Abstract
The prognostic factors for survival among patients with secondary osteosarcoma remain unclear. The aim of this study was to develop a practical nomogram for predicting cancer-specific survival (CSS) in patients with osteosarcoma as a secondary malignancy. The surveillance, epidemiology, and end results database was used for the identification of osteosarcoma cases. The total sample comprised 5860 cases of primary osteosarcoma and 268 cases of secondary osteosarcoma during the period from 1973 to 2015. The CSS and overall survival (OS) of primary and secondary osteosarcomas were analyzed. The predictors of CSS for secondary osteosarcoma were identified and integrated to build a nomogram. Validation of the nomogram was performed using concordance index (C-index) and calibration plots. The results indicated that patients with secondary osteosarcoma had poorer CSS and OS than patients with primary osteosarcoma. The nomogram model exhibited high discriminative accuracy in the training cohort (C-index = 0.826), which was confirmed in the internal validation cohort (C-index = 0.791). In addition, the calibration plots confirmed good concordance for prediction of CSS at 3, 5, and 10 years. In conclusion, we developed a practical nomogram that provided individual predictions of CSS for patients with secondary osteosarcoma. This nomogram may help clinicians with prognostic evaluations and with the development of individualized therapies for this aggressive disease.
Subject terms: Cancer, Bone cancer
Introduction
Osteosarcoma is the most common primary bone malignancy, with an age-standardized incidence rate of 2.9 per 1 million amongst men and 2.2 per 1 million amongst women. Nearly 90% of cases are classified as high-grade osteosarcoma at the time of diagnosis. Osteosarcoma is the most common primary malignant bone tumor among people of all ages1. Osteosarcoma may present as a primary malignancy or as a secondary malignancy following other primary malignancies. Secondary osteosarcomas frequently occur due to a genetic predisposition and/or as the consequence of prior cancer therapies2. Osteosarcoma is one of the most common secondary malignancies among patients with retinoblastoma, with cumulative incidence of 7% at 20 years of age. Patients often have a large number of mutations in the retinoblastoma susceptibility gene, RB13,4. Osteosarcoma is also a common secondary malignancy in childhood cancer survivors. The condition often arises as a result of exposure to radiotherapy and chemotherapy5–7. Increased incidences of osteosarcoma are associated with Ewing’s sarcoma and Paget’s bone disease2,8.
The survival of patients with osteosarcoma has improved considerably since the 1980s with the advent of multiagent chemotherapy, with overall survival of roughly 20% in metastatic patients and 70% in non-metastatic patients9,10. If patients with poor survival can be identified preoperatively, personalized treatment plans may be helpful in decision making. Therefore, there is a critical need to identify the patients who are more likely to experience poor survival and thus benefit from additional therapy. Generally, tumor site, tumor size, patient age, location of metastases, response to chemotherapy, and type of surgery are significant prognostic factors for patients with primary osteosarcoma11–13. However, because patients with secondary osteosarcoma generally have a history of prior malignances, this history may affect the speed of diagnosis, treatment intensity, and, eventually, the prognosis of secondary osteosarcoma14. Secondary osteosarcoma is rarer than primary osteosarcoma, as published by many authors. Previous studies were limited to case reports and small series4,5,14–17. Therefore, the prognostic factors of survival for secondary osteosarcoma remain poorly understood.
Nomograms have been successfully used as prognostic tools for predicting the probability of disease outcomes with a simple visualization figure that integrates the relevant variables in complex mathematical models18,19. Nomograms can improve the discriminatory accuracy of outcome predictions; these have therefore been widely used to quantify the risk of various malignancies20,21. However, no nomogram has been developed for patients with secondary osteosarcoma to date. The present study developed an elaborate nomogram for assessing individualized prognoses for secondary osteosarcoma in terms of 3-year, 5-year, and 10-year cancer-specific survival (CSS) using data from the Surveillance, Epidemiology, and End Results (SEER) database22.
Methods
Patients and selection criteria
We queried nine population-based cancer registries in the SEER program to obtain records for patients seen during the period from 1973 to 2015 (November 2017 submission) using SEER*Stat software (version 8.3.5)23. The SEER program is a population-based cancer registry system with data collected from 18 registries in 14 states across the U.S., representing nearly 30% of the U.S. population. The selection of osteosarcoma cases was done using the Histologic International Classification of Diseases (ICD)-O-3 codes 9180/3–9186/3, 9192/3–9194/3, and 9120/3. No written informed consent was obtained for this study because the data were de-identified and publicly available.
Patients were divided and classified by their sequence numbers: patients with primary osteosarcoma without any prior malignancy were assigned sequence number = 1, and patients with subsequent osteosarcoma following prior malignancies were assigned sequence numbers ≥ 2. Osteosarcomas that occurred following the primary malignancy were considered as “secondary osteosarcoma” in our study.
The exclusion criteria were missing or incomplete data including survival status and time, age, sex, race, and prior malignancies and diagnosis of osteosarcoma at the time of autopsy or on the death certificate. The demographic and clinico-pathological data of all eligible cases were collected and analyzed.
Endpoint definition
Cancer-specific death was taken as the primary endpoint of the study. The cause of death was defined as death from osteosarcoma, according to the SEER database. The primary endpoint in this study was defined as the interval between the diagnosis of osteosarcoma and the occurrence of cancer-specific death. The secondary endpoint was overall survival (OS), which was defined as the interval between the diagnosis of osteosarcoma and death from any cause or last follow-up.
Statistical analyses
Statistical analyses were performed using R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 20.0 (IBM Corporation, Armonk, NY). The t-test was used to examine differences between mean values. The χ2 or Fisher’s exact test was used to compare proportions. Survival was assessed using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazard regression analyses were performed to identify independent prognostic factors of survival (univariate and multivariate). Significant variables (P < 0.1) in univariate analyses were included in multivariate regression analyses. Variables that were significant in multivariable analyses were incorporated to formulate the nomogram.
Adequate discrimination and calibration were performed to test and validate the prognostic accuracy of the nomogram model24. Discrimination was quantified using Harrell’s concordance index (C-index), in which an absolute value close to 1 indicates that a nomogram model has strong predictive ability. The nomogram was further subjected to bootstrapping validation (1000 bootstrap replicates) to calculate the relatively corrected C-index. Calibration plots were developed to evaluate predictive accuracy and, further, to assess the concordance between predicted and observed ongoing survival probabilities. A two-sided P < 0.05 was taken to indicate statistical significance.
Results
Study cohorts
The total sample was comprised of 6128 patients, out of which 5860 patients were diagnosed with primary osteosarcoma (sequence number = 1) and 268 patients were diagnosed with secondary osteosarcoma (sequence number ≥ 2). Osteosarcoma was a second malignancy in 231 cases, third malignancy in 34 cases, fourth malignancy in 2 cases, and sixth malignancy in 1 case. Comparisons of baseline demographic and clinicopathological characteristics between patients with primary and secondary osteosarcomas are presented in Table 1. Patients with secondary osteosarcoma were older than those with primary osteosarcoma at the time of diagnosis (55.1 vs. 29.8 years, respectively; P < 0.001), 194 (72.4%) secondary osteosarcoma patients were older than 40 years at diagnosis. The ratio of females to males was higher in the secondary osteosarcoma group than in the primary osteosarcoma group (53.4% vs. 44.8%, respectively; P = 0.007). Furthermore, the primary site was less likely to be an extremity in cases of secondary osteosarcoma, the pelvis was the most commonly affected site (77 out of 268, 28.7%). Non-pagetic osteosarcoma was more common in patients with primary osteosarcoma, while pagetic osteosarcoma was more common in patients with secondary osteosarcoma, which demonstrates the significant differences in histological subtype between groups. In cases of secondary osteosarcoma, the first primary malignancies included 157 carcinomas (58.6%), 42 sarcomas (15.7%), 41 lymphomas/leukemias (15.3%), 14 retinoblastomas (5.2%), and 14 other cancers (5.2%). Among these 268 patients, 54.9% (147 cases) had received radiotherapy for prior malignancies; secondary osteosarcomas occurred within the prior radiation field in 104 patients (38.8%) and outside the radiation field in 43 patients. The median latency interval between the first primary malignancy and the diagnosis of secondary osteosarcoma was 98.5 months (2–501 months). The mean follow-up times were 90.1 and 39.3 months in the primary and secondary osteosarcoma cohorts, respectively. As primary osteosarcoma mostly occurred in children and adolescents, the prognosis of this was good. This could be the main reason for substantial variation of the follow-up period.
Table 1.
Demographics and clinicopathologic characteristics of primary and secondary osteosarcomas.
| Primary Osteosarcoma | Secondary Osteosarcoma | p value | ||
|---|---|---|---|---|
| Total cases | 5860 (100) | 268 (100) | ||
| Age at diagnosis (years) | 29.8 ± 21.5 | 55.1 ± 24.3 | < 0.001 | |
| Sex | 0.007 | |||
| Male | 3,236 (55.2) | 125 (46.6) | ||
| Female | 2624 (44.8) | 143 (53.4) | ||
| Race | 0.716 | |||
| White | 4447 (75.9) | 206 (76.9) | ||
| Others | 1413 (24.1) | 62 (23.1) | ||
| Marital status at diagnosis | < 0.001 | |||
| Married | 1504 (25.7) | 180 (67.2) | ||
| Un-married | 4356 (74.3) | 88 (32.8) | ||
| Year of diagnosis | 0.197 | |||
| 1973–1994 | 1501 (25.6) | 59(22.0) | ||
| 1995–2015 | 4359 (74.4) | 209(78.0) | ||
| Tumor location | < 0.001 | |||
| Bone | 5556 (94.8) | 221 (82.5) | ||
| Extra-skeleton | 304 (5.2) | 47 (17.5) | ||
| Primary site | < 0.001 | |||
| Extremity | 4407 (75.2) | 80 (29.9) | ||
| Trunk | 1353 (23.1) | 175 (65.2) | ||
| Unknown | 100 (1.7) | 13 (4.9) | ||
| Histology | < 0.001 | |||
| Pagetic ostosarcoma | 72 (1.2) | 9 (3.4) | ||
| Non-Pagetic ostosarcoma | 1675 (28.6) | 48 (17.9) | ||
| NOS | 4113 (70.2) | 211 (78.7) | ||
| Stage | 0.024 | |||
| Localized | 1987 (33.9) | 76 (28.4) | ||
| Regional | 2331 (39.8) | 104 (38.8) | ||
| Distant | 1133 (19.3) | 58 (21.6) | ||
| Unstaged | 409 (7.0) | 30 (11.2) | ||
| Grade | 0.266 | |||
| I | 236 (4.0) | 4 (1.5) | ||
| II | 327 (5.6) | 15 (5.6) | ||
| III | 1064 (18.2) | 50 (18.7) | ||
| IV | 2051 (35.0) | 90 (33.6) | ||
| Unknown | 2182 (37.2) | 109 (40.7) | ||
Data are expressed as n (%) unless otherwise specified.
Survival in primary and secondary osteosarcoma
Median CSS was not reached in the primary osteosarcoma cohort because the survival probability was greater than 50% at the last follow-up point, while it was 65 months (95% confidence interval [CI] 20.2–109.8) in the secondary osteosarcoma cohort (P < 0.001). The median OS was 126 months in cases of primary osteosarcoma (95% CI 101.3–150.7) and 15 months (95% CI 11.8–18.2) in cases of secondary osteosarcoma (P < 0.001).
Prognostic factors associated with CSS in patients with secondary osteosarcoma
The prognostic factors for CSS for secondary osteosarcoma are shown in Table 2. In univariable analyses, younger age at diagnosis, Caucasian ethnicity, unmarried marital status, chemotherapy for prior malignancies, later year of diagnosis, first primary malignancy other than carcinoma, extraskeletal tumor location, an extremity primary site, non-pagetic osteosarcoma histology, localized disease at presentation, surgical resection, chemotherapy and no radiation therapy for osteosarcoma were significantly associated with improved CSS. These 13 factors were submitted to multivariable analysis. The results showed that age, race, year of diagnosis, a skeletal/extraskeletal tumor location, stage and surgical resection retained significance in the multivariate analysis.
Table 2.
Univariable and multivariable analysis of each factor’s ability in predicting CSS and OS of secondary osteosarcomas.
| Characteristic | CSS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age at diagnosis | 1.025 (1.015–1.034) | < 0.001 | 1.035 (1.019–1.051) | < 0.001 | 1.024 (1.017–1.03) | < 0.001 | 1.028 (1.017–1.039) | < 0.001 |
| Race | ||||||||
| White | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| Others | 1.65 (1.072–2.540) | 0.023 | 1.708 (1.063–2.742) | 0.027 | 1.255 (0.916–1.720) | 0.158 | ||
| Sex | ||||||||
| Male | 1 (reference) | 1 (reference) | ||||||
| Female | 0.840 (0.565–1.248) | 0.388 | 1 (0.762–1.313) | 1.000 | ||||
| Marital status | ||||||||
| Married | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Un-married | 0.462 (0.295–0.723) | < 0.001 | 1.417 (0.740–2.715) | 0.293 | 0.435 (0.319–0.593) | < 0.001 | 1.136 (0.724–1.782) | 0.578 |
| Year of diagnosis | ||||||||
| 1973–1994 | 2.461 (1.63–3.714) | < 0.001 | 2.644 (1.492–4.686) | < 0.001 | 1.461 (1.07–1.995) | 0.017 | 1.680 (1.116–2.528) | 0.0129 |
| 1995–2015 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| First primary malignancy | ||||||||
| Carcinomas | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Lymphomas/leukemias | 0.672 (0.393–1.152) | 0.148 | 0.921 (0.490–1.731) | 0.798 | 0.626 (0.427–0.920) | 0.017 | 1.133 (0.723–1.776) | 0.585 |
| Sarcomas | 0.315 (0.151–0.659) | 0.002 | 0.592 (0.256–1.370) | 0.221 | 0.505 (0.333–0.767) | 0.001 | 0.913 (0.558–1.494) | 0.717 |
| Others | 0.449 (0.223–0.905) | 0.025 | 1.404 (0.589–3.346) | 0.444 | 0.421 (0.256–0.691) | < 0.001 | 1.021 (0.572–1.823) | 0.945 |
| Radiation for prior malignancies | ||||||||
| Yes | 1 (reference) | 1 (reference) | ||||||
| No | 1.102 (0.740–1.64) | 0.633 | 1.161 (0.883–1.526) | 0.285 | ||||
| Chemotherapy for prior malignancies | ||||||||
| Yes | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| No/Unknown | 1.456 (0.954–2.222) | 0.081 | 1.157 (0.688–1.945) | 0.582 | 1.321 (0.993–1.756) | 0.056 | 1.067 (0.748–1.524) | 0.719 |
| Number of primary malignancies | ||||||||
| 1 | 1 (reference) | 1 (reference) | ||||||
| ≥ 2 | 1.472 (0.819–2.648) | 0.196 | 1.411 (0.933–2.133) | 0.103 | ||||
| Latency interval | 1 (0.999–1.003) | 0.868 | 0.999 (0.998–1.001) | 0.737 | ||||
| Tumor location | ||||||||
| Bone | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| Extra-skeleton | 0.086 (0.021–0.348) | < 0.001 | 0.096 (0.023–0.406) | 0.001 | 0.734 (0.503–1.071) | 0.109 | ||
| Primary site | ||||||||
| Extremity | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| Trunk | 1.146 (0.736–1.785) | 0.546 | 1.027 (0.621–1.699) | 0.916 | 1.176 (0.870–1.590) | 0.292 | ||
| Unknown | 2.264 (1.034–4.957) | 0.041 | 1.143 (0.456–2.863) | 0.776 | 1.608 (0.866–2.986) | 0.133 | ||
| Osteosarcomas occurring within the prior radiation field | ||||||||
| No/Unknown | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| Yes | 1.076 (0.609–1.903) | 0.801 | 1.501 (0.986–2.285) | 0.058 | 1.852 (1.124–3.052) | 0.016 | ||
| No first radiation | 1.156 (0.668–1.999) | 0.604 | 1.534 (1.018–2.313) | 0.041 | 1.386 (0.874–2.197) | 0.165 | ||
| Histology | ||||||||
| Non-Pagetic osteosarcoma | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Pagetic osteosarcoma | 4.704 (1.603–13.800) | 0.005 | 2.660 (0.828–8.551) | 0.100 | 2.587 (1.238–5.406) | 0.012 | 1.680 (0.756–3.734) | 0.203 |
| NOS | 2.419 (1.253–4.668) | 0.008 | 1.826 (0.908–3.670) | 0.091 | 1.425 (0.984–2.064) | 0.061 | 1.088 (0.727–1.630) | 0.681 |
| Stage | ||||||||
| Localized | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Regional | 2.486 (1.398–4.421) | 0.002 | 3.292 (1.701–6.370) | < 0.001 | 1.437 (1.008–2.048) | 0.045 | 1.902 (1.283–2.819) | 0.001 |
| Distant | 5.919 (3.145–11.138) | < 0.001 | 5.977 (2.930–12.191) | < 0.001 | 3.696 (2.476–5.517) | < 0.001 | 4.370 (2.775–6.881) | < 0.001 |
| Unstaged | 1.971 (0.894–4.345) | 0.092 | 1.190 (0.477–2.972) | 0.709 | 1.539 (0.953–2.484) | 0.078 | 1.146 (0.663–1.979) | 0.626 |
| Grade | ||||||||
| I | 1 (reference) | 1 (reference) | ||||||
| II | 1.019 (0.106–9.800) | 0.987 | 1.505 (0.325–6.97) | 0.602 | ||||
| III | 1.857 (0.248–13.920) | 0.547 | 1.941 (0.468–8.054) | 0.361 | ||||
| IV | 2.195 (0.299–16.09) | 0.439 | 2.397 (0.587–9.792) | 0.223 | ||||
| Unknown | 2.346 (0.323–17.05) | 0.399 | 2.386 (0.587–9.696) | 0.224 | ||||
| Surgery | ||||||||
| Yes | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| No | 2.85 (1.741–4.663) | < 0.001 | 2.346 (1.351–4.075) | 0.002 | 2.417 (1.750–3.339) | < 0.001 | 1.947 (1.343–2.824) | < 0.001 |
| Unknown | 2.298 (1.421–3.716) | < 0.001 | 1.563 (0.782–3.124) | 0.207 | 1.346 (0.954–1.898) | 0.090 | 1.061 (0.654–1.722) | 0.811 |
| Radiation | ||||||||
| Yes | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| No | 0.567 (0.364–0.882) | 0.012 | 1.261 (0.664–2.394) | 0.478 | 0.610 (0.447–0.832) | 0.002 | 1.127 (0.758–1.676) | 0.553 |
| Radiation sequence with surgery | ||||||||
| Radiation prior to surgery | 1 (reference) | 1 (reference) | ||||||
| Radiation after surgery | 2.313 (0.301–17.8) | 0.421 | 2.285 (0.540–9.672) | 0.262 | ||||
| No radiation and/or cancer-directed surgery | 1.939 (0.270–13.93) | 0.51 | 2.036 (0.505–8.208) | 0.317 | ||||
| Chemotherapy | ||||||||
| Yes | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| No/unknown | 1.672 (1.122–2.491) | 0.012 | 1.363 (0.818–2.271) | 0.234 | 1.631 (1.24–2.145) | < 0.001 | 1.345 (0.958–1.887) | 0.087 |
Independent prognostic factors associated with OS in patients with secondary osteosarcoma
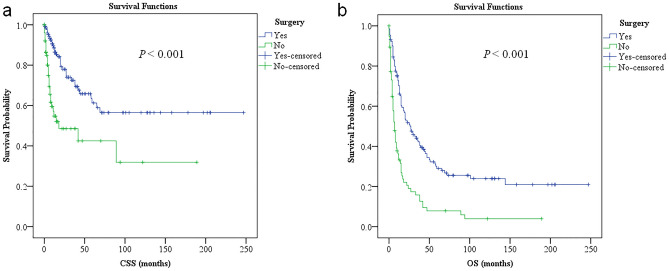
Univariable analysis suggested that younger age at diagnosis, unmarried marital status, later year of diagnosis, first primary malignancies other than carcinomas, chemotherapy for prior malignancies, osteosarcomas occurring outside the prior radiation field, non-Pagetic osteosarcoma histology, localized disease at presentation, surgical resection, chemotherapy and no radiation therapy for secondary osteosarcoma were favorable predictors of OS. Similar to CSS, age, year of diagnosis, stage, and surgical resection for osteosarcoma were independent prognostic factors associated with OS in multivariable analyses, but with the addition of osteosarcoma occurring within/outside the prior radiation field. To be noted, surgical resection was an independent favorable factor for both CSS and OS in the present cohort (Fig. 1). Unlike CSS, however, race and skeletal/extraskeletal tumor location did not have any bearing on OS among patients with secondary osteosarcoma (Table 2).
Figure 1.
Patients who underwent surgical resection for secondary osteosarcomas had longer CCS (a) and OS (b) compared with patients who didn’t. CSS cancer-specific survival, OS overall survival.
Building and validating a prognostic nomogram for CSS in patients with secondary osteosarcoma
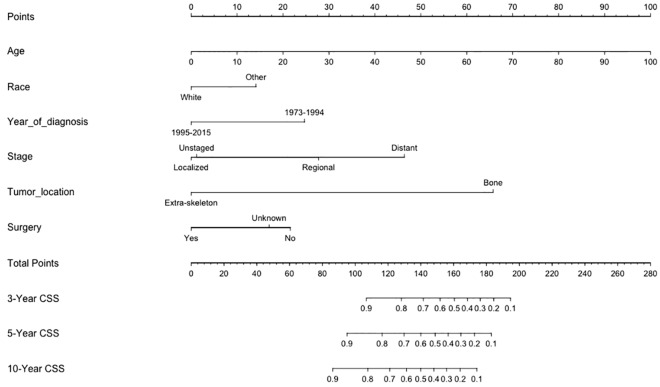
The nomogram for predicting CSS among patients with secondary osteosarcoma was formulated using the significant independent factors, including age, race, year of diagnosis, skeletal/extraskeletal tumor location, stage, and surgical resection. The nomogram showed that the largest contributions to prognosis were the location (skeletal or extraskeletal tumor) and age at diagnosis, followed by stage and year of diagnosis. Each variable was assigned a score according to the demographic and clinical features of individual patient (Table 3). By adding up these scores according to a patient’s condition, the total score was computed by summing the individual scores. Then, the total score was located on the total point line, and a straight line could be drawn to estimate the patient’s probability of 3-year, 5-year, and 10-year CSS from the nomogram (Fig. 2).
Table 3.
Score assignment for each variable included in the nomogram.
| Variables | Points |
|---|---|
| Age at diagnosis | |
| 0 | 0 |
| 10 | 10 |
| 20 | 20 |
| 30 | 30 |
| 40 | 40 |
| 50 | 50 |
| 60 | 60 |
| 70 | 70 |
| 80 | 80 |
| 90 | 90 |
| 100 | 100 |
| Race | |
| White | 0 |
| Other | 14 |
| Year of diagnosis | |
| 1995–2015 | 0 |
| 1973–1994 | 25 |
| Stage | |
| Localized | 0 |
| Regional | 28 |
| Distant | 46 |
| Unstaged | 1 |
| Tumor location | |
| Bone | 66 |
| Extra-skeleton | 0 |
| Surgery | |
| Yes | 0 |
| No | 22 |
| Unknown | 17 |
Figure 2.
Nomogram predicting 3-year, 5-year and 10-year cancer-specific survival (CSS) of patients with secondary osteosarcomas. The nomogram summed the points identified on the scale for each variable. The total points projected on the button scale indicate the probabilities of 3-year, 5-year and 10-year CSS. CSS cancer-specific survival.
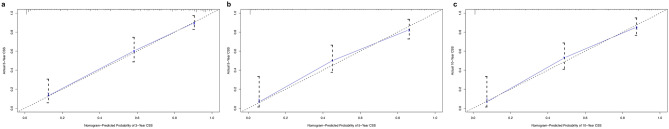
The C-index for the CSS prediction nomogram was 0.826 (95% CI: 0.787–0.865) for the training cohort and was confirmed to be 0.791 through bootstrapping validation, which suggested that the model had good discriminative ability. The calibration plots for CSS probability at 3-year, 5-year, and 10-year showed that the concordance between predicted and observed survival was optimal (Fig. 3).
Figure 3.
Calibration curves of the nomogram for predicting 3-year CSS (a), 5-year CSS (b) and 10- year CSS (c). CSS cancer-specific survival.
Discussion
The incidence of primary osteosarcoma has always been considered higher in males than in females25, while the current study revealed that in cases of secondary osteosarcoma, the majority of the patients were females. The proportions of patients with different races were consistent for primary and secondary osteosarcomas. This study showed that amongst patients with secondary osteosarcomas, 72.4% were older than 40 years at the time of diagnosis, the result was similar to previous reports16,26. Primary osteosarcoma mostly occurred in the long bones of the extremities near the metaphyseal growth plates25. However, we observed that secondary osteosarcomas were more likely to be located at non-extremity sites. In the current study, the authors found that the most common primary malignancies were carcinomas, followed by sarcomas and lymphomas/leukemias. These results were inconsistent with previous studies27,28. Distant metastases were present at diagnosis in 21.6% of secondary osteosarcoma patients. Radiation is a well-documented etiological factor of osteosarcoma, with the median interval between radiation and the occurrence of osteosarcoma reported to be 12–16 years25. This study observed a shorter post-radiation latency because the median latency interval between the diagnosis of first primary malignancies and osteosarcoma was 98.5 months, as the exact date of prior radiotherapy was not available. In this cohort, the pelvis was the most commonly affected site, 38.8% of secondary osteosarcomas occurred within the prior radiation field.
Most previous SEER studies on osteosarcoma either treated primary and secondary osteosarcoma together or were limited to osteosarcoma of specific histological subtypes29–32. There was only one study focusing on secondary osteosarcoma from SEER data, published nearly 20 years ago, which included only 133 patients and indicated that secondary osteosarcoma had poorer OS than primary osteosarcoma. However, that study did not evaluate CSS nor analyze the impact of any treatment on survival14. A later study reported that radiation-induced secondary osteosarcoma proved to have similar outcomes to primary osteosarcoma33. However, a recent study suggested that the prognosis of secondary osteosarcoma may be more favorable than that of primary osteosarcoma34. The survival and prognostic factors of secondary osteosarcoma remain unclear. So, identifying accurate prognostic factors has clinical importance for guiding personalized cancer therapy. The present study provides detailed survival data, and it could be the largest cohort study on secondary osteosarcoma reported to date. Furthermore, an optimal graphical validated nomogram was developed for predicting CSS. The nomogram model exhibited high discriminative accuracy in the training cohort (C-index = 0.826), which was further confirmed in the internal validation cohort (C-index = 0.791). This study suggests the excellent performance of this nomogram for estimating the prognosis of secondary osteosarcoma, as the calibration plots confirmed good concordance for the prediction of CSS at 3-, 5-, and 10-years. To the best of our knowledge, this is the first prognostic nomogram developed for secondary osteosarcoma.
This study revealed that patients with secondary osteosarcoma had poorer CSS and OS than patients with primary osteosarcoma. We identified age, race, year of diagnosis, skeletal/extraskeletal tumor location, stage, and surgical resection as independent factors for CSS. For OS, the independent prognostic factors included age, year of diagnosis, stage, surgical resection, and osteosarcoma occurring within/outside the prior radiation field. Notably, patients with secondary osteosarcomas occurring within the irradiated field had inferior OS compared to patients with secondary osteosarcomas occurring outside the irradiated field. CSS was similar between groups. This suggests that the difference in OS was caused by factors other than secondary osteosarcoma itself.
Previous studies have reported that surgical resection significantly improves disease-free survival and OS in patients with secondary osteosarcoma28,33. We also observed significant differences in CSS and OS between secondary osteosarcoma cases with or without surgical resection, which demonstrated that surgical resection was an independent factor significantly improving CSS and OS in the present cohort. For osteosarcomas occurring within the prior radiation field, one important issue that must be addressed is that radiation therapy can prolong postoperative complications because the condition of the operative field is entirely altered after radiotherapy33. For these patients, surgical options should be prudently adopted35. In the present study, data on the postoperative complications were not available due to the limitations of the SEER database; however, the favorable CSS and OS findings strongly justify the surgical resection of secondary osteosarcoma.
Intensive chemotherapy has considerably improved the prognosis of patients with primary osteosarcoma9. For secondary osteosarcoma, Shaheen et al. reported that patients treated aggressively with a combination of chemotherapy and surgical resection had better outcomes than patients treated with surgical resection alone33. The present study did not demonstrate significant benefits of chemotherapy on CSS or OS in multivariable analyses. However, the heterogeneous regimens and intensity of chemotherapy over more than 40 years may have limited the statistical power of this study. Prior myelosuppressive chemotherapy and/or radiotherapy may limit the tolerance of patients with secondary osteosarcoma who undergo subsequent intensive chemotherapy, and we strongly recommend the prophylactic use of myeloid growth factors after chemotherapy36.
This study had several limitations. First, due to the retrospective study design, selection bias was unavoidable. Second, the SEER dataset lacks data on doses of radiotherapy or chemotherapy regimens, and we were therefore unable to evaluate the impacts of these factors on the development and survival of secondary osteosarcoma. Third, due to the rarity of this disease, we were not able to validate the constructed nomogram using other cohorts.
Conclusion
We developed a practical nomogram that provided individual predictions of CSS for patients with secondary osteosarcoma using five clinicopathological factors and one treatment-related factor. Bootstrapping validation of the model confirmed its good performance. This nomogram may help clinicians with prognostic evaluations and with the development of individualized therapy for this aggressive disease. Future prospective studies are required to further determine the impacts of different treatment modalities on the survival of patients with secondary osteosarcoma.
Acknowledgements
This study was supported by the China National Science Foundation (Grant Nos. 81503396 and 81602004), the Shanghai Senior Talents Program (ZY(2018–2020)-RCPY-2017), and the National Key Research Project of Science and Technology Ministry (No. 2016YFC0106204).
Author contributions
Conception and design: Y.H. and J.Z. Collection and assembly of data: H.L. and S.W. Data analyses and interpretation: all authors. Manuscript preparation: Y.H. Manuscript proofing: all authors. Final approval of the manuscript: all authors.
Data availability
Data for this manuscript are available after formal request to the corresponding authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yanqi He, Email: heyq2004@126.com.
Jianjun Zhang, Email: robustzhang168@aliyun.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer Treat. Res. 2009;152:15–32. doi: 10.1007/978-1-4419-0284-9_2. [DOI] [PubMed] [Google Scholar]
- 3.Calvert GT, et al. At-risk populations for osteosarcoma: the syndromes and beyond. Sarcoma. 2012;2012:152382. doi: 10.1155/2012/152382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujiwara T, et al. Second primary osteosarcomas in patients with retinoblastoma. Jpn. J. Clin. Oncol. 2015;45:1139–1145. doi: 10.1093/jjco/hyv140. [DOI] [PubMed] [Google Scholar]
- 5.Liao LQ, et al. Radiation-induced osteosarcoma of the maxilla and mandible after radiotherapy for nasopharyngeal carcinoma. Chin. J. Cancer. 2016;35:89. doi: 10.1186/s40880-016-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behjati S, et al. Mutational signatures of ionizing radiation in second malignancies. Nat Commun. 2016;7:12605. doi: 10.1038/ncomms12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton WA, Jr, Meadows AT, Shimada H, Bunin GR, Vawter GF. Bone sarcomas as second malignant neoplasms following childhood cancer. Cancer. 1991;67:193–201. doi: 10.1002/1097-0142(19910101)67:1<193::AID-CNCR2820670132>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs B, Pritchard DJ. Etiology of osteosarcoma. Clin. Orthop. Related Res. 2002;397:40–52. doi: 10.1097/00003086-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Abarrategi A, et al. Osteosarcoma: cells-of-origin, cancer stem cells, and targeted therapies. Stem Cells Int. 2016;2016:3631764. doi: 10.1155/2016/3631764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo T, Furuta T, Johan MP, Adachi N, Ochi M. Percent slope analysis of dynamic magnetic resonance imaging for assessment of chemotherapy response of osteosarcoma or Ewing sarcoma: systematic review and meta-analysis. Skeletal. Radiol. 2016;45:1235–1242. doi: 10.1007/s00256-016-2410-y. [DOI] [PubMed] [Google Scholar]
- 11.Bacci G, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 12.Bielack SS, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari S, et al. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity: an analysis of 300 patients treated at the Rizzoli Institute. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001;12:1145–1150. doi: 10.1023/A:1011636912674. [DOI] [PubMed] [Google Scholar]
- 14.Hamre MR, et al. Osteosarcoma as a second malignant neoplasm. Radiother. Oncol. J. Eur. Soc. Therap. Radiol. Oncol. 2002;65:153–157. doi: 10.1016/S0167-8140(02)00150-0. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal G, Kochar HS, Julka PK, Bahadur S. Osteosarcoma as a second malignant disease in a case of bilateral retinoblastoma. Indian J. Otolaryngol. Head Neck Surg. Off. Publ. Assoc. Otolaryngol. India. 2011;63:115–117. doi: 10.1007/s12070-011-0254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget's disease of bone. J Bone Mineral Res Off J Am Soc Bone Miner Res. 2006;21(Suppl 2):P58–63. doi: 10.1359/jbmr.06s211. [DOI] [PubMed] [Google Scholar]
- 17.Barker JP, Monument MJ, Jones KB, Putnam AR, Randall RL. Secondary osteosarcoma: is there a predilection for the chondroblastic subtype? Orthopedics. 2015;38:e359–366. doi: 10.3928/01477447-20150504-51. [DOI] [PubMed] [Google Scholar]
- 18.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 19.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young KA, et al. External validation of a survival nomogram for non-small cell lung cancer using the national cancer database. Ann. Surg. Oncol. 2017;24:1459–1464. doi: 10.1245/s10434-017-5795-5. [DOI] [PubMed] [Google Scholar]
- 21.Zheng ZF, et al. Development and external validation of a simplified nomogram predicting individual survival after R0 resection for gastric cancer: an international multicenter study. Ann Surg Oncol. 2018;25:2383–2390. doi: 10.1245/s10434-018-6551-1. [DOI] [PubMed] [Google Scholar]
- 22.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, , Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
- 23.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence–SEER 9 Regs Research Data, Nov 2017 Sub (1973–2015) <Katrina/Rita Population Adjustment>–Linked To County Attributes–Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
- 24.Alba AC, et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA. 2017;318:1377–1384. doi: 10.1001/jama.2017.12126. [DOI] [PubMed] [Google Scholar]
- 25.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 26.Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer. 1986;57:1442–1449. doi: 10.1002/1097-0142(19860401)57:7<1442::AID-CNCR2820570734>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Joo MW, et al. Post-radiation sarcoma: a study by the Eastern Asian Musculoskeletal Oncology Group. PLoS ONE. 2018;13:e0204927. doi: 10.1371/journal.pone.0204927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra S, et al. Radiation-induced sarcomas of bone: factors that affect outcome. J. Bone Join.t Surg. 2007;89:808–813. doi: 10.1302/0301-620X.89B6.18729. [DOI] [PubMed] [Google Scholar]
- 29.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015;39:593–599. doi: 10.1016/j.canep.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng KA, Grogan T, Wang MB. Head and neck sarcomas: analysis of the SEER database. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2014;151:627–633. doi: 10.1177/0194599814545747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun HH, Chen XY, Cui JQ, Zhou ZM, Guo KJ. Prognostic factors to survival of patients with chondroblastic osteosarcoma. Medicine. 2018;97:e12636. doi: 10.1097/MD.0000000000012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaheen M, et al. Prognosis of radiation-induced bone sarcoma is similar to primary osteosarcoma. Clin. Orthop. Relat. Res. 2006;450:76–81. doi: 10.1097/01.blo.0000229315.58878.c1. [DOI] [PubMed] [Google Scholar]
- 34.Yonemoto T, et al. The prognosis of osteosarcoma occurring as second malignancy of childhood cancers may be favorable: experience of two cancer centers in Japan. Int. J. Clin. Oncol. 2015;20:613–616. doi: 10.1007/s10147-014-0729-8. [DOI] [PubMed] [Google Scholar]
- 35.Kimata Y, et al. Postoperative complications and functional results after total glossectomy with microvascular reconstruction. Plast. Reconstr. Surg. 2000;106:1028–1035. doi: 10.1097/00006534-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, et al. Impact of first-line treatment on outcomes of Ewing sarcoma of the spine. Am. J. Cancer Res. 2018;8:1262–1272. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this manuscript are available after formal request to the corresponding authors.