We read with great interest the study of Chen Y et al., who analyzed, during the Chinese epidemic peak, the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among 105 healthcare workers (HCWs) exposed to COVID-19 patients.1 They found 17.14% of seropositive asymptomatic or paucisymptomatic HCWs although their nasopharyngeal swab samples were SARS-CoV-2 RNA negative. Our purpose was to document at the end of the Belgium epidemic the seroprevalence of SARS-CoV-2 in HCWs exposed to COVID-19 at varying degrees and to compare these rates with those observed by other teams worldwide. Another objective was to highlight SARS-CoV-2 carriage in a priori healthy staff members to sensitize them to the need to respect individual protection measures and distancing to avoid patient contamination.
In Belgium, the COVID-19-outbreak peak was reached on 10 April 2020.2 At the end of May, when the epidemic spread was greatly slowed down, our management decided to offer screening tests to all staff members (n = 3145), regardless of their status and function. The campaign took place from May 25 to June 19, 2020 in the network of Iris hospitals South (HIS-IZZ, Brussels, Belgium), a 550-bed public hospital spread over 4 sites. Participation was voluntary and regardless of whether the HCW had already contracted the disease or not. A questionnaire was prepared focusing on the type of service the participant works in, the practice of medical procedures potentially at risk for SARS-CoV-2 infection, its status, function and perception of being infected or not. People with COVID-19 symptoms3 were excluded from routine screening.
On the same day, all asymptomatic HCWs who agreed to participate benefited from both serological and RT-qPCR SARS-CoV-2 tests. The quantitative analysis of IgG antibodies directed against the S1 and S2 subunits of the virus spike protein was carried out using the LIAISON®SARS-CoV-2 IgG kit (DiaSorin, Saluggia, Italy). This CLIA method was extensively evaluated in our laboratory and showed 100% sensitivity two weeks after positive qRT-PCR diagnosis using an adapted cut-off.4 Equivocal results were confirmed by a semi-quantitative ELISA method directed against the S1 subunit spike protein (Euroimmun Medizinische Labordiagnostika, Lübeck, Germany). HCWs with a previous COVID-19 documented history and a persistent positive RT-qPCR benefited from a viral culture. Statistical analyses were carried out using MedCalc version 10.4.0.0 (MedCalc Software, Ostend, Belgium). A P-value <0.05 is considered statistically significant.
During the study period, 1499 staff members participated (47.7%). Table 1 shows the participant characteristics. Among them, 215 workers (14.3%) reported having a function with no contact with patients while 1138 (75.9%) have had regular or occasional contact. This information was missing for 146 (9.7%). Among all workers, having had contact with patients, 838 had contacts with COVID patients: 205 worked in a COVID emergency department (of which 54.6% regularly), 399 worked in a COVID hospitalization unit (of which 54.9% regularly) and 234 worked in an intensive care unit (47.4% of them on a regular basis). Sixty-one performed bronchoscopies and 119 intubated patients (45.9% and 61.3% regularly). One hundred and eighty (12.0%) thought they were infected with SARS-CoV-2, 630 (42.0%) did not, 612 (40.8%) did not know and 77 (5.1%) did not answer.
Table 1.
. Population characteristics.
| Demography | |||
|---|---|---|---|
| Males (N = 414) | |||
| Age min Age max Age (median ; 95% CI) |
21.9 71.9 47.45 ; 46.00–48.84 |
||
| Females (N = 1085) | |||
| Age min Age max Age (median ; 95% CI) |
21.6 72.7 43.90 ; 42.80–45.10 |
||
| Type of occupation | N | % positive serology | |
|---|---|---|---|
| Nurse, Caregiver, Dietitian, Midwife, Occupational therapist, Psychologist, Social worker | 588 | 19.2% | |
| Medical doctor, Dentist, Physiotherapist, Logopedist | 323 | 11.8% | |
| Pharmacist, Administrative staff, IT | 320 | 9.1% | |
| Maintenance staff, Technical services | 134 | 16.4% | |
| Imaging technologist, Laboratory technologist | 61 | 6.6% | |
| Other | 17 | 11.0% | |
| Not specified | 56 | 19.6% |
| COVID-19 exposure (patient exposed personnel only) | N | % positive serology | |
|---|---|---|---|
| Workers at least once exposed to COVID-19 patients | 550 | 13.5% | |
| Workers never exposed to COVID-19 patients | 588 | 12.6% |
| Status of the workers | N | ||
|---|---|---|---|
| Employee | 1178 | ||
| Self-employed | 297 | ||
| Not specified | 24 | ||
| Fields of occupation | regular | intermittent | % positive serology |
|---|---|---|---|
| COVID inpatient unit | 219 | 180 | 24.3% |
| Intensive care unit | 111 | 123 | 13.2% |
| COVID emergencies | 112 | 93 | 14.6% |
| Non-COVID emergencies | 98 | 86 | 18.5% |
| Non-COVID inpatient unit/Other services with patient contact | 800 | 306 | 14.4% |
| Other services without patient contact | 250 | 67 | 10.5% |
| Not specified | 146 | 11.0% | |
| Procedures at risk | regular | intermittent | % positive serology |
|---|---|---|---|
| Bronchoscopies | 28 | 33 | 4.9% |
| Intubations | 73 | 46 | 9.2% |
| Other* | 174 | 39 | 17.4% |
Legend: "Regular" means being assigned to a care unit or to perform a technical act and "intermittent" means providing voluntary or on-demand assistance to work in a care unit or to perform a technical act.
Medical procedures potentially at risk of SARS-CoV-2 transmission: dental or stomatology care (N = 19); upper respiratory and digestive tract endoscopies (ENT fibroscopy, trans-esophageal ultrasound, gastroscopy) (N = 40); aerosol procedures (respiratory physiotherapy, aspirations, etc.) (N = 89); other procedures at risk (COVID smear, etc.) (N = 52).
A negative serology (<6.1 AU/mL) was observed in 1093 people, 206 were positive (>=15 AU/mL) and 200 were equivocal. Among the equivocal results re-tested with the second (ELISA) method, 11 were positive, 175 were negative and 14 remain undetermined. The overall seroprevalence reached 14.6% (217/1485). Seroprevalence was 53.9% in HCWs who thought they had the disease, 7.1% for those who did not and 9.6% for those who doubted.
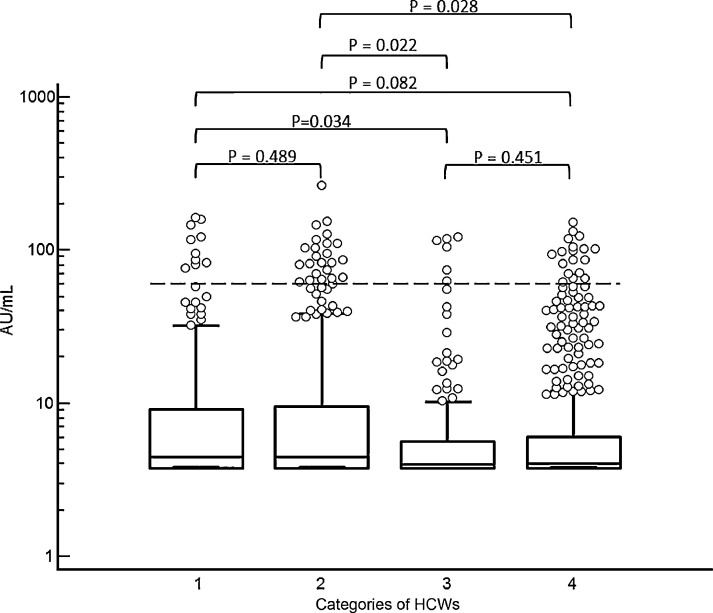
The median values [95% IC] of the UA/mL were significantly higher in HCWs who thought they had become ill (16.2 [10.6–24.2]) compared to others (4.1 [3.9–4.2]) (P<0.0001, Mann-Whitney U test). No difference was found between people with regular patient contact and those with none (P = 0.0522, Mann-Whitney U test). Seroprevalence was higher in HCWs in COVID units (COVID hospitalization units, COVID emergencies, intensive care) compared to the ones in non-COVID units: 13.5% versus 12.6% (P = 0.0007, Chi-square). Fig. 1 shows the quantitative results of serology in 4 HCW groups with various exposure levels.
Fig. 1.
Distribution of AU/mL in HCWs with varying degrees of exposure to SARS-CoV-2. 1: COVID units only; 2: Mixed COVID and non-COVID units; 3: No contact with patients; 4: Non-COVID units only. Dashline: cut-off of positivity. A P-value <0.05 is significant.
Among the 1499 samples sent for molecular diagnosis, 13 were positive, 1479 negative and 7 invalid. Amid the 13 HCWs, 5 had already a positive RT-qPCR result in the past. The median value of the delay between the first and the second RT-qPCR was 63 days (min-max: 50–67). Twelve people with positive RT-qPCR agreed to undergo a new sampling for viral-culture assessment. All came back negative meaning the RT-qPCR identified residual debris of viral RNA rather than living viruses. Among people with previously documented infection, 47 had positive serology, 5 had no antibodies and 2 were equivocal.
During the COVID-19 pandemic, several studies were conducted in HCWs, based on molecular and serological testing. Hunter et al. performed a massive PCR screening of UK healthcare personnel, which showed 14% positivity, but without serological documentation.4 Korth et al. reported low seroprevalence (1.6%) in 316 German HCWs.5 Our seroprevalence result of 14.6% is closer to that reported by Chen et al.1 To the best of our knowledge, seroprevalence in HCWs after the epidemic peak was never studied in as many participants. At the end of May, the Belgian Public Health Institute, Sciensano, assessed the seroprevalence of HCWs at 8.4% among 785 samples.6 The difference between our results and those of Sciensano can be explained by the outbreak evolution which led to seroprevalence increase.
Unexpectedly, our screening campaign failed to identify a single new case of COVID-19 among the participants. People positive to RT-qPCR were not living-virus carriers. This confirm that molecular methods can give positive results at a distance from a documented infection with an up to 67-day delay. Seroprevalence is higher than that documented by Sciensano during the epidemic peak and higher among HCWs who worked in COVID units. This shows that it is important to re-evaluate national seroprevalence in both the general population and HCWs at the end of the outbreak, especially as SARS-CoV-2 infection may be paucisymptomatic or asymptomatic and therefore infected people might ignore their status.
Ethical statement
The study design, the procedure of results communication, the information circular and the questionnaire have been submitted to and approved by our hospital's ethics committee (ethical agreement number: CEHIS/2020–19). An informed consent form has been requested from each participant, guaranteeing anonymity of the data and requesting permission to use them for statistical analysis. Out of respect for everyone's privacy, the participant was free to not answer to certain questions.
Declaration of Competing Interest
Authors state no conflict of interest.
Acknowledgments
The authors thank the general and medical managements of the Iris Hospital South for taking the lead on this massive screening; the Blood Sampling Centre, the technologists and administrative staff who contributed to the analytical, pre-analytical and post-analytical steps of the laboratory tests and all those who participated in this investigation. All molecular tests were supported by the federal COVID-19 platform.
References
- 1.Chen Y., Tong X., Wang J., Huang W., Yin S., Huang R., et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect 2020. 10.1016/j.jinf.2020.05.067 [DOI] [PMC free article] [PubMed]
- 2.Sciensano. COVID-19 Bulletin épidémiologique du 10 avril 2020.
- 3.Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020 doi: 10.1111/joim.13091. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter E., Price D.A., Murphy E., van der Loeff I.S., Baker K.F., Lendrem D. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020;395:e77–e78. doi: 10.1016/S0140-6736(20)30970-3. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desombere I., Mortgat L., Duysburgh E. COVID-19 study: 8,4% of Belgian health workers have antibodies to SARS-COV-2, Sciensano press release 26 May 2020.