Abstract
A genome-wide association study (GWAS) using 83 diverse non-heading Chinese cabbage (NHCC) accessions identified 42,526 high-quality single nucleotide polymorphism markers associated with turnip mosaic virus (TuMV) resistance. Seventeen associated loci were identified, along with the related genes that were differentially expressed between resistant and susceptible varieties, suggesting that they may be candidate genes for TuMV tolerance. Nine mutant genes of Arabidopsis were selected for inoculation with TuMV-GFP (green fluorescence protein) to further confirm the disease resistance of these genes. Quantitative polymerase chain reaction (qPCR) analysis showed that the virus content in the Arabidopsis mutants with the homologous genes of cell wall-associated proteins, pectin methyl-esterase (PME), transcription factors (TFs), resistance gene (R), VAN3/SFC protein and F-box gene were significantly higher than that in the mutants with the homologous genes of methylation and J protein. Our results provide the basis of further study of the potential function of these candidate TuMV resistance genes and demonstrate that the described diverse NHCC can be efficiently used for GWAS of various quantitative traits. Taken together, the findings of this study will be useful to improve TuMV resistance in NHCC breeding and to discover new genes related to TuMV resistance.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02344-9) contains supplementary material, which is available to authorized users.
Keywords: Non-heading chinese cabbage, Turnip mosaic virus, Genome-wide association study, Resistance genes
Introduction
Brassica rapa crops are popular in China for their diverse nutrients, short growth period and high production, and have gradually become one of the most welcomed economic vegetables globally. Brassica rapa includes many important vegetables, such as Chinese cabbage (Brassica campestris ssp. Pekinensis), non-heading Chinese cabbage (B.campestris ssp. chinensis Makino), and rape seed (B. rapa L. subsp. rapa). The non-heading Chinese cabbage (Brassica campestris L.ssp.chinensis Makino), which originated in China, is one of the most widespread economically cultivated vegetables in China (Liu et al. 2012; Song et al. 2015).
Turnip mosaic virus (TuMV) is a member of the potato Y family, which is the largest group of plant viruses, causing serious economic losses in plants. It is a positive strand RNA virus with a genome of 9,830-9,833 nucleotides (Ohshima et al. 1996). It is among the most destructive viruses that infect economic crops and is ranked second to the cucumber mosaic virus. The virus has a wide host range and can infect 156 types of plant through non-persistent spreading way by almost 89 aphid species, causing significant reductions in Brassica crop production with obvious symptoms such as mosaic patterns, stunting, and necrosis after TuMV infection (Kim et al. 2008). Our preferred TuMV control method in Brassica crops has always been to explore for resistance genes and developing resistant cultivars. However, probing and defining resistance genes are difficult because there are only a finite number of TuMV resistance genes that are found associated with Brassica, such as the R genes are TuRB01, TuRB0lb, TuRB03, TuRB04, TuRB05, Retr01, ConTR01 and TuRB02 (Jenner Wang et al. 2003).
Genome-wide association studies (GWAS) based on the linkage disequilibrium (LD) and high-throughput single nucleotide polymorphisms (SNP) markers are a powerful tool to investigate the candidate genes underlying complex mechanisms. They optimized the traditional biparental quantitative trait locus (QTL) mapping, which makes use of phenotypic variation and natural gene recombination, and overcome the limitation of populations segregation (Li Chen et al. 2016). In 1996, Risch proposed the concept of GWAS. Since then, we have studied the application of GWAS in our research (Risch et al. 1996). By studying the flowering time and pathogen resistance, In 2005, Aranzana verified the feasibility of using GWAS in Arabidopsis thaliana (Aranzana et al. 2005), which was the first fully meaningful GWAS analysis in plants. Genome-wide association studies use the genetic markers in the population combined with the phenotypic data of the target traits for the statistical analysis to identify the genetic loci associated with the target traits. With the development of sequencing techniques, GWAS was proven to be a powerful tool for use in many plants, such as rice (Huang et al. 2010),maize (Brown et al. 2011), wheat (Breseghello and Sorrells 2005), potato (Malosetti et al. 2007) and cotton (Li et al. 2017).In recent years, GWAS studies on plants have proved that it is the preferred tool for phenotypic variation studies. Several genes related to important agronomic traits have been identified by genome-wide association analysis. In rice, GWAS was applied to analyze 14 agricultural traits (Huang et al. 2010) and then 32 loci were identified related to flowering time and production analysis (Huang et al. 2012). Many genes related to maize kernel oil were identified (Li et al. 2013). Single nucleotide polymorphisms (SNPs) and population structure that influenced the phenotypic variation in flowering time were identified (Li et al. 2010). A region with SNPs strongly associated with pathotype 1 resistance on the north arm of chromosome 11 was identified in potato (Prodhomme et al. 2020). Two candidate genes, Nal1 and OsJAZ1, identified by association analysis in cotton (Li et al. 2017).
In this study, 83 different NHCC varieties were used to examine their resistance to TuMV, with a further analysis using GWAS to find resistance related genes. In this study, we aimed to determine the effectiveness of the GWAS method for identifying genes related to TuMV resistance that might improve the production and quality of NHCC.
Materials and methods
Plant materials
Eighty-three accessions of NHCC were provided by the Chinese Cabbage System Biology Laboratory of Nanjing Agricultural University, China. The specific material information can be searched for on the non-heading Chinese cabbage database website (https://nhccbase.njau.edu.cn/website/). The Arabidopsis mutants used for the identification of the candidate genes were obtained from the Arabidopsis Biological Resource Center, the USA (Table 1). All the plants were grown in plastic trays in a climatic room under controlled environmental conditions (16 h light/8 h dark photoperiod, 22 °C/18 °C and 60–65% humidity).
Table 1.
The 17 genes related to TuMV resistance with a functional annotation identified by the genome wide association study
| Gene | Chr | A. thaliana | mutants | Function annotation |
|---|---|---|---|---|
| Bra023789 | A01 | AT4G17360 | CS860079 | methyltransferase |
| Bra022153 | A02 | AT5G47730 | SALK_116953C | SEC14 cytosolic factor |
| Bra035579 | A02 | AT5G55590 | CS811343 | pectinesterase |
| Bra032093 | A04 | AT2G24450 | CS93932 | FLA3 |
| Bra017486 | A09 | AT5G47430 | zinc ion binding | |
| Bra007162 | A09 | AT3G55430 | beta-1,3-glucanase, putative | |
| Bra034191 | A09 | AT4G03190 | CS65982 | GRH1/ ubiquitin-protein ligase |
| Bra007010 | A09 | AT1G22000 | F-box family protein | |
| Bra039065 | A09 | AT3G27920 | CS3786 | ATMYB0 |
| Bra033247 | A10 | AT1G01630 | SEC14 cytosolic factor, putative | |
| Bra002184 | A10 | At5G18750 | DNAJ heat shock protein | |
| Bra009548 | A10 | AT5G03160 | SALK_140273C | ATP58IPK |
| Bra002345 | A10 | AT5G20930 | CS8168 | protein serine/threonine kinase |
| Bra008838 | A10 | AT5G13300 | CS70031 | SFC, VAN3 |
| Bra015597 | A10 | AT1G12280 | CC-NBS-LRR class | |
| Bra009141 | A10 | AT5G22690 | TIR-NBS-LRR class | |
| Bra002117 | A10 | AT5G17970 | TIR-NBS-LRR class |
Phenotyping
In order to conduct the TuMV resistance survey on the 83 accessions of the NHCC germplasm resources used in this study, the 83 plant types were first planted at the Jiangpu Experimental Base (Nan Jing, Jiang Su province, China), and then plants with the same growth potential and the same degree of health were selected and transplanted to Cabbage System Biology Laboratory artificial climate room. When the plants grew to 5–6 true leaves, the sap of tobacco leaves with the symptom of TuMV was used to inoculate the different varieties of NHCC and 10 plants for each variety. The TuMV CP level was measured 30 days after inoculation. Variance analysis were conducted by SPSS 17.0.
Virus inoculation for whole genome sequencing
Tobacco (Nicotiana benthamiana) were infiltrated with TuMV-GFP when 4–5 true leaves were formed. Two weeks later, we identified whether the tobacco plants were successfully infected, and then selected the leaves with signs of disease and grind these into a paste. The paste was rubbed in plants from the 83 NHCC accessions to inoculate them when 5–6 true leaves were formed and 10 plants from every accession were infiltrated. At 1 month post-inoculation, samples were taken for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis to confirm the virus content and for whole genome sequencing.
Identification of the candidate genes in non-heading Chinese cabbage and Arabidopsis
In order to further verify the R genes, the NHCC resistant variety NHCC001, the susceptible variety NHCC003 and the corresponding Arabidopsis mutants with candidate genes were inoculated with TuMV-GFP. The method for inoculation was similar for inoculating NHCC described above, with Arabidopsis thaliana inoculated when they grew four leaves. Columbia (Col-0) was used as the wild-type line. At 21 days post inoculation, samples were taken for qRT-PCR analysis to confirm the expression levels of the candidate genes and the virus content in the Arabidopsis mutants.
RNA extraction and qRT-PCR analysis
The total RNA was extracted from NHCC leaves using TIANGEN’s plant RNA extraction kit, with the Complementary DNA (cDNA) was synthesized with the Prime Script RT reagent Kit (Takara, Japan). The actin gene (AF111812) (F: CTCAGTCCAAAAGAGGTATTCT, R: GTAGAATGTGTGATGCCAGATC) was used as an internal control to normalize expression levels of the TuMV CP gene (F: GCTGATTACGAACTGACGGAGGA, R: ATGGTCGGTCTTGGTTACGCTTT), while the Atactin2 real-time PCR primer set was Atactin2-F (5′-CACAACAGCAGAGCGGGAAATT-3′) and Atactin2-R (5′-TTCTGGGCATCTGAATCTCTCA-3′), which were used as an internal control to count the level of TuMV in Arabidopsis. The primers were designed using Beacon Designer 7 software. Real-time quantification experiments were performed with three biological replicates and three technical replicates. The system refers to the Takara SYBR® PrimeScript® RT-PCR Kit II (TakaRra, Japan) protocol. The comparative cycle threshold (CT) value was used to calculate the relative levels of gene expression. The expression levels relative to actin were calculated as 2–∆∆CT in accordance with the method previously described (Pfaffl 2001), and the resulting data was analyzed using Microsoft Excel software.
Genome-wide association analysis and genome selection analysis
The gene sequences for NHCC were obtained from a previous study (Song et al. 2015). The SNPs with a minor allele frequency > 5% were used for the association analysis. According to previous research methods, the GWAS was carried out using two models, namely, a general linear model (GLM) and a mixed linear model (MLM) (Huang et al. 2010; Zhang et al. 2010).
Tassel 3.0 (https://www.maizegenetics.net) was used to analyze the data of GWAS (Bradbury et al. 2007). The population structure (Q matrix) was analyzed using Admixture V1.3.0 software (Alexander et al. 2009), and the K was set from 1 to 6. The kinship matrix (K matrix) was calculated using SPAGeDi (V1.5) software (Loiselle et al. 1995; Hardy and Vekemans 2002). We used four different models (general linear model, GLM and mixed linear model, MLM) for the association analysis, and then determined the optimal model according to the quantile–quantile (QQ) plot, which was conducted using R program (Turner 2014). The SNPs with p-values < 1 × 10−5 were as suggestive of a genome-wide significance level according to the previous report (Coltell et al. 2019).
Gene function annotation
The gene function annotation was gained from public protein databases, which included Iprscan (https://www.ebi.ac.uk/Tools/pfa/iprscan/),UniProtKB (https://www.ebi.ac.uk/uniprot/) (Schneider and Poux 2012), TrEMBL(https://www.ebi.ac.uk/uniprot/TrEMBLstats/) (O’Donovan et al. 2002), GO(https://www.geneontology.org/), COG(https://www.ncbi.nlm.nih.gov/COG/) and KEGG(https://www.genome.jp/kegg/).
Results
The disease resistance characters of different NHCC varieties
The 83 sequenced accessions were classified into six grades after inoculation based on the phenotypic characteristics and the expression level of the viral CP protein (Table 2). There were 22 accessions belonging to the first grade; 16 accessions belonging to the second grade; 15 accessions belonging to the third grade; 11 accessions belonging to the fourth grade; 9 accessions belonging to the fifth grade; 10 accessions belonging to the sixth grade (Table 2). The grades were:
Table 2.
Summary statistics of TuMV CP level and the disease grade of 83 accessions of non-heading Chinese cabbage infected by TuMV
| Accession | TuMV CP level | Grade | Accession | TuMV CP level | Grade |
|---|---|---|---|---|---|
| NHCC226 | 0.5 | 1 | NHCC300 | 60.45 | 3 |
| NHCC026 | 0.99 | 1 | NHCC034 | 38.36 | 3 |
| NHCC038 | 1 | 1 | NHCC037 | 38.43 | 3 |
| NHCC063 | 1.99 | 1 | NHCC036 | 38.62 | 3 |
| NHCC042 | 2.01 | 1 | NHCC052 | 61.16 | 3 |
| NHCC017 | 2.39 | 1 | NHCC047 | 63.73 | 3 |
| NHCC001 | 3.03 | 1 | NHCC028 | 63.8 | 3 |
| NHCC041 | 3.78 | 1 | NHCC081 | 76.65 | 3 |
| NHCC081 | 3.81 | 1 | NHCC076 | 77.32 | 3 |
| NHCC225 | 3.98 | 1 | NHCC060 | 97.3 | 3 |
| NHCC078 | 4.79 | 1 | NHCC033 | 97.41 | 3 |
| NHCC014 | 4.81 | 1 | NHCC056 | 121.51 | 4 |
| NHCC095 | 5.99 | 1 | NHCC223 | 127.43 | 4 |
| NHCC084 | 7.62 | 1 | NHCC057 | 152.35 | 4 |
| NHCC224 | 7.94 | 1 | NHCC053 | 152.45 | 4 |
| NHCC067 | 7.95 | 1 | NHCC002 | 153.26 | 4 |
| NHCC087 | 7.96 | 1 | NHCC040 | 153.63 | 4 |
| NHCC080 | 7.96 | 1 | NHCC021 | 193.43 | 4 |
| NHCC221 | 7.98 | 1 | NHCC006 | 193.53 | 4 |
| NHCC083 | 8 | 1 | NHCC073 | 241.2 | 4 |
| NHCC222 | 9.52 | 1 | NHCC048 | 242.35 | 4 |
| NHCC068 | 9.53 | 1 | NHCC024 | 244.87 | 4 |
| NHCC054 | 12.05 | 2 | NHCC027 | 305.4 | 5 |
| NHCC227 | 15.92 | 2 | NHCC010 | 306.23 | 5 |
| NHCC009 | 15.93 | 2 | NHCC064 | 306.59 | 5 |
| NHCC016 | 15.95 | 2 | NHCC228 | 307.2 | 5 |
| NHCC046 | 15.96 | 2 | NHCC022 | 307.58 | 5 |
| NHCC086 | 15.98 | 2 | NHCC051 | 389.01 | 5 |
| NHCC077 | 16.08 | 2 | NHCC092 | 482.15 | 5 |
| NHCC070 | 16.2 | 2 | NHCC069 | 613.66 | 5 |
| NHCC029 | 17.53 | 2 | NHCC071 | 777.3 | 5 |
| NHCC007 | 19.13 | 2 | NHCC079 | 1545.99 | 6 |
| NHCC032 | 19.17 | 2 | NHCC072 | 2432.54 | 6 |
| NHCC065 | 24.23 | 2 | NHCC050 | 2443.47 | 6 |
| NHCC210 | 30.51 | 2 | NHCC003 | 2444.94 | 6 |
| NHCC018 | 30.52 | 2 | NHCC020 | 3252.97 | 6 |
| NHCC025 | 31.78 | 2 | NHCC061 | 4082.2 | 6 |
| NHCC066 | 31.86 | 2 | NHCC088 | 4083.69 | 6 |
| NHCC015 | 38.14 | 3 | NHCC089 | 3268.67 | 6 |
| NHCC035 | 38.14 | 3 | NHCC090 | 5846.32 | 6 |
| NHCC058 | 48.49 | 3 | NHCC082 | 6513.22 | 6 |
| NHCC049 | 48.56 | 3 |
Grade 1: no symptoms (The CP content: 0–10);
Grade 2: slightly variegated leaves (The CP content: 11–35);
Grade 3: heart leaf and a few leaves were variegated (The CP content: 36–100);
Grade 4: heart leaves and most of the leaves were variegated, a few of the leaves were shriveled and deformed or had obvious veins, the petioles had brown spots and slightly necrotic tissue, and the plant dwarfism was mild (The CP content: 101–250);
Grade 5: the variegated leaves are very obvious, the diseased leaves are severely deformed, and the plants became arbuscular and even died (The CP content: 251–1000).
Grade 6: the variegated leaves were very obvious, most of the leaves are seriously shriveled, the veins were tapered and deformed, or the stems had brown spots, and the plants were severely dwarfed or dead (The CP content: 1000–7000).
Phenotypic variations of TuMV CP level after inoculation with TuMV among NHCC germplasm accessions
The results of TuMV CP level analysis indicate that the wide range of TuMV symptom in different NHCC germplasm accessions, from almost no symptom to the variegated leaves were very obvious and the TuMV CP level range from 0.5 to 6513.22 after inoculation with TuMV. ANOVA showed the TuMV CP level have highly significant variance among NHCC germplasm accessions after inoculation with TuMV (R = 0.591) (Table 3).
Table 3.
ANOVA of TuMV level of different NHCC accessions after inoculation
| N | Mean | Standard deviation | Variance | Minimum | Maximum | R |
|---|---|---|---|---|---|---|
| 83 | 518.24 | 1266.115 | 1,603,047 | 0.5 | 6513.22 | 0.591 |
Genome-wide association analysis of disease resistance traits in TuMV
In this study, 42,526 high-quality SNPs were obtained from re-sequencing of the 83 non-heading Chinese cabbages were used to perform the genome-wide association analysis on the resistance traits of TuMV. The GLM and the MLM used for genome-wide association analysis were used to obtain the Manhattan map (Fig. 1). The GLM used the Q model, and the MLM used the Q + K model, which can greatly reduce the false negatives. As can be seen from the Fig. 1, the SNP loci were mainly located on chromosomes 9 and 10, and a total of 17 resistance-related genes were screened within the 200 Kb genome region (Table 1).
Fig. 1.
The genome-wide association study results for chlorophyll content in 83 non-heading Chinese cabbage accessions. a Manhattan plots of the general linear model (GLM) for chlorophyll content. The negative log10-transformed p values from a genome-wide scan are plotted against the position of each chromosome. The imaginary horizontal line indicates the genome-wide significance threshold; b the quantile–quantile (QQ) plots of the GLM for chlorophyll content; c the Manhattan plots of the mixed linear model (MLM); The negative log10-transformed p values from a genome-wide scan are plotted against the position of each chromosome. The imaginary horizontal line indicates the genome-wide significance threshold; d the QQ plots of the MLM for chlorophyll content
We also investigated the population structure of these accessions using these high-quality SNPs across all chromosomes. The population structure analysis showed that there were six groups in these accessions based on the delta K. The most suitable population structure was K = 6, which was exactly consistent with our classification of disease resistance. The obtained SNPs were used to construct an evolutionary tree. The different groups were distinguished by different colors, and the materials with similar genetic relationships came together, most of which had the same or similar phenotypes. To some extent, it can be considered that the genotype and phenotype were consistent (Figure S1).
Identification of candidate genes controlling TuMV tolerance
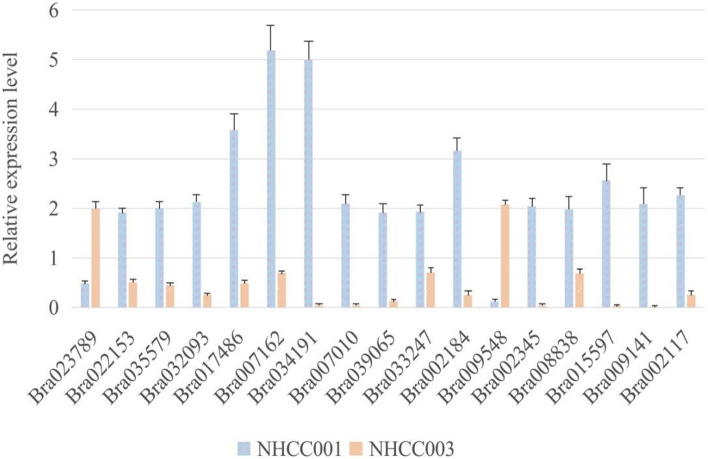
To identify these candidate genes responding to TuMV, we performed the experiment where the susceptible variety NHCC003 and the resistant variety NHCC001 were inoculated with TuMV-GFP. After 21 days past inoculation, the susceptible variety NHCC003 had obvious symptom of TuMV-GFP infection compared to the resistant variety NHCC001 (Fig. 2). The qRT-PCR analysis (Fig. 3) demonstrated that the expression level of most candidate genes (Bra022153, Bra035579, Bra032093, Bra017486, Bra007162, Bra034191, Bra007010, Bra039065, Bra033247, Bra002184, Bra002345, Bra008838, Bra015597, Bra009141, Bra002117) in the resistant variety NHCC001 was higher than that in the susceptible variety NHCC003 after inoculation. However, two genes, Bra023789 and Bra009548, were different from the other genes. The expression levels of these two genes in the resistant variety was lower than that in the susceptible variety.
Fig. 2.
The non-heading Chinese cabbage after inoculation with TuMV. a NHCC001, without apparent symptoms; b NHCC003, with obvious variegated leaves
Fig. 3.
The relative expression of candidate genes in the non-heading Chinese cabbage varieties NHCC001 and NHCC003 after inoculation with TuMV. The expression level of most candidate genes in NHCC001 are higher than in NHCC003 after inoculation with TuMV-GFP. However, the expression levels of two genes in NHCC001 are lower than in NHCC003
Resistance genes validated in Arabidopsis
To further verify the resistance genes, Arabidopsis mutants (Table 2) with corresponding candidate genes were inoculated with TuMV-GFP. After a period of inoculation, the qRT-PCR was used to analyze the viral content of these Arabidopsis mutants. The Arabidopsis gene ISO4e is a disease-resistance gene, and the viral content of the above mutants was analyzed with reference to the virus content of iso4e (the mutant of ISO4e). Through a quantitative analysis and comparison, the result indicated that the TuMV levels of CS93932, CS811343, CS3786, CS8168, CS70031, SALK_116953C and CS65982 were highly expressed. However, the TuMV levels of CS860079 and SALK_140273C, the TuMV level were lowly expressed (Fig. 4). The results showed that when the Bra023789 and Bra009548 were down-regulated, the plants were more resistant to disease, while the mutations of Bra022153, Bra035579, Bra032093, Bra034191, Bra039065, Bra002345 and Bra008838 led to easy infection of plants by TuMV. The results were consistent with those of the Chinese cabbage.
Fig. 4.
The TuMV level of Arabidopsis thaliana mutants. The TuMV level was higher in CS811343, CS3786, CS70031, SALK-116953C, CS8168, CS65982 and CS93932 than in CS860079 and SALK-140273C
Discussion
During the past 10 years, high density SNP arrays and DNA re-sequencing have provided most genotype spaces for rice, maize and wheat (Khahani et al. 2020; Li et al. 2020; Sun et al. 2017). It has provided opportunities for researchers to conduct molecular research. With the development of molecular biology research, study of genes has become increasingly extensive. In the past few years, genome-wide association components have been widely used and a growing number of loci related to various traits have been found (Huang et al. 2010; Brown et al. 2011; Breseghello and Sorrells 2005; Malosetti et al. 2007; Li et al. 2017), which provides useful clues for the study of complex traits.
In this study, we took advantage of GWAS based on high-quality SNPs to analyze TuMV resistance traits in NHCC. We identified 17 genes associated with TuMV disease resistance in NHCC. Gene annotation showed the function of these genes included methyltransferase (Bra023789), SEC14 cytosolic factor (Bra022153;Bra033247), pectin methyl-esterase (Bra035579), FLA3 (Bra032093), zinc ion binding (Bra017486), beta-1,3-glucanase (Bra007162), GRH1/ubiquitin-protein ligase (Bra034191), F-box family protein (Bra007010), ATMYB0 (Bra039065), DNAJ heat shock protein (Bra002184), ATP58IPK (Bra009548), protein serine/threonine kinase (Bra002345), SFC/VAN3 (Bra008838) and NBS-LRR class (Bra015597;Bra009141;Bra002117).
According to the previous study, these proteins and gene families participate in disease resistance. TIR and CC domains are involved in the regulation of downstream signals and the LRR domains can specifically recognize pathogens (Y et al. 2004). The S/TK is a type of receptor kinase in biological enzymes. The S/TK initiates disease resistance by participating in protein phosphorylation and cell-to-cell signal transmission (Sharma and Komatsu 2002). The ubiquitination pathway of the protein involved in the F-box protein is one of the most important regulatory systems in biology (Duplan and Rivas 2014). The F-box gene MAX2 helps the plant close the stomata to prevent pathogens from entering the plant (Piisilä et al. 2015). The SEC14 protein can induce plant resistance to Ralstonia. solanacearum and Pseudomonas syringae (Kiba et al. 2014). The P58-IPK protein inhibits the expression of the serine protein/threonine kinase (S/TK) PKR during viral infection, which will accelerate the process of viral infection of plants (Li et al. 2006). The PRs are synthesized when plants are infected with pathogenic bacteria and play a defensive role in plant disease resistance. Relevant studies have shown that the expression level of β-1,3-glucanase (PRP-2) in rice is 3.9 fold higher than that of normal plants when rice is infected with Rhizoctonia (Lee et al. 2010). The PME is a plant cell wall degrading enzyme secreted by plant pathogens (Salerno et al. 2004). Knocking out the PME gene can reduce the virulence of pathogens (Beaulieu 1993). After the rice zinc finger protein OsBIRF1 was transferred into tobacco, the resistance of tobacco increased, and the expression of other related resistance genes were promoted (Liu et al. 2008). Methylation is closely related to cell growth, senescence, and belongs to epigenetic modification (Yang et al. 2006). When Arabidopsis is invaded by a pathogen, demethylation can induce the activation of multiple disease-resistance genes (Le et al. 2014).
Although a previous study showed that the candidate genes may be involved in disease resistance, we did not validate the function of these genes. Future research will focus on identifying the effects of these candidate genes and their functional variants using genetic transformation and genetic silencing to verifying that these genes engender resistance to TuMV in NHCC.
Conclusion
This study explored some of the genes involved in the resistance of TuMV through the GWAS based on the content of viral coat proteins. These results indicated that GWAS is a powerful tool to investigate candidate genes underlying complex mechanism. This will provide a wealth of resources for the genetic breeding of NHCC and other horticultural crops with increased disease resistance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 Fig.S1. The population structure and phylogenetic tree of non-heading Chinese cabbage (NHCC). (A) The population structure; each color represents one population, and K1to K6 are shown using STRUCTURE. The y-axis quantifies thesubgroup membership, and the x-axis shows the accessions.(B) The delta K was used to define the populationstructure of all NHCC accessions.(C) The neighbor-joining phylogenetic treeof the NHCCaccessions for each population were constructed using SNPs. (DOCX 355 kb)
Acknowledgements
The authors thank Jiangsu Agricultural Industry Technology System [JATS (2019) 416] and Natural science foundation of Jiangsu Province (BK20191308) for providing support to carrying out this research work.
Abbreviations
- CMV
Cucumber mosaic virus
- CP
Coat protein
- CT
Cycle threshold
- FLA
Fasciclin-like domains of AGPs
- GFP
Green fluorescence protein
- GLM
General linear model
- GWAS
Genome-wide association study
- J protein
J-domain protein
- MLM
Mixed linear model
- NBS-LRR
Nucleotide binding site-leucine-rich repeats
- NHCC
Non-heading Chinese cabbage
- PRP
Pathogenesis-related protein
- qRT-PCR
Quantitative real-time PCR-Polymerase chain reaction
- QTL
Quantitative trait locus
- SNP
Single-nucleotide polymorphism
- S/TK
Serine-threorine kinase
- TF
Transcription factors
- TuMV
Turnip mosaic virus
Author contributions
RZ, CL, XS, FS, YW, XH and CZ conceived the study. RZ, CL, XS and YW completed the experiments. RZ, CL, XS and FS contributed to data analysis and manuscript preparation. XH and CZ participated in the planning of experiments and revising the manuscript. All authors had read and approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranzana MJ, Kim S, Zhao KY, et al. Genome-wide association mapping in Arabidopsis identifies previously known flowering time and pathogen resistance genes [J] PLoS Genet. 2005;1(5):e60. doi: 10.1371/journal.pgen.0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. Pathogenic behavior of pectinase-defective erwinia chrysanthemi mutants on different plants. Mol Plant-Mic Inter MPMI (USA) 1993;6:197. doi: 10.1094/MPMI-6-197. [DOI] [Google Scholar]
- Bradbury PJ, Zhang Z, Kroon DE. TASSEL: software for association mapping of complex traits in diverse samples [J] Bioinformatics. 2007;23(19):2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- Breseghello F, Sorrells ME (2005) Association mapping of kernal size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics. [DOI] [PMC free article] [PubMed]
- Brown PJ, Upadyayula N, Mahone GS. Distinct genetic architectures for male and female inflorescence traits of maize. PLoS Genet. 2011;7(11):e1002383. doi: 10.1371/journal.pgen.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltell O, Sorlí JV, Asensio EM, Fernández-Carrión R, Barragán R, Ortega-Azorín C, Estruch R, González JI, Salas-Salvadó J, Lamon-Fava S, Lichtenstein AH, Corella D. Association between taste perception and adiposity in overweight or obese older subjects with metabolic syndrome and identification of novel taste-related genes. Am J Clin Nutr. 2019;109:1709–1723. doi: 10.1093/ajcn/nqz038. [DOI] [PubMed] [Google Scholar]
- Duplan V, Rivas S. E3 ubiquitin-ligases and their target proteins during the regulation of plant innate immunity. Front Plant Sci. 2014;5:42. doi: 10.3389/fpls.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy O, Vekemans X. SPAGeDI: a versatile computer program to analyze spatial genetic structure at the individual or population levels. Mol Ecol Notes. 2002;2:618–620. doi: 10.1046/j.1471-8286.2002.00305.x. [DOI] [Google Scholar]
- Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, Zhang Z. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet. 2010;42:961. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, Zhao Y, Liu K, Lu H, Li W. A map of rice genome variation reveals the origin of cultivated rice. Nature. 2012;490:497–501. doi: 10.1038/nature11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khahani B, Tavakol E, Shariati V, Fornara F. Genome wide screening and comparative genome analysis for Meta-QTLs, ortho-MQTLs and candidate genes controlling yield and yield-related traits in rice. BMC Genomics. 2020;21:294. doi: 10.1186/s12864-020-6702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba A, Galis I, Hojo Y, Ohnishi K, Yoshioka H, Hikichi Y. SEC14 phospholipid transfer protein is involved in lipid signaling-mediated plant immune responses in Nicotiana benthamiana. PLoS One. 2014;9(5):e98150. doi: 10.1371/journal.pone.0098150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Masuta C, Matsuura H, Takahashi H, Inukai T. Veinal necrosis induced by turnip mosaic virus infection in Arabidopsis is a form of defense response accompanying HR-like cell death. Mol Plant-Mic Interact MPMI. 2008;21:260. doi: 10.1094/MPMI-21-2-0260. [DOI] [PubMed] [Google Scholar]
- Le TN, Schumann U, Smith NA, Tiwari S, Au PC, Zhu QH, Taylor JM, Kazan K, Llewellyn DJ, Zhang R. DNA demethylases target promoter transposable elements to positively regulate stress responsive genes in Arabidopsis. Genome Biol. 2014;15:1–18. doi: 10.1186/s13059-014-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Bricker TM, Lefevre M, Pinson SR, Oard JH. Proteomic and genetic approaches to identifying defence-related proteins in rice challenged with the fungal pathogen Rhizoctonia solani. Mol Plant Pathol. 2010;7:405–416. doi: 10.1111/j.1364-3703.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- Li S, Min JY, Krug RM, Sen GC. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology. 2006;349:13–21. doi: 10.1016/j.virol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang Y, Bergelson J, Nordborg M, Borevitz JO. Association mapping of local climate-sensitive quantitative trait loci in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2010;107:21199. doi: 10.1073/pnas.1007431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Peng Z, Yang X, Wang W, Fu J, Wang J, Han Y, Chai Y, Guo T, Yang N. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat Genet. 2013;45:43–U72. doi: 10.1038/ng.2484. [DOI] [PubMed] [Google Scholar]
- Li X, Guo Z, Yan L, Xiang C, Ding X, Hua W, Li X, Huang J, Xiong L. Genetic control of the root system in rice under normal and drought stress conditions by genome-wide association study. PLoS Genet. 2017;13:e1006889. doi: 10.1371/journal.pgen.1006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu P, Zhang X, et al. Genome-wide association studies and QTL mapping uncover the genetic architecture of ear tip-barrenness in maize. Physiol Plant. 2020 doi: 10.1111/ppl.13087. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang H, YangGJ Y, Yang Y, Wang X, Vindhya B, Basnayake S, Li D, Song F. Functional analysis reveals pleiotropic effects of rice RING-H2 finger protein gene OsBIRF1 on regulation of growth and defense responses against abiotic and biotic stresses. Plant Mol Biol. 2008;68:17–30. doi: 10.1007/s11103-008-9349-x. [DOI] [PubMed] [Google Scholar]
- Liu T, Li Y, Zhang C, Qian Y, Wang Z, Hou X. Overexpression of FLOWERING LOCUS C, isolated from non-heading chinese cabbage (Brassica campestris ssp. chinensis Makino), influences fertility in Arabidopsis. Plant Mol Biol Rep. 2012;30:1444–1449. doi: 10.1007/s11105-012-0469-8. [DOI] [Google Scholar]
- Loiselle BA, Sork VL, Nason J, Graham C. Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae) Am J Bot. 1995;82:1420–1425. doi: 10.1002/j.1537-2197.1995.tb12679.x. [DOI] [Google Scholar]
- Malosetti M, Cg VDL, Vosman B, van Eeuwijk FA. A mixed-model approach to association mapping using pedigree information with an illustration of resistance to Phytophthora infestans in potato. Genetics. 2007;175:879–889. doi: 10.1534/genetics.105.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan C, Martin MJ, Gattiker A, Gasteiger E, Bairoch A, Apweiler R. High-quality protein knowledge resource: SWISS-PROT and TrEMBL. Brief Bioinform. 2002;3:275. doi: 10.1093/bib/3.3.275. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Tanaka M, Sako N. The complete nucleotide sequence of turnip mosaic virus RNA Japanese strain. Adv Virol. 1996;141:1991–1997. doi: 10.1007/BF01718209. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piisilä M, Keceli MA, Brader G, Jakobson L, Jõesaar I, Sipari N, Kollist H, Palva ET, Kariola T. The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol. 2015;15:53. doi: 10.1186/s12870-015-0434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodhomme C, Vos PG, Paulo MJ, et al. Distribution of P1(D1) wart disease resistance in potato germplasm and GWAS identification of haplotype-specific SNP markers. Theor Appl Genet. 2020;133(6):1859–1871. doi: 10.1007/s00122-020-03559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases [J] Science. 1996;273(5281):1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Salerno MI, Gianinazzi S, Arnould C, Gianinazzipearson V. Ultrastructural and cell wall modifications during infection of Eucalyptus viminalis roots by a pathogenic Fusarium oxysporum strain. J Gen Plant Pathol. 2004;70:145–152. doi: 10.1007/s10327-004-0107-x. [DOI] [Google Scholar]
- Schneider M, Poux S. UniProtKB amid the turmoil of plant proteomics research. Front Plant Sci. 2012;3:270. doi: 10.3389/fpls.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Komatsu S. Involvement of a Ca 2+ -Dependent Protein Kinase Component Downstream to the Gibberellin-Binding Phosphoprotein, RuBisCO Activase, in Rice. Biochem Biophys Res Commun. 2002;290:690–695. doi: 10.1006/bbrc.2001.6269. [DOI] [PubMed] [Google Scholar]
- Song X, Ge T, Li Y, Hou X. Genome-wide identification of SSR and SNP markers from the non-heading Chinese cabbage for comparative genomic analyses. Bmc Genomics. 2015;16:328. doi: 10.1186/s12864-015-1534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang F, Yan X, Zhang X, Dong Z, Cui D, Chen F. Genome-wide association study for 13 agronomic traits reveals distribution of superior alleles in bread wheat from the Yellow and Huai Valley of China. Plant Biotechnol J. 2017;15(8):953–969. doi: 10.1111/pbi.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SD (2014) qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. bioRxiv:005165
- Y B, R S, JL D. Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr Opin Plant Biol. 2004;7:391–399. doi: 10.1016/j.pbi.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yuan JS, Ross J, Noel JP, Pichersky E, Chen F. An Arabidopsis thaliana methyltransferase capable of methylating farnesoic acid. Arch Biochem Biophys. 2006;448:123–132. doi: 10.1016/j.abb.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Ersoz E, Lai CQ, Todhunter RJ, Tiwari HK, Gore MA, Bradbury PJ, Yu JM, Arnett DK, Ordovas JM. Mixed linear model approach adapted for genome-wide association studies. Nat Genet. 2010;42:355–360. doi: 10.1038/ng.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Fig.S1. The population structure and phylogenetic tree of non-heading Chinese cabbage (NHCC). (A) The population structure; each color represents one population, and K1to K6 are shown using STRUCTURE. The y-axis quantifies thesubgroup membership, and the x-axis shows the accessions.(B) The delta K was used to define the populationstructure of all NHCC accessions.(C) The neighbor-joining phylogenetic treeof the NHCCaccessions for each population were constructed using SNPs. (DOCX 355 kb)