Abstract
Lactobacillus fermentum MTCC 25067 produces a hetero-exopolysaccharide (HePS) when cultured which forms supramolecular networks in the culture medium, increasing the viscosity. In the present study, the viscosity of the bacterial culture reached its maximum at 48 hr of cultivation and then decreased during a stationary growth phase lasting for up to 144 hr. The monosaccharide composition did not change during the stationary growth phase, whereas degradation of HePS molecules was noticeable, leading to partial disintegration of their supramolecular networks. The viscosity values of the HePS purified from the culture and dissolved in a fresh medium indicated little contribution of medium components to the viscosity. Absence of the apparent network structure of the HePS in the surrounding area of bacterial cells was observed during the late growth phase, supporting the idea that the decreases in culture viscosity during the prolonged period of cultivation were caused mainly by reduced interactions between bacterial cells and the intact supramolecular networks as a consequence of decreasing bacterial cell wall integrity and partial degradation of HePS molecules.
Keywords: lactic acid bacteria, polysaccharide network, slime hetero-exopolysaccharide, structure-rheology relationship, viscosity loss
INTRODUCTION
Bacterial exopolysaccharides (EPSs) are long chains of monosaccharides either associated with the external cellular environment, often covalently bound to the cell surface in the form of capsules, or secreted in the environment in the form of slime detached from the bacterial cell [1]. There are two types of bacterial EPSs: homo-EPS (HoPS), composed of a single type of monosaccharide, synthesized by the action of glycosyltransferases outside the bacterial cell represents one type, and the other is hetero-exopolysaccharide (HePS), sugar repeating units of which are synthesized in the cytoplasm and polymerized by the catalyzing action of a cell surface polymerase [2,3,4]. Besides their physiological roles, which are believed to protect bacteria themselves from environmental stresses such as drying, extreme temperature, salinity, and heavy metal ions [5,6,7], bacterial EPSs can be implemented in a wide range of biotechnological applications, including bioremediation, cosmetics, oil recovery, pharmaceuticals, and textiles [8,9,10,11,12]. Bacterial EPSs are also used for emulsification, encapsulation, film formation, gelation, stabilization, and inhibition of syneresis in food industries [13,14,15,16,17,18]. Several bacterial EPSs have also been reported to have health benefits such as antitumor, antiulcer, anti-obesity, and immune-modulating effects [19,20,21,22]. To date, curdlan (a β-1,3-glucan), dextran (an α-1,6-glucan), pullulan (an α-glucan), gellan (a HePS produced by Sphingomonas elodea), succinoglycan (a HePS produced by Agrobacterium tumefaciens), and xanthan gum (a HePS produced by Xanthomonas campestris) have been extensively used in the food industry for decades [23,24,25,26,27]. Furthermore, food scientists are keen to explore the functionality of EPS-producing dairy lactic acid bacteria (LAB) that are generally recognized as safe (GRAS), sometimes with health claims, aiming mainly to use them as starters for improving the texture and consistency of fermented dairy products [28, 29].
A noteworthy rheological feature of LAB-derived EPSs is viscosity when they are produced by dairy starters, and hence the structure-rheology relationship of the EPSs and their molecular interaction with other food components such as milk proteins have been intensively studied [14, 15, 30]. Rheological properties of pure bacterial EPSs are diverse, reflecting wide variations in their molecular weight distributions, monosaccharide compositions, and types of linkages between sugar monomers, as well as variations in the presence of side chains and modifications such as acylation, carboxylation, phosphorylation, pyruvation, and sulfation [31,32,33,34]. It is well known that a decline in the viscosity of bacterial culture, which reflects certain changes in EPS molecules and/or other components in the medium, frequently occurs during a prolonged period of cultivation of HePS-producing LAB [35, 36]. Enzymatic degradation of EPS molecules has been suggested as a possible cause of the decrease in viscosity because the decrease is accompanied by a decrease in the production yield of HePSs [35,36,37].
Lactobacillus fermentum MTCC 25067 (formerly TDS 030603) was previously isolated from a traditionally fermented Indian milk, Dahi, as a producer of a neutral HePS [38]. Solutions of the purified HePS showed high viscosities comparable to a commercially available viscosifier, xanthan gum, at lower concentrations of approximately 100 mg/L, suggesting that the HePS is a potential candidate for a novel viscosifier for the food industry. Its chemical structure consists of a main chain of 1,3-glucan, composed of α-glucose and β-glucose residues appearing alternately, and a disaccharide subunit of glucose-α-1,6-galactose-α-1,2- substituted at the C-2 position of the α-glucose [39]. It has been confirmed using proton nuclear magnetic resonance (1H NMR) spectroscopy that the average molar ratio of the terminal D-Glcp to the 3-substituted D-Glcp to the 2,3-disubstituted D-Glcp to the 6-substituted D-Galp is 1.3:1.0:1.1:1.1 [39]. The non-integer ratio is considered to reflect the heterogeneity of the distribution of side chains along the main chain. Recently, the formation of a supramolecular network has been suggested as a major reason for the high viscosity of the HePS solution [40].
Under our experimental conditions, the production yield of the purified HePS and the viscosity of the culture medium reached their maximums at 48 hr of cultivation, and this was followed by a continuous decrease in the viscosity of the culture medium until the cultivation time reached 144 hr. In the present study, to elucidate factors causing the thinning of the culture medium, we compared physicochemical and microscopic characteristics of the HePSs purified from the culture media at 48 and 144 hr of cultivation, which showed the highest and lowest viscosities, respectively. Furthermore, it was demonstrated using atomic force microscopy (AFM) whether interaction between the supramolecular network of the HePS and the bacterial cell surfaces could be an important factor for determining the viscosity of the culture medium.
MATERIALS AND METHODS
Bacterial strain and reagents
L. fermentum MTCC 25067 was obtained from the bacterial collection in our laboratory at Obihiro University of Agriculture and Veterinary Medicine. The strain was stored at −80°C in de Man-Rogosa-Sharpe (MRS) broth containing 20% (v/v) glycerol until use. MRS was obtained from Oxoid (Cambridge, UK). DEAE-Sephadex A-50 and Toyopearl HW-55F were purchased from GE Healthcare (Little Chalfont, UK) and Tosoh (Tokyo, Japan), respectively. All the chemicals used were of analytical grade.
Culture conditions and preparation of the HePSs
Bacterial culture and HePS preparation were done according to Ikeda et al. [40], with minor modifications. In brief, the bacterial cells were propagated statically and aerobically in MRS medium. An equal volume of ice-cold ethanol was added to the collected culture supernatant, and then the precipitant was purified by batch method using DEAE-Sephadex A-50 (GE Healthcare) equilibrated with 50 mM Tris-HCl buffer (pH 8.7). After that, the HePS solution was thoroughly dialyzed against Milli-Q water using Biotech CE 1000kD (Repligen, Waltham, MA, USA). The purity of the HePS solution was confirmed by 1H NMR. Viable cell numbers and the pH of the culture medium were monitored at 0, 8, 16, 24, 32, 48, 96, and 144 hr of cultivation as previously reported [41]. The glucose concentration of the culture supernatant was measured at the same time intervals as above on the basis of the phenol-sulfuric acid method using glucose as the standard [42].
After 48 and 144 hr of cultivation in a 1-liter MRS broth each at 30°C, each culture medium was diluted 5 times with distilled water, and then bacterial cells were removed using centrifugation (17,000 g, 1 hr, 4°C). Crude HePS was precipitated by adding an equal volume of ice-cold ethanol to the supernatant. The precipitate was collected using centrifugation (17,000 g, 30 min, 4°C) and dissolved thoroughly in 100 mL of ion-exchange water. In order to remove protein contaminants, a 300-mL slurry of DEAE-Sephadex A-50 equilibrated with 50 mM Tris-HCl (pH 8.7) was added to the crude HePS solution. A non-adsorbed fraction was collected using aspirating filtration. The HePS was precipitated by mixing the filtrate with an equal volume of ice-cold ethanol, collected using centrifugation (17,000 g, 30 min, 4°C), and dissolved thoroughly in 50 mL of Milli-Q water. This HePS solution was dialyzed against Milli-Q water extensively, lyophilized, and stored in a desiccator until use.
Viscosity measurements
The viscosities of the culture media collected at 0, 8, 16, 24, 32, 48, 96, and 144 hr of cultivation were measured using a VT-03F single cylinder-type rotational viscometer (Rion, Tokyo, Japan) according to the manufacturer’s instructions. Briefly, 460 mL of a culture broth was placed in an outer cup with a radius of 46 mm for measurement using an inner rotor with a radius of 30.6 mm. The apparatus was left at room temperature for 4 min before measuring viscosity at a rotational speed of 62.5 rpm. The shear rate was estimated to be 17/s using the following equation (1):
D = (2πn/60)(1 + δ2)/(δ2 − 1), (1)
where D, n, and δ represent the shear rate (1/s), the rotational speed (rpm), and the ratio of the radius of the inner rotor to the radius of the outer cup, respectively. All the experiments were done in triplicate.
Steady shear viscosities of 0.02% (w/v) and 1% (w/v) HePS dissolved in the MRS broth and ion exchanged water, respectively, were determined at 25°C as a function of the shear rate (0.1 to 100/s) using a TA Discovery HR-2 rheometer (TA Instruments, New Castle, DE, USA) equipped with a Peltier temperature control unit and a cup and bob assembly for 0.02% (w/v) HePS solutions in MRS or a cone and plate attachment (diameter 20 mm; angle 1.009°; gap 65 μm) for 1% (w/v) HePS solutions in ion exchanged water.
Gel permeation chromatography
To estimate molecular weight distribution in HePS at the maximum of viscosity and at the end of cultivation, two HePS samples were purified from the media cultivated for 48 hr (HePS48hr) and 144 hr (HePS144hr), respectively, and subjected to gel permeation chromatography. One milligram of a purified HePS was dissolved in 1 mL of water, and then a 100-μL aliquot of the HePS solution was loaded onto a TSKgel G5000PWXL column (ϕ7.8 × 300 mm) equilibrated with 50 mM Tris-HCl (pH 7.0). Isocratic elution was done at 40°C and a flow rate of 1 mL/min using the same buffer. Ultraviolet absorbance was monitored using a UV-2075 Plus spectrophotometer (Jasco, Tokyo, Japan). The eluate was collected every 1 min up to 20 min manually. The concentration of total carbohydrates in each fraction was determined on the basis of the phenol-sulphate method using glucose as the standard [42] and reported as the ratio of the carbohydrates in each fraction to the total carbohydrates. The ratio of the summation of the relative concentrations in the fractions collected at elution times of 6–8 min to that in the fractions collected at elution times of 9–15 min was calculated as a measure of the ratio of the content of high-molecular-weight (HMW) polysaccharides to that of low-molecular-weight (LMW) polysaccharides. Experiments were performed in triplicate.
1H NMR spectroscopy
Purified HePS48hr and HePS144hr (2 mg each) were dissolved in 99.96% D2O (Eurisotop, Saint-Aubin, France), lyophilized, and subjected to the following 1H NMR spectroscopy. 1H NMR spectra of HePS48hr and HePS144hr dissolved in D2O (99.999 atom %D, Acros Organics, Morris, NJ, USA) were recorded at 600 MHz using a Varian INOVA 600 spectrometer (Varian Inc., Palo Alto, CA, USA) operated at 323.1 K. Chemical shifts (δ) were expressed as those relative to internal 3-(trimethylsilyl)-1-propane sulfuric acid but were measured practically using internal acetone (δ=2.225 ppm) as a reference. Experiments were done in triplicate.
AFM
A lyophilized HePS was dissolved in Milli-Q water to obtain a 1 mg/L solution. A 2-μL aliquot of the HePS solution was deposited onto a freshly cleaved mica surface and left to dry for 30 min at an ambient temperature of around 22°C. To observe the bacterial cells and HePS in the culture medium simultaneously, 2 µL of the culture medium was collected during cultivation, diluted 100 times using ion exchanged water, deposited onto a freshly cleaved mica sheet, and left to dry for 2 hr in a desiccator. Topographical imaging was performed in air using a BioScope Catalyst atomic force microscope (Bruker, Santa Barbara, CA, USA) operated in peak force tapping mode using a NanoScope V controller (Bruker). Obtained images were flattened using NanoScope Analysis software version 1.40 (Bruker).
Scanning electron microscopy
Scanning electron microscopy (SEM) analysis was performed on a colony of L. fermentum MTCC 25067 harvested on an agar medium after glutaraldehyde fixation (2% in 0.1 M phosphate buffer, pH 7.4) for 30 min at ambient temperature. After washing 3 times using 0.1 M phosphate buffer (PB), the samples were immersed in 1% tannic acid dissolved in PB for 1 hr, and this was followed by washing with 0.1 M PB and conductive staining in 1% osmium tetraoxide dissolved in PB for 30 min at room temperature. The samples were then dehydrated 3 times in ascending series of ethanol (70, 80, 90, 95, and 100%) for 5 min each, transferred into 2-methyl-2-propanol, and dried in an ES-2030 freeze-dryer (Koki Holdings, Tokyo, Japan). The dried samples were coated with platinum-palladium in an E1010 ion sputtering device (Koki Holdings), mounted on an aluminum plate, and examined using an S-4100 field emission SEM (Hitachi High-Tech, Tokyo, Japan) operated in SE mode.
Statistical analysis
Data obtained from three independent experiments were expressed as means ± standard deviation and analyzed using the one-sided paired t-test. P values less than 0.05 were considered to be statistically significant.
RESULTS
Cultivation profiles
The time courses of the changes in the viable cell number, pH, residual glucose concentration, and viscosity of the culture were recorded during cultivation for 144 hr (Fig. 1). The viable cell number reached its maximum at around 16–24 hr of cultivation and then decreased gradually over time during cultivation for a longer period of time. The values for pH and the residual glucose concentration decreased remarkably during the first 24 hr of cultivation and remained at constant values thereafter. The viscosity of the culture medium increased in the first 24 hr, remained at relatively high values in the subsequent 24 hr, and then decreased continuously toward the end of cultivation. There was little variability among the three independent experiments in regards to the values of viable cells, pH, and the residual glucose concentration. In contrast, considerable variability of the viscosity of the culture medium was noticeable among the three independent experiments at 24–96 hr of cultivation. The maximum average viscosity of the culture medium was observed at 48 hr and was determined to be 53 mPa·s at the estimated shear rate of 17/s.
Fig. 1.
Changes in (A) viable cell counts, (B) pH, (C) residual glucose concentration, and (D) viscosity in the culture medium during cultivation of L. fermentum MTCC 25067. The error bars indicate standard deviations.
Viscosities of the purified HePSs
Typical shear-thinning behavior in response to increasing shear rate was observed in 1% (w/v) aqueous solutions of both HePS48hr and HePS144hr dissolved in ion exchanged water (Fig. 2A). Significant differences in steady shear viscosity (η) between the two HePSs were seen in the shear rate range lower than 25/s. Our general observation was that approximately 100 mg of the HePS were typically produced during a single batch (1 L) of cultivation. To simulate the conditions during cultivation, HePS48hr and HePS144hr were dissolved in MRS broth to give a polysaccharide concentration of 0.02% (w/v) and then subjected to viscosity measurement. Both solutions exhibited typical Newtonian fluid behavior (Fig. 2B). The steady shear viscosities of HePS48hr and HePS144hr at a shear rate of 15.8/s, which was close to that used in the viscosity measurement using the single cylinder-type rotational viscometer as described above, were 1.6 and 1.2 mPa·s, respectively. Differences in viscosity between the two HePSs were significant, except for the difference at a shear rate of 63/s. No reliable data were obtained from measurements at shear rates equal to or lower than 1/s because the signal-to-noise ratios were too low. Compared with the viscosities of the culture medium during cultivation (Fig. 1D), the viscosities of the HePSs dissolved in the MRS broth were significantly lower.
Fig. 2.
Steady shear viscosities of (A) 1% (w/v) HePSs dissolved in water and (B) 0.02% (w/v) HePSs dissolved in MRS broth. Circles and triangles represent the HePSs sampled at 48 and 144 hr of cultivation, respectively. The error bars indicate standard deviations. An asterisk (*) indicates statistical significance (p<0.05).
Molecular weight distributions of the HePSs
The elution profiles of HePS48hr and HePS144hr both showed their first peak at 7 min of elution (Fig. 3A). HePS48hr showed a second peak at 9–10 min, whereas HePS144hr showed second and third peaks at 9 min and 11 min, respectively. These elution profiles are similar to previously reported results [41] in which two major peaks and one shoulder were observed at retention times similar to those observed in this study. The highest peak was observed at 7 min for HePS48hr and at 9 min for HePS144hr. Among all of the fractions, only the fractions eluted at 12 min showed significant differences in relative carbohydrate concentrations between HePS48hr and HePS144hr and showed significantly higher ratios of HMW polysaccharides determined from the results obtained at elution times of 6–8 min to LMW polysaccharides determined from the results obtained at elution times of 9–15 min, indicating that HePS144hr contained more LMW polysaccharides than HePS48hr (Fig. 3B).
Fig. 3.
(A) Gel permeation chromatography profiles of the HePS48hr (circles) and HePS144hr (triangles) and (B) comparison of the ratio of the content of high-molecular-weight (HMW) polysaccharides to that of low-molecular-weight (LMW) polysaccharides in the HePSs. The error bars indicate standard deviations. An asterisk (*) indicates statistical significance (p<0.05).
Side-chain structure of the HePSs
Almost identical chemical shift profiles were observed in the 600-MHz 1D 1H NMR spectra of HePS48hr and HePS144hr (data not shown). As was reported by Gerwig et al. [39], H-1 chemical shifts of around δ=5.674, 5.325, 4.985, and 4.731 ppm were assigned to -(1→2,3)-α-D-Glcp-(1→ or 2,3-disubstituted D-Glcp, -(1→6)-α-D-Galp-(1→ or 6-substituted D-Galp, α-D-Glcp-(1→ or terminal D-Glcp, and -(1→3)-β-D-Glcp-(1→ or 3-substituted D-Glcp, respectively. The H-1 chemical shifts and the peak areas of the signals assigned to these four sugar residues in this study are summarized in Table 1. The average molar ratio of terminal D-Glcp to 3-substituted D-Glcp to 2,3-disubstituted D-Glcp to 6-substituted D-Galp was determined to be 0.9:1.0:0.8:0.7 for HePS48hr and 0.9:1.0:0.8:0.6 for HePS144hr, as a result of calculating the ratio of the peak area of each residue to that of the 3-substituted D-Glcp. Consequently, these HePSs had almost identical average molar ratios for their constituent sugars, suggesting that their chemical structures, including the distribution of side chains, were similar. In a previous report [39], the average molar ratio of terminal D-Glcp to 3-substituted D-Glcp to 2,3-disubstituted D-Glcp to 6-substituted D-Galp was determined to be 1.3:1.0:1.1:1.1 for the HePS produced by the same strain of LAB as in the present study. The differences in the molar ratio between the two studies indicate that the quantity of 3-substituted D-Glcp was overestimated, due to the presence of contaminant peaks that partially overlapped a peak assigned to 3-substituted D-Glcp in this study (data not shown).
Table 1. H-1 chemical shifts (δ) and peak areas of sugar residues consisting of the HePSs.
| Residue | HePS48hr
|
HePS144hr
|
||
|---|---|---|---|---|
| δ (ppm) | Peak area (%) | δ (ppm) | Peak area (%) | |
| -(1→2,3)- α -D-Glcp-(1→ | 5.679, 5.680, 5.682 | 3.80 ± 0.92 | 5.683, 5.684, 5.682 | 3.07 ± 0.18 |
| -(1→6)- α -D-Galp-(1→ | 5.327, 5.326, 5.326 | 3.20 ± 0.91 | 5.330, 5.330, 5.329 | 2.51 ± 0.20 |
| α -D-Glcp-(1→ | 4.979, 4.977, 4.978 | 4.56 ± 1.04 | 4.978, 4.978, 4.979 | 3.64 ± 0.18 |
| -(1→3)- β -D-Glcp-(1→ | 4.741, 4.740, 4.743 | 4.88 ± 0.30 | 4.745, 4.754, 4.744 | 4.06 ± 0.03 |
AFM and SEM imaging of the supramolecular structure of the HePSs
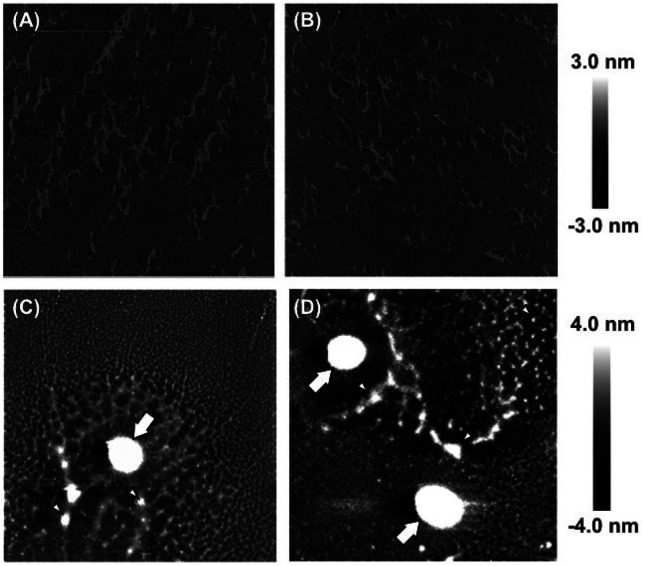
The topographical image of HePS48hr deposited from a 1 mg/L solution onto a mica surface and air-dried prior to imaging showed a network structure similar to that of the same type of HePS reported previously [40] (Fig. 4A). The average length and height of the HePS48hr fibers were 1.29 μm and 1.25 nm, respectively. HePS144hr also showed a similar network structure, but the population of fibers that were both shorter (0.36 μm on average) and thinner (1.09 nm on average) appeared to be greater than that of HePS48hr (Fig. 4B). Bacterial cell sizes were found to be in the range of 1.5 to 2.2 micrometers at a z-scale of 300 nm (Supplementary Fig. 1), which was similar to the bacterial cell sizes of Lactobacillus species observed by AFM previously [43]. Upon observation of bacterial cells grown in the MRS broth sampled at 48 hr of cultivation, it was clearly observed that the network structure of the HePS was present around the bacterial cell (Fig. 4C and Supplementary Fig. 2A). Furthermore, remarkable disintegration of the network structure was noticeable in the culture medium sampled at 144 hr of cultivation, especially in the area surrounding bacterial cells (Fig. 4D and Supplementary Fig. 2B). The network of HePS fibers appeared to be detached from the cell surfaces in the 144-hr culture medium. On average, the heights of certain cross-linking regions in the HePS network (white spots, some of which are indicated by arrowheads in Fig. 4D) were 1.9-fold greater than those in other parts of the network. There were fewer of such tall cross-linking regions in the culture medium collected at 48 hr (Fig. 4C), and they were undetectable in the purified HePS144hr (Fig. 4B). The network structure that HePS forms and its attachment to bacterial cells were observed when a bacterial colony was subjected to SEM imaging (Fig. 5A). At several locations in the colony, bacterial cells were covered by multiple layers of polysaccharide networks (Fig. 5B).
Fig. 4.
Topographical AFM images of purified (A) HePS48hr and (B) HePS144hr, (C) LAB in the culture medium collected at 48 hr and (D) 144 hr of cultivation. The white arrows indicate bacterial cells. The scan sizes are 3 μm × 3 μm (A, B) and 20 μm × 20 μm (C, D).
Fig. 5.
SEM images of (A) bacterial cells exposed to the environment and (B) bacterial cells covered by the layers of HePS in a bacterial colony grown on MRS agar.
DISCUSSION
A problem hampering industrial use of HePSs produced by LAB and purified from the culture medium is the decline of their yields and viscosity during a prolonged period of cultivation, which is a commonly observed feature of bacterial EPS production [35, 36]. In the present study, remarkable losses of the viscosity of the culture medium, up to 68%, were observed when the cultivation period was extended from 48 hr to 144 hr. It has already been reported that the production yield of the present HePS decreases during a prolonged period of cultivation [41]. As expected, it was confirmed in the present study that the ratio of the smaller molecular-weight fraction of the HePS significantly increased after a prolonged period of cultivation, as shown in Fig. 3B. This implies that HePS-degrading enzymes such as glycosidases and 1,3-glucanases, both of which are capable of hydrolyzing the main chain of the HePS consisting of 1,3-glucan, may have leaked out from dead bacterial cells into the medium, as pointed out by Pham et al. [36]. It is unlikely that polysaccharide lyases, which are capable of hydrolyzing uronic acid-containing polysaccharides via a β-elimination mechanism, participate in the degradation of the present HePS, because of the absence of uronic acid in the HePS [44]. The possibility that unknown enzymes capable of degrading HePS via a β-elimination mechanism, such as those similar to α-glucan lyases that might have occurred in the present study, cannot be excluded [45]. Our earlier attempts to isolate HePS-degrading enzymes were unsuccessful, but further investigation is needed. In our preliminary studies, enzymatic activities for releasing galactose residues from the HePS were detected in cell-surface components extracted using 0.1 M glycine (unpublished data), suggesting that the heterogeneity found in the distribution of side chains of the present HePS may have resulted from the action of such enzymes [39]. However, a prolonged period of cultivation had few effects on the chemical structures of side chains, as shown in Table 1. Therefore, enzymatic activities for modifying side chains are likely to be very weak and to have had little influence on the decline in the viscosity of the culture medium during a prolonged period of cultivation. In the case of Lactobacillus rhamnosus R, prolongation of the period of incubation for 72 hr resulted in up to an approximately 20% loss of the viscosity of the culture medium, which was accompanied by increasing ratios of the low-molecular-weight (2.5 × 104 Da) fraction of the EPS [36]. The viscosity of the EPS also decreased when it was mixed with a cell extract and incubated. Consequently, the researchers concluded that the loss of viscosity was attributed to enzymatic degradation of the EPS by the concerted action of glycohydrolases, such as α-d-glucosidase, β-d-glucosidase, α-d-galactosidase, β-d-galactosidase, β-d-glucuronidase, and α-l-rhamnosidase. Exploration of inhibitors against such EPS-degrading enzymes may provide a means for preventing viscosity losses of LAB-derived HePSs during cultivation.
The HePS produced by L. fermentum MTCC 25067 has been reported to exhibit high viscosities comparable to xanthan gum due to the formation of supramolecular networks of the polysaccharide as a result of lateral association of multiple molecular chains [40]. In accordance with these previous results, AFM images of both HePS48hr and HePS144hr revealed network structures in this study. Additionally, the population of thinner and shorter polysaccharide fibers was larger in HePS48hr than in HePS144hr. It is reasonable to relate the degradation of HePS molecules to the decrease in viscosity, whereas the influences of variabilities in side chains of HePS are controversial. Hassler and Doherty [46] reported that removal of terminal mannose from the side chain of xanthan gum led to a reduction of its viscosity, but successive removal of glucuronic acid from the side chain increased the viscosity in comparison with unmodified xanthan gum. Additionally, the loss of galactose residues in the side chain of xyloglucan derived from tamarind seeds increased the hydrophobicity of the polysaccharide molecule and enhanced its gelling ability [47]. This observation is consistent with the present results in that the chemical structure of side chains appeared to be preserved to a large degree during cultivation for 144 hr and no enhancement of the network formation was noticeable in the present study.
As shown in Fig. 1D, the 48-hr culture medium gave one order of magnitude greater viscosity (53 mPa at an estimated shear rate of 17/s) than 0.02% (w/v) solutions of HePS48hr dissolved in the fresh MRS broth (1.6 mPa at a shear rate of 15.8/s). It is therefore likely that interactions between HePS networks and bacterial cells and/or their metabolites contributed largely to the high viscosity of the 48-hr culture medium. AFM images of the culture media collected at 48 hr and 144 hr and air-dried on a mica surface revealed that the HePS networks surrounding bacterial cells in the 144-hr culture medium were totally disintegrated compared with those in the 48-hr culture medium (Fig. 4C and 4D). To the best of our knowledge, this is the first report to demonstrate the loss of HePS networks surrounding bacterial cells after prolonged cultivation. Intriguingly, the heights of several cross-linking regions of the HePS network in the 144-hr culture medium were significantly greater than those in other parts of the network (Fig. 4D), while no such tall cross-linking regions existed in the purified HePS144hr (Fig. 4B). Pletikapić et al. [48] reported similar structural features found in marine gels and presumed that tall cross-linking regions might have resulted from hydrophobic interactions between specific polysaccharide segments. In the case of the present HePS, the loss of side chains is expected to promote interchain association between main chains leading to the formation of tall cross-linking regions. In contrast, tall cross-linking regions were not seen in the purified HePS144hr, suggesting that certain components in the culture medium may be required for their formation. In addition, SEM images demonstrated the presence of HePS networks in the immediate proximity of bacterial cell surfaces as well as the formation of several polysaccharide layers covering bacterial cells. Therefore, attachment of the HePS network to the bacterial cell surface is highly likely to be the nature of the strain, although further investigation is needed to prove that it also occurs in liquid media. The formation of similar HePS networks has been reported in the case of the HePS produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2483 grown in a 10% (w/v) reconstituted skim milk [49]. Interactions between the present HePS and food matrices should be the subject of future investigations.
The viscosity of the culture medium was highly variable from the beginning to the middle of the stationary growth phase, as shown in Fig. 1D. This may reflect the occurrence of random release of HePS from bacterial cells during that period, considering the existence of several factors influencing the interaction between HePS molecules and bacterial cell surfaces. In contrast, little variability was found in the viscosity of the culture medium in the late stationary phase. This may represent the situation of most HePS molecules being detached from dead bacterial cell surfaces. As commonly recognized, the cytoplasmic membrane loses its integrity after bacterial cell death, leading to increased cell membrane permeability. This results in depolarization of the bacterial cell membrane and a change in pH within the cell wall, triggering activation of murein hydrolases, which can degrade the peptide glycan [50]. Therefore, loss of interaction between dead bacterial cell surfaces and the HePS network is also presumable. Previous whole-genome sequencing of the present strain revealed that the strain biosynthesized a slime HePS via the Wzx/Wzy-dependent pathway [51, 52]. According to the pathway, sugar repeating units, anchored to undecaprenyl-phosphate (C55-P) lipid carriers, are synthesized in the cytoplasm, transferred one by one into the periplasmic space by the Wzx protein (flippase), polymerized, and transferred outside by a cooperative action of the Wxy protein (polymerase), polysaccharide co-polymerase (PCP), and the outer membrane polysaccharide export (OPX) proteins. Rausch et al. [53] recently proved that in Staphylococcus aureus the CapA1 activator protein cleaves lipid-linked capsular polysaccharide precursors, releasing C55-P. The precise molecular mechanism of how HePS molecules are released from the lipid carriers in the present strain is still unknown. Vuong et al. [54] reported that the deacetylation of poly-N-acetylglucosamine was essential for its attachment onto the cell surface of Staphylococcus epidermidis, most likely due to the cationic character of the amine group. These pieces of evidence imply that various intermolecular interactions, such as electrostatic, hydrophobic, and hydrogen-bonding interactions, affect the release of HePS molecules from the cell surface of the present strain during the stationary growth phase. Interactions between the present HePS and extracellular DNA, frequently observed during the formation of biofilm by pathogenic bacteria, also need to be elucidated [55].
To summarize, high viscosities of the culture medium for L. fermentum MTCC 25067 were considered to arise mainly from the presence of an intact HePS network and its interaction with bacterial cells. During a prolonged period of cultivation, the HePS network was disintegrated as a result of partial degradation of HePS molecules by the action of EPS-degrading enzymes, which likely leaked out from dead bacterial cells. The present findings shed light on the importance of understanding interactions between EPS molecules and bacterial cell surfaces for enabling control of the viscosity of the culture medium as well as fermented food products. Precise control of culture conditions and exploration of inhibitors against EPS-degrading enzymes are suggested to be effective approaches to avoiding a loss of viscosity. Development of a cell-free HePS production system could be an ambitious alternative solution.
Supplementary
Acknowledgments
We deeply appreciate Professor Richard W. Hartel and Dr. Hassan Firoozmand (Department of Food Science, University of Wisconsin-Madison) for their technical assistance with rheological experiments. This work was partially supported by the USDA National Institute of Food and Agriculture, Hatch project 1012819.
REFERENCES
- 1.Kumar AS, Mody K, Jha B. 2007. Bacterial exopolysaccharides—a perception. J Basic Microbiol 47: 103–117. [DOI] [PubMed] [Google Scholar]
- 2.Monsan P, Bozonnet S, Albenne C, Joucla G, Willemot RM, Remaud-Siméon M. 2001. Homopolysaccharides from lactic acid bacteria. Int Dairy J 11: 675–685. [Google Scholar]
- 3.Notararigo S, Nácher-Vázquez M, Ibarburu I, Werning ML, de Palencia PF, Dueñas MT, Aznar R, López P, Prieto A. 2013. Comparative analysis of production and purification of homo- and hetero-polysaccharides produced by lactic acid bacteria. Carbohydr Polym 93: 57–64. [DOI] [PubMed] [Google Scholar]
- 4.Welman AD, Maddox IS. 2003. Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Biotechnol 21: 269–274. [DOI] [PubMed] [Google Scholar]
- 5.Chang WS, van de Mortel M, Nielsen L, Nino de Guzman G, Li X, Halverson LJ. 2007. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J Bacteriol 189: 8290–8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirog TP. 1997. Role of acinetobacter sp. exopolysaccharides in protection against heavy metal ions. Microbiology 66: 284–288. [Google Scholar]
- 7.Poli A, Anzelmo G, Nicolaus B. 2010. Bacterial exopolysaccharides from extreme marine habitats: production, characterization and biological activities. Mar Drugs 8: 1779–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freitas F, Alves VD, Carvalheira M, Costa N, Oliveira R, Reis MAM. 2009. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr Polym 78: 549–556. [Google Scholar]
- 9.Freitas F, Alves VD, Pais J, Costa N, Oliveira C, Mafra L, Hilliou L, Oliveira R, Reis MA. 2009b. Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour Technol 100: 859–865. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P, Diwan B. 2016. Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep (Amst) 13: 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66: 506–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moscovici M. 2015. Present and future medical applications of microbial exopolysaccharides. Front Microbiol 6: 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farris S, Unalan IU, Introzzi L, Fuentes-Alventosa JM, Cozzolino CA. 2014. Pullulan-based films and coatings for food packaging: Present applications, emerging opportunities, and future challenges. J Appl Polym Sci 131: 40539. [Google Scholar]
- 14.Hassan AN, Ipsen R, Janzen T, Qvist KB. 2003. Microstructure and rheology of yogurt made with cultures differing only in their ability to produce exopolysaccharides. J Dairy Sci 86: 1632–1638. [DOI] [PubMed] [Google Scholar]
- 15.Hess SJ, Roberts RF, Ziegler GR. 1997. Rheological properties of nonfat yogurt stabilized using Lactobacillus delbrueckii ssp. bulgaricus producing exopolysaccharide or using commercial stabilizer systems. J Dairy Sci 80: 252–263. [Google Scholar]
- 16.Jiménez-Pranteda ML, Poncelet D, Náder-Macías ME, Arcos A, Aguilera M, Monteoliva-Sánchez M, Ramos-Cormenzana A. 2012. Stability of lactobacilli encapsulated in various microbial polymers. J Biosci Bioeng 113: 179–184. [DOI] [PubMed] [Google Scholar]
- 17.Kanmani P, Satish kumar R, Yuvaraj N, Paari KA, Pattukumar V, Arul V. 2011. Production and purification of a novel exopolysaccharide from lactic acid bacterium Streptococcus phocae PI80 and its functional characteristics activity in vitro. Bioresour Technol 102: 4827–4833. [DOI] [PubMed] [Google Scholar]
- 18.Piermaria JA, de la Canal ML, Abraham AG. 2008. Gelling properties of kefiran, a food-grade polysaccharide obtained from kefir grain. Food Hydrocoll 22: 1520–1527. [Google Scholar]
- 19.Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, Motherway MO, Shanahan F, Nally K, Dougan G, van Sinderen D. 2012. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci USA 109: 2108–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaoka M, Hashimoto S, Watanabe T, Yokokura T, Mori Y. 1994. Anti-ulcer effects of lactic acid bacteria and their cell wall polysaccharides. Biol Pharm Bull 17: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. 2014. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol 63: 133–139. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Zhou Z, Li Y, Zhou L, Ding Q, Xu L. 2016. Isolated exopolysaccharides from Lactobacillus rhamnosus GG alleviated adipogenesis mediated by TLR2 in mice. Sci Rep 6: 36083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajaj IB, Survase SA, Saudagar PS, Singhal RS. 2007. Gellan gum: Fermentative production, downstream processing and applications. Food Technol Biotechnol 45: 341–354. [Google Scholar]
- 24.Cao Y, Dickinson E, Wedlock DJ. 1990. Creaming and flocculation in emulsions containing polysaccharide. Food Hydrocoll 4: 185–195. [Google Scholar]
- 25.Leemhuis H, Pijning T, Dobruchowska JM, van Leeuwen SS, Kralj S, Dijkstra BW, Dijkhuizen L. 2013. Glucansucrases: three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J Biotechnol 163: 250–272. [DOI] [PubMed] [Google Scholar]
- 26.Palaniraj A, Jayaraman V. 2011. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J Food Eng 106: 1–12. [Google Scholar]
- 27.Prajapati VD, Jani GK, Khanda SM. 2013. Pullulan: an exopolysaccharide and its various applications. Carbohydr Polym 95: 540–549. [DOI] [PubMed] [Google Scholar]
- 28.Torino MI, Font de Valdez G, Mozzi F. 2015. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front Microbiol 6: 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zannini E, Waters DM, Coffey A, Arendt EK. 2016. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl Microbiol Biotechnol 100: 1121–1135. [DOI] [PubMed] [Google Scholar]
- 30.Vaningelgem F, Zamfir M, Mozzi F, Adriany T, Vancanneyt M, Swings J, De Vuyst L. 2004. Biodiversity of exopolysaccharides produced by Streptococcus thermophilus strains is reflected in their production and their molecular and functional characteristics. Appl Environ Microbiol 70: 900–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambo-Fodje AM, Leeman M, Wahlund KG, Nyman M, Öste R, Larsson H. 2007. Molar mass and rheological characterisation of an exopolysaccharide from Pediococcus damnosus 2.6. Carbohydr Polym 68: 577–586. [Google Scholar]
- 32.Tuinier R, van Casteren WHM, Looijesteijn PJ, Schols HA, Voragen AGJ, Zoon P. 2001. Effects of structural modifications on some physical characteristics of exopolysaccharides from Lactococcus lactis. Biopolymers 59: 160–166. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt NB, Gunther CM, Liberatore MW. 2011. Increasing viscosity in entangled polyelectrolyte solutions by the addition of salt. Polymer (Guildf) 52: 2437–2444. [Google Scholar]
- 34.Zeidan AA, Poulsen VK, Janzen T, Buldo P, Derkx PMF, Øregaard G, Neves AR. 2017. Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiol Rev 41 Supp_1: S168–S200. [DOI] [PubMed] [Google Scholar]
- 35.Cerning J. 1995. Production of exopolysaccharides by lactic acid bacteria and dairy propionibacteria. Lait 75: 463–472. [Google Scholar]
- 36.Pham PL, Dupont I, Roy D, Lapointe G, Cerning J. 2000. Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl Environ Microbiol 66: 2302–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gancel F, Novel G. 1994. Exopolysaccharide production by Streptococcus salivarius ssp. thermophilus cultures. 2. Distinct modes of polymer production and degradation among clonal variants. J Dairy Sci 77: 689–695. [Google Scholar]
- 38.Leo F, Hashida S, Kumagai D, Uchida K, Motoshima H, Arai I, Asakuma S, Fukuda K, Urashima T. 2007. Studies on a neutral exopolysaccharide of Lactobacillus fermentum TDS030603. J Appl Glycosci 54: 223–229. [Google Scholar]
- 39.Gerwig GJ, Dobruchowska JM, Shi T, Urashima T, Fukuda K, Kamerling JP. 2013. Structure determination of the exopolysaccharide of Lactobacillus fermentum TDS030603—a revision. Carbohydr Res 378: 84–90. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda S, Murayama D, Tsurumaki A, Sato S, Urashima T, Fukuda K. 2019. Rheological characteristics and supramolecular structure of the exopolysaccharide produced by Lactobacillus fermentum MTCC 25067. Carbohydr Polym 218: 226–233. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda K, Shi T, Nagami K, Leo F, Nakamura T, Yasuda K, Senda A, Motoshima H, Urashima T. 2010. Effects of carbohydrate source on physicochemical properties of the exopolysaccharide produced by Lactobacillus fermentum TDS030603 in a chemically defined medium. Carbohydr Polym 79: 1040–1045. [Google Scholar]
- 42.Dubois M, Gills KA, Hamilton JK, Roberts PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356. [Google Scholar]
- 43.Dean SN, Leary DH, Sullivan CJ, Oh E, Walper SA. 2019. Isolation and characterization of Lactobacillus-derived membrane vesicles. Sci Rep 9: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linhardt RJ, Galliher PM, Cooney CL. 1986. Polysaccharide lyases. Appl Biochem Biotechnol 12: 135–176. [DOI] [PubMed] [Google Scholar]
- 45.Lee SS, Yu S, Withers SG. 2003. Detailed dissection of a new mechanism for glycoside cleavage: α-1,4-glucan lyase. Biochemistry 42: 13081–13090. [DOI] [PubMed] [Google Scholar]
- 46.Hassler RA, Doherty DH. 1990. Genetic engineering of polysaccharide structure: production of variants of xanthan gum in Xanthomonas campestris. Biotechnol Prog 6: 182–187. [DOI] [PubMed] [Google Scholar]
- 47.Reid JSG, Edwards M, Dea ICM. 1988. Enzymatic modification of matural seed gum. In Gums and stabilisers for the food industry 4, Phillips GO, Wedlock DJ, Williams PA (eds), IRL Press, Oxford, pp. 391–398. [Google Scholar]
- 48.Pletikapić G, Lannon H, Murvai Ü, Kellermayer MSZ, Svetličić V, Brujic J. 2014. Self-assembly of polysaccharides gives rise to distinct mechanical signatures in marine gels. Biophys J 107: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goh KKT, Derek Haisman R, Singh H. 2005. Examination of exopolysaccharide produced by Lactobacillus delbrueckii subsp. bulgaricus using confocal laser scanning and scanning electron microscopy techniques. J Food Sci 70: M224–M229. [Google Scholar]
- 50.Rice KC, Bayles KW. 2008. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev 72: 85–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aryantini NPD, Prajapati JB, Urashima T, Fukuda K. 2017. Complete genome sequence of Lactobacillus fermentum MTCC 25067 (formerly TDS030603), a viscous exopolysaccharide-producing strain isolated from Indian fermented milk. Genome Announc 5: e00091–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmid J, Sieber V, Rehm B. 2015. Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 6: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rausch M, Deisinger JP, Ulm H, Müller A, Li W, Hardt P, Wang X, Li X, Sylvester M, Engeser M, Vollmer W, Müller CE, Sahl HG, Lee JC, Schneider T. 2019. Coordination of capsule assembly and cell wall biosynthesis in Staphylococcus aureus. Nat Commun 10: 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, Otto M. 2004. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem 279: 54881–54886. [DOI] [PubMed] [Google Scholar]
- 55.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295: 1487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.