Abstract
The ability to detect a silent gap within a sound is critical for accurate speech perception, and gap detection has been shown to have an extended developmental trajectory. In certain conditions, the detectability of the gap decreases as the gap is placed closer to the beginning of the signal. Early in development, the detection of gaps shortly after signal onset may be especially difficult due to immaturities in the encoding and perception of rapidly changing sounds. The present study explored the development of gap detection from age 8 to 19 years, specifically when the temporal placement of the gap varied. Performance improved with age for all temporal placements of the gap, demonstrating a gradual maturation of gap detection abilities throughout adolescence. Younger adolescents did not benefit from increasing gap onset times, while older adolescents’ thresholds gradually improved as gap onset time lengthened. Regardless of age, listeners learned between the two testing days but did not improve within days. Younger adolescents had poorer thresholds for the last block of testing on the second day, returning to baseline performance despite learning between days. These data support earlier studies showing that gaps are harder to detect near stimulus onset and confirm that gap detection abilities continue to mature into adolescence. The data also suggest that younger adolescents do not receive the same benefit of increasing gap onset time and respond differently to repeated testing than older adolescents and young adults.
Keywords: gap onset, development, temporal processing, human
Introduction
Auditory perception has a gradual maturational trajectory extending well into adolescence, yet the vast majority of work has examined early rather than later development. Gap detection, the ability to detect a silent period within an otherwise continuous sound, matures late in development and is important for speech comprehension (Buss et al. 2017; Eggermont 1995; Fischer and Hartnegg 2004; Irwin et al. 1985; Steinschneider et al. 1994; Trehub et al. 1995; Walker et al. 2006). For example, voice onset time is the length of the silent period or “gap” between the release of a stop consonant and the onset of voicing of the following speech sound. Accurate gap detection thus determines the perceptual boundary between different phonemes with different voice onset times, such as /ba/ and /pa/ (Elangovan and Stuart 2008). The preponderance of such temporally varying elements in speech may explain why gap detection abilities in younger children predict later speech and language skills (Benasich and Tallal 2002; Muluk et al. 2011). Yet we still do not fully understand the full developmental course of gap detection abilities.
To gain a clearer picture of development, one strategy is to focus on how adult perceptual abilities are affected by varying specific stimulus parameters and to measure any differential effects of varying these parameters during childhood and adolescence. Gap detection is influenced by many variables, including the temporal placement of the gap in relation to the onset of the overall sound. In adults, gaps occurring close to the sound onset need to be longer than those occurring later (> 50 ms) within the sound in order to be perceptible (Phillips et al. 1997; Snell and Hu 1999). Very little is known about the effect of gap onset time, defined here as the onset of the gap relative to the onset of the sound (Fig. 1), across development. One study suggested that shorter gap onset times (e.g., 5–10 ms) may be particularly difficult for younger listeners to detect (Diedler et al. 2007). However, that study only examined listeners up until 8 years of age, a point in development where gap detection abilities (measured without varying gap placement) are still immature. It is thus unknown how the gap detection abilities of older children and adolescents are affected by varying the temporal placement of the gap.
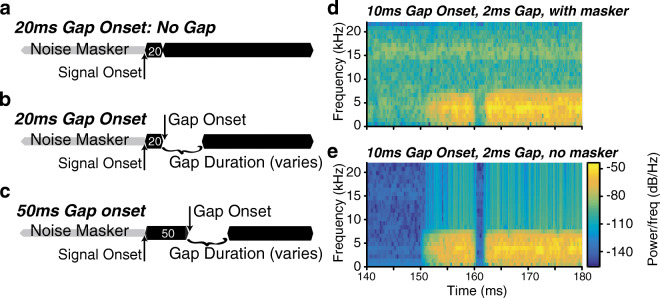
Fig. 1.
Exemplars for a subset of the stimuli: a no-gap, b 20-ms gap onset, and c 50-ms gap onset. The noise masker (40 dB SPL broadband noise) began 150 ms prior to the onset of the signal (70 dB SPL bandpass noise). Gap onsets occurred at five temporal delays relative to signal onset (10, 15, 20, 30, or 50 ms); two are depicted here. Gap duration was defined as the interval where signal amplitude was zero, excluding the 1 ms rise/fall surrounding the gap. For each gap onset condition, gap duration was varied with adaptive tracking, as indicated by the text. d Spectrogram showing the frequency (y-axis) and intensity (color) of one example computer-generated stimulus (10 ms gap onset time with a 2 ms gap) over time (x-axis) with the masker present. e To illustrate the increase in intensity information available that would have been available if we had not included the masker, this spectrogram shows the same stimulus as in D but with no masker present
Adolescents have previously been shown to have an immature response to perceptual training on some temporal processing tasks (Huyck and Wright 2011, 2013), despite the common assumption that learning is greatest in the young. In these studies, adults improve quickly and continue to learn over multiple days of training. In contrast, most adolescents either learn more slowly than adults or do not improve their perceptual abilities with daily training. Furthermore, many adolescents worsen with repeated testing, with performance deteriorating both within individual daily sessions and over multiple days of training (Huyck and Wright 2011, 2013). The reasons for these immature training outcomes in adolescents are unclear. One potentially confounding issue is that this work has only examined adolescents who started with adult-like performance, raising the possibility that these adolescents might show worsening performance because they are regressing toward the mean. Alternatively, they may have been highly engaged during initial testing, resulting in adult-like starting performance, but were less able to remain engaged as training progressed. To date, the potential outcomes for adolescents who begin with poorer-than-adult (immature) performance have not been explored.
The present study examines how the temporal placement of a gap within a sound affects gap detection from late childhood through early adulthood, an age range that is vastly underrepresented in the current literature. Given that performance on a standard gap detection task (i.e., consisting of a singular gap onset time) remains immature until ~ 11 years old (Buss et al. 2017), we predict that gap detection abilities will improve with age for all temporal placements of the gap. We further predict that since younger age groups lack precision in temporal coding abilities across a variety of tasks (Sanes and Woolley 2011), short gap onset times will be particularly detrimental to the gap detection in younger listeners, and this effect will decrease with age. Perceptual learning will be examined across the 2 days of testing. Based on previous research (Huyck and Wright 2011, 2013), we predict that older adolescents or young adults will show greater improvement between days and that performance for children and younger adolescents may actually worsen within or between the 2 days.
Methods
Subjects
Individuals ranging in age from 8 to 19 years old served as paid listeners (n = 40, 15 males, mean age ± SD = 14.3 ± 3.6 years). All listeners had hearing thresholds ≤ 20 dB HL for standard audiometric frequencies (250–8000 Hz) and either the listeners themselves (if ≥ 18 years of age) or parents/caregivers (if < 18 years) reported no history of hearing loss, pressure equalization tubes, language or learning disabilities, attention deficit/hyperactivity disorder, traumatic brain injury, or any major neurological problem. All data were collected in accordance with Kent State University’s policies on the conduct of research with human subjects and with approval of the human ethics Institutional Review Board. Written informed consent was obtained from listeners (if ≥ 18 years) or their parents or caregivers (if < 18 years) prior to the experiment. Informed assent was obtained from all listeners under 18 years of age. Listeners were compensated via gift cards.
Procedure
Listeners were tested on their ability to detect a silent gap in a gated noise with varying temporal positions of the gap relative to noise burst onset (10, 15, 20, 30, and 50 ms) (Fig. 1). Listeners were instructed that they would hear three sounds, one of which would contain a silent gap, and to indicate on a number pad which sound contained the gap. Custom Matlab scripts (MATLAB R2018a, Mathworks, Natick MA; scripts by M. Rosen, available upon request) presented the three stimuli with a 0.75-s interstimulus interval, recorded participant responses, and provided feedback for each trial in the form of either “Correct” or “Incorrect” appearing on the screen. Practice trials were given to ensure that listeners understood the task. Gap detection thresholds were determined using a 3-down 1-up adaptive rule targeting the 79.4% correct point on the psychometric function (Levitt 1971). The gap duration began at 20 ms and decreased after three consecutive correct responses or increased after one incorrect response, with an initial step size of 5 ms for the first three reversals and steps of 1 ms for all subsequent trials. Gap duration had upper and lower limits of 125 and 0 ms, respectively. Individual threshold measurements were determined based on 60 trials, with gap onset time held constant. We used a fixed number of trials per threshold measurement in order to allow assessment of learning based on equivalent experience with the task across listeners. The first three reversals were dropped from the threshold estimate and threshold was defined as the shortest gap listeners could detect, as calculated by the geometric mean of the last four reversals. Individual tracks with fewer than seven reversals were dropped from the analysis. Listeners completed two testing blocks per day on two consecutive days, with each block consisting of five threshold estimates: one for each gap onset time. This yielded four gap detection thresholds for each of the five gap onset times. Breaks were given between each block of testing. Order of these threshold estimates was counterbalanced using a digram-balanced Latin Square (Wagenaar 1969).
Stimuli
Stimuli were generated using custom Matlab scripts and presented monaurally via circumaural headphones (Sennheiser HD280) to the left ear through a Dell PC with a Fireface 800 sound interface. Gap stimuli consisted of a 200-ms bandpass noise burst (200–6000 Hz) generated from a random seed before each trial and presented at 70 dB SPL. Each gap stimulus contained a silent gap with a 1-ms fall/rise; the duration of the gap was adaptively varied with the listener’s performance. Gap duration was defined as the interval between the lead and trailing burst where the amplitude was at zero and did not include the 1-ms fall/rise time of the gap. Gap onset was defined as the elapsed time between the onset of the noise burst and the onset of the gap. The introduction of a silent gap in noise results in both a change in stimulus intensity and, when presented over headphones, spectral splatter outside the frequency range of the bandpass noise (Grose and Hall 1993; Snell and Hu 1999). We used the 1-ms fall/rise surrounding the gap to reduce the spectral splatter usually introduced by rapid onsets and offsets. To minimize the availability of intensity cues resulting from the 1-ms fall/rise (Fig. 1), both the gap and the no-gap stimuli contained the 1-ms fall/rise. For the no-gap stimuli, the rise/fall began at the same time as for the gap stimuli but without a silent period, so that there was a small change in intensity but no silent period between the leading and trailing burst. Furthermore, all gap and no-gap stimuli were presented concurrently with a 350 ms 40 dB SPL white noise masker (200–20,000 Hz) that began 150 ms prior to the onset of the lead burst (Fig. 1). Adding background noise has previously been shown to mask any spectral splatter (Moore 1993) that might be introduced through the headphones and thus reduce the extent to which listeners could base responses on non-temporal cues.
Analysis
Data were analyzed using a full-factorial mixed effects model (R Core Team 2017) with the log-transformed threshold from each track (up to 20 tracks per participant) as the dependent variable. A mixed effects model was chosen because such models account for high inter-individual variance; allow for all measurements (rather than means) to be entered into the model; are robust to missing data (e.g., such as when an adaptive track failed to converge on a threshold, which occurred 2.9% of the time); and enable the examination of continuous variables, such as age (Baayen et al. 2008; Gordon 2019). Fixed effects were chronological age (continuous), gap onset (ordinal) and testing block (ordinal), and all interactions between these variables. Gap onset and testing block were coded as ordinal rather than interval due to unequal spacing between gap onsets and testing times. The effects of these variables were modeled as polynomial contrasts (growth curves) because this approach allowed for an examination of linear and non-linear changes over these temporal variables (Curran et al. 2010). For example, instead of focusing on how performance differed between any two testing blocks, the growth curve analysis enables us to focus on the pattern with which performance changed across all four testing blocks. The previous literature on perceptual learning has documented both learning (Halliday et al. 2008; Huyck and Rosen 2018; Moore et al. 2008, b) and worsening over elapsed time (Huyck and Wright 2011, 2013; Mednick et al. 2008; Mednick et al. 2002). Because it was unknown how repeated testing would affect thresholds in this case, it was best to allow for all possible effects over the ordinal block variable. There were four measurement blocks and therefore there were three possible polynomial contrasts, indicating three patterns of change over time. A linear pattern is a steady increase or decrease in thresholds across blocks. Quadratic patterns have one pivot point and often take the form of improvement then plateau. Cubic patterns have two pivot points and could manifest as a sequence of improvement, worsening, and improvement over blocks. For gap onset, which was another temporal variable, growth curves allowed us to examine the pattern with which performance changed across the five gap onset times (i.e., “How does increasing gap onset time change performance?”). Because there are limited data on the effect of gap onset in children, we allowed for all possible changes across the five gap onset times (i.e., linear, quadratic, cubic, quartic). To account for between-participants variability, the intercepts were allowed to vary randomly across participants (n = 40) and presentation orders (10).
The Akaike information criterion (AIC), an indicator of model quality, was lower (better) when log-transformed gap thresholds were used (AIC = 5761.11 for raw thresholds and AIC = 142.91 for log-transformed thresholds). This difference in model fit was significant (ANOVA; p < 0.001) and therefore, the model with log-transformed threshold was selected. The model was also fit once with raw (linear) chronological age (AIC = 345.63) and once with log-transformed chronological age (AIC = 208.27). The model fit was significantly better for log-transformed age (ANOVA; p < 0.001). Therefore, all significant main effects and interactions with age in the model should be interpreted as a logarithmic change in age.
To further examine detection abilities for each gap onset and block, post hoc comparisons were made using Hochberg corrections (Hochberg 1988). Significant interactions with log-transformed age were interpreted using post hoc linear mixed effects models examining gap onset and block effects (including their interaction) separately for four age groups. In anticipation of this analysis, these four age groups were chosen a priori and therefore had equal numbers of listeners with the ten possible presentation orders counterbalanced across listeners within these groups (n = 10 per group): 8- to 10-year-olds (age = 9.4 ± 1.1 years), 11- to 13-year-olds (12.8 ± 1.1 years), 14- to 17-year-olds (16.0 ± 1.1 years), and 18- to 19-year-olds (18.7 ± 0.5 years). Post hoc t tests (with Hochberg corrections) were repeated for these age groups.
To assess possible contributions of attention or compliance on performance, within-listener variance was incorporated into the model. First, the arithmetic standard deviation (SD) of the reversal values within the entirety of each adaptive track (i.e., 7 or more reversals) was calculated as an indicator of within-listener variability. To estimate the extent to which this within-listener variability contributed to developmental trends, first, the correlations between the SD of reversals of a given track and each of the following variables were calculated: (1) the log-transformed age, (2) the log-transformed threshold of that track, (3) the block during which that track occurred, and (4) the gap onset time for that track. Specifically, Pearson correlations were conducted for the continuous variables (log-transformed age and threshold) and Kendall rank correlations were conducted for the ordered, discrete variables (block and gap onset time). The correlations were significant between the SD of reversals and the following variables: log-transformed age (t = −7.01, p < 0.001), log-transformed threshold (t = 14.83, p < 0.001), and gap onset time (z = 2.60, p = 0.01). There was no relationship between the SD of reversals and block (z = −0.60, p = 0.55). Based on these correlations, the mixed effects model was repeated, but including the main effect of within-listener variability (SD of reversals), the interaction between within-listener variability and log-transformed threshold, the interaction of within-listener variability with gap onset time, and the three-way interaction among within-listener variability, gap onset time, and log-transformed thresholds. Due to a lack of correlation, effects including an interaction between within-listener variability and block were excluded from the model.
Results
Our model revealed significant main effects, along with both two- and three-way interactions. While significant main effects are meaningful, they are most easily interpreted in the context of their interactions with other variables. Below, and in the tables, we focus on the effects that were statistically significant at α ≤ 0.05. The de-identified data from this study and tables with the full model outcomes (including effects that were not significant) are openly available at https://osf.io/xqs3c/?view_only=432215e410bf48eca525897a7719d275.
Development and Learning
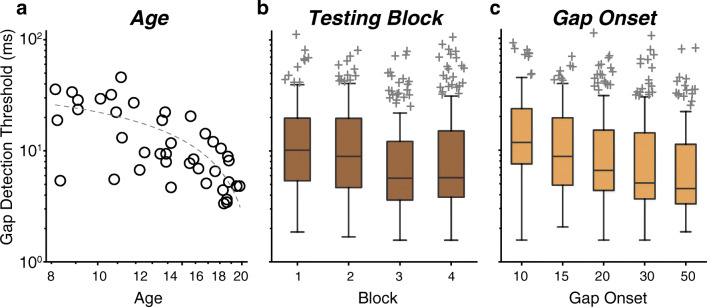
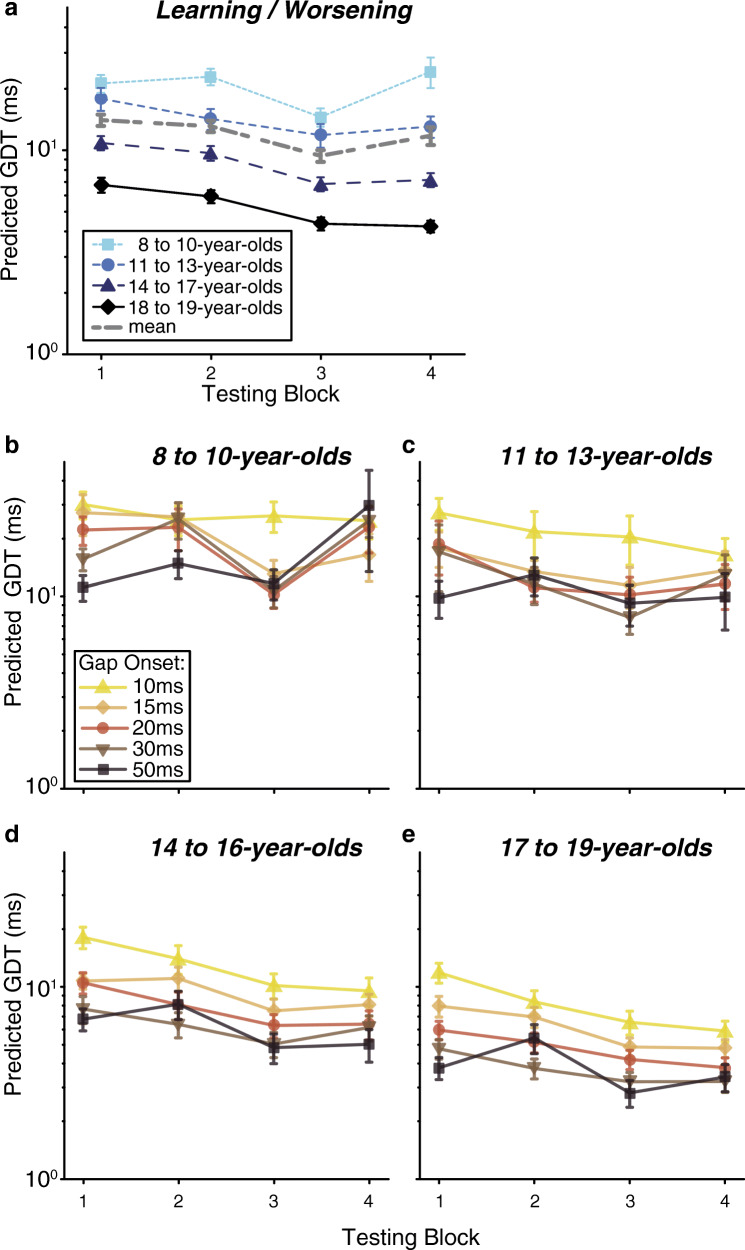
With regard to development and learning, there were two key outcomes: (1) thresholds improved with log-transformed age and (2) older (11–19 years) but not younger listeners (8–10 years) learned across the four blocks. First, gap detection thresholds improved linearly with increasing log-transformed age (Fig. 2a; main effect, t = −5.59, p < 0.001), demonstrating that gap detection matures gradually throughout adolescence. Second, there was a cubic main effect of block (Fig. 2b; t = 2.78, p = 0.01), reflecting learning between the two testing days (block 1 vs. 3 and 4: t ≥ 5.09, p < 0.0001; block 2 vs. 3 and 4: t ≥ 3.92, p ≤ 0.0003) and worsening within the second day (block 3 vs. 4; t = −2.69, p = 0.02). Thresholds did not change within day 1 (block 1 vs. 2; t = 1.19, p = 0.24). The cubic contrast best fit these data because there were two pivot points: flat performance until after block 2, a pivot after block 2 to improve, and a pivot after block 3 to get worse. This effect differed with age, as shown by a log-transformed age by block (cubic) interaction (t = −2.31, p = 0.02). Overall, post hoc comparisons revealed that the oldest three age groups improved between blocks 1 and 4 (t ≥ 3.14, p ≤ 0.01) but the 8- to 10-year-olds did not (t = −1.06, p = 0.71) (Fig. 3). Similar to the main effect of block, thresholds were steady within day 1 for all age groups (block 1 vs. 2; t ≤ 2.01, p ≥ 0.09) and then improved between the 2 days (block 1 and 2 vs. 3; t ≥ 2.68, p ≤ 0.03). Nevertheless, within day 2, the 8- to 10-year-olds showed worsening performance across blocks 3 and 4 (t = −3.70, p = 0.001), while the older three age groups did not show any change (t ≤ −0.49, p ≥ 0.62). Thus, while all ages are capable of learning across days, the 8- to 10-year-old listeners did not maintain these improvements and in fact worsened by the end of the second day. This worsening diminished the overall effect of learning such that 8- to 10-year-olds learned between days and yet still finished the experiment with similar performance as when they started.
Fig. 2.
There were significant main effects of age, testing block, and gap onset. a Gap detections thresholds improve with log-transformed age. Circles represent individual subject thresholds, with the dashed line showing a linear fit of the threshold onto log-transformed age. b Gap detection threshold improves between days, and worsens within the second day, resulting in a cubic main effect of testing block. c Gap detection improves the most for gap onsets between 10 and 15 ms, has slower improvement between 15 and 30 ms, and no improvement between 30 and 50 ms, resulting in a cubic main effect of gap onset. On each box in b and c, the central mark is the median gap threshold, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points not considered outliers (± 2.7σ), and outliers are plotted individually
Fig. 3.
Collapsed across gap onset time, gap detection thresholds improve with increasing age. Thresholds also change over the course of the four testing blocks in an age-dependent manner. The interaction of block with age was driven by a combination of worsening in the youngest adolescents (8- to 10-year-olds) for the last block, while the older three adolescent groups (≥ 11 years of age) improved between the 1st and 4th block. No significant improvement occurred within a single day (block 1 vs. 2 or block 3 vs 4) regardless of age. Error bars represent SEM
Effects of Gap Onset
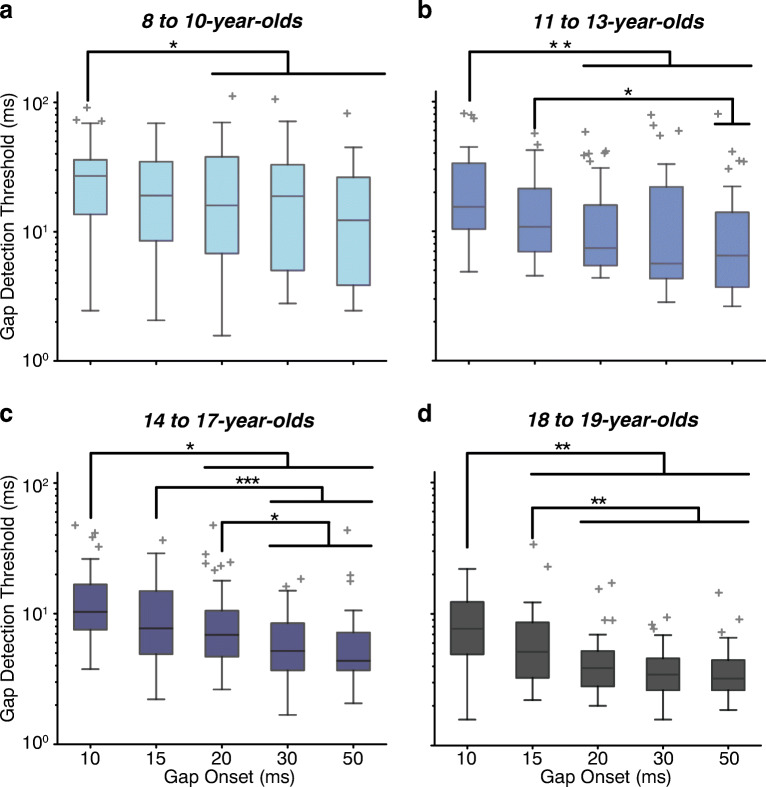
In general, across all ages, gaps that started later relative to signal onset were easier to detect; however, the interactions revealed that only the older listeners (14–19 years) benefitted from longer gap onsets. The main effect of gap onset was significant but not linear (Fig. 2c; linear main effect t = 0.40, p = 0.69; cubic main effect t = −2.26, p = 0.024). The effect was cubic because there were two pivot points: thresholds improved with increasing the gap onset between 10 and 15 ms, continued to improve but at a slower rate between 15 and 30 ms, thus creating a pivot point at 15 ms, and then stopped improving beyond 30 ms, creating a pivot point at 30 ms. Post hoc analyses were consistent with this interpretation: thresholds were poorer for the 10-ms gap onset conditions than for any other condition (all t ≥ 4.90, p < 0.0001); poorer for the 15-ms gap onset condition than for the 20-, 30-, and 50-ms condition (t ≥ 2.88, p ≤ 0.012); and poorer for the 20-ms gap onset condition than for the 30- and 50-ms condition (t ≥ 2.39, p ≤ 0.03). There was a significant interaction between gap onset time (cubic) and log-transformed age (Fig. 4, t = 2.22, p = 0.03), demonstrating that the benefit of having longer gap onset times differed with age. The 8- to 10-year-olds had similar thresholds for gap onset times of 10 vs. 15 ms (post hoc comparisons t = 2.27, p = 0.17), and poorer thresholds for the 10-ms gap onset condition than for any gap onset of 20 ms or longer (all t ≥ 2.83, p ≤ 0.04), but otherwise did not benefit from increasing gap onset times (Fig. 4, all comparisons |t| ≤ 0.07, p ≥ 0.14). The 11- to 13-year-olds also had poorer thresholds for the 10-ms gap onset than for gap onsets ≥ 20 ms (t ≥ 3.49, p ≤ 0.005) and further had poorer thresholds for the 15-ms versus the 50-ms gap onset (t = 2.80, p = 0.025), suggesting that increasing gap onset was slightly more beneficial for this age group than for the youngest group. Like the younger groups, the 14- to 17-year-olds had the poorest thresholds for the 10-ms gap onset compared with those for gap onsets ≥ 20 ms (t ≥ 3.47, p ≤ 0.003). However, unlike the younger groups, their thresholds further improved with increasing gap onset time such that they were poorer for 15-ms and 20-ms conditions than for the 30- and 50-ms conditions (Fig. 4, t ≥ 2.83, p ≤ 0.02; all other comparisons t ≤ 1.03, p ≥ 0.30). Finally, the 18- to 19-year-olds had the poorest thresholds for the 10-ms gap onset compared with those for all other gap onset times, including the 15-ms gap onset (10 ms vs. all others all t ≥ 3.72, p ≤ 0.001), and poorer thresholds for the 15-ms gap onset than for all longer gap onset times (t ≥ 3.33, p ≤ 0.004). Together, it appears that listeners of all ages benefitted from extending the gap onset beyond 10 ms, but only the listeners that were 14 years of age and older benefitted from further increasing the gap onset time. The exact pattern differed slightly between the two oldest age groups, likely due to ceiling effects in the adult group (thresholds could not get any better (lower) than they were with the 15-ms gap onset time).
Fig. 4.
There was a significant interaction between age and gap onset times, demonstrating higher thresholds for shorter vs longer gaps for younger listeners than older listeners. a Gap detection thresholds for 8- to 10-year-olds, b 11- to 13-year-olds, c 14- to 17-year-olds, and d 18- to 19-year-olds. Significance values indicated by asterisks: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. The central mark on each box is the median gap threshold, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points not considered outliers (± 2.7σ), and outliers are plotted individually
Three-Way Interaction: Age by Block by Gap Onset
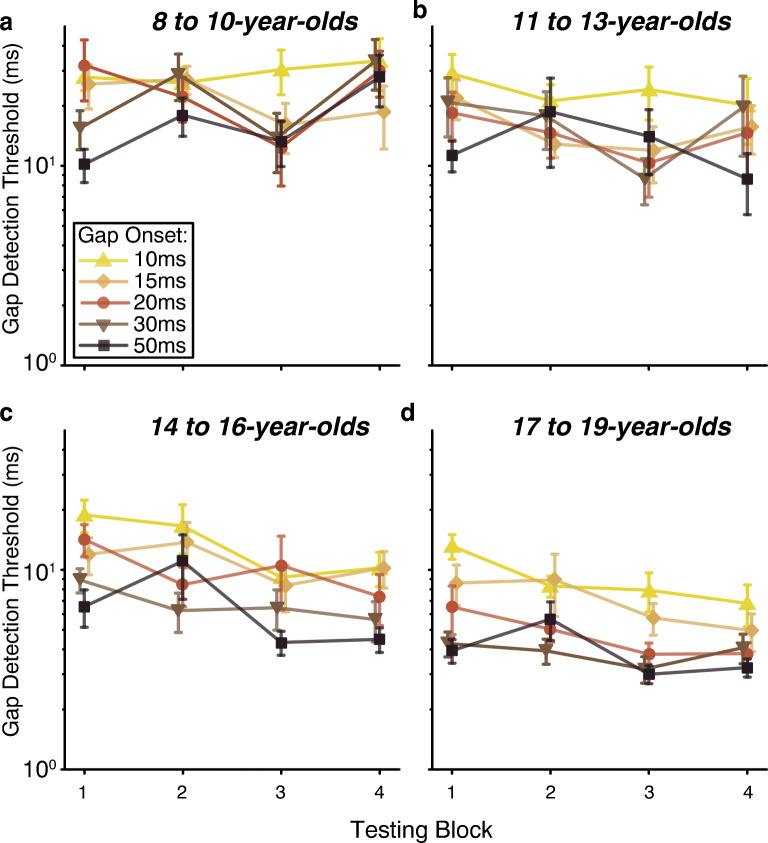
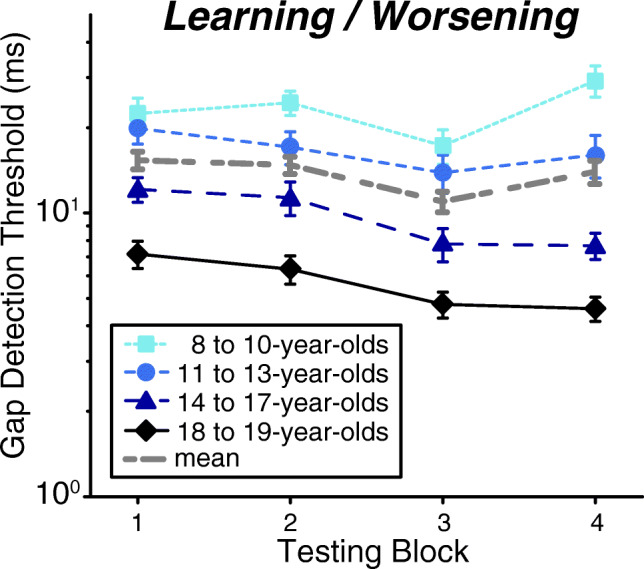
Figure 5 depicts, for each age group, how performance changed across testing blocks as gap onset time increased. These plots illustrate the significant three-way interaction between log-transformed age, cubic block, and quadratic gap onset (t = 2.36, p = 0.02). All two-way interactions underlying this three-way interaction were significant: log-transformed age by block (described above), age by gap onset (described above), and cubic block by quadratic gap onset (t = − 2.42, p = 0.02). The cubic block by quadratic gap onset interaction likely reflects that, collapsed across age, there was a tendency for performance to improve and then asymptote as gap onset time increased, but that the patterns of learning and worsening described above differed across the gap onset times.
Fig. 5.
Overall performance changed across the four testing blocks and this effect differed with both age and gap onset time, resulting in a three-way interaction between age, cubic block, and quadratic gap onset. The two older age groups (n = 10 per group) had consistent improvement across gap onsets, resulting in significant linear growth functions. The younger age groups (n = 10 per group) had inconsistent performance across blocks, as demonstrated by either a lack of significant growth functions, or significant cubic or quadratic functions. This effect was driven by a combination of worsening in the youngest adolescents (8- to 10-year-olds) for the last block, particularly for gap onsets > 15 ms, while the older three adolescent groups (≥ 11 years of age) improved between the 1st and 4th block for almost all gap onsets. No improvement occurred within a single day (block 1 vs. 2 or block 3 vs. 4) regardless of age. Error bars represent SEM
Post hoc comparisons separated by age group examined, for each gap onset, whether performance changed in a linear, quadratic, or cubic way across testing blocks. The 8- to 10-year-olds showed no significant changes on the 10-ms and 15-ms gap onset conditions, a quadratic change on the 20-ms gap onset condition reflecting learning followed by worsening (p = 0.04), and a cubic change on the 30-ms and 50-ms gap onset conditions, illustrating worsening within day 1, learning between days, and worsening or a plateau within day 2 (p ≤ 0.03). Despite learning overall across blocks (main effect of block, see above), the 11- to 13-year-olds did not demonstrate consistent changes in threshold across block for any single gap onset time except for 15 ms, which improved linearly (p = 0.04). The older two age groups had significant linear improvement (p ≤ 0.05) at most gap onsets, indicating overall learning at most gap onsets across blocks. The only exceptions were the 15-ms gap onset condition for 14- to 17-year-olds (p = 0.06) and the 30-ms condition for the 18- to 19-year-olds (p = 0.39). Interestingly, these two older groups also had significant cubic growth functions for the 50-ms gap onset condition (p ≤ 0.03), reflecting worsening within day 1, learning between days, and a plateau during day 2. These post hoc analyses indicate (1) that the 8- to 10-year-olds’ combination of learning and worsening occurred mostly for the gap onset times of 20 ms and later, (2) that the 11- to 13-year-olds’ learning was driven the most by the 15-ms gap onset condition, and (3) that listeners who were 14 years of age or older tended to learn linearly across blocks for gap onset times of 10 to 30 ms, but showed a different pattern for the 50-ms gap onset condition, on which they worsened within day 1, learned between days, and then showed steady performance within day 2 (Fig. 5).
Attention and Other Non-Cochlear Factors
Given the prolonged developmental course observed here, as well as the way that short temporal gaps are encoded by the central auditory system (Kelly et al. 1996; Moreno-Paublete et al. 2017), it is likely that the present results are attributable to a combination of processes occurring above the level of the cochlea, including auditory encoding, attention, and other cognitive processes. Ideally, we would be able to separate contributions of these actors to the development of gap perception performance during adolescence. However, this is difficult given the available data and the fact that domain-general cognitive and attentive processes are known to modulate sensory encoding, for example, in auditory cortex (Caras and Sanes 2017; Fritz et al. 2003; Niwa et al. 2012; Winkowski et al. 2018). Others have used measures of within-listener variance as a proxy for attention or compliance on auditory tasks (Buss et al. 2017; Dawes and Bishop 2008; Moore et al. 2008; Wightman et al. 1989; Wightman et al. 2010). To begin to determine the potential contribution of attention or compliance to the present results, the mixed effects model was repeated while including the main effect of within-listener variability (SD of reversals) and its interactions with all variables with which it correlated (see “Methods” section).
As expected based on the correlations, all effects involving within-listener variability were significant (|t| ≥ 2.27, p ≤ 0.02). In this way, the effect of within-listener variability (a proxy for attention or compliance) was accounted for by the model (Table 2; Fig. 6). The biggest difference from the main model was that the main effect of gap onset time and the interaction of age and gap onset time were not significant when the contribution of within-listener variability was accounted for, suggesting that these effects could potentially be attributed to attention or compliance. Most outcomes remained consistent with those seen with the main model (compare Tables 1 and 2). The cubic main effect of block remained significant (t = 2.12, p = 0.03), reflecting a plateau during session 1, improvements between sessions 1 and 2, and worsening within session 2. The main effect of age (t = −3.88, p < 0.01) continued to show improvements in threshold as log-transformed age increased. The age by block interaction (linear, t = −2.24, p = 0.03) still reflected that, while all four age groups learned between sessions (block 1 vs. 3: p ≤ 0.02), only the youngest age group worsened within session 2 (p = 0.05; all other groups p ≥ 0.58). The block by gap onset interaction (linear block by cubic gap onset t = −2.02, p = 0.04; cubic block × quadratic gap onset t = −1.91, p = 0.06) also remained. The robustness of these outcomes even when the standard deviation of reversals was included in the model suggests that these effects might be attributed to factors that differ from those that contribute to within-listener variability, such as cortical encoding, intensity discrimination abilities, or other (non-attentional) modulatory processes, as discussed below. The three-way interaction between age, block (cubic), and gap onset (quadratic) became a non-significant trend (t = 1.88, p = 0.06), suggesting that the factors underlying within-listener variability contribute to but may not fully explain this interaction.
Table 2.
Significant effects from full-factorial mixed effect model (as in Table 1) with within-listener variability (SD of reversals) added as a factor
| Testing variable | Estimate | Std. error | df | t value | p value |
|---|---|---|---|---|---|
| (Intercept) | 2.14 | 0.34 | 40.72 | 6.29 | < 1e-04 |
| logAge | − 1.15 | 0.30 | 39.60 | − 3.88 | < 1e-04 |
| Block.Cubic | 0.33 | 0.16 | 664.48 | 2.12 | 0.03 |
| SDrev | 0.12 | 0.03 | 678.08 | 3.36 | < 1e-04 |
| logAge × Block.Linear | − 0.31 | 0.14 | 664.65 | − 2.24 | 0.03 |
| Block.Linear × GapOnset.Cubic | − 0.73 | 0.36 | 664.42 | − 2.02 | 0.04 |
| logAge × SDrev | − 0.08 | 0.03 | 677.71 | − 2.39 | 0.02 |
| GapOnset.Quadratic × SDrev | − 0.17 | 0.07 | 669.86 | − 2.27 | 0.02 |
| logAge × GapOnset. Quadratic × SDrev | 0.16 | 0.07 | 669.72 | 2.29 | 0.02 |
Fig. 6.
Predicted gap detection thresholds (generated by including within-listener variability). The adjusted model eliminated the main effect of gap onset and its interactions, suggesting that attention may have contributed to the effects of gap onset time. By extension, the remaining effects can be attributed at least in part to non-attentional processes. a Predicted gap detection thresholds plotted as in Fig. 3, indicating that thresholds change over the course of testing in an age-dependent manner. b–e Predicted gap detection thresholds after accounting for within-listener variation (plotted as in Fig. 5) indicate maintained main effects of age and testing block along with their interaction, as well as an interaction of gap onset time and testing block
Table 1.
Significant effects from full-factorial mixed effect model with log-transformed threshold as the dependent variable, participant and order number as random effects, and the following fixed effects (including all interactions): log-transformed age, gap onset, and testing block
| Testing variable | Estimate | Std. error | df | t value | p value |
|---|---|---|---|---|---|
| (Intercept) | 2.85 | 0.34 | 29.30 | 8.29 | < 1e-04 |
| logAge | − 1.67 | 0.30 | 28.31 | − 5.59 | < 1e-04 |
| Block.Linear | 0.36 | 0.17 | 674.77 | 2.18 | 0.03 |
| Block.Cubic | 0.45 | 0.16 | 674.35 | 2.78 | 0.01 |
| GapOnset.Cubic | − 0.42 | 0.18 | 674.33 | − 2.26 | 0.02 |
| logAge × Block.Linear | − 0.42 | 0.14 | 674.70 | − 2.91 | 0.00 |
| logAge × Block.Cubic | − 0.33 | 0.14 | 674.33 | − 2.31 | 0.02 |
| logAge × GapOnset.Cubic | 0.36 | 0.16 | 674.30 | 2.21 | 0.03 |
| Block.Cubic × GapOnset.Quadratic | − 0.87 | 0.36 | 674.22 | − 2.42 | 0.02 |
| logAge × Block. Cubic × GapOnset.Quadratic | 0.74 | 0.31 | 674.20 | 2.36 | 0.02 |
Discussion
The goal of this study was to examine how gap detection develops across adolescence. We predicted that older adolescents and young adults would be able to detect shorter gaps and that gaps occurring closer to sound onset would be particularly difficult at younger ages due to immature processing of stimuli over short time scales. As predicted, gap detection thresholds improved with age for all temporal placements of the gap. The effect of age remained significant even after accounting for the effect of within-listener variability, a proxy for attention or compliance. This suggests that poor performance in younger listeners results, at least in part, from higher-level auditory encoding or cognitive processes unrelated to this measure of attention or compliance. The younger age groups showed minimal improvement for gap onset times greater than 10 ms, while older adolescents gradually improved with increasing gap onset time. Listeners of all ages learned between but not within days, implying that memory consolidation may contribute to perceptual learning on this task. The thresholds of the youngest group worsened between blocks within the second testing day. This worsening remained even after within-listener variability was added to the model, demonstrating that noncompliance or inattention may not be sufficient to explain perceptual worsening in 8- to 10-year-olds.
Development of Gap Detection
The present study documents the maturation of gap detection abilities well into adolescence, extending previous work in which 8- to 10-year-olds did not perform as well as adults on some gap detection tasks (Buss et al. 2017; Diedler et al. 2007). The results add to a growing literature showing that many late-developing auditory skills involve the perception of time-varying sounds, both in humans (Buss et al. 1999, 2017; Dawes and Bishop 2008; Huyck and Wright 2011, 2017; Huyck and Wright 2013; Moore et al. 2011) and animal models (Dean et al. 1990; Friedman et al. 2004; Green et al. 2016; Sarro and Sanes 2010). When gap detection was measured using auditory brainstem responses in infants, the encoding of gaps appeared adult-like by 3 months of age (e.g., Werner et al. 2001). When mismatch negativity measured infants’ gap sensitivity, infants had thresholds comparable with those of adults but the morphology of the P2 component differed, suggesting some differences in cortical processing (Trainor et al. 2001). Thus, the encoding of gaps by brainstem centers may mature early in life, and additional maturation may be attributable either to encoding in auditory cortex, which plays a role in gap detection in non-human animals (Kelly et al. 1996; Moreno-Paublete et al. 2017; Threlkeld et al. 2008) or to higher-level modulatory processes. This is consistent with research showing that brainstem regions mature earlier than central regions (Ceponiene et al. 2002; Chang et al. 2005; Fitzgerald and Sanes 2001; Ryan and Woolf 1988; Smith and Kraus 1987).
All temporal processing tasks require the encoding of a stimulus attribute that changes over time. As such, measures of temporal processing depend on both sensitivities to that stimulus attribute and temporal resolution. In the case of gap detection, the changing stimulus attribute is the intensity of the stimulus; therefore, intensity resolution also contributes to gap detection. Nevertheless, maturational changes in intensity resolution were not likely responsible for the developmental effects observed here, as intensity discrimination is mature by age 4 to 6 years (Buss et al. 2013; Maxon and Hochberg 1982) and our listeners were aged 8 to 19 years.
Here, within-listener variability, as measured using the standard deviation of reversals of the adaptive tracks, decreased with increasing age throughout adolescence. This measure of variability is typically associated with attention. Auditory attention develops throughout childhood and remains immature beyond 10 years of age (Cherry 1981; Geffen and Sexton 1978; Gomes et al. 2000; Jones et al. 2015; Leek et al. 1991; Moore et al. 2008, b; Passow et al. 2012; Pearson and Lane 1991; Wetzel and Schröger 2007; Wetzel et al. 2006; Wightman and Kistler 2005). While we did not have sufficient data to measure full psychometric functions for our listeners, the psychometric functions of the children and adolescents presumably would have been shallower due to higher variability in those age groups. Nevertheless, the younger listeners performed more poorly than the older adolescents and young adults even when within-listener variability was included in the model, suggesting that poorer gap performance at younger ages was at least partially due to immature cortical sensory processing or other cognitive functions unrelated to this measure of performance variability. Note, however, that adding standard deviation of reversals to the model did not account for any variance within an individual listener but between adaptive tracks, perhaps due to fatigue. Thus, performance variability may still have played a role in younger adolescents’ performance. Overall, the present results support the idea that processing efficiency contributes to poorer performance on temporal tasks (Cabrera et al. 2019; Hartley and Moore 2002). Processing efficiency refers to all higher-level (non-cochlear) sensory and cognitive factors (e.g., listening strategies or attention) that affect the ability to detect acoustic signals (Bargones et al. 1995; Cabrera et al. 2019; Hartley and Moore 2002; Patterson et al. 1982).
Neural Mechanisms Underlying Immature Gap Detection
The brain regions involved in both bottom-up auditory encoding and top-down cognitive access of encoded stimuli develop gradually (Cai et al. 2017; Chang et al. 2005; Klingberg et al. 2002; Moyer et al. 2016; Oswald and Reyes 2011; Sowell et al. 2001) and thus might contribute to the present results. Regarding auditory encoding, multiple neural inactivation studies in adult animals demonstrate that perceptual gap detection relies on central auditory structures: detection of short, but not long (> 50 ms), gaps is impaired when the auditory cortex is inactivated or ablated (Ison et al. 1991; Kelly et al. 1996; Moreno-Paublete et al. 2017; Syka et al. 2002; Threlkeld et al. 2008). Notably, increased magnitude and more precise timing of inhibitory synaptic inputs in relation to excitatory activity can enhance neural representations of temporally varying sounds, including short gaps in noise (Cai et al. 2017; Gay et al. 2014; Keller et al. 2018; Kurt et al. 2006; Razak et al. 2008; Ye et al. 2010). Cortical inhibition has been directly demonstrated to affect behavioral gap detection, as suppression of inhibitory interneurons in the auditory cortex immediately following a gap improves gap detection thresholds in adult animals (Weible et al. 2014). In young animals, inhibition in the auditory cortex has a less precise onset and longer duration in response to a stimulus, which impairs the temporal accuracy of sound-evoked neural responses (Cai et al. 2017; Oswald and Reyes 2011; Takesian et al. 2012). As auditory cortex activity is causally linked with gap detection performance, it is plausible that immature cortical inhibition contributes to perceptual deficits in gap detection.
In the present study, within-listener track variability decreased with age, indicating a maturational trajectory of attention and implicating top-down influences on auditory regions. Task engagement enhances auditory cortex responses to sound in both humans and animals (Buran et al. 2016; Caras and Sanes 2017; Downer et al. 2017; Engell et al. 2016; Fritz et al. 2003; Hayes et al. 2003; Strait et al. 2014) via modulation arising from the frontal cortex and other regions (Fritz et al. 2010; Froemke et al. 2007; Weinberger 2007; Winkowski and Kanold 2013; Xiong et al. 2015; Zhou et al. 2014). Yet in humans, the frontal lobe and its associated cognitive control functions remain immature until late adolescence or young adulthood (Best and Miller 2010; Giedd 2008; Klingberg et al. 2002; Larsen and Luna 2018; Sowell et al. 2001). Indeed, top-down enhancement of responses in the auditory cortex is immature until at least 14 years of age for humans (Strait et al. 2014). Thus, immaturities in cortical modulation from higher neural regions could have caused increased difficulty with gap detection in the present study. The reduced interaction of age and gap onset time when within-listener variability was added to the model implies that this effect may have been at least partially attributable to attention. Other immature top-down modulatory processes may have contributed to the main effects of age and block, the interaction of age and block, and the interaction of block and gap onset time.
Gap Onset
All age groups had better performance for longer gap onset times, consistent with previous work with inexperienced adult listeners (Snell and Hu 1999). Thresholds decreased until gap onset was ~30 ms for older adolescents. This outcome is consistent with neural data showing that the benefit of delayed onset time for gaps in noise plateaus between 30 and 50 ms for adult animals (Eggermont 2000). We hypothesized that the known gradual maturation of temporal processing would induce particular difficulty with short gap onset times for younger adolescents. Based on recordings in animals, the length of the inhibitory response in the auditory cortex to an initial sound sets the window for how long a subsequent gap must be for neurons to encode the sound following the gap (Cai et al. 2017; Eggermont 2000). The time course of recovery from inhibition in young to adolescent animals is much longer than for adults, ranging from 94 to 50 ms across development versus 20 ms in adults (Cai et al. 2017). For gaps occurring shortly after sound onset, the slower time course of inhibition in the immature auditory cortex could impair the detection of gaps occurring after a short onset by not allowing enough time for cortical neurons to recover from inhibition and respond reliably to the post-gap sound. Indeed, the one human study that examined this effect in children did find that children up to 8 years of age were differentially affected by shorter gap onset times (Diedler et al. 2007). In contrast, the younger listeners (8 to 14 years) in the present study showed very little benefit of gap onset time increasing past 10 ms. It is possible that temporal resolution is still maturing in this age range, and that the longest gap onset time presented (50 ms) was too short for younger listeners to benefit from increased gap onset time. However, including within-listener variability in the model eliminated the main effect of gap onset and its interaction with age. This implies that these effects in the main model were likely due to central processes associated with within-subject variation, i.e., attention and its known effects on cortical excitation and inhibition.
Perceptual Learning
In all age groups, performance improved between the two days, but not within them. Perceptual learning has at least two phases: an acquisition phase, often corresponding to within-day performance, and a consolidation phase which usually leads to between-day gains (Ahissar et al. 2009; Amitay et al. 2014; Irvine 2018). Our results suggest that learning on gap detection does not occur rapidly during acquisition (i.e., within days) but instead requires consolidation between days, shown by the improvement between both blocks on day 1 and the initial performance on day 2. Contrary to our predictions, we did not observe any developmental differences in between-day learning. One possible reason is that the present experiment only included two days of testing, whereas previous work showing differences in across-day learning between adolescents and adults have included ten daily training sessions, and the across-day differences were more evident later in the training phase (Huyck and Wright 2011, 2013).
Within the second day, the performance of the youngest group worsened. Worsening is not uncommon on perceptual tasks and is usually associated with some form of fatigue, either due to top-down factors such as attention (Moore et al. 2008, b; Rowan and Lutman 2006) or bottom-up factors such as perceptual over-stimulation (Huyck and Wright 2013), where worsening has been associated with changes in primary sensory cortical responses (Mednick et al. 2008; Mednick et al. 2002). Consistent with this literature, worsening remained significant even when within-listener variability was added to the model, suggesting that this phenomenon may not be solely attributable to maturational differences in attention or compliance.
Summary
In summary, perceptual gap detection has a prolonged developmental trajectory that extends well into adolescence. Furthermore, younger adolescents did not benefit from increasing gap onset times to the same extent as older adolescents and young adults. All age groups improved between days; however, the 8- to 10-year-olds worsened within the second testing day, demonstrating that their response to perceptual training may also be immature at this age. This adds to a growing literature on adolescent auditory perception showing that temporal processing develops late, suggesting that adolescents process everyday sounds differently than adults. The youngest listeners showed less benefit from increasing gap onset time than predicted, but the behavioral results are still consistent with neural data that predict difficulty with short gap onsets during development. Additionally, the behavioral results predict that cortical sensitivity to gaps should be worse for shorter gap onset times during development, with sensitivity potentially improving even beyond 50 ms.
Acknowledgments
We are grateful to all the subjects for their participation in this study. Cheyanne Allen, Madison Hayes, Kaitlyn Mazzola, Sarah McKim, Amanda Moss, Anna Okun, Cora Paolino, Katrina Potter, and Victoria Scarnati assisted with data collection.
Compliance with Ethical Standards
All data were collected in accordance with Kent State University’s policies on the conduct of research with human subjects and with approval of the human ethics Institutional Review Board. Written informed consent was obtained from listeners (if ≥ 18 years) or their parents or caregivers (if < 18 years) prior to the experiment. Informed assent was obtained from all listeners under 18 years of age.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jennifer D. Gay, Email: jgay1@neomed.edu
Merri J. Rosen, Email: mrosen@neomed.edu
Julia Jones Huyck, Email: jhuyck@kent.edu.
References
- Ahissar M, Nahum M, Nelken I, Hochstein S. Reverse hierarchies and sensory learning. Philos Trans R Soc B Biol Sci. 2009;364(1515):285–299. doi: 10.1098/rstb.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitay S, Zhang YX, Jones PR, Moore DR. Perceptual learning: top to bottom. Vis Res. 2014;99:69–77. doi: 10.1016/j.visres.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59(4):390–412. doi: 10.1016/j.jml.2007.12.005. [DOI] [Google Scholar]
- Bargones JY, Werner LA, Marean GC. Infant psychometric functions for detection: mechanisms of immature sensitivity. J Acoust Soc Am. 1995;98(1):99–111. doi: 10.1121/1.414446. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136(1):31–49. doi: 10.1016/S0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81(6):1641–1660. doi: 10.1037/a0035216.Family. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buran BN, von Trapp G, Semple MN, Sanes DH, Sen K. A decline in response variability improves neural signal detection during auditory task performance. J Neurosci. 2016;36(43):11097–11106. doi: 10.1523/jneurosci.1302-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Hall JW, Grose JH, Dev MB. Development of adult-like performance in backward, simultaneous, and forward masking. J Speech Lang Hear Res. 1999;42(4):844–849. doi: 10.1044/jslhr.4204.844. [DOI] [PubMed] [Google Scholar]
- Buss E, Hall JW, Grose JH. Factors affecting the processing of intensity in school-aged children. J Speech Lang Hear Res. 2013;56(1):71–80. doi: 10.1044/1092-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Porter HL, Hall JW, Grose JH. Gap detection in school-age children and adults: center frequency and ramp duration. J Speech Lang Hear Res. 2017;60(1):172–181. doi: 10.1044/2016_JSLHR-H-16-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera L, Varnet L, Buss E, Rosen S, Lorenzi C. Development of temporal auditory processing in childhood: changes in efficiency rather than temporal-modulation selectivity. J Acoust Soc Am. 2019;146(4):2415–2429. doi: 10.1121/1.5128324. [DOI] [PubMed] [Google Scholar]
- Cai D, Han R, Liu M, Xie F, You L, Zheng Y, Yuan K. A critical role of inhibition in temporal processing maturation in the primary auditory cortex. Cereb Cortex. 2017;28:1–15. doi: 10.1093/cercor/bhx057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras ML, Sanes DH. Top-down modulation of sensory cortex gates perceptual learning. Proc Natl Acad Sci. 2017;114(37):201712305–201719977. doi: 10.1073/pnas.1712305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceponiene R, Rinne T, Näätänen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113(6):870–882. doi: 10.1016/S1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U S A. 2005;102(45):16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry RS. Development of selective auditory attention skills in children. Percept Mot Skills. 1981;52(2):379–385. doi: 10.2466/pms.1981.52.2.379. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. J Cogn Dev. 2010;11(2):121–136. doi: 10.1080/15248371003699969.Twelve. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes P, Bishop DVM. Maturation of visual and auditory temporal processing in school-aged children. J Speech Lang Hear Res. 2008;51(4):1002–1015. doi: 10.1044/1092-4388(2008/073). [DOI] [PubMed] [Google Scholar]
- Dean KF, Sheets LP, Crofton KM, Reiter LW. The effect of age and experience on inhibition of the acoustic startle response by gaps in background noise. Psychobiology. 1990;18(1):89–95. doi: 10.3758/BF03327220. [DOI] [Google Scholar]
- Diedler J, Pietz J, Bast T, Rupp A. Auditory temporal resolution in children assessed by magnetoencephalography. Neuroreport. 2007;18(16):1691–1695. doi: 10.1097/WNR.0b013e3282f0b6e2. [DOI] [PubMed] [Google Scholar]
- Downer JD, Rapone B, Verhein J, O’Connor KN, Sutter ML. Feature-selective attention adaptively shifts noise correlations in primary auditory cortex. J Neurosci. 2017;37(21):5378–5392. doi: 10.1523/JNEUROSCI.3169-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ. Representation of a voice onset time continuum in primary auditory cortex of the cat. J Acoust Soc Am. 1995;98(2):911–920. doi: 10.1121/1.413517. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Neural responses in primary auditory cortex mimic psychophysical, across-frequency-channel, gap-detection thresholds. J Neurophysiol. 2000;84(3):1453–1463. doi: 10.1152/jn.2000.84.3.1453. [DOI] [PubMed] [Google Scholar]
- Elangovan S, Stuart A. Natural boundaries in gap detection are related to categorical perception of stop consonants. Ear Hear. 2008;29(5):761–774. doi: 10.1097/AUD.0b013e318185ddd2. [DOI] [PubMed] [Google Scholar]
- Engell A, Junghöfer M, Stein A, Lau P, Wunderlich R, Wollbrink A, Pantev C. Modulatory effects of attention on lateral inhibition in the human auditory cortex. PLoS One. 2016;11(2):1–15. doi: 10.1371/journal.pone.0149933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Hartnegg K. On the development of low-level auditory discrimination and deficits in dyslexia. Dyslexia. 2004;10(2):105–118. doi: 10.1002/dys.268. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K, Sanes D (2001) The development of stimulus coding in the auditory system. In Physiology of the Ear, 2nd Edition (pp. 215–240)
- Friedman JT, Peiffer AM, Clark MG, Benasich AA, Fitch RH. Age and experience-related improvements in gap detection in the rat. Dev Brain Res. 2004;152(2):83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6(11):1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Fritz JB, David SV, Radtke-Schuller S, Yin P, Shamma SA. Adaptive, behaviorally gated, persistent encoding of task-relevant auditory information in ferret frontal cortex. Nat Neurosci. 2010;13(8):1011–1019. doi: 10.1038/nn.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450(7168):425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Gay JD, Voytenko SV, Galazyuk AV, Rosen MJ. Developmental hearing loss impairs signal detection in noise : putative central mechanisms. Front Syst Neurosci. 2014;8:1–11. doi: 10.3389/fnsys.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen G, Sexton MA. The development of auditory strategies of attention. Dev Psychol. 1978;14(1):11–17. doi: 10.1037/0012-1649.14.1.11. [DOI] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42(4):335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Gomes H, Molholm S, Christodoulou C, Ritter W, Cowan N. The development of auditory attention in children. Front Biosci. 2000;5:108–120. doi: 10.1093/toxsci/kfs057. [DOI] [PubMed] [Google Scholar]
- Gordon KR. How mixed-effects modeling can advance our understanding of learning and memory and improve clinical and educational practice. J Speech Lang Hear Res. 2019;62:507–524. doi: 10.1044/2018_JSLHR-L-ASTM-18-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DB, Ohlemacher J, Rosen MJ. Benefits of stimulus exposure: developmental learning independent of task performance. Front Neurosci. 2016;10:1–13. doi: 10.3389/fnins.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Hall JW. Gap detection in a narrow-band of noise in the presence of a flanking band of noise. J Acoust Soc Am. 1993;93(3):1645–1648. doi: 10.1121/1.406825. [DOI] [Google Scholar]
- Halliday LF, Taylor JL, Edmondson-Jones AM, Moore DR. Frequency discrimination learning in children. J Acoust Soc Am. 2008;123(6):4393–4402. doi: 10.1121/1.2890749. [DOI] [PubMed] [Google Scholar]
- Hartley DEH, Moore DR. Auditory processing efficiency deficits in children with developmental language impairments. J Acoust Soc Am. 2002;112(6):2962–2966. doi: 10.1121/1.1512701. [DOI] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, Zecker SG, Kraus N. Neural plasticity following auditory training in children with learning problems. Clin Neurophysiol. 2003;114(4):673–684. doi: 10.1016/S1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. doi: 10.1093/biomet/75.4.800. [DOI] [Google Scholar]
- Huyck JJ, Rosen MJ. Development of perception and perceptual learning for multi-timescale filtered speech. J Acoust Soc Am. 2018;144(2):667–677. doi: 10.1121/1.5049369. [DOI] [PubMed] [Google Scholar]
- Huyck JJ, Wright BA. Late maturation of auditory perceptual learning. Dev Sci. 2011;14(3):614–621. doi: 10.1111/j.1467-7687.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyck JJ, Wright BA. Learning, worsening, and generalization in response to auditory perceptual training during adolescence. J Acoust Soc Am. 2013;134(2):1172–1182. doi: 10.1121/1.4812258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyck JJ, Wright BA. Transient sex differences during adolescence on auditory perceptual tasks. Dev Sci. 2017;21(3):1–8. doi: 10.1111/desc.12574. [DOI] [PubMed] [Google Scholar]
- Irvine DRF. Auditory perceptual learning and changes in the conceptualization of auditory cortex. Hear Res. 2018;366:3–16. doi: 10.1016/j.heares.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Irwin R, Ball A, Kay N, Stillman J, Rosser J. The development of auditory temporal acuity in children. Child Dev. 1985;56(3):614–620. doi: 10.2307/1129751. [DOI] [PubMed] [Google Scholar]
- Ison JR, O’Connor K, Bowen GP, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci. 1991;105(1):33–40. doi: 10.1037/0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- Jones PR, Moore DR, Amitay S. Development of auditory selective attention: why children struggle to hear in noisy environments. Dev Psychol. 2015;51(3):353–369. doi: 10.1037/a0038570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CH, Kaylegian K, Wehr M. Gap encoding by parvalbumin-expressing interneurons in auditory cortex. J Neurophysiol. 2018;120(1):105–114. doi: 10.1152/jn.00911.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Rooney BJ, Phillips DP. Effects of bilateral auditory cortical lesions on gap-detection thresholds in the ferret (Mustela putorius) Behav Neurosci. 1996;110(3):542–550. doi: 10.1037/0735-7044.110.3.542. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kurt S, Crook JM, Ohl FW, Scheich H, Schulze H. Differential effects of iontophoretic in vivo application of the GABA A-antagonists bicuculline and gabazine in sensory cortex. Hear Res. 2006;212(1–2):224–235. doi: 10.1016/j.heares.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Larsen B, Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev. 2018;94:179–195. doi: 10.1016/j.neubiorev.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek MR, Hanna TE, Marshall L. An interleaved tracking procedure to monitor unstable psychometric functions. J Acoust Soc Am. 1991;90(3):1385–1397. doi: 10.1121/1.401930. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(2):467–477. doi: 10.1121/1.1912375. [DOI] [PubMed] [Google Scholar]
- Maxon B, Hochberg I. Development of psychoacoustic behavior: sensitivity and discrimination. Ear Hear. 1982;3(6):301–308. doi: 10.1097/00003446-198211000-00003. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, Stickgold R. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5(7):677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Drummond SPA, Arman AC, Boynton GM. Perceptual deterioration is reflected in the neural response: Fmri study of Nappers and non-Nappers. Perception. 2008;37(7):1086–1097. doi: 10.1068/p5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ. Temporal analysis in Normal and impaired hearing. Ann N Y Acad Sci. 1993;682(1 temporal info):119–136. doi: 10.1111/j.1749-6632.1993.tb22964.x. [DOI] [PubMed] [Google Scholar]
- Moore DR, Ferguson MA, Halliday LF, Riley A. Frequency discrimination in children: perception, learning and attention. Hear Res. 2008;238(1–2):147–154. doi: 10.1002/lipi.19680700112. [DOI] [PubMed] [Google Scholar]
- Moore DR, Cowan JA, Riley A, Edmondson-Jones AM, Ferguson MA. Development of auditory processing in 6- to 11-yr-old children. Ear Hear. 2011;32(3):269–285. doi: 10.1097/AUD.0b013e318201c468. [DOI] [PubMed] [Google Scholar]
- Moreno-Paublete R, Canlon B, Cederroth CR. Differential neural responses underlying the inhibition of the startle response by pre-pulses or gaps in mice. Front Cell Neurosci. 2017;11(February):1–11. doi: 10.3389/fncel.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer CE, Erickson SL, Fish KN, Thiels E, Penzes P, Sweet RA. Developmental trajectories of auditory cortex synaptic structures and gap-prepulse inhibition of acoustic startle between early adolescence and young adulthood in mice. Cereb Cortex. 2016;26(5):2115–2126. doi: 10.1093/cercor/bhv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muluk NB, Yalçinkaya F, Keith RW. Random gap detection test and random gap detection test-expanded: results in children with previous language delay in early childhood. Auris Nasus Larynx. 2011;38(1):6–13. doi: 10.1016/j.anl.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Niwa M, Johnson JS, O’Connor KN, Sutter ML. Active engagement improves primary auditory cortical neurons’ ability to discriminate temporal modulation. J Neurosci. 2012;32(27):9323–9334. doi: 10.1523/JNEUROSCI.5832-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AMM, Reyes AD. Development of inhibitory timescales in auditory cortex. Cereb Cortex. 2011;21(6):1351–1361. doi: 10.1093/cercor/bhq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passow S, Müller M, Westerhausen R, Hugdahl K, Wartenburger I, Heekeren HR, Lindenberger U, Li S-C. Development of attentional control of verbal auditory perception from middle to late childhood: comparisons to healthy aging. Dev Psychol. 2012;49(10):1982–1993. doi: 10.1037/a0031207. [DOI] [PubMed] [Google Scholar]
- Patterson RD, Nimmo-Smith I, Weber DL, Milroy R. The deterioration of hearing with age: frequency selectivity, the critical ratio, the audiogram, and speech thresholda. J Acoust Soc Am. 1982;72(6):1788–1803. doi: 10.1121/1.388652. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Lane DM. Auditory attention switching: a developmental study. J Exp Child Psychol. 1991;51(2):320–334. doi: 10.1016/0022-0965(91)90039-U. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Taylor TL, Hall SE, Carr MM, Mossop JE. Detection of silent intervals between noises activating different perceptual channels: some properties of “central” auditory gap detection. J Acoust Soc Am. 1997;101(6):3694–3705. doi: 10.1121/1.419376. [DOI] [PubMed] [Google Scholar]
- Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci U S A. 2008;105(11):4465–4470. doi: 10.1073/pnas.0709504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2017) R: A language and environment for statistical computing. Vienna, Austria
- Rowan D, Lutman ME. Learning to discriminate interaural time differences: an exploratory study with amplitude-modulated stimuli. Int J Audiol. 2006;45(9):513–520. doi: 10.1080/14992020600801434. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Woolf NK. Development of tonotopic representation in the Mongolian gerbil: a 2-deoxyglucose study. Dev Brain Res. 1988;41(1–2):61–70. doi: 10.1016/0165-3806(88)90169-1. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Woolley SMN. A behavioral framework to guide research on central auditory development and plasticity. Neuron. 2011;72(6):912–929. doi: 10.1016/j.neuron.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Sanes DH. Prolonged maturation of auditory perception and learning in gerbils. Dev Neurobiol. 2010;70(9):636–648. doi: 10.1002/dneu.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DI, Kraus N. Postnatal development of the auditory in the unanesthetized brainstem response (ABR) gerbil. Brain. 1987;27:157–164. doi: 10.1016/0378-5955(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Snell KB, Hu H-L. The effect of temporal placement on gap detectability. J Acoust Soc Am. 1999;106(6):3571–3577. doi: 10.1121/1.428210. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider M, Schroeder CE, Arezzo JC, Vaughan HG. Speech-evoked activity in primary auditory cortex: effects of voice onset time. Electroencephalogr Clin Neurophysiol Evoked Potentials. 1994;92(1):30–43. doi: 10.1016/0168-5597(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Strait DL, Slater J, Abecassis V, Kraus N. Cortical response variability as a developmental index of selective auditory attention. Dev Sci. 2014;17(2):175–186. doi: 10.1111/desc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Mazelova J, Druga R. Gap detection threshold in the rat before and after auditory cortex ablation. Hear Res. 2002;172(1–2):151–159. doi: 10.1016/S0378-5955(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Age-dependent effect of hearing loss on cortical inhibitory synapse function. J Neurophysiol. 2012;107(3):937–947. doi: 10.1152/jn.00515.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Penley SC, Rosen GD, Fitch RH. Detection of silent gaps in white noise following cortical deactivation in rats. NeuroReport. 2008;19(8):893–898. doi: 10.1097/WNR.0b013e3283013d7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor LJ, Samuel SS, Desjardins RN, Sonnadara RR. Measuring temporal resolution in infants using mismatch negativity. NeuroReport. 2001;12(11):2443–2448. doi: 10.1097/00001756-200108080-00031. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Schneider BA, Henderson JL. Gap detection in infants, children, and adults. J Acoust Soc Am. 1995;98(5 Pt 1):2532–2541. doi: 10.1121/1.414396. [DOI] [PubMed] [Google Scholar]
- Wagenaar A. Note on the construction of digram-balanced Latin squares. Psychol Bull. 1969;72(6):384–386. doi: 10.1037/h0028329. [DOI] [Google Scholar]
- Walker KMM, Hall SE, Klein RM, Phillips DP. Development of perceptual correlates of reading performance. Brain Res. 2006;1124(1):126–141. doi: 10.1016/j.brainres.2006.09.080. [DOI] [PubMed] [Google Scholar]
- Weible AP, Moore AK, Liu C, Deblander L, Wu H, Kentros C, Wehr M (2014) Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr Biol 24(13):1447–1455. 10.1016/j.cub.2014.05.031 [DOI] [PMC free article] [PubMed]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229(1–2):54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner LA, Folsom RC, Mancl LR, Syapin CL. Human auditory brainstem response to temporal gaps in noise. J Speech Lang Hear Res. 2001;44(1–4):737–750. doi: 10.1044/1092-4388(2001/058). [DOI] [PubMed] [Google Scholar]
- Wetzel N, Schröger E. Cognitive control of involuntary attention and distraction in children and adolescents. Brain Res. 2007;1155(1):134–146. doi: 10.1016/j.brainres.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Wetzel N, Widmann A, Berti S, Schröger E. The development of involuntary and voluntary attention from childhood to adulthood: a combined behavioral and event-related potential study. Clin Neurophysiol. 2006;117(10):2191–2203. doi: 10.1016/j.clinph.2006.06.717. [DOI] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. Informational masking of speech in children: effects of ipsilateral and contralateral distracters. J Acoust Soc Am. 2005;118(5):3164–3176. doi: 10.1121/1.2082567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman F, Allen P, Dolan T, Kistler D, Jamieson D. Temporal resolution in children. Child Dev. 1989;60(3):611–624. doi: 10.1111/j.1467-8624.1989.tb02742.x. [DOI] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ, O’Bryan A. Individual differences and age effects in a dichotic informational masking paradigm. J Acoust Soc Am. 2010;128(1):270–279. doi: 10.1121/1.3436536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Kanold PO. Laminar transformation of frequency organization in auditory cortex. J Neurosci. 2013;33(4):1498–1508. doi: 10.1523/JNEUROSCI.3101-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Nagode DA, Donaldson KJ, Yin P, Shamma SA, Fritz JB, Kanold PO. Orbitofrontal cortex neurons respond to sound and activate primary auditory cortex neurons. Cereb Cortex (New York, N.Y. : 1991) 2018;28(3):868–879. doi: 10.1093/cercor/bhw409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Znamenskiy P, Zador AM. Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature. 2015;521(7552):348–351. doi: 10.1038/nature14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye CQ, Poo MM, Dan Y, Zhang XH. Synaptic mechanisms of direction selectivity in primary auditory cortex. J Neurosci. 2010;30(5):1861–1868. doi: 10.1523/jneurosci.3088-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Liang F, Xiong XR, Li L, Li H, Xiao Z, Zhang LI. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat Neurosci. 2014;17(6):841–850. doi: 10.1038/nn.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]