Abstract
Background and Objective
The temporal bone window (TBW) for transcranial Doppler (TCD) often fails to insonate the anterior cerebral artery (ACA). The frontal bone window (FBW) has never been evaluated in intensive care units (ICU). The main objective was to determine the ability of the FBW to assess ACA velocities in critically ill patients.
Methods
A prospective study was conducted in two ICUs of the Montpellier University Hospital (France), between November 2014 and September 2016. Adult patients admitted to ICU for brain injury, with a Glasgow Coma Scale score ≤ 13, were enrolled within 3 days after admission. A first TCD examination was carried out bilaterally through the TBW and FBW by an intensivist expert in TCD, repeated by the same examiner, and 15 min later by an intensivist certified in TCD, designated as non-expert, blinded. The success of the FBW examinations was defined by the ability to measure the ACA velocities. Intra- and interobserver agreements were analyzed according to the Bland and Altman method.
Results
A total of 147 patients were analyzed. The FBW succeeded in insonating the ACA in 66 patients [45%, CI (37–53)], 45 bilaterally and 21 unilaterally. For 16 patients (11%), the FBW was the only way to measure ACA velocities. By combining the two techniques, the ACA success rate increased from 62% CI (54–70) to 73% CI (65–79) (P = 0.05). Intra- and interobserver mean biases and 95% limits of agreement for ACA systolic velocity measurements through the FBW were 1 (− 33 to 35) and 2 (− 34 to 38) cm s−1, respectively. For paired TBW and FBW measures of ACA velocities, mean biases (± SD) for ACA systolic, and mean and diastolic velocities were relatively close to zero, but negatives (− 7 ± 33, − 2 ± 19, − 1 ± 15 cm s−1, respectively), highlighting that ACA velocities were lower with the FBW (A2 segment) than TBW (A1 segment). The correlation coefficient for ACA systolic velocities measured by the FBW and TBW was R = 0.47, CI (0.28–0.62). No risk factors for failure of the FBW were identified.
Conclusions
In ICU, the FBW was able to insonate the ACA in 45% of patients admitted for brain injury, without the use of contrast agents. The FBW could improve the detection of ACA vasospasms.
Electronic supplementary material
The online version of this article (10.1007/s12028-019-00869-3) contains supplementary material, which is available to authorized users.
Keywords: Transcranial Doppler ultrasonography (D017585), Anterior cerebral artery (D020771), Cerebral vasospasm (D020301), Neurophysiological monitoring (D064926), Reliability and validity (D015203), Frontal acoustic bone window
Introduction
Transcranial Doppler ultrasonography (TCD) is a crucial monitoring tool in neurocritical care units [1–3]. TCD is particularly useful in patients suffering from aneurysmal subarachnoid hemorrhage (SAH) [4–6], traumatic brain injury (TBI) [7–9], or cerebral stroke [10, 11]. TCD is reputed to be a low-cost and readily repeatable diagnostic imaging test, offering a noninvasive real-time monitoring of cerebral circulation at bedside, which suits perfectly for the intensive care unit (ICU) setting [1–3].
In order to insonate basal cerebral arteries and Willis polygon, the ultrasound beams have to penetrate the skull through a proper acoustic bone window. The temporal bone window (TBW) described by Aaslid et al. [12] in 1982 has become the standard approach for TCD examination in adults. However, the conventional TBW has two main limits. First, velocity measurement is not feasible in approximately 10–30% of patients by TBW due to a lack of echogenicity [2, 13, 14]. Second, the TBW is most often inadequate to insonate the anterior cerebral artery (ACA), especially the A2 segment, because of unfavorable angle of insonation [2, 23]. A segmental disease of the ACA, such as a vasospasm occurring in case of SAH, will be consequently undetectable using TBW [6, 15–18]. At times, patients receive delayed catheter-based therapies due to the fact that the ACA could not be insonated.
First described in neuropediatric and neuroradiology, the frontal bone window (FBW) is a poorly known alternative approach for TCD [19–23]. By positioning the probe above the supraorbital arcade, the FBW allows to measure the ACA velocities with low-angle correction. Previous studies in non-critically ill patients showed that the FBW allowed to assess cerebral artery velocities when the TBW failed [23]. Nevertheless, these observations were not established in critically ill patients, who are more likely to develop cerebral vasospasms.
The main purpose of the present study was to determine the ability of the FBW to assess ACA velocities in patients admitted to ICU for brain injury. Secondary goals were to define risk factors for failure, intra- and interobserver agreement, and correlation between TBW and FBW measures of ACA velocities.
Materials and Methods
Study Design
A prospective study was conducted in the two critical care units of the Montpellier University Hospital (France), the trauma ICU and the neurological ICU. The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. The institutional review board waived the need for informed consent (IDRCB-2014A0143641). The registration number was NCT02832895.
Eligibility Criteria
Patients 18 years of age or older, admitted to ICU for brain injury (i.e., TBI; SAH; intracranial hematoma; cerebral stroke; post-cardiac arrest syndrome; encephalitis; or craniotomy for brain tumor) with an initial Glasgow Coma Scale score less than or equal to 13, were eligible for the present study. Patients were enrolled within the first 3 days after admission, once they were clinically stable. Patients with clinical suspicion of brain death, or refusing to participate (or refusal of the legally authorized representative), were excluded from analysis.
Data Collection
Clinical characteristics and biological parameters upon inclusion were noted, such as body temperature, mean arterial pressure, glycemia, natremia, hemoglobin level, and arterial blood gas values. The hospital length of stay and survival on hospital discharge were collected.
TCD Protocol
According to a standardized protocol, a first TCD examination was carried out bilaterally by the TBW and FBW in all studied patients by an intensivist expert in TCD imaging (with more than 10 years of experience in TCD imaging in ICU, designated as referent for TCD in the center, and teaching the new FBW). Subsequently, the same examiner performed 15 min later a second TCD examination through the FBW. Afterward, a third TCD examination was performed 15 min later through the FBW by a second examiner. The second examiner, blinded to the results of the expert, was an intensivist certified in ultrasound imaging and trained to the new technique (i.e., more than 30 TCD examinations through the FBW), designated as non-expert. All TCD examinations were achieved in clinically stable patients, postured in supine position with 15-30° head up. The maximal duration of each examination was arbitrarily fixed to 10 min. No clinical or therapeutic interventions were allowed during the study procedure.
TCD examinations were performed using ultrasound machines Vivid-i™ or Vivid-q™ (GE Healthcare®, Chicago, USA), connected to a 3S-RS® (GE Healthcare®) adult probe (1.7-4 MHz). The settings of ultrasound machines are as follows: 2D mode, emission frequency of 2.5 MHz; color mode, emission frequency of 1.8 MHz, gain of 15 dB, and scale of 2 kHz. Ultrasound contrast agents were not allowed. Once the targeted blood vessel was insonated with the two-dimensional color-coded image, velocities were measured using angle-corrected pulsed wave Doppler. According to guidelines [2], Doppler tracing lasting at least 10 cardiac cycles was recorded after a 30-s stabilized period and the cycle with the highest systolic velocity was studied. Peak systolic velocity, end-diastolic velocity, and mean velocity (respectively, SV, DV, and MV, expressed in cm s−1) were measured, and the pulsatility index (corresponding to [SV–DV]/MV) was determined. Depth of measurement (expressed in cm) and angle correction (expressed in degrees) were also collected.
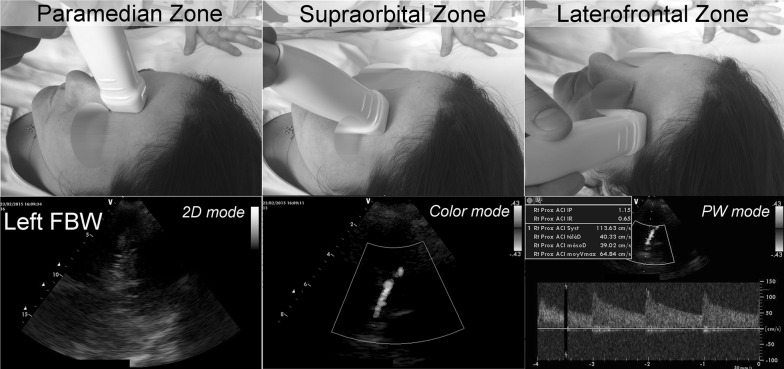
The FBW examinations were conducted as previously described [19–23]: The transducer was positioned vertically at the paramedian frontal zone (index mark at the top), rotated 90° outward, and moved horizontally (index mark laterally) to the supraorbital zone at the top of the orbital arcade, up to the laterofrontal zone (Fig. 1 and Supplementary video). Only the A2 segments of the ACA were recorded by the FBW, while only the A1 segments were measured by the TBW. If ACA was insonated with multiple approaches, the one with maximal velocities was reported.
Fig. 1.
Description of the FBW technique for TCD ultrasonography. The transducer is positioned vertically at the paramedian frontal zone (index mark at the top), rotated 90° outward and shifted horizontally (index mark laterally) to the supraorbital zone at the top of the orbital arcade, up to the laterofrontal zone. Visualization of the brain parenchyma in B-mode (two-dimensional mode), insonation of the A2 segment of the ACA in color mode, and blood flow velocity measurements using the pulsed Doppler (PW) mode. [ACA anterior cerebral artery, PW pulsed waved Doppler]
Main study Endpoints
The success of the TBW and FBW examinations was defined by the ability to measure the ACA velocities on the first TCD examination. Patients were thus categorized into two groups: the FBW success group (unilateral or bilateral success of FBW examination) or the FBW failure group (bilateral failure of FBW examination). The diagnostic contribution of the FBW was expressed as the increase in the proportion of patients with a TCD success for the ACA, from the TBW examination to the combination of TBW + FBW.
Statistical Analysis
The study population was first described, and characteristics of the groups FBW failure and FBW success were compared. Qualitative variables were expressed by number (percentage) and tested using the Chi-squared test. For quantitative variables, the normality of distribution was tested using Shapiro–Wilk test and expressed as mean (SD) or median (IQR). Unpaired quantitative data were compared using Student’s t test or Mann–Whitney test. Paired quantitative data were compared using paired Student’s t test or Wilcoxon test.
The TBW and FBW success was described as percentages with 95% confidence intervals (CI). As recent literature reported a FBW success rate of about 40% in non-critically ill patients [23], a sample size of 150 subjects would have approximately 80% power to determine a success rate arbitrarily estimated to 40%, using a 95% CI with a ± 8% accuracy, considering an alpha risk of 0.05. Risk factors for FBW failure were investigated. Intra- and interobserver concordance was evaluated by the kappa coefficient. Agreement between ACA measurements was subsequently studied according to the Bland and Altman method [24], expressed as mean bias with 95% limits of agreement (LOA). The statistical relationship between A1 (TBW) and A2 (FBW) velocities was expressed using the Pearson’s correlation coefficient (r).
Calculated P values less than or equal to 0.05 were considered statistically significant for all two-sided tests. Statistical analysis was performed using Prism 6™ software (GraphPad®, La Jolla, USA).
Results
Study Population
Between November 2014 and September 2016, 152 patients were included in the present study (Fig. S1). Five were secondarily excluded (two for consent withdrawal and three for missing data). Therefore, a total of 147 patients were analyzed, 60 (41%) in the trauma ICU and 87 (59%) in the neurological ICU.
The cohort consisted of 96 men (65%), with mean age 51 ± 19 years (Table 1). The main causes of ICU admission were TBI (27%) and SAH (25%). Eleven patients (8%) had frontotemporal decompressive craniectomy. At the time of the study, clinical and biological parameters were stabilized. The mean length of stay was 21 ± 20 days, simplified acute physiology score (SAPS II) was 43 ± 17, and mortality rate on day 28 was 27%.
Table 1.
Study population (n = 147 patients)
| Characteristics | All patients (n = 147) |
| Demographic data | |
| Age, mean ± SD, y | 51 ± 19 |
| Gender, no. of men (%)/women (%) | 96 (65)/51 (35) |
| Body mass index, mean ± SD, kg/m2 | 26 ± 5 |
| Severity | |
| Initial GCS score, mean ± SD | 7 ± 4 |
| SAPS score, mean ± SD | 43 ± 17 |
| Length of stay in ICU, mean ± SD, d | 21 ± 20 |
| Mortality on day 28, No. (%) | 40 (27%) |
| Pathology, no. (%) | |
| Traumatic brain injury | 39 (27) |
| Subarachnoid hemorrhage | 37 (25) |
| Spontaneous intracranial hematoma | 18 (12) |
| Ischemic stroke | 17 (12) |
| Post-cardiac arrest syndrome | 15 (10) |
| Metabolic encephalopathy | 14 (9) |
| Brain tumor | 7 (5) |
| Decompressive craniectomy, no. (%) | 11 (7) |
| Inclusion day, no. (%) | |
| Day 1 | 35 (24) |
| Day 2 | 48 (33) |
| Day 3 | 64 (43) |
| Sedation at inclusion day, no. (%) | |
| No sedative drugs | 29 (20) |
| Midazolam + Sufentanil | 100 (68) |
| Other drugs | 18 (12) |
| Clinical parameters and laboratory values at inclusion, mean ± SD | |
| Body temperature, °C | 36.9 ± 1.1 |
| Mean arterial pressure, mmHg | 85 ± 13 |
| Glycemia, g/L | 1.3 ± 0.3 |
| Arterial SO2, % | 98 ± 2 |
| Arterial PCO2, mmHg | 37 ± 6 |
| Arterial pH | 7.41 ± 0.07 |
| Natremia, mmol/L | 141 ± 4 |
| Hemoglobin, g/L | 117 ± 20 |
GCS Glasgow Coma Scale, PCO2 partial pressure of carbon dioxide, SAPS simplified acute physiology score, SD standard deviation, SO2 oxygen saturation
TBW and FBW Success Rates
Among the studied population, the TBW and FBW success rates were 62% (95% CI 54–70%) and 45% (95% CI 37–53%), respectively. The FBW success rate did not differ significantly between the two participating units: 48% (95% CI 35–60%) in the trauma ICU vs. 41% (95% CI 30–51%) in the neurological ICU (P = 0.41).
Diagnostic Contribution of the FBW
In our population, the TBW did not provide bilateral assessment of ACA velocities in 77 patients (52%), including 56 (38%) with bilateral TBW failure (Fig. S1). Among them, the FBW was a success in 25 (29%). Moreover, for 16 of these patients, the FBW was the only way to measure ACA velocities. The ACA success rate increased therefore from 62% (95% CI 54–70%) using only the TBW to 73% (95% CI 65–79%) using the combination TBW + FBW (P = 0.05) (Fig. 2). Thus, the diagnostic contribution of the FBW for ACA was estimated to a + 11% increase of the ACA insonation rate.
Fig. 2.
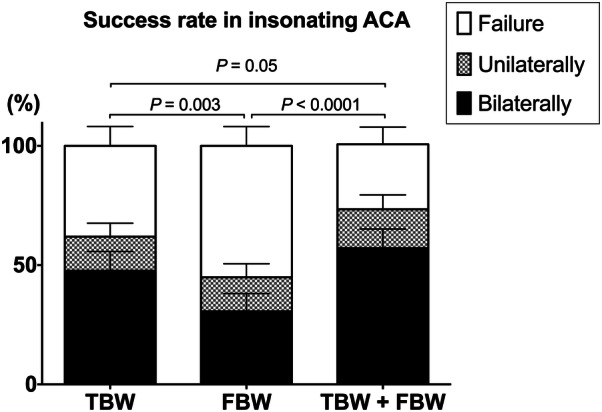
Success rates in insonating the ACA by the TBW, FBW and the combination of both techniques. The success is the ability to insonate the ACA, uni- or bilaterally (stacked percentage bar plot and upper range of the 95% confidence interval). One-way ANOVA, P values for Tukey’s multiple comparisons tests. [ACA anterior cerebral artery, FBW frontal bone window, TBW temporal bone window]
Risk Factors for the FBW Failure
The univariate analyses did not reveal a variable significantly associated with an increased risk of FBW failure. The relative risks (RR) of the most commonly known risk factors, an age over 60 and the female gender, were 1.2 (95% CI 0.9–1.5) and 1.2 (95% CI 0.9–1.6), respectively. Craniectomy was not significantly associated with a lower FBW failure rate (RR = 0.6, 95% CI 0.3–1.4). The FBW success rate did not differ significantly between non-craniectomized patients (n = 136) and the entire cohort (Table S2 and Figure S2).
Agreement Between TBW and FBW Measures
All data derived from the first TCD examination are presented in Table S1. Notably, the ACA systolic velocities were lower on average when measured by the FBW (A2 segment) than TBW (A1), 79 ± 31 vs. 93 ± 38 cm s−1, respectively (P = 0.04), as well as pulsatility index. The other parameters did not differ significantly between the two windows.
Agreement between paired TBW and FBW measures of ACA velocities is presented in Table 2. Mean biases (± SD) for ACA systolic and mean and diastolic velocities were relatively close to zero, but negatives (− 7 ± 33, − 2 ± 19, − 1 ± 15 cm s−1, respectively), confirming that ACA velocities were lower using the FBW (A2 segment).
Table 2.
Intra- and interobserver agreements for the FBW, and correlation between TBW and FBW measures
| FBW | FBW vs. TBW | ||
|---|---|---|---|
| ACA measurements | Intraobserver agreement | Interobserver agreement | Agreement between A2 (FBW) and A1 (TBW) measures |
| Systolic velocity (cm s−1) | |||
| Mean bias ± SD | 1 ± 17 | 2 ± 18 | -7 ± 33 |
| Range (95% LOA) | 68 (− 33 to 35) | 72 (− 34 to 38) | 131 (− 73 to 58) |
| R (95% CI) | 0.84 (0.77–0.88) | 0.64 (0.47–0.77) | 0.47 (0.28–0.62) |
| R2 | 0.70 | 0.41 | 0.22 |
| Mean velocity (cm s−1) | |||
| Mean bias ± SD | 1 ± 9 | 3 ± 14 | -2 ± 19 |
| Range (95% LOA) | 35 (− 17 to 18) | 54 (− 24 to 30) | 75 (− 39 to 36) |
| R (95% CI) | 0.90 (0.85–0.93) | 0.70 (0.57–0.79) | 0.42 (0.23–0.59) |
| R2 | 0.80 | 0.49 | 0.18 |
| Diastolic velocity (cm s−1) | |||
| Mean bias ± SD | 0 ± 6 | -2 ± 9 | -1 ± 15 |
| Range (95% LOA) | 22 (− 11 to 11) | 34 (− 19 to 15) | 60 (− 31 to 29) |
| R (95% CI) | 0.87 (0.82–0.91) | 0.69 (0.56–0.78) | 0.17 (-0.06–0.39) |
| R2 | 0.76 | 0.48 | 0.03 |
| Pulsatility index | |||
| Mean bias ± SD | 0 ± 0.18 | 0 ± 0.22 | -0.07 ± 0.28 |
| Range (95% LOA) | 0.72 (− 0.36 to 0.36) | 0.84 (− 0.42 to 0.42) | 1.10 (− 0.62 to 0.48) |
| R (95% CI) | 0.78 (0.70–0.85) | 0.71 (0.59–0.80) | 0.53 (0.35–0.67) |
| R2 | 0.62 | 0.50 | 0.28 |
ACA anterior cerebral artery, A1 segment A1 of the ACA, A2 segment A2 of the ACA, CI confidence interval; FBW frontal bone window, LOA limits of agreement, R Pearson’s correlation coefficient; R2 determination coefficient, SD standard deviation, TBW temporal bone window, TCD transcranial Doppler
Intra- and Interobserver Agreement of FBW Measures
In 100% of cases, the second TCD examination was a success when the first TCD examination succeeded, leading to an intraobserver kappa coefficient of 1.00 (95% CI 0.89–1.11). Besides, the third TCD examination performed by blinded examiners was a success in 90% of cases when the first TCD examination succeeded, leading to an interobserver kappa coefficient of 0.80 (95% CI 0.69–0.91).
The assessment of intra- and interobserver agreement for ACA velocities and pulsatility index measurements is presented in Table 2. Intra- and interobserver agreements for ACA systolic and mean and diastolic velocities were optimal, with biases close to zero and a limited dispersion (1 ± 17, 1 ± 9, 0 ± 6, and 2 ± 18, 3 ± 14, − 2 ± 9 cm s−1, respectively). Likewise, a strong correlation between measures was found. The corresponding Bland and Altman plots are presented in Fig. 3. It is noteworthy that the intraobserver and interobserver agreements for systolic velocity were comparable in our series, meant by similar 95% LOA (− 33 to 35 cm s−1 and − 34 to 38 cm s−1, respectively).
Fig. 3.
Intra- and interobserver agreement for measurements made by the FBW. Bland and Altman plots representing intra- and interobserver 95% LOA for ACA systolic, mean, diastolic velocities and pulsatility index measured by the FBW. [ACA anterior cerebral artery, FBW frontal bone window, LOA limits of agreement]
Technical Considerations
Technically, the ACA was more frequently insonated in the paramedian and supraorbital areas (39 and 37% of measurements, respectively), while the laterofrontal zone was the less performing (24%) (Table S3). Duration of FBW examinations was 5 ± 3 min on average. The angle correction for measuring ACA velocities tended to be lower through FBW than TBW, 9 ± 14° vs. 22 ± 20°, without reaching significance (P = 0.27).
Discussion
The FBW for TCD was evaluated for the first time in 147 critically ill patients, the largest cohort studied to date. Our population consisted of 65% men, mean age of 51 years, and TBI and SAH were the leading causes of ICU admission. In the present population, the FBW succeeded in insonating the ACA in about one in two patients. The reproducibility was excellent; intra- and interobserver mean biases for ACA velocities were close to zero with limited dispersion. The ACA detection rate increased from 62 to 73% using the combination TBW + FBW, an absolute gain of 11%.
A Crucial Need for ACA Evaluation
In patients with SAH, cerebral velocities are monitored regularly by TCD to detect vasospasm [2–5]. To be efficient, this monitoring requires a specific segmental evaluation of cerebral velocities. The TBW, standard approach for TCD, does not allow in most cases to insonate the A2 segment of the ACA, which is perpendicular to the probe. A segmental vasospasm of A2 will be consequently undetectable by the TBW [15–18], which decreases the chances of receiving appropriate catheter-based therapies, highlighting the need for complementary TCD approaches. Therefore, by adding the FBW approach, treatable lesions of the ACA could be identified which would have gone unnoticed with an isolated TBW approach.
The FBW: A Complementary TCD Technique for Insonating the ACA
First described in a pediatric population [20], the FBW provided ACA measures in 80% of cases. Nevertheless, it is well known that echogenicity in adults is much lower. In a cohort of 163 adults with stroke [23], the FBW success rate was 37% without the use of contrast agents. In line with these results, the FBW success rate was 45% in our cohort. It has been previously shown that the FBW success rate is improved by intravenous contrast agents. Stolz et al. [21] described a success rate of 73% in 75 healthy volunteers. Three years later, the same authors published a detection rate of 86% in 40 control patients with echocontrast enhancement [22]. The use of echocontrast agents could certainly improve the success rate of the FBW in ICU, but further investigations are needed.
Risk Factors for FBW Failure
It is well known that elderly people and female gender are risk factors for failure of the TBW examination [2, 14]. The same risk factors for FBW failure were previously described. Stolz et al. [21] reported that echogenicity of the FBW decreased in women and elderly people, with a strong decrease of ACA insonation rate, 85–22%, when comparing patients younger than 40 and those older than 60 years. In our study, older age and female gender trended to be associated with FBW failure, without reaching significance. Our success rates were 55% in patients younger than 40 and 45% over 60. It is likely that the improvement of echogenicity by echocontrast agents is more marked in young than elderly people. Craniectomized patients tended to have a higher success rate than the non-craniectomized. But this subgroup was small (n = 11) and did not influence the results of the entire cohort (Table S2 and Figure S2). The impact of the frontal bone thickness and calcium content on the FBW echogenicity rate and 2D-imaging quality could be investigated in future studies. Ethnicity was not investigated to comply with the French legislation.
Agreement Between the FBW and TBW Measures
The agreement between ACA velocities measured by the two techniques has already been studied, showing contrasting results. Stolz et al. [22] reported that ACA systolic velocity was higher when measured by the FBW than TBW in healthy patients, 92 ± 23 and 78 ± 16 cm s−1. Our study showed contrasting results, with ACA systolic velocities lower when measured by the FBW (A2 segment) than TBW (A1). To our understanding, this difference is mainly explainable by the fact that A2 is more distal along the arterial tree. Furthermore, the Doppler angle correction was higher for the A1 segment (TBW) than A2 (FBW), which could have led to measurement biases.
Reproducibility of the FBW Technique
To our knowledge, the reproducibility of the FBW had never been evaluated before. In our study, intra- and interobserver agreements of ACA systolic, diastolic, mean velocities, and pulsatility index measurements were excellent with biases close to zero and limited dispersions. Remarkably, intra- and interobserver limits of agreement for systolic velocity measurements were similar, meaning that the expert and non-expert examiners found closely the same values for ACA systolic velocities. For mean and diastolic velocities, larger interobserver LOA was noted, but without clinical relevance. A plausible explanation for this finding is that a small absolute error of ± 5 cm s−1 could have induced a larger relative error for low velocities (diastolic and mean velocity) than high velocities (systolic velocity). Nevertheless, in both healthy patients and SAH, Staalsø et al. [25] found larger intra- and interobserver LOA for MCA mean velocity measured by the TBW than those we observed for ACA by the FBW. Reproducibility of angle correction was not investigated.
The Added Value of the FBW: Improving the ACA Detection Rate
In the study of Yoshimura et al. [23], the combined application of TBW and FBW improved the detection rates of A1 segment from 46 to 59%, and A2 segment from 7 to 44% compared to the TBW alone. The limit of the TBW examination was highlighted in our study, since the TBW was unable to provide bilateral ACA measurements in 52% of patients, which is consistent with previous observations [23]. The FBW provided measurements in about a third of these patients, representing one in six patients of the entire cohort. Thus, by combining the FBW with the standard TBW evaluation, the ACA success rate increased significantly from 62 to 73% of patients. In order to investigate the additional clinical benefit of the FBW, it will be important to study whether abnormal findings are identified by either or both methods, and whether either improves diagnostic certainty or results in changed management. Until then, the importance of an 11% increase in yield for identifying the ACA (any segment) will remain uncertain.
Clinical Perspectives
In clinical practice, the intended use of the FBW examination could be a complement to the TBW examination, not a replacement, as is the case with other alternative approaches [26–28]. Even if focused on different segments, the FBW might be important as a way to make sure at least some of the ACA is identified, or maybe more important as a way to study the A2, which is rarely identified by TBW. Therefore, the new FBW examination could have decisive implications for the bedside monitoring in critically ill patients at risk of vasospasm, such as patients with SAH, in whom a segmental monitoring of cerebral blood flow velocities is crucial.
Limitations of the Study
First, our TBW success rate for insonating the ACA was lower than expected despite the concordance with the findings of Yoshimura et al. [23]. There is a lack of published literature about TCD success rates in ICU, varying from one center to another. Success rates would probably be higher if examinations were performed by expert sonographers rather than intensivists, or with the use of contrast agents. We assume that FBW is a technically difficult test that should be performed by experts, or at least certified intensivists. Even if our results could certainly be transposed to ICU with high-performance ultrasound machines, the interest of FBW is probably more limited in centers performing TCD without imaging assistance. The B-mode also allowed angle correction which is a parameter that must be taken into account when comparing our results with previous literature.
Second, the diagnostic accuracy of the FBW examination was not easy to determine using our study design. There is no gold standard for assessing flow velocities in the A2 segment of the ACA. Test results were not categorized as either positive or negative, so the familiar diagnostic accuracy statistics such as sensitivity, specificity, and predictive values were not estimated [29, 30]. Determining a test positivity cutoff was not possible in our heterogeneous study population. The diagnostic gain of the FBW has to be investigated in further studies in larger cohorts of ICU patients with SAH.
Conclusions
The FBW was able to insonate the ACA in 45% of patients admitted to ICU for brain injury, without the use of contrast agents. The reproducibility was excellent. Combining the TBW with the FBW significantly enhanced the insonation rate of the ACA when compared to the TBW alone. The FBW for TCD could have major implications for clinical practice, mainly improve detection of cerebral vasospasms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Sophie Bringuier for planning the statistical investigations. The authors thank Marie Grillet-Garcia for his contribution to Fig. 1.
Author Contributions
PS designed the study, performed acquisition analysis and interpretation of data, and drafted the manuscript. JC contributed to design the study, performed acquisition analysis, and revised the manuscript critically. CM, PB, GD, and FG performed acquisition analysis and revised the manuscript. XC and PFP helped to design the study and revised the manuscript critically. All authors give their final approval of the version to be published and agree to be accountable for all aspects of the work, by ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Source of Support
Support for this study was provided solely from institutional and/or departmental sources.
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical Approval
The manuscript adheres to ethical guidelines; ethical approval (ID-RCB 2014-A01436-41) was obtained.
Informed Consent
The institutional review board waived the need for informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moheet AM, Livesay SL, Abdelhak T, et al. Standards for neurologic critical care units: a statement for healthcare professionals from The Neurocritical Care Society. Neurocrit Care. 2018;29(2):145–160. doi: 10.1007/s12028-018-0601-1. [DOI] [PubMed] [Google Scholar]
- 2.D’Andrea A, Conte M, Scarafile R, et al. Transcranial Doppler ultrasound: physical principles and principal applications in neurocritical care unit. J Cardiovasc Echography. 2016;26(2):28–41. doi: 10.4103/2211-4122.183746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saqqur M, Zygun D, Demchuk A. Role of transcranial Doppler in neurocritical care. Crit Care Med. 2007;35(5 Suppl):S216–S223. doi: 10.1097/01.CCM.0000260633.66384.FB. [DOI] [PubMed] [Google Scholar]
- 4.Bederson JB, Connolly ES, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 5.Vora YY, Suarez-Almazor M, Steinke DE, Martin ML, Findlay JM. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44(6):1237–1247. [PubMed] [Google Scholar]
- 6.Mastantuono J-M, Combescure C, Elia N, Tramèr MR, Lysakowski C. Transcranial Doppler in the diagnosis of cerebral vasospasm: an updated meta-analysis. Crit Care Med. 2018;46(10):1665. doi: 10.1097/CCM.0000000000003297. [DOI] [PubMed] [Google Scholar]
- 7.Chan KH, Dearden NM, Miller JD, Andrews PJ, Midgley S. Multimodality monitoring as a guide to treatment of intracranial hypertension after severe brain injury. Neurosurgery. 1993;32(4):547–552. doi: 10.1227/00006123-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Ract C, Moigno S, Bruder N, Vigué B. Transcranial Doppler ultrasound goal-directed therapy for the early management of severe traumatic brain injury. Intensive Care Med. 2007;33(4):645–651. doi: 10.1007/s00134-007-0558-6. [DOI] [PubMed] [Google Scholar]
- 9.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007;24 Suppl 1:S1–106. [DOI] [PubMed]
- 10.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 11.D’Andrea A, Conte M, Riegler L, et al. Transcranial Doppler ultrasound: incremental diagnostic role in cryptogenic stroke part II. J Cardiovasc Echography. 2016;26(3):71–77. doi: 10.4103/2211-4122.187947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57(6):769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 13.Marinoni M, Ginanneschi A, Forleo P, Amaducci L. Technical limits in transcranial Doppler recording: inadequate acoustic windows. Ultrasound Med Biol. 1997;23(8):1275–1277. doi: 10.1016/s0301-5629(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y-P, Fu M-H, Tan T-Y. Factors associated with no or insufficient temporal bone window using transcranial color-coded sonography. J Med Ultrasound. 2015;23(3):129–132. [Google Scholar]
- 15.Sloan MA, Alexandrov AV, Tegeler CH, et al. Assessment: transcranial Doppler ultrasonography report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2004;62(9):1468–1481. doi: 10.1212/wnl.62.9.1468. [DOI] [PubMed] [Google Scholar]
- 16.Wozniak MA, Sloan MA, Rothman MI, et al. Detection of vasospasm by transcranial Doppler sonography. The challenges of the anterior and posterior cerebral arteries. J Neuroimaging Off J Am Soc Neuroimaging. 1996;6(2):87–93. doi: 10.1111/jon19966287. [DOI] [PubMed] [Google Scholar]
- 17.Lysakowski C, Walder B, Costanza MC, Tramèr MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm a systematic review. Stroke. 2001;32(10):2292–2298. doi: 10.1161/hs1001.097108. [DOI] [PubMed] [Google Scholar]
- 18.Suarez JI, Qureshi AI, Yahia AB, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30(6):1348–1355. doi: 10.1097/00003246-200206000-00035. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ora A, Eddy L, Hatch G, Solida B. The anterior fontanelle as an acoustic window to the neonatal ventricular system. J Clin Ultrasound JCU. 1980;8(1):65–67. doi: 10.1002/jcu.1870080116. [DOI] [PubMed] [Google Scholar]
- 20.Wang HS, Kuo MF. Supraorbital approach of the anterior cerebral artery: a new window for transcranial Doppler sonography. J Ultrasound Med Off J Am Inst Ultrasound Med. 1995;14(4):259–261. doi: 10.7863/jum.1995.14.4.259. [DOI] [PubMed] [Google Scholar]
- 21.Stolz E, Kaps M, Kern A, Dorndorf W. Frontal bone windows for transcranial color-coded duplex sonography. Stroke. 1999;30(4):814–820. doi: 10.1161/01.str.30.4.814. [DOI] [PubMed] [Google Scholar]
- 22.Stolz E, Mendes I, Gerriets T, Kaps M. Assessment of intracranial collateral flow by transcranial color-coded duplex sonography using a temporal and frontal axial insonation plane. J Neuroimaging Off J Am Soc Neuroimaging. 2002;12(2):136–143. doi: 10.1111/j.1552-6569.2002.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura S, Koga M, Toyoda K, et al. Frontal bone window improves the ability of transcranial color-coded sonography to visualize the anterior cerebral artery of Asian patients with stroke. AJNR Am J Neuroradiol. 2009;30(6):1268–1269. doi: 10.3174/ajnr.A1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 25.Staalsø JM, Edsen T, Romner B, Olsen NV. Transcranial Doppler velocimetry in aneurysmal subarachnoid haemorrhage: intra- and interobserver agreement and relation to angiographic vasospasm and mortality. Br J Anaesth. 2013;110(4):577–585. doi: 10.1093/bja/aes458. [DOI] [PubMed] [Google Scholar]
- 26.Geeraerts T, Launey Y, Martin L, et al. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med. 2007;33(10):1704–1711. doi: 10.1007/s00134-007-0797-6. [DOI] [PubMed] [Google Scholar]
- 27.Harper C, Cardullo PA, Weyman AK, Patterson RB. Transcranial Doppler ultrasonography of the basilar artery in patients with retrograde vertebral artery flow. J Vasc Surg. 2008;48(4):859–864. doi: 10.1016/j.jvs.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 28.Geeraerts T, Thome W, Tanaka S, Leblanc PE, Duranteau J, Vigué B. An alternative ultrasonographic approach to assess basilar artery flow. 2 Suppl Operative. 2011;68(2):276–281. doi: 10.1227/NEU.0b013e3182124835. [DOI] [PubMed] [Google Scholar]
- 29.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess Winch Engl. 2007;11(50):iii, ix–51. doi: 10.3310/hta11500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.