Abstract
Objective
Major depressive disorder (MDD) arises from a combination of genetic and environmental risk factors and DNA methylation is one of the molecular mechanisms through which these factors can manifest. However, little is known about the epigenetic signature of MDD in brain tissue. This study aimed to investigate associations between brain tissue-based DNA methylation and late-life MDD.
Methods
We performed a brain epigenome-wide association study (EWAS) of late-life MDD in 608 participants from the Religious Order Study and the Rush Memory and Aging Project (ROS/MAP) using DNA methylation profiles of the dorsal lateral prefrontal cortex generated using the Illumina HumanMethylation450 Beadchip array. We also conducted an EWAS of MDD in each sex separately.
Results
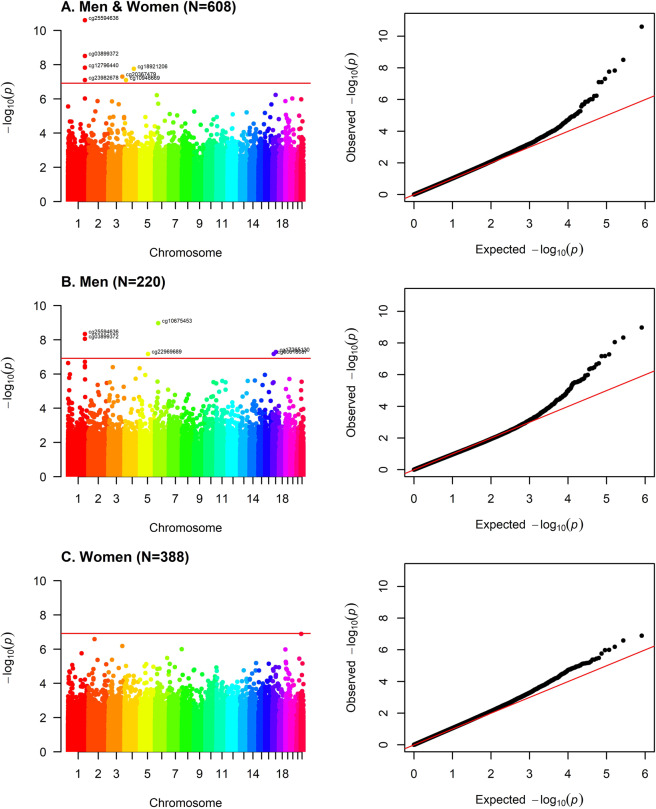
We found epigenome-wide significant associations between brain tissue-based DNA methylation and late-life MDD. The most significant and robust association was found with altered methylation levels in the YOD1 locus (cg25594636, p value = 2.55 × 10−11; cg03899372, p value = 3.12 × 10−09; cg12796440, p value = 1.51 × 10−08, cg23982678, p value = 7.94 × 10−08). Analysis of differentially methylated regions (p value = 5.06 × 10−10) further confirmed this locus. Other significant loci include UGT8 (cg18921206, p value = 1.75 × 10−08), FNDC3B (cg20367479, p value = 4.97 × 10−08) and SLIT2 (cg10946669, p value = 8.01 × 10−08). Notably, brain tissue-based methylation levels were strongly associated with late-life MDD in men more than in women.
Conclusions
We identified altered methylation in the YOD1, UGT8, FNDC3B, and SLIT2 loci as new epigenetic factors associated with late-life MDD. Furthermore, our study highlights the sex-specific molecular heterogeneity of MDD.
Subject terms: Depression, Genetics
Introduction
Major depressive disorder (MDD) severely limits psychosocial functioning, diminishes quality of life, and is a leading cause of disability worldwide1. The 12-month prevalence of MDD is ~6%2 and similar when comparing high-income countries with low-income and middle-income countries, indicating that MDD is neither a simple consequence of modern day lifestyle in developed countries, nor poverty3,4. Furthermore, although social and cultural factors such as socioeconomic status can have a role in major depression, genomic and other underlying biological factors ultimately drive the occurrence of this condition5. Twin studies have provided heritability estimates of the MDD of ~30–40%6. One of the molecular mechanisms through which environmental and genetic factors can modulate a disease outcome is epigenetics, with DNA methylation being one of the most studied modifications of the genome.
Recent epigenome-wide association studies (EWAS) showed an association of whole blood DNA methylation levels with depressive symptoms7,8 as well as MDD9–11, but little is known about brain epigenetic markers of depression or MDD. The EWAS of depressive symptoms were both conducted in late middle-aged and elderly people from the general population (mean age 70 years8 and 65 years7), an age group with an increased risk of developing dementia12. However, both studies could not determine whether their findings were confounded by dementia, which is known to be highly associated with late-life depression13. On the other hand, recent EWAS of MDD were performed in younger participants (mean age 42 years)9–11 and it is unclear if their findings can be generalized across age groups. Furthermore, most previous EWAS on depression were limited owing to measuring DNA methylation changes in blood7–10. Two recent studies conducted EWAS of MDD in 206 post mortem brain samples, but the MDD diagnosis was based on information obtained from a family member and there was no information on dementia9,11. Thus, there is need to understand the epigenetic changes in the human brain that are associated with late-life MDD and to determine whether these changes are independent of dementia.
In this study, we investigated associations between both brain tissue-based individual CpGs as well as regions of differential methylation and late-life MDD in 608 participants from the Religious Order Study and Rush Memory and Aging Project (ROS/MAP) cohorts. To reduce the risk of confounding by cognitive status, we excluded participants with a diagnosis of dementia at the time of MDD assessment and adjusted for cognitive status at the last follow-up visit (closest to methylation assessment) in our analyses. Furthermore, we performed a stratified analysis for men and women to investigate the sex-specific methylation patterns of MDD.
Methods
Study design and study population
The study population included deceased subjects from two large, prospectively followed cohorts recruited by investigators at Rush Alzheimer’s Disease Center in Chicago, IL: The Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP)14,15. Participants provided informed consent, an Anatomic Gift Act for organ donation, and a repository consent to allow their data to be repurposed. Both studies were approved by an Institutional Review Board of Rush University Medical Center. To be included in the present study, participants must have been assessed for MDD and have available genotype data and methylation profiles derived from the dorsolateral prefrontal cortex. Furthermore, we excluded participants with a diagnosis of dementia at the time of MDD assessment (at baseline evaluation). As in previous publications, the ROS and MAP data were analyzed jointly since much of the phenotypic data collected are identical at the item level in both studies and collected by the same investigative team14,16.
DNA methylation
DNA methylation was measured from the dorsolateral prefrontal cortex (dPFC; Broadman area 46) as previously described in 737 ROS/MAP participant samples14. DNA was extracted from cortically dissected sections of dPFC and DNA methylation was measured using the Illumina HumanMethylation450 Beadchip array. Initial data processing, including color channel normalization, and background removal, was performed using the Illumina GenomeStudio software. The raw IDAT files were obtained from Synapse (www.synapse.org; Synapse ID: syn7357283) and the following probes were removed: (1) probes with a detection p value > 0.01 in any sample, (2) probes annotated to the X and Y chromosomes by Illumina, (3) probes that cross-hybridize with other probes owing to sequence similarity, (3) non-CpG site probes, and (4) probes that overlap with common SNPs. After this filtering, the remaining CpG sites were normalized using the BMIQ algorithm in Watermelon R package17, and the ComBat function from the sva R package was used to adjust for batch effects18. After quality control, 408,689 discrete CpG dinucleotides in 608 subjects were used for analysis.
Genotype data
Genotyping data were generated using two microarrays, Affymetrix GeneChip 6.0 (Affymetrix, Inc, Santa Clara, CA, USA), and Illumina HumanOmniExpress (Illumina, Inc, San Diego, CA, USA) as described previously19. Genotyping was imputed to the 1000 Genome Project Phase 3 using the Michigan Imputation Server20, and the following filtering criteria were applied minor allele frequency (MAF) > 5%, Hardy–Weinberg p value > 10−5 and genotype imputation R2 > 0.3. Principal components were estimated using common (MAF > 0.05) unlinked (R2 < 0.1) autosomal markers by EIGENSTRAT21.
Diagnosis of MDD
A clinical diagnosis of current MDD was rendered by an examining clinician. The diagnosis was based on clinical interview using the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised (DSM-III-R)22. The MDD diagnosis included present versus not present. In this study, we focused on the diagnosis of MDD at the baseline assessment to reduce the risk that our findings are confounded by dementia.
Clinical diagnosis of cognitive status
A clinical diagnosis of dementia status was rendered based on a three-stage process including computer scoring of cognitive tests, clinical judgment by a neuropsychologist, and diagnostic classification by a clinician. All participants undergo a uniform, structured, clinical evaluation including a battery of 21 cognitive tests of which 19 are in common. These tests were scored by computer using a decision tree designed to mimic clinical judgment and a rating of severity of impairment was given for five cognitive domains. A neuropsychologist, blinded to participant demographics, reviews the impairment ratings and other clinical information and renders a clinical judgment regarding the presence of impairment and dementia. A clinician (neurologist, geriatrician, neuropsychologist, or geriatric nurse practitioner) then reviews all available data and examines the participant and renders a final diagnostic classification. Clinical diagnosis of dementia and clinical Alzheimer’s disease (AD) are based on criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA). The diagnosis of AD requires evidence of a meaningful decline in cognitive function relative to a previous level of performance with impairment in memory and at least one other area of cognition. Diagnosis of mild cognitive impairment (MCI) is rendered for persons who are judged to have cognitive impairment by the neuropsychologist but are judged to not meet criteria for dementia by the clinician. Persons without dementia or MCI are categorized as having no cognitive impairment.
Statistical analysis
For the brain EWAS of MDD, we ran a multivariate robust linear regression model with empirical Bayes from the R package limma (version 3.40.6)23 using clinical diagnosis of MDD at baseline as the independent variable and each CpG methylation as a dependent variable, adjusting for age at death, sex, post mortem interval (PMI), proportion of neurons, and the first three genetic principal components. The effect estimates from the adjusted models (Δ beta) refer to the difference in mean DNA methylation beta values between groups (with and without MDD). We applied a Bonferroni threshold to correct for multiple testing based on the number of tested CpG sites (threshold: 0.05/408,689 = 1.22 × 10−07). Fine mapping of our epigenome-wide associations was done with coMET24, which is a visualization tool of EWAS results with functional genomic annotations and estimation of co-methylation patterns. We conducted the following sensitivity analyses: (1) We included the cognitive status at the last follow-up visit (closest to methylation assessment) as a covariate to investigate if our findings were confounded by dementia, (2) We confirmed our associations using linear regression with p values obtained from normal theory (lm() function in R) as well as from a permutation test, (3) we corrected the p values for inflation and bias using a Bayesian method for estimation of the empirical null distribution as implemented in the R/Bioconductor package bacon25, and (4) We adjusted our association models for a polygenic risk score (PRS) for MDD (calculated with PRSice26 and UK Biobank summary statistics from27 with a p value < 0.05) to test if our EWAS findings were independent of genetic risk for MDD. Furthermore, we investigated the overlap between CpG sites associated with MDD and those associated with the PRS for MDD.
CpG sites that reach epigenome-wide significance were replicated using the summary statistics from a cell type-specific EWAS of MDD, which is based on methylation enrichment-based sequencing data from three collections of human post mortem brain (n = 206)11. This replication was used to validate our findings and to provide mechanistic insights about the most relevant cell types for our associations.
Differentially methylated regions (DMRs) in MDD were identified using DMRcate, that identifies DMRs from tunable kernel smoothing process of association signals28. Input files were our single-CpG EWAS results on MDD including regression coefficients, standard deviations, and uncorrected p values. DMRs were defined based on the following criteria: (a) a DMR should contain more than one probe; (b) regional information can be combined from probes within 1000 bp; (c) the region showed FDR corrected p value < 0.05.
To identify plausible pathways associated with MDD, we performed an over-representation analysis based on the 1000 CpGs with the lowest p values for the association with MDD. We used the R Bioconductor package missMethyl (version 1.18.0 gometh function), which performs one-sided hypergeometric tests taking into account and correcting for any bias derived from the use of differing numbers of probes per gene interrogated by the array29.
Results
Description of study participants
There were 608 ROS/MAP participants included in this study with an average age at baseline visit of 81 years and an average age of death of 86 years (Table 1). Sixty-four percent of the participants were female. At baseline, 5% of the participants were diagnosed with MDD, which is consistent with the 12-month prevalence rate of MDD in the general population2. Women showed a slightly higher prevalence of MDD than men (5.4% versus 4.1%, difference not significant).
Table 1.
Study characteristics.
| All | Male | Female | |
|---|---|---|---|
| N | 608 | 220 | 388 |
| Age at baseline visit | 80.55 ± 6.51 | 78.76 ± 6.72 | 81.57 ± 6.17 |
| Age at death, mean ± sd | 86.31 ± 4.73 | 84.94 ± 5.41 | 87.09 ± 4.11 |
| Female, n (%) | 388 (63.82%) | 0 (0.00%) | 388 (100.00%) |
| Post mortem interval (PMI), mean ± sd | 7.55 ± 6.01 | 7.80 ± 7.50 | 7.41 ± 4.98 |
| Proportion of neurons, mean ± sd | 0.45 ± 0.06 | 0.44 ± 0.06 | 0.45 ± 0.06 |
| Clinical diagnosis of cognitive status at baseline visit | |||
| No cognitive impairment, n (%) | 396 (65.13%) | 142 (64.55%) | 254 (65.46%) |
| Mild cognitive impairment (MCI), n (%) | 212 (34.87%) | 78 (35.45%) | 134 (34.54%) |
| Alzheimer’s disease dementia (AD)a, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Other dementiaa, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Clinical diagnosis of cognitive status at last follow-up visit | |||
| No cognitive impairment, n (%) | 232 (38.16%) | 93 (42.27%) | 139 (35.82%) |
| Mild cognitive impairment (MCI), n (%) | 177 (29.11%) | 66 (30.00%) | 111 (28.61%) |
| Alzheimer’s disease dementia (AD), n (%) | 184 (30.26%) | 53 (24.09%) | 131 (33.76%) |
| Other dementiaa, n (%) | 8 (1.32%) | 5 (2.27%) | 3 (0.77%) |
| Clinical diagnosis of MDD at baseline visit | 30 (4.93%) | 9 (4.09%) | 21 (5.41%) |
aParticipants with a clinical diagnosis of dementia at baseline were excluded from the analysis sample.
Differentially methylated CpG sites in brain tissue are associated with late-life depression
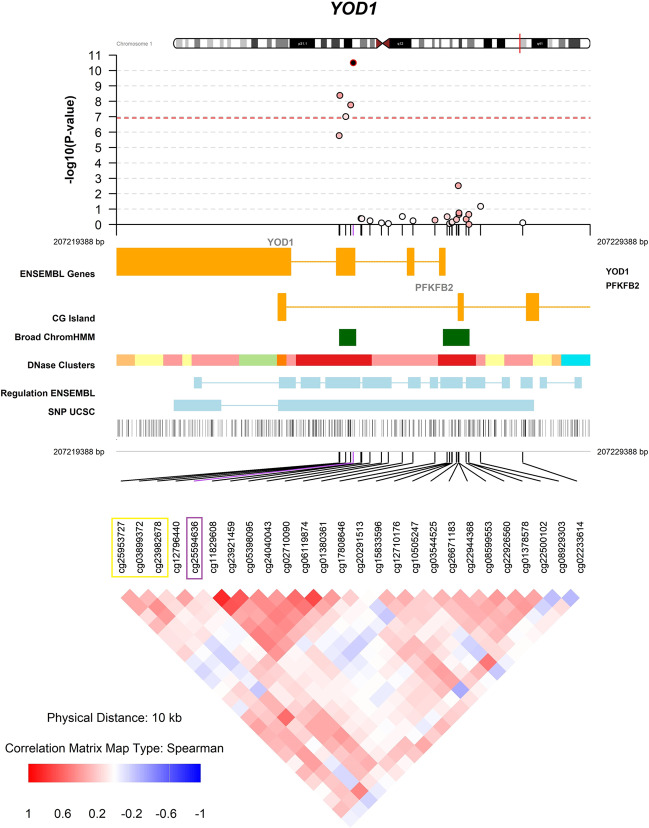
Differentially methylated CpG sites in the YOD1 (cg25594636, p value = 2.55 × 10−11; cg03899372, p value = 3.12 × 10−09; cg12796440, p value = 1.51 × 10−08, cg23982678, p value = 7.94 × 10−08), UGT8 (cg18921206, p value = 1.75 × 10−08), FNDC3B (cg20367479, p value = 4.97 × 10−08) and SLIT2 (cg10946669, p value = 8.01 × 10−08) loci were associated with MDD at the epigenome-wide significance level (Bonferroni-adjustment) after adjusting for sex, PMI, proportion of neurons, first three genetic principal components, and age at death (Table 2, Fig. 1a, Table S1). These associations were robust to additional adjustment for dementia diagnosis assessed at the last follow-up visit (Table 2). Overall, four CpG sites in YOD1 were significantly associated with late-life MDD (Table 2) and these were all located in the same CpG island, but only moderately correlated with each other (Fig. 2). This CpG island is located in an exon of YOD1 and in an intron of PFKFB2. The distribution of the DNA methylation beta values of the four most significant CpG sites in the YOD1 locus stratified by MDD diagnosis is shown in Fig. S1 and the distribution of the DNA methylation beta values of the other three significant CpG sites (cg18921206, cg20367479, and cg10946669) is shown in Fig. S2. The significant associations were confirmed in sensitivity analyses using linear regression models, permutation tests (Table S2) as well as correcting p values for potential inflation and bias (Fig. S3). Furthermore, associations were robust to additional adjustment for a PRS for MDD, which shows that our EWAS findings were independent of a genetic risk for MDD (Table S3).
Table 2.
Significant associations between DNA methylation and MDD.
| Main modela | Additionally adjusted for dementia at last follow-up | ||||||
|---|---|---|---|---|---|---|---|
| cpg | chr | Position | Nearest gene | Δ beta | p value | Δ beta | p value |
| cg25594636 | 1 | 207224388 | YOD1 | 0.013 | 2.55E-11 | 0.013 | 2.98E-11 |
| cg03899372 | 1 | 207224102 | YOD1 | 0.020 | 3.12E-09 | 0.020 | 3.76E-09 |
| cg12796440 | 1 | 207224331 | YOD1 | 0.022 | 1.51E-08 | 0.022 | 1.34E-08 |
| cg18921206 | 4 | 115320920 | UGT8 | −0.067 | 1.75E-08 | −0.068 | 9.45E-09 |
| cg20367479 | 3 | 171873675 | FNDC3B | −0.032 | 4.97E-08 | −0.032 | 3.89E-08 |
| cg23982678 | 1 | 207224227 | YOD1 | 0.021 | 7.94E-08 | 0.021 | 7.91E-08 |
| cg10946669 | 4 | 20253130 | SLIT2 | 0.013 | 8.01E-08 | 0.013 | 6.65E-08 |
Bonferroni threshold: 1.22 × 10−07.
aAdjusted for age at death, sex, PMI, neuron subtype proportion, and the first three principal components from the genotype data.
Δ beta: this coefficient represents the mean difference of DNA methylation beta values between participants with and without MDD. Negative coefficients refer to smaller mean DNA methylation beta values in participants with MDD and positive coefficients refer to larger mean DNA methylation beta values in participants with MDD.
Fig. 1. Manhattan and QQ-plots EWAS on clinical diagnosis of MDD.
Adjusted for age at death, sex, PMI, neuron proportion and the first three principal components from the genotype data. Bonferroni threshold: 1.22 × 10−07.
Fig. 2. Fine mapping of the association between DNA methylation in YOD1 and MDD.
EWAS results of the association between CpG sites and MDD adjusted for age at death, sex, PMI, neuron proportions and the first three principal components from the genotype data. The most significant CpG site (cg25594636) is marked in purple. The three CpG sites marked in yellow belong to a DMR (p value = 5.06 × 10−10, Table S3). The y axis indicates the strength of association in terms of negative logarithm of the association P value. Each circle represents a CpG site. Red dashed line within the graph indicates the genome-wide significance threshold (Bonferroni threshold: 1.22 × 10−07). The regulatory information and correlation matrix of other CpG sites in the region with the top hit are shown below the x axis. Color intensity marks the strength of the correlation and color indicates the direction of the correlation.
Only two of the seven CpG sites that were significantly associated with MDD in ROS/MAP were included in the cell type-specific EWAS published in ref. 11 (cg18921206 and cg20367479, Table S4). Of these, only cg20367479 was nominally significant for bulk brain (p value = 0.022) and the effects were not robust across different cell types in the replication cohort11 and not in the same direction as in ROS/MAP.
Analyses of differentially methylated regions
We identified one significant DMR from our EWAS results on late-life MDD that is located in the YOD1/PFKFB2 locus (Fig. 2, Table S5, minimum FDR p value for the region = 5.06 × 10−10), which is not surprising given the differential CpG site analysis. This DMR includes three CpG sites that are located downstream of the most significant CpG site from our EWAS on late-life MDD (cg25594636, Table 2).
Associations are stronger in men than in women
Interestingly, we found more methylation sites associated with MDD in men than in women (Fig. 1, Table 3, Table S1, Fig. S4), although the sample size was much smaller in men (N = 220 men vs N = 388 women). Differentially methylated CpG sites in YOD1 were more strongly associated with late-life MDD in men than in women (e.g., for cg03899372, men: beta = 0.041, p value = 8.80 × 10−09; women: beta = 0.010, p value = 0.0024; p value sex interaction = 4.51 × 10−06; Table 3). Methylation in PRICKLE4 (p value sex interaction = 1.26 × 10−09), GFAP (p value sex interaction = 6.88 × 10−05), RP11-1E3.1 (p value sex interaction = 1.11 × 10−07) and UBB (p value sex interaction = 1.54 × 10−11) was only associated with MDD in men, but not in women or in both men and women (Table 3).
Table 3.
Significant associations between DNA methylation and MDD in male.
| Men | Women | Interactiona | Men and women | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cpg | chr | Position | Nearest gene | Δ beta | p value | Δ beta | p value | p value | Δ beta | p value |
| cg10675453 | 6 | 41754588 | PRICKLE4 | 0.036 | 1.07E-09 | 0.003 | 0.2796 | 1.26E-09 | 0.013 | 1.92E-06 |
| cg25594636 | 1 | 207224388 | YOD1 | 0.023 | 4.58E-09 | 0.008 | 6.03E-05 | 0.0001 | 0.013 | 2.55E-11 |
| cg03899372 | 1 | 207224102 | YOD1 | 0.041 | 8.80E-09 | 0.010 | 0.0024 | 4.51E-06 | 0.020 | 3.12E-09 |
| cg17265120 | 17 | 42987382 | GFAP | −0.054 | 5.22E-08 | −0.006 | 0.3968 | 6.88E-05 | −0.021 | 0.0002 |
| cg22969689 | 5 | 96845117 | RP11-1E3.1 | −0.036 | 6.74E-08 | 0.000 | 0.8953 | 1.11E-07 | −0.011 | 0.0004 |
| cg00618087 | 17 | 16282382 | UBB | −0.049 | 6.79E-08 | 0.002 | 0.5535 | 1.54E-11 | −0.013 | 0.0009 |
Bonferroni threshold: 1.22e-07.
Adjusted for age at death, PMI, neuron subtype proportion and the first three principal components from the genotype data.
aInteraction between sex and MDD diagnosis is tested for each CpG site.
Δ beta: this coefficient represents the mean difference of DNA methylation beta values between participants with and without MDD. Negative coefficients refer to smaller mean DNA methylation beta values in participants with MDD and positive coefficients refer to larger mean DNA methylation beta values in participants with MDD.
Associations in men, women, and all participants are ordered by the p values from the analysis of male participants.
Pathway analysis
No significantly enriched pathway was found among the 1000 most significant CpG sites from the EWAS of late-life MDD (Table S6). The smallest p value (6 × 10−5) was reached for calmodulin-dependent protein phosphatase activity (GO:0033192). The genes that belong to this pathway are PPM1A (chr12), PPM1F (chr16), PPP3CA (chr3), PPP3CB (chr14), and PPP3CC (chr14) (Table S7).
To identify biological pathways associated with MDD across both sexes, we conducted a pathway analysis of the CpG sites that reached at least nominal significance in both sex-specific EWAS (p value < 0.05). In total, 807 CpG sites reached at least nominal significance in both sex-specific EWAS (Fig. S5), but no significantly enriched pathway was found among these (Table S8).
Differential DNA methylation in association with polygenic burden for MDD
DNA methylation was not associated with the PRS for MDD at the epigenome-wide significance level (Bonferroni-adjustment) after adjusting for sex, PMI, proportion of neurons, first three genetic principal components, age at death and additional unmeasured confounding using the R package “cate” (Fig. S6, Table S9). Furthermore, the PRS for MDD was not associated with late-life MDD in our study (Fig. S7) and there was only minimal overlap between MDD-associated differentially methylated positions and those associated with the PRS for MDD (Fig. S8).
Discussion
In this study, we found epigenome-wide significant associations between brain tissue-based DNA methylation and late-life MDD in >600 participants from the ROS/MAP cohorts. The most significant and robust association was found with altered methylation levels in the YOD1/PFKFB2 loci. This association was not confounded by dementia or a genetic risk for MDD and significant in both the single site and region-based analysis. Interestingly, brain tissue-based methylation levels were stronger associated with late-life MDD in men than in women.
The most significant CpG sites were found in a region covering an exon of YOD1 and an intron of PFKFB2. YOD1 is a highly conserved deubiquitinase similar to yeast OTU130 that is associated with regulation of the endoplasmic reticulum-associated degradation to maintain the proper folded state of proteins31. In addition, YOD1 is a negative regulator of TRAF6/p62-triggered IL-1 signaling32 and IL-1 has an important role in the regulation of inflammatory responses as well as in depression33–35. Together, these suggest that YOD1 is associated with depression perhaps via influencing the inflammatory responses. At last, previous studies suggest that YOD1 contributes to pathogenesis of neurodegenerative disease like Huntington disease and Parkinson’s disease30. PFKFB2 has been studied in the context of brain tumors36,37, but there is no evidence for an association with neuropsychological disease. Therefore, we hypothesize that the CpG sites we found to be associated with late-life MDD are most likely linked to YOD1 regulation.
Further associations with MDD were found for CpG sites in the UGT8, FNDC3B, and SLIT2 loci. UGT8 is a known blood biomarker gene for low mood with evidence of differential expression in human post mortem brains from mood disorder subjects38. In addition, lower expression of UGT8 have been shown in brain tissue from subjects with MDD compared with normal controls39. Therefore, our study extends the current literature by highlighting that not only gene expression, but also brain tissue-based methylation in UGT8 is linked to MDD. FNDC3B and SLIT2 have been discussed in association with brain tumors40, but there is no evidence for an association with neuropsychological disease.
We found stronger associations between brain tissue-based methylation levels and late-life MDD in men than in women. This finding is in line with previous studies showing sex-specific differences in serum biomarkers, mRNA expression, and brain activity of MDD cases, demonstrating that sex has an important role in the molecular heterogeneity of MDD41–43. Our findings expand the existing literature by adding DNA methylation from brain tissue to the list of biological patterns that differ between women and men with MDD, which may have important implications for diagnosis as well as treatment strategies.
This is to our knowledge, the first brain tissue-based epigenome-wide study of late-life MDD in a community-based study, and the first EWAS of MDD, which incorporates cognitive status at time of MDD diagnosis as well as at time of death. Two previous EWAS investigated the association with depressive symptoms in middle-aged and elderly people using methylation levels from whole blood7,8. Beside the difference in phenotype definition, the biggest difference between these studies and ours is the tissue in which methylation was measured. In line with a previous study comparing signals from blood and brain tissue44, we could not replicate the whole blood methylation signals from ref. 7,8 in our brain tissue-based EWAS (Tables S10 and S11). In two recent brain tissue-based EWAS of MDD, differential methylation was measured in 206 post mortem brain samples by enrichment-based sequencing9,11. However, as in ROS/MAP methylation was measured with the Illumina HumanMethylation450 Beadchip array, loci overlapping between blood and brain in9 (chr2: 208,230,169; chr9: 101,119,679; chr4: 71,632,888) were not available in ROS/MAP; therefore, we could not use the data set from Aberg et al. for replication purposes. Using the same samples, Chan et al.11 conducted a cell type-specific EWAS, in which none of the CpGs reached epigenome-wide significance for neurons and glia or for bulk brain. Owing to the different assessment of methylation (array-based versus sequencing), only two of our seven significant CpG sites were available in ref. 11 and both of them were not successfully replicated.
Strengths of this study include the ROS/MAP cohort itself, which is notable for its longitudinal nature with very high follow-up rates, prospective collection of data, a community-based cohort design, and high autopsy rates. Furthermore, the 12-month prevalence of MDD in our study population matches that in the general population2, which makes our findings generalizable beyond our study population. Another strength of our study is the analysis of methylation levels from brain tissue, which is the most relevant tissue for the pathophysiology of depression. In addition, we reduced the risk of confounding by cognitive status by excluding participants with a diagnosis of dementia at time of MDD assessment and by adjusting our analyses for cognitive status at the last follow-up visit (closest to methylation assessment).
The study is potentially limited by the use of bulk tissue analysis, which might obscure signals from different cell populations. This problem was mitigated in our analysis by adjusting for cell type composition. Future studies should investigate the role of YOD1, UGT8, FNDC3B, and SLIT2 in specific cell types from brain and investigate whether there is a causal relationship between gene dysregulation and MDD in animal models. Up to now, there is only one study analyzing cell type-specific associations between DNA methylation and MDD. However, the authors did not find any significant associations with MDD and owing to the different approach of assessing differential DNA methylation (array-based versus sequencing), our most significant CpG sites were not available in their data11. Therefore, there is an urgent need for more large-scale brain tissue-based EWAS of MDD to validate our and the previous9,11 findings and to better understand the consequences of MDD on the human brain. Another limitation of our EWAS was the small number of MDD cases in our study population. However, to reduce the risk of false positive findings owing to the imbalanced study design, we validated our findings by using different modelling approaches (limma, linear regression, permutation tests, DMR analysis).
In conclusion, we have presented evidence for brain-based DNA methylation in association with late-life MDD. We identified methylation in YOD1, UGT8, FNDC3B, and SLIT2 as new epigenetic factors associated with late-life MDD, which are not confounded by cognitive status or a genetic risk for MDD and stronger associated with MDD in male than in female.
Supplementary information
Acknowledgements
The authors are grateful to the participants of the Rush Memory and Aging Project and Religious Orders Study and the Medical Research Counsel Brain Bank. Furthermore, the authors would like to thank Dr. Yiyi Ma (Columbia University Medical Center) for her valuable feedback on the manuscript. AH was supported by a research fellowship from the Deutsche Forschungsgemeinschaft (DFG; HU 2731/1-1) and by the HERCULES Center (NIEHS P30ES019776). MPE was supported by NIH grant R01 GM117946. APW is supported by NIH grants R01 AG056533, VA I01 BX003853, and NIH U01 MH115484. TSW was supported by NIH grants P50 AG025688, R56 AG062256, R56 AG060757, and R01 AG056533. CR was supported by NIH grant T32 NS007480. DAB was supported by P30AG10161, R01AG15819, R01AG17917, R01AG16042, R01AG36042, U01AG61356. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael P. Epstein, Email: mpepste@emory.edu
Thomas S. Wingo, Email: thomas.wingo@emory.edu
Aliza P. Wingo, Email: aliza.wingo@emory.edu
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-00948-6).
References
- 1.Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev. Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 7.Jovanova OS, et al. DNA methylation signatures of depressive symptoms in middle-aged and elderly persons: meta-analysis of multiethnic epigenome-wide studies. JAMA Psychiatry. 2018;75:949–959. doi: 10.1001/jamapsychiatry.2018.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starnawska A, et al. Epigenome-wide association study of depression symptomatology in elderly monozygotic twins. Transl. Psychiatry. 2019;9:214. doi: 10.1038/s41398-019-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aberg KA, et al. Methylome-wide association findings for major depressive disorder overlap in blood and brain and replicate in independent brain samples. Mol. Psychiatry. 2018;25:1344–1354. doi: 10.1038/s41380-018-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark SL, et al. A methylation study of long-term depression risk. Mol. Psychiatry. 2019;25:1334–1343. doi: 10.1038/s41380-019-0516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan RF, et al. Cell type–specific methylome-wide association studies implicate neurotrophin and innate immune signaling in major depressive disorder. Biol. Psychiatry. 2020;87:431–442. doi: 10.1016/j.biopsych.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prince M, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dement. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Steffens DC. Late-life depression and the prodromes of dementia. JAMA Psychiatry. 2017;74:673–674. doi: 10.1001/jamapsychiatry.2017.0658. [DOI] [PubMed] [Google Scholar]
- 14.De Jager PL, et al. Alzheimer’s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat. Neurosci. 2014;17:1156–1163. doi: 10.1038/nn.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DA, et al. Religious orders study and rush memory and aging project. J. Alzheimer’s Dis. 2018;64:S161–S189. doi: 10.3233/JAD-179939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann. Neurol. 2012;72:599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teschendorff AE, et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29:189–196. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Jager PL, et al. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol. Aging. 2012;33:1017.e1–15. doi: 10.1016/j.neurobiolaging.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S, et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016;48:1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price AL, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, et al. Late-life depression is not associated with dementia-related pathology. Neuropsychology. 2016;30:135–142. doi: 10.1037/neu0000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin TC, Yet I, Tsai PC, Bell JT. coMET: Visualisation of regional epigenome-wide association scan results and DNA co-methylation patterns. BMC Bioinformatics. 2015;16:1–5. doi: 10.1186/s12859-015-0568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Iterson M, van Zwet EW, the BIOS Consortium, Heijmans BT. Controlling bias and inflation in epigenome- and transcriptome-wide association studies using the empirical null distribution. Genome Biol. 2017;18:1–13. doi: 10.1186/s13059-016-1131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Euesden J, Lewis CM, O’Reilly PF. PRSice: polygenic risk score software. Bioinformatics. 2015;31:1466–1468. doi: 10.1093/bioinformatics/btu848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard DM, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019;22:343–352. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters TJ, et al. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin. 2015;8:6. doi: 10.1186/1756-8935-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phipson B, Maksimovic J, Oshlack A. missMethyl: an R package for analyzing data from Illumina’s HumanMethylation450 platform. Bioinformatics. 2016;32:286–288. doi: 10.1093/bioinformatics/btv560. [DOI] [PubMed] [Google Scholar]
- 30.Tanji K, et al. YOD1 attenuates neurogenic proteotoxicity through its deubiquitinating activity. Neurobiol. Dis. 2018;112:14–23. doi: 10.1016/j.nbd.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Rumpf S, Jentsch S. Functional division of substrate processing cofactors of the ubiquitin-selective Cdc48 chaperone. Mol. Cell. 2006;21:261–269. doi: 10.1016/j.molcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Schimmack G, et al. YOD1/TRAF6 association balances p62-dependent IL-1 signaling to NF-$κ$B. Elife. 2017;6:1–24. doi: 10.7554/eLife.22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 34.Ellul P, Boyer L, Groc L, Leboyer M, Fond G. Interleukin-1 β-targeted treatment strategies in inflammatory depression: toward personalized care. Acta Psychiatr. Scand. 2016;134:469–484. doi: 10.1111/acps.12656. [DOI] [PubMed] [Google Scholar]
- 35.Khazim K, Azulay EE, Kristal B, Cohen I. Interleukin 1 gene polymorphism and susceptibility to disease. Immunol. Rev. 2018;281:40–56. doi: 10.1111/imr.12620. [DOI] [PubMed] [Google Scholar]
- 36.Zakrzewska M, et al. Expression-based decision tree model reveals distinct microRNA expression pattern in pediatric neuronal and mixed neuronal-glial tumors. BMC Cancer. 2019;19:1–11. doi: 10.1186/s12885-019-5739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Z, You C, Zhao D. Long non-coding RNA UCA1/miR-182/PFKFB2 axis modulates glioblastoma-associated stromal cells-mediated glycolysis and invasion of glioma cells. Biochem. Biophys. Res. Commun. 2018;500:569–576. doi: 10.1016/j.bbrc.2018.04.091. [DOI] [PubMed] [Google Scholar]
- 38.Le-Niculescu H, et al. Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol. Psychiatry. 2009;14:156–174. doi: 10.1038/mp.2008.11. [DOI] [PubMed] [Google Scholar]
- 39.Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol. Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- 40.Stangeland B, et al. Combined expressional analysis, bioinformatics and targeted proteomics identify new potential therapeutic targets in glioblastoma stem cells. Oncotarget. 2015;6:26192–26215. doi: 10.18632/oncotarget.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J. Biological sex differences in depression: a systematic review. Biol. Res. Nurs. 2018;20:383–392. doi: 10.1177/1099800418776082. [DOI] [PubMed] [Google Scholar]
- 42.Yang X, et al. Sex differences in the clinical characteristics and brain gray matter volume alterations in unmedicated patients with major depressive disorder. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-02828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramsey JM, et al. Sex differences in serum markers of major depressive disorder in the Netherlands Study of Depression and Anxiety (NESDA) PLoS ONE. 2016;11:e0156624. doi: 10.1371/journal.pone.0156624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hüls, A. et al. Brain DNA methylation patterns in CLDN5 associated with cognitive decline. bioRxiv Prepr.10.1101/857953 (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.