Abstract
Dysbiosis, defined as an imbalance in the gut microbiota caused by too few beneficial bacteria and an overgrowth of bad bacteria, yeast, and/or parasites, is now being associated with several diseases, including the development of colorectal carcinoma (CRC). In this study, the potential association of Clostridioides difficile (formerly Clostridium difficile) with CRC was investigated. Plasma samples obtained from preoperative histologically confirmed CRC patients (n=39) and their age- and sex-matched clinically healthy controls (n=39) were analyzed for antibodies to toxin B of C. difficile (anti-tcdB) by enzyme-linked immunosorbent assay (ELISA). A significantly greater number (p=0.012) of CRC cases (n=26/39, 66.7%) had anti-tcdB IgG levels above the cutoff value compared with controls (n=12/39, 30.8%). Eight cases (8/39, 20.5%) and none of the controls registered anti-tcdB IgA levels above the cutoff value (p=0.0039). Anti-tcdB IgG and IgA levels were not shown to be significantly associated with tumor grade or tumor stage. Anti-tcdB IgG showed 66.7% sensitivity and 69.2% specificity. For anti-tcdB IgA, sensitivity and specificity were 20.5% and 100%, respectively. The positive predictive values for anti-tcdB IgA and IgG were 100% and 68.4%, respectively. The anti-tcdB IgA and IgG negative predictive values were 55.7% and 67.5%, respectively. The results suggest the potential association of C. difficile with CRC and anti-tcdB levels, particularly the IgA level. Hence, anti-tcdB antibodies can be candidate serologic markers for CRC.
Keywords: colorectal cancer, Clostridioides difficile, ELISA, dysbiosis, gut microbiome, tcdB, diagnostic performance
INTRODUCTION
Colorectal cancer (CRC) is one of the most prevalent cancers in the world [1]. Diet, particularly the consumption of poultry and animal products, has been linked to increased cases of CRC [2]. Imbalance in chromosome number, genomic amplifications in the subchromosomal region, and high frequency of heterozygous loss also lead to mutation and malignant transition of cells in the colon [3, 4].
In addition, the potential role of gut microbes in CRC development has been a hot topic lately. Several hypotheses have emerged on how these bacteria promote carcinogenesis. Dysbiosis and alterations in the normal microbial community remodel the whole microbiome, initiating inflammation and cell differentiation which could eventually lead to cancer [5]. The “driver-passenger” model proposes that some bacteria classified as “bacterial drivers” initiate the development of colonic tumors through gene damage, stimulating the colonization of passenger bacteria in the tumoral microenvironment [5, 6]. Some “keystone pathogens” that emerge during dysbiosis and are likely to be part of carcinogenesis include Bacteroides, Enterococcus, Fusobacterium, Streptococcus, Escherichia coli, and Clostridium [5, 7].
Clostridioides difficile (formerly Clostridium difficile) is a gram-positive, anaerobic, motile endospore-forming bacterium that is part of the normal gut microbiota and present in 2–5% of the adult population [8]. Few studies have been conducted on the potential role of C. difficile in the malignant transformation of cells in the colon [9,10,11,12]. Supposedly, the toxin B of C. difficile (tcdB) deregulates Rho-GTPases, leading to increased expression of proto-oncogenes [13]. Hence, this study was conducted to determine any association of C. difficile with CRC development among preoperative Filipino patients by analyzing their antibody levels against tcdB.
MATERIALS AND METHODS
This study was approved by the Institutional Review Boards of the University of Santo Tomas Hospital (USTH) in Manila (IRB-2016-11-191-IS-A1/CR2) and Mariano Marcos Memorial Hospital and Medical Center (MMMHMC) in Ilocos Norte (RERC-17-001). All participants gave their written informed consent.
Preoperative patients with histologically confirmed CRC (n=39) seen at USTH and MMMHMC between April 2018 and March 2019 were enrolled as cases. They were age (± 2 years) and sex-matched with physician-assessed clinically healthy controls (n=39) living in the same locality. All clinical data were retrieved from medical records. Blood was collected in EDTA tubes from all participants. Plasma was immediately separated by centrifugation at 2,500 RPM for 15 min and stored at −20°C until analysis.
Plasma samples were analyzed for anti-tcdB IgG and IgA using a commercial enzyme-linked immunosorbent assay (ELISA; tgcBiomics, Bingen am Rhein, Germany) according to the manufacturer’s protocol. Negative and positive controls provided by the manufacturer were run in parallel with the samples for each plate. Absorbance readings equal to or above the cutoff value (OD450-620=0.200) as set by the manufacturer were considered positive. Each sample was analyzed in duplicate, and ELISA was run twice to assess reproducibility of results.
Anti-tcdB IgG and IgA levels of CRC cases and matched clinically healthy controls were compared using paired t-test, and p<0.05 was considered significant. Logistic regression analyses followed by one-way ANOVA and two-sample t-test with equal variances were performed to determine any association of anti-tcdB IgG or IgA levels with tumor grade and tumor stage, respectively. Diagnostic performance (sensitivity, specificity, positive and negative predictive values) of the anti-tcdB ELISA was also computed. All statistical analyses were conducted using the Stata 14 software (StataCorp, College Station, TX, USA).
RESULTS
Clinical and serologic profiles of CRC cases and matched clinically healthy controls
There were more males (22/39, 56.4%) than females, and the mean age at initial diagnosis was 58 years old. Most of the cases had well (28.2%) or moderately (35.9%) differentiated tumors and were in an advanced stage (stage III or IV, 48.7%) at presentation (Table 1).
Table 1. Clinical characteristics of the cases.
| Characteristics | n=39 | % | |
|---|---|---|---|
| Sex | |||
| Male | 22 | 56.4 | |
| Female | 17 | 43.6 | |
| Age at initial diagnosis | |||
| <50 years | 11 | 28.2 | |
| ≥50 years | 28 | 71.8 | |
| Mean age at initial diagnosis (years ± SD) | 58 ± 12 | ||
| Median age at initial diagnosis (years ± SD) | 61 ± 12 | ||
| Tumor grade | |||
| G1 (well-differentiated) | 11 | 28.2 | |
| G2 (moderately-differentiated) | 14 | 35.9 | |
| G3 (poorly-differentiated) | 3 | 7.7 | |
| No information available | 11 | 28.2 | |
| Tumor stage | |||
| T1/2 (early) | 8 | 20.5 | |
| III/IV (advanced) | 19 | 48.7 | |
| No information available | 12 | 30.8 | |
| Tumor location | |||
| Colon | 17 | 43.6 | |
| Rectum | 17 | 43.6 | |
| Rectosigmoid | 3 | 7.7 | |
| Cecum | 1 | 2.6 | |
| Sigmoid | 1 | 2.6 | |
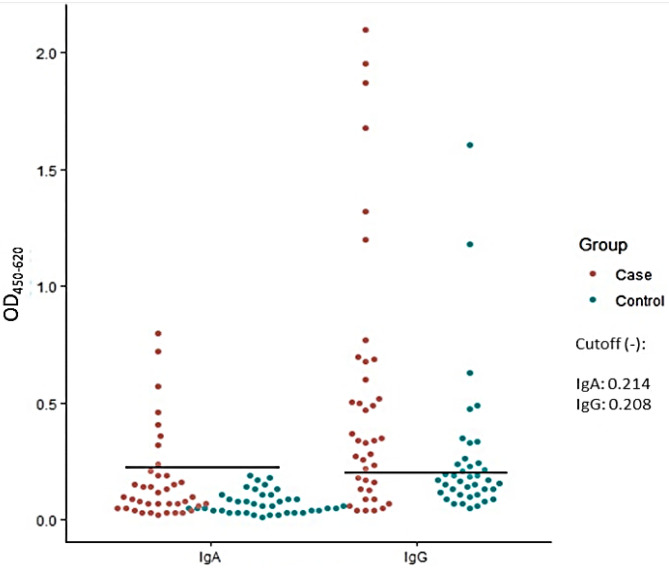
Significantly higher numbers of CRC cases had anti-tcdB IgG (p=0.012) and IgA (p=0.004) levels above the cutoff values compared with controls. Among the study participants, 26 (66.7%) cases and 12 (30.8%) controls tested positive for the anti-tcdB IgG. For IgA, only 8 (20.8%) of the cases and none of the controls were positive. Mean absorbances of the cases (IgG=0.520; IgA=0.171) were significantly higher (p=0.0041 for IgG; p=0.0051 for IgA) than those of the controls (IgG=0.260; IgA=0.073; Fig. 1).
Fig. 1.
Anti-tcdB IgG and IgA levels of CRC patients versus healthy controls. Significantly higher numbers of CRC cases had anti-tcdB IgG (p=0.012) and IgA (p=0.004) titers above the cutoff values compared with controls. The mean IgG absorbance of the cases (0.520) was significantly higher (p=0.0041) than that of the controls (0.260). The mean IgA absorbance of the cases (0.171) was also significantly higher (p=0.0051) than that of the controls (0.073).
Association of anti-tcdB IgG and IgA levels with tumor grade and stage
Mean anti-tcdB IgG and IgA levels of CRC cases with well-differentiated, moderately differentiated, and poorly differentiated tumors were not significantly different (IgG, p=0.5337; IgA, p=0.7506) from each other. Likewise, those diagnosed with early (I/II) stages of CRC had mean anti-tcdB IgG and IgA levels that were not significantly (IgG, p=0.4845; IgA, p=0.2522) different from those who presented at an advanced (III/IV) stage of the disease (Table 2).
Table 2. Association of tumor grade and tumor stage with anti-tcdB levels.
| Tumor grade | n=28 | Mean IgG | SD | p-value* | Mean IgA titer | SD | p-value* |
|---|---|---|---|---|---|---|---|
| Well-differentiated | 11 | 0.54 | 0.46 | 0.5337 | 0.21 | 0.23 | 0.7506 |
| Moderately differentiated | 14 | 0.63 | 0.64 | 0.19 | 0.22 | ||
| Poorly differentiated | 3 | 0.23 | 0.25 | 0.10 | 0.04 | ||
| Tumor stage | n=27 | Mean IgG | SD | p-value# | Mean IgA titer | SD | p-value# |
| Early stages (I/II) | 8 | 0.62 | 0.63 | 0.4845 | 0.22 | 0.27 | 0.2522 |
| Advanced stages (III/IV) | 19 | 0.46 | 0.48 | 0.13 | 0.11 | ||
SD: standard deviation. *One-way ANOVA. #Two-sample t-test with equal variances.
It should be noted that information was not available for the tumor stages and grades of 11 and 12 cases, respectively.
Diagnostic value of anti-tcdB IgG and IgA levels
The performance of the anti-tcdB ELISA in discriminating CRC was computed using the histologically confirmed CRC cases who tested positive for anti-tcdB IgG or IgA as true positives and the physician-assessed clinically healthy and malignancy-free volunteer controls who tested negative for anti-tcdB IgG or IgA as true negatives. Table 3 shows that anti-tcdB IgG is fairly sensitive (66.7%) and moderately specific (69.2%) for detection of CRC. Meanwhile, anti-tcdB IgA was very specific (100%) but showed very low sensitivity (20.5%). Since the positive predictive value for anti-tcdB IgA was 100%, a positive test could be equated with having CRC. Since the negative predictive value was 55.7%, a negative or normal test for anti-tcdB IgA would require other tests to confirm the results.
Table 3. Diagnostic performance of anti-tcdB levels in detecting colorectal carcinoma.
| Parameters* | IgG (%) | IgA (%) |
|---|---|---|
| Sensitivity | 66.7 | 20.5 |
| Specificity | 69.2 | 100.0 |
| Positive predictive value | 68.4 | 100.0 |
| Negative predictive value | 67.5 | 55.7 |
*At 95% confidence interval (CI).
DISCUSSION
The results of this study show that a significantly higher number of preoperative histologically confirmed CRC cases were positive for the anti-tcdB IgG and IgA antibodies compared with their age- and sex-matched clinically healthy controls. Based on the above observations, it can be inferred that there is a potential association of C. difficile with CRC among selected Filipino patients.
A high prevalence of C. difficile among preoperative CRC patients was previously reported in China [10]. It was also observed that a significantly higher quantity of Fusobacterium nucleatum and C. difficile were present in fecal samples of CRC patients compared with healthy controls in Brazil, suggesting that these bacteria may play a significant role in colon carcinogenesis [9]. Whole-genome sequencing of the gut microbiota further showed that Bacteroides, Fusobacterium, Streptococcus, and Clostridium were the most abundant genera in tumor versus normal samples [12]. Another study unexpectedly discovered that Clostridium, Fusobacterium, and Lactobacillus species were more abundant in the gut than Helicobacter pylori. The study also found that these bacteria demonstrated certain cancer-specific bacterial signatures [11].
Members of the human intestinal microbiota have been implicated in the development of CRC. Enterotoxigenic Bacteroides fragilis (ETBF) induces colonic tumors by triggering a Th17 inflammatory response [14] and activating signal transducer and activator of transcription 3 (STAT3) [15]. F. nucleatum is thought to promote inflammation and tumorigenesis by modulating the tumor immune microenvironment via expansion of myeloid-derived immune cells [16]. A study found intraepithelial E. coli in the tumors of CRC cases, specifically in the colonic mucosa [17]. The prevalence of E. coli in the colon could have induced chronic inflammatory responses [18], contributing to CRC development, as has been observed [19]. Streptococcus gallolyticus subsp. gallolyticus has also been demonstrated to promote malignant transformation of colon cells depending on cell context, bacterial growth phase, and direct contact between bacteria and colon cancer cells [20,21,22].
The potent toxin B enterotoxin of C. difficile (tcdB) has been proven to induce the inflammatory response that occurs in pseudomembranous colitis [23]. First, it binds to the receptors of the target-specific host cells when released into the environment. The toxin-receptor complex is endocytosed into the host cells, and the toxin is translocated into the cytosol, passing through the acidic endosomal membrane. As the active toxin moiety (glucosyltransferase) is released into the environment, it transfers glucose into the Rho proteases, preventing the normal binding of GTP to the GDP-bound form of Rho protein, thus making it inactive. Any deregulation of the activities of these proteins promotes pathogenic effects such as the disruption of epithelial integrity and opening of tight junctions [24]. Immune mechanisms such as vascular permeability, tumor necrosis α activation, and pro-inflammatory interleukin production are then stimulated, leading to chronic inflammation-induced colitis and inflammatory bowel disease, which are major risk factors for growth and proliferation of tumor cells in the colorectal tract [25,26,27].
C. difficile has been proven to proliferate during antibiotic-induced dysbiosis in the gut microenvironment [28,29,30,31]. Antibiotic treatments deplete the commensal bacteria in the gut, which are known to metabolize primary bile acids into secondary bile acids that, in turn, inhibit the proliferation of C. difficile. Hence, depletion of commensal bacteria leads to accumulation of primary bile acids, which serve as energy sources of C. difficile for growth and proliferation [32].
Records show that the Philippines has a history of prevalent antibiotics misuse [33] and self-medication [34]. Antibiotics are widely available in convenience stores, and Filipinos have been observed to often share them with family members, even those without prescriptions [33]. Moreover, agriculture, aquaculture, and horticulture businesses in the Philippines commonly incorporate antibiotics in their products [35]. Hence, a longitudinal study of long-term antibiotic use in patients answer questions related to antibiotic-induced C. difficile proliferation and CRC development.
While previous studies [9, 10] analyzed fecal samples by molecular techniques, the current study compared the anti-tcdB IgG and IgA levels of preoperative CRC patients with matched physician-assessed clinically healthy controls. The results show that the anti-tcdB IgA titer was more associated with CRC than the anti-tcdB IgG titer and hence was a more valuable serologic marker. The elevated anti-tcdB IgA levels may be attributed to the invasion of the mucosal barrier of the colon or rectum by C. difficile [36]. Meanwhile, the presence of this bacterium in 2–5% of the adult population [8] may explain why a number of clinically healthy cases also tested positive for anti-tcdB IgG.
Similarly, higher levels of anti-F. nucleatum (anti-Fn) IgA have been recorded in preoperative CRC patients compared with healthy controls [37]. In the current study, anti-tcdB IgG and IgA levels were not seen to be associated with the tumor stage or tumor grade. In comparison, anti-Fn IgG, but not IgA, levels were associated with the tumor grade [37]. But similar to the current study, tumor stage was not also associated with anti-Fn IgG or IgA levels [37]. Serologic markers have been useful in evaluating other infection-associated cancers, such as those caused by the human papillomavirus [38, 39], Epstein Barr virus [40], and H. pylori [41].
While the results show that a significantly greater number of preoperative CRC patients were positive for the anti-tcdB antibodies, it is not certain whether this organism directly or indirectly induced the malignant transformation of colon cells. It could be that the increase in anti-tcdB IgG and IgA levels was due to chronic infection with C. difficile or that the presence of malignancy weakened the beneficial members of the gut microbiota, thereby increasing the risk of C. difficile colonizing the large intestine. Whichever is the underlying reason, anti-tcdB antibodies have proven to be valuable in evaluating CRC.
This study contributes to the limited data on the high occurrence of C. difficile in CRC cases. However, it was only able to enroll a small number of preoperative CRC cases, since the majority of the patients from the study sites had already undergone surgery and other forms of treatment when the study was initiated. Future studies should include information on long-term antibiotics use and history of inflammatory bowel disease. Molecular analysis of tissues and fecal samples must also be done to confirm the results of the current study. Finally, assays for carcinoembryonic antigen (CEA) and CA 19-9 can be run in parallel with anti-tcdB IgG and IgA to determine if the sensitivity of the latter can be significantly improved without compromising the specificity, as has been observed with anti-Fn antibodies [37].
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
We thank Ms. Caren Joy Bacsid for assistance in the statistical analysis and the Department of Science and Technology—Science Education Institute for funding this study.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Kang M, Martin A. 2017. Microbiome and colorectal cancer: unraveling host-microbiota interactions in colitis-associated colorectal cancer development. Semin Immunol 32: 3–13. [DOI] [PubMed] [Google Scholar]
- 3.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. 2009. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. 2009. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457: 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. 2012. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol 10: 575–582. [DOI] [PubMed] [Google Scholar]
- 6.Sears CL, Pardoll DM. 2011. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J Infect Dis 203: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boleij A, Tjalsma H. 2012. Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer. Biol Rev Camb Philos Soc 87: 701–730. [DOI] [PubMed] [Google Scholar]
- 8.Hsu MS, Wang JT, Huang WK, Liu YC, Chang SC. 2006. Prevalence and clinical features of Clostridium difficile-associated diarrhea in a tertiary hospital in northern Taiwan. J Microbiol Immunol Infect 39: 242–248. [PubMed] [Google Scholar]
- 9.Fukugaiti MH, Ignacio A, Fernandes MR, Ribeiro Júnior U, Nakano V, Avila-Campos MJ. 2015. High occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Braz J Microbiol 46: 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, Luo Y, Lv Y, Huang C, Sheng Q, Zhao P, Ye J, Jiang W, Liu L, Song X, Tong Z, Chen W, Lin J, Tang YW, Jin D, Fang W. 2017. Clostridium difficile colonization in preoperative colorectal cancer patients. Oncotarget 8: 11877–11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh YY, Tung SY, Pan HY, Yen CW, Xu HW, Lin YJ, Deng YF, Hsu WT, Wu CS, Li C. 2018. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci Rep 8: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo M, Xu E, Ai D. 2019. Inferring bacterial infiltration in primary colorectal tumors from host whole genome sequencing data. Front Genet 10: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahai E, Marshall CJ. 2002. RHO-GTPases and cancer. Nat Rev Cancer 2: 133–142. [DOI] [PubMed] [Google Scholar]
- 14.Kostic M, Dzopalic T, Zivanovic S, Zivkovic N, Cvetanovic A, Stojanovic I, Vojinovic S, Marjanovic G, Savic V, Colic M. 2014. IL-17 and glutamate excitotoxicity in the pathogenesis of multiple sclerosis. Scand J Immunol 79: 181–186. [DOI] [PubMed] [Google Scholar]
- 15.Yu YN, Fang JY. 2015. Gut microbiota and colorectal cancer. Gastrointest Tumors 2: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keku TO, McCoy AN, Azcarate-Peril AM. 2013. Fusobacterium spp. and colorectal cancer: cause or consequence? Trends Microbiol 21: 506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. 2012. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338: 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan S. 2015. Potential role of Escherichia coli DNA mismatch repair proteins in colon cancer. Crit Rev Oncol Hematol 96: 475–482. [DOI] [PubMed] [Google Scholar]
- 19.Raisch J, Buc E, Bonnet M, Sauvanet P, Vazeille E, de Vallée A, Déchelotte P, Darcha C, Pezet D, Bonnet R, Bringer MA, Darfeuille-Michaud A. 2014. Colon cancer-associated B2 Escherichia coli colonize gut mucosa and promote cell proliferation. World J Gastroenterol 20: 6560–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdulamir AS, Hafidh RR, Abu Bakar F. 2011. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res 30: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt J, Romero-Hernández B, Pérez-Gómez B, Willhauck-Fleckenstein M, Holzinger D, Martin V, Moreno V, Linares C, Dierssen-Sotos T, Barricarte A, Tardón A, Altzibar JM, Moreno-Osset E, Franco F, Requena RO, Huerta JM, Michel A, Waterboer T, Castaño-Vinyals G, Kogevinas M, Pollán M, Boleij A, de Sanjosé S, Del Campo R, Tjalsma H, Aragonés N, Pawlita M. 2016. Association of Streptococcus gallolyticus subspecies gallolyticus with colorectal cancer: serological evidence. Int J Cancer 138: 1670–1679. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Herold JL, Schady D, Davis J, Kopetz S, Martinez-Moczygemba M, Murray BE, Han F, Li Y, Callaway E, Chapkin RS, Dashwood WM, Dashwood RH, Berry T, Mackenzie C, Xu Y. 2017. Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development. PLoS Pathog 13: e1006440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 18: 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aktories K, Schwan C, Jank T. 2017. Clostridium difficile toxin biology. Annu Rev Microbiol 71: 281–307. [DOI] [PubMed] [Google Scholar]
- 25.Kim ER, Chang DK. 2014. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol 20: 9872–9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triantafillidis JK, Nasioulas G, Kosmidis PA. 2009. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res 29: 2727–2737. [PubMed] [Google Scholar]
- 27.Park CH, Eun CS, Han DS. 2018. Intestinal microbiota, chronic inflammation, and colorectal cancer. Intest Res 16: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagier JC. 2016. Gut microbiota and Clostridium difficile infections. Hum Microbiome J 2: 10–14. [Google Scholar]
- 29.Milani C, Ticinesi A, Gerritsen J, Nouvenne A, Lugli GA, Mancabelli L, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Lauretani F, De Vos W, van Sinderen D, Meschi T, Ventura M. 2016. Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci Rep 6: 25945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burdet C, Sayah-Jeanne S, Nguyen TT, Hugon P, Sablier-Gallis F, Saint-Lu N, Corbel T, Ferreira S, Pulse M, Weiss W, Andremont A, Mentré F, de Gunzburg J. 2018. Antibiotic-induced dysbiosis predicts mortality in an animal model of Clostridium difficile Infection. Antimicrob Agents Chemother 62: e00925-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phalak P, Henson M. 2019. Metabolic modeling of Clostridium difficile associated dysbiosis of the gut microbiota. Processes (Basel) 7: 97. [Google Scholar]
- 32.Abt MC, McKenney PT, Pamer EG. 2016. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol 14: 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber DA, Casquejo E, Ybañez PL, Pinote MT, Casquejo L, Pinote LS, Estorgio M, Young AM. 2017. Prevalence and correlates of antibiotic sharing in the Philippines: antibiotic misconceptions and community-level access to non-medical sources of antibiotics. Trop Med Int Health 22: 567–575. [DOI] [PubMed] [Google Scholar]
- 34.Kim SA, Capeding MRZ, Kilgore PE. 2014. Factors influencing healthcare utilization among children with pneumonia in Muntinlupa city, the Philippines. Southeast Asian J Trop Med Public Health 45: 727–735. [PubMed] [Google Scholar]
- 35.Verraes C, Van Boxstael S, Van Meervenne E, Van Coillie E, Butaye P, Catry B, de Schaetzen MA, Van Huffel X, Imberechts H, Dierick K, Daube G, Saegerman C, De Block J, Dewulf J, Herman L. 2013. Antimicrobial resistance in the food chain: a review. Int J Environ Res Public Health 10: 2643–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Twigg HL., 3rd2005. Humoral immune defense (antibodies): recent advances. Proc Am Thorac Soc 2: 417–421. [DOI] [PubMed] [Google Scholar]
- 37.Wang HF, Li LF, Guo SH, Zeng QY, Ning F, Liu WL, Zhang G. 2016. Evaluation of antibody level against Fusobacterium nucleatum in the serological diagnosis of colorectal cancer. Sci Rep 6: 33440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro KB, Levi JE, Pawlita M, Koifman S, Matos E, Eluf-Neto J, Wunsch-Filho V, Curado MP, Shangina O, Zaridze D, Szeszenia-Dabrowska N, Lissowska J, Daudt A, Menezes A, Bencko V, Mates D, Fernandez L, Fabianova E, Gheit T, Tommasino M, Boffetta P, Brennan P, Waterboer T. 2011. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol 40: 489–502. [DOI] [PubMed] [Google Scholar]
- 39.Frazer IH. 2010. Measuring serum antibody to human papillomavirus following infection or vaccination. Gynecol Oncol 118 Suppl: S8–S11. [DOI] [PubMed] [Google Scholar]
- 40.Gulley ML. 2001. Molecular diagnosis of Epstein-Barr virus-related diseases. J Mol Diagn 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiota S, Matsunari O, Watada M, Yamaoka Y. 2010. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol 5: 1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]