Abstract
Background and Aims
Diarrhoea is a common, debilitating symptom of gastrointestinal disorders. Pathomechanisms probably involve defects in trans-epithelial water transport, but the role of aquaporin [AQP] family water channels in diarrhoea-predominant diseases is unknown. We investigated the involvement of AQPs in the pathobiology of collagenous colitis [CC], which features chronic, watery diarrhoea despite overtly normal intestinal epithelial cells [IECs].
Methods
We assessed the expression of all AQP family members in mucosal samples of CC patients before and during treatment with the corticosteroid drug budesonide, steroid-refractory CC patients and healthy controls. Samples were analysed by genome-wide mRNA sequencing [RNA-seq] and quantitative real-time PCR [qPCR]. In some patients, we performed tissue microdissection followed by RNA-seq to explore the IEC-specific CC transcriptome. We determined changes in the protein levels of the lead candidates in IEC by confocal microscopy. Finally, we investigated the regulation of AQP expression by corticosteroids in model cell lines.
Results
Using qPCR and RNA-seq, we identified loss of AQP8 expression as a hallmark of active CC, which was reverted by budesonide treatment in steroid-responsive but not refractory patients. Consistently, decreased AQP8 mRNA and protein levels were observed in IECs of patients with active CC, and steroid drugs increased AQP8 expression in model IECs. Moreover, low APQ8 expression was strongly associated with higher stool frequency in CC patients.
Conclusion
Down-regulation of epithelial AQP8 may impair water resorption in active CC, resulting in watery diarrhoea. Our results suggest that AQP8 is a potential drug target for the treatment of diarrhoeal disorders.
Keywords: Microscopic colitis, permeability, RNA sequencing
1. Introduction
Diarrhoea is a common symptom of gastrointestinal disorders, the management of which presents a major challenge in clinical practice due to its high impact on the patients’ quality of life. Targeted treatment options are often lacking, especially in cases of chronic watery diarrhoea with idiopathic origin, such as the inflammatory bowel disease [IBD] collagenous colitis [CC].1 Consequently, clinicians frequently resort to unspecific anti-diarrhoeal or anti-inflammatory medication that may provoke major undesirable side effects.1,2 In CC, for example, the corticosteroid drug budesonide is highly effective at inducing and maintaining clinical remission, including the rapid cessation of diarrhoea.3 However, relapse after discontinuation of budesonide is common, and patients can become steroid-dependent or treatment-refractory.4,5 Thus, specific anti-diarrhoeal drugs are an unmet clinical need.
Because of the complexity of intestinal electrophysiology and water homeostasis, the pathomechanisms of diarrhoea remain incompletely understood. Trans-epithelial ion transport plays a central role in intestinal water homeostasis, in particular the regulated absorption of sodium ions from the gut lumen.6,7 Following epithelial Na+ uptake, water is passively absorbed into the lamina propria via a paracellular route along the osmotic gradient. The exchange of sodium and other osmotically active ions is facilitated by various channel proteins, including the sodium-hydrogen exchanger isoforms 2/3 [SLC9A2/3 or NHE2/3], downregulated in adenoma [SLC26A3/DRA], putative anion transporter 1 [SLC36A1/PAT1], epithelial sodium channel [SCNN1/ENaC], and the cystic fibrosis transmembrane conductance regulator [CFTR].6 Accordingly, loss-of-function of several of these proteins causes congenital diarrhoea,7 and dysregulated ion transport has been observed in diarrhoeal disorders, including CC.8,9 However, given that intestinal epithelia use both paracellular and transcellular water transport in parallel, and that the surface area of epithelia far exceeds that of their intercellular space, impaired ion exchange is probably insufficient to explain the massive fluid loss observed in chronic watery diarrhoea.6
Transcellular water flux, including in the intestinal mucosa, primarily occurs via aquaporin [AQP] family water channels.10,11 Despite the critical role of AQPs in maintaining water homeostasis in virtually all organs, their tissue-specific function and regulation is largely unresolved. Of the 13 human isoforms, eight AQPs have been detected in the intestine, where they are distributed in a distinct regional and subcellular manner. In particular, AQPs 7, 8, 10 and 11 localize to the apical membrane of colonic intestinal epithelial cells [IECs], while AQPs 3 and 4 are mainly found in the basolateral membrane.12,13 A general decrease in AQP expression has been observed in the colonic mucosa of patients with the IBDs Crohn’s disease and ulcerative colitis, and it has been suggested that altered water transport may exacerbate symptoms in these diseases.14–17 However, in contrast to diarrhoea-predominant disorders such as CC, these IBDs typically feature fulminant inflammation and frank ulceration of the mucosa; it is thus unclear if loss of AQPs indeed contributes to IBD pathogenesis, or whether it is rather just an epiphenomenon of epithelial erosion.
To address this question, we investigated the regulation of AQPs and intestinal ion transporters in the model diarrhoeal disorder CC, which is characterized by frequent watery bowel movements despite an overtly normal intestinal epithelium. Our data suggest that defective water transport contributes to IBD pathogenesis, and that AQP8 is a promising therapeutic target in CC as well as other diarrhoeal diseases.
2. Materials And Methods
2.1. Study population
Biopsy samples from the descending colon were collected during scheduled sigmoidoscopy from adult collagenous colitis [CC] patients at the Division of Gastroenterology at Linköping University Hospital, Sweden. CC was diagnosed according to current guidelines,18 primarily clinical history and histopathological features, including a subepithelial collagen band of > 10 µm thickness. Active CC was defined as more than three bowel movements/day or at least one watery bowel movement/day during a 1-week registration period. Clinical remission was defined as fewer than three bowel movements/day and no watery bowel movement within a 1-week period. A diagnosis of steroid-refractory CC was reached if patients did not achieve clinical remission after treatment for 12 weeks with 6–9 mg/day budesonide.18,19 Healthy volunteers were recruited from the local colon cancer screening programme at Linköping University Hospital (Sweden) or St Olav University Hospital (Norway); these individuals showed normal macro- and microscopic findings upon histopathological assessment, had normal bowel movements, and were not taking any medication at the time of colonoscopy. In total, we enrolled 26 treatment-responsive patients with active CC, 18 o whom agreed to have additional biopsies taken after reaching remission during budesonide treatment [after 6 weeks of treatment on average]. We also obtained samples from 14 steroid-refractory CC patients and 27 healthy controls following the same bowel preparation procedure and biopsy taken from the descending colon as mentioned above. An additional group of 14 healthy volunteers agreed to have biopsy samples taken from both ascending and descending colon for site-specific expression analysis. Detailed patient characteristics are given in Table 1. Adjacent biopsy samples were stored in AllProtect tissue reagent for subsequent RNA extraction, or in PBS for embedding and microscopy analyses. Informed written consent was obtained from all subjects, and their data were handled according to current regulations [EU2016/679, corrigendum May 23, 2018]. Ethical approval was issued by Linköping’s regional ethical committee to conduct studies in microscopic colitis, including CC [Dnr 2015/31-31].
Table 1.
Clinical and demographic characteristics of CC patients and controls
| Variable | Hc | auCC | itCC* | aRCC |
|---|---|---|---|---|
| Total number of subjects | 27 | 26 | 18 | 14 |
| On budesonide treatment | No | No | Yes | No |
| Steroid-responders | – | Yes | Yes | No |
| Sex, % females | 42 | 73 | 78 | 93 |
| Average age, years [range] | 61 [60–71] | 60 [27–86] | 60 [27–86] | 55 [25–79] |
| Average stools/day [range] | – | 6.84 [3–12] | 1.39 [1–2]† | 9.21 [4–20] |
| Average watery stools/day [range] | – | 6.72 [2–12] | 0 [N/A]† | 8.71 [4–20] |
| Average collagenous band, µm [range] | − | 32.58 [12–52] | 26.28 [2–72] † | 35.71 [10–72] |
*Matched samples from itCC patients were collected before and during treatment with budesonide. Note that samples before treatment [active disease] were included in the group of auCC samples, whereas samples during treatment were included as itCC samples.
†Note that the average stool frequencies and collagenous band thickness before treatment of itCC patients are nearly the same as the auCC patient group.
au, active/untreated; aR, active/steroid-refractory; CC, collagenous colitis; Hc, healthy controls; it, inactive/treated; N/A, not applicable.
2.2. Quantitative PCR (qPCR)
Biopsies preserved in AllProtect were homogenized in RLT buffer from the RNeasy Mini Kit supplemented with 1% 2-mercaptoethanol in a TissueLyser II instrument [all from Qiagen]. Total RNA from homogenized biopsy samples or cell lines was isolated using an RNeasy Mini Kit [Qiagen] following the manufacturer’s instructions. RNA was quantified using a NanoDrop ND-2000 device [ThermoFisher Scientific] and reverse-transcribed with a High Capacity cDNA Reverse Transcription Kit [Thermo Fisher Scientific]. Relative gene expression was quantified by real-time [RT]-PCR with iTaq Universal SYBR Green Supermix [BioRad] following the manufacturer’s instructions and using the primer pairs given in Supplementary Table 1. Primers were designed to amplify all transcript coding variants of the selected gene in the Reference Sequence [RefSeq] collection of the National Center for Biotechnology Information [NCBI] taking the longest transcript sequence as a reference, with primers annealing in different exons for all transcript variants using Primer3Plus v2.4.2 software.20 Quantitative analysis was carried out in a CFX96 Touch Real-Time PCR detection system [Bio-Rad] using the relative quantification [ΔΔCt] method. Hypoxanthine phosphoribosyltransferase [HPRT] 1 was used as a reference gene, and each sample was analysed in duplicate.
2.3. Analysis of public datasets
AQP protein levels in the normal human intestine were based on annotations in the Human Protein Atlas [www.proteinatlas.org].21 Immunohistochemical staining annotations for glandular cells and endothelium were coded as ++ [medium], + [low], – [not detected] and N/A [not available]. We analysed differential gene expression in lymphocytic colitis samples curated in GEO dataset GSE65107.22 This study comprises RNA-sequencing data from four lymphocytic colitis patients and four healthy controls. Pre-processed, adjusted RNA transcript levels were obtained through the recount2 resource,23 and data were analszed in R.
2.4. Genome-wide mRNA sequencing [RNA-seq]
Total RNA was isolated from full mucosal biopsies [n = 13 healthy controls, and n = 9 per CC group] stored in AllProtect, using an RNeasy mini kit [Qiagen] according to the supplier’s instructions. RNA from laser capture microdissection [LCM] material [n = 8–9 per group, see next section] was isolated with an RNeasy FFPE kit [Qiagen]. RNA integrity was assessed using an Agilent RNA 6000 Pico kit on a 2100 Bioanalyzer [Agilent Technologies]. The DV200 value, representing the percentage of RNA fragments more than 200 nucleotides long, was used as a measure of RNA quality. The range of DV200 values was 30–70% [Supplementaryl Table 2]. RNA-seq libraries were constructed with SENSE totalRNA with Ribo cop rRNA depletion [Lexogene], and single-read sequenced for 75 cycles to a depth of 25 million base reads on a HiSeq4000 instrument [Illumina], according to the manufacturer’s recommendations. FASTQ files were generated using bcl2fastq software v2.18. Data were analysed using the R Bioconductor software v3.5.1 [R Core Team 2017], including SARTools v1.6.6 and DESeq2 v1.22.1 packages.24–26 Reads were aligned to the Ensembl GRCh38 genome version, release 92. Differential gene expression was identified by linear models using DESeq2 v1.22.1 and significance was decided by Benjamini–Hochberg false discovery rate adjusted p-values < 0.05.
2.5. Laser capture microdissection
Colonic biopsy samples collected in PBS were fixed in paraformaldehyde and embedded in paraffin [FFPE samples]. Matched samples from eight steroid-responsive CC patients before and during budesonide treatment, nine steroid-refractory CC patients, and nine healthy controls were used for microdissection. LCM was performed as previously described.27 Briefly, samples were cut into 10-µm sections and mounted on RNAse-free MMI Membrane Slides [Molecular Machines and Industry, MMI]; samples were then stained with haematoxylin following standard protocols. The sections were dehydrated with 100% ethanol and xylenes, followed by air-drying in a desiccator for at least 30 min. Intestinal epithelial cells [area of 106 µm2, corresponding to ~104 cells] were isolated from all samples with a UV-LCM MMI Cellcut device connected to an Olympus IX71 microscope, and collected in MMI isolation caps with diffuser [all from MMI AG], following the manufacturer’s recommendations. Examples of the microdissection process and details of isolated area for each sample set can be found in Supplementary Figure 1 and Table 2, respectively. Isolated cells were kept in PKD lysis buffer from the RNeasy FFPE kit [Qiagen] at −80°C until RNA was isolated.
2.6. Confocal microscopy
Paraffin-embedded sections [4 µm] were cut in a microtome and deparaffinated with Histolab Clear [Histolab Products AB]. Antigen retrieval was performed in 10 mM citric acid pH 6.0 containing 0.05% Tween20 [Sigma-Aldrich] in a 2100 Retriever [Aptum Biologics]. Samples were blocked in 1% bovine serum albumin [BSA, Sigma-Aldrich] in phosphate buffered saline [PBS] supplemented with 22.52 mg/mL glycine [Bio-Rad], and permeabilized in 0.1% Triton X-100 in PBS [Sigma-Aldrich]. Anti-AQP1 [ab15080, Abcam], anti-AQP7 [bs-2506R, Bioss Antibodies], anti-AQP7 [NBP1-30862, Novus Biologicals], anti-AQP8 antibody [HPA046259, Human Protein Atlas Antibodies], anti-AQP11 [HPA042879, Human Protein Atlas Antibodies], rabbit IgG isotype antibody [Thermo Fisher Scientific] and secondary donkey anti-rabbit IgG DyLight550 conjugated antibody [Invitrogen] were used to stain the samples. ProLong Glass Antifade Mountant with NucBlue [Invitrogen] was used both to stain cell nuclei and to mount the slides. Images were acquired on an LSM800 laser scanning confocal microscope with Airyscan module [Zeiss], using identical detector settings. Three and 14 z-stacks covering 1.0 and 3.5 µm of tissue thickness from six different regions of the mucosa were obtained per sample for AQP1 and AQP8 stainings, respectively, with epithelial glands in cross-sectional [‘doughnut’] orientation. AQP7 and AQP11 pictures were obtained as 14 z-stack images covering 3.5 µm of tissue thickness of representative areas of the mucosa. AQP1 and AQP8 median fluorescence intensity in the apical side of intestinal epithelial cells was analysed using the segmented line tool in FIJI-ImageJ v1.52e software [National Institutes of Health] following z median projection. Two healthy control samples were included in every experiment for normalization. A minimum of five well-orientated images per sample were used for subsequent statistical analysis. Image acquisition and analysis were performed in a blinded manner.
2.7. Cell cultures and in vitro assays
Authenticated HTC116 [DSMZ – German Collection of Microorganisms and Cell Cultures, cat. no ACC581], and HT-29 [American Type Culture Collection, HT29 HTB38] cell lines were maintained at 5% CO2/37°C in Dulbecco’s modified Eagle’s medium [DMEM] supplemented with 10% fetal bovine serum [FBS], 2 mM l-glutamine, and 1% penicillin-streptomycin [all from Life Technologies]. Caco-2 [Health Protection Agency, collection number 86010202] cell lines were maintained at 5% CO2/37°C in DMEM supplemented with 15% FBS, 2 mM l-glutamine, 1% non-essential amino acids and 1% penicillin-streptomycin [all from Life Technologies]. All experiments were performed using low passage cells from confirmed mycoplasma-free frozen stocks. Cells were cultured at a concentration of 5 × 104 cells in 24-well plates [BD] and medium was replaced every other day. Once cells formed a monolayer [confluence in ~7 days], cells were treated with 1 µM corticosteroids [budesonide or dexamethasone, both from Sigma-Aldrich] for 24 h. Unstimulated cells grown simultaneously were used as controls. Experiments were carried out in biological triplicates. For RNA extraction, cells were directly lysed in RLT buffer from the RNeasy Mini Kit [Qiagen] supplemented with 1% 2-mercaptoethanol [Sigma-Aldrich].
2.8. Statistical analyses
Quantitative PCR data [ΔCt values] and AQP8 median fluorescence intensity values from human biopsy samples were analysed with the non-parametric Mann-–Whitney test when different groups were compared to healthy controls. The non-parametric Wilcoxon test was used to compare paired samples from CC patients before and during treatment. Both tests were run with the Monte Carlo algorithm for 100 000 sample sets. Spearman’s test was used to correlate clinical values with experimental data. A false discovery rate (FDR)-adjusted p-value < 0.05 was considered as statistically significant. Quantitative PCR data [ΔCt values] from in vitro assay samples were analysed with an ANOVA variance test. Statistical analyses were performed in SPSS v15.0 [IBM], and data were plotted in GraphPad Prism v8.0.1 [GraphPad] or RStudio v1.2.1335 [RStudio]. Differential gene expression from RNA-seq data was determined with linear models using DESeq2 v1.22.1 and significance was baxed on Benjamini–Hochberg FDR-adjusted p-values < 0.05.
3. Results
3.1. AQPs 7, 8 and 11 are the primary water channels in the intestinal epithelium
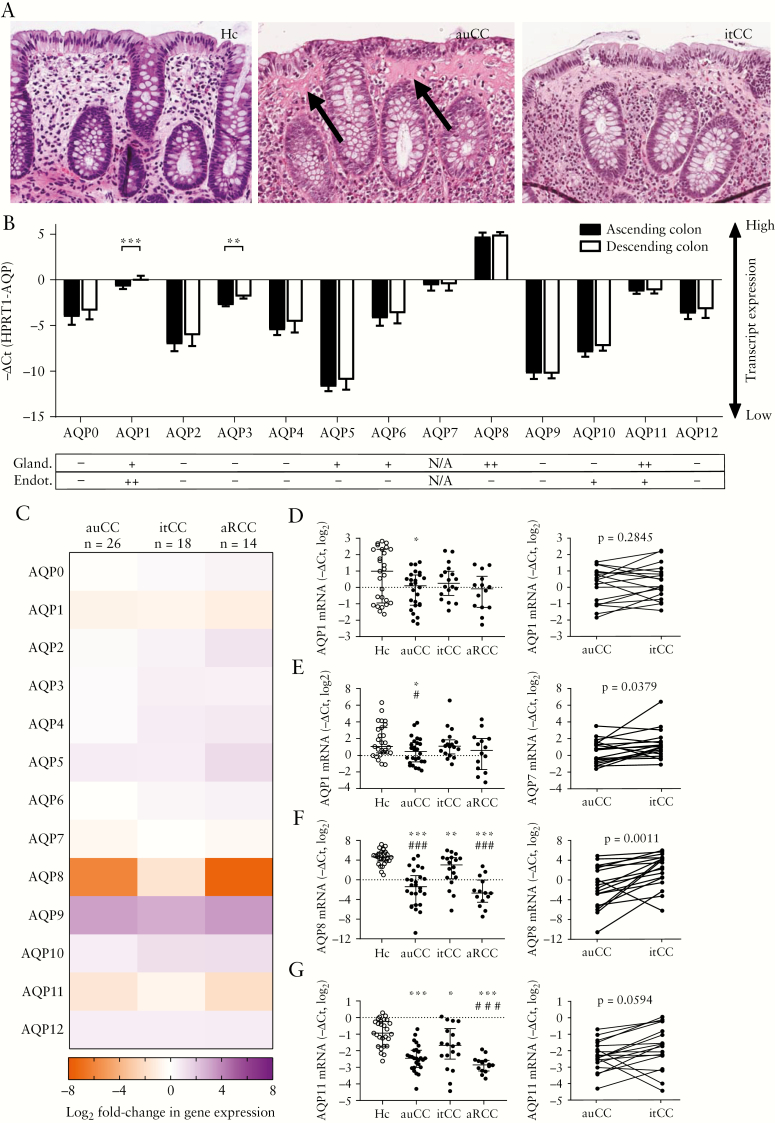
The regulation of intestinal water homeostasis in diarrhoea-predominant diseases is incompletely understood. However, it has been suggested that impaired trans-epithelial fluid absorption by AQP family water channels is a major pathomechanism in these disorders.11,28 We therefore investigated the regulation of AQPs in the chronic diarrhoeal disorder CC, which is characterized by frequent watery bowel movements in the absence of intestinal epithelial dysfunction and frank mucosal inflammation [Figure 1A]. To establish a baseline for the subsequent studies, we first determined mRNA and protein levels of all AQP family members in the healthy colon [Figure 1B]. qPCR analysis of matched ascending and descending colon samples from healthy volunteers revealed broad and largely comparable AQP mRNA expression in both parts of the colon. Consistent with earlier observations,15 AQPs 1, 3, 7, 8 and 11 were found to be most highly expressed in the colonic mucosa, as indicated by high −ΔCt values. Moreover, we matched these results with public data from the Human Protein Atlas,21 and observed that AQPs 8 and 11 also exhibited high protein levels in colonic IECs [Figure 1B]. Of note, although AQP7 protein data are not available from the Human Protein Atlas, Ricanek et al. previously reported robust staining of this molecule in colonic epithelia.15 Collectively these results suggest that AQPs 7, 8 and 11 are the primary water channels in the intestinal epithelium.
Figure 1.
Aquaporin [AQP] gene expression is altered in collagenous colitis [CC]. [A] Representative histology of haematoxylin-eosin-stained paraffin-embedded sections of the human colonic mucosa in a healthy control [left], an active untreated CC [centre], and an inactive treated CC [right] sample. Note the increased collagenous band thickness [arrows] but unremarkable epithelial architecture changes in the auCC sample, and the histological resemblance between auCC and itCC samples even if the latter sample was collected from a patient after achieving clinical remission due to treatment. Original magnification 200×. [B] AQP mRNA abundance as determined by quantitative PCR in matched ascending [black bars] and descending [white bars] colonic mucosa of healthy donors [n = 14]. Data are represented as median with interquartile range. The table below shows corresponding AQP protein levels in colonic glandular and endothelial cells, based on summary annotations from the Human Protein Atlas [HPA]. Protein levels are indicated as medium [++], low [+], not detected [–] and not available [N/A]. [C] Heatmap showing AQP gene expression changes in CC colonic mucosa compared to healthy controls [Hc, n = 27]. Data are displayed as log2-transformed fold-changes of the −ΔΔCt values, with HPRT1 as a housekeeping control. [D–G] Detailed analysis of gene expression of AQP1 [D], AQP7 [E], AQP8 [F] and AQP11 [G] in all CC groups [left, median with interquartile range]. Paired data of CC patients [n = 18] before [auCC] and during [itCC] successful treatment with budesonide are shown on the right. Note that all primers detect all coding transcript variants of the indicated gene. Statistically significant differences relative to Hc samples are shown as *p < 0.05, **p < 0.01 and ***p < 0.001; statistically significant differences relative to itCC samples are shown as #p < 0.05, # #p < 0.01 and # # #p < 0.001. AQP, aquaporin; au, active/untreated; aR, active/refractory; CC, collagenous colitis; Hc, healthy controls; HPA, human protein atlas; it, inactive/treated.
3.2. The colonic AQP expression profile is altered in CC
We next investigated the regulation of AQP expression in mucosal biopsies from CC patients [Table 1]. Our samples included 26 active, subsequently steroid-responsive individuals [active/untreated, auCC], of which 18 agreed to have additional biopsies taken after achieving clinical remission with budesonide treatment [inactive/treated, itCC]. Moreover, we obtained samples from 14 individuals with active disease despite treatment with steroids [active/Refractory, aRCC], as well as 27 healthy controls. qPCR analysis revealed differential expression of multiple AQPs in active CC [Figure 1C–G, Table 2; Supplementary Figure 2]. In particular, mRNA expression of AQPs 1, 7, 8 and 11 was consistently decreased in auCC and aRCC samples compared to controls. Importantly, detailed analysis showed that the expression of these molecules was restored almost back to control levels in patients in clinical remission, especially in the case of AQP8 [Figure 1D–G]. Conversely, AQP9 expression was increased in all patient samples, and was slightly lower after budesonide treatment [Figure 1C; Supplementary Figure 2]. However, this result should be interpreted with caution due to the low levels of AQP9 mRNA expression. In summary, these data show that the expression of multiple AQP family members is altered in active CC, and that clinical remission is associated with a normalization of epithelial AQP levels.
Table 2.
Relative expression of AQPs 1, 7, 8 and 11 in CC samples; expression values are shown as median [interquartile range: Q1, Q3] of relative fold-changes [2−Δ ΔCt values] in gene expression
| Gene | Hc | auCC | itCC | aRCC |
|---|---|---|---|---|
| AQP1 | 1.00 | 0.54* | 0.61 | 0.48 |
| [0.28, 2.51] | [0.24, 0.82] | [0.40, 0.94] | [0.22, 0.68] | |
| AQP7 | 1.00 | 0.67* | 1.03 | 0.75 |
| [0.61, 4.49] | [0.29, 1.31] | [0.60, 1.57] | [0.19, 1.81] | |
| AQP8 | 1.00 | 0.02*** | 0.34** | 0.01*** |
| [0.56, 1.74] | [0.00, 0.05] | [0.05, 0.86] | [0.00, 0.03] | |
| AQP11 | 1.00 | 0.35*** | 0.60* | 0.27*** |
| [0.69, 1.61] | [0.25, 0.48] | [0.39, 1.05] | [0.21, 0.32] |
Statistically significant differences related to Hc samples are shown as *p < 0.05, **p < 0.01 and ***p < 0.001. auCC, active untreated collagenous colitis; AQP, aquaporin; CC, collagenous colitis; Hc, healthy controls; aRCC, active refractory collagenous colitis; itCC, inactive treated collagenous colitis; Q, quartile.
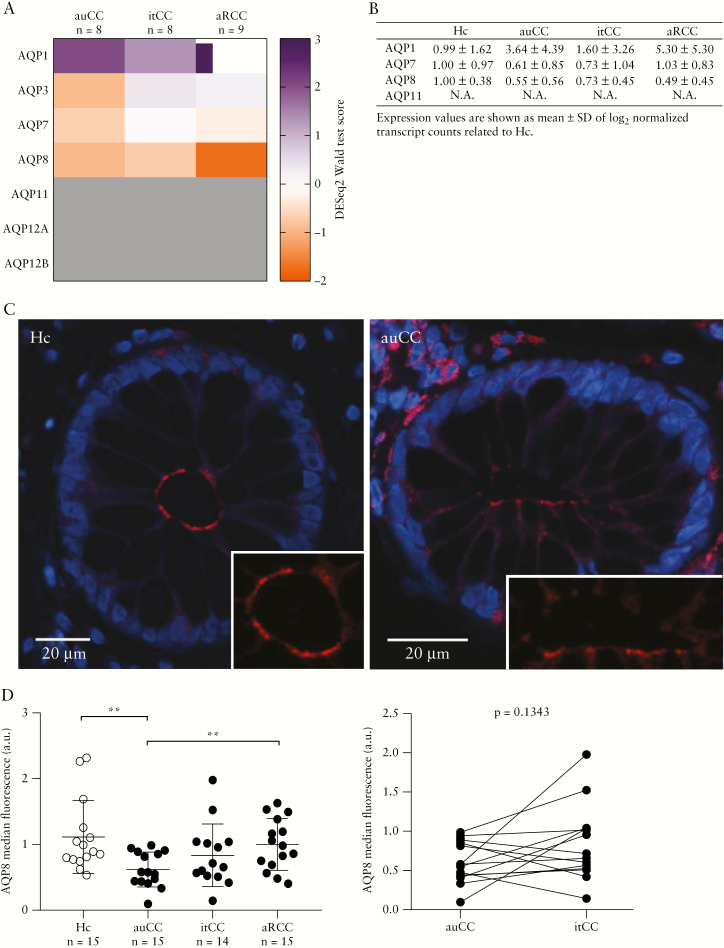
3.3. RNA-seq confirms loss of AQPs 7 and 8 in microscopic colitis
To validate these findings, we additionally analysed a subset of patient samples using unbiased, genome-wide RNA-seq of full mucosal biopsies [Figure 2]. Compared to our qPCR data, RNA-seq results exhibited much higher variability across samples, and multiple AQPs with low mRNA expression were not reliably detected using this method. Importantly, however, we observed considerably lower transcript counts of AQPs 7 and 8 in both auCC and aRCC samples, whereas the levels of AQPs 1 and 11 were largely unchanged or even slightly elevated [Figure 2]. Moreover, in good agreement with the qPCR data, transcript levels of AQPs 7 and 8 were partially restored after budesonide treatment, especially in the case of AQP8 [Figure 2C, D]. We additionally analysed publicly available RNA-seq data of lymphocytic colitis,22 the other major form of microscopic colitis [Supplementary Figure 3]. As in our CC samples, transcript levels of AQPs 7 and 8, but not AQPs 1 and 11, were decreased in lymphocytic colitis patients compared to healthy controls. We conclude that transcript levels of select colonic AQPs are decreased in microscopic colitis, dependent on disease activity.
Figure 2.
RNA-seq analysis confirming AQP8 modulation in CC colonic mucosa. [A] Heatmap showing normalized, log2-transformed read counts [DESeq2 Wald statistic test scores] for the indicated AQP genes in CC mucosal biopsy samples [n = 13 Hc, and n = 9 per CC group]. Samples were analysed by transcriptome-wide mRNA sequencing [RNA-seq]. Datasets with < 10 total reads with a value of ≥ 1 read were omitted. [B–E] Transcript counts [TC] of AQP1 [B], AQP7 [C], AQP8 [D] and AQP11 [E] in all CC groups [left, median with interquartile range], and paired CC samples [n = 18] before [auCC] and during [itCC] successful treatment with budesonide [right]. p-values are FDR-adjusted; values above the significance threshold of 0.05 are shown as not significant [NS]. Sample number in all panels was n = 13 for Hc and n = 9 for CC cohorts. [F] Summarized AQP transcript counts, normalized to Hc. Expression values are shown as mean ± SD of log2-transformed TC. Statistically significant differences relative to Hc samples are shown as *p < 0.05, **p < 0.01 and ***p < 0.001; statistically significant differences relative to itCC samples are shown as #p < 0.05, and # # #p < 0.001. AQP, aquaporin; au, active untreated; aR, active/refractory; CC, collagenous colitis; Hc, healthy controls; it, inactive/treated; NS, not significant; RNA-seq, RNA-sequencing; SD, standard deviation; TC, transcript counts.
3.4. Colonic epithelial AQP8 levels are reduced in CC
Our data thus suggested that AQP8 is the major intestinal epithelial water channel, whose expression is dysregulated in CC. Indeed, previous studies using primary rat colonocytes suggested that AQP8 may facilitate more than one-third of the total trans-epithelial water transport.29 Because our earlier analyses of full mucosal biopsies are unable to distinguish gene expression changes in epithelial vs non-epithelial cells, we next investigated the regulation of AQPs specifically in IECs. To this end, we first isolated intestinal epithelia from paraffin-embedded tissue sections using laser capture microdissection, and analysed the samples by RNA-seq. As expected, epithelial cell markers [KRT18, KRT19] were increased in epithelial isolates compared to full biopsies, whereas stromal markers [ENG, ACTA2] were reduced [Supplementary Figure 1]. Owing to the limited amount of RNA recovered from the FFPE sections, we did not obtain sufficient high-quality reads for most AQPs. Importantly, however, we were able to recapitulate the reduction in gene expression for AQPs 7 and especially 8 in active CC, and this was again partially rescued in patients in clinical remission [Figure 3A, B]. To confirm and expand on these observations, we additionally assessed colonic epithelial AQP protein levels in a subset of CC patients by confocal microscopy. In agreement with previous studies,15,29 AQP8 localized to the apical membrane of colonic IECs, and we noted an apparent loss of AQP8 immunostaining in active CC [Figure 3C]. Blinded analysis of all samples revealed a significant reduction of AQP8 protein in auCC, which was partially restored by budesonide treatment [Figure 3D]. Somewhat surprisingly, AQP8 protein levels in aRCC samples were comparable to healthy controls, suggesting that steroid-refractory individuals constitute a pathophysiologically distinct patient group. We also detected AQP1 protein in the apical membrane of colonic IECs [Supplementary Figure 4A]. However, its distribution was highly heterogeneous within crypts of the same sample, and was not significantly altered in CC [Supplementary Figure 4A, B]. AQP7 and AQP11 were not detected in IECs [Supplementary Figure 4C]. Taken together, our data show that CC is associated with reduced AQP8 mRNA and protein levels in IECs, which are restored by corticosteroid treatment.
Figure 3.
AQP8 levels are decreased in intestinal epithelial cells [IECs] of steroid-responsive CC patients. [A] Heatmap showing normalized, log2-transformed read counts [DESeq2 Wald statistic test scores] for the indicated AQP genes in CC IECs. IECs were isolated by laser capture microdissection of FFPE mucosal biopsy samples, and analysed using RNA-seq. AQPs 11, 12A and 12B [grey] did not reach the minimum threshold of 10 total reads. n = 8–9 per group. [B] Summarized AQP transcript counts [TC] in IECs, normalized to healthy control [Hc, n = 9]. Expression data are shown as mean ± SD of log2-transformed TC. n = 8–9 per group. [C] Representative confocal microscopy images of cross-sectioned epithelial glands stained for AQP8 protein [red] in FFPE sections of Hc and auCC colonic mucosa. Note that AQP8 is mainly located at the apical membrane of IECs, facing the intestinal lumen [magnified in the lower right corner]. Nuclei are shown in blue. [D] Analysis of AQP8 median fluorescence intensity, as determined by confocal microscopy [left, median with interquartile range]. Paired CC samples before [auCC] and during [itCC] successful treatment with budesonide are shown on the right. n = 14–15 per group. Statistically significant differences are shown as **p < 0.01. AQP, aquaporin; au, active untreated; aR, active/refractory; a.u., arbitrary units; CC, collagenous colitis; FFPE, formalin-fixed, paraffin-embedded; Hc, healthy controls; IEC, intestinal epithelial cell; it, inactive/treated; RNA-seq, RNA-sequencing; SD, standard deviation; TC, transcript counts.
3.5. AQP8 expression is directly regulated by steroid drugs and correlates with stool frequency in CC
Although CC is an inflammatory disorder, it lacks gross tissue ulceration; however, aberrant activation of T cells and eosinophils is thought to contribute to disease progression.1 Thus, the normalization of AQP levels after budesonide treatment may be an epiphenomenon of reduced tissue inflammation, rather than a direct regulatory process. To discern between these hypotheses, we investigated the effect of corticosteroids on AQP expression in model IEC cell lines grown in vitro, i.e. in the absence of inflammatory stimuli [Table 3; Supplementary Table 3]. Treatment of three different IEC lines [HCT116, HT-29 and Caco-2] with the clinically used steroid drugs budesonide and dexamethasone was sufficient to consistently increase AQP8 expression within 24 h, compared to vehicle control. In particular, corticosteroids significantly induced AQP8 expression in Caco-2 cells, which represent absorptive enterocytes.30 In contrast, corticosteroids decreased the expression of AQP0 and AQP1 in Caco-2 cells, and altered AQP2 expression in both HT-29 and Caco-2 cells [Supplementary Table 3]. Finally, we investigated if AQP expression in our CC patient cohorts was associated with stool frequency, the primary clinical variable in this disease. Indeed, AQP8, and to a lesser extent AQP11, mRNA levels were strongly negatively correlated with the frequency of bowel movements per day [Figure 4A, B]. In contrast, we observed no correlation between stool frequency and the levels of AQPs 1 and 7 [Figure 4C, D]. Previous studies have shown that corticosteroids increase water uptake in the gut.31 Our data suggest that steroid drugs directly and rapidly increase AQP8 levels in IECs, which may restore intestinal water homeostasis and thereby improve bowel frequency and consistency irrespective of additional anti-inflammatory steroid effects.
Table 3.
AQP8 mRNA expression profile in IEC monolayers after 1 µM corticosteroid treatment for 24 h; expression values are shown as median [interquartile range: Q1, Q3] of relative log2 fold-changes [2–ΔΔCt values] in gene expression
| Cell line | Control | Budesonide | Dexamethasone |
|---|---|---|---|
| HCT116 | 1.04 [0.93, 1.10] | 1.27 [1.15, 1.34] | 1.17 [1.16, 1.32] |
| HT-29 | 0.97 [0.93, 1.06] | 1.24 [0.99, 1.32] | 1.47 [1.13, 2.19] |
| Caco-2 | 0.94 [0.86, 1.16] | 2.54 [2.15, 2.69] * | 1.90 [1.84, 2.20] * |
Statistically significant differences related to unstimulated control cells are shown as *p < 0.05. AQP, aquaporin; IEC, intestinal epithelial cell; Q, quartile.
Figure 4.
High AQP8 mRNA expression is associated with lower frequency of bowel movements in CC. [A–D] Scatter plots showing the correlation of stool frequency per day with AQP8 [A], AQP11 [B], AQP1 [C] and AQP7 [D] log2-transformed fold-changes of the −ΔCt values [with HPRT1 as a housekeeping control], as determined by quantitative PCR, in all CC samples. Higher −ΔCt values indicate greater mRNA abundance. Spearman’s rho and the adjusted p-value [FDR-corrected] are shown in the graph. The trend-line indicates the linear regression model with 95% confidence interval [grey]. AQP, aquaporin; au: active/untreated, aR: active/refractory; CC, collagenous colitis; FDR, false discovery rate; it: inactive/treated, qPCR, quantitative PCR.
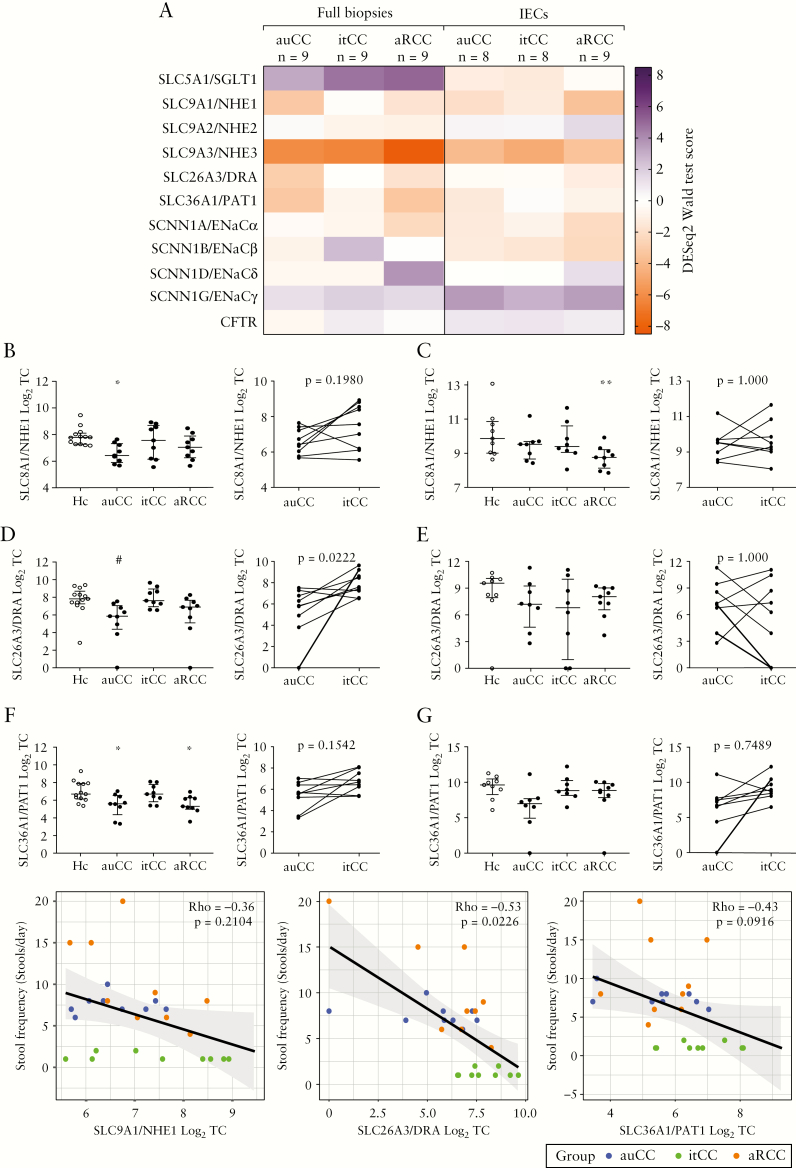
3.6. Expression of selected ion channels is modestly altered in CC
Defective ion transport, primarily driven by dysregulation of epithelial ion channels, has been posited as the primary cause of diarrhoea in CC.8,32 Therefore, we explored the regulation of major ion channels in RNA-seq data from our CC patient cohort [Figure 5A]. Among all investigated ion channels, only SLC9A1/NHE1, SLC26A3/DRA and SLC36A1/PAT1 exhibited a similar expression pattern to AQP8, i.e. their transcript levels were decreased in active CC and restored following budesonide treatment in both full biopsies and IEC isolates [Figure 5A–G]. Moreover, as with AQP8, SLC26A3/DRA and to a lesser extent SLC36A1/PAT1, transcript levels were negatively correlated with stool frequency [Figure 5H–J]. In contrast, other channel molecules implicated in diarrhoeal disorders, such as NHE2/3, ENaC α-γ and CFTR, were inconsistently changed in these samples and are thus unlikely to contribute to the pathogenesis of CC. In summary, these data suggest that dysregulation of epithelial ion transport may exacerbate diarrhoea in CC. We note, however, that the expression changes of SLC9A1/NHE1, SLC26A3/DRA and SLAC36A1/PAT1 were much more modest than those observed for AQP8, which argues against secondary secretory diarrhoea as the main pathomechanism in CC.
Figure 5.
Ion channel expression is modestly decreased in CC colonic mucosa. [A] Heatmap showing normalized, log2-transformed read counts [DESeq2 Wald statistic test scores] for the indicated channel genes in CC full biopsy samples and microdissected IECs. Samples were analysed by transcriptome-wide mRNA sequencing [RNA-seq]. SCNN1D did not reach the minimum threshold of 10 total reads in IEC samples [grey]. n = 8–13 per group. [B–G] Transcript counts [TC] of NHE1 [B, C], DRA [D, E], and PAT1 [F, G] in all CC groups [left, median with interquartile range], and paired CC samples before [auCC] and during [itCC] successful treatment with budesonide [right]. Panels B, D and F correspond to full biopsy RNA-seq data, and panels C, E and G to IEC RNA-seq data. Sample number for full biopsy RNA-seq was n = 13 for Hc, and n = 9 for the CC full biopsy cohort; and for IEC RNA-seq was n = 9 for Hc and aRCC, and n = 8 for auCC and itCC. [H–J] Scatter plots showing the correlation of stool frequency per day with NHE1 [H], DRA [I] and PAT1 [J] log2-transformed TC, as determined by RNA-seq, in all CC full biopsy samples. Spearman’s rho and the adjusted p-value [FDR-corrected] are shown in the graph. The trend-line indicates the linear regression model with 95% confidence interval [grey]. Statistically significant differences relative to Hc samples are shown as *p < 0.05 and **p < 0.01; statistically significant differences relative to itCC samples are shown as #p < 0.05. au, active/untreated; aR, active/refractory; CC, collagenous colitis; Hc, healthy controls; it, inactive/treated; RNA-seq, RNA-sequencing; TC, transcript counts.
4. Discussion
Chronic diarrhoea is a major indication for specialist referral in clinical practice, and affects up to 5% of the population at any given time.33 Management of watery diarrhoea in particular is challenging due to the unresolved physiology of intestinal water homeostasis. The current paradigm is that impaired water resorption is a secondary effect resulting from dysregulated ion exchange in the colonic epithelium.34,35 However, it is generally accepted that water is also taken up actively by intestinal epithelia, although the magnitude of this effect is unclear due to a lack of high-quality studies.11 It therefore remains to be determined whether altered transcellular water contributes to the pathobiology of diarrhoeal disorders.
We observed that clinical disease activity in the model diarrhoeal disorder CC specifically correlated with expression levels of AQP8, a major epithelial water channel. AQP8 is located in the apical membrane of intestinal epithelia, and exhibits increased protein levels in surface colonocytes.29 RNA interference studies in intestinal epithelia suggest that AQP8 may facilitate more than one-third of the total trans-epithelial water transport.29 In agreement with this hypothesis, exogenous expression of AQP8 in model cell lines dramatically increased their osmotic water permeability.36 Thus, loss of APQ8 expression, as observed in active CC, may severely impair water resorption and thereby stool formation. It should be noted, however, that AQP8-knockout mice do not exhibit spontaneous diarrhoea, possibly due to functional compensation by other AQPs in unchallenged animals.36
Reduced AQP8 expression has also been observed in IBDs, infectious colitis and diarrhoea-predominant irritable bowel syndrome [IBS],15,17,37 suggesting that loss of AQP8 may be a shared pathomechanism in gastrointestinal disorders. Indeed, Wang and Hou reported that decreased colonic AQP8 levels were associated with increased faecal water content in IBS patients,37 positing AQP8 as a key regulator of intestinal fluid resorption whose dysregulation drives malabsorptive diarrhoea. Consistently, we found that induction of clinical remission in CC patients led to a restoration of AQP8 expression to near-normal levels. This observation is in contrast to, for example ulcerative colitis, where mucosal AQP8 levels were inconsistent across studies and did not follow disease activity, possibly due to secondary, inflammation-induced changes in intestinal epithelia.16,38
In addition, we observed dysregulation of AQPs 1, 7 and 11 mRNA expression in CC, which followed disease activity. In contrast to AQP8, however, the biology and [patho]-physiological role of these AQPs is less well understood, and results were generally more variable across different assays. Reduced AQP1 and AQP7 expression has been observed in intestinal infection and inflammation,15,17,39 but was less pronounced in our CC cohort, and even increased in a study on IBS.40 AQP1 protein, which is primarily located in erythrocytes and might function as a CO2 channel,12 was also expressed in IECs, but remained unchanged in CC. On the other hand, AQP7 belongs to the aquaglyceroporin family of proteins, i.e. it transports glycerol and other molecules in addition to water.12 Similarly, loss of AQP11 expression has been observed in duodenal epithelia of coeliac disease, which was restored by induction of clinical remission.41 However, AQP11 exhibits poor water conductance compared to other AQPs, and is thought to contribute little to transcellular water transport.42 Moreover, our data indicate that these AQPs are expressed at considerably lower levels than AQP8 in intestinal epithelia. Impaired water homeostasis in diarrhoeal disorders may result from changes in single water channels (i.e. AQP8). We thus consider it unlikely that other water channels contribute significantly to impaired water resorption in diarrhoea.
Of note, Camilleri et al. recently reported an increase in AQP8 and AQP7 expression in IBS, which is at odds with our observations.40 Although we cannot fully explain this apparent discrepancy, we note that these authors examined the rectosigmoidal mucosa rather than the descending colon. In contrast to the colon proper, basal AQP8 and AQP7 levels are considerably lower in the sigma and rectum based on expression data from, for example, the Human Protein Atlas [https://www.proteinatlas.org/] and the Genotype-Tissue Expression [GTEx] project [https://www.gtexportal.org/home/], which may suggest a different mode of transcription regulation of these water channels in the distal intestine.
Steroid-refractory CC is a rare condition, but represents a major clinical challenge.1,19 We observed that decreased AQP8 mRNA expression in aRCC was not reflected in concomitantly altered AQP8 protein levels. Interestingly, similar inconsistencies between mRNA and protein levels have previously been observed in CC, especially for some cytokines and chemokines.43–45 It has been suggested that these observations may be explained by differential post-transcriptional modifications, including micro-RNA regulation, and that detection of proteins with a short half-life might prevent mRNA–protein correlation.44–46 Why this might be the case in steroid-refractory but not responsive patients is currently unclear. However, as with similar observations in treatment-resistant UC patients,47 an in-depth analysis of our RNA-seq data suggests that refractory CC is a transcriptionally distinct disease entity with potentially unique pathomechanisms [C.E.H. and A.M., manuscript in preparation]. Indeed, alternative therapies such as immunomodulators and anti-tumour necrosis factor [TNF]-α treatments suggest favourable outcomes in the management of aRCC, possibly due to a stronger immune reaction in these patients.1
In summary, our results suggest that dysregulated transcellular water transport, specifically a loss of AQP8 in intestinal epithelia, is a causative mechanism in chronic diarrhoea which can be termed ‘malabsorptive’. We note that this model is compatible with the current paradigm of secondary fluid loss due to altered osmoregulation in the gut,34,35 and may explain the massive, rapid changes in stool frequency and consistency in disorders such as CC. Indeed, our own data indicate changes in the expression of some ion channels in CC [e.g. SLC26A3/DRA], indicating that multiple disease processes occur in parallel with potentially additive effects.48 However, further investigation is clearly warranted to substantiate this conclusion. Nonetheless, we believe that direct targeting of water channels, particularly AQP8, may aid in the clinical management of common diarrhoeal diseases.
Supplementary Material
Acknowledgments
We thank Lena Svensson for support with collecting human samples, and Dr Vesa Loitto and the Microscopy Unit at Linköping University’s core facilities for their technical support. We also thank Arnar Flatberg at the Genomics Core Facility (GCF) at the Norwegian University of Science and Technology (NTNU), where the gene expression analysis of RNA sequencing data and bioinformatics analysis was performed. GCF is funded by the Faculty of Medicine and Health Sciences at NTNU, and the Central Norway Regional Health Authority. We are grateful to all investigators who have made materials and data available through public repositories, and volunteer patients who agreed to participate in the study.
Funding
This work was supported by grants from Ferring Pharmaceuticals [Switzerland]; ALF [Region Östergötland, Sweden]; the Magtarmfonden [Swedish Society of Gastroenterolgy]; the Mucosal Infection and Inflammation Centre [MIIC, Linköping University] [postdoctoral fellowship to C.E.H.]; Norwegian Research Council [Norway]; Liaison Committee between St Olav’s University Hospital and the Faculty of Medicine and Health Sciences at NTNU [Norway] and the Knut and Alice Wallenberg Foundation [KAW, Sweden] [grant to S.K.]. These institutions had no role in study design, data collection and analysis, or manuscript preparation. Ferring Pharmaceuticals reviewed and approved the manuscript.
Conflicts of Interest
C.E.H., S.K. and A.M. received financial support from Ferring Pharmaceuticals [Switzerland]. A.M. has received salary for consultancies from Tillotts, Ferring, Vifor and Dr Falk Pharma, and speaker’s honoraria from Tillotts and Vifor.
Author Contributions
C.E.H. designed, performed and analysed most experiments. S.K. analysed publicly available data. A.B.G. contributed to design and analysis of LCM and RNA-seq experiments. S.K., A.E.Ø. and A.M. conceived, and S.K. and A.M. supervised the study. A.M. and A.E.Ø. enrolled and sampled patients and volunteers that participated in the study. C.E.H. and S.K. wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
References
- 1. Miehlke S, Verhaegh B, Tontini GE, Madisch A, Langner C, Münch A. Microscopic colitis: pathophysiology and clinical management. Lancet Gastroenterol Hepatol 2019;4:305–14. [DOI] [PubMed] [Google Scholar]
- 2. Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med 2016;9:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Münch A, Bohr J, Miehlke S, et al.; BUC-63 investigators Low-dose budesonide for maintenance of clinical remission in collagenous colitis: a randomised, placebo-controlled, 12-month trial. Gut 2016;65:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pardi DS. After budesonide, what next for collagenous colitis? Gut 2009;58:3–4. [DOI] [PubMed] [Google Scholar]
- 5. Tripathi K, Dunzendorfer T. Budesonide-related iatrogenic Cushing’s syndrome in microscopic colitis. ACG Case Rep J 2017;4:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camilleri M, Sellin JH, Barrett KE. Pathophysiology, evaluation, and management of chronic watery diarrhea. Gastroenterology 2017;152:515–32 e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 2003;111:931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology 2002;123:433–43. [DOI] [PubMed] [Google Scholar]
- 9. Protic M, Jojic N, Bojic D, et al.. Mechanism of diarrhea in microscopic colitis. World J Gastroenterol 2005;11:5535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laforenza U. Water channel proteins in the gastrointestinal tract. Mol Aspects Med 2012;33:642–50. [DOI] [PubMed] [Google Scholar]
- 11. Masyuk AI, Marinelli RA, LaRusso NF. Water transport by epithelia of the digestive tract. Gastroenterology 2002;122:545–62. [DOI] [PubMed] [Google Scholar]
- 12. Day RE, Kitchen P, Owen DS, et al.. Human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta 2014;1840:1492–506. [DOI] [PubMed] [Google Scholar]
- 13. Zhu C, Chen Z, Jiang Z. Expression, distribution and role of aquaporin water channels in human and animal stomach and intestines. Int J Mol Sci 2016;17:1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Te Velde AA, Pronk I, de Kort F, Stokkers PC. Glutathione peroxidase 2 and aquaporin 8 as new markers for colonic inflammation in experimental colitis and inflammatory bowel diseases: an important role for H2O2? Eur J Gastroenterol Hepatol 2008;20:555–60. [DOI] [PubMed] [Google Scholar]
- 15. Ricanek P, Lunde LK, Frye SA, et al.. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin Exp Gastroenterol 2015;8:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Planell N, Lozano JJ, Mora-Buch R, et al.. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut 2013;62:967–76. [DOI] [PubMed] [Google Scholar]
- 17. Hardin JA, Wallace LE, Wong JF, et al.. Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn’s disease and infectious colitis. Cell Tissue Res 2004;318:313–23. [DOI] [PubMed] [Google Scholar]
- 18. Münch A, Aust D, Bohr J, et al.; European Microscopic Colitis Group [EMCG] Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis 2012;6:932–45. [DOI] [PubMed] [Google Scholar]
- 19. Münch A, Langner C. Microscopic colitis: clinical and pathologic perspectives. Clin Gastroenterol Hepatol 2015;13:228–36. [DOI] [PubMed] [Google Scholar]
- 20. Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 2007;35:W71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uhlén M, Fagerberg L, Hallström BM, et al.. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 22. Barmeyer C, Erko I, Fromm A, et al.. ENaC dysregulation through activation of MEK1/2 contributes to impaired Na+ absorption in lymphocytic colitis. Inflamm Bowel Dis 2016;22:539–47. [DOI] [PubMed] [Google Scholar]
- 23. Collado-Torres L, Nellore A, Kammers K, et al.. Reproducible RNA-seq analysis using recount2. Nat Biotechnol 2017;35:319–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varet H, Brillet-Guéguen L, Coppée JY, Dillies MA. SARTools: A DESeq2- and EdgeR-based R pipeline for comprehensive differential analysis of RNA-seq data. PLoS One 2016;11:e0157022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thorsvik S, van Beelen Granlund A, Svendsen TD, et al. Ulcer-associated cell lineage expresses genes involved in regeneration and is hallmarked by high neutrophil gelatinase-associated lipocalin (ngal) levels. J Pathol 2019;248:316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ikarashi N, Kon R, Sugiyama K. Aquaporins in the colon as a new therapeutic target in diarrhea and constipation. Int J Mol Sci 2016;17:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laforenza U, Cova E, Gastaldi G, et al.. Aquaporin-8 is involved in water transport in isolated superficial colonocytes from rat proximal colon. J Nutr 2005;135:2329–36. [DOI] [PubMed] [Google Scholar]
- 30. Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989;96:736–49. [PubMed] [Google Scholar]
- 31. Black HE. The effects of steroids upon the gastrointestinal tract. Toxicol Pathol 1988;16:213–22. [DOI] [PubMed] [Google Scholar]
- 32. Barmeyer C, Erko I, Fromm A, et al.. Ion transport and barrier function are disturbed in microscopic colitis. Ann N Y Acad Sci 2012;1258:143–8. [DOI] [PubMed] [Google Scholar]
- 33. Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: diagnosis and management. Clin Gastroenterol Hepatol 2017;15:182–193.e3. [DOI] [PubMed] [Google Scholar]
- 34. Schulzke JD, Siegmund B, Günzel D. New insights into intestinal secretion. Gut 2014;63:1371–2. [DOI] [PubMed] [Google Scholar]
- 35. Rao MC. Physiology of electrolyte transport in the gut: implications for disease. Compr Physiol 2019;9:947–1023. [DOI] [PubMed] [Google Scholar]
- 36. Yang B, Zhao D, Solenov E, Verkman AS. Evidence from knockout mice against physiologically significant aquaporin 8-facilitated ammonia transport. Am J Physiol Cell Physiol 2006;291:C417–23. [DOI] [PubMed] [Google Scholar]
- 37. Wang JP, Hou XH. Expression of aquaporin 8 in colonic epithelium with diarrhoea-predominant irritable bowel syndrome. Chin Med J (Engl) 2007;120:313–6. [PubMed] [Google Scholar]
- 38. Zahn A, Moehle C, Langmann T, et al.. Aquaporin-8 expression is reduced in ileum and induced in colon of patients with ulcerative colitis. World J Gastroenterol 2007;13:1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taman H, Fenton CG, Hensel IV, Anderssen E, Florholmen J, Paulssen RH. Transcriptomic landscape of treatment-naïve ulcerative colitis. J Crohns Colitis 2018;12:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Camilleri M, Carlson P, Chedid V, Vijayvargiya P, Burton D, Busciglio I. Aquaporin expression in colonic mucosal biopsies from irritable bowel syndrome with diarrhea. Clin Transl Gastroenterol 2019;10:e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laforenza U, Miceli E, Gastaldi G, et al.. Solute transporters and aquaporins are impaired in celiac disease. Biol Cell 2010;102:457–67. [DOI] [PubMed] [Google Scholar]
- 42. Yakata K, Tani K, Fujiyoshi Y. Water permeability and characterization of aquaporin-11. J Struct Biol 2011;174:315–20. [DOI] [PubMed] [Google Scholar]
- 43. Kumawat AK, Strid H, Tysk C, Bohr J, Hörnquist EH. Microscopic colitis patients demonstrate a mixed Th17/Tc17 and Th1/Tc1 mucosal cytokine profile. Mol Immunol 2013;55:355–64. [DOI] [PubMed] [Google Scholar]
- 44. Günaltay S, Kumawat AK, Nyhlin N, et al.. Enhanced levels of chemokines and their receptors in the colon of microscopic colitis patients indicate mixed immune cell recruitment. Mediators Inflamm 2015;2015:132458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carrasco A, Esteve M, Salas A, et al.. Immunological differences between lymphocytic and collagenous colitis. J Crohns Colitis 2016;10:1055–66. [DOI] [PubMed] [Google Scholar]
- 46. Zhang C, Zhao Z, Osman H, Watson R, Nalbantoglu I, Lin J. Differential expression of miR-31 between inflammatory bowel disease and microscopic colitis. Microrna 2014;3:155–9. [DOI] [PubMed] [Google Scholar]
- 47. Haberman Y, Karns R, Dexheimer PJ, et al.. Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanisms underlying disease severity and treatment response. Nat Commun 2019;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fuller PJ, Brennan FE, Burgess JS. Acute differential regulation by corticosteroids of epithelial sodium channel subunit and Nedd4 mRNA levels in the distal colon. Pflugers Arch 2000;441:94–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.