Abstract
Aims
CERAMENT|G is an absorbable gentamicin-loaded biocomposite used as an on-site vehicle of antimicrobials for the treatment of chronic osteomyelitis. The purpose of the present study was to investigate the sole effect of CERAMENT|G, i.e. without additional systemic antimicrobial therapy, in relation to a limited or extensive debridement of osteomyelitis lesions in a porcine model.
Methods
Osteomyelitis was induced in nine pigs by inoculation of 104 colony-forming units (CFUs) of Staphylococcus aureus into a drill hole in the right tibia. After one week, the pigs were allocated into three groups. Group A (n = 3) received no treatment during the study period (19 days). Groups B (n = 3) and C (n = 3) received limited or extensive debridement seven days postinoculation, respectively, followed by injection of CERAMENT|G into the bone voids. The pigs were euthanized ten (Group C) and 12 (Group B) days after the intervention.
Results
All animals presented confirmatory signs of bone infection post-mortem. The estimated amount of inflammation was substantially greater in Groups A and B compared to Group C. In both Groups B and C, peptide nucleic acid fluorescence in situ hybridization (PNA FISH) of CERAMENT|G and surrounding bone tissue revealed bacteria embedded in an opaque matrix, i.e. within biofilm. In addition, in Group C, the maximal measured post-mortem gentamicin concentrations in CERAMENT|G and surrounding bone tissue samples were 16.6 μg/ml and 6.2 μg/ml, respectively.
Conclusion
The present study demonstrates that CERAMENT|G cannot be used as a standalone alternative to extensive debridement or be used without the addition of systemic antimicrobials.
Cite this article: Bone Joint Res 2020;9(7):394–401.
Keywords: Osteomyelitis, Animal model, Antimicrobial carrier, Calcium sulphate, Hydroxyapatite
Article focus
This study aimed to describe the sole effect of CERAMENT|G in relation to a limited or extensive debridement of osteomyelitis lesions in a porcine model.
Key messages
The estimated amount of infection and inflammation was substantially reduced for extensive debridement and application of CERAMENT|G.
Despite type of debridement, biofilm formation was found around and inside CERAMENT|G ten to 12 days after revision surgery.
Strengths and limitations
This is the first study to describe the penetration of CERAMENT|G into the surrounding bone tissue.
The present study is a descriptive study and, therefore, a restricted number of animals were included.
Introduction
Bacterial contamination of implants and bone tissue is a serious complication in orthopaedic surgery and may result in development of bone infections.1 The most common causative bacteria are Staphylococcus aureus and Staphylococcus epidermidis, which are known for their ability to form biofilms.2 Bacteria living in biofilms show a high degree of tolerance towards the host immune system and antimicrobials.3 Therefore, treatment of bone infections comprises a combination of both surgical debridement and long-term antimicrobial therapy, usually based on a multidisciplinary treatment protocol.4-6
Local antimicrobial carriers have evolved as a promising tool for treatment of orthopaedic infections both as bone void fillers and by delivering high antimicrobial concentrations at the surgical site.7,8 The ideal antimicrobial-loaded bone void filler possesses properties such as biodegradability, biocompatibility, providing an osteoconductive and osteoinductive scaffold for healing, and elution of high levels of antimicrobials.6,9,10 CERAMENT|G (BoneSupport AB, Lund, Sweden) is an injectable bone void filler composed of 60 wt% calcium sulphate and 40 wt% calcium hydroxyapatite (HA).9-11 Calcium sulphate (CS) is resorbed quickly and is thereby quickly replaced by newly formed osteoid and is effective at delivering high levels of antimicrobials.6,11 CERAMENT|G contains 175 mg gentamicin per 10 ml paste. The biodegradability of the product allows single-stage surgery and has previously been demonstrated to be highly efficient for treatment of chronic bone infections when combined with debridement and systemic antimicrobials.9,11,12 In relation to a limited or extensive debridement of osteomyelitis lesions in a porcine model, the purpose of the present study was to elucidate the histopathological and microbiological impact of CERAMENT|G when used without simultaneously systemic antimicrobial treatment.
Methods
Experiment design
The experimental protocol was approved by The Danish Animal Experiments Inspectorate (license no. 2013/15-2934-00946). A well-established and characterized porcine model of implant-associated osteomyelitis was used.13 The present study is descriptive and, therefore, a restricted number of experimental animals were included. To establish an infection, a small steel implant was inserted into the right tibial bone together with S. aureus bacteria. One week after surgery, the pigs were allocated into three groups (Table I). Group A (n = 3) received no treatment during the study period (19 days). One pig from this group (A1) was euthanized after one week to confirm osteomyelitis at the time of revision surgery. Group B (n = 3) and Group C (n = 3) animals received revision surgery seven days after inoculation with either limited or extensive debridement, respectively, followed by filling of the bone void with CERAMENT|G. None of the animals were treated systemically with antimicrobials.
Table I.
Experimental design.
| Group | Animals | Inoculum | Revision surgery 7 days after inoculation | Time of euthanasia PI |
|---|---|---|---|---|
| A | A1 | S. aureus (104 CFUs) | No revision | 7 days |
| A2, A3 | S. aureus (104 CFUs) | No revision | 19 days | |
| B | B1, B2, B3 | S. aureus (104 CFUs) | Limited debridement+ CERAMENT|G | 19 days, 4 days (B3) |
| C | C1, C2, C3 | S. aureus (104 CFUs) | Extensive debridement+ CERAMENT|G | 17 days |
CFU, colony forming unit; PI, postinoculation; S. aureus, Staphylococcus aureus.
Inoculum
A high-virulence gentamicin-sensitive S. aureus strain S54F9 (spa-type t1333), originally isolated from a chronic porcine lung abscess, was used to induce infection.14 This strain has been characterized by whole-genome sequencing,15 and has the ability to form biofilm both in vitro and in vivo.16 The final inoculum was 104 colony-forming units (CFUs) in 10 μl.
CERAMENT|G
An injectable ceramic bone graft substitute (CERAMENT|G, PART NO: A0450-01, LOT: MLOT0599 and MLOT0621), was supplied by BoneSupport. Each CERAMENT|G syringe included 10 ml paste and one syringe was used for each animal. Approximately 1 ml to 2 ml of CERAMENT|G was administrated in Group B (limited debridement) and 4 ml to 6 ml in Group C (extensive debridement), respectively.
Surgical procedure and inoculation
A skin incision down to the periosteum was made 10 mm distal to and parallel with the growth plate of the right proximal tibia.13 An additional incision was made through the periosteum. An implant cavity (IC) was created by drilling a Kirschner wire (K-wire; 4 mm in diameter) 20 mm into the trabecular bone tissue at the site of the periosteal incision.13 Afterwards, the inoculum was injected before insertion of a steel implant (K-wire of 2 mm × 10 mm). Finally, the periosteum, subcutaneous tissue, and skin were closed separately.13
Intervention (revision surgery)
The infection was allowed to progress for one week before intervention (Groups B and C). Each animal received an intramuscular injection of 6.84 mg/kg gentamicin (Genta-Equine, 100 mg/ml; Dechra Veterinary Products SLU, Barcelona, Spain) prior to surgery in order to avoid development of surgery-related sepsis. Through the scar of the first surgery, the wound was opened. Soft-tissue abscesses were emptied and the IC was located. The implants were removed and placed in centrifuge tubes containing 2 ml sterile isotonic saline (0.9%). Implants and swabs from IC were sampled for microbiological evaluation. Limited debridement was performed in Group B animals. Limited debridement involved curettage of membranes and necrotic bone tissue followed by rinsing of the bone void and subcutaneous tissue with isotonic sterile saline (0.9%). Extensive debridement (Group C) included complete removal of subcutaneous granulation tissue followed by curettage and excision of the necrotic bone and membranes until pint point bleedings from healthy bone tissue were present. Afterwards, the bone void and subcutaneous tissue were irrigated with isotonic sterile saline (0.9%). The definitions of debridement, i.e. limited or extensive, were defined in the protocol before revision surgery by the research team. Samples of the debrided tissue (bone and subcutaneous tissue) were saved for microbiological evaluation. After debridement (limited or extensive), the bone void was filled with CERAMENT|G. Finally, the periosteum, subcutaneous tissue, and skin were closed separately. The pigs were euthanized ten (Group C) or 12 (Group B) days after the intervention by an intravenous overdose of pentobarbital (Euthanimal, 365 mg/ml; ScanVet Animal Health A/S, Fredensborg, Denmark). All animals received daily oral analgesia with meloxicam (0.3 mg/kg BW, Metacam; Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany) and were monitored as described by Jensen et al13
CT scanning
After euthanasia, the right hind leg was scanned with a single slide CT scanner (Siemens Somatom Emotion; Siemens, Erlangen, Germany) to assess the development of osteomyelitis. Tibia was scanned in the craniocaudal direction (slide thickness = 2 mm, kV = 130, and mAs = 55).13 A standard soft tissue algorithm (B80) was used. All scans were evaluated with Osirix Lite (OsiriX, Bernex, Switzerland).13
Pathology
The surgical wounds were macroscopically evaluated and opened down to the bone. The implants from animals in Group A were removed and placed in centrifuge tubes containing 2 ml sterile isotonic saline (0.9%). In all animals, the right tibia was sagitally sectioned and macroscopically evaluated before being placed in 10% neutral formalin buffer for approximately two weeks. Formalin fixation of the tibial bones was followed by decalcification. The bone lesions were cut into representative pieces and embedded in paraffin. Tissue sections of 4 μm to 5 μm were stained with haematoxylin and eosin (H&E), Masson’s trichome, and immunohistochemistry (IHC) towards S. aureus.14 Samples from Group C were also stained with a biofilm-specific stain based on a combination of IHC towards S. aureus and Alcian blue pH3.16 In all groups, the bone voids were localized and the pathological bone area (PBA) was measured.13 PBA was defined as the maximum perpendicular distance from the edge of the bone void (the IC in Group A) and until normal pattern of trabecular bone and marrow.13 The result was determined as the mean of two or more measurements in one histological section. Based on a method developed by Morawietz et al,17 the number of neutrophil granulocytes (NGs) and osteoclasts/giant cells were counted within PBA. A maximum of ten NGs or osteoclasts/giant cells were counted in ten high power fields resulting in a maximum count of 100 per pig.17 To quantify the amount of collagen in PBA, a method described by Street et al18 was used on the Masson’s trichome-stained sections. Areas rich in collagen were selected and using a percentage value, the amount of collagen in each field was scored (< 5%, 5% to 10%, 11% to 25%, 26% to 50%, 51% to 75%, and 76% to 100%). These percentages were converted into a six-point scoring system, and a final mean score was calculated. Finally, slides were evaluated for bacterial/biofilm aggregates with IHC.13 To increase validation, all histological evaluations were carried out blinded.
Microbiology
Implants within sterile centrifuge tubes were placed in an ultrasonic bath and sonicated as previously described.19 After euthanasia, swabs were taken from the following areas: IC of Group A, the centre of CERAMENT|G, the bone surface towards CERAMENT|G, and subcutaneous tissue. In addition, bone samples and samples of CERAMENT|G were collected. All swabs and samples (including samples obtained at revision surgery) were smeared over Luria-Bertani agar plates and incubated at 37 °C for 24 hours. Furthermore, samples from the lungs were also obtained in order to analyze for systemic bacterial spreading. Any growth on the plates was spa-typed.20 Evaluations of swabs, bone samples, spa-typing, and sonication were performed blinded.
Peptide nucleic acid fluorescence in situ hybridization
Bone lesions from pigs receiving CERAMENT|G (Groups B and C) were selected for peptic nucleic acid fluorescence in situ hybridization (PNA FISH) analysis as described by Bay et al21 In brief, sections were stained with TexasRed-labelled S. aureus-specific PNA probes (Advandx, Woburn, Massachusetts, USA) and counter-stained with a 4',6-diamidino-2-phenylindol (DAPI) DNA stain solution. Sections were imaged using a Zeiss LSM 880 (Zeiss, Oberkochen, Germany) running ZEN 2.1. Images were obtained using a 63 × /1.4 oil objective and 405 nm and 594 nm lasers with an emission band of 410 nm to 465 nm and 600 nm to 660 nm. Images were processed in Imaris 8.5 (Bitplane, Zürich, Switzerland).
Quantification of total homogenate gentamicin concentrations
From Group C pigs, small pieces of CERAMENT|G located in subcutaneous tissue and inside the bone were sampled together with adjacent bone tissue located distally, medially, and proximally to CERAMENT|G. All samples were kept at -80 °C until analysis. During preparation, sample storage was kept in liquid nitrogen and all equipment kept cold. The samples were weighed, and sterile 0.9% sodium chloride (NaCl) 2 to 10× weight volume was added. The material was homogenized using a Retsch titanium ball three times in succession for 30 seconds with a frequency of 30 times per second. Then, the samples were spun for 1,000× g for 30 seconds, before being incubated at 4 °C for 24 hours. Before the supernatant was transferred to a new vial for analysis, the samples were spun at 5,000× g for five minutes. The gentamicin concentrations in the supernatants were quantified using liquid chromatography tandem mass spectrometry.22 The lower limit for quantitation was defined as the lowest concentration with a total CV of 20% and was found to be 0.04 μg/ml.
Results
Apart from one pig (B3, limited debridement), all animals completed the study. Pig B3 was euthanized four days after revision surgery due to fever and apathy. Based on the necropsy, the pig was diagnosed with chronic, apostematous disseminated pneumonia and pericarditis.
CT scanning
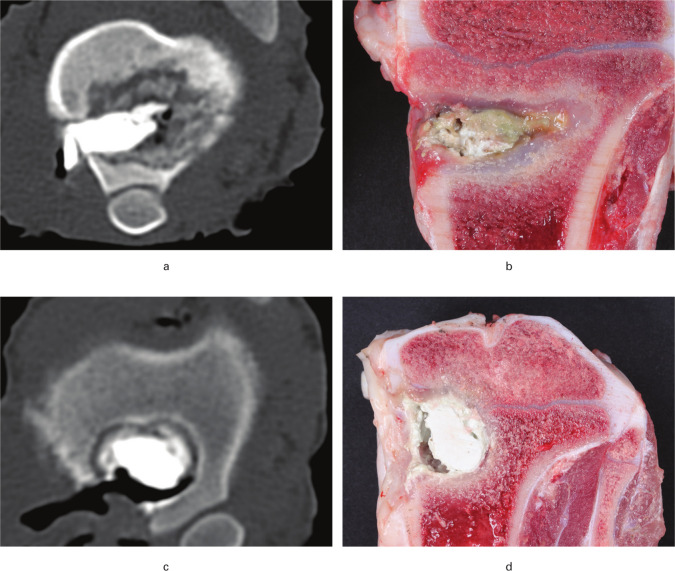
CT was not performed for pig A1. Signs of osteomyelitis like osteolysis and periosteal bone formation were seen in all animals (Table II and Figures 1a and 1c). In general, the bone lesions in animals receiving limited debridement (Group B) were irregular, with cortical sequesters and with no clear sclerotic border (Figure 1a). In contrast, the pigs from Group C demonstrated CERAMENT|G surrounded by a regular sclerotic border. However, in pig C3 the cavity with CERAMENT|G was communicating with a small osteolytic lesion without a sclerotic border.
Table II.
Results of the CT scans, macroscopic pathology, and gentamicin concentrations ten (Group C animals) and 12 (Group B animals) days after revision surgery in a porcine bone infection model. No revision surgery was performed for Group A. Revision surgery, Group B = limited debridement+ CERAMENT|G (CG). Revision surgery, Group C = extensive debridement+ CG.
| Animal | CT | Macroscopic pathology | Gentamicin concentration, μg/ml | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Osteolysis | Cortical sequester | Periosteal ossification | Sclerotic border | Sinus tract | Purulent osteomyelitis | CG - inside bone | CG - subcutis | Bone tissue distal to CG | Bone tissue proximal to CG | Bone tissue in level with CG | |
| A1* | N/A | N/A | N/A | N/A | Yes | Yes | N/A | N/A | N/A | N/A | N/A |
| A2† | Yes | No | Yes | Yes | Yes | Yes | N/A | N/A | N/A | N/A | N/A |
| A3† | Yes | No | Yes | No | Yes | Yes | N/A | N/A | N/A | N/A | N/A |
| B1 | Yes | Yes | Yes | Partly | Yes | Yes | N/A | N/A | N/A | N/A | N/A |
| B2 | Yes | No | Yes | Partly | Yes | Yes | N/A | N/A | N/A | N/A | N/A |
| B3‡ | Yes | Yes | Yes | Partly | Yes | Yes | N/A | N/A | N/A | N/A | N/A |
| C1 | Yes | No | Yes | Yes | Yes | Yes | 14.550 | 16.600 | 1.570 | 2.710 | 5.260 |
| C2 | Yes | No | Yes | Yes | Yes | Yes | 13.950 | 1.090 | 0.681 | 0.465 | 4.660 |
| C3§ | Yes | No | Yes | Partly | Yes | Yes | 1.420 | 6.240 | 0.704 | 0.653 | 0.533 |
Pig euthanized seven days after inoculation.
Pig euthanized 19 days after inoculation.
Pig euthanized four days after revision surgery.
Pig with unrevised bone lesion, thereby representing limited debridement/Group B.
CG, CERAMENT|G; N/A, not available.
Fig. 1.
CT scans (cranio-caudal direction) and macroscopic images obtained ten and 12 days after revision surgery in a porcine model of Staphylococcus aureus osteomyelitis. Pigs were treated with either limited debridement (A + B) or extensive debridement (C + D) followed by injection of CERAMENT|G into the bone voids. a) Osteomyelitis with osteolysis and irregular borders of the lesion. b) CERAMENT|G surrounded by pus and fibrosis. c) Regular sclerotic border of the bone void. d) CERAMENT|G surrounded by a rim of fibrosis.
Bone pathology
All Group A pigs had a sinus tract draining the bone lesions. The bone lesion in pig A1 showed bone necrosis, pus, and fibrosis surrounding an enlarged and irregular IC. Comparable lesions were seen in pigs A2 and A3, although additional massive periosteal bone formation was observed around the cortical bone void.
The extraosseous findings in animals B1 and B2 were comparable to those in Group A pigs. In pig B3 (euthanized four days after minor revision surgery), the subcutaneous tissue over the bone lesion was found to be necrotic. In all Group B pigs a white/grey substance, assumed to be CERAMENT|G, intermingled with yellow exudation appeared within the subcutaneous tissue adjacent to the filled bone void. Following sagittal sectioning of the tibial bones, Group B animals demonstrated CERAMENT|G surrounded by pus and fibrosis (Figure 1b). In cases of extensive debridement (animals C1 and C2) the extraosseous and osseous macroscopic findings were comparable to limited debridement, although with remarkably less periosteal bone formation, pus, and fibrosis around CERAMENT|G (Figure 1d). In pig C3, most of the CERAMENT|G was located outside the bone, i.e. in the subcutaneous tissue. Furthermore, pig C3 had a purulent bone lesion communicating with the CERAMENT|G-filled bone void.
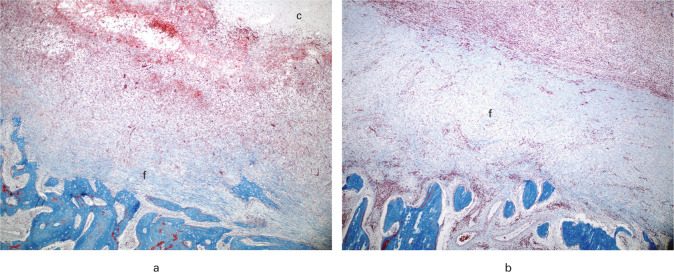
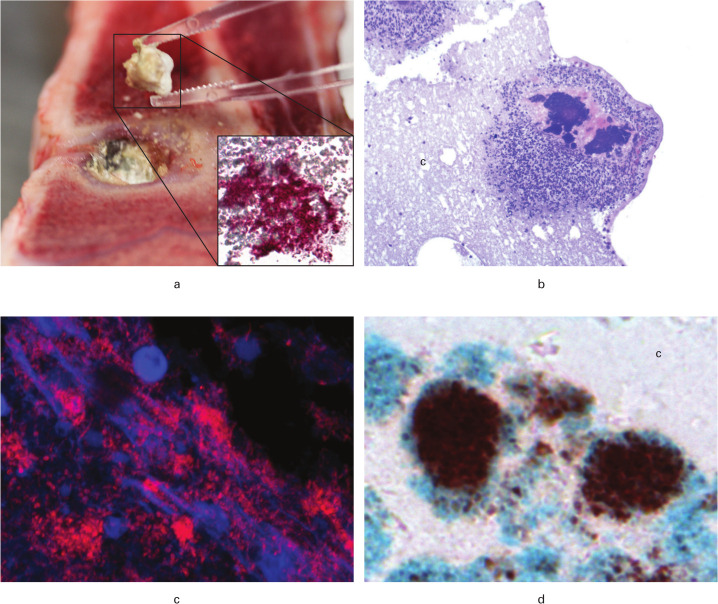
Histopathologically, all bone lesions showed necrotic bone tissue and cell debris adjacent to the CERAMENT|G filled bone voids or IC, intermingled/surrounded by a cellular layer of NG, mononuclear cells, giant cells, and fibroblasts (granulation tissue and/or connective tissue). Furthermore, areas of newly formed osteoid and osteoclasts were occasionally seen outside this cellular layer. All animals had a NG count of 100 and commonly, microabscesses (dense focal accumulation of neutrophils) were seen (Table III). No differences in osteoclast/giant cell count were observed between the three groups. Trabecular sequesters were only observed in Groups A and B (Table III). The distance of bone pathology (PBA) surrounding the CERAMENT|G-filled bone voids was enlarged in Group B compared to Group C (Table III). In addition, a more manifest fibrotic response was also seen in Group B (Table III, Figures 2a and 2b). The localization of bacteria is reported in Table III and demonstrated in Figures 3a to 3d. In Group C animals, positive biofilm double staining of matrix and bacterial cells was present (Figure 3d).
Table III.
Results of histology ten (Group C animals) and 12 (Group B animals) days after revision surgery in a porcine bone infection model. No revision surgery was done for Group A. Treatment Group B = limited debridement+ CERAMENT|G (CG). Treatment Group C = extensive debridement+ CG.
| Animal | Histology | PNA FISH/IHC positive bacteria within | ||||||
|---|---|---|---|---|---|---|---|---|
| Trabecular sequester | NG, n | Micro-abscesses | Fibrosis score | Mean PBA, μm (SD) | CERAMENT|G (CG) | Adjacent bone to CG | Distant located to CG | |
| A1* | Yes | 100 | Yes | 5 | 1385.84 (477.31) | N/A | N/A | Yes |
| A2† | No | 100 | Yes | 5 | 2132.83 (2018.26) | N/A | N/A | Yes |
| A3† | Yes | 100 | Yes | 4.4 | 1478.69 (1085.54) | N/A | N/A | Yes |
| B1 | Yes | 100 | Yes | 4.3 | 1424.84 (433.32) | Yes | Yes | Yes |
| B2 | Yes | 100 | Yes | 5 | 1851.9 (913.69) | Yes | Yes | Yes |
| B3‡ | No | 100 | Yes | 4.8 | 1372.83 (580.3) | Yes | Yes | Yes |
| C1 | No | 100 | No | 3.9 | 841.9 (348.97) | Yes | Yes | No |
| C2 | No | 100 | Yes | 3.6 | 889.63 (427.64) | Yes | Yes | No |
| C3§ | No | 100 | Yes | 5 | 2586.68 (1129.42) | Yes | Yes | No |
Pig euthanized seven days after inoculation.
Pig euthanized 19 days after inoculation.
Pig euthanized four days after revision surgery.
Pig with unrevised bone lesion, thereby representing limited debridement/Group B.
IHC, immunohistochemistry; N/A, not applicable; NG, neutrophil granulocyte; PBA, pathological bone area; PNA FISH, peptide nucleic acid fluorescence in situ hybridization.
Fig. 2.
Histological findings within the pathological bone area (PBA) ten and 12 days after revision surgery in a porcine model of Staphylococcus aureus osteomyelitis. Pigs were treated with either a) extensive debridement or b) limited debridement followed by injection of CERAMENT|G into the bone voids. Fibrous tissue was located adjacent to the border of the bone void. A stronger fibrotic response was seen in the pig receiving limited debridement compared to the pig receiving extensive debridement. Masson’s trichome stain, magnification × 40. c, CERAMENT|G; f, fibrous tissue.
Fig. 3.
Visualization of bacteria ten and 12 days after revision surgery in a porcine model of Staphylococcus aureus osteomyelitis. Pigs were treated with either limited (A + C) or extensive (B + D) debridement followed by injection of CERAMENT|G into the bone voids. a) Macroscopic picture showing removal of CERAMENT|G. Insert: Immunohistochemistry (IHC) towards S. aureus of the CERAMENT|G removed in Figure 3a; red bacteria are demonstrated here (× 200). b) Bone void border showing bacterial aggregates surrounded by pink matrix and neutrophil granulocytes inside CERAMENT|G; haematoxylin and eosin (HE) stain (× 100). c) Peptide nucleic acid fluorescence in situ hybridization (PNA FISH) of CERAMENT|G showing red aggregates of S. aureus (× 630). d) Biofilm forming S. aureus in CERAMENT|G; red bacteria surrounded by a blue matrix is seen here, image produced with IHC combined with Alcian blue pH3 (× 400). c, CERAMENT|G.
Microbiology
All located implants were positive for bacterial attachment and the mean bacterial load was 105 CFUs/ml. All swabs and bone samples taken at the time of revision surgery and euthanasia were positive for S. aureus. Furthermore, all CERAMENT|G samples and bone samples surrounding CERAMENT|G were positive for S. aureus in both Groups B and C. In addition, S. aureus was observed in the lung tissue of one pig (Group A1). All S. aureus positive samples were confirmed to contain S. aureus spa type t1333, i.e. identical to the spa type used for inoculation.
PNA FISH
All bone lesions from Groups B and C were positive for S. aureus by PNA FISH (Table III). The bacteria were present as single cells as well as small aggregates of cocci embedded in an opaque matrix, indicating biofilm formation.
Gentamicin concentrations
The gentamicin concentrations are reported in Table II. The maximal measured gentamicin concentrations of CERAMENT|G samples located in subcutaneous tissue and bone voids were 16.6 μg/ml and 14.5 μg/ml, respectively. However, in pig C3, which had a small purulent lesion communicating with the bone void of revision surgery, all the CERAMENT|G gentamicin concentrations were below 6.2 μg/ml (Table II).
Discussion
Local administration of CERAMENT|G, without additional systemic antimicrobial therapy, could not eradicate infection following either limited or extensive debridement in a porcine osteomyelitis model inoculated with a high-virulence gentamicin-susceptible S. aureus strain. In both groups of debridement, the inoculated strain could be isolated from CERAMENT|G and surrounding bone tissue. Moreover, biofilm formation was evident due to bacteria in clusters surrounded by extracellular matrix and persistent infection despite exposure to an antimicrobial agent to which the causative organism was sensitive.23 However, the amount of inflammation, or expansion of lesions into the bone tissue surrounding the CERAMENT|G-filled bone voids (created during revision surgery), was highly different between limited and extensive debridement. The bone lesions in Group B (limited debridement) were progressing, resulting in substantial bone loss. Signs of expansion of lesions in Group B included irregular contour of bone lesions, no sclerotic border, distant located bacteria, and high PBA values and collagen scores. In contrast, pathology and CT scan indicated that the bone voids created with successful extensive debridement and filled with CERAMENT|G (pigs C1 and C2) were contained/unchanged in the second postoperative period. Thus, the combination of extensive debridement and CERAMENT|G holds the remnant infection in an initial non-progressing state. However, in one Group C pig the bone void formed during revision surgery was connected to an unrevised bone lesion, thereby representing a limited debridement (artificial).
In a recent preclinical study, the effect of CERAMENT|G was evaluated in a murine fracture model of implant-related S. aureus osteitis.10 Lavage and debridement was performed before implantation of the product, and a final lavage was obtained again on the day of euthanization.10 Although based on lavage, and not bone tissue samples, all samples were positive for bacteria. Therefore, it was concluded that the application of the antimicrobial-impregnated material in an acute implant-associated infection did not cure the lesion.10 This corresponds to the findings of the present preclinical study. In accordance with our observations, biofilm formation in antimicrobial-loaded carriers has also been described in the clinic. In a paper by Stoodley et al,24 viable biofilm growing bacteria were demonstrated in tobramycin-impregnated bone cement from a patient with total joint arthroplasty. In the present study, it can be speculated that the bone tissue concentration of gentamicin obtained from CERAMENT|G was too low to eradicate remnants of mature biofilms left behind after debridement, thereby allowing the carrier product to be colonized. This is supported by the low gentamicin concentrations measured in the bone tissue surrounding CERAMENT|G. Another study based on a noninfectious rabbit model also demonstrated low gentamicin tissue concentrations released from gentamicin-loaded calcium sulphate.25 In both the rabbit model25 and the present porcine model, the total gentamicin concentrations were estimated based on homogenized bone samples. However, it is important to appreciate that only the unbound gentamicin concentrations from the interstitial fluid are considered to be microbiologically active. As with most local antimicrobials, the true in vivo pharmacokinetic behaviour of CERAMENT|G remains dubious. It is unknown whether gentamicin is actually able to penetrate into the peri-carrier-tissue in anti-biofilm efficient concentrations. The gentamicin concentration released from CERAMENT|G is highly dependent on local factors such as bleeding, suppuration, increased vascular permeability, increased blood flow, and exudation of plasma proteins.19 All these factors may contribute to an uncontrolled dilution of the gentamicin concentration, resulting in a fast gentamicin removal from the local site as seen in pig C3.
Several clinical studies support the use of antimicrobial-impregnated CS/HA as an on-site vehicle in the treatment of chronic osteomyelitis,8,9,11,12,26 but essentially combined with systemic antimicrobials and extensive debridement.9,12 The present study emphasizes that the bone tissue which envelopes the antimicrobial carriers can act as a reservoir for biofilms even after extensive debridement. In conclusion, CERAMENT|G cannot be used as an alternative to extensive debridement, i.e. CERAMENT|G should not be used in an effort to save bone tissue. Extensive debridement and application of CERAMENT|G reduced progression of infection and inflammation and, therefore, CERAMENT|G currently may be used as a complementary to systemic antimicrobial therapy and the immune system in eradication of bone infections.12
Author contributions
S. A. Blirup-Plum: Performed the animal experiments, Evaluated the histology, Collected the data, Wrote the manuscript.
T. Bjarnsholt: Sonicated the implants and FISH analyses, Edited the manuscript.
H. E. Jensen: Designed the study, Evaluated the histology, Edited the manuscript.
K. N. Kragh: Sonicated the implants and FISH analyses, Edited the manuscript.
B. Aalbæk: Prepared the inoculum, Performed the microbiological examinations, Edited the manuscript.
H. Gottlieb: Designed the study, Performed the experimental revision surgery, Edited the manuscript.
M. Bue: Designed the study, Performed the experimental revision surgery, Recorded the gentamicin measurements, Edited the manuscript.
L. K. Jensen: Designed the study, Performed the animal experiments, Wrote the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
S.A. Blirup-Plum and L. K. Jensen report institutional payments from BoneSupport AB to fund the animal experiments and supply the CERAMENT|G for the purposes of this study.
Acknowledgements
We thank Betina Andersen, Elizabeth Petersen, and Andreas Petersen for excellent laboratory assistance.
Ethical review statement
The experimental protocol was approved by The Danish Animal Experiments Inspectorate (license no. 2013/15-2934-00946).
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Montanaro L, Testoni F, Poggi A, et al. . Emerging pathogenetic mechanisms of the implant-related osteomyelitis by Staphylococcus aureus. Int J Artif Organs. 2011;34(9):781–788 [DOI] [PubMed] [Google Scholar]
- 2.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2008;52(1):13–22 [DOI] [PubMed] [Google Scholar]
- 3.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013;121(136):1–51 [DOI] [PubMed] [Google Scholar]
- 4.Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med. 2014;276(2):111–119 [DOI] [PubMed] [Google Scholar]
- 5.García-Gareta E, Davidson C, Levin A, Coathup MJ, Blunn GW. Biofilm formation in total hip arthroplasty: prevention and treatment. RSC Advances. 2016;6(83):80244–80261 [Google Scholar]
- 6.Ferguson J, Diefenbeck M, McNally M. Ceramic biocomposites as biodegradable antibiotic carriers in the treatment of bone infections. J Bone Jt Infect. 2017;2(1):38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metsemakers WJ, Fragomen AT, Moriarty TF, et al. . Evidence-based recommendations for local antimicrobial strategies and dead space management in fracture-related infection. J Orthop Trauma. 2020;34(1):18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stravinskas M, Horstmann P, Ferguson J, et al. . Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: in vitro and clinical release studies. Bone Joint Res. 2016;5(9):427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson J, Athanasou N, Diefenbeck M, McNally M. Radiographic and histological analysis of a synthetic bone graft substitute eluting gentamicin in the treatment of chronic osteomyelitis. J Bone Jt Infect. 2019;4(2):76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oezel L, Büren C, Scholz AO, Windolf J, Windolf CD. Effect of antibiotic infused calcium sulfate/hydroxyapatite (CAS/HA) insets on implant-associated osteitis in a femur fracture model in mice. PLoS One. 2019;14(3):e0213590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logoluso N, Drago L, Gallazzi E, et al. . Calcium-based, antibiotic-loaded bone substitute as an implant coating: a pilot clinical study. J Bone Jt Infect. 2016;1:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNally MA, Ferguson JY, Lau ACK, et al. . Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. Bone Joint J. 2016;98-B(9):1289–1296 [DOI] [PubMed] [Google Scholar]
- 13.Jensen LK, Koch J, Dich-Jorgensen K, et al. . Novel porcine model of implant-associated osteomyelitis: A comprehensive analysis of local, regional, and systemic response. J Orthop Res. 2017;35(10):2211–2221 [DOI] [PubMed] [Google Scholar]
- 14.Johansen LK, Frees D, Aalbaek B, et al. . A porcine model of acute, haematogenous, localized osteomyelitis due to Staphylococcus aureus: a pathomorphological study. APMIS. 2011;119(2):111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aalbæk B, Jensen LK, Jensen HE, Olsen JE, Christensen H. Whole-genome sequence of Staphylococcus aureus S54F9 isolated from a chronic disseminated porcine lung abscess and used in human infection models. Genome Announc. 2015;3(5):e01207–e01215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen LK, Henriksen NL, Bjarnsholt T, Kragh KN, Jensen HE. Combined staining techniques for demonstration of Staphylococcus aureus biofilm in routine histopathology. J Bone Jt Infect. 2018;3(1):27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morawietz L, Tiddens O, Mueller M, et al. . Twenty-three neutrophil granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology. 2009;54(7):847–853 [DOI] [PubMed] [Google Scholar]
- 18.Street JM, Souza ACP, Alvarez-Prats A, et al. . Automated quantification of renal fibrosis with Sirius Red and polarization contrast microscopy. Physiol Rep. 2014;2(7):e12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen LK, Bjarnsholt T, Kragh KN, et al. . In vivo gentamicin susceptibility test for prevention of bacterial biofilms in bone tissue and on implants. Antimicrob Agents Chemother. 2019;63(2):e01889–e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stegger M, Andersen PS, Kearns A, et al. . Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA(LGA251). Clin Microbiol Infect. 2012;18(4):395–400 [DOI] [PubMed] [Google Scholar]
- 21.Bay L, Kragh KN, Eickhardt SR, et al. . Bacterial aggregates establish at the edges of acute epidermal wounds. Adv Wound Care (New Rochelle). 2018;7(4):105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomassen MB, Hanberg P, Stilling M, et al. . Local concentrations of gentamicin obtained by microdialysis after a controlled application of a GentaColl sponge in a porcine model. J Orthop Res. 2020;38(8):1793–1799 [DOI] [PubMed] [Google Scholar]
- 23.Høiby N, Bjarnsholt T, Moser C, et al. . ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(suppl 1):S1–S25. [DOI] [PubMed] [Google Scholar]
- 24.Stoodley P, Nistico L, Johnson S, et al. . Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am. 2008;90-A(8):1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pförringer D, Obermeier A, Kiokekli M, et al. . Antimicrobial formulations of absorbable bone substitute materials as drug carriers based on calcium sulfate. Antimicrob Agents Chemother. 2016;60(7):3897–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgenstern M, Vallejo A, McNally MA, et al. . The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Joint Res. 2018;7(7):447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]