Abstract
BACKGROUND:
Fetal bovine serum is widely used as a growth supplement for cell culture medium; however, animal-borne pathogens increase the risk of transmitting infectious agents. Platelet-rich fibrin is recently considered as a successful alternative but leukocytes present limits to its allogeneic feasibility. The aim of this study was to explore the effects of allogeneic fibrin clot (AFC) without leukocytes on inducing odontogenic/cementogenic differentiation of human dental pulp stem cells (hDPSCs) and human periodontal ligament stem cells (hPDLSCs) in vitro and in vivo.
METHODS:
AFC was prepared by high-speed centrifugation and leukocytes were almost removed, and AFC serum was obtained through three freeze–thaw cycles. hDPSCs and hPDLSCs were treated with AFC serum to investigate the odontogenic or cementogenic associated markers by real-time polymerase chain reaction. hDPSCs were treated with AFC serum and placed inside of dentin canal, hPDLSCs were treated with AFC serum to wrap outside of dentin, the mixture was then transplanted into the subcutaneous of nude mice for 12 weeks.
RESULTS:
AFC serum exhibited enough growth factors and cytokines to induce odontogenic/cementogenic differentiation of hDPSCs and hPDLSCs in vitro. Furthermore, AFC seurum could induce hDPSCs to differentiate into odontoblasts-like cells and pulp-like tissues, and hPDLSCs to regenerate cementum-like tissues.
CONCLUSION:
AFC could be an alternative safe source with growth factors for the expansion of human dental mesenchymal stem cells (hDMSCs).
Keywords: Allogeneic fibrin clot, Human dental mesenchymal stem cells, Platelet-rich fibrin, Leukocyte, Odongtogenic/cementogenic
Introduction
Complete or partial loss of tooth structures can be caused by mechanical trauma, periodontal diseases, endodontic complications, and the therapeutic approaches include restorations, fixed or removable prosthesis, and implants. Regeneration of the lost structures of the tooth received concerns among the cell biologists in the recent years, as this results in protecting the integrity of the natural tooth structure and preventing tooth loss [1]. Cell transplantation became the principal strategy of choice in regenerative medicine. Stem cell technology can induce mesenchymal stem cells to differentiate into a variety of different cells in vitro, which can be used to treat several kind of diseases such as leukemia, dementia, Parkinson”s disease [2].
Human dental mesenchymal stem cells (hDMSCs), which exhibit multilineage differentiation abilities, could be obtained from different dental tissues such as pulp, periodontal ligaments, apical papilla, periapical follicles, and so on [3–6]. Human dental pulp stem cells (hDPSCs) could differentiate into odontoblasts, and generate dentin or pulp tissues [7]. Human periodontal ligament cells (hPDLSCs) also had the ability to differentiate into osteoblasts, cementoblasts, adipocytes, and chondrocytes [8]. Recent studies indicated that hPDLSCs could regenerate cementum-like tissues and periodontal ligament-like tissues, when hPDLSCs were transplanted into rodents with growth factors [9]. When culturing human stem cells, the culture medium is usually supplemented with xenogeneic additives such as bovine thrombin or fetal bovine serum (FBS) [10]. In the early stage, autologous platelet-rich plasma (PRP) was developed and used as a replacement for cell culture [11–13], but PRP with anticoagulants showed the limited effects on bone and hard tissue regeneration [14, 15]. To overcome this disadvantage, platelet-rich fibrin (PRF) was found to induce the differentiation of mesenchymal stem cells (MSCs) without any anticoagulants [16, 17]. PRF is a fibrin clot with a solid structure, including platelets, leukocytes, and concentrated red blood cells (RBCs), and these cells mostly get distributed in the buffy coat [16]. However, concentrated RBCs may promote inflammation because of several pro-inflammatory interleukins [18].
Recently, the low-speed centrifugation concept (LSCC) was found to separate a new type of liquid PRF without anticoagulant capacity [19, 20]. Liquid PRF, obtained using a low speed relative centrifugal force (RCF), is mainly used as an autologous carrier system. It was reported that PRF contains higher concentrations of leukocytes, lymphocytes, and neutrophils compared to high RCF [21, 22]. However, the biggest problem is to mitigate the immune response triggered by the residual white blood cells in PRF.
In this study, we applied the allogeneic serum for tissue regeneration of hDPSCs and hPDLSCs, because of the limitations of autologous serum. Allogenic Fibrin Clot (AFC) was prepared for odontogenic/cementogenic differentiation of hDPSCs and hPDLSCs, which removed almost leukocytes. To determine the optimal concentrations of AFC serum for hDPSCs and hPDLSCs culturing, we investigated the effects of AFC serum on the differentiation of hDPSCs and hPDLSCs compared to 10% FBS in vitro. Then, to explore the effects of AFC serum on odontogenic/cementogenic differentiation of hDPSCs and hPDLSCs in vivo, we mixed the cells with AFC serum and transplanted into the mice.
Materials and methods
Samples
Human third molars and blood samples were collected from three healthy women aged 18–25 years. The use of anonymous tooth samples and blood for research purposes was approved by the local ethics, and informed consent was acquired from all donors. The study was approved by the institutional review board (IRB) of Seoul National University Dental Hospital, Seoul, Korea (IRB no. 05004). The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Seoul National University (No. SNU-190426-13).
Fabrication of AFC, and AFC serum
The collected blood samples were placed in a sterile tube without anticoagulants and immediately centrifuged at 950 g using a Union 32R Plus centrifuge (Hanil Science Industrial Co., Ltd., Incheon, Korea) for 16 min at room temperature (Fig. 1). The blood was then stratified in the following three layers (Fig. 1B): a yellow-colored liquid serum (top), a red portion full of RBCs (bottom), and a middle part between these two areas (leukocyte pellet). The liquid serum (top layer) was carefully collected in a new polypropylene tube, avoiding RBC contamination (Fig. 1C) and, after a few minutes, separated into AFC and supernatant serum (SS) (Fig. 1D). To break the platelet membrane and release growth factors, AFC was passed through at least three freeze–thaw cycle. Then, the AFC was divided into fibrin membrane (Fig. 1E) and serum AFC extraction, then all of the serum collected and named AFC serum and stored at −70 °C for 24 h and immersed in a 50 °C water bath for 30 min. Finally, AFC serum obtained was sterilized using a 0.2-µm GVS filter (Filter Technology, Inc., Chicago, IL, USA) for cell culture (Fig. 1F). In conventional PRF, after centrifugation at 400 g for 12 min, it separates into three layers and is left at room temperature for a while. When it clots in a gel state, the upper portion is usually called PRF (Fig. 2A) [23, 24].
Fig. 1.
Fabrication of AFC. A 10 mL of blood; B Separated 3 layers by high-speed centrifugation; C AFC (Liquid serum); D AFC (a: supernatant serum, b: AFC); E After three freeze–thaw cycles, AFC more compacted and called fibrin membrane; F AFC serum after filtered. AFC, allogeneic fibrin clot
Fig. 2.
AFC compared with PRF. A PRF. B and F Representative histological images of a fibrin clot along its longitudinal axis. A and B Buffy coat (arrows). C Low magnification of PRF. D High magnification (a: leukocytes, b: RBCs). E AFC. G Low magnification of AFC. H Fibrin network structure. I and J Evaluation of human MMP array for AFC serum as compared with PRF serum. Three positive controls (red circle) were designated in two corners. H&E staining: magnifications: × 40 (C, G), × 200 (D, H); Scale bars: 100μm, 20μm. PRF, platelet-rich fibrin; MMP, matrix metalloproteinase; RBCs, red blood cells; H&E, hematoxylin and eosin
Histological analysis of AFC and PRF
AFC and PRF were histologically examined to determine their microstructure. PRF was prepared via the protocol previously described. Briefly, the specimens were fixed in 3.7% paraformaldehyde (PFA) at 4 °C for one day. Samples were embedded in paraffin and 4-mm-thick serial sections were transversely cut at the center of the exposed. Then, slice site dehydration using an alcohol series of various concentrations (70%, 80%, 90%, 95%, 100%) every 2 min was performed and processed for haematoxylin and eosin (H&E) staining.
Scanning electron microscopy (SEM) analysis
To observe cell bodies trapped in the AFC and the overall architecture of the fibrin network, samples were washed with 0.1 M of phosphate-buffered saline (PBS) and fixed in modified karnovsky’s fixative buffer at 4 °C for 2 h. The AFC was washed three times using 0.1 M of PBS at room temperature for 15 min and fixed in 0.1 M of cacodylate buffer (pH: 7.4) containing 1% osmium tetroxide for 1 h. After rapid dehydration through an ethanol gradient (70, 80, 90, 95, and 100% v/v), the samples were coated with Platinum prior to cell shape observation with field emission S-4700 scanning electron microscope (Hitachi, Tokyo, Japan) using an acceleration voltage of 15kv.
Human matrix metalloproteinase (MMP) and cytokine antibody array
Focused protein array analysis was performed using the human MMP and cytokine antibody array panel Proteome Profiler Array (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The human MMP antibody array panel was used for AFC serum, and PRF serum, detected MMP-1, MMP-2, MMP3, MMP-8, MMP-9, MMP-10, MMP-13, metallopeptidase inhibitor 1 (TIMP-1), TIMP-2, and TIMP-4. While human cytokine array panels were used for AFC serum, detected angiogenin, brain-derived neurotrophic factor (BDNF), B lymphocyte chemoattractant (BLC), Bone morphogenetic protein 4 (BMP-4), BMP-6, casein kinase β8-1 (CK β8-1), ciliary neurotrophic factor (CNTF), epidermal growth factor (EGF), Eotaxin, Eotaxin-2, Eotaxin-3, fibroblast growth factor 6 (FGF-6), FGF-7, Fit-3 Ligand, Fractalkine, granulocyte chemotactic protein 2 (GCP-2), glial cell-derived neurotrophic factor (GDNF), granulocyte–macrophage colony-stimulating factor (GM-CSF), chemokine (C–C motif) ligand 1 (I-309), interferon gamma (IFN-γ), insulin-like growth factor–binding protein 1 (IGFBP-1), IGFBP-2, IGFBP-4, IGF-I, interleukin 10 (IL-10), IL-13, IL-15, IL-16, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, Leptin, LIGHT, monocyte chemoattractant protein 1 (MCP-1), MCP-2, MCP-3, MCP-4, M-CSF, Macrophage-derived chemokine (MDC), monokine induced by gamma interferon (MIG), macrophage inflammatory protein-1δ (MIP-1δ), MIP-3α, neutrophil-activating peptide-2 (NAP-2), neurotrophin-3 (NT-3), pulmonary and activation-regulated chemokine (PARC), platelet-derived growth factor-BB (PDGF-BB), regulated upon activation normal T cell expressed, and secreted (RANTES), stem cell factor (SCF), stromal cell-derived factor 1 (SDF-1), thymus and activation regulated chemokine (TARC), transforming growth factor beta 1 (TGF-β1), TGF-β3, Tumor necrosis factor alpha (TNF-α), and TNF-β.
Isolation and proliferation of hDPSCs and hPDLSCs
Dental pulp was separated from root canal of extracted human third molars to for culturing hDPSCs, and periodontal ligament was obtained from the surface of tooth roots for culturing hPDLSCs. Each separated tissues were digested in a solution containing 3 mg/mL of collagenase type I (Worthington Biochem, Freehold, NJ, USA) and 4 mg/mL of dispase (Boehringer Ingelheim, Ingelheim am Rhein, Germany) at 37 °C for 1 h. At this point, single-cell suspensions were obtained. Briefly, the cells were passed through a 40-mm strainer (BD Labware, Franklin Lakes, NJ, USA), cultured in an alpha modification of Minimum Essential medium (Alpha MEM) (WELGENE, GS, Gyeong San, Korea) supplemented with AFC serum, 100 × Antibiotic (GIBCO USA), and incubated at 37 °C in 5% CO2 [25]. hDPSCs and hPDLSCs were randomly selected pool of these colonies and used for in vitro proliferation and differentiation studies respectively. All primary cells used in this study were from passages 2 through 5.
To study the effects of AFC serum on cell proliferation, hDPSCs and hPDLSCs were plated in 96-well plates at a density of 5 × 103 cells per well. AFC serum was added to FBS-free α-MEM at different final concentrations of 1%, 2%, 5%, 8%, and 10%. For comparison, cells with 10% FBS complete medium were prepared separately. At 1, 4, 7, and 14 days after culture, cell proliferation was measured using a WST-8 assay Viability Assay Kit (MediFab Co., Ltd., Rolleston, New Zealand) and enzyme-linked immunosorbent assay reader at an optical density of 450 nm.
Flow cytometric analysis
To characterize the immunophenotype of hDPSCs and hPDLSCs, the expression of MSCs-associated surface markers were analyzed by flow cytometry as described previously (Becton–Dickinson Immunocytometry Systems, San Jose, CA, USA) [25]. Briefly, cells (1 × 106 cells) were collected and fixed in 4% PFA for 10 min and resuspended in PBS containing 1% bovine serum albumin (BSA) for 30 min for blocking, respectively. Both of cells were then incubated with specific antibodies CD13, CD34, CD90, or CD146 (BD Biosciences, San Jose, CA, USA) at 4 °C for 1 h, followed by incubation with fluorescence secondary antibodies at room temperature for 1 h. The percentage of CD13-, CD90-, and CD146-positive and CD34-negative cells was measured with a FACSCalibur flow cytometer (Becton–Dickinson Immunocytometry Systems, San Jose, CA, USA) and the results were analyzed using CellQuest Pro software (Becton–Dickinson Immunocytometry Systems, San Jose, CA, USA).
Multi-differentiation
For osteogenic differentiation, hDPSCs and hPDLSCs were seeded into 24-well plates at a density of 4 × 104 cells per well with α-MEM containing 1%, 2%, 5%, and 10% AFC serum, compared with 10% FBS, supplemented with 10 mM of β-glycerophosphate, 50 mg/mL of ascorbic acid, and 1 mM of dexamethasone. After osteogenic differentiation induction, calcium deposition was assessed using Alizarin Red S (Sigma Aldrich Corp., St. Louis, MO, USA) staining. For induction of chondrogenic and adipogenic differentiation of hDPSCs and hPDLSCs, the cells (4 × 104 cells) were seeded into 24-well plates and induced with Stempro Chondrogenic and Stempro Adipogenic induction media Kit (Lonza, Walkersville, MD, USA) supplemented with the appropriate supplements (Lonza, Walkersville, MD, USA), respectively. The medium was changed after the first 24 h and then every 3 days. After chondrogenic and adipogenic induction, the cells were washed with PBS and fixed in 3.7% paraformaldehyde for 10 min. The cells were stained with 1% Alcian Blue (Sigma Aldrich Corp.) and 0.3% Oil Red O dye (Sigma Aldrich Corp.) for detection of proteoglycans and fat vacuoles as indicators of chondrogenic and adipogenic differentiation. Cell photographs were visualized using the same Olympus U-SPT microscope (Olympus, Tokyo, Japan).
Real-time polymerase chain reaction (PCR)
To evaluate gene expression, 5.0 × 105 cells were seeded in a 6-well plate and cultured under differentiation-inducing conditions. hDPSCs were cultured for 7 and 18 days, while hPDLSCs, 7 and 12 days. Total RNA was prepared using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions, and cDNA was synthesized from 2 µg of total RNA using reverse transcriptase (Superscript II Preamplification System, Invitrogen, Gaithersburg, MD, USA). Real-time PCR was performed using SYBR Green PCR Master Mix in a Step One Plus real-time PCR detection system (Applied Biosystems, Foster City, CA, USA). All reactions were run in triplicates and normalized to the reference gene glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH). Table 1 lists the specific primer sets used for this analysis.
Table 1.
Primer sequences for real-time polymerase chain reaction
| Target cDNA | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) |
|---|---|---|
| ALP | TAAGGACATCGCCTACCAGCTC | TCTTCCAGGTGTCAACGAGGT |
| COL1 | AACATGGAGACTGGTGAGACCT | CGCCATACTCGAACTGGAATC |
| Runx2 | CTTTACTTACACCCCGCCAGTC | AGAGATATGGAGTGCTGCTGGTC |
| DMP-1 | CTCGCACACACTCTCCCACTCAAA | TGGCTTTCCTCGCTCTGACTCTCT |
| DSPP | GCAGCAATAGCAGTGAGAGCAGTGA | GCTGCTGTCACTATCGCTGCTGTTA |
| CEMP1 | TCAAGGCAGAGGTGGGTATC | GGAAATGTCTCCAGGTCCAA |
| GAPDH | CTTTGGTATCGTGGAAGGACTC | GTAGAGGCAGGGATGATGTTCT |
Preparation of human dentin matrix
The dental pulp tissue and the periodontal tissues were completely removed using a surgical bur, grinding along the tooth root profile for removing pre-dentin (inside) and cementum (outside). The final size of the dentin was 5 mm length, 5 mm diameter, with thickness up to 1.0 mm. The dentin was immersed in deionized water and mechanically cleaned using an ultrasonic cleaner for 20 min. The dentin was washed 3-times with ethylene diamine tetra-acetic acid (EDTA) (Sigma, USA), before it was used as human dentin matrix. Human dentin matrix were stored in sterile PBS with 100 U/mL penicillin and 100 mg/mL streptomycin (Biofluids, Rockville, MD, USA) for 72 h, and washed in sterile deionized water for 10 min with an ultrasonic cleaner, and stored in Alpha MEM at 4 °C [26].
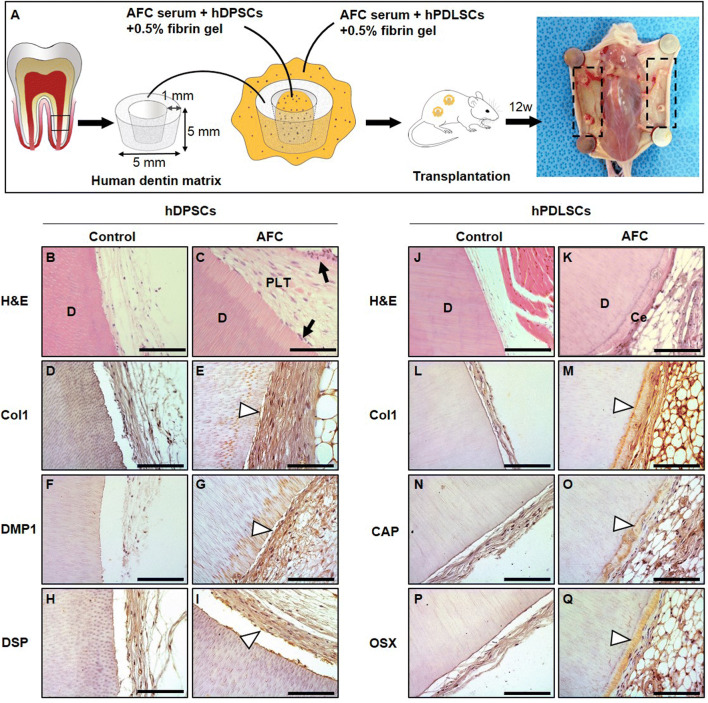
Transplantation and histological analysis
To assess the effects of AFC on the regeneration of dentin and cementum, hDPSCs or hPDLSCs were treated with AFC serum and mixed with human dentin matrix, then subcutaneously transplanted into immunocompromised (BALB/c-nu) mice. hDPSCs (1.0 × 107 cells) were first treated with AFC serum under the role of 0.5% fibrin gel and put into the cavity of human dentin matrix. Next, hPDLSCs (1.0 × 107 cells) were also treated with AFC serum and wrapped around human dentin matrix under the role of 0.5% fibrin gel. After 12 weeks of transplantation, nude mice were euthanized, and the complexes were removed for analysis. Samples were retrieved, fixed with 4% phosphate-buffered paraformaldehyde, and decalcified in a 12% EDTA (pH 7.4) solution at 4 °C. All samples were embedded in paraffin, sectioned, and stained with H&E for histological analysis. Some of the sectioned samples were also used for immunohistochemistry.
The immunohistochemistry (IHC) analysis
Some section of the samples were further processed as described previously [9]. The deparaffinized sections were immersed in 0.6% H2O2/methanol for 20 min and pre-incubated with 1% bovine serum albumin in PBS for 30 min, and then, sections incubated overnight at 4 °C with rabbit polyclonal antibodies against type I collagen (Col1; 1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), dentin matrix protein 1 (DMP1; 1:200, Santa Cruz Biotechnology), dentin sialoprotein (DSP; 1:200, Santa Cruz Biotechnology), cementum attachment protein (CAP; 1:200, Santa Cruz Biotechnology), osterix (OSX; 1:100, Abcam, Cambridge, UK). Sections were incubated for 1 h at room temperature with the appropriate secondary antibodies (1:1000) and then reacted with avidin–biotin–peroxidase complexes (VECTASTAIN ABC Systems; Vector Laboratories, Inc., Burlingame, CA, USA) in PBS for 30 min. After color development with 0.05% 3, 3′-diaminobenzidine tetrahydrochloride (DAB Peroxidase Substrate; Vector Laboratories), the stained sections were counterstained with Hematoxylin.
Statistical analysis
All experiments were repeated at least three times, and data were expressed as means and standard deviations (SD). Statistical significance was evaluated using unpaired Student’s t-tests (two-tailed) with MS-Excel (Microsoft, Inc., Redmond, WA, USA). Statistical significance was considered at *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
The characterization of AFC
H&E staining results encompassed a large number of dark-purple concentrated leukocytes, pink cross-linking mesh-like fibers, and a moderate number of pink-colored RBCs in PRF (Fig. 2C, D). Total blood cell numbers (i.e., leukocytes and RBCs) of AFC were significantly decreased relative to those of PRF (Figs. 2G, H), but AFC still exhibited fibrin network structure (Fig. 2H). Furthermore, human MMP antibody array showed the level of MMP-8 and MMP-9 was significantly decreased in AFC serum compared with PRF serum (Figs. 2I, J).
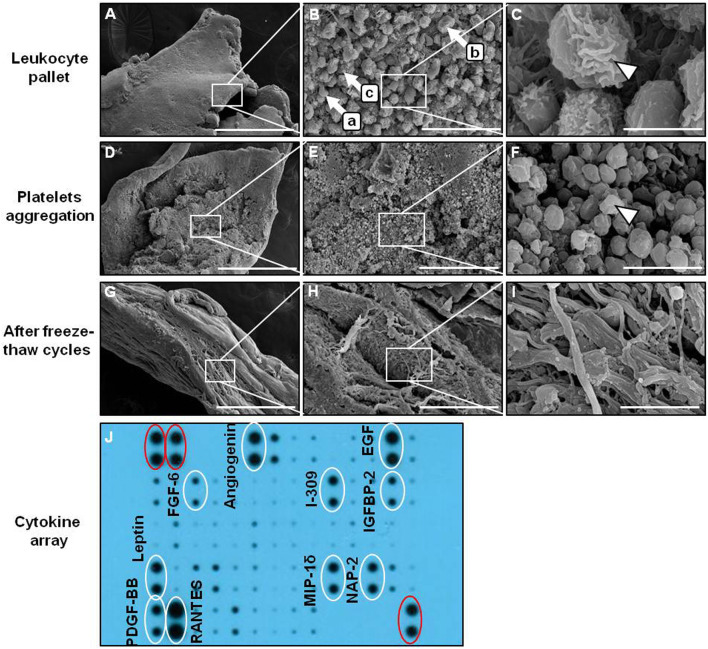
SEM analysis found that a large-size cell complex (~ 4–8 µm in diameter) containing platelet aggregates, RBCs, and leukocytes (Fig. 3A, B) was observable in the middle layer (leukocyte pallet) at a low magnification, and single leukocytes exhibited spherical structures with irregular surfaces (Fig. 3C). Centrifugation of the abovementioned serum was conducted again for 15 min at 1880 g before fibrin coagulation (Fig. 3D), where residual cells were enmeshed into the fibrin network by matrix shrinkage (Fig. 3E). At a low magnification, platelets were observed in the supernatant, and the fibrin was denser with a solid and thick mesh due to extensive aggregation and clotting (Fig. 3E). The typical size of the cells clearly became smaller (~ 1–2 µm in diameter) (Fig. 3F). The size and morphology of the Platelets were different from the RBCs or large leukocytes. This was centrifuged at a higher rotational force and time to identify residual cells in the yellow liquid serum of the upper layer. The results show that the middle layer we discarded contained a large number of platelets, but the upper layer liquid serum also contained some platelets. AFC serum was subjected after three freeze–thaw cycles, AFC was condensed and compacted as a thin fibrin membrane shrunk to a compact size (Fig. 3G, H). The fibrin membrane almost had no platelets or leukocytes observed by SEM (Fig. 3I).
Fig. 3.
SEM of AFC. A Leukocyte pallet (middle layer). B (a: RBCs, b: leukocytes, c: platelet). C Leukocyte with irregular surface (arrowhead). D Fibrin coagulation after liquid serum again for 15 min at 1880 g. E Platelets aggregation. F Platelet (arrowhead). G AFC become solid fibrin mambrane after freeze–thaw for three cycles. H AFC membrane more shrinks. I No platelets can be observed in the fibrin membrane. J Evaluation of human cytokine array for AFC serum. Three positive controls (red circle) were designated in two corners. Three different magnifications: × 100, × 1000, and × 10,000; Scale bars: 500 μm, 50 μm, and 5 μm. SEM, scanning electron microscopy; EGF, epidermal growth factor; FGF6, fibroblast growth factor 6; IGFBP2, insulin-like growth factor–binding protein 2; MIP-1δ, macrophage inflammatory protein-1δ; NAP-2, neutrophil-activating peptide-2; PDGF-BB, platelet-derived growth factor-BB; RANTES, regulated upon activation, normal T-cell expressed, and secreted
Detection of cytokines in AFC serum
The cytokine levels of AFC serum were detected by a human cytokine antibody array. The results revealed that 10 cytokines were detectable in AFC serum, including angiogenin, EGF, FGF6, I-309, IGFBP2, leptin, MIP-1δ, NAP-2, PDGF-BB, and RANTES (Figs. 3J). These results indicate that AFC serum still exhibit enough cytokines to induce the differentiation of hDPSCs and hPDLSCs.
Characterization of hDPSCs and hPDLSCs in AFC serum
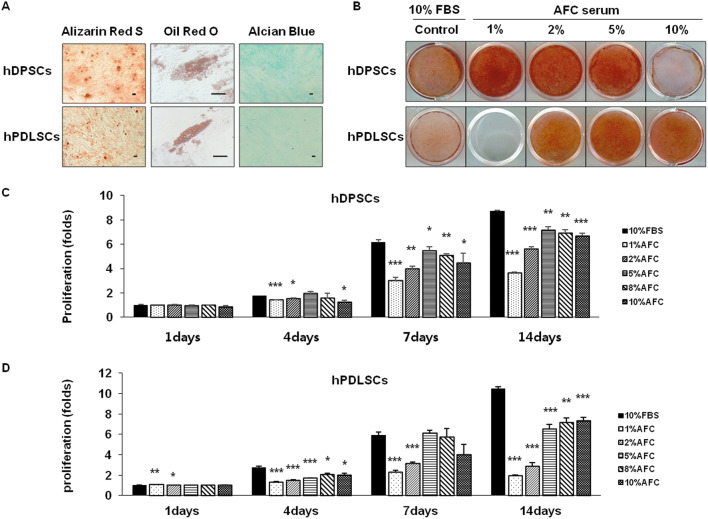
At passage 5, hDPSCs and hPDLSCs were cultured with AFC serum, and the expression of MSC-related markers was confirmed by fluorescence-activated cell sorting analysis. The expressions of CD13 and CD90 were strongly positive in hDPSCs and hPDLSCs to level of more than 95%. CD146 was also expressed in the majority of hDPSCs and hPDLSCs over 70%. CD34, a hematopoietic stem cell marker, was negatively expressed in both of the stem cells to level of less than 3% (Fig. 4). These results suggested that the isolated cells were mainly hDMSCs and stil preserve the stemness of hDPSCs and hPDLSCs up to passage 5. Next, we investigated the multi-differentiation potential of hDPSCs and hPDLSCs, after mineral induction, hDPSCs and hPDLSCs formed an extensive amount of Alizarin Red S–positive mineral deposits throughout adherent layers, respectively. Furthermore, after treatment with adipogenic-inductive supplements for 3 weeks, hDPSCs and hPDLSCs demonstrated the ability to differentiate into adipogenic cells. In addition, hDPSCs and hPDLSCs appeared as Alcian Blue–positive nodules after 3 weeks of chondrogenic induction (Fig. 5A). hDPSCs and hPDLSCs treated with AFC serum formed a lot of mineral nodules of alizarin red S—positive throughout adherent layers after 18 days and 12 days, respectively (Fig. 5B). According to the different characteristics of differentiation of hDPSCs and hPDLSCs, 18 days and 12 days were selected as the time points of later differentiation for hDPSCs and hPDLSCs, respectively.
Fig. 4.
Characterization of hDPSCs and hPDLSCs at passage 5, by flow cytometry assay. Negative of hematopoietic cell surface markers, CD34 and high expression levels of multiple MSC surface markers, CD13, CD90, and CD146 on hDPSC and hPDLSC surface
Fig. 5.
Demonstrating functional differentiation of hDPSCs, hPDLSCs in specialized differentiation media. A Multi-differentiation stained Alizarin red S, Oil red O, and Alcian Blue staining, respectively. B hDPSCs were differentiated for day-18, hPDLSCs were differentiated for day-12 stained with Alizarin red S. C, D Proliferation of hDPSCs, hPDLSCs in AFC serum. N = 4; Significant differences versus the 10% FBS; *p < 0.05; **p < 0.01; ***p < 0.001
Effects of AFC serum on hDPSCs and hPDLSCs in vitro
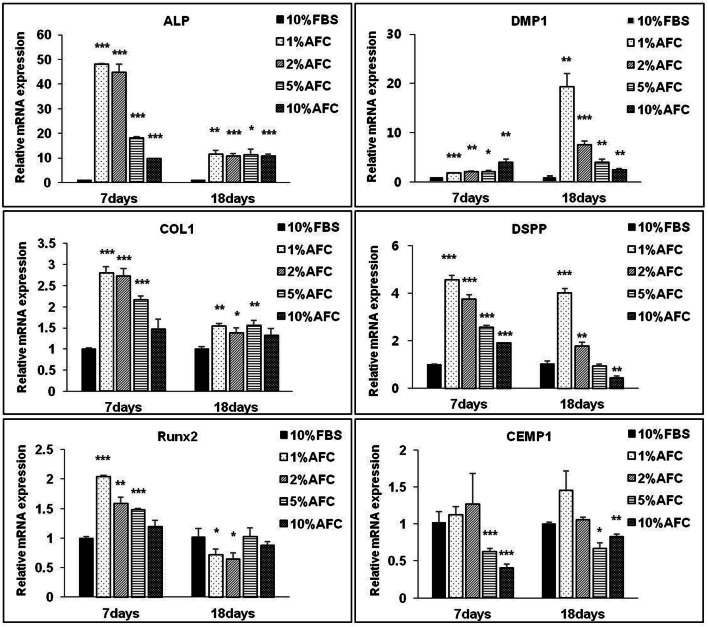
The optimized concentrations of AFC serum for the proliferation of hDPSCs and hPDLSCs were 5% and 10%. The effects of AFC serum on proliferation were inferior to 10% FBS (Figs. 5C, D). The expressions of odontogenic-related genes alkaline phosphatase (ALP), COL1, DMP1, and dentin sialophosphoprotein (DSPP) were significantly increased after treatment with AFC serum in hDPSCs compared to FBS at 7 and 18 days. An increase in the concentration of AFC serum downregulated the DMP1 and DSPP gene expressions as compared with involvement of FBS. Interestingly, treatment with a low concentration (1% AFC serum) significantly increases the expression of odontogenic-related genes ALP, COL1, DMP1, and DSPP during both early and later stages but decreases an index of osteogenic runt-related transcription factor 2 (RUNX2) and cementogenic-related gene cementum protein 1 (CEMP1) expression at later stages (Fig. 6).
Fig. 6.
Effects of AFC serum on odontogenic/cementogenic influence of hDPSCs in vitro. The mRNA levels assay of ALP, COL1, RUNx2, DMP1, DSPP, CEMP1 on day-7, day-18. N = 3; Significant differences versus the 10% FBS; *p < 0.05; **p < 0.01; ***p < 0.001
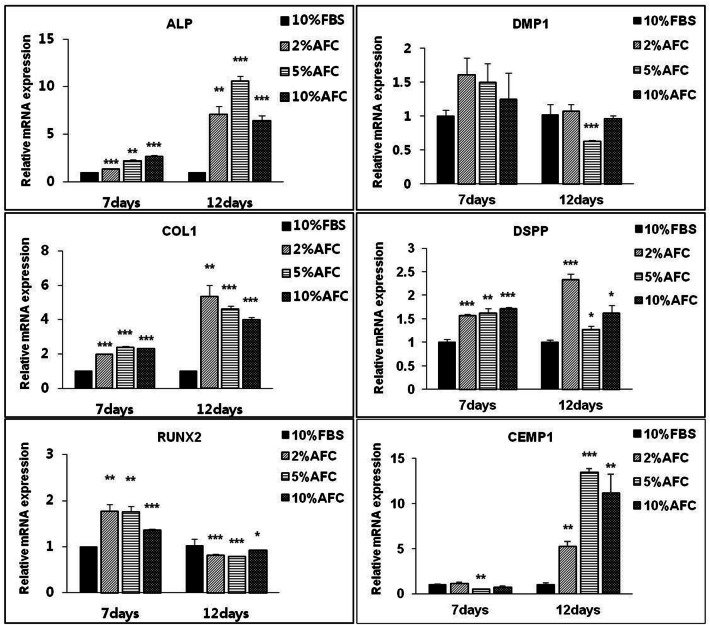
We also checked the gene expression patterns of hPDLSCs. The expressions of cementogenic-related genes ALP, COL1, and DSPP were significantly increased in the 5% AFC serum-culture group, compared with in the FBS-culture group. In contrast, treatment with 5% AFC serum significantly decreased the gene expression of RUNX2 and DMP1 and significantly facilitates CEMP1 expression at later stages (Fig. 7).
Fig. 7.
Effects of AFC serum on odontogenic/cementogenic influence of hPDLSCs in vitro. The mRNA levels assay of ALP, COL1, RUNx2, DMP1, DSPP, CEMP1 on day-7, day-18. N = 3; Significant differences versus the 10% FBS; *p < 0.05; **p < 0.01; ***p < 0.001
The histological analysis of human dentin matrix surface in vivo
The H&E staining showed that AFC serum induced hDPSCs to differentiate into odontoblast-like cells layer (Fig. 8), which are evenly distributed on the pulp-like tissues, while, more cells are concentrated on the inner boundary of human dentin matrix, similar to the odontoblastic layer. On surface of human dentin matrix, new cementum-like tissues were generated (Fig. 8K). In the control group, cells were not found, only sparse collagen fibers in the cavity of human dentin matrix were present, and obvious new hard tissue were not observed on the outer side. It was suggested that AFC serum could promoted cementogenic differentiation of hPDLSCs. IHC analysis was used to identify the characteristics of generated tissues. The results showed that dental pulp-like tissues were also positive for Col-1, DMP-1, and DSP (Fig. 8E, G, I), Meanwhile, the cementum-like tissues were positive for Col-1, CAP, and OSX. But all of the tissues were negative for the markers in control group (Fig. 8M, O, Q).
Fig. 8.
Effects of AFC serum on human dentin matrix surface in vivo. A Image of individual components of the transplantation composite and schematic of final construct. B and C Histological analysis showed that AFC serum promoted pulp-like tissue in vivo compared with control on the inside of human dentin matrix. In hDPSCs, dental odontoblast (arrows)–like cells are evenly distributed on the pulp-like tissues. D–I IHC showed that hDPSCs + AFC serum were all positive for COL1, DMP1, DSP antibody staining in human dentin matrix cavity, respectively (arrowhead). J and K In hPDLSCs + AFC serum, Cementum-like tissue in representative H&E staining compared with control on the outside of human dentin matrix. L–Q IHC analysis demonstrated identify the characteristics of cementum-like tissues were positive for Col-1, CAP and OSX (arrowhead). AFC, allogeneic fibrin clot; H&E, hematoxylin and eosin; IHC, immunohistochemistry; D, dentin; PLT, pulp-like tissue; Ce, cementum; Scale bar, 100 μm
Discussion
With the aim of developing a xenofree cell culture medium to ameliorate the quality and safety of the expanded hDMSCs for clinical applications, the present study explored the feasibility of AFC serum as an alternative to FBS. Our results revealed that AFC could maintain the characteristics of hDMSCs and promote odontogenic/cementogenic differentiation of hDPSCs and hPDLSCs.
MSC research, more popular now than ever, is used to identify the characteristics of stem cells and treat various diseases [27, 28]. In dentistry, hDMSCs have been largely focused on because of its potential ability to differentiate into dental tissues, which were considered irreparable before. Riecke et al. report that a sufficient number of hDMSCs play a critical role in the thickness of the floor of maxillary sinus [29]. As FBS contains various infectious agents, such as animal-borne pathogens, attention was sought to human serum supplement, which contains platelet derivatives such as PRF and can be used to culture hDMSCs. Because of its autologous origin and without anticoagulants, PRF can accelerate wound healing and tissue regeneration [28]. However, the PRF matrix contains a very high number of host immune cells (platelets, leukocytes, and T-lymphocytes) leading to different unwanted effects in MSC [30, 31]. Leukocytes can affect the mechanical properties of their fibrin scaffolds and are directly related to cell inflammation and proliferation [32]. The functions of leukocytes in PRF were always a matter of debate.
The allogeneic serum is especially important for tissue regeneration because of the deficiency of autologous serum for in vitro expansion of a sufficient number of cells. In this study, AFC was prepared using different donors for the culture of hDPSCs and hPDLSCs. The reduced leukocytes could result in a decreased anti-immune reaction to inflammation for allogeneic application. Cell-specific properties such as weight, size, and density can be controlled by changing the centrifugal force and time [23]. We prepared different PRFs via different centrifugal forces and time durations and compared the structure and composition of PRF matrices. The results showed that leukocytes were collected in the middle layer and changed shape into a pellet, thus making it easier to remove the upper layer without leukocytes before the hardening of the serum. Moreover, the histological staining of AFC convincingly showed the absence of leukocytes when compared with PRF (Figs. 2D, H). Additionally, the fibrin mass structure of AFC is more uniform compared with PRF. MMP-8 is in large part put out by neutrophils [33]. The levels of MMP8 are significantly lower in AFC serum compared with conventional PRF serum (Figs. 2I, J). Our observations were in accordance with the results obtained by Wend et al., who reported that high-speed RCF significantly decreased the levels of growth factors [21].
Another method of reducing the potential risk for alloimmunization is freeze–thawing, which can reduce the presence of platelet antigens against major histocompatibility complex class Ι [13]. We observed that most leukocytes and a portion of platelets were arrested in the middle pellet layer in high-speed centrifuged RCF (Fig. 3A–C). The residual platelets release various growth factors such as angiogenin, EGF, FGF-6, IGFBP-2, and PDGF-BB, which are sufficient to maintain hDMSCs growth (Fig. 3J). Those findings supported previous reports that platelets release a variety of growth factors including IGF-1, FGF, transforming growth factor beta, VEGF, PDGF, and EGF which promote cell growth [34].
Bieback et al. proposed that human platelet lysate-containing media could be used as a substitute for FBS-containing media to expand MSCs [12]. Some studies provided that allogeneic serum could be an alternative to FBS in supporting in vitro cell expansion [35–37]. However, none of them used the latest high-speed allogeneic liquid PRF to compare and investigate the hDPSCs and hPDLSCs most significant to the dentists. In this study, hDPSCs and hPDLSCs treated with AFC serum indicated that the isolated cells are mainly hDMSCs. The MSC properties of hDMSCs were characterized by their cell surface markers and by evaluating their multi-differentiation potential under AFC serum conditions in passage 5.
In proliferation assays, hDPSCs and hPDLSCs were cultured in AFC serum, and then cell proliferation was evaluated. The results showed that no significant effect was found on cell proliferation by treatment with low concentration of AFC serum (less than 5%), but a concentration of AFC serum between 5% and 10% promoted cell proliferation; however, cell viability was reduced by treatment with AFC serum in high concentration (> 10%). Previous studies of other types of stem cells also recommended concentration of 10% PRF [38]. The optimal concentration of AFC serum on proliferation of hDPSCs and hPDLSCs is 5% and 10%, respectively. The results showed that the effects of AFC serum on cell proliferation are different depending on the stem cell type. However, the effects of AFC serum on cell proliferation are lower than those on FBS, and this finding is consistent with the results published by Arpornmaeklong et al. which stated that human serum is inferior to FBS in its ability to support in vitro MSC expansion [35]. The AFC mechanism about cell proliferation is unclear, and the response varies in different kinds of stem cells, at which further research is needed.
The potential of differentiation is closely associated with hDPSC and hPDLSC based root regeneration, and this study showed that the optimum concentration exhibited a positive effect on the odontogenic/cementogenic differentiation of variety types of hDMSCs in vitro. Simultaneously, osteogenic differentiation of hDPSCs and hPDLSCs are inhibited on AFC serum, which is thought to exhibit a conflict to relationship with odonto/cementogenic differentiation. Our results are same with some previous studies, which reported that allogeneic serum could promote the differentiation of some MSCs [39]. The appropriate concentration of AFC serum is important for different cell types quite, suggesting that exploring the optimum concentration of AFC serum is of great worth to its application in tissue engineering. The present study showed that it provides guidance for the application of AFC in hDPSCs and hPDLSCs based root regeneration.
Cementum and dentin are mineralized tissues that share some common characteristics with bone in terms of composition and mineralization processes. The expressions of ALP, COL1, and DSPP were all increased in hDPSCs and hPDLSCs. These genes are all shared by osteoblasts, odontoblasts, and cementoblasts and all play an important role in hard tissue mineralization. As suggested by the above results, hDPSCs were induced to display odontogeneic differentiation, and the hPDLSCs were induced to display cementogenic differentiation, under AFC serum conditions. Different AFC serum concentrations induce differentiation of hDMSCs into odontogeneic/cementogeneic in different ways. AFC secretes growth factors and shows a benefit for differentiating hDPSCs and hPDLSCs into odontogenic and cementogenic differentiation. However, the mechanism of the interaction between the molecules has yet to be elucidated and should be studied further.
The in vivo implantation for 12 weeks in nude mice showed that both pulp-like tissue and cementum-like tissue complexes were formed expressing root-related antibodies. IHC results demonstrated that dental pulp-like tissues were positive for Col-1, DMP1, and DSP throughout the hDPSCs treated with AFC serum. Col-1 is the major extracellular matrix protein in the pulp tissues and cementum [40]. At the same time, DMP-1 as extracellular matrix proteins, is essential for mineralization of dentin [41]. DSP is always expressed by new odontoblasts and is related to the secretion of pre-dentin matrix [42]. The expression of COL1, DMP1 and DSP showed that the cavity of human dentin matrix inside successfully formed a dentin-pulp complex, meanwhile, the expression of COL1, CAP and OSX showed that the surface of human dentin matrix outside successfully formed cementum-like tissues. CAP is a cementum specific protein and is not expressed in other periodontal or other component tissues [40]. OSX plays a critical role for postnatal tooth root development [43] and is present in cementum. These results suggested that AFC serum could induce hDPSCs and hPDLSCs to construct complete tooth root tissues including dental pulp/dentin and cementum complexes, when transplanted into the subcutaneous of nude mice. However, cementum was not uniformly regenerated around the dentin.
This study proved that high-speed centrifugation removes many host immune cells with a small amount of platelets in the supernatant serum. In addition, when different types of hDMSCs were exposed to allogeneic serum conditions, they maintained their characteristic stemness. However, hDPSC and hPDLSC required different concentrations in proliferation and different directions for promoting odontogenic and cementogenic differentiation depending on the cell type. Therefore, AFC should be useful in the application of hDMSCs in root regeneration. These conditions are suitable for cultivating hDMSCs under animal-serum-free conditions/human-derived medium additives and donor-to-donor variability and provide a method of cultivating stem cells for tooth root regeneration without FBS.
Acknowledgements
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (116135-3) and the National Research Foundation of Korea (2017M3A9B403364).
Compliance with ethical standards
Conflicts of interest
The authors have no financial conflicts of interest.
Ethical statement
The study was approved by the institutional review board (IRB) of Seoul National University Dental Hospital, Seoul, Korea (IRB no. 05004). Informed consent was confirmed by the IRB. The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Seoul National University (No. SNU-190426-13).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jong Hoon Chung, Email: jchung@snu.ac.kr.
Pill-Hoon Choung, Email: choungph@snu.ac.kr.
References
- 1.Marei MK, El Backly RM. Dental mesenchymal stem cell-based translational regenerative dentistry: from artificial to biological replacement. Front Bioeng Biotechnol. 2018;6:49. doi: 10.3389/fbioe.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleifel D, Rahmoon MA, AlOkda A, Nasr M, Elserafy M, El-Khamisy SF. Recent advances in stem cells therapy: a focus on cancer, Parkinson’s and Alzheimer’s. J Genet Eng Biotechnol. 2018;16:427–432. doi: 10.1016/j.jgeb.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 4.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei M, Li K, Li B, Gao LN, Chen FM, Jin Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials. 2014;35:6332–6343. doi: 10.1016/j.biomaterials.2014.04.071. [DOI] [PubMed] [Google Scholar]
- 6.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo B, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 9.Jin H, Choung HW, Lim KT, Jin B, Jin C, Chung JH, et al. Recombinant human plasminogen activator inhibitor-1 promotes cementogenic differentiation of human periodontal ligament stem cells. Tissue Eng Part A. 2015;21:2817–2828. doi: 10.1089/ten.tea.2014.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinzebach S, Bieback K. Expansion of mesenchymal stem/stromal cells under xenogenic-free culture conditions. Adv Biochem Eng Biotechnol. 2013;129:33–57. doi: 10.1007/10_2012_134. [DOI] [PubMed] [Google Scholar]
- 11.Lee UL, Jeon SH, Park JY, Choung PH. Effect of platelet-rich plasma on dental stem cells derived from human impacted third molars. Regen Med. 2011;6:67–79. doi: 10.2217/rme.10.96. [DOI] [PubMed] [Google Scholar]
- 12.Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 13.Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 14.Schlegel KA, Donath K, Rupprecht S, Falk S, Zimmermann R, Felszeghy E, et al. De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials. 2004;25:5387–5393. doi: 10.1016/j.biomaterials.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 15.Thorwarth M, Rupprecht S, Falk S, Felszeghy E, Wiltfang J, Schlegel KA. Expression of bone matrix proteins during de novo bone formation using a bovine collagen and platelet-rich plasma (prp)—an immunohistochemical analysis. Biomaterials. 2005;26:2575–2584. doi: 10.1016/j.biomaterials.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Kang YH, Jeon SH, Park JY, Chung JH, Choung YH, Choung HW, et al. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A. 2011;17:349–359. doi: 10.1089/ten.TEA.2010.0327. [DOI] [PubMed] [Google Scholar]
- 18.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 19.El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept) Eur J Trauma Emerg Surg. 2019;45:467–479. doi: 10.1007/s00068-017-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, et al. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig. 2017;21:2619–2627. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]
- 21.Wend S, Kubesch A, Orlowska A, Al-Maawi S, Zender N, Dias A, et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med. 2017;28:188. doi: 10.1007/s10856-017-5992-6. [DOI] [PubMed] [Google Scholar]
- 22.Abd El Raouf M, Wang X, Miusi S, Chai J, Mohamed AbdEl-Aal AB, Nefissa Helmy MM, et al. Injectable-platelet rich fibrin using the low speed centrifugation concept improves cartilage regeneration when compared to platelet-rich plasma. Platelets. 2019;30:213–221. doi: 10.1080/09537104.2017.1401058. [DOI] [PubMed] [Google Scholar]
- 23.Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–689. doi: 10.1563/aaid-joi-D-14-00138. [DOI] [PubMed] [Google Scholar]
- 24.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate: Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51–e55. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Jin H, Park JY, Choi H, Choung PH. HDAC inhibitor trichostatin a promotes proliferation and odontoblast differentiation of human dental pulp stem cells. Tissue Eng Part A. 2012;19:613–624. doi: 10.1089/ten.TEA.2012.0163. [DOI] [PubMed] [Google Scholar]
- 26.Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32:4525–4538. doi: 10.1016/j.biomaterials.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 28.Anitua E, Prado R, Orive G. Endogenous morphogens and fibrin bioscaffolds for stem cell therapeutics. Trends Biotechnol. 2013;31:364–374. doi: 10.1016/j.tibtech.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Riecke B, Heiland M, Hothan A, Morlock M, Amling M, Blake FA. Primary implant stability after maxillary sinus augmentation with autogenous mesenchymal stem cells: a biomechanical evaluation in rabbits. Clin Oral Implants Res. 2011;22:1242–1246. doi: 10.1111/j.1600-0501.2010.02043.x. [DOI] [PubMed] [Google Scholar]
- 30.Portela GS, Cerci DX, Pedrotti G, Araujo MR, Deliberador TM, Zielak JC, et al. L-PRP diminishes bone matrix formation around autogenous bone grafts associated with changes in osteocalcin and PPAR-γ immunoexpression. Int J Oral Maxillofac Surg. 2014;43:261–268. doi: 10.1016/j.ijom.2013.07.739. [DOI] [PubMed] [Google Scholar]
- 31.Giovanini AF, Deliberador TM, Tannuri Nemeth JE, Crivellaro VR, Portela GS, De Oliveira Filho MA, et al. Leukocyte-platelet-rich plasma (L-PRP) impairs the osteoconductive capacity of the autograft associated to changes in the immunolocalization of TGF-β1 and its co-expression with Wnt10b and CD34 cells. J Craniomaxillofac Surg. 2013;41:e180–e186. doi: 10.1016/j.jcms.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS One. 2015;10:e0121713. doi: 10.1371/journal.pone.0121713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, et al. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 34.Giannini S, Cielo A, Bonanome L, Rastelli C, Derla C, Corpaci F, et al. Comparison between PRP, PRGF and PRF: lights and shadows in three similar but different protocols. Eur Rev Med Pharmacol Sci. 2015;19:927–930. [PubMed] [Google Scholar]
- 35.Arpornmaeklong P, Sutthitrairong C, Jantaramanant P, Pripatnanont P. Allogenic human serum, a clinical grade serum supplement for promoting human periodontal ligament stem cell expansion. J Tissue Eng Regen Med. 2018;12:142–152. doi: 10.1002/term.2379. [DOI] [PubMed] [Google Scholar]
- 36.Muraglia A, Nguyen VT, Nardini M, Mogni M, Coviello D, Dozin B, et al. Culture medium supplements derived from human platelet and plasma: cell commitment and proliferation support. Front Bioeng Biotechnol. 2017;5:66. doi: 10.3389/fbioe.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Blanc K, Samuelsson H, Lönnies L, Sundin M, Ringdén O. Generation of immunosuppressive mesenchymal stem cells in allogeneic human serum. Transplantation. 2007;84:1055–1059. doi: 10.1097/01.tp.0000285088.44901.ea. [DOI] [PubMed] [Google Scholar]
- 38.Fekete N, Gadelorge M, Fürst D, Maurer C, Dausend J, Fleury-Cappellesso S, et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active comp. Cytotherapy. 2012;14:540–554. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verboket R, Herrera-Vizcaíno C, Thorwart K, Booms P, Bellen M, Al-Maawi S, et al. Influence of concentration and preparation of platelet rich fibrin on human bone marrow mononuclear cells (in vitro) Platelets. 2019;30:861–870. doi: 10.1080/09537104.2018.1530346. [DOI] [PubMed] [Google Scholar]
- 40.Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix—based scaffold. Biomaterials. 2012;33:2449–2461. doi: 10.1016/j.biomaterials.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 41.Nam S, Won JE, Kim CH, Kim HW. Odontogenic differentiation of human dental pulp stem cells stimulated by the calcium phosphate porous granules. J Tissue Eng. 2011;2011:812547. doi: 10.4061/2011/812547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasad M, Butler WT, Qin C. Dentin sialophosphoprotein in biomineralization. Connect Tissue Res. 2010;51:404–417. doi: 10.3109/03008200903329789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Jiang Y, Qin C, Liu Y, Ho SP, Feng JQ. Essential role of osterix for tooth root but not crown dentin formation. J Bone Miner Res. 2015;30:742–746. doi: 10.1002/jbmr.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]