Abstract
BACKGROUND:
Hair loss is a prevalent medical problem in both men and women. Maintaining the potential hair inductivity of dermal papilla cells (DPCs) during cell culture is the main factor in hair follicle morphogenesis and regeneration. The present study was conducted to compare the effects of different concentrations of human hair outer root sheath cell (HHORSC) and platelet lysis (PL) exosomes to maintain hair inductivity of the human dermal papilla cells (hDPCs).
METHODS:
In this study, hDPCs and HHORSCs were isolated from healthy hair samples. Specific markers of hDPCs (versican, α-SMA) and HHORSCs (K15) were evaluated using flow cytometric and immunocytochemical techniques. The exosomes were isolated from HHORSCs and PL with ultracentrifugation technique. Western blot was used to detect specific markers of HHORSCs and PL exosomes. Particle size and distribution of the exosomes were analyzed by NanoSight dynamic light NanoSight Dynamic Light Scattering. Different methods such as proliferation test (MTS assay), migration test (Transwell assay) were used to evaluate the effects of different concentrations of exosomes (2,550,100 µg/ml) derived from HHORSC and PL on hDPCs. Expression of specific genes in the hair follicle inductivity, including ALP, versican and α-SMA were also evaluated using real time-PCR.
RESULTS:
The flow cytometry of the specific cytoplasmic markers of the hDPCs and HHORSCs showed expression of versican (77%), α-SMA (55.2%) and K15 (73.2%). The result of particle size and distribution of the exosomes were analyzed by NanoSight dynamic light NanoSight Dynamic Light Scattering, which revealed the majority of HHORSC and PL exosomes were 30–150 nm. For 100 µg/ml of HHORSC exosomes, the expressions of ALP, versican and α-SMA proteins respectively increased by a factor of 2.1, 1.7and 1.3 compared to those in the control group.
CONCLUSION:
In summary, we applied HHORSC exosomes as a new method to support hair inductivity of dermal papilla cells and improve the outcome for the treatment of hair loss.
Electronic supplementary material
The online version of this article (10.1007/s13770-020-00266-4) contains supplementary material, which is available to authorized users.
Keywords: Dermal papilla cells, Exosome, Hair inductivity, Hair loss, Hair outer root sheath cell
Introduction
Hair loss is one of the most common complaints for which both male and female patients seek treatment [1]. In general, hair is a feature of mammalians that plays a great role in their beauty, social acceptance and self-esteem. Hair loss is therefore a threat to beauty and a psychological challenge as well. Over the last decade, patent-pending statistics have shown increasing costs. The common treatments for hair loss include herbal extracts [2–4] platelet rich plasma [5–8], adipose-derived stem cells [9–11], keratinocyte-conditioned media [12] and hair transplantation [13, 14]. None of these methods, however, have been able to create satisfactory results [15]. The current advances in the field of tissue engineering and regenerative medicine have brought new hope for hair loss treatment. Different research groups have carried out projects to create the hair organoid structure in the laboratory [16–19]. Although research findings suggests that using stem cells and mice dermal papilla cells help create the hair structure, these studies have not been successful in humans [20]. The main reasons for the failure of human studies include the loss of trichogenic ability of the hair dermal papilla cells, the insufficient number of hair in people with severe hair loss and the lack of appropriate medical culture media [21, 22]. The dermal papilla consists of mesenchymal cells in the hair follicle that plays the main role in the regulation of hair growth [23]. Maintaining the potential hair inductivity of the dermal papilla cells and the dermal sheath cells during cell culture is the most important factor in in-vitro hair follicle morphogenesis and regeneration [18, 24]. The use of common methods for the cultivation of human dermal papilla reduces the inductive capacity of the dermal papilla and the expression of specific dermal papilla biomarkers and it is therefore essential to optimize the culture conditions for the dermal papilla cells [18]. In recent years, the therapeutic potential of different sources of conditioned medium and exosomes [25, 26], such as keratinocyte stem/progenitor cells (KSCs) [12], has been evaluated in the proliferation of hDPCs and outer root sheath (ORS) cells. The result of studies has shown KSC-CM contain growth-factor cocktail and proteins such as (amphiregulin, insulin-like growth factor binding protein-2, insulin-like growth factor binding protein-5, granulocyte macrophage-colony stimulating factor, platelet-derived growth factor-AA, and vascular endothelial growth factor) that enhanced the hair growth [12].Among the different effective approaches for maintaining the in-vitro and in-vivo hair inductivity of the dermal papilla cells, exosome-based treatment may be the most efficient method [27]. The advantages of using exosomes in hair experiments and their clinical application include induction endogenous mechanisms, simple processing, long-term storage and reduced risk of immune reactions [28]. Studies have shown that exosomes can induce cell proliferation, migration and angiogenesis and promote tissue repair. Nevertheless, the effect of exosomes on the trichogenic ability of the hDPCs cells and hair regrowth is unknown.
The main reasons for the failure of human studies include the loss of trichogenic ability of the hair dermal papilla cells, the insufficient number of hair in people with severe hair loss and the lack of appropriate medical culture media. Maintaining the potential hair inductivity of the dermal papilla cells is the most important factor in in-vitro hair follicle morphogenesis. In an attempt to reach the goal of maintaining the potential hair inductivity of the hDPCs during cell culture, we isolated and characterized exosomes from two sources including HHORSC and PL and tested different concentration of exosomes, which support maintenance of potential hair inductivity of the hDPCs. Finally we demonstrated HHORSC exosomes as new method to support hair inductivity of dermal papilla cells and improve the outcome for the treatment of hair loss.
Materials and methods
The isolation and culture of normal hDPCs and HHORSCs from the human scalp
After receiving informed consent from the donors, the healthy human hair samples were transferred to the cell culture laboratory of the Skin and Stem Cell Research Center of Tehran University of Medical Sciences. To investigate the ability of the hDPCs and HHORSCs in hair production, these cells were isolated from healthy hair samples. This section describes the procedures of isolating and cultivating the cells:
The hair samples were transferred to the laboratory in Dulbecco's modified Eagle's medium/F-12 (DMEM/F12) (1:1) (Thermo Fisher Scientific, Waltham, MA, USA) + 10% FBS (Hyclone, Logan, UT, USA) + 50 µg/ml penicillin/streptomycin + 50 µg/ml GlutaMAX™ Supplement (Thermo Fisher Scientific) solution. The hair samples were rinsed in Hanks buffered salt saline (HBSS) (Gibco, Grand Island, NY, USA) + 50 µg/ml penicillin/streptomycin, 70% alcohol and HBSS + 50 µg/ml penicillin/streptomycin (Gibco), respectively. The hair samples were placed in dispase II (Gibco) 1.2 unit/ml at 4 °C for 16 h. Part of the hair (the lower one-third) was rinsed as the hDPCs in collagenase I (0.1%) (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 4 h. Collagenase I, inactivated with medium containing 10% FBS and the cells, was passed through a 70-µm mesh filter and then centrifuged at 1500 rpm for 5 min. Part of the hair (the upper two-third) was rinsed as HHORSC in trypsin–EDTA (0.05%) (Gibco) at 37 °C for 20 min. Trypsin/EDTA enzyme, inactivated with medium containing 10% FBS and the cells, was passed through a 70-µm mesh filter and then centrifuged at 200 rpm for 5 min. The epithelial cells were cultivated on 6-well (TPP, Trasadingen, Switzerland) coated with Matrigel (Sigma) with DMEM/F12 and a gold KGM kit (KGMTM Gold Keratinocyte Growth Medium SingleQuotsTM Supplements and Growth Factors, Basel, Switzerland) at 37 °C in a humidified atmosphere of 5% CO2. The duration of cultivation was 14 days. The hDPCs were cultivated on 6-well (TPP) with DMEM/F12 + 10% FBS + 50 µg/ml penicillin/streptomycin + 50 µg/ml GlutaMAX™ Supplement (Thermo Fisher) solution at 37 °C in a humidified atmosphere of 5% CO2. The cell culture medium was changed every 3 days.
Flow cytometry for hDPCs and HHORSC detection
The hDPCs and HHORSCs were collected and incubated with primary antibodies against (R & D system, Minneapolis, MN, USA), K15 (Abcam, Boston, MA, USA), Versican (Abcam) at 4 °C for 15 h, and were then incubated with secondary antibodies against goat anti-human-FITC (Abcam), goat anti-human-FITC (abcam), and goat anti-human-FITC (Abcam), in respective order. After washing collected cells with PBS, the fluorescence cells were analyzed by flow cytometry (FC500; Beckman Coulter, Brea, CA, USA).
Characterization of hDPCs and HHORSC with immunohistochemical (IHC) staining
For immunohistochemical (IHC) staining, the DP and hair keratinocyte cells were fixed with 4% paraformaldehyde and permeabilized using Triton X-100 (Merck, Darmstadt, Germany, 0.2%). After washing with PBS/Tween (PBST) 0.05% (Sigma), primary antibodies including versican (Abcam), α-SMA (R & D system) and K15 (Abcam) were diluted overnight at 4 °C. After the washing step, the DPC and hair keratinocyte cells were incubated with secondary antibodies for 1 h at 37 °C. Finally, specific cell markers were checked under a fluorescent microscope. Dapi dye was used for nuclear staining.
Assessment of alkaline phosphatase and toluidine blue staining in hDPCs
The expression of alkaline phosphatase in the hDPCs cells suggests that these cell types are able to induce hair growth. For evaluating ALP activity in the hDPCs in-vitro, the hDPCs cultivated on the 6 wells were fixed with paraformaldehyde 4% and then stained according to the ALP kit instructions (Sigma). The hDPCs extracellular matrix contains parts such as chondroitin 6-sulfate, heparan sulfate proteoglycans Laminin and type-IV collagen. One of the ways to identify hDPCs is to determine the metachromatic features of these cells with toluidine blue staining. Consequently, the hDPCs were stained after fixation, and washed with toluidine blue (Merck), and then examined under a light microscope.
Isolation of PL of human cord blood
A sample of 200 mL of pooled human cord blood was collected in a 50-ml conical tube containing an average of 2 × 1011 platelets. The pooled human cord blood was centrifuged at 300 g for 30 min at room temperature to obtain the plasma. In the next step, the plasma obtained from each tube was transferred to a new 50-ml conical tube and centrifuged at 200 g for 15 min. Then, the platelet rich plasma (PRP) was stored at − 80 °C. When bacterial tests were negative, PL was thawed at 37 °C. In the next step, PL solution was centrifuged at 4000 g for 15 min at 4 °C. Aliquots of PL were frozen again at least − 80 °C and stored for isolation exosomes.
Isolation of conditioned medium from HHORSCs
HHORSCs were isolated as previously described. In brief, HHORSCs at passage 3 were cultured in DMEM/F12, supplemented with 10% FBS exosome free and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere of 5% CO2 for 48 h before collecting the conditioned medium. The conditioned medium was then stored − 80 °C for exosome isolation.
Exosome isolation from PL and HHORSCs
The protocol of exosome isolation from PL and HHORSCs comprised a serial low speed centrifugation and ultracentrifugation. The HHORSCs conditioned medium and PL were centrifuged at 300 g for 10 min to remove the cells. The dead cells were removed by centrifuging at 2000 g for 10 min. The supernatant was transferred to a 15-ml conical tube and then centrifuged at 10,000 g to remove the cell debris. In the next step, the supernatant was again centrifuged at 100,000 g for 2 h in an ultracentrifuge (Model: BECKMAN COULTER, MODEL: Optima L-100XP- Indianapolis, IN, USA) to obtain the exosome-containing proteins. The supernatant was again collected in a new tube and centrifuged at 100,000 g for 2 h at 4 °C. The pellet including exosomes was diluted in 1 × PBS and stored at − 80 °C for future application.
Identification of the exosomes derived from PL and HHORSCs
The quantitate concentration of the exosomes derived from HHORSCs and PL were determined by BCA protein assay kit (Thermo Fisher). NanoSight LM10 (Nanosight) and Nanoparticle Tracking Analysis software version 2.2 were used to determine the particle size and distribution of the exosomes. The expression of CD9, CD63, CD81, ITG101 and Calnexin proteins in the collected exosomes was analyzed by western blotting.
Western blot analysis
In brief, the proteins extracted from the exosomes derived from HHORSCs and PL were isolated using SDS-PAGE gel 10% and then transferred to nitrocellulose membrane. The blots were blocked with skim milk 5% and then washed three times with TBST buffer. The blots were incubated with primary antibodies such as (anti-CD81, 1:100; Thermofisher Scientific, Waltham), (anti-CD9, 1:100; Thermofisher Scientific), (monoclonal anti TSG101, 1:100; Thermofisher Scientific), (anti-CD63, 1:100; Thermofisher Scientific) and (anti-calnexin, 1:100; Thermofisher Scientific) at 4 °C for 15 h. In the next step, the blots were indicated by their respective secondary antibodies. Biorad Chemidoc MP Imaging System was applied for band detection.
Analysis of the exosomes derived from HHORSCs and PL by nanosight dynamic light scattering
The pellet of exosomes derived from HHORSCs and PL was diluted in 100 μl of PBS and the particle size and distribution of the exosomes were analyzed by NanoSight dynamic light scattering (632.8-nm laser) using a BI 200SM Research Goniometer System (Brookhaven Instruments Corporation, Holtsville, NY, USA).
Internalization of the exosomes by hDPCs
This study examined whether the HHORSCs -Exo and PL-Exo can enter dermal papilla cells.
The HHORSCs -Exo and PL -Exo (100,000 g) were centrifuged for 60 min. The 200 µl of Diluent C from the PKH26 kit (Sigma-Aldrich) was added to the HHORSCs -Exo and PL -Exo pellets. Then 200 µl of Diluent C was added to the PBS. In this step, 200 µl of dye solution was prepared by adding 4 µl of the PKH26 dye solution to 200 µl of Diluent C in a conical tube and mixing them well. The 200 µl of HHORSCs -Exo and PL -Exo was added to 200 µl of dye solution and mixed by pipetting. The HHORSCs -Exo and PL -Exo solution and the dye solution were incubated for 5 min at RT. The staining procedure was stopped by adding equal volumes of 1% BSA for 1 min. The HHORSCs -Exo and PL -Exo were centrifuged at 100,000 g for 60 min and then were suspend in 200 µl of PBS. The uptake of the HHORSCs -Exo and PL -Exo by DP were analyzed with fluorescence microscopy.
In-vitro migration assay
Approximately a 1 × 105 suspension of hDPCs was seeded on the upper section of the Transwell with 8-um pore filters (Corning® Gorilla® Glass, New York, NY, USA) and different concentrations of HHORSCs -Exo and PL -Exo (0, 25, 50, 100 μg/ml) were added to the lower part of the Transwell for 24 h. After washing the upper part of Transwell three times, the migrated cells stained with 0.4% crystal violet were counted with an invert microscope.
Cell proliferation assay (MTS test)
This study analyzed the effect of exosomes on cell proliferation using an MTS assay. Dermal papilla cells were cultivated (104 cells/well) in 96-well plates with different groups of exosomes for 72 h at 37 ˚C. The conditioned medium and MTS solution (Promega, Madison, WI, USA) were applied with a ratio of 5:1 for the MTS assessment. An ELISA reader (Thermo Fisher Scientific, Waltham, MA, USA) then measured absorbance at 490 nm in the experimental groups.
Scanning electron microscopy (SEM) analysis of HHORSCs and PL exosomes
The pellet of exosomes derived from HHORSCs and PL was suspended in 0.2–1 ml of PBS. The samples were fixed in a 2.5% paraformaldehyde solution and 0.1 M of sodium phosphate buffer (pH = 7.2) for 12 h. One drop of the exosome suspension (5 μl) was added onto the clean slides. The slides were immersed in 1% osmium tetroxide for 1 h, dehydrated in ethanol and distilled water and then dried under a ventilation hood. The samples were coated with gold in a sputter coater (Hitachi, Tokyo, Japan) before examination under an SEM (KYKYEM3200, Beijing, China) with an accelerating voltage of 24 kV. The exosome sizes were evaluated using the SEM images via ImageJ.
Real-Time PCR
The total RNA was extracted from the hDPCs cultivated with HHORSCs -Exo and PL–Exo using an RNeasy Mini Kit (Qiagen, Germantown, MD, USA) and then reverse-transcribed into complementary DNA (cDNA), and the appropriate primers for the PCR were then designed as shown in Table 1.
Table 1.
Hair qRT-PCR primers
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| Versican | tgagcatgacttccgttggactga | ccactggccattctcatgccaaat |
| α-SMA | gtttgagaccttcaatgtccc | cgatctcacgctcagcagtga |
| ALP | caacagggtagatttctcttgg | ggtcagatccagaatgttcc |
Statistical analysis
In-vitro assays were performed three times to validate the experimental data. All the data were expressed as mean ± standard deviation (SD) from three replicates using one-way test by MINITAB software. P values of less than or equal to 0.05 (one star), 0.01 (two stars), 0.005 (three stars) and 0.0001(four stars) were considered as statistically significant.
Results
Characterization of the cultivated hDPCs and HHORSCs
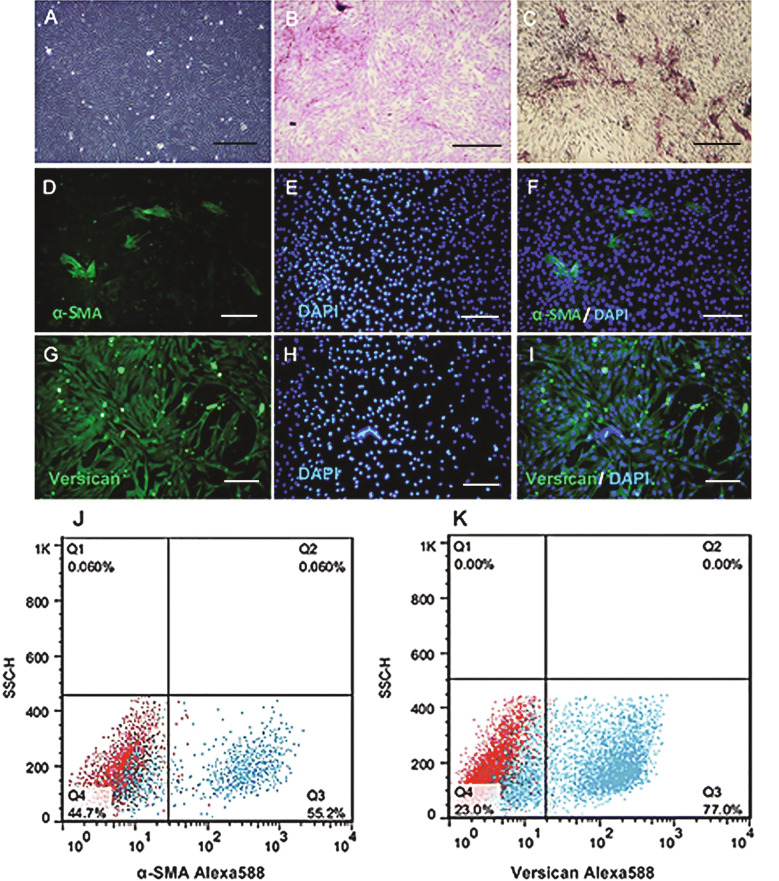
Figure 1A presents the spindle-shaped cultivated dermal papilla cells with a sunflower pattern morphology. The ability of hDPCs to produce an extracellular matrix is demonstrated by the toluidine blue staining (Fig. 1B).The results of ALP staining of the cultivated hDPCs confirm their ability to induce hair growth (Fig. 1C). Immunofluorescent Imaging data was presented as α-SMA (Fig. 1D–F) and versican (Fig. 1G–I) expression after culture. The flow cytometry analysis of the specific cytoplasmic markers of the hDPCs revealed α-SMA (55.2%) (Fig. 1J) and versican (77%), as shown in (Fig. 1K).
Fig. 1.
The morphology and cell profile markers of hDPCs. A hDPCs showed a typical morphology of fibroblast cell, with spindle like appearance. B Dermal papilla colony tested strongly positive for toluidine blue staining (Red). C Alkaline phosphatase activity (red) showed in hDPCs passage two into a conventional culture medium. D Immunofluorescent imaging data was presented as α-SMA expression after culture (green). E Nuclei were stained with DAPI (blue) and F pseudo colored merged image. G Immunofluorescent imaging data was presented as vimentin expression after culture (green). H Nuclei were stained with DAPI (blue) and I pseudo colored merged image. Flow data are presented as dot plot and histograms from the specific markers (blue) overlaid with control (red) from hDPCs. The dot plot flow cytometry analysis showed that hDPC was positive for Jα-SMA and K versican. Scale bars are 200 μm
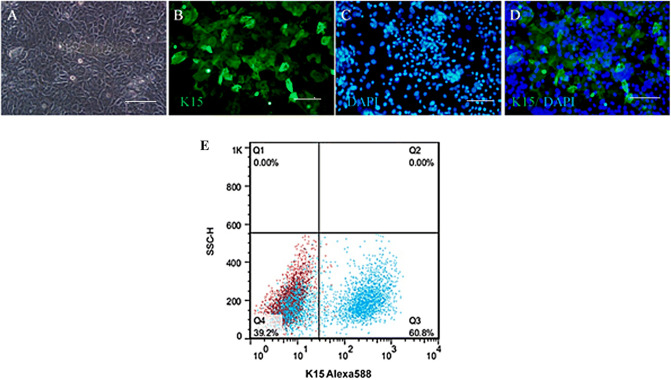
The morphology of cultivated HHORSCs with polygonal-shaped are shown in Fig. 2A. The results of the immunostaining of HHORSCs showed the expression of K15 (Green) and nuclei were stained with DAPI (blue) (Fig. 2B–D). The flow cytometry of the specific cytoplasmic markers of the HHORSCs showed K15 (60.8%) (Fig. 2E).
Fig. 2.
The morphology and cell profile markers of HHORSCs. A HHORSCs showed a typical morphology of polygonal-shaped. B Immunofluorescent imaging data was presented as K15 expression after culture (green). C Nuclei were stained with DAPI (blue) and D pseudo colored merged image. E Flow data is presented as dot plot from the specific markers (blue) overlaid with control (red) from HHORSCs. The dot plot flow cytometry analysis showed that HHORSCs were positive for K15. Scale bars are 200 μm
Characterization of HHORSCs -Exo and PL -Exo
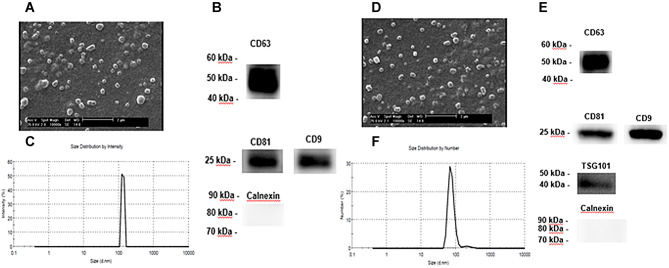
The morphology of the exosomes was examined by electron microscopic evaluation after isolating the exosomes from the supernatants of HHORSCs and PL. The diameter range of the exosomes was 30–100 nm. The distribution and concentration of the exosomes were evaluated using NanoSight analysis. A few exosomal markers, such as CD9, CD63, CD81, ITG101 and calnexin were illustrated by western blotting. The results of the NanoSight Dynamic Light Scattering analysis demonstrated that the size of most HHORSCs- Exo and PL-Exo were in the range of 30–170 nm (Fig. 3). The analysis of the SEM revealed that the HHORSCs-Exo and PL-Exo had a round shape and a size ranging from 50 to 160 nm (Fig. 3A, D). A HHORSCs- exosomes and PL-Exo markers such as CD9, CD63, CD81 and calnexin were illustrated by western blotting (Fig. 3B, E). The results of the exosome isolation showed the appropriate purity of the specific exosomes (Fig. 3).
Fig. 3.
A–F Characterization of exosomes derived from PL and HHORSC. A, D SEM analysis of exosomes. Scale bars are 2 μm. B, E Nanoparticle analysis of HHORSC-Exo and PL-Exo. The mean diameter was 167 and 147 nm for HHORSC-Exo and PL-Exo, respectively. C, F Immunoblotting for CD63, CD81, CD9, TSG101 and Calnexin in exosomes
Internalization of the exosomes by hDPCs
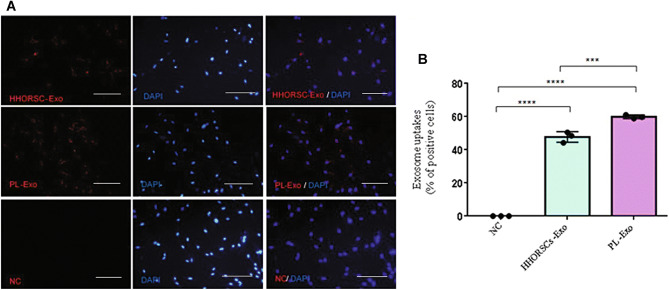
According to previous studies, exosomes can enter different types of cells. Therefore, this study examined the entrance of HHORSCs and PL into the hDPCs. The uptake of HHORSCs and PL by hDPCs was evaluated by fluorescence microscopy (Fig. 4). The results showed that exosomes can enter the hDPCSs cytoplasm and be localized in the perinuclear area. When uptake efficiency was calculated (total number of cells taken up exosomes divided by amount of total cells, quantitated by the ImageJ software), we found that, (47.73% ± 3.21) of HHORSCs- Exo were uptake by cells, whereas (59.93 ± % 1.12) of PL-Exo were uptake by hDPCs. All data are expressed as mean ± standard deviation (SD) from three replicates.
Fig. 4.
Internalization of exosomes by human dermal papilla: A Verification of the uptake of exosomes in hDPCs. HHORSC-Exo or PL-Exo (20 μg/mL) were stained with PKH26 (red) and incubated with hDPCs for 24 h. Scale bars are 200 μm. PBS- was used as negative control (NC). Nuclei were stained with DAPI (blue) for counterstaining. B The quantitative analysis of exosomes internalization by the ImageJ software were showed (47.73% ± 3.21) of HHORSCs- Exo were uptake by cells, whereas (59.93 ± % 1.12) of PL-Exo were uptake by hDPCs
HHORSCs and PL induced hDPCs migration, proliferation and in vitro ALP activity
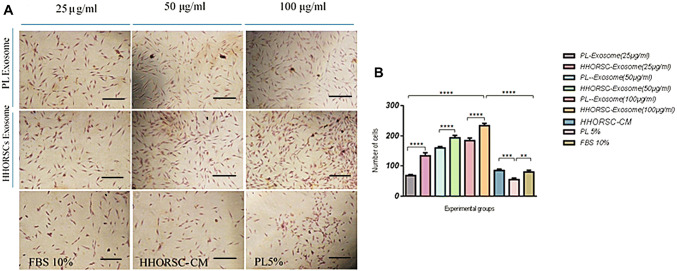
Based on previous research findings, this study assumed that exosomes may promote the migration and proliferation of hDPCs. The results of the transwell migration assay showed that HHORSCs -Exo at a concentration of 100 µg/ml significantly promoted DP migration compared to other experimental groups at 48 h (P < 0.001; Fig. 5).
Fig. 5.
Transwell migration assays. A Representative microscopic images of cells that migrated through the transwell in the migration assay. (Crystal violet stain, magnification × 200). B HHORSC-Exo at a dose of 100 µg/ml significantly increased cell migration compared to other experimental groups. ns: not significant,*p < 0.05, **p < 0.01, *** < 0.005, **** < 0.0001
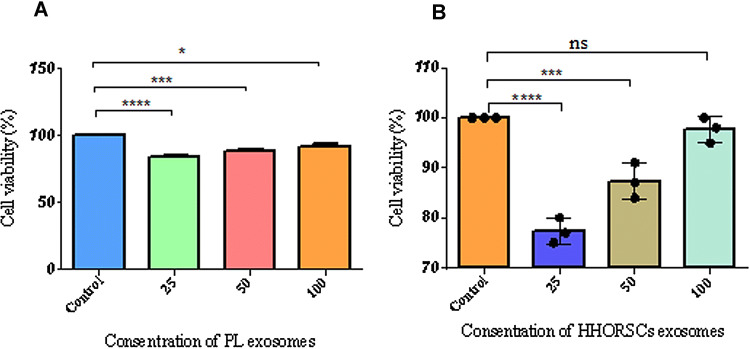
The cell proliferation of hDPCs was evaluated in different concentrations of exosomes at 3 days based on the MTS assay (Fig. 6A, B). The results of the metabolic activity of different sources of exosomes on the 2D culture of hDPCs showed a significant increase in HHORSCs -Exo at the 100-µg/ml dose group. However, the metabolic activity of different concentrations of PL-Exo on the 2D culture of hDPCs was lower than that of the control group. These results may indicate that higher concentrations of PL-Exo are needed to increase cell proliferation and viability of hDPCs than the HHORSCs –Exo group. The decrease in cell viability may be due to inadequate components of the PL-Exo including growth factors, cytokines, and proteins compared to the control group.
Fig. 6.
Cell proliferation and cell viability by MTS assay. hDPCs were plated onto 96-well plates for 24 h without or with different concentration (25, 50, 100 mg/mL) of HHORSCs exosomes A and PL exosomes B. HHORSC exosomes at a dose of 100 µg/ml significantly increased proliferation and viability of hDPCs compared to other experimental groups. But PL exosomes decreased proliferation and viability of hDPCs compared to the control group. The quantitative analysis of exosome internalization showed (47.73% ± 3.21) of HHORSCs- Exo were uptake by cells, whereas (59.93 ± % 1.12) of PL-Exo were uptake by hDPCs. NS: not significant,*p < 0.05, **p < 0.01, *** < 0.005, **** < 0.0001. All data are expressed as mean ± standard deviation (SD) from three replicates
HHORSCs- Exo induce ALP, versican and α-SMA expression in cultivated hDPCs
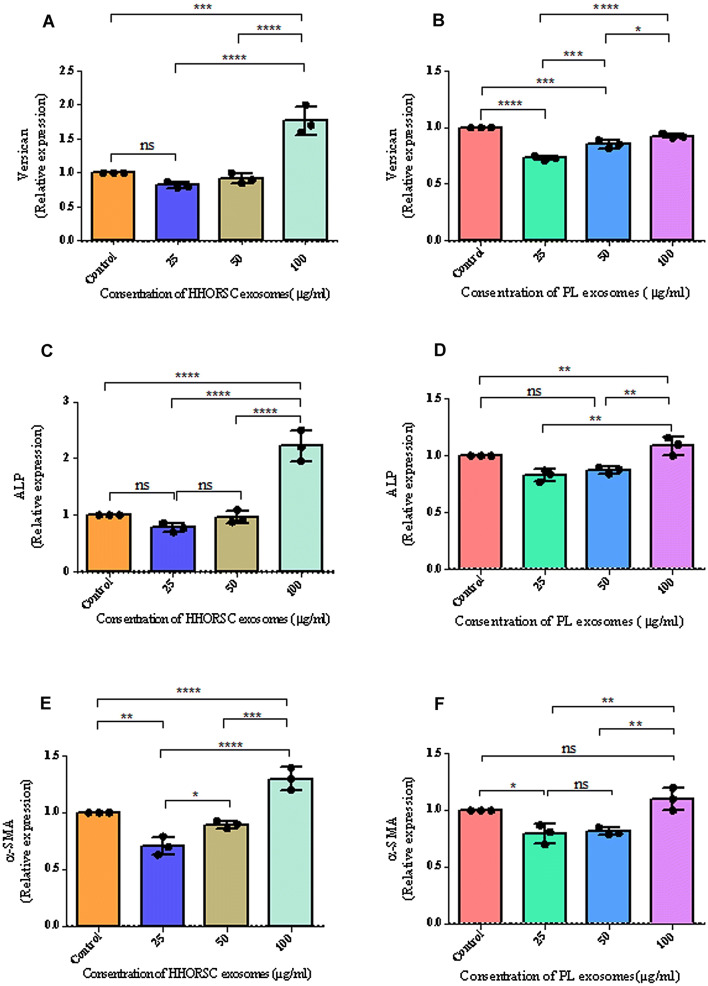
The cultivation of hDPCs with HHORSCs- Exo in comparison with PL-Exo increased the expression of ALP, versican and α-SMA significantly (Fig. 7). At the concentration of 100 µg/ml of the HHORSCs- Exo, the expression of ALP, versican and α-SMA proteins increased 2.1, 1.7 and 1.3-fold, respectively, compared to the control group (Fig. 7A–F). This evidence suggests that HHORSCs- Exo can be effective in maintaining the trichogenic ability of the hDPCs. These results suggest that the HHORSCs-Exo approach to the culture of hDPCs might provide a suitable method for maintaining the trichogenic ability of these cells; however, more studies are needed to confirm this finding.
Fig. 7.
A–F Comparison of the versican (A, B), ALP (C, D) and α-SMA (E, F) relative mRNA expressions following different concentration of HHORSCs and PL exosomes treatment. Different experimental groups such as 100, 50 and 25 μg/mL of HHORSCs -Exo or PL -Exo were incubated with hDPC for 48 h, respectively. Exosome-treated hDPC were subjected to qRT-PCR analysis and the expression of each gene was normalized against the expression in the cells cultured with serum (10%) were used as control group. All data are expressed as mean ± standard deviation (SD) from three replicates. *P < 0.05, **p < 0.01, *** < 0.005, **** < 0.0001
Discussion
Dermal papilla cells play a major role in regulating the behavior of the HHORSCs in HF morphogenesis and function [29, 30]. hDPCs singling and growth factors contribute to the maintenance of hair growth and induction of new hair formation [10]. The transplantation of a combination of hDPCs and keratinocytes onto nude mice induce hair follicle. The major problem with the transplantation of dermal papilla cells is the loss of hair follicle inductivity after several passages in culture and also the limitations faced in hair follicle (HF) sampling [31–33]. Therefore, we evaluate the ability of our new method to maintain hair follicle inductivity of dermal papilla cells during 2D cell culture condition with HHORSCs and PL exosomes. Our results illustrated combination HHORSCs secretory growth factors in hDPCs culture maintain hair follicle inductivity after several passages compared to conventional methods. The results of recent studies have demonstrated that a paracrine mechanism such as conditioned medium and exosomes play a major role in cell-based therapies [34]. Adipose derived stromal cells (ADSC) derived secretory growth factors, such as keratinocyte growth factor, vascular endothelial growth factor, platelet-derived growth factor and hepatocyte growth factor, are effective in hair regeneration [35, 36]. The paracrine mechanism of ADSC-conditioned medium is meliorated by hypoxia [35, 37]. The secretion of growth factors was detected in Keratinocyte stem/progenitor cells (KSCs) containing amphiregulin (AREG), insulin-like growth factor binding protein-2 (IGFBP2), insulin-like growth factor binding protein-5 (IGFBP5), granulocyte macrophage-colony stimulating factor (GM-CSF), PDGF-AA, and VEGF that promote hair regeneration via a paracrine mechanism in the HF [12]. PL also contains different growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor (TGF), and epidermal growth factor and bFGF which have been used in the treatment of chronic wounds. The result of experimental study showed PL acts as a growth-promoting factor in the mesenchymal stem cells (MSCs) cultures and increase proliferation rate of MSCs cultures, especially at late passages [38]. Despite the good results of stem cell-derived conditioned media for the treatment of various diseases, the short half-life of cytokines and growth factors of CM is a disadvantage in regenerative therapy [25]. In the field of dermatology, exosomes have been shown to play an effective role in wound healing, preventing scarring and inducing hair growth. Therefore, exosomes may offer more efficient and safer treatment approaches for patients compared to CM and stem cell therapy [39]. Different compartments of exosomes, such as RNAs, miRNAs and proteins, mediate cell–cell communication and function. One of the specific advantages of the therapeutic application of exosome instead of CM is protection from degradation [34]. Other advantages of exosomes are their long shelf life, long-range intracellular communications, simple storage conditions and lower risk of immune system reaction [40]. Therefore, two groups of exosomes were used in the present study to evaluate of maintenance thricogenic ability of dermal papilla in 2D cell culture condition. Our results revealed that the culture of human dermal papilla cells with HHORSCs-Exo exhibited significantly increased migration, proliferation and hair inductivity compared to other experimental groups. In the hair cycle, MSC exosomes can stimulate the conversion of the HF telogen phase to anagen phase [1]. The results of experimental studies have demonstrated that the activation PL may afford activation of additional platelets and/or initiation of relevant hair stimulation pathways more effectively than PRP since the platelet cell membranes are removed [41]. A recent study showed that hDPCs- exosomes can delay the conversion of the hair anagen phase to catagen phase through the activation of β-catenin and Shh signaling pathways. The results of our study showed that a concentration of 100 µg/ml of HHORSCs-Exo promotes hDPCs proliferation, migration and expression of ALP, versican and α-SMA significantly compared to PL-Exo. As a result, HHORSCs-Exo offers a new effective method for the treatment of hair loss. The mechanism of exosomes is still unclear; therefore, the researchers recommend future studies to investigate this issue, since exosomes have a rich combination of different RNAs, growth factors and proteins, which are dependent on the function of cells. In summary, we successfully applied exosomes as a new method to support hair inductivity of dermal papilla cells and improve the outcome for the treatment of hair loss. Using a different strategy for the maintenance of hair inductive capacity of hDPCs may provide an appropriate method for discovering new therapeutic goals for hair regeneration. Further studies should not only aim to use a different strategy with different compounds and adjuvants but also expand the existing knowledge on the maintenance of dermal papilla hair inductivity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Skin and Stem Cell Research Center and Royan Institute for financially supporting this project. This research was the thesis of PhD student from Tehran University of Medical Science.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
The study protocol was approved by the ethics committee of Tehran University of Medical Sciences with ethics approval code (of IR.TUMS.VCR.REC.1395.624) Informed consent was confirmed (or waived) by the ethics committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolff H, Fischer TW, Blume-Peytavi U. The diagnosis and treatment of hair and scalp diseases. Dtsch Arztebl Int. 2016;113:377–386. doi: 10.3238/arztebl.2016.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rho SS, Park SJ, Hwang SL, Lee MH, Kim CD, Lee IH, et al. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J Dermatol Sci. 2005;38:89–97. doi: 10.1016/j.jdermsci.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Tong T, Kim N, Park T. Topical application of oleuropein induces anagen hair growth in telogen mouse skin. PLoS One. 2015;10:e0129578. doi: 10.1371/journal.pone.0129578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S, Sharma V, Chauhan NS, Thakur M, Dixit VK. Hair growth: focus on herbal therapeutic agent. Curr Drug Discov Technol. 2015;12:21–42. doi: 10.2174/1570163812666150610115055. [DOI] [PubMed] [Google Scholar]
- 5.Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107–110. doi: 10.4103/0974-2077.138352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li ZJ, Choi HI, Choi DK, Sohn KC, Im M, Seo YJ, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040–1046. doi: 10.1111/j.1524-4725.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 7.Godse K. Platelet Rich Plasma in Androgenic Alopecia: Where do we Stand? J Cutan Aesthet Surg. 2014;7:110–111. [PMC free article] [PubMed] [Google Scholar]
- 8.Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491–497. doi: 10.1097/DSS.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 9.Stefanis AJ, Groh T, Arenbergerova M, Arenberger P, Bauer PO. Stromal vascular fraction and its role in the management of alopecia: a review. J Clin Aesthet Dermatol. 2019;12:35–44. [PMC free article] [PubMed] [Google Scholar]
- 10.Gentile P, Garcovich S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells. 2019;8:E466. doi: 10.3390/cells8050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein GK, Epstein JS. Mesenchymal stem cells and stromal vascular fraction for hair loss: current status. Facial Plast Surg Clin North Am. 2018;26:503–511. doi: 10.1016/j.fsc.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Won CH, Jeong YM, Kang S, Koo TS, Park SH, Park KY, et al. Hair-growth-promoting effect of conditioned medium of high integrin alpha6 and low CD 71 (alpha6bri/CD71dim) positive keratinocyte cells. Int J Mol Sci. 2015;16:4379–4391. doi: 10.3390/ijms16034379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma R, Ranjan A. Follicular unit extraction (FUE) hair transplant: curves ahead. J Maxillofac Oral Surg. 2019;18:509–517. doi: 10.1007/s12663-019-01245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosati P, Barone M, Alessandri Bonetti M, Giorgino R, Panasiti V, Coppola R, et al. A systematic review of outcomes and patient satisfaction following surgical and non-surgical treatments for hair loss. Aesthetic Plast Surg. 2019;43:1523–1535. doi: 10.1007/s00266-019-01480-9. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka H, Narita K, Suga H. Hair regeneration therapy: application of adipose-derived stem cells. Curr Stem Cell Res Ther. 2017;12:531–534. doi: 10.2174/1574888X12666170522114307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann R, Lee CM, Shugart EC, Benedetti M, Charo RA, Gartner Z, et al. Human organoids: a new dimension in cell biology. Mol Biol Cell. 2019;30:1129–1137. doi: 10.1091/mbc.E19-03-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta AC, Chawla S, Hegde A, Singh D, Bandyopadhyay B, Lakshmanan CC, et al. Establishment of an in vitro organoid model of dermal papilla of human hair follicle. J Cell Physiol. 2018;233:9015–9030. doi: 10.1002/jcp.26853. [DOI] [PubMed] [Google Scholar]
- 18.Kalabusheva E, Terskikh V, Vorotelyak E. Hair germ model in vitro via human postnatal keratinocyte-dermal papilla interactions: impact of hyaluronic acid. Stem Cells Int. 2017;2017:9271869. doi: 10.1155/2017/9271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Böscke R, Tang PC, Hartman BH, Heller S, Koehler KR. Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep. 2018;22:242–254. doi: 10.1016/j.celrep.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohyama M. Use of human intra-tissue stem/progenitor cells and induced pluripotent stem cells for hair follicle regeneration. Inflamm Regen. 2019;39:4. doi: 10.1186/s41232-019-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi BY. Hair-growth potential of ginseng and its major metabolites: a review on its molecular mechanisms. Int J Mol Sci. 2018;19:E2703. doi: 10.3390/ijms19092703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orasan MS, Roman II, Coneac A, Muresan A, Orasan RI. Hair loss and regeneration performed on animal models. Clujul Med. 2016;89:327–334. doi: 10.15386/cjmed-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan BA. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med. 2014;4:a015180. doi: 10.1101/cshperspect.a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:965849. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakhtyar N, Jeschke MG, Herer E, Sheikholeslam M, Amini-Nik S. Exosomes from acellular Wharton's jelly of the human umbilical cord promotes skin wound healing. Stem Cell Res Ther. 2018;9:193. doi: 10.1186/s13287-018-0921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansoor H, Ong HS, Riau AK, Stanzel TP, Mehta JS, Yam GH. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int J Mol Sci. 2019;20:E2853. doi: 10.3390/ijms20122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira ADF, Gomes DA. Stem cell extracellular vesicles in skin repair. Bioengineering. 2018;6:4. doi: 10.3390/bioengineering6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu N, Huang K, Liu Y, Zhang H, Lin E, Zeng Y, et al. miR-195-5p regulates hair follicle inductivity of dermal papilla cells by suppressing Wnt/beta-Catenin activation. Biomed Res Int. 2018;2018:4924356. doi: 10.1155/2018/4924356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abaci HE, Coffman A, Doucet Y, Chen J, Jacków J, Wang E, et al. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun. 2018;9:5301. doi: 10.1038/s41467-018-07579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilforoushzadeh M, Rahimi Jameh E, Jaffary F, Abolhasani E, Keshtmand G, Zarkob H, et al. Hair follicle generation by injections of adult human follicular epithelial and dermal papilla cells into nude mice. Cell J. 2017;19:259–268. doi: 10.22074/cellj.2016.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao J, Zawadzka A, Philips E, Turetsky A, Batchelor S, Peacock J, et al. Hair follicle neogenesis induced by cultured human scalp dermal papilla cells. Regen Med. 2009;4:667–676. doi: 10.2217/rme.09.50. [DOI] [PubMed] [Google Scholar]
- 34.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:E1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park BS, Kim WS, Choi JS, Kim HK, Won JH, Ohkubo F, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31:27–34. doi: 10.2220/biomedres.31.27. [DOI] [PubMed] [Google Scholar]
- 36.Owczarczyk-Saczonek A, Krajewska-Wlodarczyk M, Kruszewska A, Banasiak L, Placek W, Maksymowicz W, et al. Therapeutic potential of stem cells in follicle regeneration. Stem Cells Int. 2018;2018:1049641. doi: 10.1155/2018/1049641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9:e96161. doi: 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barsotti MC, Losi P, Briganti E, Sanguinetti E, Magera A, Al Kayal T, et al. Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS One. 2013;8:e84753. doi: 10.1371/journal.pone.0084753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Jiao Y, Pan Y, Zhang L, Gong H, Qi Y, et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating notch signaling. Stem Cells Int. 2019;2019:2402916. doi: 10.1155/2019/2402916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Sun X, Zhao J, Yang Y, Cai X, Xu J, et al. Exosomes: a novel strategy for treatment and prevention of diseases. Front Pharmacol. 2017;8:300. doi: 10.3389/fphar.2017.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole JP, Cole MA, Insalaco C, Cervelli V, Gentile P. Alopecia and platelet-derived therapies. Stem Cell Investig. 2017;4:88. doi: 10.21037/sci.2017.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.