Abstract
AbstractTargeted therapy has gained mainstream attention with notable successes against specific genetic mutations in many cancers. One particular mutation, the BRAF V600E mutation, is present in a small subset of gliomas in adults. Although clinical experience and trial data of RAF-targeted therapy in adults with glioma are lacking at this time, the poor prognosis of adult high-grade glioma has led neuro-oncology practitioners to consider the use of targeted therapy in these patients. In this manuscript, we describe the use of RAF and MEK inhibitors in adults with recurrent glioma. We discuss the utility of these agents, describe their toxicities, and give examples of management strategies. Given the significant toxicities of RAF and MEK inhibitors, along with the long potential duration of treatment, neuro-oncology providers should counsel patients carefully before initiating therapy and monitor them closely while undergoing treatment with RAF-targeted therapy.
Keywords: adult, BRAF, glioma, management, MEK, RAF, toxicity
BRAF mutations have gained significant attention because of the success of FDA-approved drugs targeting the BRAF V600E mutation in melanoma and other cancers.1,2 Some neuro-oncology providers consider prescribing these agents off-label for patients with BRAF-mutated gliomas. The purpose of this case-based overview is to familiarize neuro-oncology providers with prescribing practices, side-effect management, and screening for serious adverse events for patients on BRAF-targeted therapy.
BRAF Mutations in Primary Brain Tumors
BRAF is a proto-oncogene that functions as a serine/threonine kinase, regulating the extracellular signal–regulated kinase (ERK) pathway. In physiologic ERK signaling, extracellular growth signaling pathways interact via H-, K-, or N-RAS leading to the activation of BRAF. Activated BRAF dimerizes and phosphorylates mitogen-activated protein kinase kinase (MEK1 or MEK2), thereby activating ERK1 or ERK2, and leading to cellular proliferation and growth. BRAF can be constitutively activated by point mutations removing the need for dimerization or by fusions with other kinases (eg, KIAA1549-BRAF).3,4 The BRAF V600E point mutation, in particular, is located within the kinase domain and allows constitutive activation by removing the inhibitory domain and necessity of dimerization for activity.5 This results in increased activation of the ERK signaling pathway, a critical regulator of cell division and differentiation. BRAF V600E mutations are found in many cancers, most commonly melanoma, papillary thyroid cancer, and colorectal cancers.3,4
BRAF V600E mutations are common in some brain tumors such as pleomorphic xanthoastrocytomas (60%) and pediatric gliomas (20%), and present at a low but measurable frequency in adult low-grade (5%-15%) and high-grade (3%) gliomas (HGG).6–8BRAF V600E is relatively enriched in the epithelioid subtype of glioblastoma.9 It is also found in gangliogliomas (20%-70%) and sporadic pilocytic astrocytomas (10%).6 Other mutations in the BRAF gene, as well as gene fusions, can also be present in brain tumors.4,10,11 Given the FDA-approved RAF inhibitors are efficacious only against tumors with the BRAF V600E mutation, this case-based overview will not discuss the function of or potential targeted treatments against other BRAF mutations.
Use of RAF and Mitogen-Activated Protein Kinase Kinase Inhibitors in Brain Tumors
Data guiding the use of RAF and MEK inhibitors (RAFi and MEKi, respectively) in brain tumors is limited, given the rarity of these tumors. An ongoing trial of the RAFi, dabrafenib, in pediatric patients with low-grade gliomas (LGG) showed a preliminary response rate of 72%.12 A trial of the RAFi, vemurafenib, in adults with glioma (n = 24) reported an overall response rate of 25%, though 5 out of 6 responders later progressed, supporting the necessity for dual inhibition as realized in melanoma.13 Preliminary results from a study of 37 patients with BRAF-mutated HGG treated with dabrafenib and trametinib combination therapy reported some positive responses.14 This is an area of active research with several ongoing clinical trials in adults evaluating the response of BRAF V600E–mutated HGG to combination treatment with RAFi/MEKi.
Currently there are 3 pairs of RAFi/MEKi approved by the FDA: vemurafenib and cobimetinib, dabrafenib and trametinib, and encorafenib and binimetinib. Side effects of the RAF and MEK inhibitors differ slightly when given as monotherapy (Table 1) and may be better tolerated in combination. The combination of dabrafenib and trametinib is best studied in brain tumors, though a clinical trial of encorafenib and binimetinib in recurrent HGG is in progress (NCT03973918). The appropriate dose of these drugs for optimal brain tumor penetration is unknown, so prescribers have used them at their FDA-approved dose in clinical trials and anecdotal case reports in adults with brain tumors.
Table 1.
Common and Serious Side Effects of BRAF and MEK Inhibitors
| BRAF Inhibitors | MEK Inhibitors | |||||
|---|---|---|---|---|---|---|
| Common side effects | Vemurafenib • Arthralgia • Rash • Alopecia • Fatigue • Photosensitivity reaction • Nausea • Pruritus • Skin papilloma • Elevations in AST/ALT | Dabrafenib • Hyperkeratosis • Headache • Pyrexia • Arthralgia • Papilloma • Alopecia • Palmar-plantar erythrodysesthesia | Encorafenib • Fatigue • Nausea • Vomiting • Abdominal pain • Arthralgia | Cobimetinib • Diarrhea • Photosensitivity reaction • Nausea • Pyrexia • Vomiting | Trametinib • Rash • Diarrhea • Lymphedema | Binimetinib • Fatigue • Nausea • Diarrhea • Vomiting • Abdominal pain |
| Serious side effects | Vemurafenib • QT prolongation • Uveitis • Primary cutaneous malignancies • Severe dermatologic reactions | Dabrafenib • Primary cutaneous malignancies • Hemorrhage • Cardiomyopathy • Uveitis | Encorafenib • Primary cutaneous malignancies • Hemorrhage • QT prolongation • Uveitis | Cobimetinib • Hemorrhage • Cardiomyopathy • Serous retinopathy and retinal vein occlusion • Elevations in AST/ALT • Severe dermatologic reactions • Rhabdomyolysis | Trametinib • Hemorrhage • Cardiomyopathy • Venous thromboembolism • Serous retinopathy and retinal vein occlusion | Binimetinib • Hemorrhage • Cardiomyopathy • Venous thromboembolism • Serous retinopathy and retinal vein occlusion • Interstitial lung disease • Rhabdomyolysis |
Abbreviation: MEK, mitogen-activated protein kinase kinase.
Although RAFi have effected remarkable tumor responses in some cancers with BRAF V600E mutations, resistance to RAFi frequently emerges following initial sensitivity, prompting the addition of a MEK inhibitor (MEKi) to therapy as another blockade point further downstream.15–18 Administering a MEKi after the emergence of resistance to a RAFi is minimally effective19; however, giving both drugs simultaneously improves survival in patients with melanoma and is now the standard of care.1,2 Even with combined RAFi/MEKi, emergent resistance is common in systemic cancers and glioma.20–23 The appropriate timing for therapy initiation in gliomas is unclear. Current practice in patients with HGG is to treat with standard-of-care therapy up front and to reserve RAFi/MEKi for recurrent disease. In LGG and pleomorphic xanthoastrocytoma, in which the disease course is more indolent and long-term side effects of radiation therapy may be more noticeable, some providers will give patients a trial of RAFi/MEKi in the first-line setting. The duration of therapy is also unclear, with some long-term responders progressing after treatment is stopped. The decision to use RAFi/MEKi therapy must be informed by detailed discussion of the potential side effects of daily targeted therapy and the risk for progression vs the long-term side effects of radiation or other therapies.
Given the rarity of these cases in routine neuro-oncology practice, we present several cases with typical treatment courses and adverse events.
Cases
Case 1: Pyrexia Due to Dabrafenib and Trametinib Combination Therapy
A 23-year-old woman was diagnosed with a glioblastoma (isocitrate dehydrogenase wild-type, BRAF V600E mutated) after developing right-sided numbness and weakness. She initially received concurrent radiation therapy and temozolomide, but the tumor recurred 6 years later, at which time she received another surgery with Gliadel (carmustine, Eisai, Inc.) placement in the tumor cavity. She experienced progression of her glioblastoma 14 months later and started dabrafenib 150 mg twice daily, followed by the addition of trametinib 2 mg once daily 2 weeks later. She developed grade 1 nausea (by Common Terminology Criteria for Adverse Events version 4.03) after starting dabrafenib that resolved within 3 weeks. Two weeks after starting trametinib, she developed grade 1 pyrexia, which progressed to grade 2 pyrexia the next week, despite daily treatment with acetaminophen. Her provider held dabrafenib and trametinib for 1 week, during which time the patient defervesced. The patient started 2 mg dexamethasone daily, and restarted the same doses of dabrafenib and trametinib, but again developed grade 2 pyrexia. She stopped treatment for 1 week and then she started dose-reduced dabrafenib (100 mg twice daily) and trametinib at 2 mg daily after defervescing, while continuing dexamethasone at 2 mg daily. She again developed grade 2 pyrexia, both drugs were held, and she defervesced. Her provider dose-reduced dabrafenib a second time to 50 mg twice daily and continued trametinib at 2 mg daily. The patient tolerated this well and stopped dexamethasone after a few months, remaining on dabrafenib 50 mg twice daily and trametinib 2 mg daily.
Case 2: Acneiform Rash Due to Dabrafenib/Trametinib Combination Therapy
A 22-year-old man was diagnosed with a pleomorphic xanthoastrocytoma (PXA; BRAF V600E mutated) and underwent a gross total resection. The tumor recurred radiographically 16 years later and progressed slowly over 3 years under closer surveillance, at which time it was partially resected and found to be an anaplastic PXA. Owing to the patient’s reluctance to undergo irradiation, he received dabrafenib and trametinib on a research study. In the first month of treatment he developed grade 1 pyrexia and chills, and grade 2 fatigue and malaise. These symptoms resolved within 6 to 8 weeks of treatment after 1 dose reduction of dabrafenib to 100 mg twice daily. At the initiation of treatment with dabrafenib and trametinib, the patient received prophylactic treatment with doxycycline 100 mg by mouth twice daily for rash prevention. He discontinued this after 4 weeks because of gastrointestinal distress. He then developed a grade 2 acneiform rash on his face, chest, back, and upper arms (25% body surface area). The patient administered clindamycin 1% solution and hydrocortisone 1% cream twice daily to affected body areas. The rash improved significantly within 2 weeks. The patient later decreased the frequency of cream application to clindamycin alone once daily and continued to experience adequate control of the rash.
Case 3: Severe Rash Due to Trametinib Therapy
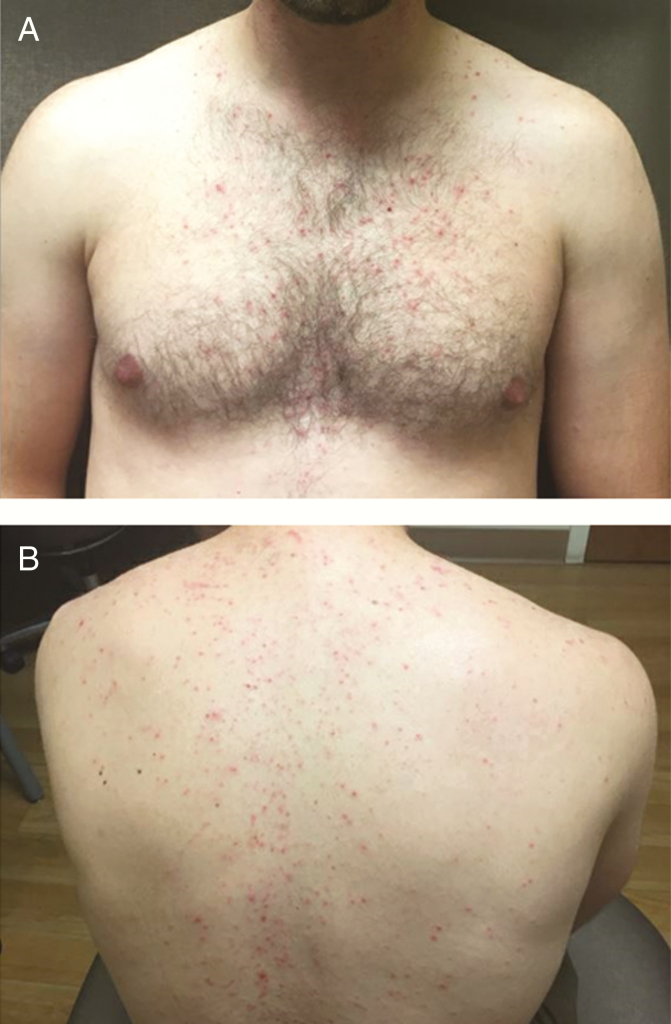
A 24-year-old man presented to an ophthalmologist for headaches and diplopia. MRI of the brain revealed a midline abnormality involving the right thalamus, midbrain, and tectum. Pathology was consistent with pilocytic astrocytoma (BRAF V600E mutated). He was managed with observation, but developed disease progression after 3 years and received concurrent radiation therapy and temozolomide. Three years later he again experienced a clinical and radiographic progression (development of a new fourth-nerve palsy). At this time he received trametinib 1 mg orally once daily for the first 9 doses, then increased to 2 mg orally once daily. After the increase to 2 mg daily, he developed pruritis without rash on his scalp and a pruritic maculopapular rash starting on his forehead then spreading to his nose, cheeks, neck, chest, and back (Fig. 1). Despite treatment with doxycycline 100 mg orally twice a day and clindamycin 1% gel topically applied twice a day, the rash progressed over the course of 1 week, then slowly improved over 1 month. He discontinued topical clindamycin after 6 months of trametinib, but continued doxycycline with good control of his symptoms.
Fig. 1.
Rash on the A, Front and B, Back of the Patient from Case 3 Treated With 2 mg Daily Trametinib
RAF/Mitogen-Activated Protein Kinase Kinase Inhibitor Toxicity
The toxicities of RAF- and MEK-targeted therapy are not insignificant. Vemurafenib was the first-approved second-generation RAFi and is used in combination with cobimetinib in patients with metastatic melanoma. In a study evaluating this combination compared to vemurafenib alone in BRAF-mutated melanoma patients, many adverse effects were mild to moderate and the regimen was well tolerated with mostly grade 1 and 2 toxicities.1 The most common toxicities with this combination include diarrhea, nausea, vomiting, retinopathy, elevated AST and ALT, increased creatinine phosphokinase (CPK), and dermatologic toxicities such as rash and photosensitivity, most of which occurred early in treatment and diminished over time.1,24 It is noteworthy that the photosensitivity associated with vemurafenib is ultraviolet A dependent and patient education on sun protection is advised.25 The following monitoring is recommended for vemurafenib plus cobimetinib: dermatologic evaluation, cardiac function tests, hepatic function tests, retinal evaluation, as well as routine serum chemistries and CPK (Table 2).26,27 Providers should advise patients to watch for signs and symptoms of skin toxicities, uveitis, and bleeding.
Table 2.
Monitoring Recommendations for BRAF and MEK Inhibitor Combinations
| Drug Names | Monitoring Parameter | Frequency of Monitoring |
|---|---|---|
| Vemurafenib (Zelboraf) and Cobimetinib (Cotellic) | Dermatologic evaluation | Baseline, every 2 mo during treatment and up to 6 mo after discontinuation |
| Electrocardiogram | Baseline, 15 d after initiation, monthly for 3 mo, then every 3 mo | |
| Cardiac function (left ventricular ejection fraction) | Baseline, 1 mo, and every 3 mo until discontinuation | |
| Basic metabolic panel | Baseline, then after dosage modifications and routinely during treatment | |
| Hepatic function and bilirubin | Baseline, then monthly | |
| Creatinine Phosphokinase | Baseline, then as clinically indicated | |
| Retinal evaluation | Baseline, then periodically during treatment and with visual disturbances | |
| Dabrafenib (Tafinlar) and Trametinib (Mekinist) | Dermatologic evaluation | Baseline, every 2 mo during treatment and up to 6 mo after discontinuation |
| Serum glucose | Routinely, particularly in patients with preexisting diabetes | |
| Cardiac function (left ventricular ejection fraction) | Baseline, 1 mo after initiation, then every 2 to 3 mo | |
| Blood pressure | Routinely during therapy | |
| Complete blood count | Baseline, then routinely during treatment | |
| Hepatic function tests | Baseline, then routinely during treatment | |
| Retinal evaluation | Periodically during treatment and with visual disturbances | |
| Encorafenib (Braftovi) and Binimetinib (Mektovi) | Dermatologic evaluation | Baseline, every 2 mo during treatment and up to 6 mo after discontinuation |
| Electrocardiogram | Baseline, then as clinically indicated | |
| Cardiac function (left ventricular ejection fraction) | Baseline, 1 mo, and every 2 to 3 mo during treatment | |
| Basic metabolic panel | Baseline, then routinely during treatment | |
| Hepatic function tests | Baseline, then monthly | |
| Creatinine phosphokinase | Baseline, then as clinically indicated |
Abbreviation: MEK, mitogen-activated protein kinase kinase.
Dabrafenib plus trametinib is the most common RAFi/MEKi regimen used in brain tumor patients, likely because of its relatively good efficacy in melanoma brain metastases.28 In a study comparing this combination to placebo in stage III melanoma, the following toxicities occurred in more than 20% of patients: pyrexia (63%), fatigue (47%), nausea (40%), headache (39%), chills (37%), diarrhea (33%), vomiting (28%), arthralgia (28%), and rash (24%).29 The most common grade 3 or 4 toxicities of combination therapy included pyrexia (5%), fatigue (4%), elevated ALT (4%) and AST (4%), and hypertension (6%), compared to less than 1% of these grade 3 or 4 toxicities in the placebo arm with all toxicities except for hypertension (2%). Recommended monitoring for dabrafenib plus trametinib includes the following: dermatologic evaluation, cardiac function tests, hepatic function tests, retinal evaluation, as well as routine blood pressure evaluation, complete blood count, and serum glucose (Table 2).30,31 Additionally, providers should observe patients for signs and symptoms of skin toxicities and secondary infections, uveitis, bleeding, hemolytic anemia, and lung toxicity.
The newest RAFi, encorafenib, is approved in combination with the MEKi, binimetinib, in the setting of advanced melanoma. The toxicity profile of this combination regimen was well tolerated in a phase 3 trial with the following grade 1 and 2 adverse effects seen in more than 20% of patients: gastrointestinal side effects (nausea, diarrhea, vomiting, constipation), fatigue, arthralgia, and headache.32 Increased CPK was also observed in 18% of cases, with some grade 3 (6%) or 4 (2%) toxicity. Encorafenib is unique from the previously discussed RAFi in that it has an increased dissociation half-life, high specificity, and higher potency.33 The increased specificity may result in improved tolerability and fewer side effects such as pyrexia and photosensitivity.34 Monitoring recommendations for this regimen include the following: dermatologic evaluation, cardiac function tests, hepatic function tests, serum chemistries, and CPK (Table 2).35,36 Providers should monitor patients for signs and symptoms of vision changes, bleeding, muscle pain or tenderness, skin changes, interstitial lung disease/pneumonitis, or venous thromboembolism.
Toxicity Management
The majority of patients treated with RAFi/MEKi combination develop mild (grade 1-2) toxicities. In general, side effects occur in 2 stages: either within a few days of initiating treatment, or after chronic exposure over several months. Patient education in the form of written documentation and instruction by a specialized nurse before initiating treatment are critical to support patients through the initial period. Although some providers consider starting the medications in a staggered fashion to facilitate symptom management in the initial phase (see cases 1 and 2), this is not necessary. Encorafenib, in particular, should not be started as monotherapy because it is better tolerated in combination with binimetinib, and it should be dose-reduced to 300 mg daily (from 450 mg daily) when binimetinib is held.32
There are several excellent published resources for management of specific toxicities due to RAFi/MEKi in other cancers.37,38 The general approach for mild symptoms is to provide supportive therapy in the form of supportive care and other pharmacologic agents to ameliorate symptoms (Table 3; see cases 2 and 3). For moderate or severe toxicities, treatment of the culprit drug should be interrupted until the reaction resolves (Table 3; see case 1). If it is unclear which drug is responsible, both drugs should be held. Dose reductions may be needed as described in cases 1 and 2, and standard dose reduction recommendations are available from manufacturers.26,27,30,31,35,36 Once mild or moderate toxicities resolve, patients may be able to reescalate therapy with proper prophylaxis in place. Side effects of some drugs, particularly trametinib and encorafenib, can be more profound if the companion RAFi or MEKi is held temporarily (Table 1). Providers should take this into account and may need to dose-reduce while the patient is on monotherapy, with resumption of the full dose on combination therapy again.
Table 3.
Management of Common Adverse Events Related to BRAF and MEK Inhibitors
| Adverse Event | Management Recommendations |
|---|---|
| Rash | Implement preventive measures when initiating therapy: avoid excessive sunlight, apply sunscreen daily, topical mild steroid (eg, hydrocortisone 1% cream) or topical antibiotic (eg, clindamycin cream) applied 2×/d. Consider oral antibiotics (eg, doxycycline 100 mg 2×/d or minocycline 100 mg 2×/d). If no improvement within 2 wk, consider holding MEKi until rash improves and then resuming at a reduced dose. |
| Diarrhea | Institute supportive care (dietary modification, hydration, loperamide). Continue BRAFi/MEKi for uncomplicated diarrhea, but consider holding both medications for grade ≥ 2 diarrhea that continues > 48 h, or complicated diarrhea. |
| Nausea/Vomiting | Promptly institute antiemetic measures. If AE is grade 1 to 2, can generally continue BRAFi/MEKi, but if higher grade should hold BRAFi and MEKi until symptoms improve. |
| Vision change | If AE is grade 1, continue drugs while obtaining ophthalmology consultation within 1 wk. If grade ≥ 2, obtain urgent consult and hold MEKi. Dose modification or discontinuation depends on diagnosis (uveitis, serous neuroretinal detachment, or retinal vein occlusion). |
| Fevers | Clinical evaluation and workup for infection. Implement antipyretics at first occurrence (acetaminophen, ibuprofen, etc). Hydration as required. Consider oral corticosteroids (eg, dexamethasone 2 mg for 5 d). Consider dose reduction of BRAFi. |
| Left ventricular function, decreased | Hold MEKi and reevaluate LVEF closely. Consider resuming MEKi at a reduced dose if LVEF improves; otherwise discontinue. |
| Liver enzyme elevation | Continue BRAFi/MEKi for asymptomatic patients with mild elevation and observe closely for improvement. If grade 3 to 4, hold BRAFi and MEKi, consider workup for other etiologies of liver injury, and resume drugs at a reduced dose if patient improves to grade ≤ 1. |
| Interstitial lung disease | For AE grade ≥ 2, hold MEKi while pursuing workup (consider chest CT, pulmonary function tests, infection workup, pulmonology consult). Consider symptomatic therapy with corticosteroids and resume MEKi at a reduced dose if AE improves to grade ≤ 1. |
Abbreviations: AE, adverse event; BRAFi, BRAF inhibitor; LVEF, left ventricular ejection fraction; MEK, mitogen-activated protein kinase kinase; MEKi, MEK inhibitor.
Conclusions
Given the very limited treatment options available for recurrent glioma in adult patients, RAF inhibitors are a powerful therapy being considered in patients whose tumors contain BRAF V600E mutations. Although this mutation is more common in pediatric glioma, it also occurs in adult patients, underscoring the importance of routine molecular testing for BRAF mutations. The data on RAF-targeted therapy in adults with glioma are limited, but neuro-oncologists are using off-label RAFi for patients with progressive/recurrent HGG. In some cases, patients with LGG are treated with a trial of targeted therapy rather than radiation in an attempt to delay the long-term toxicity of radiation therapy. Although RAF and MEK inhibitors can produce remarkable responses in some cases, they also have significant toxicities that warrant appropriate patient counseling and monitoring. Neuro-oncology providers need to understand the dosing, monitoring, and toxicity management for these drugs, and convey that information clearly to patients, to make an informed decision to treat with RAFi/MEKi-targeted therapy.
Funding
This work was supported by the Musella Foundation.
Conflict of interest statement. None declared.
Acknowledgments
The authors would like to acknowledge the patients who contributed to this study and to our understanding of the effects of RAFi and MEKi in glioma.
References
- 1. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. [DOI] [PubMed] [Google Scholar]
- 2. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. [DOI] [PubMed] [Google Scholar]
- 3. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. [DOI] [PubMed] [Google Scholar]
- 4. Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG.. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67(9):878–887. [DOI] [PubMed] [Google Scholar]
- 5. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N.. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464(7287):427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]
- 7. Dahiya S, Emnett RJ, Haydon DH, et al. BRAF-V600E mutation in pediatric and adult glioblastoma. Neuro Oncol. 2014;16(2):318–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Behling F, Barrantes-Freer A, Skardelly M, et al. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol. 2016;11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kleinschmidt-DeMasters BK, Aisner DL, Foreman NK. BRAF VE1 immunoreactivity patterns in epithelioid glioblastomas positive for BRAF V600E mutation. Am J Surg Pathol. 2015;39(4):528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pratt D, Camelo-Piragua S, McFadden K, et al. BRAF activating mutations involving the β3-αC loop in V600E-negative anaplastic pleomorphic xanthoastrocytoma. Acta Neuropathol Commun. 2018;6(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kieran MW, Bouffet E, Tabori U, et al. The first study of dabrafenib in pediatric patients with BRAF V600-mutant relapsed or refractory low-grade gliomas. Ann Oncol. 2016;27(suppl 6):vi552–vi587. [Google Scholar]
- 13. Kaley T, Touat M, Subbiah V, et al. BRAF inhibition in BRAF(V600)-mutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36(35):3477–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen P, Alexander S, Yung-Jue B, et al. Efficacy and safety of dabrafenib + trametinib in patients with recurrent/refractory BRAF V60E-mutated high-grade glioma (HGG). Neuro Oncol. 2018;20(suppl 6):vi238. [Google Scholar]
- 15. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. [DOI] [PubMed] [Google Scholar]
- 16. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chapman PB, Hauschild A, Robert C, et al. ; BRIM-3 Study Group Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chamberlain MC. Recurrent ganglioglioma in adults treated with BRAF inhibitors. CNS Oncol. 2016;5(1):27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31(4):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schreck KC, Guajardo A, Lin DDM, Eberhart CG, Grossman SA.. Concurrent BRAF/MEK inhibitors in BRAF V600-mutant high-grade primary brain tumors. J Natl Compr Canc Netw. 2018;16(4):343–347. [DOI] [PubMed] [Google Scholar]
- 21. Chamberlain MC. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J Neurooncol. 2013;114(2):237–240. [DOI] [PubMed] [Google Scholar]
- 22. Johanns TM, Ferguson CJ, Grierson PM, Dahiya S, Ansstas G.. Rapid clinical and radiographic response with combined dabrafenib and trametinib in adults with BRAF-mutated high-grade glioma. J Natl Compr Canc Netw. 2018;16(1):4–10. [DOI] [PubMed] [Google Scholar]
- 23. Yaeger R, Corcoran RB. Targeting alterations in the RAF-MEK pathway. Cancer Discov. 2019;9(3):329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dréno B, Ribas A, Larkin J, et al. Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann Oncol. 2017;28(5):1137–1144. [DOI] [PubMed] [Google Scholar]
- 25. Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemurafenib therapy. N Engl J Med. 2012;366(5):480–481. [DOI] [PubMed] [Google Scholar]
- 26. Zelboraf. Package insert. Genentech USA, Inc; 2017. [Google Scholar]
- 27. Cotellic. Package insert. Genentech USA, Inc; 2016. [Google Scholar]
- 28. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. [DOI] [PubMed] [Google Scholar]
- 30. Tafinlar. Package insert. Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 31. Mekinist. Package insert. Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 32. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(10):1315–1327. [DOI] [PubMed] [Google Scholar]
- 33. Sun J, Zager JS, Eroglu Z. Encorafenib/binimetinib for the treatment of BRAF-mutant advanced, unresectable, or metastatic melanoma: design, development, and potential place in therapy. Onco Targets Ther. 2018;11:9081–9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trojaniello C, Festino L, Vanella V, Ascierto PA.. Encorafenib in combination with binimetinib for unresectable or metastatic melanoma with BRAF mutations. Expert Rev Clin Pharmacol. 2019;12(3):259–266. [DOI] [PubMed] [Google Scholar]
- 35. Braftovi. Package insert. Array BioPharma Inc; 2019. [Google Scholar]
- 36. Mektovi. Package insert. Array BioPharma Inc; 2019. [Google Scholar]
- 37. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7(2):122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daud A, Tsai K. Management of treatment-related adverse events with agents targeting the MAPK pathway in patients with metastatic melanoma. Oncologist. 2017;22(7):823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]