEM technology. CrossBeam FIB-SEM gives a nominal isotropic resolution of 5 nm in XY and Z compared to 7 nm XY and 35 nm Z in our SBF-SEM image sets. As shown in Figure 4, both FIB- and SBF-SEM imaging of washed platelets revealed a mostly interconnected CS system with small diameter interconnections being more obvious in the FIB-SEM rendering examples (arrowheads, Figure 4H, J). Thus, the imaging sets from the two methods report similar results with only minor differences in detail, as revealed by the higher resolution FIB-SEM technique. Since the differences between the two techniques appeared inconsequential to organelle segmentation, all quantitative data reported are from SBF-SEM imaging, the quicker of the two techniques.
To illustrate further the comparative activation state of immediately fixed, resting platelets versus washed mouse platelets, full cell surface renderings of 2 resting and 3 washed platelets are shown in Figure 5, and rotated in 3D in the supplemental movies (Supplemental Figures 1–4). The representative washed platelets exhibited cell surface extensions, short to longer pseudopods, relative to immediate-fixed, resting platelets.
Figure 5. Surface features of rendered resting and inhibitor treated washed platelets.
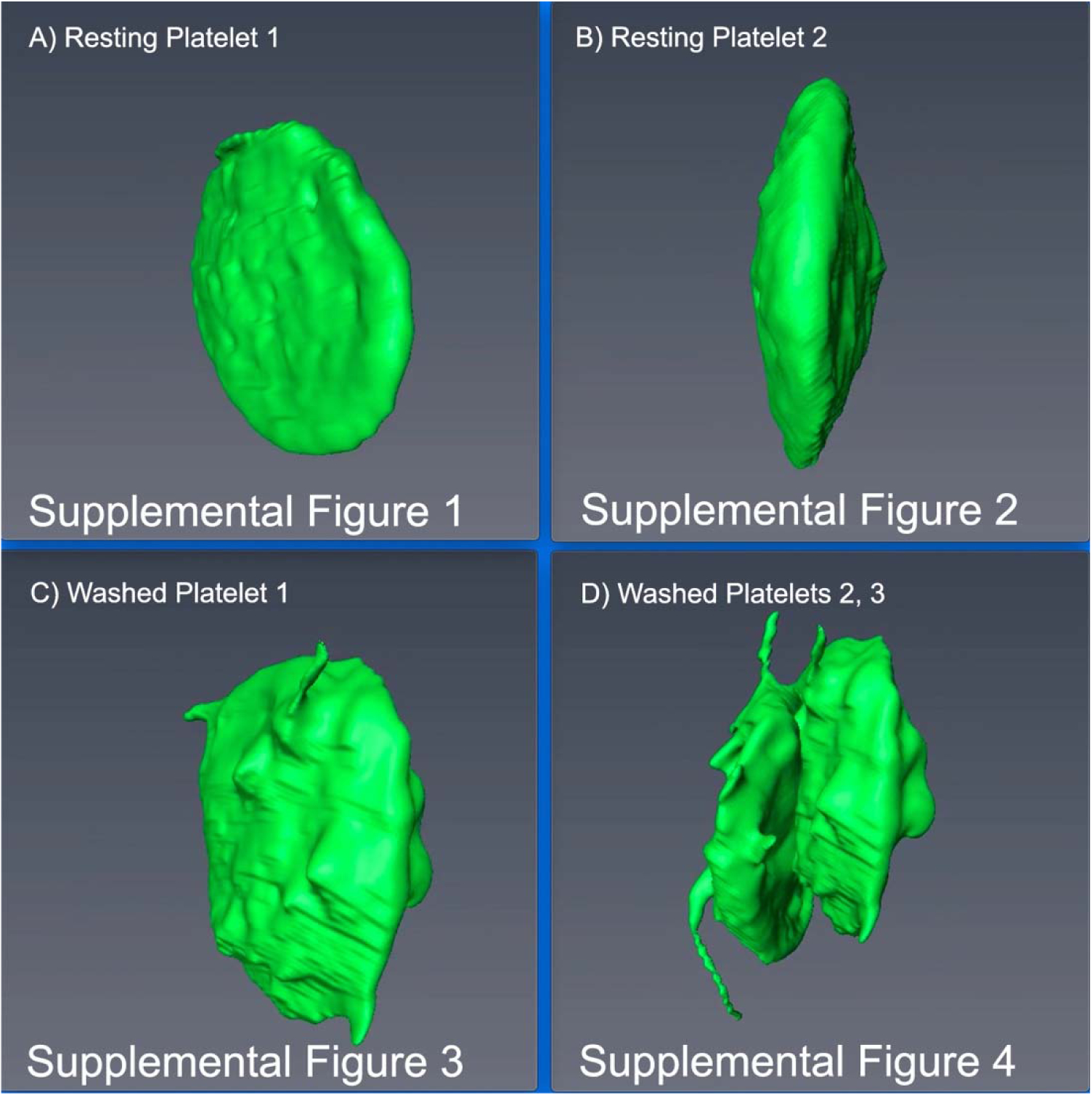
A, B resting platelets. C, D washed platelets. In D, two platelets adherent to one another are shown. For a 3D rotation in space of each of the representative examples, the reader is referred to the respective supplemental figure (movie).
CS reorganization during platelet activation
In vitro, PM surface area expanded during agonist-induced activation with the extension of pseudopods amongst other observed changes in platelet shape. The CS has been repeatedly proposed as the major source of membrane for this surface area expansion. By SBF-SEM imaging and 3D rendering, thrombin-induced (0.1 U/mL) platelet activation produced a progressive change in platelet shape, pseudopod extension (Figure 1B–D, F–H) and loss of CS (Figure 1J–L). The loss of OCS appeared to be faster than that of CCS. Quantitatively (Figure 6 and Table 2), the OCS loss tended to be more rapid than that of CCS with loss of OCS being almost complete over the 300 sec activation period, whereas less than two-thirds of the CCS was lost. Activation differences for CCS and total CS had P values of 0.04 and 0.03 when changes in membrane surface area were compared between 0 time and 300 sec. At both 90 and 300 sec, following thrombin addition, putative CS mobilization to the platelet plasma was insufficient to account for the full extent of platelet surface area increase (Table 2). At 300 sec, the surface area data indicate that only approximately 50% of the surface area increase was attributed to CS, be it OCS or CCS (Table 2). Since, under these conditions, there is no collapse of α-granule membrane into the platelet plasma membrane [10] and lysosomes and dense granules are minor membrane compartments [e.g., 10,14], we suggest that other intracellular membranes, perhaps portions of DTS or Golgi apparatus, must also contribute to the expanded platelet surface area.
Figure 6. Kinetics of surface change for PM (platelet surface), OCS, and CCS following thrombin stimulation.
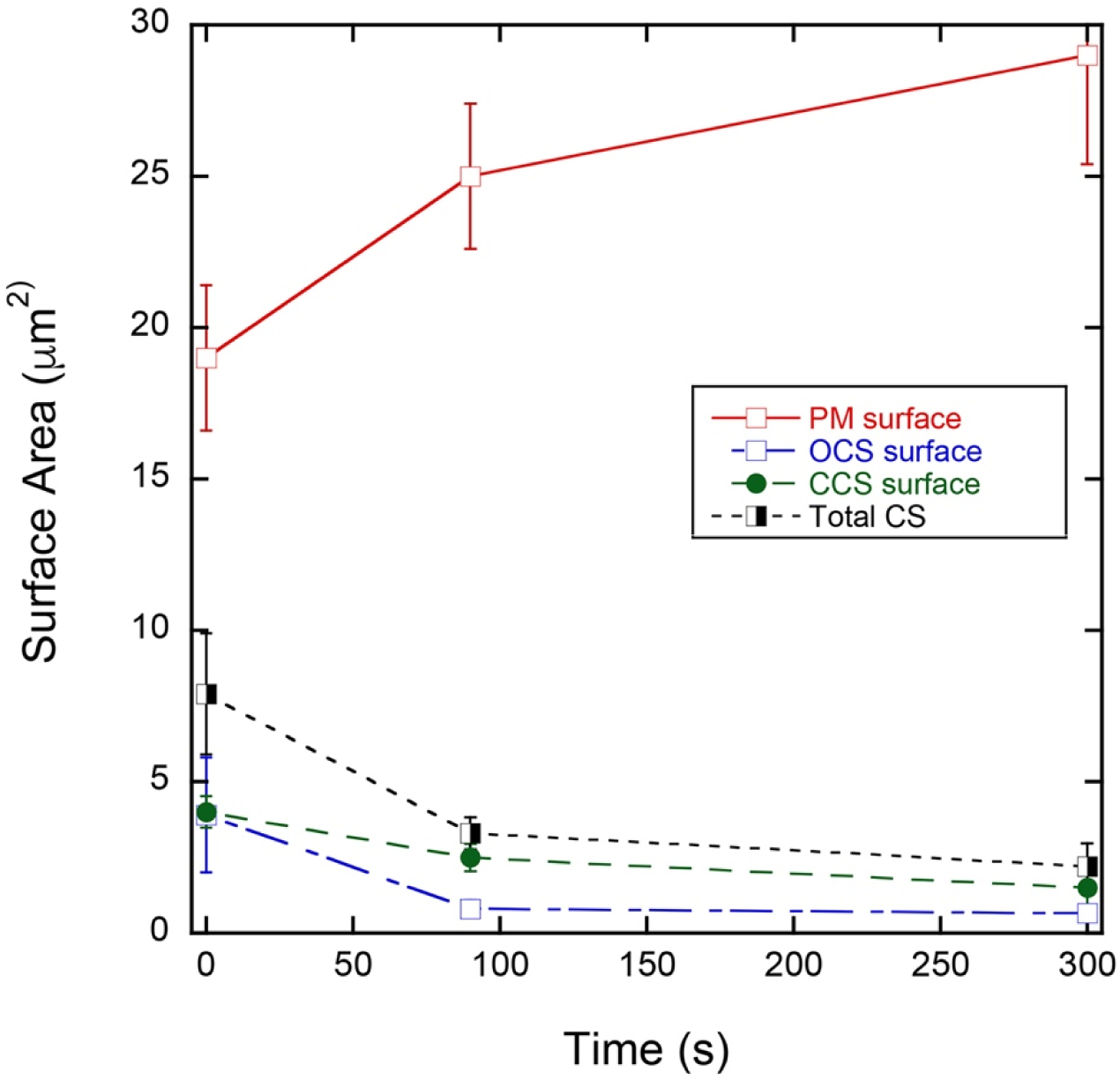
Values shown are ± Standard Error of the Mean. Each data point represents the pooled blood of 6 mice. N = 5 platelets each for the respective data point. P values are given in Table 2. Where an error bar is not visible, the error is less than the symbol size.
Table 2.
Quantitative Summary of CS Changes in Response to Thrombin-Induced Platelet Activation and Their Putative Contribution to an Expanded Platelet Surface Area
| Plasma Membrane Surface Area (μm2) | Closed Canalicular System Surface Area (μm2) | Open Canalicular System Surface Area (μm2) | Total Canalicular System Surface Area (μm2) | Putative CS Mobilized for PM Expansion (μm2) | CS Surface Area Needed for PM Expansion (μm2) | |
|---|---|---|---|---|---|---|
|
0 seconds Activated () |
19 ± 2.4 | 4.0 ± 0.52 | 3.9 ± 1.9 | 7.9 ± 2.0 | N/A | N/A |
|
90 seconds Activated () |
25 ± 2.4 | 2.5 ± 0.45 | 0.81 ± 0.28 | 3.3 ± 0.53 | 4.6 ± 2.0 | 6.0 ± 3.4 |
|
300 seconds Activated () |
29 ± 3.6 (P = 0.089, 0 sec vs. 300 sec) | 1.5 ± 0.72 (P = 0.04, 0 sec vs. 300 sec) | 0.67 ± 0.24 (P = 0.14, 0 sec vs. 300 sec) | 2.2 ± 0.76 (P = 0.03, 0 sec vs. 300 sec) | 5.6 ± 2.1 | 10 ± 4.3 |
Cell surface and CS parameters were quantified for 5 fully rendered platelets at each time point. The blood from 6 mice, mixed gender, was pooled for each time point preparation.
DISCUSSION
We used state-of-the-art, 3D ultrastructural imaging techniques to examine the reorganization of the canalicular system (CS) in mouse platelets during their isolation and thrombin-induced activation. The CS has been assumed for decades to be 1) an inward, invaginated tubular membrane channel that supports protein and virus entry into and exit from the platelet, 2) a structure that is static during platelet isolation, and 3) the predominant, if not exclusive, source of membrane for platelet surface area expansion during platelet activation. Morphologically, in electron micrographs of thin sections, there is little that intrinsically distinguishes CS from other small, electron lucent, single membrane enclosed structures within the platelet cytoplasm. Emphasis has been placed on interconnections, network properties, and connection to the plasma membrane; all properties that are difficult to detect in electron micrographs of thin sections. Previously studies used mordants (e.g., tannic acid) to coat the platelet plasma membrane and its invaginations to establish cell surface connectivity. That criterion led to ambiguity in assigning platelet fusion events to the plasma membrane vs. canalicular system, since mordants can incompletely stain structures or can be endocytosed. The use of newer 3D-methodologies, recent work with STEM tomography [9] or serial block face imaging [10,11] has yielded results that question the relative importance of CS in granule release during platelet activation. Here, we have used serial block face imaging to generate 3D data sets to test the other assumptions about CS, namely, its stability during platelet isolation and the extent to which it is the membrane source for platelet surface area expansion. Because our image sets were collected in 3D, we were able to filter our quantification of SBF-SEM images on the basis of the added parameter of spatial extension over at least 3 Z-planes, ~100 nm, and hence excluded membrane trafficking and/or other small vesicles from our analysis. Together our qualitative analysis from both SBF- and FIB-SEM images and our quantitative SBF-SEM data strongly indicate that the CS, even in the presence of activation inhibitors, reorganizes during platelet isolation and that the CS redistribution into the plasma membrane can account for only about half the plasma membrane, cell surface expansion seen with thrombin induced platelet activation in vitro.
Putative CS elements were defined as CCS or OCS based on connections to the platelet plasma membrane detected in sequential analysis of consecutive images found in SBF-or FIB-SEM image stacks. Open CS was connected, albeit sometimes by a circuitous path, and closed CS was not. Our ability to detect connections was limited by the Z-resolution of serial block face SEM resolution. Higher resolution, <5 nm, is achievable with STEM or electron tomography, techniques restricted to one quarter to three-quarters platelet volume [e.g., 9,18,20 ]. The qualitative outcome of previous STEM, shown for 1 platelet each, immediately fixed and washed platelet, appear consistent with quantitative results reported here for full volumes of multiple platelets at full platelet volume, We reasoned that our identification of CCS might include non-CS structures such as endosomes or Golgi apparatus. If non-CS elements were a significant portion of this class, then we would expect that there would be significant divergence in the response of CCS and OCS to platelet activation. Surprisingly, we found that CCS and OCS behaved similarly during mouse platelet purification. Both classes appeared swollen in isolated, washed platelets prepared in the presence of activation inhibitors, compared to “resting”, immediately fixed platelets. Both also appeared more interconnected; such interconnections were rare in resting platelets. As swelling is normally associated with altered ionic properties in a closed compartment, we suggest that CS connections to the plasma membrane may be dynamic during the preparation of purified washed platelets and that CS fusion in general may be differentially regulated in resting vs. washed platelets. We note that platelet and α-granule surface area also appeared greater in washed than in resting platelets. Qualitatively, washed platelets were less discoid than resting platelets and exhibited occasional pseudopods. Based on the surprisingly similar swelling and increased connectivity of open and putative closed CS during platelet isolation, we suggest that there must be shared transport and regulatory processes between the two classes of CS. To give adequate sample volume and sample across multiple mice for each data point, blood was pooled from 6 mice for each time point.
As might be expected for separate organelle populations with some intermixing, thrombin-induced activation of washed mouse platelets led to a rapid, 90 sec, decrease in OCS levels (~ 80%) while the levels of CCS decreased by less than 50%. Much of the early increase in platelet surface area could be accounted for by the decreased CS surface area, in particular OCS. However, as activation time was extended to 300 sec, the slower release of CCS was insufficient to account for the further increase in platelet surface. In total, the release of putative CS elements could account for no more than 50% of the increase in platelet cell surface area with total OCS release accounting for greater than half, ~60%, and CCS accounting for the remainder, ~40%. If OCS alone is considered as a membrane source, then CS would account for about one-third of the platelet membrane expansion. An additional membrane source in these ex vivo experiments with thrombin stimulated washed platelets is α-granules. However, although there is extensive α-granule cargo release occurring under the ex vivo conditions of these experiments [e.g., 10,21], there is no detectable collapse of α-granule membrane into the platelet cell surface by quantitative 3D SBF-SEM [10]. Ex vivo platelets retain decondensed α-granule “ghosts” that contain residual granule matrix. The surface area of the α-granule population remains constant over a 0, 90 s, and 300 s of 0.1 U/ml thrombin stimulation sequence (Table 2, [10]). Platelet lysosomes and dense granules are known to be secretory organelles; however, they are less abundant organelles in mammalian platelets [14] and unlikely to be a significant membrane reservoir. A remaining major organelle system in mammalian platelets is the dense tubular system (DTS, aka: endoplasmic reticulum in other cell systems) and its putative subcompartment, the T-granule [22]. Protein disulfide isomerase (PDI), a normal constituent of the endoplasmic reticulum lumen in many cell types, is secreted from platelets [e.g., 22,23]; however, there is a limited quantitative understanding of DTS membrane exocytosis or PDI release [23,24]. The morphological analysis of van Nispen et al. [25] indicates that little, if any, DTS collapses into the plasma membrane during platelet activation. Biochemical analysis indicates that PDI is released but the extent of PDI release is low [23]. A third possible membrane source is small vesicle sized structures, a size class that we deliberately excluded from our consideration of CS and did not otherwise analyze.
In sum, our quantitative 3D data indicate that CS can account for no more than half of agonist-induced cell surface expansion in a mouse platelet and together with our qualitative imaging call into question our ability to maintain CS as a stable compartment during platelet isolation. Physiologically, CS appears to be sensitive to even small changes in the platelet activation state. Based on the evidence from the new techniques of serial block face imaging, be they SBF- or FIB-SEM, we conclude that there is indeed reason to revisit our assumptions regarding CS structure and function in the light of improved technology. However, in so doing, we note that a major outcome of the present analysis is to reaffirm the role of CS as a distinctive platelet structure contributing to cell surface expansion during platelet activation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Carl Zeiss, Inc (Thornwood, NY) for performing the FIB-SEM imaging and Joel Mancusco of Zeiss for arranging this.
FUNDING
The Storrie laboratory was supported in part by National Institutes of Health grants R01 HL119393 and R56 HL119393. The Leapman laboratory was supported by the intramural program at NIBIB at the National Institutes of Health, Bethesda, MD. The Whiteheart laboratory was supported in part by National Institutes of Health grants HL56652 and HL138179, American Heart Association grant AHA16GRNT27620001, and a Veterans Affairs Merit Award to SWW and an American Heart Association predoctoral grant AHA15PRE25550020 to SJ.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors have no conflict of interest to declare.
References
- 1.Behnke O. Electron microscope observations on the membrane systems of the rat blood platelet. Anat Rec 1967;158(2):121–137. [DOI] [PubMed] [Google Scholar]
- 2.Behnke O. An electron microscope study of the megakaryocyte of the rat bone marrow I. The development of the demarcation membrane system and the platelet surface coat. J. Ultrastruct Res 1968;24(5);5412–433. [DOI] [PubMed] [Google Scholar]
- 3.White JG. A search for the platelet secretory pathway using electron dense tracers. Am J Pathol 1970;58(1):31–49. [PMC free article] [PubMed] [Google Scholar]
- 4.White JG. Transfer of thorium particles from plasma to platelets and platelet granules. Am J Pathol 1968;53(4):567–575. [PMC free article] [PubMed] [Google Scholar]
- 5.White JG. Fine structural alterations induced in platelets by adenosine diphos-phate. Blood 1968;31(5):604–622. [PubMed] [Google Scholar]
- 6.Slavem P, Stromme J, Bull O. Immunological studies in a cases of gold salt induced thrombocytopenia. Scand J Haematol 1968;5(4):271–277. [DOI] [PubMed] [Google Scholar]
- 7.Escolar G, Leistikow E, White JC. The fate of the open canalicular sysem in surface and suspension-activated platelets. Blood 1989;74(11):1983–1988. [PubMed] [Google Scholar]
- 8.Morgenstern E, Neumann K, Patschke H. The exocytosis of human blood platelet. A fast freezing and free-substitution analysis. Eur J Cell Biol 1987;43:273–282. [PubMed] [Google Scholar]
- 9.Pokrovskaya ID, Aronova MA, Kamykowski JA, et al. STEM tomography reveals that the canalicular system and α-granules remain separate compartments during early secretion stages in blood platelets. J Thromb Haemost 2016;14:572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pokrovskaya ID, Joshi S, Tobin M, et al. SNARE-dependent membrane fusion initiates α-granule matrix decondensation in mouse platelets. Blood Advances 2018;2(21):2947–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckly A, Rinckel JY, Proamer F, et al. Respective contributions of single and compound granule fusion to secretion by activated platelets. Blood 2016;120(26):2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee M, Whiteheart SW. The ins and outs of endocytic trafficking in patelet functions. Curr Opin Hematol 2017;467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvadurai MV, Hamilton JR. Structure and function of the open canalicular system – the platelet’s specialized internal membrane network. Platelets 2018;29(4):319–325. [DOI] [PubMed] [Google Scholar]
- 14.Pokrovskaya ID, Yadav S, Rao A, et al. 3D ultrastructural analysis of α-granule, dense granule, mitochondria and canalicular system arrangement in resting human platelets. Res Practice Thromb Haemost, early publication, journal website. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav S, Williamson JK, Aronova MA, Prince AA, Pokrovskaya ID, Leapman RD, Storrie B. Golgi proteins in circulating human platelets are distributed across non-stacked, scattered structures. Platelets 2017;28:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wandall HH, Rumjantseva V, Sørensen AL, et al. The origin and function of platelet glycosyltransferases. Blood 2012;120(3):626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride EL, Rao A, Zhang G, et al. Comparison of 3D cellular imaging techniques based on scanned electron probes: serial block face SEM vs. axial bright-field STEM tomography. J Struct Biol. 2018; 202:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Nispen tot Pannerden H, de Haas F, Geerte W, Posthuma P, van Dijk S, Heijnen HFG. The platelet interior revisited: electron tomographjy reveals tubular α-granule subtypes. Blood 2010;116(7):1147–1156. [DOI] [PubMed] [Google Scholar]
- 19.Yadav S, Storrie B. The cellular basis of platelet secretion: Emerging structure/function relationships. Platelets 2017;28(2):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi S, Banerjee M, Zhang J, et al. Alterations in platelet secretion differentially affect thrombosis and hemostasis. Blood Advances 2018; 2(17):2187–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Q, Barber HK, Crawford GL, et al. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol Biol Cell 2007;18(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thon JN, Peters CG, Machlus KR, et al. T granules in human platelets function in TLR9 organization and signaling. J Cell Biol 2012;198(4):561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Dewiler TC, Essex DW. Characterization of protein disulphide isomerase released from activated platelets. Br J Haematol 1995;90(2):425–431. [DOI] [PubMed] [Google Scholar]
- 24.Flaumenhaft R. Advances in vascular thiol isomerase function. Curr Opin Hematol 2017;24(5):438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Nispen tot Pannerden H, van Dijk AM, Du V, Heijnen HFG. Platelet protein disulfide isomerase is localizaed in the dnese tubular system and does not become surface expressed after activation. Blood 2009;114(21):4738–4740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


