Abstract
Certain strains of lactic acid bacteria (LAB) have beneficial effects on Japanese cedar pollinosis (JCPsis), which is a major concern in Japan. Heat-killed Lactobacillus plantarum YIT 0132 (LP0132), selected for its ability to induce interleukin (IL)-10, has been shown to suppress JCPsis symptoms. Lactobacillus casei Shirota (LcS), a popular probiotic, potentially induces a high level of IL-12 and is reported to delay the onset of JCPsis symptoms. However, it is unclear whether a combination of different types of LAB exerts additional effects without interfering with the benefits of each individual LAB. Thus, we conducted a pilot study to investigate the effects of LP0132-fermented citrus juice on JCPsis during simultaneous consumption of LcS-fermented milk. Fifty-nine subjects with JCPsis were allocated to two groups after a 2-week preconsumption period: one group consumed LP0132-fermented citrus juice and LcS-fermented milk (LcS+LP0132 group) for 12 weeks, while the other consumed LcS-fermented milk alone (LcS group). JCPsis symptoms, JCPsis-associated quality of life (QOL) impairment, and bowel movements were assessed by questionnaires. Compared with the LcS group, the LcS+LP0132 group showed significant alleviation of total symptoms and total ocular symptoms during the consumption period, as well as relief of impaired QOL. Bowel movements were significantly improved during the consumption period compared with the baseline in a combined analysis of all subjects in the two groups. In conclusion, LP0132-fermented citrus juice appears to have positive effects on some JCPsis symptoms and QOL in a population consuming immunomodulating probiotics such as LcS-fermented milk.
Keywords: Lactobacillus casei Shirota, QOL, seasonal allergic rhinitis, bowel movements
INTRODUCTION
Japanese cedar pollinosis (JCPsis), one of the most common forms of seasonal allergic rhinitis, is sometimes aggravated by Japanese cypress pollen (JCyP) and has become increasing common over the past few decades, with more than 30% of Japanese now affected [1]. Due to its severe symptoms and impact on quality of life (QOL), JCPsis is a major social problem in Japan. Although standard treatments such as antihistamines and topical steroids are available, concerns remain about their safety. Thus, alternative means such as functional foods are required.
Recently, attention has been focused on the beneficial effects of lactic acid bacteria (LAB) on human health resulting from multiple functions, including modulation of the host’s intestinal microbiota and cytokine profile [2], and the utilization of LAB in foods has been increasing. It is widely known that certain strains of LAB, such as Bifidobacterium and Lactobacillus, show anti-allergic activity [3]. Some strains stimulate interleukin (IL)-10 production to induce regulatory T (Treg) cells and suppress Th2 responses [4], whereas others stimulate IL-12 production to induce Th1 development and inhibit Th2 responses [5,6,7]. In several clinical trials, specific LAB were reported to have beneficial effects on JCPsis [8].
Lactobacillus plantarum YIT 0132 (LP0132) potentially induces a high level of IL-10 secretion in vitro, and previous clinical trials showed that LP0132-fermented citrus juice containing heat-killed LP0132 attenuated JCPsis and other allergic symptoms [9,10,11,12]. In contrast, Lactobacillus casei Shirota (LcS) has high IL-12 productivity in macrophages [13], and daily intake of fermented milk containing LcS delays the onset of JCPsis symptoms [14]. Although the two strains are considered to exert beneficial effects on JCPsis in different ways, it is unclear whether the combination of them would have additional effects. Moreover, fermented milk containing LcS is widely consumed as a probiotic drink to improve intestinal microbiota and bowel movements, as well as for its immunomodulatory effects [15, 16]. There is a concern that LP0132 may have some negative effects on the benefits of LcS when the two LABs are consumed together. Hence, with consumption of LcS-fermented milk as a base, we investigated the effects of LP0132-fermented citrus juice on JCPsis symptoms in this pilot study.
MATERIALS AND METHODS
Subjects
Enrollment was conducted from late January to early February of 2018. The enrolled subjects met the following inclusion criteria: men and women aged 20 to 65 years old with symptoms of JCPsis in the last pollen season. Subjects were excluded if they met any of the following exclusion criteria: 1) specific IgE for Japanese cedar pollen (JCP) <0.35 UA/mL in a blood test performed at the end of the preconsumption period; 2) maximum total symptom score (TSS) in the last season < 1; 3) citrus allergy; 4) pregnancy or breastfeeding. During the study, subjects were instructed to avoid intake of LAB products except for kimchi, pickles, and cheese and to refrain from excessive intake of Satsuma mandarin (Citrus unshiu) fruit or juice.
This study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethics committee of the Yakult Central Institute (Kunitachi, Tokyo, Japan). Written informed consent was obtained from all subjects prior to enrollment.
Test beverages
Fermented milk and fermented citrus juice were used as the test beverages. Fermented milk was prepared as in a previous report [15] and contained more than 4.0 × 1010 CFU of L. casei strain Shirota in an 80-ml bottle (LcS-fermented milk). Fermented Satsuma mandarin juice was prepared based on a previous report [11] and contained more than 6.0 × 1010 cells of heat-killed LP0132, as measured by the DAPI counting method, in a 125-ml bottle (LP0132-fermented juice). LcS-fermented milk was stored in a refrigerator, whereas LP0132-fermented juice was stored at room temperature.
Study protocol
The study was conducted at the Yakult Central Institute. An outline of the study is shown in Fig. 1. After a 2-week preconsumption period, eligible subjects were randomly assigned to two groups based on the allocation factors of sex, age, specific IgE level (JCP, JCyP, mite 1, and house dust 1), total IgE, eosinophil count, JCPsis symptoms and medication use in the last pollen season, bowel movements, and body mass index. One group took both the LP0132-fermented juice and the LcS-fermented milk (LcS+LP0132 group), while the other took the LcS-fermented milk alone (LcS group). They consumed the assigned beverages daily for 12 weeks after the 2-week preconsumption period, from March 1 to May 23, 2018. The subjects were asked to record daily medications and bowel movements throughout the study period, including during the preconsumption period. They also recorded a weekly assessment of their JCPsis symptoms, JCPsis-associated QOL impairment, and abdominal symptoms on a questionnaire. Blood samples were collected at the end of the preconsumption period and at the middle and end of the consumption period (weeks 4 and 12).
Fig. 1.
Study outline.
JCPsis symptoms, JCPsis-associated QOL impairment, and medication score
Subjects rated their JCPsis symptoms and JCPsis-associated QOL impairment on a 5-point scale from 0 (none) to 4 (extremely severe) based on the Japanese Rhinoconjunctivitis Quality of Life Questionnaire (JRQLQ No. 1) [17]. The 6 items for JCPsis symptoms and 17 items for JCPsis-associated QOL impairment are described in Tables 1 and 2. The TSS is the average score of the 6 items for JCPsis symptoms; the total nasal symptom score (TNSS) is the sum of the scores for sneezing, runny nose, blocked nose, and itchy nose; and the total ocular symptom score (TOSS) is the sum of the scores for itchy eyes and watery eyes. JCPsis symptoms were evaluated using each of the symptom scores: the TSS, TNSS, and TOSS. Items for JCPsis-associated QOL impairment comprised 6 categories, which were calculated as the average scores of the items in each category.
Table 1. Scoring system for each JCPsis symptom.
| Items | Score |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Sneezing1 | 0 | 1 to 5 | 6 to 10 | 11 to 20 | >21 |
| Runny nose2 | 0 | 1 to 5 | 6 to 10 | 11 to 20 | >21 |
| Blocked nose | None | Mild | Moderate | Severe | Extremely severe |
| Itchy nose | None | Mild | Moderate | Severe | Extremely severe |
| Itchy eyes | None | Mild | Moderate | Severe | Extremely severe |
| Watery eyes | None | Mild | Moderate | Severe | Extremely severe |
1Number of sneezes per day.
2Number of runny nose episodes per day.
Table 2. Scoring system for JCPsis-associated QOL impairment.
| Category | Items |
|---|---|
| Usual daily activities | Reduced productivity at work/home/school |
| Poor mental concentration | |
| Reduced thinking power | |
| Impaired reading book/paper | |
| Memory loss | |
| Outdoor activities | Limitation of outdoor life |
| Limitation of going out | |
| Social functioning | Hesitation visiting friends or relatives |
| Reduced contact with friends or others by telephone or conversation | |
| Difficult to interact with | |
| Sleep problem | Impaired sleeping |
| General physical problems | Tiredness |
| Fatigue | |
| Emotional function | Frustration |
| Irritability | |
| Depression | |
| Unhappiness | |
Each item is scored from 0 to 4 (0, none; 1, mild; 2, moderate; 3, severe, 4, extremely severe).
The medication score (MS) was assessed weekly as the average daily MS, calculated as the sum of the scores for each medicine used. The scores for each medicine were as follows: 1=antihistamine, histamine release inhibitor, leukotriene receptor antagonists; 2=topical corticosteroids; 3=systemic corticosteroids.
Bowel movements and abdominal symptoms
Bowel movements were assessed by the number of days with bowel movements per week. For abdominal symptoms, we assessed the frequency of abdominal pain and diarrhea per week as follows: none, a few days, or every day.
Blood parameters
Blood samples were collected three times via the brachial vein. The serum levels of total IgE and specific IgEs for JCP, JCyP, mite 1, and house dust 1 were determined by fluorescent enzyme immunoassay. The eosinophil count was determined by flow cytometry using an automated analyzer (XE-2100; Sysmex Corp., Ltd., Kobe, Japan). General serum biochemical parameters and hematological parameters were also determined. All analyses of blood parameters were outsourced to LSI Medience Corporation (Tokyo, Japan).
Outcomes
Primary outcomes were JCPsis symptom scores during the consumption period. Secondary outcomes included JCPsis-associated QOL impairment scores, MS, peripheral blood parameters, and bowel movements.
Statistical analysis
Symptom scores and QOL impairment scores during the consumption period were analyzed by two-way ANOVA. Each symptom and QOL impairment score at each time point was analyzed by the Mann-Whitney U test, whereas TSS, TNSS, TOSS, and categorized QOL impairment were analyzed by Student’s t-test. Blood data and bowel movements were compared between groups by Student’s t-test and within groups by the paired t-test with Bonferroni correction. The male to female ratio and the number of subjects responding to each option on the questionnaire were compared between groups by the χ2 test. Changes in bowel movements in the combined subjects of the two groups were analyzed using the paired t-test. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). All p values less than 0.05 were considered statistically significant.
RESULTS
Subjects and baseline characteristics
Sixty-six subjects with JCPsis were enrolled, and 7 were excluded based on the exclusion criteria: 6 who had specific IgE for JCP <0.35 UA/mL at the end of the preconsumption period and 1 who had a maximum TSS in the last season <1. The 59 eligible subjects were randomly assigned to either the LcS+LP0132 group (n=30) or the LcS group (n=29). Baseline characteristics and JCPsis symptoms in the last season were not significantly different between the two groups (Table 3). All subjects completed the study, and the consumption rate of the test beverages was over 80%.
Table 3. Baseline characteristics of the subjects.
| Items | LcS+LP0132 | LcS | p value | ||
|---|---|---|---|---|---|
| n=30 | n=29 | ||||
| At enrollment | |||||
| Sex, n | male:female | 13:17 | 12:17 | 0.879 | |
| Age, mean (SD) | year | 39.1 (9.2) | 40.6 (10.3) | 0.553 | |
| BMI, mean (SD) | kg/m2 | 21.3 (2.3) | 21.4 (2.7) | 0.800 | |
| Maximum TSS in the last pollen season, mean (SD) | 2.2 (0.5) | 2.2 (0.6) | 0.784 | ||
| At baseline | |||||
| Number of days with bowel movements, mean (SD) | days/week | 6.0 (1.4) | 5.9 (1.2) | 0.580 | |
| Frequency of abdominal pain per week, n | none | 26 | 25 | 1.000 | |
| a few times | 4 | 4 | |||
| Frequency of diarrhea per week, n | none | 29 | 25 | 0.195 | |
| a few times | 1 | 4 | |||
| Total IgE, mean (SD) | IU/mL | 359 (1,284) | 128 (118) | 0.333 | |
| Log-total IgE, mean (SD) | 4.1 (1.7) | 4.4 (1.0) | 0.376 | ||
| JCP-specific IgE, mean (SD) | UA/mL | 11.3 (13.9) | 12.8 (19.5) | 0.732 | |
| JCyP-specific IgE, mean (SD) | UA/mL | 1.5 (1.9) | 1.4 (1.8) | 0.788 | |
| House dust 1-specific IgE, mean (SD) | UA/mL | 8.6 (25.3) | 3.8 (9.9) | 0.341 | |
| Mite 1-specific IgE, mean (SD) | UA/mL | 9.1 (25.4) | 5.0 (13.6) | 0.451 | |
| Eosinophil count, mean (SD) | /μL | 201 (242) | 182 (121) | 0.704 | |
LcS+LP0132: Lactobacillus plantarum YIT 0132-fermented citrus juice and Lactobacillus casei Shirota-fermented milk consumption group; LcS: Lactobacillus casei Shirota-fermented milk consumption group; SD: standard deviation; BMI: body mass index; TSS: total symptom score; JCP: Japanese cedar pollen; JCyP: Japanese cypress pollen.
Number of subjects responding to each option on the questionnaire was analyzed by the χ2 test; all other data were analyzed by Student’s t-test.
Pollen dispersion
The Bureau of Social Welfare and Public Health in Tokyo reported that, in 2018, JCP and JCyP were dispersed in Tachikawa (a city adjacent to Kunitachi) from mid-February to late April and from mid-March to early May, respectively, and the peaks of these pollen seasons were the first half of March and late March to early April, respectively.
JCPsis symptoms, QOL impairment scores, and MS
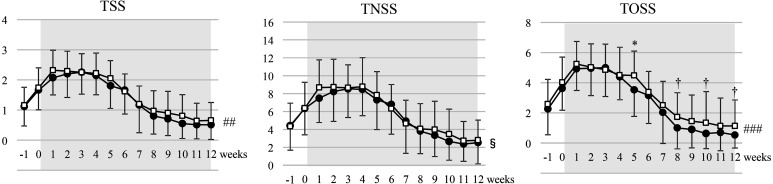
Regarding the primary outcomes, TSS and TOSS during the consumption period and TOSS at week 5 were significantly lower in the LcS+LP0132 group than in the LcS group, whereas TNSS was not different between the two groups (Fig. 2). Regarding the symptom scores, the LcS+LP0132 group had significantly lower scores for blocked nose, itchy nose, itchy eyes, and watery eyes during the consumption period (Fig. 3).
Fig. 2.
Changes in total symptom score (TSS), total nasal symptom score (TNSS), and total ocular symptom score (TOSS) during the study period.
Data are expressed as the mean ± SD. ● LcS+LP0132; □ LcS. Data were compared between groups at each time point by Student’s t-test (†p<0.10; *p<0.05) and between groups during the entire consumption period by two-way ANOVA (§p<0.10; ##p<0.01; ###p<0.001).
Fig. 3.
Changes in Japanese cedar pollinosis (JCPsis) symptom scores for sneezing, runny nose, blocked nose, itchy nose, itchy eyes, and watery eyes during the study period.
Data are expressed as the mean ± SD. ● LcS+LP0132; □ LcS. Data were compared between groups at each time point by Student’s t-test (†p<0.10; *p<0.05) and between groups during the entire consumption period by two-way ANOVA (#p<0.05; ##p<0.01).
QOL impairment scores for usual daily activities, outdoor activities, and emotional function during the consumption period and for usual daily activities at week 12 were significantly lower in the LcS+LP0132 group than in the LcS group (Fig. 4). MS was not significantly different between the two groups during the study period or at any time point (data not shown).
Fig. 4.
Changes in QOL impairment scores for usual daily activities, outdoor activities, social functioning, sleep problems, general physical problems, and emotional function during the study period.
Data are expressed as the mean ± SD. ● LcS+LP0132; □ LcS. Data were compared between groups at each time point by Student’s t-test (†p<0.10; *p<0.05) and between groups during the entire consumption period by two-way ANOVA (§p<0.10; ##p<0.01; ###p<0.001).
Blood parameters
No blood parameters tested were significantly different between the groups. Total IgE, JCP-specific IgE, and JCyP-specific IgE were significantly increased at weeks 4 and 12 of the consumption period compared with the preconsumption period (baseline) in the two groups (Table 4). The eosinophil count was also significantly increased at week 4 in both groups. No major adverse changes in biochemical and hematological test results were noted during the study period (data not shown).
Table 4. Changes in allergy-related blood parameters.
| Items | Group | Baseline |
Consumption period (12 weeks) |
|||||
|---|---|---|---|---|---|---|---|---|
| week –1 | week 4 | week 12 | ||||||
| Total IgE | IU/mL | LcS+LP0132 | n=30 | 359.3 (1,284.1) | 356.6 (1,156.3) | 408.2 (1,300.7) | # | |
| LcS | n=29 | 127.8 (117.6) | 153.3 (148.1) | # | 178.3 (170.7) | ## | ||
| Log-total IgE | IU/mL | LcS+LP0132 | n=30 | 4.1 (1.7) | 4.3 (1.6) | ### | 4.5 (1.5) | ### |
| LcS | n=29 | 4.4 (1.0) | 4.6 (1.0) | ## | 4.7 (1.0) | ### | ||
| JCP-specific IgE | UA/mL | LcS+LP0132 | n=30 | 11.3 (13.9) | 26.6 (27.1) | ### | 31.4 (30.5) | ### |
| LcS | n=29 | 12.8 (19.5) | 24.2 (25.7) | ### | 30.9 (27.3) | ### | ||
| JCyP-specific IgE | UA/mL | LcS+LP0132 | n=30 | 1.5 (1.9) | 2.1 (2.5) | ## | 4.2 (5.1) | ### |
| LcS | n=29 | 1.4 (1.8) | 1.7 (2.0) | # | 3.2 (3.3) | ### | ||
| House dust 1-specific IgE | UA/mL | LcS+LP0132 | n=30 | 8.6 (25.3) | 7.1 (19.8) | 7.2 (21.5) | ||
| LcS | n=29 | 3.8 (9.9) | 3.4 (8.3) | 3.2 (8.2) | ||||
| Mite 1-specific IgE | UA/mL | LcS+LP0132 | n=30 | 9.1 (25.4) | 8.3 (23.2) | 7.9 (22.4) | ||
| LcS | n=29 | 5.0 (13.6) | 4.4 (10.9) | 4.0 (10.4) | ||||
| Eosinophil count | /μL | LcS+LP0132 | n=30 | 201.0 (241.8) | 305.3 (286.4) | ### | 225.7 (295.6) | |
| LcS | n=29 | 182.1 (120.8) | 268.6 (182.5) | ## | 187.6 (95.6) | |||
LcS+LP0132: Lactobacillus plantarum YIT 0132-fermented citrus juice and Lactobacillus casei Shirota-fermented milk consumption group; LcS: Lactobacillus casei Shirota-fermented milk consumption group; IgE: immunoglobulin E; IU: international units; JCP: Japanese cedar pollen; JCyP: Japanese cypress pollen.
Values are shown as mean (standard deviation). Data were compared between baseline and week 4 and week 12 by the paired t-test with Bonferroni correction (#p<0.025; ##p<0.005, ###p<0.0005).
Bowel movements and abdominal symptoms
The number of days with bowel movements per week significantly increased during the consumption period compared with the baseline in the combined analysis of all subjects in the two groups (p=0.021; Fig. 5). Bowel movements showed an increasing trend in the LcS+LP0132 group (p=0.095) and the LcS group (p=0.116) but were not significantly different between the groups. The frequencies of abdominal pain and diarrhea were not significantly different between the groups (data not shown).
Fig. 5.
Changes in number of days with bowel movements per week.
Dark gray bar, baseline (week 0); black bar, during the consumption period (average of weeks 1 to 12). Data were analyzed by the paired t-test within groups (†p<0.10; *p<0.05).
DISCUSSION
In this pilot study, we investigated the effects of LP0132-fermented citrus juice on JCPsis with consumption of LcS-fermented milk in the pollen season. Both JCPsis symptoms and impaired QOL were alleviated in the LP0132-supplemented group. The results suggest that subjects with JCPsis symptoms who consumed LcS-fermented milk also received the same benefits from LP0132-fermented juice as those who did not consume LcS-fermented milk [9]. A previous study showed that ingestion of LcS-fermented milk by JCPsis patients delayed the occurrence of nasal symptoms [14]. Another placebo-controlled trial involving patients with pollen allergy in the United Kingdom showed that LcS consumption reduced the serum level of pollen-specific IgE and the IL-5 response in peripheral blood mononuclear cells [18]. Considering such effects of LcS, the benefits of LP0132-fermened juice over LcS-fermented milk observed in the present study might be add-on effects, though we did not evaluate the effects of LcS-fermented milk alone in this study.
Although the pollen season in 2018 had almost twice the level of JCP and JCyP as the average of the past 10 years, the results clearly showed that JCPsis symptoms and impaired QOL were alleviated in the LcS+LP0132 group compared with the LcS group. In particular, at week 5 of the consumption period, which is when JCyP dispersal peaked, the itchy eye score and several QOL impairment scores were significantly lower in the LcS+LP0132 group than in the LcS group (Fig. 6), suggesting that the effect of LP0132-fermented juice was not limited to pollinosis triggered by JCP but also included pollinosis triggered by JCyP.
Fig. 6.
JCPsis symptom scores and JCPsis-associated QOL impairment scores at week 5 of the consumption period.
● LcS+LP0132; □ LcS. Data were compared between groups by the Mann-Whitney U test (†p<0.10; *p<0.05).
Almost all allergy-related blood parameters examined in this study worsened during the test period in the two groups, but no significant differences were detected between the groups. The amount of pollen in the 2018 season might have been too large to assess the effects of LP0132-fermented juice on blood parameters, or the LP0132-fermented juice might have affected other blood parameters that were not measured in this study, such as cytokines and other mediators from mast cells and eosinophils. Moreover, consumption of LcS-fermented milk as a base might also complicate the detection of intergroup differences.
The number of bowel movements increased during the consumption period compared with the baseline in the combined analysis of the subjects in the two groups. Previous studies have shown that consumption of LcS-fermented milk improves bowel movements in subjects with a low defecation frequency [15]. Our results indicate that consumption of LcS-fermented milk has the potential to slightly increase the number of bowel movements in subjects with relatively good bowel function (average defecation frequency of 6 days per week) and that LP0132-fermented juice does not interfere with the benefits of LcS.
We conducted exploratory fecal microbiota analysis to examine the impact of LP0132-fermented citrus juice on the intestinal environment. Fecal samples were obtained from subjects who agreed to collect their feces in the preconsumption and consumption periods, and 16S rRNA gene amplicon sequencing analysis was performed as previously reported [19]. Although no significant difference was detected in the relative abundances of bacterial families between the groups, an increase in Lactobacillaceae was observed in both groups, indicating good adherence to sample intake (Supplementary Table 1). Bifidobacteriaceae increased significantly in the combined analysis of the two groups, which is consistent with previous results with LcS-fermented milk [15]. In addition, because some bacterial families tended to be increased in the LP0132 group, LP0132-fermented juice might be able to affect intestinal microbiota.
This study has some limitations. Self-evaluated items such as JCPsis symptoms and QOL impairment might have been affected by a placebo effect because the study was conducted as an open-label study. Because there was no control group free from beneficial probiotics, it was difficult to detect a difference when the LcS+LP0132 group was compared with the LcS group. In addition, another concern is whether the dose of LcS was optimal for achieving the greatest effect. We expect that LP0132-fermented juice would have more benefits in people consuming larger amounts of LcS-fermented milk because the mechanisms of the two bacteria are considered to be different. Moreover, LP0132-fermented juice contains not only heat-killed LP0132 but also several beneficial citrus juice-derived components, such as hesperidin and narirutin, which have anti-degranulation activities in mast cells and basophils [20, 21]. Thus, the beneficial effects of LP0132-fermented juice observed in this study might derive from citrus juice-derived flavonoids and their fermented products, in addition to LP0132. Further study with a blinded, placebo-controlled design is needed to confirm the effects of LP0132-fermented juice, especially LP0132, on JCPsis during probiotic consumption. In addition, the impact of LP0132-fermented juice on intestinal microbiota needs to be further elucidated.
In this pilot study, we demonstrated that LP0132-fermented citrus juice exerts positive effects on JCPsis symptoms and QOL impairment during consumption of LcS-fermented milk. The findings provide valuable information for people in modern society, many of whom regularly consume a variety of immunomodulating probiotics.
Supplementary
Acknowledgments
We would like to thank all the participants in this study. We would also like to acknowledge Kouji Miyazaki, Chiaki Kaga, and Erika Kotaki of the Yakult Central Institute for their helpful advice and technical support.
REFERENCES
- 1.Yamada T, Saito H, Fujieda S. 2014. Present state of Japanese cedar pollinosis: the national affliction. J Allergy Clin Immunol 133: 632–9.e5. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh T, Beniwal A, Semwal A, Navani NK. 2019. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front Microbiol 10: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajavi J, Esmaeili SA, Varasteh AR, Vazini H, Atabati H, Mardani F, Momtazi-Borojeni AA, Hashemi M, Sankian M, Sahebkar A. 2019. The immunomodulatory role of probiotics in allergy therapy. J Cell Physiol 234: 2386–2398. [DOI] [PubMed] [Google Scholar]
- 4.Dwivedi M, Kumar P, Laddha NC, Kemp EH. 2016. Induction of regulatory T cells: a role for probiotics and prebiotics to suppress autoimmunity. Autoimmun Rev 15: 379–392. [DOI] [PubMed] [Google Scholar]
- 5.Murosaki S, Yamamoto Y, Ito K, Inokuchi T, Kusaka H, Ikeda H, Yoshikai Y. 1998. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J Allergy Clin Immunol 102: 57–64. [DOI] [PubMed] [Google Scholar]
- 6.Shida K, Makino K, Morishita A, Takamizawa K, Hachimura S, Ametani A, Sato T, Kumagai Y, Habu S, Kaminogawa S. 1998. Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol 115: 278–287. [DOI] [PubMed] [Google Scholar]
- 7.Sagitani A. 2010. Anti-allergic effects of Lactobacillus acidophilus L-92 strain. Japanese J Lact Acid Bact 21: 207–213. [Google Scholar]
- 8.Güvenç IA, Muluk NB, Mutlu FŞ, Eşki E, Altıntoprak N, Oktemer T, Cingi C. 2016. Do probiotics have a role in the treatment of allergic rhinitis? A comprehensive systematic review and meta-analysis. Am J Rhinol Allergy 30: 157–175. [DOI] [PubMed] [Google Scholar]
- 9.Harima-Mizusawa N, Iino T, Onodera-Masuoka N, Kato-Nagaoka N, Kiyoshima-Shibata J, Gomi A, Shibahara-Sone H, Kano M, Shida K, Sakai M, Miyazaki K, Ishikawa F. 2014. Beneficial effects of citrus juice fermented with Lactobacillus plantarum YIT 0132 on Japanese cedar pollinosis. Biosci Microbiota Food Health 33: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harima-Mizusawa N, Kamachi K, Kano M, Nozaki D, Uetake T, Yokomizo Y, Nagino T, Tanaka A, Miyazaki K, Nakamura S. 2016. Beneficial effects of citrus juice fermented with Lactobacillus plantarum YIT 0132 on atopic dermatitis: results of daily intake by adult patients in two open trials. Biosci Microbiota Food Health 35: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harima-Mizusawa N, Kano M, Nozaki D, Nonaka C, Miyazaki K, Enomoto T. 2016. Citrus juice fermented with Lactobacillus plantarum YIT 0132 alleviates symptoms of perennial allergic rhinitis in a double-blind, placebo-controlled trial. Benef Microbes 7: 649–658. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Kubota N, Kakiyama S, Miyazaki K, Sato K, Harima-Mizusawa N. 2020. Effect of Lactobacillus plantarum YIT 0132 on Japanese cedar pollinosis and regulatory T cells in adults. Allergy 75: 453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shida K, Kiyoshima-Shibata J, Nagaoka M, Watanabe K, Nanno M. 2006. Induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J Dairy Sci 89: 3306–3317. [DOI] [PubMed] [Google Scholar]
- 14.Tamura M, Shikina T, Morihana T, Hayama M, Kajimoto O, Sakamoto A, Kajimoto Y, Watanabe O, Nonaka C, Shida K, Nanno M. 2007. Effects of probiotics on allergic rhinitis induced by Japanese cedar pollen: randomized double-blind, placebo-controlled clinical trial. Int Arch Allergy Immunol 143: 75–82. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Takada T, Shimizu K, Kado Y, Kawakami K, Makino I, Yamaoka Y, Hirano K, Nishimura A, Kajimoto O, Nomoto K. 2006. The effects of a probiotic milk product containing Lactobacillus casei strain Shirota on the defecation frequency and the intestinal microflora of sub-optimal health state volunteers: a randomized placebo-controlled cross-over study. Biosci Microflora 25: 39–48. [Google Scholar]
- 16.Miyazaki K, Matsuzaki T. 2008. Health properties of milk fermented with Lactobacillus casei strain Shirota (LcS). In Handbook of Fermented Functional Foods, Second Edition, Farnworth ER (ed), CRC Press, Boca Raton, pp. 165–208. [Google Scholar]
- 17.Okubo K, Kurono Y, Ichimura K, Enomoto T, Okamoto Y, Kawauchi H, Suzaki H, Fujieda S, Masuyama K, Japanese Society of Allergology.2017. Japanese guidelines for allergic rhinitis 2017. Allergol Int 66: 205–219. [DOI] [PubMed] [Google Scholar]
- 18.Ivory K, Chambers SJ, Pin C, Prieto E, Arqués JL, Nicoletti C. 2008. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin Exp Allergy 38: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 19.Nagino T, Kaga C, Kano M, Masuoka N, Anbe M, Moriyama K, Maruyama K, Nakamura S, Shida K, Miyazaki K. 2018. Effects of fermented soymilk with Lactobacillus casei Shirota on skin condition and the gut microbiota: a randomised clinical pilot trial. Benef Microbes 9: 209–218. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi S, Tanabe S. 2006. Evaluation of the anti-allergic activity of Citrus unshiu using rat basophilic leukemia RBL-2H3 cells as well as basophils of patients with seasonal allergic rhinitis to pollen. Int J Mol Med 17: 511–515. [PubMed] [Google Scholar]
- 21.Murata K, Takano S, Masuda M, Iinuma M, Matsuda H. 2013. Anti-degranulating activity in rat basophil leukemia RBL-2H3 cells of flavanone glycosides and their aglycones in citrus fruits. J Nat Med 67: 643–646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.