Abstract
Background
Talaromyces marneffei (TM) is a dimorphic fungus mainly prevalent in Southeast Asian countries, which often causes disseminated life-threatening infection. TM infection often occurs in HIV/AIDS patients even in the antiretroviral therapy (ART) era. However, there has as yet, not been a systematic analysis of the prevalence of TM infection in HIV-infected populations in Asia.
Methods
In this study, we searched Pubmed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), and WanFang from inception to 21 November 2018 for studies reporting TM infection in people living with HIV/AIDS (PLWHA). Our meta-analysis included studies investigating the prevalence of TM infection in PLWHA. Reviews, duplicate studies, and animal studies were excluded. A random effects model was used to estimate pooled prevalence, and meta-regression analysis was conducted to explore potential factors for heterogeneity.
Results
159,064 patients with HIV infection in 33 eligible studies were included in our meta-analysis. The pooled prevalence of TM infection in PLWHA was 3.6%. Vietnam had the highest prevalence (6.4%), followed by Thailand (3.9%), China (3.3%), India (3.2%) and Malaysia (2.1%). In China, TM infection was most prevalent in South China (15.0%), while the burden in Southwest China was not very heavy (0.3%). CD4+ T-cell counts below 200 cells/mm3 contributed to the increased risk of TM infection in PLWHA (OR 12.68, 95%CI: 9.58–16.77). However, access to ART did not significantly decrease the risk of TM infection in PLWHA.
Conclusions
The burden of TM infection in Asia is heavy, and varies from region to region. PLWHA in lower latitude areas are more likely to suffer from TM infection. Optimization of diagnostic tools and universal screening for TM in vulnerable people to ensure early case detection and prompt antifungal treatment should be considered.
Keywords: HIV/AIDS, Talaromyces marneffei, Prevalence, Meta-analysis
Background
Talaromyces marneffei (TM), previously known as Penicillium marneffei, is a dimorphic and pathogenic fungus that often causes invasive infection in immunocompromised patients. Southeast Asia, South China and Northeast India are the main endemic areas of TM infection, while there are a few case reports outside these endemic areas [1–5]. As an AIDS-defining illness, the prevalence of TM infection has increased significantly in the past decades due to the epidemic of HIV infection [6, 7].
TM infection has a higher mortality than most of AIDS-related illnesses [8] in people living with HIV/AIDS (PLWHA), which ranges from 8 to 40% [9–12]. The common clinical manifestations in TM infection include fever, weight loss, anemia, weakness, skin lesions, lymphadenopathy and hepatosplenomegaly [13]. Diagnosis of TM infection primarily depends on culture-based methods. Although there have been some highly specific sero-diagnostic methods developed, false-negative results remain an obstacle to rapid diagnosis of TM infection [14].
Although TM infection continues to pose a serious public health problem to PLWHA in many world regions, we still do not have a comprehensive understanding of the severity of the issue, since results and data from different studies vary greatly, even when different studies have been conducted in the same country, or in the same endemic area. Furthermore, the impact of ART on the prevalence of TM infection still remains unclear. Previous studies have analyzed the burden of Toxoplasma gondii infection, Cryptosporidium infection and pneumocystis pneumonia [15–17]. However, there has been no meta-analysis on the burden of TM infection in PLWHA thus far. We therefore conducted a systematic review and meta-analysis in this study, aiming to understand the overall burden of the disease in different countries and regions, and the impact of ART on the disease burden.
Methods
Search strategy and inclusion criteria
We systematically searched Pubmed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI) and WanFang from inception to 21 November 2018, for research articles published in both the English and Chinese languages. Search terms included “Talaromyces marneffei”, “Penicillium marneffei”, “marneffei” or “penicilliosis” cross-referenced with “HIV”, “AIDS”, “human immunodeficiency virus”, or “acquired immune deficiency syndrome”. We conducted the meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. The protocol of our study was registered at the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42018115645).
An article was included in our meta-analysis if it satisfied the following criteria: (1) participants were PLWHA; and (2) available data was sufficient to estimate the prevalence of TM infection in PLWHA. We excluded articles if (1) they were reviews, or duplicate studies; (2) research objects were animals; (3) the sample size was less than 50; and (4) the methodology for TM infection diagnosis was not clearly stated. TM infection was diagnosed once Talaromyces marneffei has been isolated from the organs, tissues, blood, bone marrow, or other sterile body fluids by culture. We did not include studies in which subjects were only diagnosed solely by clinical symptoms or by serological testing. All titles and abstracts retrieved were attentively examined by two reviewers (Y-YQ and YL).
Data extraction
We extracted the following information from each eligible article: name of the first author, publication year, location of the study, study design, sample size, number of TM co-infection individuals, diagnostic methods, and demographic characteristics. Two reviewers (YL and Y-YQ) extracted the data independently. If there was any disputed finding, we achieved consensus through discussions with another two authors (X-JH and Y-KC). For duplicate data, we made a judgment according to known information about the original author, corresponding author, sample size and sample source.
Quality assessment and publication bias test
Two authors (J-HH and HC) used the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool to assess the quality of included articles [18]. We defined some signaling questions for our study according to the QUADAS-2 user guidelines. The item “Was a case-control design avoided?” was substituted by “Was the sample size large enough (≥200)?” in Domain 1 (Patient Selection). The items “Were the index test results interpreted without knowledge of the results of the reference standard?” and “If a threshold was used, was it pre-specified?” were replaced by the item “Was the diagnostic method of TM described?” in Domain 2 (Index Test). The reason for this was that the replaced items were designed for meta-analysis of diagnostic methodologies, whereas we aimed to investigate the prevalence of TM infection. The Domain 3 (Reference Standard) was omitted. In Domain 4 (Flow and Timing), the item “Was there an appropriate interval between index test and reference standard?” was omitted, and the item “Were all participants tested for TM?”, replaced the item, “Did all patients receive the same reference standard?”. Each item in all included articles was assessed according to the following criteria: high risk of bias, low risk of bias, or unclear (Additional file 1: Fig. S1-S2) [15, 19]. The symmetry of funnel plots and the Egger test were used to assess the presence of publication and selective reporting bias. A p value of less than 0.10 was considered indicative of statistically significant publication bias.
Statistical analysis
We calculated the pooled prevalence of TM infection in PLWHA in the included studies. Sub-group analyses were performed based on countries, geographic regions and provinces (only studies in China), latitude, ART eras (excluded studies whose research duration spans the limited ART era and the widespread ART era). Forest plots and heterogeneity analysis were performed by Stata (Version 15.1, Stata Corporation, College Station, TX, USA). Data were entered into ArcGIS (Version 10.2, ESRI Inc., Redlands, CA, USA) to generate maps, which shows the estimated prevalence of TM infection in PLWHA at the national level and provincial level. To measure the association between CD4 counts or ART status and TM infection, we performed meta-analyses of available data using a random- or a fixed-effects method to pool weighted odds ratios (OR) of TM infection risk estimates.
We used Cochran’s Q (χ2 and p values) and the I2 statistic to assess the heterogeneity between studies. Random-effects models were used for summary statistics, due to the high heterogeneity (I2 > 50%, p < 0.1) of studies. We performed both univariate meta-regression and multivariate meta-regression to determine the factors contributing to the heterogeneity in the studies. The investigated factors included latitude (studies in areas intersecting the Tropic of Cancer or south of the Tropic of Cancer versus studies in North of the Tropic of Cancer), income levels (low-income countries versus middle-income countries) and publication year. We also analyzed sensitivity in order to determine whether large differences in sample sizes would have a statistically appreciable impact on results, which resulted in the exclusion of one study with a sample size much larger than the rest of the included studies.
Results
Characteristics of original studies
Initially, 2026 articles were identified. After deduplicating, 1201 unduplicated articles were obtained. 1121 articles unrelated to this analysis were removed, the remaining 80 articles were examined by full text reading. Among these 80 publications, 47 were excluded for various reasons (Fig. 1). Finally, 33 articles assessing TM infection in HIV-infected individuals, with a total subject population of 159,064 PLWHA, were included in our study. As shown in Table 1, the identified studies were conducted in five countries in Asia. There were 47,644 participants (19 studies) from China; 108,285 participants (7 studies) from Thailand, 2370 participants (3 studies) from Vietnam; 620 participants (3 studies) from India, and 145 participants (1 study) from Malaysia (Fig. 1, Table 1). Twenty-five articles were reported in English, and 8 studies were published in Chinese. Publication bias was examined by funnel plots (Fig. 3 in Additional file 2), while statistical significance was assessed by the Egger’s regression asymmetry test. There is as yet no indication of significant bias in the measurements (p = 0.889).
Fig. 1.
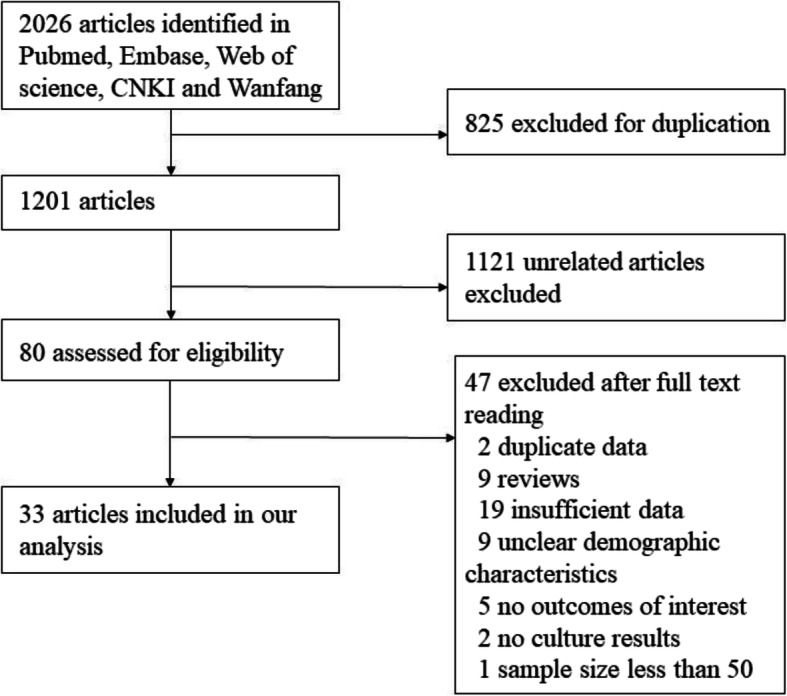
Flow chart of included studies
Table 1.
The detailed characteristics of included studies
| Study | Latitude | Country | No. of TM infection patients | No. of PLWHA | Prevalence |
|---|---|---|---|---|---|
| Jiang et al. (2018) [8] | Lower | China | 1093 | 6791 | 16.09% |
| Pang et al. (2018) [20] | Higher | China | 5 | 2298 | 0.22% |
| Li et al. (2018) [21] | Lower | China | 2 | 200 | 1.00% |
| Ni et al. (2018) [22] | Higher | China | 8 | 852 | 0.94% |
| Yen et al. (2017) [23] | Lower | China | 126 | 21,375 | 0.59% |
| Kaur et al. (2016) [24] | Higher | India | 4 | 280 | 1.43% |
| Qi et al. (2016) [25] | Higher | China | 43 | 2442 | 1.76% |
| Zhai et al. (2016) [26] | Higher | China | 2 | 827 | 0.24% |
| Zheng et al. (2015) [27] | Higher | China | 47 | 981 | 4.79% |
| Kolalapudi et al. (2014) [28] | Lower | India | 1 | 142 | 0.70% |
| Son et al. (2014) [9] | Lower | Vietnam | 103 | 2100 | 4.90% |
| Nguyen et al. (2013) [29] | Lower | Vietnam | 14 | 170 | 8.24% |
| Xiao et al. (2013) [10] | Higher | China | 12 | 1104 | 1.09% |
| Han et al. (2013) [30] | Lower | China | 40 | 348 | 11.49% |
| Su et al. (2012) [31] | Lower | China | 17 | 177 | 9.60% |
| Xie et al. (2012) [32] | Lower | China | 389 | 3905 | 9.96% |
| Huang et al. (2011) [33] | Higher | China | 5 | 796 | 0.63% |
| Huang et al. (2010) [34] | Lower | China | 136 | 762 | 17.85% |
| Lin et al. (2009) [35] | Lower | China | 18 | 1790 | 1.01% |
| Zeng et al. (2009) [36] | Lower | China | 19 | 71 | 26.76% |
| Tang et al. (2009) [37] | Lower | China | 99 | 1559 | 6.35% |
| Manosuthi et al. (2007) [38] | Lower | Thailand | 1 | 793 | 0.13% |
| Tang et al. (2007) [39] | Lower | China | 50 | 319 | 15.67% |
| Sun et al. (2006) [11] | Lower | China | 25 | 1047 | 2.39% |
| Chierakul et al. (2004) [40] | Lower | Thailand | 57 | 2602 | 2.19% |
| Louie et al. (2004) [12] | Lower | Vietnam | 7 | 100 | 7.00% |
| Subsai et al. (2004) [41] | Lower | Thailand | 19 | 155 | 12.26% |
| Ranjana et al. (2002) [13] | Lower | India | 36 | 198 | 18.18% |
| Chariyalertsak et al. (2001) [42] | Lower | Thailand | 3054 | 101,945 | 3.00% |
| Wananukul et al. (1999) [43] | Lower | Thailand | 3 | 91 | 3.30% |
| Jing et al. (1999) [44] | Lower | Malaysia | 3 | 145 | 2.07% |
| Tansuphasawadikul et al. (1999) [45] | Lower | Thailand | 50 | 2261 | 2.21% |
| Supparatpinyo et al. (1994) [1] | Lower | Thailand | 86 | 438 | 19.63% |
Fig. 3.
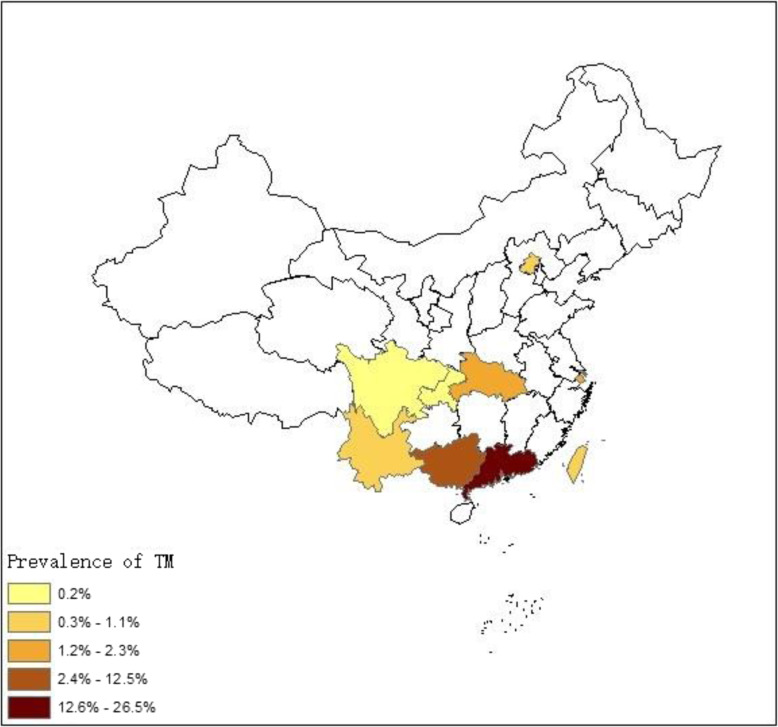
Prevalence of TM infection in PLWHA in China at the provincial level. Overall, the estimated pooled prevalence of TM infection in China was 3.3% (95%CI:1.8–5.8). The prevalence by province was as follows: 26.5% (95%CI: 16.2–43.5) in Guangdong, 12.5% (95%CI: 8.7–17.9) in Guangxi, 1.8% (95%CI: 1.3–2.4) in Shanghai, 1.1% (95%CI: 0.5–2.8) in Taiwan, and 1.0% (95%CI: 0.3–4.1) in Yunnan, 0.9% (95%CI: 0.6–1.5) in Beijing, 0.2% (95%CI: 0.1–1.0) in Chongqing and 0.2% (95%CI: 0.1–0.5) in Sichuan respectively. Map image is the intellectual property of Esri and is used herein under license. Copyright© 2019 Esri and its licensors. All rights reserved
Prevalence of TM infection in Asian countries
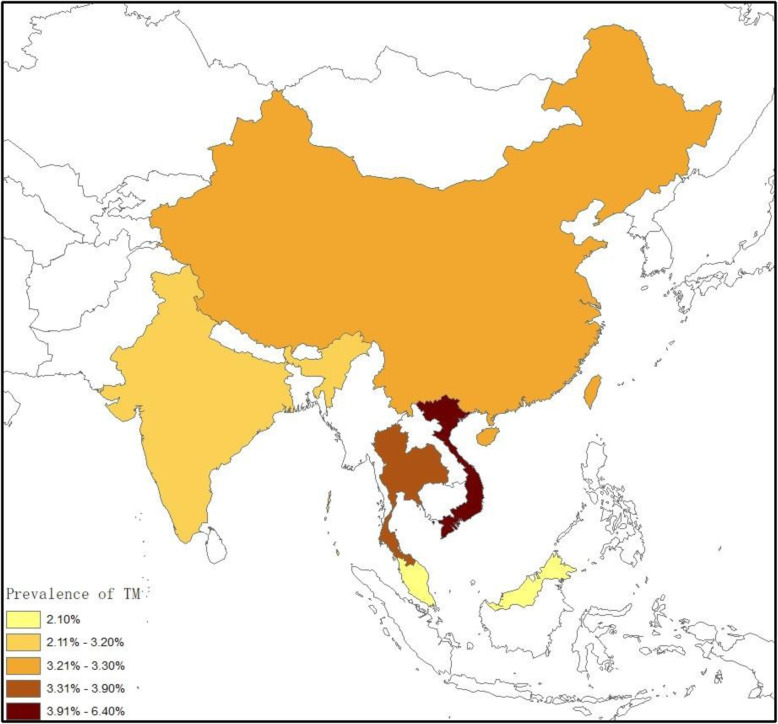
The prevalence of TM infection in PLWHA reported in the included articles ranged from between 0.13 to 19.63% in different regions (Table 1). Overall, the estimated pooled prevalence of TM infection in Asia was 3.6% (95% CI:2.4–5.4, n = 159,064 participants, I2 = 98%, p < 0.001). The prevalence by country was as follows: 6.4%(95%CI: 4.4–9.5) in Vietnam, 3.9% (95%CI:1.8–8.3) in Thailand, 3.3%(95%CI:1.8–5.8) in China, 3.2%(95%CI:0.3–32.6) in India, and 2.1%(95%CI:0.7–6.6) in Malaysia, respectively (Fig. 2). The sensitivity analysis showed that our results are stable. After excluding the study of Chariyalertsak et al. [42] with the much larger sample size, the pooled prevalence of TM infection in Asia was 3.6% (95%CI: 2.4–5.5, n = 57,119). And in Thailand, the pooled prevalence was 3.6% (95%CI, 1.2–11.4, n = 6340).
Fig. 2.
Prevalence of TM infection in PLWHA in different countries in Asia. Overall, the estimated pooled prevalence of TM infection in Asia was 3.6% (95% CI: 2.4–5.4). The prevalence by country was as follows: 6.4% (95%CI: 4.4–9.5) in Vietnam, 3.9% (95%CI: 1.8–8.3) in Thailand, 3.3% (95%CI: 1.8–5.8) in China, 3.2% (95%CI: 0.3–32.6) in India, and 2.1% (95%CI: 0.7–6.6) in Malaysia. Map image is the intellectual property of Esri and is used herein under license. Copyright© 2019 Esri and its licensors. All rights reserved
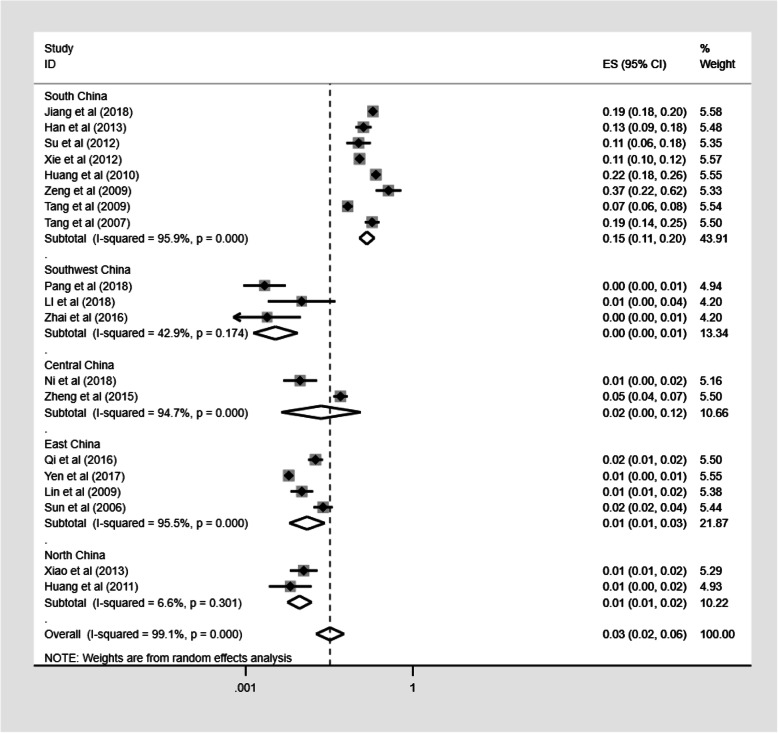
We also assessed the geographical distribution of TM infection in China. The prevalence of TM infection in PLWHA in China ranged from 0.2% (95%CI: 0.1–0.5) to 26.5% (95%CI: 16.2–43.5%; Fig. 3). South China had the highest prevalence, estimated at 15.0% (95%CI: 11.0–20.4), while Southwest China had the lowest prevalence, estimated at 0.3% (95%CI: 0.1–0.9; Fig. 4). Detailed information of these studies is presented in Additional file 3: Table S1.
Fig. 4.
Pooled prevalence of TM infection in different regions of China. Forest plot shows the estimated prevalence of TM infection in PLWHA in five regions of China with 95%CI. CI = confidence interval; ES = effect size (the prevalence of TM infection in PLWHA); I2 = I2 statistic was used to assess the heterogeneity between studies
Prevalence of TM infection in different latitudes
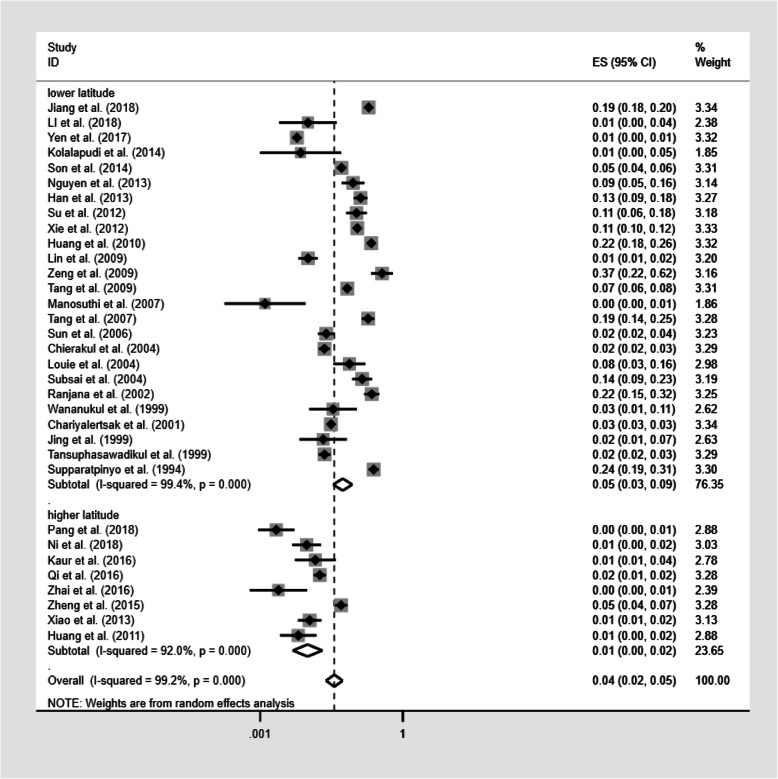
The sub-group analysis based on different latitudes was performed in 33 studies. As depicted in Fig. 5, The prevalence of TM infection was 5.5% (95%CI: 3.4–8.7; n = 149,484) in lower latitude regions and 1.0% (95%CI: 0.5–2.0; n = 9580) in higher latitude regions. The prevalence of TM infection after excluding the study with the much larger sample size [42] was 5.6% (95%CI: 3.5–9.0; n = 47,539) in lower latitude regions and 1.0% (95%CI: 0.5–2.0; n = 9580) in higher latitude regions.
Fig. 5.
Pooled prevalence of TM infection according to latitude. Forest plot shows the estimated prevalence of TM infection in PLWHA in different latitude areas with 95%CI. CI = confidence interval; ES = effect size (the prevalence of TM infection in PLWHA); I2 = I2 statistic was used to assess the heterogeneity between studies
Analysis of data heterogeneity
We observed substantial heterogeneity (I2 = 99.2%, p < 0.001; Table 2) in the included studies. We further analyzed the source of the heterogeneity. Our univariate meta-regression analyses indicated that latitude (OR 5.616, 95%CI: 1.941–16.246, p = 0.002) was a source of heterogeneity, and that there was no influence on heterogeneity associated with publication year (OR 0.930, 95%CI: 0.862–1.004, p = 0.057) and income levels (OR 0.466, 95%CI: 0.077–2.813, p = 0.393). Subsequently, multivariate meta-regression analysis results showed that latitude was a possible cause of heterogeneity (OR 4.442, 95%CI: 1.213–16.268, p = 0.026).
Table 2.
The influence of variables on the heterogeneity of prevalence (n = 159,064)
| No. of study | No. of TM infection patients | No. of PLWHA | Prevalence of TM infection | Heterogeneity | Univariate meta-regression | ||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | p value | I2 | OR (95%CI) | p value | |||||
| Latitude | 5.616 (1.941 to 16.246) | 0.002 | |||||||
| Higher | 8 | 126 | 9580 | 1.0% (0.5–2.0) | 87.65 | 0.000 | 92.0% | ||
| Lower | 25 | 5448 | 149,484 | 5.5% (3.4–8.7) | 3960.18 | 0.000 | 99.4% | ||
| Income level | 0.466 (0.771 to 2.813) | 0.393 | |||||||
| Low | 3 | 124 | 2370 | 6.4% (4.4–9.5) | 4.09 | 0.129 | 51.1% | ||
| Middle | 30 | 5450 | 156,694 | 3.4% (2.2–5.2) | 4148.53 | 0.000 | 99.3% | ||
| Publication year | 33 | 5574 | 159,064 | – | – | – | – | 0.930 (0.862 to 1.004) | 0.057 |
| Total | 33 | 5574 | 159,064 | ||||||
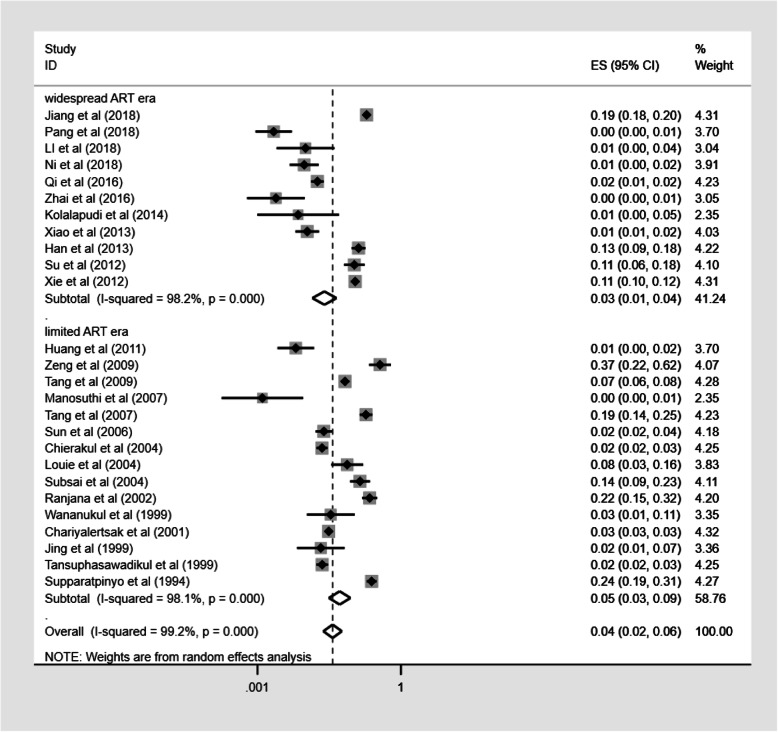
Prevalence of TM infection in different ART eras
In this meta-analysis, we divided HIV treatment into two eras: limited ART era (before 2008) and widespread ART era (2008 and thereafter) and compared the prevalence of TM infection in the two different eras. Seven studies were excluded from the sub-group analysis of the different ART eras, because the study period spanned the widespread ART era and the limited ART era. The prevalence of TM infection was 5.2% (95%CI: 3.1–8.8; n = 112,520) in limited ART era and 2.5% (95%CI: 1.4–4.5; n = 19,086) in widespread ART era (Fig. 6); however, we did not observe significant statistical difference in the prevalence of TM infection between the different ART eras (Table 3).
Fig. 6.
Pooled prevalence of TM infection in different ART eras. Forest plot shows the estimated prevalence of TM infection within PLWHA in widespread ART era (2008 and thereafter) and limited ART era (before 2008) with 95%CI. CI = confidence interval; ES = effect size (the prevalence of TM infection within PLWHA); I2 = I2 statistic was used to assess the heterogeneity between studies
Table 3.
The influence of ART eras on the heterogeneity of prevalence (n = 131,606)
| No. of study | No. of TM infection patients | No. of PLWHA | Prevalence of TM infection | Heterogeneity | Univariate meta-regression | ||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | p value | I2 | OR (95%CI) | p value | |||||
| ART era | 0.453(0.130 to 1.578) | 0.203 | |||||||
| Limited | 11 | 3514 | 112,520 | 5.2% (3.1–8.8) | 727.31 | 0.000 | 98.1% | ||
| Widespread | 15 | 1612 | 19,086 | 2.5% (1.4–4.5) | 565.88 | 0.000 | 98.2% | ||
We did perceive the large difference in sample size between the limited-ART era and the widespread-ART era groups, and in order to determine whether the large difference in sample size would have an impact on our results, we further did a sensitivity analysis for comparability, which excluded the study of Chariyalertsak et al. [42] with the large sample size. Subsequent sensitivity analysis showed that our results were stable. The prevalence of TM infection after excluding the study with the much larger sample size was 5.3% (95%CI: 2.9–9.8; n = 10,575) in limited ART era and 2.5% (95%CI: 1.4–4.5; n = 19,086) in widespread ART era.
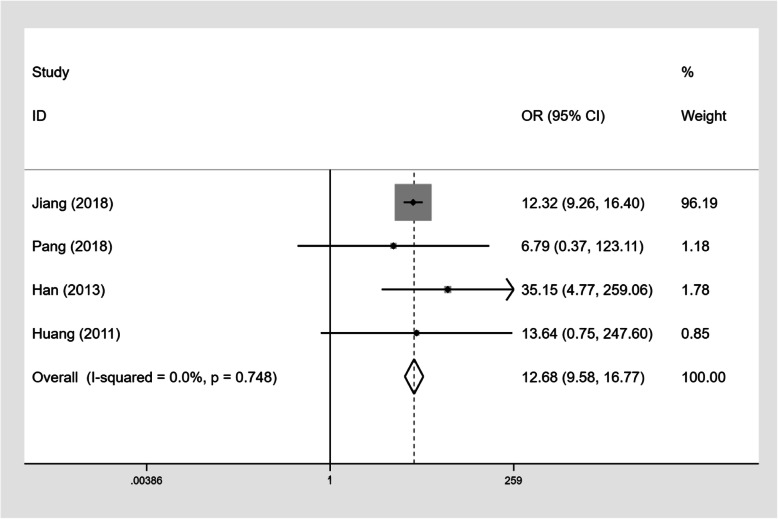
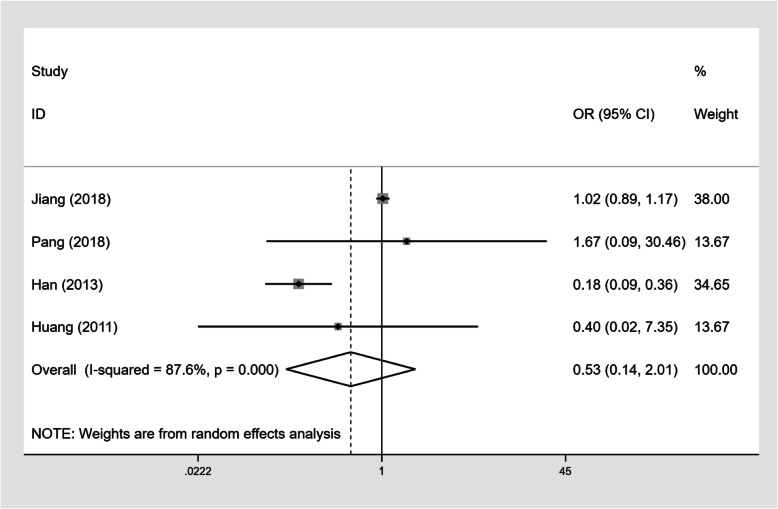
The impact of CD4+ T-cell counts and on-ART treatment on TM infection
Four studies (n = 7809) described the number of PLWHA with CD4+ T-cell counts below 200 cells/mm3 and the number of PLWHA on ART (Table 4). Our results showed that PLWHA with CD4+ T-cell counts below 200 cells/mm3 had a higher TM infection prevalence than those with CD4+ T-cell counts≥200 cells/mm3 (OR 12.68, 95%CI: 9.58–16.77, Fig. 7). However, there was no statistically significant difference in TM infection prevalence rates between PLWHA on ART and PLWHA not on ART (OR 0.53, 95%CI: 0.14–2.01, Fig. 8).
Table 4.
Influence of CD4 counts and ART treatment on TM infection
| CD4 < 200 group | CD4 ≥ 200 group | OR | ART group | Without ART group | OR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TM infection | Without TM infection | TM infection | Without TM infection | TM infection | Without TM infection | TM infection | Without TM infection | |||
| Jiang [8] | 873 | 2760 | 52 | 2026 | 12.32 | 393 | 2021 | 700 | 3677 | 1.02 |
| Pang [20] | 5 | 587 | 0 | 362 | 6.79 | 5 | 824 | 0 | 125 | 1.67 |
| Han [30] | 39 | 162 | 1 | 146 | 35.15 | 16 | 243 | 24 | 65 | 0.18 |
| Huang [33] | 5 | 353 | 0 | 438 | 13.64 | 0 | 145 | 5 | 646 | 0.40 |
Fig. 7.
Forest plot of odds ratios and 95% confidence interval for the association between CD4+ T-cell counts and TM infection in PLWHA (CD4+ T-cell counts < 200 cells/mm3 versus ≥200 cells/mm3)
Fig. 8.
Forest plot of odds ratios and 95% confidence interval for the association between ART and TM infection in PLWHA (with ART versus without ART)
Articles ranked by year of publication. The lower latitude is defined as intersecting the tropic of cancer or south of the tropic of cancer and the higher latitude is defined as north of the tropic of cancer.
Discussion
Our analysis found that the pooled prevalence of TM infection in Asia was 3.6%, which appears not as high as expected, compared with the prevalence of Toxoplasma gondii infection and Cryptosporidium infection globally [15, 16]. However, actual prevalence rates may not be as optimistic as what the results show. Firstly, the diagnosis of TM infection in our included studies was based on culture of clinical specimens, which is considered to be the gold standard for diagnosis of TM infection, but is not available in many TM endemic areas [46]. Therefore, patients with TM infection may not be reliably identified in these areas. Secondly, we excluded studies in which patients were diagnosed by serological and molecular methods, because of issues related to the specificity of these methods [47, 48]. Thirdly, symptoms of TM infection are complex and non-specific, and misdiagnosis and missed diagnosis are not uncommon in clinical practice. As the most populous continent in the world, Asia has approximately 5,900,000 (5100000–7,100,000) PLWHA according to data from UNAIDS from 2018 [49]. Based on our pooled prevalence, the approximate number of PLWHA with TM co-infection may approach 236,000 (102000–355,000), suggesting that the burden of disease inflicted by TM infection is significantly heavy in this region.
Univariate meta-regression on publication year, income level, and latitude were conducted. No statistically significant difference was found in TM infection prevalence among different income level countries in our meta-analysis, suggesting that income level is not a factor in the prevalence of TM infection. This differs from prevalence rates for Toxoplasma gondii infection and Cryptosporidium infection, which was found to be related to differing national income levels [15, 50]. In our study, both univariate and multivariate meta-regression indicated that latitude may be the main source of heterogeneity. Most lower latitude areas in Asia have a tropical monsoon climate and relatively high temperatures throughout the year. The annual average temperature of those regions is above 22 °C, and the temperature is generally above 16 °C, even in the coldest month. A study by Philips et al. [51] found that TM hospital admission was closely associated with environmental humidity, but not to other environmental variables. This may explain the association between lower latitudes and higher TM prevalence, in that the prevailing climate in Southeast Asia and South China may provide favorable environmental conditions for the growth and spread of TM.
In China, the prevalence of TM infection varied greatly from province to province, with the lowest prevalence in Sichuan (0.22%) [20], and the highest prevalence in Guangdong (26.76%) [52]. Overall, the prevalence of TM infection in South China was significantly higher than that in other regions. The lowest monthly temperature in south China averages 10 °C or above, and the annual precipitation in this region is 1400-2000 mm. In addition to warm and humid weather, some farmers collect livestock excreta as fertilizer, and people also hunt wild bamboo rats for food, and each of these activities may also be a potential pathway for human infection [53, 54].
As reported by researchers in the past, AIDS patients with low baseline CD4+ T-cell counts were more likely to develop new OIs than others [38, 55]. TM infection is not an exception to this rule. Our study found that patients with CD4+ T-cell counts below 200cells/mm3 had a higher risk of TM infection.
Notwithstanding the broader coverage of antiretroviral therapy in Asia, we found no significant statistical difference in TM infection prevalence rates in the widespread ART era compared with the limited ART era. In 2003, only 70,000 people in South and Southeast Asia were accepting ART, and by the end of 2008, 565,000 people were accepting ART, which was eight times the number in 2003 [56]. Although ART use is more widespread than in the past, it is far from the 90% goal set by WHO, which envisions that, by 2020, 90% of people who are HIV infected will be diagnosed, 90% of people who are diagnosed will be on ART, and 90% of those who receive ART will be virally suppressed. This may be the principal reason that the difference in TM infection prevalence rates between the two eras was not calculated to be statistically significant in our analysis. Additionally, delayed diagnosis of HIV infection remains common in this resource-limited region, and this may be another reason for the lack of statistical significance noted above. It is hoped that as ART use gradually becomes more universal in Asia in the future, the prevalence of TM infection will likely decline.
To the best of our knowledge, this is the first systematic review and meta-analysis reporting the burden of TM infection in PLWHA. However, limitations to our meta-analysis should be mentioned. Firstly, our study only included patients diagnosed by TM culture results, and excluded diagnoses by other methods. This may result in underestimation of TM prevalence. Secondly, all data are from East Asia, South Asia and Southeast Asia, and data from other parts of Asia are not available. However, patients are increasingly diagnosed with TM infection outside of epidemic areas after acquiring the fungus during travel within epidemic areas [57]. Thirdly, this study only analyzed articles published in English and Chinese. Research articles published in other languages may have been omitted, which could potentially be a factor that may have resulted in different outcomes.
Conclusions
Although the prevalence of TM infection in Asia is not exceedingly high, the large population in Asia means that numerous people are still at risk, especially the number of susceptible people in vulnerable sub-populations, e.g. patients with low CD4+ T-cell counts and patients living in endemic areas. Owing to the fact that this fungus poses considerable risk to PLWHA in Asia, our results support the optimization of diagnostic tools and universal screening for TM in vulnerable people to enable early case detection, and thus facilitate prompt, appropriate antifungal treatment.
Supplementary information
Additional file 1: Fig. S1-S2 showing bias and quality assessment.
Additional file 2: Fig. S3 showing the funnel plot for publication bias.
Additional file 3: Table S1 showing the prevalence of TM infection in different provinces of China.
Acknowledgements
Not applicable.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- ART
Antiretroviral therapy
- CI
Confidence interval
- CNKI
China National Knowledge Infrastructure
- HIV
Human immunodeficiency virus
- OR
Odds ratios
- PLWHA
People living with HIV/AIDS
- PRISMA
Reporting Items for Systematic Reviews and Meta-Analysis
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies 2
- TM
Talaromyces marneffei
- UNAIDS
Joint United Nations Programme on HIV/AIDS
- WHO
World Health Organization
Authors’ contributions
Y-YQ, X-JH and Y-KC designed the study. Y-YQ and YL performed the literature search. J-HH and HC extracted and analyzed the data, and assessed the methodology quality of included studies. Y-YQ, X-JH and A-XL performed the meta-analysis, generated the figures and edited the manuscript with assistance from Y-KC, X-CL, and X-FY. All authors read and approved the final manuscript.
Funding
This study was supported by the National Science and Technology Major Project of China during the 13th Five-year plan period (2018ZX10302104, 2017ZX10201101), Major Project of Beijing Municipal Science and Technology Committee (D161100000416003, D171100000517003), the National Natural Science Foundation of China (No. 81701984), Beijing Key Laboratory (BZ0089), 2018 Beijing Youan Hospital Scientific Research Project for Young & Middle-aged Talent’s Cultivation (YNKTTS20180107), and Chongqing Municipal Science and Technology Bureau TCM Key Research Project (ZY201801003). The funders provided financial support for statistical consultation and English editing.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuanyuan Qin and Xiaojie Huang contributed equally to this work.
Contributor Information
Xiaofeng Yan, Email: 2429918342@qq.com.
Yaokai Chen, Email: yaokaichen@hotmail.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12879-020-05260-8.
References
- 1.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344(8915):110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 2.Thuy L, Wolbers M, Nguyen HC, Vo MQ, Nguyen TC, Nguyen PHL, Pham SL, Kozal MJ, Shikuma CM, Day JN, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis. 2011;52(7):945–952. doi: 10.1093/cid/cir028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gugnani H, Fisher MC, Paliwal-Johsi A, Vanittanakom N, Singh I, Yadav PS. Role of Cannomys badius as a natural animal host of Penicillium marneffei in India. J Clin Microbiol. 2004;42(11):5070–5075. doi: 10.1128/JCM.42.11.5070-5075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh PN, Ranjana K, Singh YI, Singh KP, Sharma SS, Kulachandra M, Nabakumar Y, Chakrabarti A, Padhye AA, Kaufman L, et al. Indigenous disseminated Penicillium marneffei infection in the state of Manipur, India: report of four autochthonous cases. J Clin Microbiol. 1999;37(8):2699–2702. doi: 10.1128/jcm.37.8.2699-2702.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Z, Ribas JL, Gibson DW, Connor DH. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis. 1988;10(3):640–652. doi: 10.1093/clinids/10.3.640. [DOI] [PubMed] [Google Scholar]
- 6.Duong TA. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23(1):125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 7.Vanittanakom N, Cooper CR, Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang J, Meng S, Huang S, Ruan Y, Lu X, Li JZ, Wu N, Huang J, Xie Z, Liang B, et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect. 2019;25(2):233–41. [DOI] [PubMed]
- 9.Son VT, Khue PM, Strobel M. Penicilliosis and AIDS in Haiphong, Vietnam: evolution and predictive factors of death. Medecine Et Maladies Infectieuses. 2014;44(11–12):495–501. doi: 10.1016/j.medmal.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Xiao J, Gao GJ, Li YM, Zhang W, Tian YF, Huang YX, Su WJ, Han N, Yang D, Zhao HX. Spectrums of Opportunistic Infections and Malignancies in HIV-Infected Patients in Tertiary Care Hospital, China. PLoS One. 2013;8(10):e75915. [DOI] [PMC free article] [PubMed]
- 11.Sun HY, Chen MY, Hsiao CF, Hsieh SM, Hung CC, Chang SC. Endemic fungal infections caused by Cryptococcus neoformans and Penicillium marneffei in patients infected with human immunodeficiency virus and treated with highly active anti-retroviral therapy. Clin Microbiol Infect. 2006;12(4):381–388. doi: 10.1111/j.1469-0691.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 12.Louie JK, Chi NLT, Quang VM, Campbell J, Chau NV, Rutherford GW, Farrar JJ, Parry CM. Opportunistic infections in hospitalized HIV-infected adults in Ho Chi Minh City, Vietnam: a cross-sectional study. Int J STD AIDS. 2004;15(11):758–761. doi: 10.1258/0956462042395159. [DOI] [PubMed] [Google Scholar]
- 13.Ranjana KH, Priyokumar K, Singh TJ, Gupta CC, Sharmila L, Singh PN, Chakrabarti A. Disseminated Penicillium marneffei infection among HIV-infected patients in Manipur state, India. J Infect. 2002;45(4):268–271. doi: 10.1053/jinf.2002.1062. [DOI] [PubMed] [Google Scholar]
- 14.Ning C, Lai J, Wei W, Zhou B, Huang J, Jiang J, Liang B, Liao Y, Zang N, Cao C, et al. Accuracy of rapid diagnosis of Talaromyces marneffei: A systematic review and meta-analysis. PLoS One. 2018;13(4):e0195569. [DOI] [PMC free article] [PubMed]
- 15.Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, Zhu XQ, Liu Q. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. 2017;4(4):e177–e188. doi: 10.1016/S2352-3018(17)30005-X. [DOI] [PubMed] [Google Scholar]
- 16.Horman A, Korpela H, Sutinen J, Wedel H, Hanninen ML. Meta-analysis in assessment of the prevalence and annual incidence of Giardia spp. and Cryptosporidium spp. infections in humans in the Nordic countries. Int J Parasitol. 2004;34(12):1337–1346. doi: 10.1016/j.ijpara.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman S, Engel ME, Griesel R, Mendelson M. Burden of pneumocystis pneumonia in HIV-infected adults in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:482. doi: 10.1186/s12879-016-1809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, Group Q QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 19.Sobanski V, Dauchet L, Lefevre G, Lambert M, Morell-Dubois S, Sy T, Hachulla E, Hatron PY, Launay D, Dubucquoi S. Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: new data from a French cohort and a systematic review and meta-analysis. Arthritis Rheumatol. 2014;66(2):407–417. doi: 10.1002/art.38219. [DOI] [PubMed] [Google Scholar]
- 20.Pang W, Shang P, Li Q, Xu J, Bi L, Zhong J, Pei X. Prevalence of opportunistic infections and causes of death among hospitalized HIV-infected patients in Sichuan, China. Tohoku J Exp Med. 2018;244(3):231–242. doi: 10.1620/tjem.244.231. [DOI] [PubMed] [Google Scholar]
- 21.Li YH, Li Q, Zhai YJ, Li YY, Bai JS, Huang YL, Cao YK. Drug sensitivity study and risk factor analysis of deep fungal infection among HIV/AIDS patients. J Dermatol Venereol. 2018;40(2):168–171. [Google Scholar]
- 22.Ni W, Yang L, Liu GZ. Distribution and drug resistance of deep fungus from AIDS patients with infection in hospital of traditional Chinese medicine. China Medical Herald. 2018;15(19):139–142. [Google Scholar]
- 23.Yen YF, Chen M, Jen I, Lan YC, Chuang PH, Liu YL, Lee Y, Chen YMA. Association of HIV and opportunistic infections with incident stroke: a Nationwide population-based cohort study in Taiwan. Jaids J Acquired Immune Deficiency Syndromes. 2017;74(2):117–125. doi: 10.1097/QAI.0000000000001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur R, Dhakad MS, Goyal R, Bhalla P, Dewan R. Spectrum of opportunistic fungal infections in HIV/AIDS patients in tertiary care hospital in India. Can J Infect Dis Med Microbiol. 2016;2016. [DOI] [PMC free article] [PubMed]
- 25.Qi T, Zhang R, Shen Y, Liu L, Lowrie D, Song W, Chen J, Wang Z, Shen J, Cai R, et al. Etiology and clinical features of 229 cases of bloodstream infection among Chinese HIV/AIDS patients: a retrospective cross-sectional study. Eur J Clin Microbiol Infect Dis. 2016;35(11):1767–1770. doi: 10.1007/s10096-016-2724-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhai ZF, Cheng LJ, Zhou CJ, Song ZQ, Yang H, Shen Z. Facial skin findings are indicators of human immunodeficiency virus infection and acquired immune deficiency syndrome: a retrospective study. J Public Health Heidelberg. 2016;24(6):505–511. [Google Scholar]
- 27.Zheng JD, Gui X, Cao Q, Yang RR, Yan YJ, Deng LP, Lio J. A Clinical Study of Acquired Immunodeficiency Syndrome Associated Penicillium marneffei Infection from a NonEndemic Area in China. PLoS One. 2015;10(6):e0130376. [DOI] [PMC free article] [PubMed]
- 28.Kolalapudi SA, Guttikonda A. Cutaneous fungal infections in HIV infection: an Indian experience. J Am Acad Dermatol. 2014;70(5):AB108. [Google Scholar]
- 29.Nguyen LT, Nguyen MV, Trinh HTX. Etiology of respiratory tract infection in HIV/AIDS patients at the national hospital of tropical diseases (NHTD) Hanoi, Vietnam. Sex Transm Infect. 2013;89(Suppl 1):A95-A95.
- 30.Han J, Lun WH, Meng ZH, Huang K, Mao Y, Zhu W, Lian S. Mucocutaneous manifestations of HIV-infected patients in the era of HAART in Guangxi Zhuang autonomous region, China. J Eur Acad Dermatol Venereol. 2013;27(3):376–382. doi: 10.1111/j.1468-3083.2011.04429.x. [DOI] [PubMed] [Google Scholar]
- 31.Su GS, Wei SQ, Luo XL, Xie N, Li JY. Opportunistic infection of HIV/AIDS patients in Nanning: clinical analysis of 177 cases. Guangxi Med J. 2012;34(8):1000–1001. [Google Scholar]
- 32.Xie N. Distribution of pathogens in blood culture of 3905 AIDS patients. Hainan Med J. 2012;23(9):89–91. [Google Scholar]
- 33.Huang XJ, Li HY, Chen DX, Wang XC, Li ZC, Wu YS, Zhang T, Gao YQ, Wu H. Clinical analysis of skin lesions in 796 Chinese HIV-positive patients. Acta Derm Venereol. 2011;91(5):552–556. doi: 10.2340/00015555-1107. [DOI] [PubMed] [Google Scholar]
- 34.Huang LF, Tang XP, Cai WP, Chen XJ, Lei CL, Li LH, Zhang FC. An analysis of opportunistic infection in 762 inpafients with human immunodeficiency virus infection in Guangdong areas. Chin J Intern Med. 2010;49(8):653–656. [PubMed] [Google Scholar]
- 35.Lin CY, Sun HY, Chen MY, Hsieh SM, Sheng WH, Lo YC, Hung CC, Chang SC. Aetiology of cavitary lung lesions in patients with HIV infection. Hiv Medicine. 2009;10(3):191–198. doi: 10.1111/j.1468-1293.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- 36.Zeng CH, Xie T, Li XQ, Liu CD, Li J, Zeng LJ, Shen SJ. Investigation and analysis of fungal infection in patients with HIV/AIDS. South China J Prev Med. 2009;35(2):46–47,50. [Google Scholar]
- 37.Tang GL, Liu AM, Li SP, Tang LS. Penicilliosis marneffei in people with HIV/AIDS: analysis of 99 cases. Ch in J Misdiagn. 2009;9(7):1652–1653. [Google Scholar]
- 38.Manosuthi W, Chaovavanich A, Tansuphaswadikul S, Prasithsirikul W, Inthong Y, Chottanapund S, Sittibusaya C, Moolasart V, Termvises P, Sungkanuparph S. Incidence and risk factors of major opportunistic infections after initiation of antiretroviral therapy among advanced HIV-infected patients in a resource-limited setting. J Infect. 2007;55(5):464–469. doi: 10.1016/j.jinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Tang ZR, Lu ZZ, Liu W, Chen J, Hu YY, Deng XE, Liang WX, Yang JY, Lu GG. Treatment of AIDS patients with Penicilliosis marneffei in Guangxi. Applied Prev Med. 2007;01:28–30. [Google Scholar]
- 40.Chierakul W, Rajanuwong A, Wuthiekanun V, Teerawattanasook N, Gasiprong M, Simpson A, Chaowagul W, White NJ. The changing pattern of bloodstream infections associated with the rise in HIV prevalence in northeastern Thailand. Trans R Soc Trop Med Hyg. 2004;98(11):678–686. doi: 10.1016/j.trstmh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Subsai K, Kanoksri S, Siwaporn C, Helen L. Neurological complications in AIDS patients: the 1-year retrospective study in Chiang Mai University, Thailand. Eur J Neurol. 2004;11(11):755–759. doi: 10.1111/j.1468-1331.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 42.Chariyalertsak S, Sirisanthana T, Saengwonloey O, Nelson KE. Clinical presentation and risk behaviors of patients with acquired immunodeficiency syndrome in Thailand, 1994-1998: regional variation and temporal trends. Clin Infect Dis. 2001;32(6):955–962. doi: 10.1086/319348. [DOI] [PubMed] [Google Scholar]
- 43.Wananukul S, Thisyakorn U. Mucocutaneous manifestations of HIV infection in 91 children born to HIV-seropositive women. Pediatr Dermatol. 1999;16(5):359–363. doi: 10.1046/j.1525-1470.1999.00093.x. [DOI] [PubMed] [Google Scholar]
- 44.Jing W, Ismail R. Mucocutaneous manifestations of HIV infection: a retrospective analysis of 145 cases in a Chinese population in Malaysia. Int J Dermatol. 1999;38(6):457–463. doi: 10.1046/j.1365-4362.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 45.Tansuphasawadikul S, Amornkul PN, Tanchanpong C, Limpakarnjanarat K, Kaewkungwal J, Likanonsakul S, Eampokalap B, Naiwatanakul T, Kitayaporn D, Young NL, et al. Clinical presentation of hospitalized adult patients with HIV infection and AIDS in Bangkok, Thailand. J Acquir Immune Defic Syndr. 1999;21(4):326–332. doi: 10.1097/00126334-199908010-00011. [DOI] [PubMed] [Google Scholar]
- 46.Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017;17(11):e334–e343. doi: 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- 47.Lu S, Li X, Calderone R, Zhang J, Ma J, Cai W, Xi L. Whole blood nested PCR and real-time PCR amplification of Talaromyces marneffei specific DNA for diagnosis. Med Mycol. 2016;54(2):162–168. doi: 10.1093/mmy/myv068. [DOI] [PubMed] [Google Scholar]
- 48.Hien HTA, Thanh TT, Thu NTM, Nguyen A, Thanh NT, Lan NPH, Simmons C, Shikuma C, Chau NVV, Thwaites G, et al. Development and evaluation of a real-time polymerase chain reaction assay for the rapid detection of Talaromyces marneffei MP1 gene in human plasma. Mycoses. 2016;59(12):773–780. doi: 10.1111/myc.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Global HIV & AIDS statistics — 2019 fact sheet. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 26 Sept 2019.
- 50.Wang ZD, Liu Q, Liu HH, et al. Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasit Vectors. 2018;11(1):28. doi: 10.1186/s13071-017-2558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bulterys PL, Le T, Quang VM, Nelson KE, Lloyd-Smith JO. Environmental predictors and incubation period of AIDS-associated penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis. 2013;56(9):1273–1279. doi: 10.1093/cid/cit058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Changhong Zeng TX, Li X, Liu C, Li J, Zeng L, Shen S. Investigation and analysis of HIV/AIDS complicated with fungal infection. South China J Prev Med. 2009;35(2):46–50. [Google Scholar]
- 53.Hu Y, Zhang J, Li X, Yang Y, Zhang Y, Ma J, Xi L. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175(1–2):57–67. doi: 10.1007/s11046-012-9577-0. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Lin Z, Shi X, Mo L, Li W, Mo W, Yang Z. Retrospective analysis of 15 cases of Penicillium marneffei infection in HIV-positive and HIV-negative patients. Microb Pathog. 2017;105:321–325. doi: 10.1016/j.micpath.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 55.Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clin Infect Dis. 2002;34(2):277–284. doi: 10.1086/338154. [DOI] [PubMed] [Google Scholar]
- 56.Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. Available from: https://www.who.int/hiv/pub/tuapr_2009_en.pdf?ua=1. Accessed 26 Sept 2019.
- 57.Antinori S, Gianelli E, Bonaccorso C, Ridolfo AL, Croce F, Sollima S, Parravicini C. Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. J Travel Med. 2006;13(3):181–188. doi: 10.1111/j.1708-8305.2006.00039.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1-S2 showing bias and quality assessment.
Additional file 2: Fig. S3 showing the funnel plot for publication bias.
Additional file 3: Table S1 showing the prevalence of TM infection in different provinces of China.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.