Abstract
We examined whether oral administration of a hop-derived prenylflavonoid isoxanthohumol (IX) would show anti-obesity activity and the underlying mechanism of the potential activity using a high-fat diet (HFD)-induced obese mouse model. Oral administration of 180 mg/kg IX for 8 weeks suppressed HFD-induced accumulation of visceral fat and body weight gain in mice. Simultaneously, IX changed the composition of the microbiome, as determined by a significant increase in the relative abundances of Akkermansia muciniphila, Blautia, and Escherichia coli. A. muciniphila accounted for 23% and 24% of the total microbiome in the HFD+60 mg/kg and 180 mg/kg IX groups, respectively, while it was undetectable in the normal diet (ND) and HFD groups. Similarly, Blautia accounted for 8% and 10% of the total microbiome in the HFD+60 mg/kg and 180 mg/kg IX groups, respectively, while it accounted for less than 1% in the ND and HFD groups. In contrast, a significant decrease in the relative abundance of Oscillospira was observed in the HFD+60 mg/kg and 180 mg/kg IX groups compared with the HFD group. We further examined the anti-obesity effect of IX using a germ-free (GF) mouse model to clarify the relationship between the microbiome and the effect of IX. IX showed no significant anti-obesity effect on fat accumulation and weight gain in GF mice. These results suggest that the anti-obesity effect of IX may involve microbial changes.
Keywords: isoxanthohumol, hop, obesity, microbiome, Akkermansia
INTRODUCTION
Dietary habits are changing worldwide, and an increasing number of people, especially in developed countries, are suffering from obesity. Obesity contributes to the occurrence of metabolic syndrome, which is characterized by abdominal obesity, atherogenic dyslipidemia, increased blood pressure, insulin resistance, proinflammatory, and prothrombotic states [1,2,3]. According to the latest survey in 2015, approximately 604 million adults and 108 million children were diagnosed with obesity in 195 countries [4]. In a review of 11 studies, obesity in childhood and adolescence had adverse effects on mortality and morbidity (diabetes, hypertension, ischemic heart disease, and stroke) in adulthood [5, 6]. Thus, it is clear that preventive measures are needed to reduce the prevalence of obesity [7].
Although pharmaceutical companies are working to develop effective anti-obesity drugs, these drugs are only covered by insurance in patients who have difficulty dieting or exercising. Because obesity results from an imbalance between calories consumed and calories expended [8], preventing obesity by changing dietary habits is important. Some natural ingredients obtained from raw food materials may help address the obesity epidemic [9]. For example, a clinical trial showed that quercetin glycosides were effective for reducing visceral fat in healthy adults [10]. Quercetin expresses lipolytic activity in adipose tissue by enhancing glycerol release and phosphorylation of hormone-sensitive lipase in 3T3-L1 adipocytes [11]. Similarly, tea catechins improve lipid metabolism through beta-oxidation activity in the liver, suggesting they may help suppress obesity [12].
Recently, several reports have suggested that the microbiome is associated with the progression of obesity. For example, the Firmicutes to Bacteroidetes ratio is higher in obese Japanese subjects compared with nonobese subjects [13]. An animal study showed that transplantation of microbiota from obese mice into germ-free (GF) mice contributed to the pathophysiology of obesity [14]. When fecal microbiota from female obese mice were transplanted into GF mice, total body weight and fat mass increased [15]. These reports support the significance of microbiomes when considering the underlying mechanism of obesity.
Some food ingredients, including fibers and polyphenols, have the potential to change the microbiome [16]. For example, in a clinical study, epigallocatechin-3-gallate (EGCG) and resveratrol were given to volunteers for 12 weeks. After the intervention, Bacteroidetes levels in the microbiome decreased [17]. Fat oxidation levels, which can be measured by indirect calorimetry, were associated with a baseline abundance of Bacteroidetes [17]. These results support the relationship between the microbiome and phenotypes and raise the possibility that food ingredients may affect the microbiome.
Xanthohumol, a hop (Humulus lupulus)-derived prenylflavonoid, has been reported to be beneficial for health. Xanthohumol inactivates sterol regulatory element-binding proteins (SREBPs) and reduces fatty acid synthesis, suggesting it can regulate lipid metabolism and improve obesity [18]. It also inhibits angiogenesis in human pancreatic adenocarcinoma cell lines and BALB/c mice, suggesting it has potential as an anti-cancer drug [19]. Xanthohumol exhibits a wide spectrum of anti-pathogenic activity [20]. However, although it is a candidate therapeutic compound for various health issues, under certain conditions (e.g., high temperatures), it can be isomerized into a prenylflavonoid called isoxanthohumol (IX) [21]. IX is more resistant to heat than xanthohumol, and it is a typical compound produced during the brewing process [22]. Although IX has desirable traits as a functional compound, little information is available on its bioactivity. To date, it has been reported that IX inactivates the degradation of precursor forms of SREBPs in Huh-7 cells, suggesting it could also regulate fatty acid synthesis [23]. However, it is not clear whether IX has any anti-obesity effects in vivo or changes the composition of the microbiome.
This study aimed to investigate whether IX could affect the progression of obesity in a mouse model and if changes in the microbiome could be relevant to the effect of IX. For this purpose, we evaluated the accumulation of visceral fat and body weight gain using a high-fat diet-induced obese mouse model after oral treatment with IX for 8 weeks. Then, we assessed the composition of the microbiome using 16S rRNA gene sequencing. To clarify the relationship between the microbiome and anti-obesity effects of IX, we evaluated the anti-obesity effect of IX using GF mice. We also analyzed the hepatic gene expression of Acyl-CoA oxidase (Acox1) and Carnitine palmitoyltransferase 1a (Cpt1a) after 2 weeks of administration of IX. We selected these two genes, Acox1 and Cpt1a, because they are rate-limiting enzymes of fatty acid oxidation and are related to anti-obesity [12, 24].
MATERIALS AND METHODS
Preparation of IX and xanthohumol
IX and xanthohumol were purified from a commercial hop extract purchased from Asama Chemical Co., Ltd. (Tokyo, Japan), using normal and reversed phase column chromatography. The purities of IX and xanthohumol were confirmed based on ultraviolet absorbance at both 280 nm and 350 nm, using high-performance liquid chromatography. The purified products with a peak area of IX above 95% relative to the overall absorbance were used in the subsequent experiments.
Anti-obesity effect of IX in the high-fat diet-induced obese mouse model
Male C57BL/6 J mice (7 weeks of age) were purchased from CLEA Japan, Inc. (Tokyo, Japan). Throughout the experiment, mice were allowed access to food and water ad libitum and were maintained at 25 ± 1°C and 60 ± 5% humidity under a 12 hr light-dark cycle. Animals were fed a commercial diet (D12450B [control]; Research Diets, New Brunswick, NJ, USA) for 1 week. After that, the mice were randomly divided into 7 groups (n=8 per group) based on body weight and allocated to the following conditions: normal diet (ND), high-fat diet (HFD) consisting of 60% of kcal from fat (D12492; Research Diets), HFD and tea catechins 300 mg/kg (HFD+C; as a positive control group), HFD and medium-dose (60 mg/kg) xanthohumol (HFD+XN-M), HFD and low-dose (L; 20 mg/kg) IX (HFD+IX-L), HFD and medium-dose (M; 60 mg/kg) IX (HFD+IX-M), and HFD and high-dose (H; 180 mg/kg) IX (HFD+IX-H).
IX, xanthohumol, and the formulation for tea catechins (polyphenon 70A, Mitsui Norin Co., Ltd., Tokyo, Japan) were diluted into 0.5 w/w% carboxymethyl cellulose sodium salt aqueous solution (Kanto Chemical Co., Inc., Tokyo, Japan) and orally given to C57BL/6 J mice once a day for 8 weeks using a feeding needle. Body weights were measured twice a week using an electronic scale (GX-6000, A&D Company, Ltd., Tokyo, Japan). On the final day of administration, mice were sacrificed under anesthesia of isoflurane, and the following tissues were collected: visceral adipose tissue (epididymal fat, perirenal fat, and mesenteric fat) and cecal contents. Each sample was collected separately into a 1.5 mL tube, freeze-dried, and stored at around −80°C until analysis. Similarly, soon after dissection, we measured the weights of each visceral adipose tissue including epididymal fat, perirenal fat, and mesenteric fat. All experiments were conducted in 2017. All protocols for animal procedures were approved by the Ethics Committee of Animal Experiments in accordance with the Internal Regulations on Animal Experiments at Suntory, which are based on the Law for the Humane Treatment and Management of Animals (Law No. 105, 1 October 1973, as amended on 2 June 2017).
Gut microbiome analysis using cecal contents
MiSeq-based high-throughput 16S rRNA gene sequencing (Illumina Inc., San Diego, CA, USA) was performed to analyze the composition of the microbiome for cecal contents. Cecal samples from ND, HFD, HFD+C, HFD+IX-M, and HFD+IX-H obese mice were used for analyses. NucleoSpin Microbial DNA (Macherey-Nagel Inc., Bethlehem, PA, USA) was used to extract genomic DNA. Approximately 500 µL of stored fecal samples were placed into a microcentrifuge tube containing 100 µL Elution Buffer BE. The mixture was then placed into a NucleoSpin Bead Tube with Proteinase K, which was subjected to mechanical bead-beating for 12 min at 30 Hz in a TissueLyser LT (QIAGEN, Hilden, Germany). The subsequent extraction procedure was performed per the manufacturer’s instructions. We purified the polymerase chain reaction (PCR) products using AMPure XP (Beckman Coulter, Inc., Brea, CA, USA). Then, we generated the sequencing library by amplifying the V3–V4 region of 16S rRNA with specific primers, 341F and 806R, corresponding to the V3–V4 region of the 16S rRNA gene. After that, we performed phylogenetic analysis of the microbiome using MiSeq systems (Illumina Inc., San Diego, CA, USA). Microbiome analysis was performed using an open-source bioinformatics pipeline, QIIME (Quantitative Insights Into Microbial Ecology; Version 1.8.0). In the representative sequence preparation step, CD-HIT-OTU (Version 0.0.1) was used to create an operational taxonomic unit (OTU). First, sequenced paired-end reads were assembled to construct contigs. Then, chimeric contigs were removed by applying the CD-HIT-OTU algorithm. After removing chimeric contigs, the remaining contigs were clustered into OTUs with 97% sequence similarity. To acquire taxonomic information for each OTU, representative sequences were assigned to the Greengenes 16S rRNA database (g_13.8) by the RDP classifier (Version 2.2, Ribosomal Database Project II).
Hepatic gene expression related to fatty acid beta-oxidation
An additional experiment was performed to investigate hepatic gene expression related to fatty acid beta-oxidation. To evaluate short-term effects, daily oral administration of IX was repeated for 2 weeks using the HFD-induced obese mouse model. Animal conditions and the pre-feeding treatment were the same as the experiments above. Mice were randomly divided into 4 groups (n=8 per group) and allocated to the following conditions: ND, HFD, HFD and low-dose (30 mg/kg) IX (HFD+IX-L’), and HFD+IX-M. The doses of IX were 30 and 60 mg/kg. After repeated administration for 2 weeks, all mice were sacrificed under anesthesia with isoflurane, and liver tissues were collected and subjected to real-time quantitative PCR. Total RNA was isolated from liver tissues using QIAzol reagent and an RNeasy kit (QIAGEN). Then, a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to synthesize cDNA. Real-time quantitative PCR was performed using TaqMan Fast Universal PCR Master Mix (Thermo Fisher Scientific Inc.) and specific primers of 2 genes: Acox1 and Cpt1a. The gene expression level was correlated with that of 18S rRNA genes.
Anti-obesity effect of IX on GF mice
An experiment using male C57BL/6N GF mice was conducted at Sankyo Labo Service Corporation, Inc. (Ibaraki, Japan). Throughout the experiment, mice were reared inside an isolator to control their exposure to viral, bacterial, or parasitic agents. Mice were allowed access to food and water ad libitum and were maintained at 23 ± 3°C and 55 ± 15% humidity under a 12 hr light-dark cycle. To explore the involvement of the microbiome in fat accumulation and weight gain, we investigated the possible anti-obesity effect of IX in GF mice for 8 weeks (starting at 7 weeks of age). GF mice were randomly divided into 5 groups (n=8 per group) based on body weight and allocated to the following conditions: ND, HFD, HFD+C, HFD+IX-M, and HFD+IX-H. The doses of IX were 60 and 180 mg/kg. On the final day, body weights were measured. Then, the mice were sacrificed under anesthesia with isoflurane, and visceral adipose tissues, epididymal fat, perirenal fat, and mesenteric fat were collected to measure weights.
Statistical analysis
All data are presented as means ± SE. IBM SPSS Statistics version 23 was used for statistical analysis (IBM, Armonk, NY, USA). Dunnett’s test was used to compare more than 2 groups. Student’s t-test was used for 2 independent groups (e.g., ND and HFD). Differences were considered significant at p<0.05. For genus levels of the microbiome in the 16S rRNA gene sequencing, differences were considered significant at p<0.01.
RESULTS
Anti-obesity effect of IX on the high-fat diet-induced obese mouse model
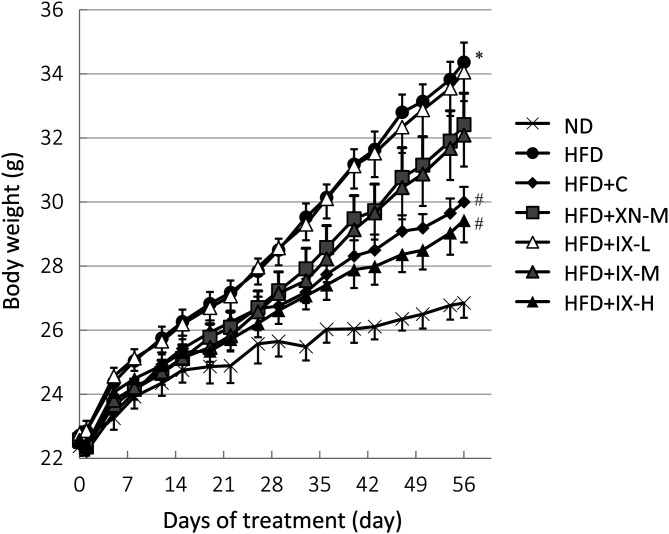
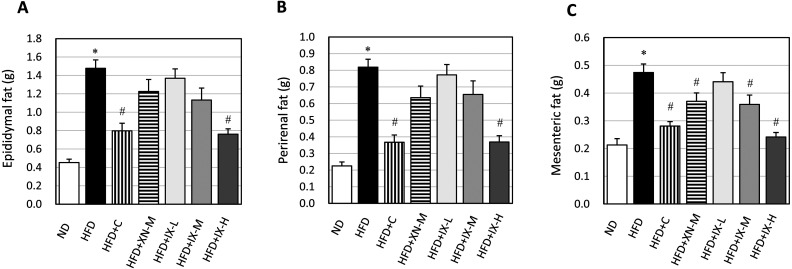
To investigate whether IX could affect the progression of obesity, we measured the accumulation of visceral fat and body weight gain. On the final day, body weight (Fig. 1) and the weights of all adipose tissues—epididymal fat (Fig. 2A), perirenal fat (Fig. 2B), and mesenteric fat (Fig. 2C)—of mice fed the HFD were significantly higher than those fed the ND. In the HFD+C and HFD+IX-H groups, body weight and the weights of all adipose tissues were significantly lower than those in the HFD group. In the HFD+IX-M and HFD+XN-M groups, only the weight of mesenteric fat was significantly lower than in the HFD group. No significant differences were observed in the amounts of consumed diets between the HFD group and any other groups. Oral administration of IX, as well as xanthohumol and tea catechins, suppressed HFD-induced accumulation of visceral fat and body weight gain in the HFD-induced obese mouse model.
Fig. 1.
Effect of oral administration of isoxanthohumol, xanthohumol, and tea catechins for 8 weeks on change in body weight in a high-fat diet-induced obese mouse model. Data are presented as means ± SE of 8 mice. *p<0.05 between the ND and HFD groups (Student’s t-test). #p<0.05 between the HFD and other groups (Dunnett’s test). IX: isoxanthohumol; ND: normal diet; HFD: high-fat diet; HFD+C: HFD and tea catechins 300 mg/kg; HFD+XN-M: HFD and medium-dose (60 mg/kg) xanthohumol; HFD+IX-L: HFD and low-dose (20 mg/kg) IX; HFD+IX-M: HFD and medium-dose (60 mg/kg) IX; HFD+IX-H: HFD and high-dose (180 mg/kg) IX.
Fig. 2.
Effect of oral administration of isoxanthohumol, xanthohumol, and tea catechins on the final weight of each adipose tissue in a high-fat diet-induced obese mouse model: (A) epididymal fat, (B) perirenal fat, and (C) mesenteric fat. Data are presented as means ± SE of 8 mice. *p<0.05 between the ND and HFD groups (Student’s t test). #p<0.05 between the HFD and other groups (Dunnett’s test). IX: isoxanthohumol; ND: normal diet; HFD: high-fat diet; HFD+C: HFD and tea catechins 300 mg/kg; HFD+XN-M: HFD and medium-dose (60 mg/kg) xanthohumol; HFD+IX-L: HFD and low-dose (20 mg/kg) IX; HFD+IX-M: HFD and medium-dose (60 mg/kg) IX; HFD+IX-H: HFD and high-dose (180 mg/kg) IX.
Gut microbiome analysis using cecal contents
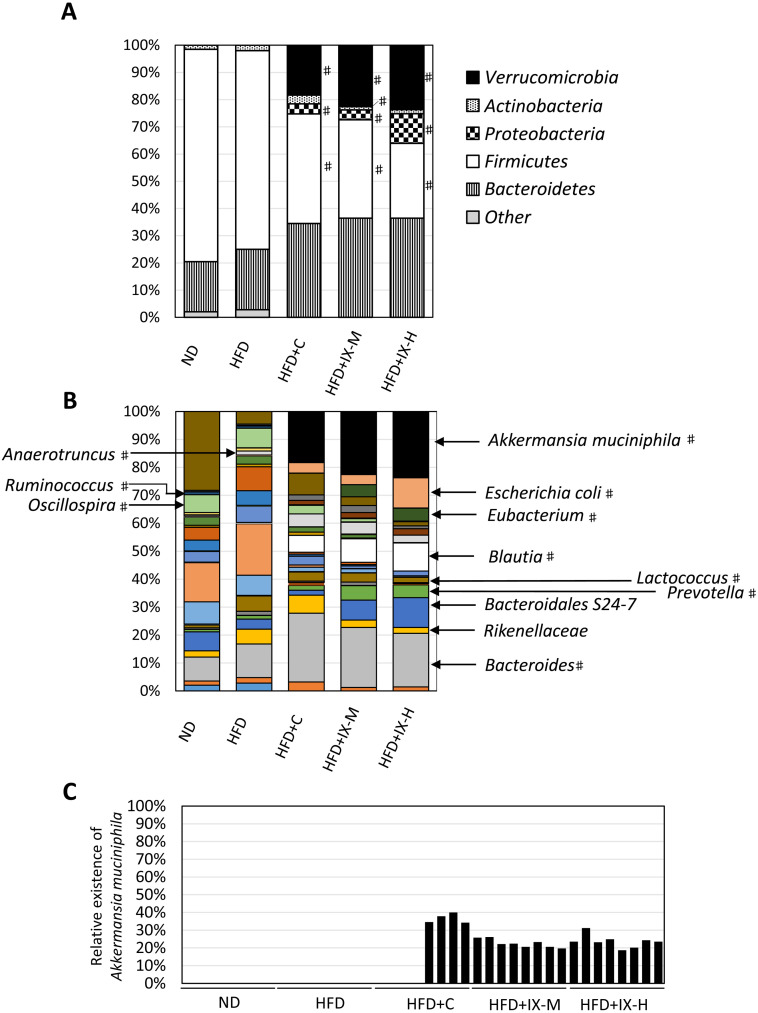
We assessed the composition of the microbiome using 16S rRNA gene sequencing. In the HFD+IX-M and HFD+IX-H groups, the relative abundances of Verrucomicrobia, Bacteroidetes, and Proteobacteria were significantly higher than those of the HFD group at the phylum level, while those of Firmicutes were significantly lower (Fig. 3A). At the genus level, the relative abundances of Akkermansia muciniphila, Blautia, Escherichia coli, Bacteroides, Prevotella, and Eubacterium were significantly higher in the HFD+IX-M and HFD+IX-H groups than in the HFD group (Fig. 3B). Relative abundances of A. muciniphila accounted for 23% and 24% of the total microbiome in the HFD+IX-M and HFD+IX-H groups, respectively, whereas A. muciniphila was undetectable in the ND and HFD groups (limit of detection for relative abundance: 0.005%). Variations within subgroups were observed in the HFD+C group. A. muciniphila was confirmed in only 4 of the 8 mice, with it being under the limit of detection in the other 4 mice (Fig. 3C). The relative abundances of Blautia accounted for 8% and 10% of the total microbiome in the HFD+IX-M and HFD+IX-H groups, respectively, whereas Blautia accounted for less than 1% in the ND and HFD groups. In contrast, the relative abundances of Oscillospira, Lactococcus, Dehalobacterium, Anaerotruncus, and Ruminococcus were significantly lower in the HFD+IX-M and HFD+IX-H groups than in the HFD group (Fig. 3B). Overall, oral treatment with IX for 8 weeks changed the composition of the microbiome.
Fig. 3.
Microbial composition of the cecal contents after oral administration of isoxanthohumol and tea catechins for 8 weeks at the (A) phylum and (B) genus levels for Akkermansia muciniphila as well as (C) individual data. Data are presented as means of 8 mice. For (A) phylum level, #p<0.05 between the HFD and other groups (Dunnett’s test). For (B) genus level, #p<0.01 between the HFD and other groups (Dunnett’s test). IX: isoxanthohumol; ND: normal diet; HFD: high-fat diet; HFD+C: HFD and tea catechins 300 mg/kg; HFD+IX-M: HFD and medium-dose (60 mg/kg) IX; HFD+IX-H: HFD and high-dose (180 mg/kg) IX.
Hepatic gene expression related to fatty acid beta-oxidation
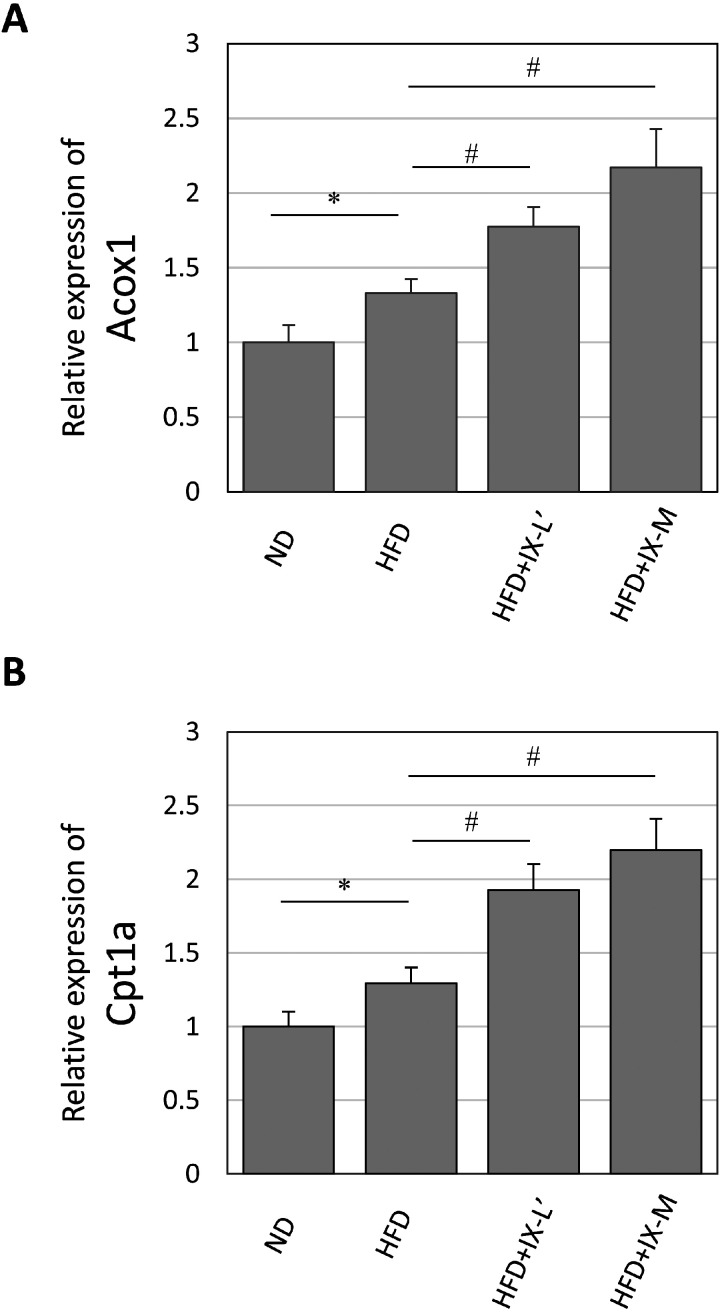
We analyzed the hepatic gene expression of rate-limiting enzymes of fatty acid oxidation after oral treatment with IX for 8 weeks. In the HFD+IX-L’ and HFD+IX-M groups, the relative expression levels of Acox1 and Cpt1a were significantly increased compared with the HFD group (Fig. 4A, 4B). The relative expression levels of Acox1 showed 1.3- and 1.6-fold increases in the HFD+IX-L’ and HFD+IX-M groups compared with the HFD group, respectively. The relative expression levels of Cpt1a showed 1.5- and 1.7-fold increases in the HFD+IX-L’ and HFD+IX-M groups compared with the HFD group, respectively.
Fig. 4.
mRNA expression levels of (A) Acyl-CoA oxidase (Acox1) and (B) Carnitine palmitoyltransferase 1a (Cpt1a) in the liver after oral administration of isoxanthohumol for 2 weeks. Data are presented as means ± SE of 8 mice. *p<0.05 between the ND and HFD groups (Student’s t-test). #p<0.05 between the HFD and other groups (Dunnett’s test). ND: normal diet; HFD: high-fat diet; HFD+IX-L’: HFD and low-dose (30 mg/kg) IX; HFD+IX-M: HFD and medium-dose (60 mg/kg) IX.
Anti-obesity effect of IX on GF mice
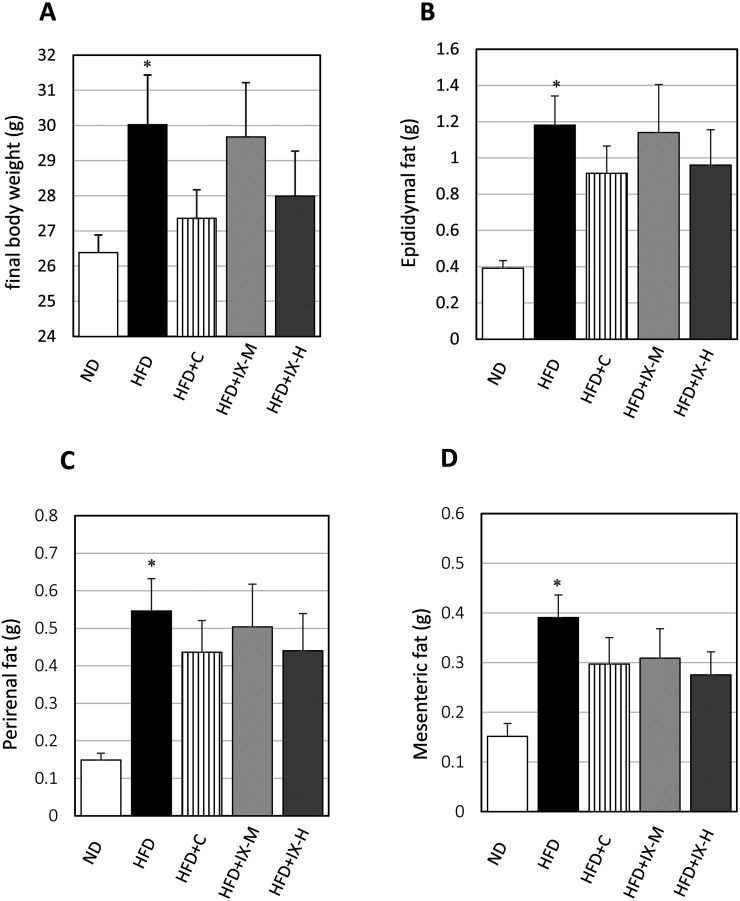
To clarify the relationship between the microbiome and the anti-obesity effect of IX, we measured the accumulation of visceral fat and body weight gain in GF mice. Final body weight (Fig. 5A) and the weights of all adipose tissues—epididymal fat (Fig. 5B), perirenal fat (Fig. 5C), and mesenteric fat (Fig. 5D)—were significantly higher in the HFD group than in the ND group. However, there were no significant differences in the final body weight and the weights of any adipose tissue between the HFD group and other intervention groups (HFD+C, HFD+IX-M, and HFD+IX-H). Overall, oral administration of IX had little effect on HFD-induced accumulation of visceral fat and body weight gain in the GF mouse model.
Fig. 5.
Effect of oral administration of isoxanthohumol and tea catechins on the final body weight and the weight of each adipose tissue in the GF mouse model: (A) final body weight, (B) weight of epididymal fat, (C) weight of perirenal fat, and (D) weight of mesenteric fat. Data are presented as means ± SE of 7 to 8 mice. *p<0.05 between the ND and HFD groups (Student’s t-test). ND: normal diet; HFD: high-fat diet; HFD+C: HFD and tea catechins 300 mg/kg; HFD+IX-M: HFD and medium-dose (60 mg/kg) IX; HFD+IX-H: HFD and high-dose (180 mg/kg) IX.
DISCUSSION
Although there is little information regarding the bioactivity of IX, especially in vivo, we hypothesized that IX would have anti-obesity activity for 2 reasons. First, in vitro experiments using Huh-7 cells demonstrated that IX inactivates the degradation of precursor forms of SREBPs, which are involved in the regulation of fatty acid synthesis [23]. Second, because its precursor, xanthohumol, has broad-spectrum anti-pathogenic activity [20], there is a possibility that IX also shows such activity. The progression of obesity can be triggered by certain intestinal pathogens. For example, Enterobacter cloacae B29, isolated from an obese volunteer’s gut, induced obesity in high-fat diet-fed GF mice [25]. Our hypothesis is that if IX affects the survival of such a pathogen, it can affect the composition of the microbiome, which is associated with the progression of obesity.
In the present study, oral administration of IX for 8 weeks suppressed the increase of body weight and accumulation of each adipose tissue (Figs. 1 and 2). Oral treatment with IX also changed the composition of the microbiome (Fig. 3). In addition to a decrease in the Firmicutes to Bacteroidetes ratio, enhanced relative abundances of A. muciniphila and Blautia were observed after treatment with IX (Fig. 3B). A. muciniphila is a mucin-degrading bacterium of the phylum Verrucomicrobia, and its relative abundance is inversely associated with worsened metabolic status, such as subcutaneous adipocyte diameter, waist-to-hip ratio, and high fasting glucose levels in humans [26]. Repeated intake of A. muciniphila suppresses obesity and insulin resistance in mice [27]. This activity is also seen with Amuc_1100, a specific protein isolated from the outer membrane of the bacterium. In a clinical trial with overweight and obese human volunteers, supplementation with pasteurized A. muciniphila for 3 months slightly decreased body weight, although the number of participants was small [28]. Therefore, an increasing relative abundance of A. muciniphila can have an anti-obesity effect. Likewise, the relative abundance of Blautia is also inversely associated with visceral fat accumulation [29]. The relative abundance of A. muciniphila is low in subjects with diabetes [30]. However, the underlying mechanisms regarding the growth of A. muciniphila and Blautia have not been clarified. One possible strategy to explore these mechanisms may include conducting an in vitro experiment to evaluate the effects of IX on mono-cultured bacteria. However, the growth of specific bacteria is complex and may be affected by competing bacteria in the intestine. Moreover, the absolute amount of the bacteria was not clear in the present study, which is a limitation of this study. Further studies are needed in this area. In the present study, we did not detect A. muciniphila (<0.005%) in the ND and HFD groups without IX (Fig. 3B). We did not check the abundance of A. muciniphila on the first day of the experiment. Therefore, it is difficult to conclude whether A. muciniphila was eliminated after 8 weeks or if only negligible abundances of A. muciniphila could have remained in the ND and HFD groups.
Although it has been shown that IX regulates the composition of the microbiome, it does not necessarily mean that a change in microbiome is related to the suppression of obesity. To confirm this relationship, we investigated the effect of IX using a GF mouse model, in which the effect of the microbiome can be ignored. In GF mice, body weight gain and visceral fat accumulation were more moderate than in non-GF mice (Figs. 1, 2 and 5). These data are consistent with a previous report demonstrating the contribution of the microbiome to body weight gain and fat accumulation [31]. Consequently, IX did not show a significant anti-obesity effect in GF mice. Based on these findings, we can hypothesize that the anti-obesity effect of IX is related to changes in the microbiome. While some intestinal bacteria can contribute to obesity when comparing the results of GF and non-GF mice, A. muciniphila or Blautia may represent anti-obesity bacteria. Tea catechins had no significant anti-obesity effects in GF mice, although there were variations in the microbiome within subgroups, such as in the relative abundance of A. muciniphila. It is not clear whether the underlying mechanism of the anti-obesity effect of tea catechins is the same as that of IX.
Administration of IX for 2 weeks raised gene expression levels related to hepatic fatty acid oxidation, Acox1 and Cpt1a (Fig. 4). Because Acox1 and Cpt1a are rate-limiting enzymes of fatty acid oxidation, their modulation contributes to the anti-obesity effects [12, 24]. Regarding the significance of transcriptional regulations, hepatic gene transfer of Cpt1a using adeno-associated viruses suppressed the progression of obesity in mice [32]. In addition, a higher expression level of Cpt1a was observed in HFD-fed rats than in those fed a control diet, which could be attributed to the epigenetic DNA methylation change [33, 34]. In the present study, comparison of gene expression between the ND and HFD-fed mice revealed similar transcriptional regulation (Fig. 4). This phenomenon may be a response to the growing necessity for fat oxidation. We hypothesize that a change in the composition of the microbiome is also involved in gene expression. In GF mice, phosphorylation levels of adenosine monophosphate kinase (AMPK) are higher than in conventionally raised mice, and AMPK upregulates the activity of its downstream target Cpt [35]. This report connects the relationship between the microbiome and fatty acid oxidation. Still, we have not identified which microorganism plays a key role in the regulation of fatty acid oxidation and, eventually, suppression of obesity. In the future, transplantation of a candidate microorganism into GF mice may help reveal its contribution to fatty acid oxidation.
In conclusion, a hop-derived prenylflavonoid, IX, showed anti-obesity activity and the ability to change the composition of the microbiome in mice. The composition of the microbiome is associated with the anti-obesity effect of IX, as IX showed no significant anti-obesity effects in GF mice. It remains unclear whether increases in the relative abundances of A. muciniphila and Blautia are directly involved in the anti-obesity effect. Because IX is more resistant to heat than xanthohumol, it may be useful in the development of food products that have anti-obesity benefits. Future clinical studies are needed to confirm our findings.
REFERENCES
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart AssociationNational Heart, Lung, and Blood Institute.2004. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109: 433–438. [DOI] [PubMed] [Google Scholar]
- 2.Wahba IM, Mak RH. 2007. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2: 550–562. [DOI] [PubMed] [Google Scholar]
- 3.Han TS, Lean ME. 2016. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis 5: 2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, et al., GBD 2015 Obesity Collaborators.2017. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruby A, Hu FB. 2015. The epidemiology of obesity: a big picture. Pharmacoeconomics 33: 673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reilly JJ, Kelly J. 2011. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes 35: 891–898. [DOI] [PubMed] [Google Scholar]
- 7.Stephens SK, Cobiac LJ, Veerman JL. 2014. Improving diet and physical activity to reduce population prevalence of overweight and obesity: an overview of current evidence. Prev Med 62: 167–178. [DOI] [PubMed] [Google Scholar]
- 8.Fock KM, Khoo J. 2013. Diet and exercise in management of obesity and overweight. J Gastroenterol Hepatol 28 Suppl 4: 59–63. [DOI] [PubMed] [Google Scholar]
- 9.Rajitha S, Martha V. 2014. Functional foods for obesity management. Food Nutr Sci 5: 1359–1369. [Google Scholar]
- 10.Saito K, Tanaka T, Obata H, Nakamura J, Fukui N, Tonozuka N. 2015. Body fat reducing effect and safety evaluation of long-term consumption of tea containing quercetin glucosides in obese subjects. Jpn Pharmacol Ther 43: 181–194. [Google Scholar]
- 11.Tateishi N, Egawa K, Kanzaki N, Kitagawa Y, Shibata H, Kiso Y, Enomoto S, Fukuda D, Nagai R, Sata M. 2009. Effects of quercetin glucosides on diet-induced obesity in mice. Jpn Pharmacol Ther 37: 123–131. [Google Scholar]
- 12.Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. 2002. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord 26: 1459–1464. [DOI] [PubMed] [Google Scholar]
- 13.Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y, Takase K. 2015. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol 15: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 15.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341: 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin D, Peters BA, Friedlander C, Freiman HJ, Goedert JJ, Sinha R, Miller G, Bernstein MA, Hayes RB, Ahn J. 2018. Association of dietary fibre intake and gut microbiota in adults. Br J Nutr 120: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Most J, Penders J, Lucchesi M, Goossens GH, Blaak EE. 2017. Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur J Clin Nutr 71: 1040–1045. [DOI] [PubMed] [Google Scholar]
- 18.Miyata S, Inoue J, Shimizu M, Sato R. 2015. Xanthohumol improves diet-induced obesity and fatty liver by suppressing sterol regulatory element-binding protein (srebp) activation. J Biol Chem 290: 20565–20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito K, Matsuo Y, Imafuji H, Okubo T, Maeda Y, Sato T, Shamoto T, Tsuboi K, Morimoto M, Takahashi H, Ishiguro H, Takiguchi S. 2018. Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer. Cancer Sci 109: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stompor M, Żarowska B. 2016. Antimicrobial activity of xanthohumol and its selected structural analogues. Molecules 21: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kami, ski DM, Gaweda K, Arczewska M, Senczyna B. 2017. A kinetic study of xanthohumol cyclization to isoxanthohumol—a role of water. J Mol Struct 1139: 10–16. [Google Scholar]
- 22.Stevens JF, Taylor AW, Clawson JE, Deinzer ML. 1999. Fate of xanthohumol and related prenylflavonoids from hops to beer. J Agric Food Chem 47: 2421–2428. [DOI] [PubMed] [Google Scholar]
- 23.Inoue J, Miyata S, Shimizu M, Sato R. 2018. Isoxanthohumol stimulates ubiquitin-proteasome-dependent degradation of precursor forms of sterol regulatory element-binding proteins. Biosci Biotechnol Biochem 82: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 24.Kuhajda FP, Ronnett GV. 2007. Modulation of carnitine palmitoyltransferase-1 for the treatment of obesity. Curr Opin Investig Drugs 8: 312–317. [PubMed] [Google Scholar]
- 25.Fei N, Zhao L. 2013. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 7: 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K, MICRO-Obes Consortium.2016. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436. [DOI] [PubMed] [Google Scholar]
- 27.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD. 2017. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23: 107–113. [DOI] [PubMed] [Google Scholar]
- 28.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. 2019. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, Sawada K, Katsuragi Y, Kakuta M, Imoto S, Ihara K, Nakaji S. 2019. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. 2010. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5: e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. 2010. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 24: 4948–4959. [DOI] [PubMed] [Google Scholar]
- 32.Orellana-Gavaldà JM, Herrero L, Malandrino MI, Pañeda A, Sol Rodríguez-Peña M, Petry H, Asins G, Van Deventer S, Hegardt FG, Serra D. 2011. Molecular therapy for obesity and diabetes based on a long-term increase in hepatic fatty-acid oxidation. Hepatology 53: 821–832. [DOI] [PubMed] [Google Scholar]
- 33.Moody L, Xu GB, Chen H, Pan YX. 2019. Epigenetic regulation of carnitine palmitoyltransferase 1 (Cpt1a) by high fat diet. Biochim Biophys Acta Gene Regul Mech 1862: 141–152. [DOI] [PubMed] [Google Scholar]
- 34.Zhou D, Hlady RA, Schafer MJ, White TA, Liu C, Choi JH, Miller JD, Roberts LR, LeBrasseur NK, Robertson KD. 2017. High fat diet and exercise lead to a disrupted and pathogenic DNA methylome in mouse liver. Epigenetics 12: 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]