Abstract
Heavy metals are harmful to human health. Therefore, we investigated the biosorption of heavy metals by lactic acid bacteria (LAB). Of all the tested heavy metals, biosorption by LAB was highest for mercury, followed by lead, cadmium, and finally arsenic. The viability of HCT-116 cells was reduced by half in the presence of 7.5 µg/mL mercury but recovered after the addition of selected LAB strains. HCT-116 cells showed increased superoxide dismutase and catalase activities, whereas glutathione peroxidase activities decreased significantly. Addition of Lactobacillus sakei TOKAI 57m recovered all antioxidant enzyme activities. Our results suggest that this strain can be used for cellular detoxification.
Keywords: mercury, heavy metal, lactic acid bacteria, antioxidant enzyme
INTRODUCTION
Reactive oxygen species (ROS) such as superoxide anion radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen are highly reactive byproducts generated during the process of utilizing oxygen in aerobic organisms. ROS are used in the immune system to prevent bacterial and viral infections [1]. However, excessive ROS oxidize important biological components such as DNA, lipids, and proteins. This oxidative damage plays a major role in the acceleration of senescence and the development of various diseases, including lifestyle diseases such as cancer, diabetes, hypertension, and arteriosclerosis [2]. The living body has various mechanisms that remove ROS and prevent oxidative damage, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) processes. However, in modern times, it is difficult to completely eliminate excessive ROS due to environmental factors, such as ultraviolet rays, radiation, and air pollution, and lifestyle factors, such as smoking and stress, producing high levels of ROS. Heavy metals also induce cellular damage and oxidative stress, through ROS generation and inhibition of antioxidant enzymes [3].
Presently, heavy metal contamination is especially serious in developing countries [4]. Food contaminated by heavy metals may have detrimental effects on human and animal health, even at low concentrations because of gradual accumulation. Mercury (Hg), lead (Pb), cadmium (Cd), and arsenic (As) are specified by the Ministry of Agriculture, Forestry and Fisheries (MAFF) of Japan as harmful factors requiring risk management. Recently, reports have shown that lactic acid bacteria (LAB) are good candidate biosorbents for cadmium and lead and can protect cells, thereby decreasing the accumulation of heavy metals in the kidney and liver [5, 6]. With regard to mercury, reports have shown lactobacilli derived from humans, animal intestines, or dairy products to reduce mercury toxicity [7, 8], whereas there are only a few reports showing that LAB derived from plants can bind to mercury. Previous studies have shown that various species of LAB with different origins can strongly bind mercury [9, 10]. However, the species were selected based on biosorption ability for cadmium, not mercury, so the effect of reducing mercury toxicity has not been fully elucidated. In this study, the biosorption capacities of LAB for heavy metals were evaluated. Mercury-binding LAB were selected, and the protective effects on intestinal epithelial cells against oxidative stress caused by mercury were investigated.
MATERIALS AND METHODS
Bacterial strains and culture conditions
Ten LAB strains derived from plants were used in this study (Table 1). One strain of Weissellaviridescens TOKAI 205m (formerly MYU 205, designated as TK 205m in this study) derived from the bovine intestine (called horumon in Japan) was used as a positive control [9, 10] (Table 1). The LAB were pre-cultured twice at 37°C for 24 hr in MRS broth (Difco Laboratories, Detroit, MI, USA) using 2% (v/v) inoculum.
Table 1. Lactic acid bacteria strains used in this study.
| Strain number | Species | Isolated source |
|---|---|---|
| TOKAI 51m | Leuconostoc mesenteroides | Kimchi |
| TOKAI 57m | Lactobacillus sakei | Rice |
| TOKAI 60m | Leuconostoc mesenteroides | Kimchi |
| TOKAI 65m | Lactobacillus sakei | Kimchi |
| TOKAI 77m | Leuconostoc sp.* | Branch of apple |
| TOKAI 89m | Pediococcus pentosaceus | Picled celery |
| TOKAI 95m | Pediococcus pentosaceus | Bed of salted rice bran used for pickling |
| TOKAI 119m | Lactobacillus plantarum or Lactobacillus pentosus* | Dipped Japanese radish in soy sauce |
| TOKAI 758m | Pediococcus pentosaceus | Rice |
| TOKAI 759m | Pediococcus pentosaceus | Rice |
| TOKAI 205m | Weissella viridescens | Borine intestine (positive control) |
*Not fully identified.
Biosorption assay
Standard solutions (1,000 µg/mL) of Pb(II) (Pb (NO3)2 in 0.1 M HNO3; Wako Pure Chemical Industries, Ltd., Osaka, Japan), As(III) (As2O3 and sodium chloride (0.05%) in 1 M HCl solution; Kanto Chemical Co., Inc., Saitama, Japan), and Hg(II) (HgCl2 in 0.1 M HNO3; Kanto Chemical Co., Inc.) were prepared and diluted with 10 mM sodium citrate buffer (pH 7.0) to appropriate concentrations. Biosorption assays were performed as previously described [9, 10]. Briefly, cultured bacterial cells were washed three times with sterile distilled water. After washing, the pellets were suspended in 1 mL of 1 µg/mL metal solution adjusted to 1.5 × 108 cells/mL and incubated at 37°C for 1 hr. Following incubation, the bacterial cells were centrifuged (5,800 g, 5 min, 4°C), and the concentrations of metal in the supernatant were determined using an inductively coupled plasma mass spectrometer (ICP-MS; ELAN DRC-e, Perkin Elmer SCIEX, Boston, MA, USA).
Cytotoxicity test of mercury
The cytotoxicity of mercury was measured using HCT-116 cells (ATCC® CCL-247™, ATCC, Manassas, VA, USA) as a cell model of the intestine. HCT-116 cells were cultured in McCoy’s 5A culture medium (ATCC) with 10% fetal bovine serum (FBS, Gibco, Burlington, ON, Canada) and penicillin-streptomycin (Gibco) at 37°C under 5% CO2 conditions. For measurement of cytotoxicity, 1 mL of 5 × 105 HCT-116 cells were added to wells of a 12-well plate (Tissue Culture Test Plate 12, TPP, Trasadingen, Switzerland) and incubated at 37°C under 5% CO2 for 24 hr. Cells were washed twice with PBS (pH 7.4), and 1 mL of culture media with Hg(II) solution was added to the wells at final concentrations of 1, 2.5, 5, 7.5, 10, 50, and 100 µg/mL, respectively, before culturing at 37°C with 5% CO2 for 24 hr. As a control, 0.5 mM nitric acid solution was used. The survival rate was expressed as a percentage of the number of viable cells in 0.5 mM of nitric acid solution (set to 100%). After treatment, cells were washed twice with PBS, and the number of viable cells was counted by trypan blue staining.
Cytotoxicity suppression test for mercury using LAB
To suppress the cytotoxicity of mercury using LAB, heat-killed LAB (100°C, 30 min) were used because living cells produce lactic acid that may decrease the pH of the culture medium. HCT-116 cells were washed twice with PBS (pH 7.4), to which 1.5 × 108 cells/mL of heat-killed LAB and Hg(II) solution (final concentrations of 7.5 or 10 µg/mL) were added, and cultured at 37°C under 5% CO2 for 24 hr. As a control, nitic acid (0.5 mM) was added to 1.5 × 108 cells/mL of heat-killed TK 57m. The survival rate was expressed as a percentage of the number of viable cells in 0.5 mM nitric acid solution (set to 100%).
Measurement of antioxidant enzyme activities
For measurement of antioxidant enzyme activities, cell extracts were prepared from 9 × 106 HCT-116 cells after 24 hr of cultivation with 0.5 mM nitric acid (control), 5 µg/mL of mercury (did not affect cell viability), or 5 µg/mL of mercury plus the heat-killed TK 57 m strain (LAB treatment). SOD activity was measured using SOD Assay Kit-WST (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s protocol. One unit of SOD was defined as the amount of the enzyme in 20 µL of sample solution that inhibits the reduction reaction of WST-1 with superoxide anion by 50%. CAT activity was assessed using the method of Luck [11], with the breakdown of hydrogen peroxide (H2O2) measured at 240 nm. One unit of CAT will decompose 1 µmol of H2O2 per min at pH 7.6 at room temperature. GPx activity was measured according to the method of Paglia and Valentine [12]. One unit was defined as the amount of GPx that produces 1 µmol of glutathione disulfide (GSSG) per min at pH 7.6 at room temperature. All enzyme activities were normalized to total protein (mg).
Statistical analyses
The assays were performed in triplicate or more. The data are expressed as means ± standard deviation (SD). Multiple comparison tests were performed using Tukey honest significant difference (HSD) tests. IBM SPSS Statistics ver. 22 was used for the statistical analysis (IBM Corp., Armonk, NY, USA).
RESULTS AND DISCUSSION
Heavy metals cause various health hazards, with food and water contaminated with heavy metals potentially having detrimental effects on human and animal health, even at low concentrations because of gradual accumulation. Therefore, we examined the biosorption ability of plant-derived LAB for heavy metals and performed a mercury cytotoxicity suppression test using selected LAB.
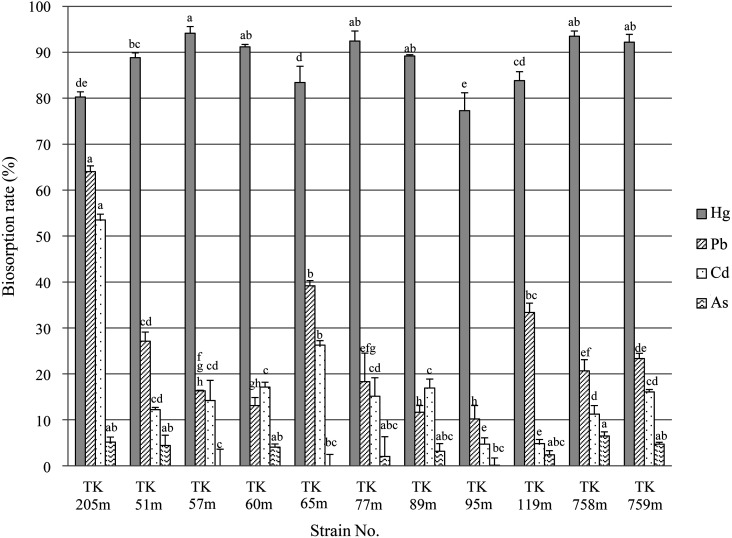
Figure 1 illustrates the heavy metal biosorption assay for LAB. Of all the tested heavy metals, biosorption by LAB was highest for mercury, followed by lead, cadmium, and finally arsenic. These results agree with previous findings that LAB strongly bind to mercury rather than lead, cadmium, and arsenic [9, 10]. The average mercury biosorption rate was 87.8 ± 1.7%. Lactobacillussakei TOKAI 57m (TK 57m) showed the highest biosorption rate at 94.1 ± 0.8%. The lowest biosorption rate, 77.3 ± 2%, was for Pediococcus pentosaceus TOKAI 95m (TK 95m). In previous studies, Lb. sakei TOKAI 10m (formerly MYU 10) isolated from Japanese takuan pickles [9] and the TK 205m strain used as a positive control [10] showed 99.1% and 90.0% biosorption in 1 µg/mL of Hg(II), respectively. In this study, many strains showed high biosorption of mercury, the rates of which were equal to or greater than those of these strains.
Fig. 1.
Biosorption assays for heavy metals with various lactic acid bacteria (LAB).
LAB strains (1.5 × 108 cells/mL) were suspended in 1 µg/mL of the metal solutions. The bacterial suspensions were incubated at 37°C for 1 hr. After incubation, the bacterial cells were removed, and the heavy metal concentrations were measured using ICP-MS. Differences in mean values were assessed using the Tukey honest significant difference (HSD) multiple comparison procedure. Different letters indicate significant differences for each group (p<0.05).
A previous study showed that 1 µg/mL of Cd(II), Pd(II), or As(III) does not affect the growth of LAB, but growth delay was observed with Hg(II) addition [9]. However, the live or dead status of LAB does not affect the biosorption ability for mercury, with mercury binding immediately to LAB after addition [10]. This strongly suggested that mercury bound to the bacterial cell surface. In fact, mercury-binding proteins were found on the bacterial cell surface in TK 205m strains [9], and a crystal structure analysis showed that an SH group of cysteine (Cys) of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) localized on the bacterial cell surface has a covalent bond with mercury [13]. The expression levels of proteins on the bacterial cell surface, including GAPDH, are different among bacterial strains [9]. Thus, LAB strains showing high binding to mercury might have many cell-surface proteins, including Cys. Jadán-Piedra et al. [14] reported that adding bovine serum albumin (BSA) and Cys to lactobacilli prevented the biosorption of mercury by lactobacilli, suggesting the formation of mercury–Cys complexes. On the other hand, Alcántara et al. [7] reported that biosorption of lactobacilli was not affected by chelating agents or protein digestion treatment and that lipoteichoic acid itself or the physicochemical characteristics of the cell wall play a major role in mercury complexation. The finding that mercury binds to the bacterial cell surface even of dead cells supports the usefulness of bacteria as potential biosorbents in removal of mercury from the human body.
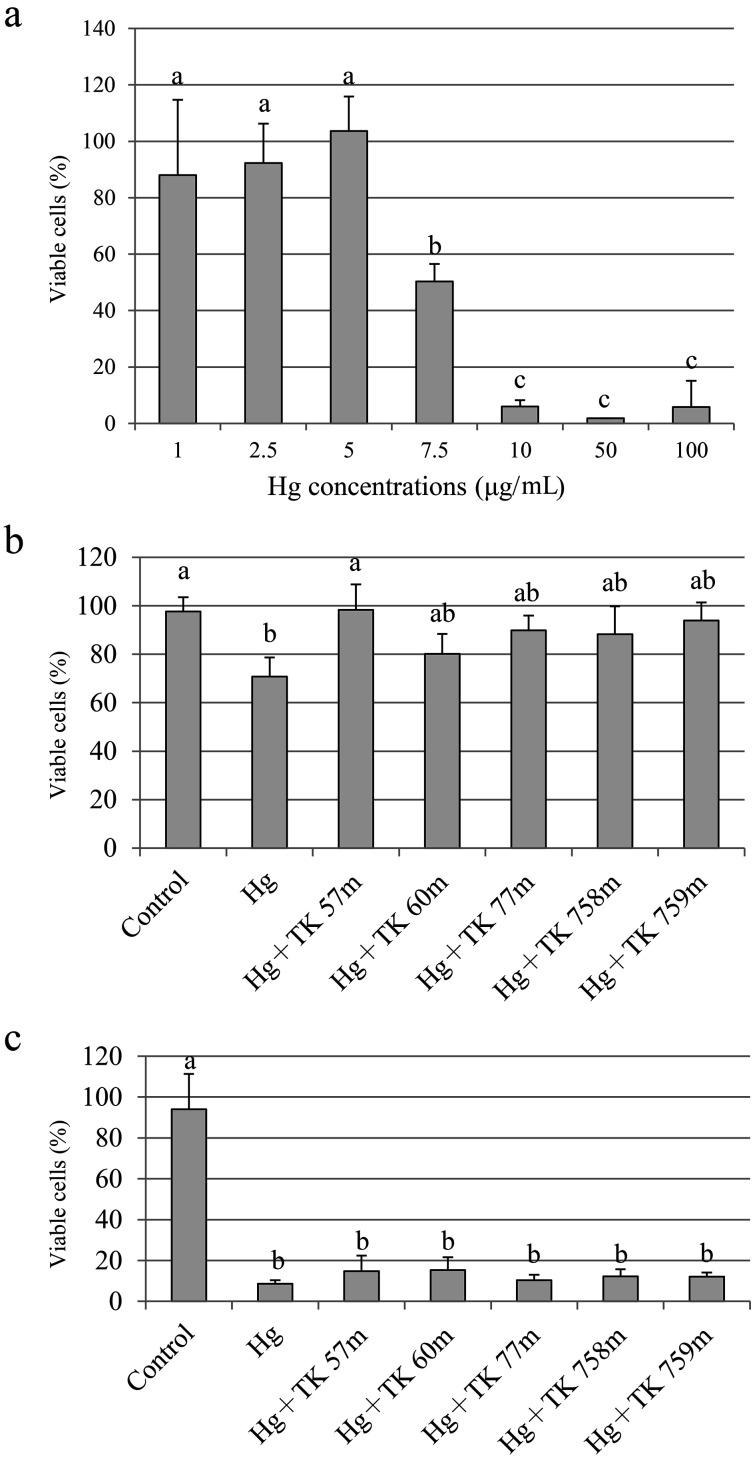
Subsequent studies focused on suppressing the cytotoxicity of mercury, and the top five strains that showed high biosorption to mercury were selected. Figure 2a shows the results for mercury cytotoxicity. Cells remained viable until 5 µg/mL of mercury addition. Addition of 7.5 µg/mL mercury decreased viability to 50%. Treatment with more than 10 µg/mL mercury resulted in almost all the cells dying. A cytotoxicity suppression test was then performed for mercury using LAB. When treated with 7.5 µg/mL of mercury, cell viability recovered after the addition of selected LAB (Fig. 2b), whereas no change was observed when cells were treated with 10 µg/mL of mercury (Fig. 2c). The concentration of mercury remaining in the culture medium was calculated to be 9.1 µg/mL in cells treated with 10 µg/mL mercury if the LAB showed 90% biosorption in 1 µg/mL mercury solution. These results suggest that the biosorption capacity of LAB is not sufficient at 10 µg/mL of mercury. Therefore, if the concentration of LAB is higher than 1.5 × 108 cells/mL, a cytoprotective effect may be observed.
Fig. 2.
Cytotoxicity of mercury (a) and suppression effects on the cytotoxicity of mercury using heat-killed LAB (b, 7.5 µg/mL mercury; c, 10 µg/mL mercury).
One milliliter of 5 × 105 HCT-116 cells was added to a 12-well plate and incubated at 37°C under 5% CO2 for 24 hr. Cells were washed twice with PBS (pH 7.4), (a) 1 mL of culture media with 1 to 100 µg/mL of Hg(II) solution, (b) 1.5 × 108 cells/mL of heat-killed LAB and Hg(II) solution at a final concentration of 7.5 µg/mL, or (c) 1.5 × 108 cells/mL of heat-killed LAB and Hg(II) solution at a final concentration of 10 µg/mL was added, and the cells were then cultured at 37°C under 5% CO2 for 24 hr. Nitric acid (final concentration of 0.5 mM) was used as a control. After culture, the cells were washed twice with PBS, and the number of viable cells was counted by trypan blue staining.
For the test of suppression by LAB, nitric acid (final concentration of 0.5 mM) along with 1.5 × 108 cells/mL of heat-killed TK 57 m was used as a control. The survival rate was expressed as a percentage of the number of viable cells in 0.5 mM nitric acid solution (set to 100%). Differences in mean values were assessed using the Tukey HSD multiple comparison procedure. Different letters indicate significant differences (p<0.05).
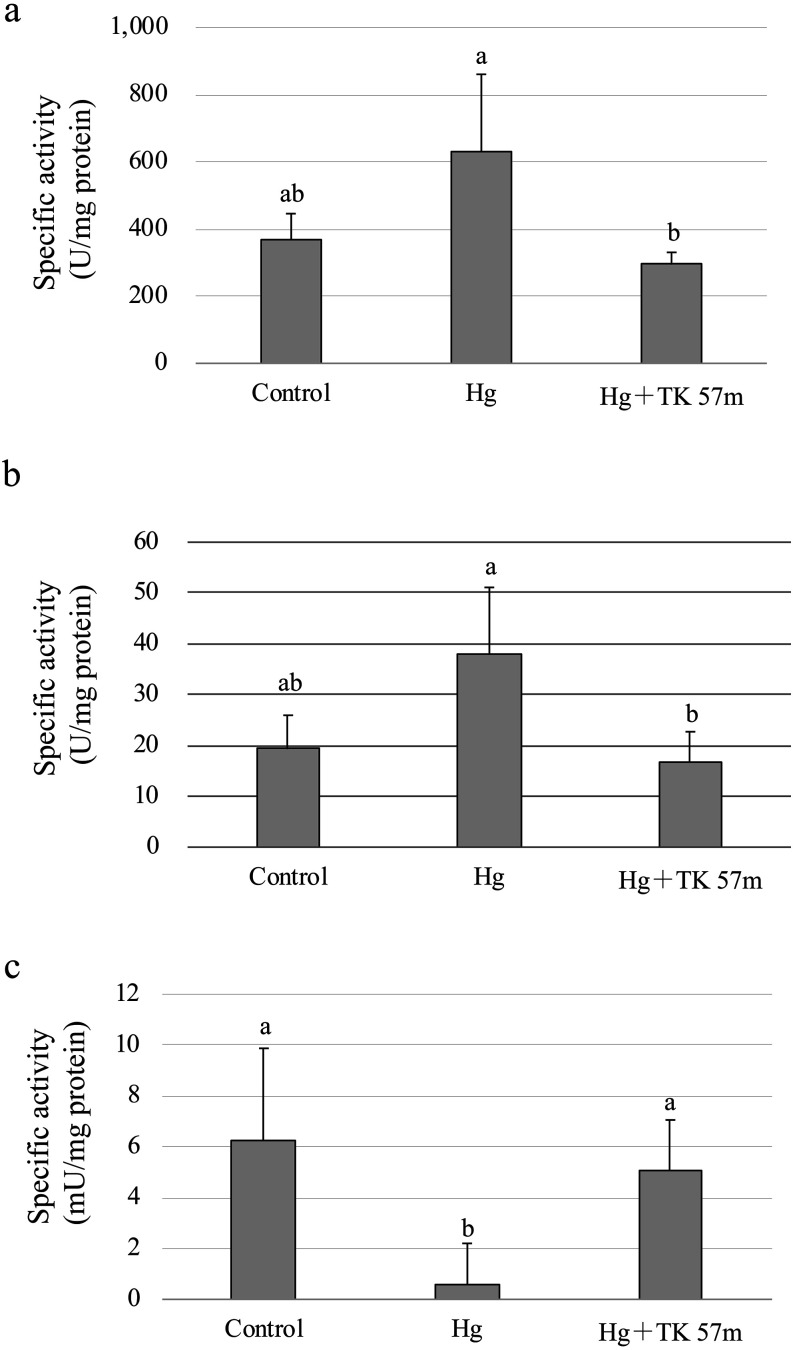
Finally, the antioxidant enzyme activities were measured. The SOD activity following the control treatment was 368.24 ± 77.57 U/mg protein, and this increased to 629.97 ± 203.88 U/mg protein after mercury treatment. LAB treatment decreased the SOD activity to levels similar to the control (294.09 ± 36.40 U/mg protein; Fig. 3a). CAT showed similar trends as for SOD activity (Fig. 3b); the activities were 19.29 ± 6.66 U/mg protein after the control treatment, 16.60 ± 6.12 U/mg protein after the LAB treatment, and 37.89 ± 13.20 U/mg protein after the mercury treatment. These activities obtained in this study were comparable to those in previous reports [15, 16]. The reason for the increased SOD and CAT activities noted after mercury treatment may be a biological defense against oxidative stress caused by mercury. GPx activity after mercury treatment (0.58 ± 1.62 mU/mg protein) was significantly decreased compared with the control (6.23 ± 3.65 mU/mg protein; p<0.01; Fig. 3c).
Fig. 3.
Effect of mercury on antioxidant enzyme activities.
After washing 9 × 106 cells of HCT-116 cells twice with PBS, a culture medium and the mercury solution with a final concentration of 5 µg/mL were added with or without 1.5 × 108 cells/mL of heat-killed L. sakei TOKAI 57m, and the cells were then cultured under the same conditions for 24 hr. Nitric acid (final concentration of 0.5 mM) was added to the control instead of mercury. After washing with PBS twice, the cells were collected. One milliliter of the enzyme extract solution was added to the cells, and the mixture was mixed by pipetting and incubated for 30 min with shaking on ice. After centrifugation, the supernatant was used as the sample. SOD (a), CAT (b), and GPx (c) activities of extracted samples were measured. Differences in mean values were assessed using the Tukey HSD multiple comparison procedure. Different letters indicate significant differences (p<0.05).
Ample evidence suggests that selenoproteins are important targets of Hg toxicity. It has been demonstrated in different experimental models that mercury can inhibit the activity of GPx [17]. Moreover, mercury binds to available intracellular selenium (Se) to form an insoluble Hg–Se, methylmercury–Se, or Hg–Se–Cys complex. This produces an intracellular Se deficiency state and reduces the availability of Se for de novo thioredoxin reductase and GPx production, thereby further inhibiting restoration of thioredoxin and glutaredoxin systems [18]. In the LAB treatment, GPx activity recovered to 5.04 ± 2.02 mU/mg protein, which was similar to the control levels, as in the case of SOD and CAT. It is thought that mercury incorporation into cells may decrease because LAB bind to mercury.
Our findings show that the TK 57m strain possesses a high biosorption ability for mercury and can protect against the cytotoxicity of mercury and suppress oxidative stress in HCT-116 cells. Jiang et al. [19] reported the protective effects of Lactobacillus brevis 23017 against acute mercury toxicity in mice. Moreover, this strain blocked oxidative stress and inflammation through the MAPK and NF-kB pathways. Majlesi et al. [20] conducted an in vivo experiment using Lactobacillus plantarum and Bacillus coagulans to alleviate mercury toxicity in rats. Their results showed that either probiotic provided significant protection against mercury toxicity by decreasing the mercury level in the liver and kidney and preventing alterations in the levels of GPx and SOD. Jadán-Piedra et al. [8] reported that oral administration of Hg(II)- and MeHg-binding lactobacilli significantly decreased of the bioavailability of MeHg in both strains and increased excretion of Hg in mouse feces, while Hg(II) bioavailability or excretion and both Hg accumulation in the liver and kidney were not affected. These findings suggest that biosorbents using the TK 57m strain showing high affinity to mercury can be used as efficient cellular detoxification tools. Future in vivo experiments are needed. In Japan, the risk of exposure to high concentrations of mercury as used in this study is low, whereas in developing countries, exposure to high concentrations of mercury around gold mines [21] and around the Amazon [17] is a serious problem. Therefore, LAB may be useful in reducing the health risk caused by mercury in these areas.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Schwarz KB. 1996. Oxidative stress during viral infection: a review. Free Radic Biol Med 21: 641–649. [DOI] [PubMed] [Google Scholar]
- 2.Waris G, Ahsan H. 2006. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero A, Ramos E, de Los Ríos C, Egea J, Del Pino J, Reiter RJ. 2014. A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res 56: 343–370. [DOI] [PubMed] [Google Scholar]
- 4.Orisakwe OE. 2014. Lead and cadmium in public health in Nigeria: physicians neglect and pitfall in patient management. N Am J Med Sci 6: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian F, Zhai Q, Zhao J, Liu X, Wang G, Zhang H, Zhang H, Chen W. 2012. Lactobacillus plantarum CCFM8661 alleviates lead toxicity in mice. Biol Trace Elem Res 150: 264–271. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Q, Wang G, Zhao J, Liu X, Tian F, Zhang H, Chen W. 2013. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl Environ Microbiol 79: 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcántara C, Jadán-Piedra C, Vélez D, Devesa V, Zúñiga M, Monedero V. 2017. Characterization of the binding capacity of mercurial species in Lactobacillus strains. J Sci Food Agric 97: 5107–5113. [DOI] [PubMed] [Google Scholar]
- 8.Jadán-Piedra C, Crespo Á, Monedero V, Vélez D, Devesa V, Zúñiga M. 2019. Effect of lactic acid bacteria on mercury toxicokinetics. Food Chem Toxicol 128: 147–153. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita H, Sohma Y, Ohtake F, Ishida M, Kawai Y, Kitazawa H, Saito T, Kimura K. 2013. Biosorption of heavy metals by lactic acid bacteria and identification of mercury binding protein. Res Microbiol 164: 701–709. [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita H, Ohtake F, Ariga Y, Kimura K. 2016. Comparison and characterization of biosorption by Weissella viridescens MYU 205 of periodic group 12 metal ions. Anim Sci J 87: 271–276. [DOI] [PubMed] [Google Scholar]
- 11.Luck H. 1971. “Catalase,” in methods of enzymatic analysis, Academic Press, New York. [Google Scholar]
- 12.Paglia DE, Valentine WN. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158–169. [PubMed] [Google Scholar]
- 13.Yoneda K, Ogata M, Nishiyama K, Fukuda K, Yasuda S, Igoshi K, Kinoshita H. 2019. Crystal structure of cell surface glyceraldehyde-3-phosphate dehydrogenase from Lactobacillus plantarum: insight into the mercury binding mechanism. Milk Science 68: 3–11. [Google Scholar]
- 14.Jadán-Piedra C, Alcántara C, Monedero V, Zúñiga M, Vélez D, Devesa V. 2017. The use of lactic acid bacteria to reduce mercury bioaccessibility. Food Chem 228: 158–166. [DOI] [PubMed] [Google Scholar]
- 15.Ben Salem I, Boussabbeh M, Kantaoui H, Bacha H, Abid-Essefi S. 2016. Crocin, the main active saffron constituent, mitigates dichlorvos-induced oxidative stress and apoptosis in HCT-116 cells. Biomed Pharmacother 82: 65–71. [DOI] [PubMed] [Google Scholar]
- 16.Buldak RJ, Gowarzewski M, Buldak L, Skonieczna M, Kukla M, Polaniak R, Zwirska-Korczala K. 2015. Viability and oxidative response of human colorectal HCT-116 cancer cells treated with visfatin/eNampt in vitro. J Physiol Pharmacol 66: 557–566. [PubMed] [Google Scholar]
- 17.da Conceição Nascimento Pinheiro M, do Nascimento JLM, de Lima Silveira LC, da Rocha JBT, Aschner M. 2009. Mercury and selenium—a review on aspects related to the health of human populations in the Amazon. Environ Bioindic 4: 222–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiller HA. 2018. Rethinking mercury: the role of selenium in the pathophysiology of mercury toxicity. Clin Toxicol (Phila) 56: 313–326. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X, Gu S, Liu D, Zhao L, Xia S, He X, Chen H, Ge J. 2018. Lactobacillus brevis 23017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-kappa B signaling cascades. Front Microbiol 9: 2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majlesi M, Shekarforoush SS, Ghaisari HR, Nazifi S, Sajedianfard J, Eskandari MH. 2017. Effect of probiotic Bacillus coagulans and Lactobacillus plantarum on alleviation of mercury toxicity in rat. Probiotics Antimicrob Proteins 9: 300–309. [DOI] [PubMed] [Google Scholar]
- 21.Bose-O’Reilly S, Drasch G, Beinhoff C, Tesha A, Drasch K, Roider G, Taylor H, Appleton D, Siebert U. 2010. Health assessment of artisanal gold miners in Tanzania. Sci Total Environ 408: 796–805. [DOI] [PubMed] [Google Scholar]