Abstract
We sought to determine whether repeated vestibulo-ocular reflex (VOR) adaptation training to increase the VOR gain (eye/head velocity) had a lasting effect in normal subjects and whether there was a retinal image slip tolerance threshold for VOR adaptation. We used the unilateral incremental VOR adaptation technique and horizontal active (self-generated, predictable) head impulses as the vestibular stimulus. Both active and passive (imposed, unpredictable) head impulse VOR gains were measured before and after unilateral incremental VOR adaptation training. The adapting side was pseudo-randomized for left or right. We tested ten normal subjects over one block (10 sessions over 12 days) of VOR adaptation training and testing, immediately followed by a second block (5 sessions over 19 days) of testing only without training. Our findings show robust short-term VOR adaptation of ~ 10 % immediately after each 15-min training session, but that the daily pre-adaptation gain was most different on days 1 and 2, and for subsequent training days before saturating to ~ 5 % greater than the pre-adaptation gain on day 1. This increase was partially retained for 19 days after regular training stopped. The data suggest that stable vision in normal subjects is maintained when there is < 5 % deviation in VOR gain from the original baseline, which corresponds to < 9°/s retinal image slip. Below this threshold, there is poor adaptive drive to return the gain to its original baseline value.
Keywords: vestibulo-ocular reflex (VOR), VOR adaptation, VOR error tolerance, VOR training repetition
INTRODUCTION
The vestibulo-ocular reflex (VOR) maintains images stable on the retina during head movement by rotating the eyes in the opposite direction to the head. The VOR can be increased or decreased in magnitude using a visual-vestibular mismatch stimulus to control the retinal image slip signal (Gauthier and Robinson 1975; Gonshor and Melvill 1976a, 1976b; Paige and Sargent 1991). For example, if the VOR does not have sufficient magnitude to stabilise the image of the visual world on the retina during head motion, a blurred world image appears on the retina, which in turn generates a signal to increase the response or VOR gain (eye/head velocity) to minimise blur (e.g. Gauthier and Robinson 1975). Presumably, the VOR is constantly adjusting its gain to maintain visual stability of the world during rapid head movements, yet prior studies have shown that the VOR gain is remarkably stable across testing separated by as much as 537 days (Schubert and Migliaccio 2016). Additionally, an under-compensatory VOR does not always result in blurred vision, presumably due to central image processing, which is especially true for the roll VOR (Schubert et al. 2012). A recent study also showed that a retinal image slip error of ~ 17°/s is sufficient to drive horizontal VOR adaptation (Todd et al. 2019), but it is not clear how much image slip is tolerated by the horizontal VOR before adaptation occurs. This is critical to know when developing treatment regimens that serve to improve a reduced VOR gain, as occurs in pathology or with age. We hypothesise that repeated exposure to VOR adaptation training in normal subjects will help answer this question. After adaptation training that increases the VOR gain to > 1 (the ideal far-viewing VOR gain is ~ 1), exposure to normal viewing conditions should result in the VOR gain returning to its original baseline value. However, if the VOR is able to tolerate some amount of retinal image slip without adaptation occurring, then the VOR gain may not have a need to return to the original baseline. We hypothesise that a physiologically efficient VOR would only drive the minimum change needed to ensure stable vision. Therefore, repeated exposure to VOR adaptation training and testing in normal subjects should result in the VOR daily baseline shifting and perhaps saturating to just within the threshold required for adaptation to occur.
In the present study, a visual-vestibular mismatch training stimulus was generated by coupling the movement of a visual laser target to active (self-generated, predictable) head impulses (Halmagyi and Curthoys 1988) to gradually increase the VOR gain unilaterally (Schubert et al. 2008; Migliaccio and Schubert 2013, 2014; Fadaee and Migliaccio 2016; Mahfuz et al. 2018a, 2018b, 2018c, 2018d; Todd et al. 2018). Head impulses were used as the vestibular training stimulus because these have the same frequency and velocity content of head motion during everyday activities that require the VOR for visual stability (Grossman et al. 1988). This 15-min exercise is known as incremental VOR adaptation training and has been used to significantly increase the horizontal VOR gain for rotations towards the training/adapting side (left or right) by ~ 10 % in healthy controls.
Ten subjects participated in 15 experimental sessions over 31 days. The first 10 sessions (over 12 days) consisted of active VOR gain training with pre- and post-adaptation testing involving both active and passive (imposed, unpredictable) head impulses. The last 5 sessions (over 19 days) consisted of active and passive VOR gain testing only, i.e. no adaptation training. The focus of the study was on tracking the active and passive daily pre-adaptation gains over both the training (12-day) and retention (19-day) periods to determine the retinal image slip tolerance threshold before VOR adaptation occurs.
METHODS
Subjects
Ten healthy controls (mean age 34 years, range 27–47 years; 2 females, 8 males) participated in 15 VOR gain adaptation sessions over separate days. None of these subjects had any history or clinical signs of vestibular hypofunction. These subjects did not participate in any other VOR adaptation studies for at least 3 months prior to this study. All subjects were students or employees based at Neuroscience Research Australia and were financially compensated for their time. All participants attended all 15 sessions. Participation in this study was voluntary and informed consent was obtained as approved by the University of New South Wales Human Ethics Committee.
Recording System
An EyeSeeCam system (Denmark) was used to measure eye and head rotation. The system consisted of a lightweight goggle frame strapped tightly to the head. Slip between the goggles and the head was minimised due to a tight fit and the use of silicon putty (Surgipack, Australia), which also improved comfort. The goggles contained a 220-Hz video camera mounted over the left eye, which tracked pupil position. The eye was illuminated by two infrared LEDs and reflected back to the camera via an infrared hot mirror. A laser, mounted to the goggles, projected a set of targets at known angles. These angles were used to calibrate the eye measurements transforming pupil position to eye rotation. The goggles also contain a gyroscope and accelerometer, which directly measured 3D (yaw, pitch and roll) head velocity. All data were subject to a 50-Hz 50-tap zero-phase low pass digital FIR filter.
VOR Adaptation Training Protocol
We used the unilateral incremental VOR adaptation training protocol to increase the VOR gain towards one side (for a detailed description, see Migliaccio and Schubert 2013). We used the head impulse test (Halmagyi and Curthoys 1988) to measure the active and passive VOR gains before and after active VOR adaptation training. Passive head impulses, applied for passive VOR testing, were delivered manually in the horizontal canal plane and were unpredictable in direction and timing. Subjects were trained to perform active (i.e. self-generated) head impulses so that they had the same velocity profile as passive head impulses (see Figure 1 in Migliaccio and Schubert 2013). For VOR testing, with the head at neutral (facing straight ahead), subjects were instructed to fixate a stationary visual target located on midline at eye level. The target was extinguished once the head rotated 0.6 degrees away from neutral.
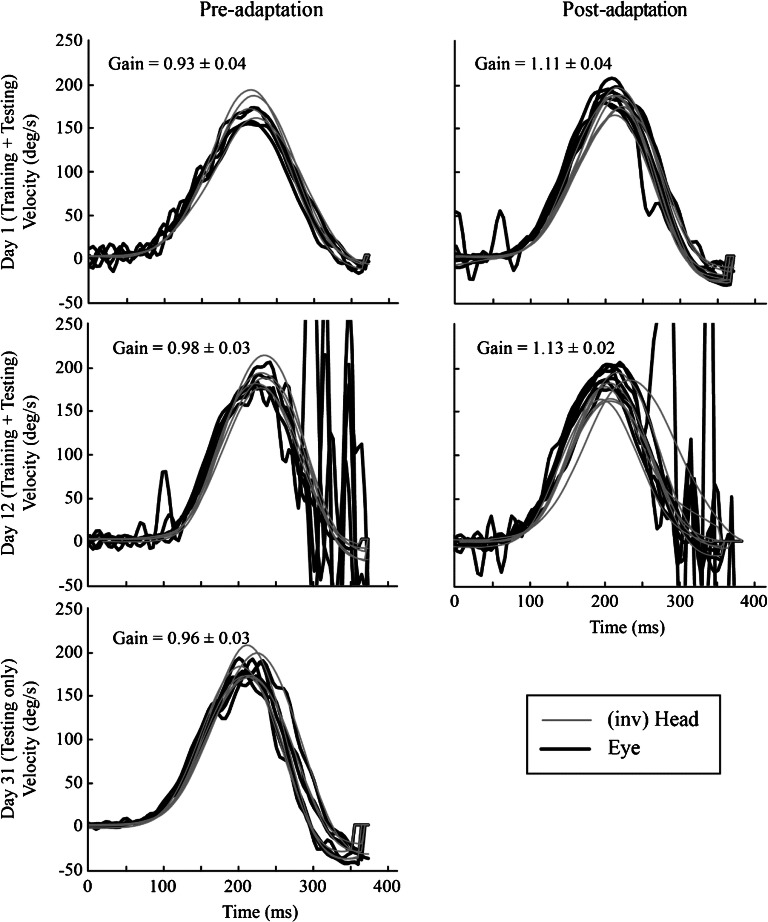
Fig. 1.
Pre- (left column) and post-adaptation (right column) training passive VOR gains towards the adapting side of one typical subject that underwent VOR adaptation training on day 1 (first day of adaptation training; first row), day 12 (last day of adaptation training; second row) and day 31 (last day of the 19-day retention period; last row). For day 31, VOR adaptation training did not occur, so only the passive VOR gain measured that day is shown. Note that for VOR gains > 1 eye velocity (black trace) has a higher peak than inverted head velocity (grey trace). The opposite is true when the gain is < 1
For unilateral VOR adaptation training, subjects were instructed to perform sequential active head impulses, starting from the neutral position, alternating leftward and rightward while maintaining visual fixation of the laser target that moved as a function of head rotation. The laser target was extinguished once head peak velocity was detected and reappeared only after the head returned to its neutral position. For rotations towards the non-adapting side, the VOR gain demand was always unity, i.e. the target was stationary, whereas for rotations towards the adapting side (either left or right, pseudo-randomly evenly assigned), the visual-vestibular mismatch stimulus was set so that the VOR gain demand required to track the target increased from unity (visual laser target velocity matched head velocity in magnitude, but synchronously moved in the opposite direction to the head) for epoch 1, to 1.9 (visual laser target velocity was set 90 % greater than head velocity in magnitude, but opposite in direction) for epoch 10. The total training took 15 min.
Data Collection
The visual fixation target was generated by a wearable digital laser device (Analogue version: Migliaccio and Schubert 2014; Digital version: Mahfuz et al. 2018a, 2018b, 2018c, 2018d, Todd et al. 2019). The device projected a laser onto a matte white screen (2.4 × 2.4 m) placed 1 m in front of the subject. The device was strapped firmly to the subject’s forehead and contained an IMU, which measured 3D angular head position at 250 Hz to within 0.1° by fusion of gyroscope, accelerometer and magnetometer measurements (STMicroelectronics, USA). The device contained a laser directed at a MEMS micro mirror (Mirrorcle Technologies Inc., USA). Real-time head orientation was used to drive the mirror angle which produced a visual laser target on the matte screen relative to the head. The device also consisted of a hand-held unit which provided audio feedback when the peak velocity of head impulses was out of the 120 to 180°/s range.
Each subject underwent 10 sessions of VOR adaptation training over 12 days, i.e. training did not occur over the 2-day weekend between sessions 5 and 6. The incremental VOR gain adaptation demand always started at the same magnitude for each of the 10 sessions. The active and passive VOR gains were measured pre- and post-adaptation training during all 10 training sessions and during an additional 5 sessions (assigned as pre-adaptation or pre- training data) over the 19 days immediately following the last training session, i.e. days 15, 17, 19, 26 and 31.
Data Analysis
Horizontal angular eye position was differentiated. The start of each head impulse was determined by fitting a polynomial curve that modelled horizontal angular head velocity magnitude versus time. The time point where the magnitude of the polynomial curve was 2 % of the curve’s peak magnitude was defined as the impulse start. Head impulses with peak magnitude < 150°/s were excluded from the analysis. Eye traces (and corresponding head traces) with saccades between head impulse start and peak velocity were removed in addition to those containing blinks and other artefacts. The instantaneous VOR gain was calculated as the absolute value of eye velocity divided by head velocity. The VOR gain was defined as the median of the instantaneous VOR gains calculated during the 30-ms period (at 220 Hz, this corresponds to 6 to 7 instantaneous gain values) immediately prior to impulse peak velocity.
The VOR gain change for each side (adapting or non-adapting) as a percentage was calculated as:
A positive result indicated an increase in VOR gain due to the adaptation training. The VOR gain asymmetry between sides (adapting or non-adapting) as a percentage was calculated as:
A positive result indicated that the adapting side VOR gain was larger than the non-adapting side gain.
Statistical Analysis
Statistical analysis was performed using Excel 2013 (Microsoft, USA), G*Power 3.1.9.4 (Universität Kiel, Germany) and SPSS version 23 (IBM, USA). Datasets were complete for all except three corrupted data files (different subjects and conditions). The VOR gain data was analysed using a linear mixed model with repeated measures (LMM), which reduced to a general linear model (GLM) when only the original baseline (day 1) data were analysed. Q-Q plots of VOR gain, gain and asymmetry percentage showed normal distributions. For this statistical model, a sample size of n = 10 was sufficient to detect a change in VOR gain of 0.03 with 92.3 % power (with assumptions SD = 0.05, α = 0.05, two-sided; G*Power analysis). The independent variables included in the model were head rotation side (‘adapting’, ‘non-adapting’), head impulse type (‘active’, ‘passive’), testing time (‘pre-training’, ‘post-training’) and testing day (1, 2, 3, 4, 5, 8, 9, 10, 11, 12, 15, 17, 19, 26 and 31). For the VOR response analysis, the dependent variable was gain, whereas for the VOR stimulus analysis, it was head peak velocity. The VOR gain increase and gain asymmetry percentage data were also analysed with a LMM using the same independent factors above but excluding the time and side variables, respectively. All variables were included in the LMM initially, with those found insignificant subsequently removed. Only significant interaction effects were included in the results. Pooled data were described as mean ± 1 SD, whereas pooled means were described as mean ± 1 SE.
RESULTS
Analysis of the VOR Stimulus (Impulse Head Peak Velocity) Data
There were differences in the head impulse peak velocities between subjects (LMM F9,214 = 14.6, P < 0.001) and between rotation sides (adapting or non-adapting) (LMM F1,177 = 4.2, P < 0.05). However, head impulse peak velocity was not affected by test day (LMM F14,134 = 1.4, P = 0.17), test time (pre-training or post-training) (LMM F1,172 = 1.8, P = 0.18), impulse type (active or passive) (LMM F1,177 = 0.2, P = 0.63) or whether the head rotation was leftward or rightward (LMM F1,76 = 0.5, P = 0.48). Pooled across all conditions for each subject, the mean head impulse peak velocity between subjects ranged from 164 ± 11 to 180 ± 18°/s with grand mean 171 ± 18°/s. The mean head impulse peak velocity towards the adapting and non-adapting sides was 172 ± 13°/s and 169 ± 12°/s, respectively.
Original Baseline (Day 1) VOR
There were no differences in the original baseline VOR gains (pre-adaptation gains on day 1) between subjects (GLM F9,39 = 0.85, P = 0.58), active and passive head impulses (GLM F1,39 = 1.3, P = 0.27) and leftward and rightward head impulses (GLM F1,39 = 1.4, P = 0.25). The mean original baseline VOR gain across subjects was 0.96 ± 0.05. However, there was a borderline significant difference between head impulses towards the (randomly assigned) adapting and non-adapting sides (GLM F1,39 = 4.1, P = 0.05), so that the mean pre-adaptation VOR gains towards the adapting and non-adapting sides were 0.94 ± 0.04 and 0.97 ± 0.05, respectively. Towards the adapting side, the mean pre-adaptation active and passive VOR gains were 0.95 ± 0.03 and 0.93 ± 0.04, respectively.
Repetition Effect on a Typical Subject
Figure 1 displays the pre- and post-adaptation training passive VOR gains towards the adapting side of one typical subject that underwent VOR adaptation training and testing over 12 days, followed by VOR testing (only) over 19 days. On day 1 (original baseline), the pre-adaptation gain was 0.93 ± 0.04 and increased by day 12 to 0.98 ± 0.05. On day 31, i.e. 19 days after the last training session, the gain (0.96 ± 0.03) did not return to the original baseline, but rather was closer to the day 12 gain, suggesting partial retention. For this subject, the mean VOR gain increase per adaptation training session over the first 12 days was 14.0 ± 3.5 %.
Repetition Effect Across Subjects
Figure 2a shows the pre- (white boxplots) and post-adaptation training (grey boxplots) VOR gains towards the adapting (left column) and non-adapting (right column) sides for both active (top row) and passive (bottom row) VOR responses across days. After day 12 (10 sessions), training no longer occurred.
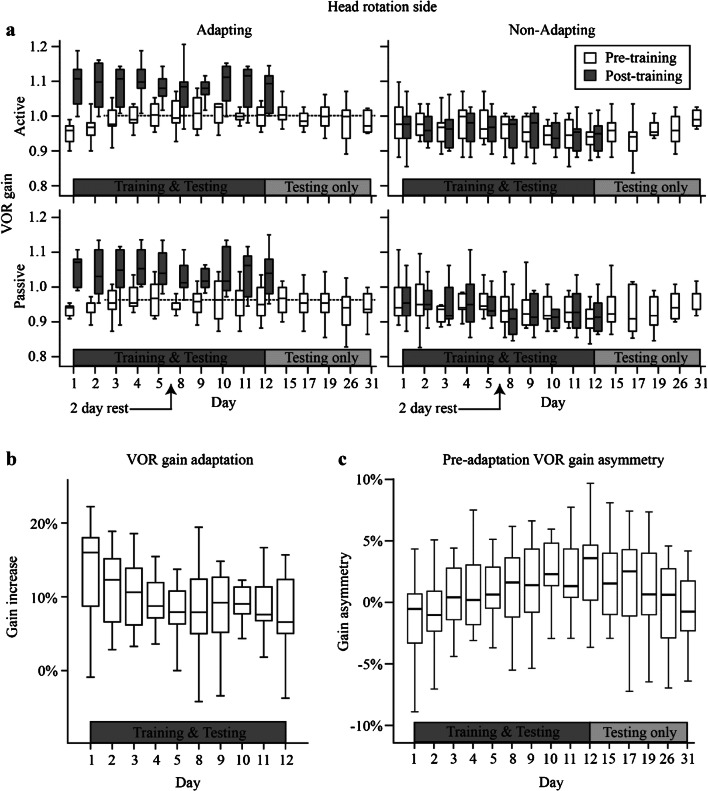
Fig. 2.
a Pre- (white boxplots) and post-adaptation training (grey boxplots) VOR gains towards the adapting (left column) and non-adapting (right column) sides for both active (top row) and passive (bottom row) VOR responses as a group across days. After day 12 (10 sessions), training no longer occurred, but the VOR continued to be tested over 19 days (5 sessions) and reported as pre-adaptation training gains only. The boxplots show the median with 2nd and 3rd quartiles and whiskers showing the minimum and maximum gains. The dashed lines in left column panels denote the mean pre-adaptation VOR gains across days 3 to 12 for the active (top panel) and passive (bottom panel) VOR, respectively. b Pooled active and passive VOR gain percentage increases towards the adapting side on training days 1 to 12. c Pre-adaptation training VOR gain asymmetry for the pooled active and passive VOR from days 1 to 31
Analysis of the group pre-adaptation VOR gain data, i.e. from days 1 to 31, showed a significant difference between head impulse type (active or passive) VOR gains (LMM F1,62 = 12.6, P < 0.001, a difference that was not significant on day 1—see above) and head rotation side (adapting or non-adapting) (LMM F1,62 = 17.6, P < 0.05). There was a significant interaction between head rotation side and day (GLM F14,156 = 1.8, P < 0.05), suggesting that the pre-adaptation VOR gain on the adapting side was most affected by the testing day. On the adapting side, the mean active and passive VOR gains were 0.99 ± 0.04 and 0.95 ± 0.04, respectively.
A sub-analysis of the pre-adaptation VOR gain data towards the adapting side only showed that between days 1 and 12, i.e. the training days, the effect of day was significant (LMM F9,155 = 2.1, P < 0.03), but it was not significant between days 12 and 31 (LMM F4,76 = 2.1, P = 0.08; day 31 active VOR gain = 0.98 ± 0.03, passive VOR gain = 0.94 ± 0.05), suggesting that the changes in pre-adaptation gain occurred during training only and that these were retained beyond the cessation of the training. Further analysis showed that the pre-adaptation VOR gain was most different on days 1 and 2, because once these 2 days were removed, the effect of training day was no longer significant (LMM F7,131 = 0.68, P = 0.69). Towards the adapting side, the mean pre-adaptation active and passive VOR gains between days 3 and 12 were 1.0 ± 0.04 and 0.96 ± 0.04, respectively.
Analysis of the post-adaptation gain data revealed a similar pattern to the pre-adaptation data, i.e. significant gain differences between head impulse type (LMM F1,39 = 5.6, P < 0.03) and head rotation side (LMM F1,39 = 78.8, P < 0.001); however, day did not affect the post-adaptation gain either directly (LMM F9,197 = 1.8, P = 0.07) or through interaction. The mean post-adaptation active and passive gains towards the adapting side were 1.09 ± 0.05 and 1.04 ± 0.05, respectively.
We normalised the data for each subject during the 12 days of training by calculating the pre- to post-adaptation training VOR gain percentage increase for each side (adapting, non-adapting). There was a significant difference in percentage VOR gain increase between head rotation side (LMM F1,62 = 228, P < 0.0001). There was no difference in the percentage increase in gain between the active or passive VOR (LMM F1,62 = 0.02, P = 0.88). Figure 2b shows the pooled active and passive VOR gain percentage increases towards the adapting side on training days 1 to 12. The mean increase across days towards the adapting side was 9.4 ± 5.1 %, whereas towards the non-adapting side (not shown) it was − 0.67 ± 3.2 %.
We calculated the VOR gain asymmetry between adapting and non-adapting sides pre- and post-adaptation training. There was a significant difference in VOR gain asymmetry between pre- and post-adaptation training time (LMM F1,49 = 31.5, P < 0.001) and day (GLM F14,103 = 2.0, P < 0.02). The pre-adaptation gain asymmetry was affected by day (GLM F14,76 = 1.9, P < 0.05), because gain asymmetry increased with day (day 1 − 1.7 ± 3.9 %; day 12 2.8 ± 3.7 % (peak asymmetry); day 31 − 0.7 ± 3.1 %). Figure 2c shows the pre-adaptation training VOR gain asymmetry for the pooled active and passive VOR from days 1 to 31. In contrast, post-adaptation asymmetry (not shown) was not affected by day (LMM F9,93 L3, P 0.22) and averaged 5.8 ± 3.9 %.
DISCUSSION
Our findings suggest that in healthy controls, the daily baseline VOR gain can increase at least up to ~ 5 % (active VOR increased 5 % from 0.95 to 1.0) with repeated training. Most of this increase occurred during the first 2 days of training and were partially retained for 19 days after regular training had stopped. Although VOR adaptation of 9.4 % occurred throughout the training sessions, the pre-adaptation gain seemed to saturate after day 2, suggesting that once VOR gain deviation was > 5 %, adaptive mechanisms reduced the gain back to the ~ 5 % threshold. Taken together, these data suggest that stable vision is maintained as long as any change in VOR gain is within 5 % of its original baseline or is within 9°/s retinal image slip (adapting side peak head velocity times percentage tolerance = 172 × 0.05 = 8.6°/s). Below this threshold, there is poor adaptive drive to return the gain to its original baseline value measured on day 1. Above this threshold, the VOR gain begins to show adaptation.
All the subjects in this study had normal VOR function, so the increase in VOR gain due to adaptation training would have been over-compensatory for normal viewing and activity. Prior studies have shown that in this situation, de-adaptation occurs quickly, i.e. ~ 3 min (see Mahfuz et al. 2018b, showing that significant de-adaptation required 3 test periods lasting 60 s each), which is appropriate given that an over-compensatory (or under-compensatory) VOR results in unstable vision. Although de-adaptation occurred between test sessions during the training period (i.e. first 10 sessions over 12 days), pre-adaptation gains on days 1 and 2 were different to all subsequent days, suggesting that the pre-adaptation gain had saturated and that the image slip signal provided a weak adaptive drive for further gain change below that value. These findings support three ideas. First, there exists a threshold for the image slip signal below which the drive for adaptive change significantly weakens. This is subtly different from the idea that as the image slip signal becomes weaker so does the adaptive drive, because under that circumstance, one would have expected that over 24 h (between training sessions), the pre-adaptation gain would have returned to its original value. This is also consistent with the observation that the mean original (day 1) baseline VOR gain was slightly different between rotations sides and was close to 0.95, which is ~ 5 % less than the ideal VOR gain of unity. Other studies have reported similar findings, which are often attributed to geometric or eye measurement technique artefacts, but could also be due in part to some tolerance for retinal image slip (e.g. Mahfuz et al. 2018a, 2018b, 2018c, 2018d). Second, central visual mechanisms do not increasingly compensate for changes in the VOR. Presumably, when the VOR gain is not perfectly compensatory, some of the resultant retinal image slip can be compensated by central mechanisms, so that visual blur is not perceived. This likely happens to the roll VOR, which is highly under-compensatory (gain ~ 0.7), yet blurring is not perceived because the image remains on the fovea (Schubert et al. 2012). If central processes had contributed further and further to vision stabilisation, then the pre-adaptation gain would have steadily increased, rather it seems that central visual processing can only compensate for a fixed amount of image slip. Third, if the daily pre-adaptation VOR gain had continued to rise over the 12-day training period, then that would have suggested that in-between training subjects were not being exposed to vestibular stimuli similar to those during training. Given that human VOR adaptation training is somewhat frequency specific (Rinaudo et al. 2019a), an increasing pre-adaptation gain would have indicated that the training stimulus was sufficiently different to the vestibulo-visual stimulus during normal viewing activity so that de-adaptation would not be evident during testing, i.e. because our testing and training stimuli were similar. However, this was not the case. After day 2, the daily pre-adaptation VOR gain seemed to saturate, suggesting that head impulse training and real-world vestibulo-visual stimuli were similar.
Limitations
The focus of this study was on repeated head impulse gain-increase training in healthy controls, although our primary focus is to improve vestibular rehabilitation methods that seek to increase the gain of the VOR in patients with peripheral vestibular hypofunction. Additionally, we have only studied the tolerance of retinal slip without a concurrent adapted change for gain-increase training. We did not study the tolerance of repeated incremental VOR gain-decrease training, as might be beneficial in conditions of cerebellar disinhibition (e.g. Walker and Zee 1999). It is possible that repeated gain-decrease training would have resulted in daily pre-adaptation gains saturating at a threshold lower than the original (day 1) pre-adaptation gain. On the other hand, the VOR could prefer to maintain an under-compensatory solution that is more energy/movement efficient if the image slip could be compensated for centrally. Therefore, the original (day 1) baseline gain may already be close to the lower-threshold gain.
Another limitation of this study is that there was not a statistically significant decrease in VOR gain after the training stopped. A larger sample size or better yet extending the number of days tested after the last training session to several months might have resulted in the daily baseline VOR gain returning to the original baseline level. We hypothesise that the de-adaptation time would be highly variable between healthy subjects, because the adaptive drive is weak and potentially random.
Implications for VOR Rehabilitation
Our data reveal that despite repeated VOR adaptation training that drives the gain above unity (1), healthy controls have a 5 % saturation tolerance—and that any retinal slip with magnitude larger than this tolerance is lost once exposure to a real-world vestibulo-visual stimulus drives the VOR gain back towards unity. In other words, VOR adaptation training in healthy controls drives the VOR gain away from unity, but exposure to real-world viewing opposes this change and drives the gain towards unity with a 5 % tolerance for retinal slip. This suggests that the closer the VOR gain can be driven to unity in hypofunction, the less likely the gain is to reduce once training has completed and the individual returns to real-world viewing. In fact, when repeated gain-increase training is reinforced by real-world viewing, the daily baseline gain should continue to increase as was shown in two recent patient case studies (Gimmon et al. 2019; Rinaudo et al. 2019b).
CONCLUSIONS
Repeated VOR adaptation gain-increase training resulted in an increase in daily baseline gain that saturated after 2 days and was partially retained for at least 19 days after the last training session. These findings suggest that stable vision in healthy controls is maintained as long as exposure to eye and head motion stays within a 5 % tolerance of the original baseline VOR gain, which corresponds to < 9°/s retinal image slip. Under that circumstance, there is a poor adaptive drive to alter the daily baseline VOR gain. These data further suggest that VOR adaptation training in patients that attempts to increase the gain towards unity will be reinforced by real-world viewing and such learning will not return to its original pathological value, but instead be retained.
Acknowledgements
A.A. Migliaccio was supported by The Garnett Passe and Rodney Williams Memorial Foundation Senior/Principal Research Fellowship in Otorhinolaryngology and Project Grant (2013-15), and NHMRC Development Grant APPI05550.
Compliance with Ethical Standards
Participation in this study was voluntary and informed consent was obtained as approved by the University of New South Wales Human Ethics Committee.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Fadaee SB, Migliaccio AA. The effect of retinal image error update rate on human vestibule-ocular reflex gain adaptation. Exp Brain Res. 2016;234:1085–1094. doi: 10.1007/s00221-015-4535-y. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Robinson DA. Adaptation of the human vestibuloocular reflex to magnifying lenses. Brain Res. 1975;92:331–335. doi: 10.1016/0006-8993(75)90279-6. [DOI] [PubMed] [Google Scholar]
- Gimmon Y, Migliaccio AA, Kim KJ, Schubert MC (2019) VOR adaptation training and retention in a patient with profound bilateral vestibular hypofunction. Laryngoscope. 10.1002/lary.27838 [DOI] [PubMed]
- Gonshor A, Melvill JG. Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol. 1976;256:361–379. doi: 10.1113/jphysiol.1976.sp011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonshor A, Melvill JG. Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol. 1976;256:381–414. doi: 10.1113/jphysiol.1976.sp011330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- Mahfuz MM, Schubert MC, Todd CJ, Figtree WVC, Khan SI, Migliaccio AA. The effect of visual contrast on human vestibulo-ocular reflex training. J Assoc Res Otolaryngol. 2018;19:113–122. doi: 10.1007/s10162-017-0644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfuz MM, Schubert MC, Figtree WVC, Todd CJ, Khan SI, Migliaccio AA. Optimal human passive vestibulo-ocular reflex adaptation does not rely on passive training. J Assoc Res Otolaryngol. 2018;19:261–271. doi: 10.1007/s10162-018-0657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfuz MM, Schubert MC, Figtree WVC, Todd CJ, Migliaccio AA. Human vestibulo-ocular reflex adaptation: consolidation time between repeated training blocks improves retention. J Assoc Res Otolaryngol. 2018;19:601–610. doi: 10.1007/s10162-018-00686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfuz MM, Schubert MC, Figtree WVC, Todd CJ, Migliaccio AA. Human vestibulo-ocular reflex adaptation training: time beats quantity. J Assoc Res Otolaryngol. 2018;19:729–739. doi: 10.1007/s10162-018-00689-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Schubert MC. Unilateral adaptation of the human angular vestibulo-ocular reflex. J Assoc Res Otolaryngol. 2013;14:29–36. doi: 10.1007/s10162-012-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Schubert MC. Pilot study of a new rehabilitation tool: improved unilateral short-term adaptation of the human angular vestibulo-ocular reflex. Otol Neurotol. 2014;35:310–316. doi: 10.1097/MAO.0000000000000539. [DOI] [PubMed] [Google Scholar]
- Paige GD, Sargent EW. Visually-induced adaptive plasticity in the human vestibulo-ocular reflex. Exp Brain Res. 1991;84:25–34. doi: 10.1007/BF00231759. [DOI] [PubMed] [Google Scholar]
- Rinaudo CN, Schubert MC, Cremer PD, Figtree WVC, Todd CJ, Migliaccio AA (2019a) Improved oculomotor physiology and behavior after unilateral incremental adaptation training in a person with chronic vestibular hypofunction: case report. Phys Ther. 10.1093/ptj/pzz083 [DOI] [PubMed]
- Rinaudo CN, Schubert MC, Figtree WVC, Todd CJ, Migliaccio AA. Human vestibulo-ocular reflex adaptation is frequency-selective. J Neurophysiol. 2019;122:984–993. doi: 10.1152/jn.00162.2019. [DOI] [PubMed] [Google Scholar]
- Schubert MC, Migliaccio AA. Stability of the aVOR to repeat head impulse testing. Otol Neurotol. 2016;37:781–786. doi: 10.1097/MAO.0000000000001055. [DOI] [PubMed] [Google Scholar]
- Schubert MC, Della Santina CC, Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008;191:435–446. doi: 10.1007/s00221-008-1537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Migliaccio AA, Ng T, Shaikh AG, Zee DS. The under-compensatory roll aVOR does not affect dynamic visual acuity. J Assoc Res Otolaryngol. 2012;13:517–525. doi: 10.1007/s10162-012-0330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd CJ, Hübner PP, Hübner P, Schubert MC, Migliaccio AA (2018) StableEyes–a portable vestibular rehabilitation device. IEEE Trans Neural Syst Rehabil Eng 26:1223–1232 [DOI] [PubMed]
- Todd CJ, Schubert MC, Figtree WVC, Migliaccio AA. Incremental vestibulo-ocular reflex adaptation training dynamically tailored for each individual. J Neurol Phys Ther. 2019;43(Suppl 2 Supplement, Special Supplement: International Conference on Vestibular Rehabilitation):S2–S7. doi: 10.1097/NPT.0000000000000269. [DOI] [PubMed] [Google Scholar]
- Walker MF, Zee DS. Directional abnormalities of vestibular and optokinetic responses in cerebellar disease. Ann N Y Acad Sci. 1999;871:205–220. doi: 10.1111/j.1749-6632.1999.tb09186.x. [DOI] [PubMed] [Google Scholar]